Abstract
We investigated the sensitivity of embryonic murine neural stem cells exposed to 10 pM – 10 μM concentrations of three heavy metals (Cd, Hg, Pb), continuously for 14 days within 3D collagen hydrogels. Critical endpoints for neurogenesis such as survival, differentiation and neurite outgrowth were assessed. Results suggest significant compromise in cell viability within the first four days at concentrations ≥ 10 nM, while lower concentrations induced a more delayed effect. Mercury and lead suppressed neural differentiation at as low as 10 pM concentration within 7 days, while all three metals inhibited neural and glial differentiation by day 14. Neurite outgrowth remained unaffected at lower cadmium or mercury concentrations (≤ 100 pM), but was completely repressed beyond day 1 at higher concentrations. Higher metal concentrations (≥ 100 pM) suppressed NSC differentiation to motor or dopaminergic neurons. Cytokines and chemokines released by NSCs, and the sub-cellular mechanisms by which metals induce damage to NSCs have been quantified and correlated to phenotypic data. The observed degree of toxicity in NSC cultures is in the order: lead > mercury > cadmium. Results point to the use of biomimetic 3D culture models to screen the toxic effects of heavy metals during developmental stages, and investigate their underlying mechanistic pathways.
Keywords: Neurotoxicity, heavy metals, neural stem cells, 3D cultures, mechanistic pathways
1. Introduction
Environmental distribution, availability and bioaccumulation of heavy metals (ρ > 5 g/cm3) have been increasing rapidly after industrialization and agriculture revolution. While some of these metal ions (e.g., transition metals such as Fe, Zn, Cu) are essential for normal physiological metabolism in mammalian cells, a majority of them (e.g., Pb, Hg, Cd and As) are toxic even at minute concentrations (Matés et al., 2010). Since exposure to heavy metals cause oxidative stress which could affect the central nervous system (CNS) (Migliore and Coppedè, 2009), speculation of a strong correlation between elevated environmental levels of these metals and epidemiological increases in neurodegenerative disorders has been gaining momentum (Grandjean, 2013; Grandjean and Landrigan, 2006). An epidemiological review article published in 2006 identified lead, methylmercury and arsenic among the five industrial chemicals contributing to developmental neurotoxicity (Grandjean and Landrigan, 2006). These toxic metals can mimic the role of various biomolecules required for routine homeostatic cellular functions, gain access to important molecular targets (Rana, 2008), compromise CNS signaling pathways, and finally result in permanent neurological defects (Rice and Barone, 2000). Numerous studies documented that the developmental neurotoxicity of heavy metals in animal models and humans occur at concentrations much lower than that deemed “safe” (Grandjean et al., 2010; Landrigan and Goldman, 2011; Needleman, 2000). Furthermore, prenatal exposure to heavy metals often has more adverse developmental effects compared to postnatal exposure (Annau and Eccles, 1986; Gottlieb, 1983; Vorhees and Mollnow, 1987). For example, prenatal exposure to even low concentrations of lead, cadmium or mercury, as measured within amniotic fluid, has been shown to result in infant health deficiencies as well as impaired cognitive abilities (Lewis et al., 1992). Therefore, identifying the concentrations at which these heavy metals begin to induce damage in the CNS is critical to properly assess their role in the embryonic and fetal development. Furthermore, there has been a critical need to classify which among these environmental toxicants have a greater risk to human health, and therefore should be prioritized for further evaluation.
Given the permeability and vulnerability of the blood-brain barrier during fetal and infant stages (Needham et al., 2011; Zheng et al., 2003), numerous environmental toxicants could enter the developing CNS possibly via amino acid transporters to induce cellular and tissue damage (Ek et al., 2012). Neural stem cells (NSCs) are among such affected cells as they are highly sensitive to neurotoxicants compared to mature adult cells (Ceccatelli et al., 2010). NSCs are an important, self-renewing and multi-potent class of precursor cells in the embryonic, fetal and adult (e.g., subventricular zone, hippocampus) CNS, which can rapidly commit to specific neural and glial cell populations in response to various guidance cues (Gage, 2000; Pevny and Rao, 2003; Zhao et al., 2008). NSCs typically assume a quiescent phenotype until they receive exogenous signals from their microenvironment to proliferate, migrate or differentiate into a specific lineage (Clarke et al., 2000). For example, during rat embryogenesis, NSCs comprise almost half of the spinal neural tube cells, ~ 5-20% cell density within the telencephalon, and most of the cells within pre-migratory neural crest (Kalyani et al., 1998; Kilpatrick and Bartlett, 1993; Qian et al., 2000; White et al., 2001). NSC population declines dramatically (≤ 1%) post-birth, highlighting the need for critical maintenance of non-toxic conditions during embryonic development (Temple, 2001).
A few recent studies investigated the toxic effects of cadmium, mercury or lead on NSC and neural progenitor cell (NPC) viability, proliferation, migration, and differentiation into various neural and glial lineages (Bose et al., 2012; Buzanska et al., 2009; Cedrola et al., 2003; Chang et al., 2013; Gulisano et al., 2009; Guo et al., 2013; Huang and Schneider, 2004; Kermani et al., 2008; Moors et al., 2009; Tamm et al., 2006; Watanabe et al., 2013; Xu et al., 2010). Some of these reports also identified the mechanisms and signaling pathways underlying the metal ion induced toxicity, leading to apoptosis and cell death. In lieu of expensive and time-consuming in vivo experiments, these studies provided valuable information on the susceptibility of these specialized cells, isolated from rodent or human CNS, upon exposure to varying concentrations of metal ions. Such a reproducible in vitro culture model also helps in simplifying the complexity and cellular heterogeneity of the developing CNS, and identifies direct response of NSCs to toxic metal ions. However, in a majority of such studies, NSCs were cultured on 2D (adherent or suspension) substrates, and tested for acute metal ion toxicity (24 - 48 h exposure). In contrast, NSCs in vivo could be experiencing continuous exposure of a broad range of metal ion concentrations, over extended periods of time, within 3D extracellular matrix microenvironments (Grandjean and Landrigan, 2006, 2014). Thus, to better mimic such conditions (e.g., metal exposure by food uptake), the objective of this study is to investigate the standalone effects of cadmium, mercury or lead on embryonic rat NSCs, within a 3D culture system, under continuous exposure to a wide range of metal ion concentrations, and over longer culture periods. We hypothesize that even sub-nanomolar concentrations of metal ions might influence NSC survival, behavior and phenotype in a time-dependent manner. The 3D biomimetic NSC culture model employed here might present an alternative tool for heavy metal neurotoxicology studies. Specifically, we evaluated the influence of metal types and concentrations on NSC survival, differentiation into various specific neural and glial lineages, as well as the neurite outgrowth, at regular time intervals during two weeks. The results were correlated to the key mechanistic pathways involved and inflammatory markers released by NSCs under such culture conditions.
2. Materials and Methods
2.1. Preparation of metal solutions
Stock Solutions (1 mM) were prepared for each metal (Alfa Aesar; 99% pure) by dissolving calculated amount of specific metal salt (CdCl2, HgCl2, Pb(CH3CO2)2.3H2O) in ACS reagent grade water (TraceSELECT® Ultra, Sigma). Lower concentrations (10 μM - 10 pM) of these metal ions were prepared by serial-dilution of the stock solutions with NSC differentiating media. Although a few studies have tested the effects of organic forms of heavy metals, we tested their inorganic counterparts in this study to minimize experimental variables, and exclude complex interactions between leached organic moieties and cells.
2.2. NSC 3D culture
Two types of NSC media were prepared: one containing basic fibroblast growth factor (b-FGF; non-differentiating medium) and the other without b-FGF (differentiating medium). Differentiating medium contained 100× L-glutamate, 100× N2 Supplement, 100× B27 supplement and 100× N-acetyl cysteine in DMEM containing sodium pyruvate. Non-differentiating medium additionally contained 10 μg/mL b-FGF. Embryonic murine NSCs (Neuracell, NY) were seeded on poly-L-lysine -coated flasks for 5 days until the formation of neural rosettes, which were detached using trypsin-EDTA, suspended in differentiating medium, and seeded within a reconstituted 2 mg/mL collagen hydrogel (rat-tail derived type-I collagen; 9.46 mg/mL stock solution; BD Biosciences) at a density of 10,000 cells/100 μL. The differentiating medium containing various concentrations of metals ions was added to these hydrogels within respective culture wells. The metal-containing medium was replaced daily. Further details are available in supplementary methods.
2.3. Cell viability measurement
A LIVE/DEAD® viability/cytotoxicity kit (Life Technologies) was used to quantify the number of surviving cells within each culture condition (n = 6 wells/concentration/metal/time point) as we explained earlier (Gishto et al., 2014). Experimental details are available in supplementary methods. To produce a conventional sigmoidal dose-response curve, the green fluorescence intensities of cells were normalized to the fluorescence intensity of 100% live cells (e.g., control cells exposed to no heavy metal ion) and then plotted against the logarithm of metal ion concentrations. The sigmoidal dose-response curves (variable slope) and IC50 values for each metal ion tested were obtained using the following equation:
where IC50 is the midpoint of the curve, H is the hill slope, X is the logarithm of test concentration, and Y is the response (% live cells), starting at Bottom and going to Top with a sigmoid shape. The IC50 value represents the concentration of a compound at which cell growth is inhibited by 50%.
2.4. Quantification of NSC differentiation
At the end of 7 or 14 day cultures (n = 6 wells/concentration/metal/time point), cell cultures were processed for differentiation analysis, as we detailed elsewhere (Kothapalli and Kamm, 2013). At least 10 images were taken at random locations in each well, and the number of cells expressing TUJ1 (immature-neuronal-specific), GFAP (astrocyte-specific), MBP2 (oligodendrocyte-specific), HB9 (motor neuron-specific) and TH (dopaminergic neuron-specific) markers were counted (at least 300 cells per test case). Details are available in supplementary methods.
2.5. Neurite outgrowth measurement
NSCs cultured within 3D collagen hydrogels for varying durations (1, 4, 7 or 14 days; n = 6 wells/ concentration/ metal/ time point) were stained with Alexa Flour® 488 Phalloidin (Life Technologies) for imaging and quantifying neurite outgrowth, as we detailed (Kothapalli and Honarmandi, 2014; Kothapalli et al., 2011). Additional details are available in supplementary methods.
2.6. JC-1 mitochondrial membrane potential assay to assess mitochondrial function
Cell health in the presence of the three metal ions was assessed using a JC-1 mitochondrial membrane potential assay kit (Cayman Chemical Company, Ann Arbor, MI). NSCs were cultured in the presence (10 nM - 100 pM) or absence (0 μM; controls) of metal solutions for 14 days as described above (n = 6 wells/ concentration/ metal), and the cultures processed with JC-1 staining solution as per vendor’s protocols. Cells were imaged using the Zeiss Axiovert A1 florescence inverted microscope, with healthy cells detected using a Texas Red filter and apoptotic cells detected with a FITC filter. Details are available in supplementary methods.
2.7. Cytokines/ Chemokines release by NSCs
Cell culture supernatant (100 μL/metal/concentration) pooled over the entire 14-day culture duration was frozen at −20 °C and analyzed using an multiplexing laser bead assay (Mouse Cytokine/Chemokine Array 31-Plex, Eve Technologies, Alberta, Canada). The following analytes were targeted: Eotaxin, G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNFα, VEGF and LIX. The analyte amounts detected in a 100 μL aliquot was pro-rated to the total volumes of supernatant collected in each respective case.
2.8. Hoechst staining to assess DNA damage
At the end of 14 day cultures (n = 3 wells/concentration/metal), live cells were stained using Hoechst 33342 in water (10 mg/mL dilution), using protocols described by vendor. Nuclei were immediately imaged using a Zeiss Axiovert A1 inverted fluorescence microscope (40×, DAPI channel, at least 40 nuclei per case). Details are available in supplementary methods.
2.9. SYTOX® Green assay for quantifying compromise in cell membrane
Cell membrane permeability in the presence of heavy metal ions was assessed using SYTOX® Green nucleic acid stain (Life technologies). SYTOX® Green has a high-affinity for nucleic acid and penetrates only cells that have compromised plasma membranes, emitting a bright florescent signal. At the end of 14 days (n = 3 wells/concentration/metal), live cells were processed as per the vendor’s protocol, and at least 10 images were taken at random locations in each well using an inverted fluorescence microscope (Zeiss Axiovert A1). The percentage of cells with compromised membranes was calculated by dividing SYTOX® Green positive cells by total number of cells in region as calculated from the seeding density.
2.10. YO-PRO®-1 assay to identify apoptotic cells
YO-PRO®-1 (Life technologies) is a proprietary carbocyanine nucleic acid which specifically identifies apoptotic cells via nucleic acid binding. Apoptotic cells are permeant to YO-PRO®-1 but not to propidium iodide which stains dead cells. At the end of 14 day cultures (n = 3 wells/concentration /metal), live cells were exposed to the dye at 1 nM concentration, and incubated for 30 min (37°C, 5% CO2). Immediately post-incubation, at least 10 images were taken at random locations in each well using an inverted fluorescence microscope. The percentage of apoptotic cells was calculated in the same manner described previously.
2.11. Statistical analysis
All assays were repeated at least 3 times within three independent cultures (i.e., n = 18 wells/concentration/metal/time point). The results are expressed as mean ± SEM. Comparison between groups was performed by the non-parametric Bonferroni-corrected Mann-Whitney U test. The following notation was used to indicate statistical significance of data compared to controls: * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05. For the SYTOX Green®, YO-PRO®-1, and Hoechst 33342 staining assays, differences in data between various concentrations within the same metal type were deemed statistically significant for p < 0.05 and denoted by #.
3. Results
3.1. NSC survival
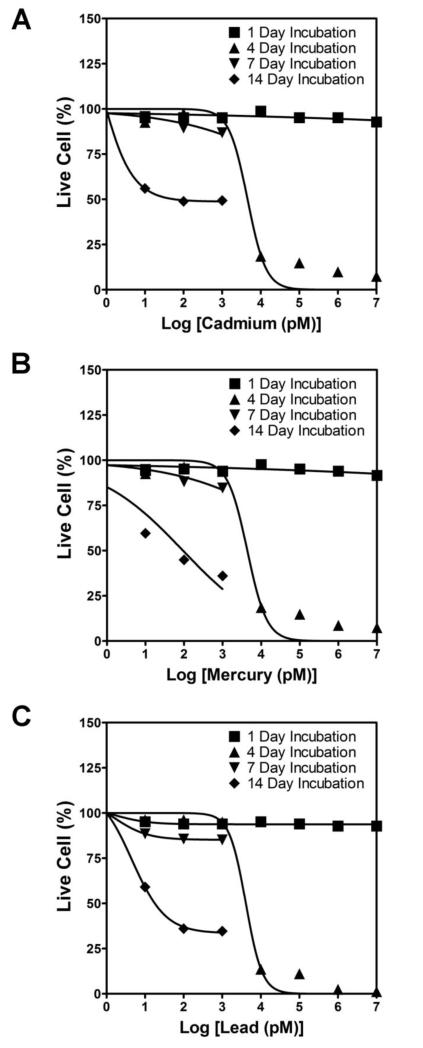
Embryonic murine NSC survival exposed to a wide range of metal concentrations was shown in Fig. 1. It could be seen that on day 1, there was no statistical difference between controls (0 M) and metal-supplemented cases at all concentrations, except at 10 μM concentration where a modest decrease in the cell survival compared to control was noted. By day 4, no significant differences were noted between controls and 10 pM to 1 nM metal supplemented cases, irrespective of the metal type. However, there was an overall dramatic decline in cell survival to less than 15% within 10 nM - 10 μM metal supplemented cultures, and with increasing concentration, for all the metals tested (p < 0.0001 vs. day 1 within respective metal types). Among the three metals, lead was more toxic at the same dosage level, compared to cadmium or mercury. The IC50 values for various culture conditions are shown in Table 1. These results suggest that higher levels (10 nM - 10 μM) of these three heavy metal ions are toxic to NSC survival even within a relatively short time frame (< 4 days).
Figure 1.
Respective dose-response curves calculated for embryonic rat NSCs exposed to broad ranges of cadmium chloride (A), mercury chloride (B), or lead acetate (C), over 1 - 14 days.
Table 1.
IC50 values (pM) of heavy metals tested on rat NSCs. N.T denotes nontoxic at the test concentrations.
| Heavy metal ions tested |
Incubation time with heavy metals | |||
|---|---|---|---|---|
| 1 day | 4 day | 7 day | 14 day | |
| Cadmium | N.T. | 4550 | 698 | 240 |
| Mercury | N.T. | 4370 | 330 | 90 |
| Lead | N.T. | 4090 | 574 | 60 |
On day 7, there was no statistically significant difference in cell survival between controls and 10 pM to 1 nM metal ion supplied NSC cultures. Compared to day 4, for all the three metals tested, a significant reduction in cell survival was noted at 100 pM and 1 nM concentrations. At higher concentrations (10 nM - 10 μM), no quantifiable cell survival was noted for all the three metal types. By day 14, cell survival within control cultures was lower compared to that on day 1 or day 4 (p < 0.001 for day 14 vs. day 1 or day 4). Compared to day 7, a significant decrease in cell viability was observed at day 14 with increasing metal concentration (p < 0.001), irrespective of the metal type. By day 14, lead seemed to adversely affect cell survival at as low as 100 pM concentration (p < 0.0001 vs. 10 pM). From these results, we conclude that the toxic effects of these heavy metals increased with increasing exposure time, and at any given time point, the cell viability is lower at higher metal concentrations.
3.2. NSC differentiation
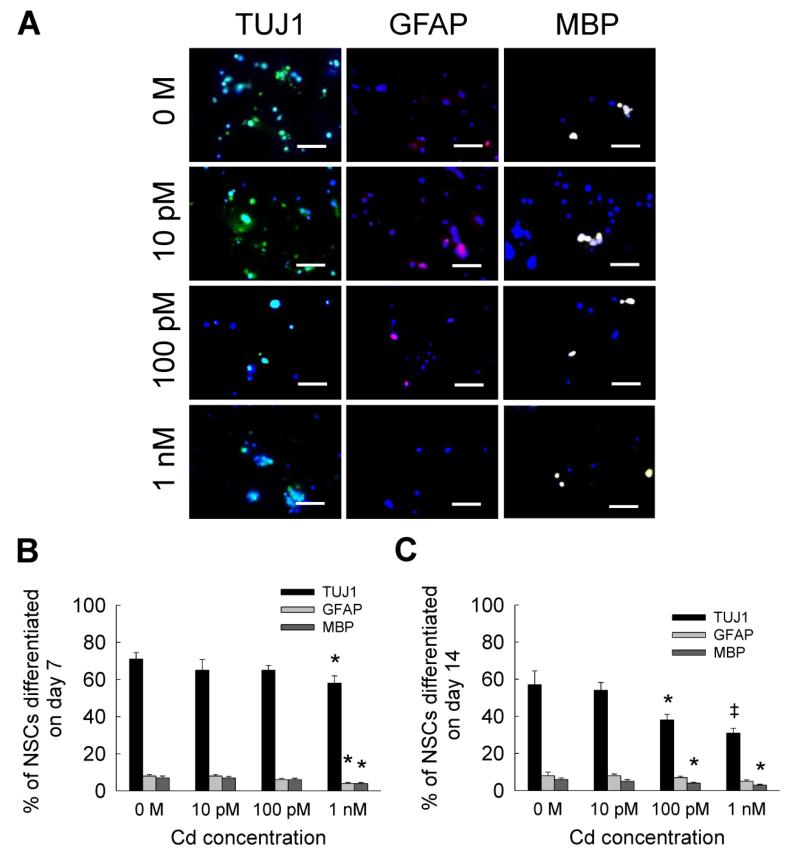
NSC differentiation studies were performed at relatively lower concentrations (10 pM - 1 nM) of metal ion solutions, as the cell viability decreased by more than 90% at concentrations higher than 10 nM. Representative immunofluorescence images for NSC differentiation into neural and glial lineages, on day 7, in the presence of low cadmium levels are shown in Fig. 2A. Quantitative data showed that by day 7, the expression of TUJ1 marker was not significantly different from controls at 10 pM and 100 pM, but it significantly decreased at 1 nM (Fig. 2B). Similarly, compared to controls, cell differentiation into glial lineages significantly decreased at 1 nM on day 7, but no difference was noted at lower cadmium concentrations. For reasons unclear at this stage, NSC differentiation to neural lineage was significantly lower at day 14 compared to that at day 7 at all concentrations tested (p < 0.01 for day 14 vs. day 7), although no such differences were noted within glial lineages (Fig. 2C). By day 14, compared to controls, differentiation to neuronal and oligodendrocyte lineages was found to be significantly lowered with the addition of 100 pM or 1 nM cadmium. In general, cadmium did not seem to significantly affect NSC differentiation to astrocyte lineage at the concentrations tested.
Figure 2.
(A) Representative immunofluorescence images of NSC cultures at day 7, differentiating into neural (TUJ1 staining) and glial (GFAP or MBP staining) lineages, in the presence of 10 pM to 1 nM cadmium chloride. Metal-free cultures served as controls. Cultures were counterstained with DAPI for cell identification. Scale bar: 50 μm. Blue dots indicate nuclei, green dots indicate neuron (TUJ1), pink dots indicate astrocyte (GFAP), and white dots indicate oligodendrocyte (MBP). Quantification of cells in each lineage was performed on day 7 (B) and day 14 (C). * indicates p < 0.05, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
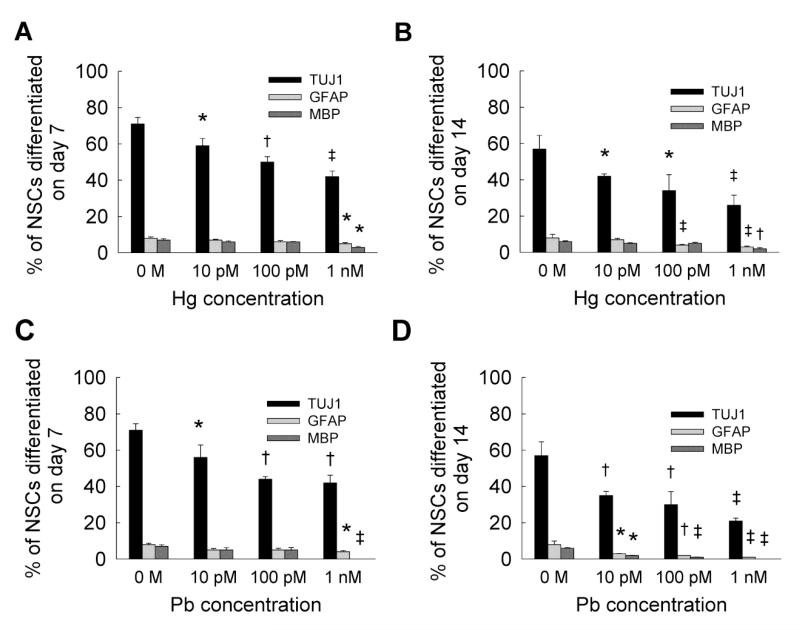
Quantitative data analysis of NSC differentiation within mercury or lead supplemented cultures (Fig. 3) suggests that TUJ1 expression was significantly affected at all the concentrations tested at both day 7 and day 14. Compared to controls, TUJ1 expression significantly decreased with time (p < 0.001 for day 14 vs. day 7) at all concentrations of mercury or lead. On the other hand, GFAP expression was suppressed only at 1 nM on day 7 (Fig. 3A), and at both 100 pM and 1 nM mercury concentrations at day 14 (Fig. 3B). Similarly, although MBP expression decreased on days 7 and 14 within 1 nM mercury-supplemented cultures, no significant differences were noted in glial lineage formation between days 7 and 14, at a given mercury concentration. Although GFAP and MBP expressions were significantly inhibited only at 1 nM lead concentration by day 7 (Fig. 3C), they were significantly suppressed in all the test cases by day 14 (Fig. 3D). However, no MBP staining was observed within 1 nM lead-supplemented cultures, at both day 7 and day 14. On day 7, the presence of mercury or lead significantly suppressed TUJ1 expression at 100 pM and 1 nM concentrations compared to cadmium. However, compared to cadmium, glial cell formation remained unchanged with the addition of mercury or lead, except for oligodendrocyte formation at 1 nM lead dosage. Among all the three metals tested, lead appeared to significantly inhibit neuronal and glial lineages formation (p < 0.01 for Cd vs. Pb; p < 0.01 for Hg vs. Pb, at all tested dosages).
Figure 3.
Quantified data from immunofluorescence images of NSC cultures, at day 7 (A) and day 14 (B), differentiating into neural (TUJ1 staining) and glial (GFAP or MBP staining) lineages, in the presence of 10 pM to 1 nM mercury chloride. Similarly, data was quantified from immunofluorescence images of NSC cultures, at day 7 (C) and day 14 (D), differentiating into neural (TUJ1 staining) and glial lineages, in the presence of 10 pM to 1 nM lead acetate. Metal-free cultures served as controls. * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
3.3. Average neurite outgrowth
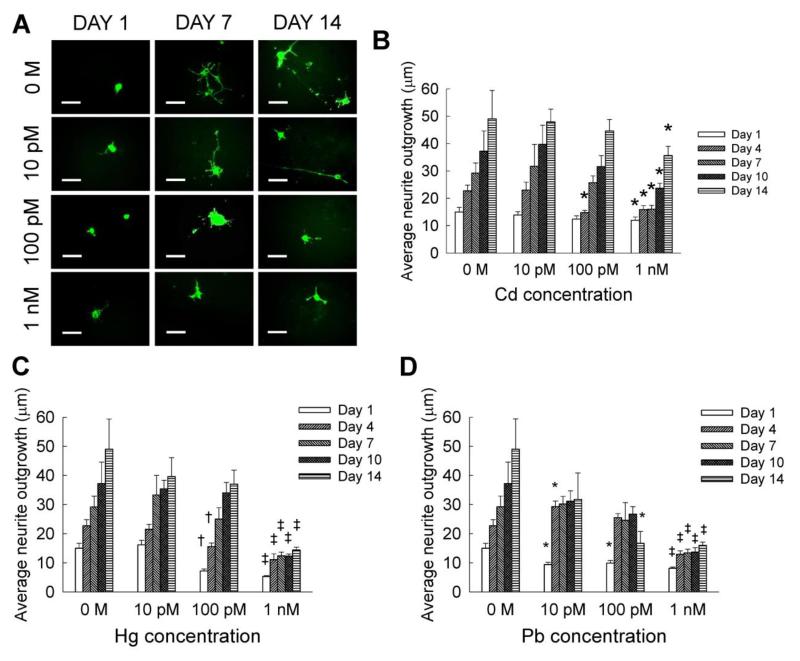
Figure 4A shows representative immunofluorescence images of neurite outgrowth within Cd-supplemented cultures, at selective time points. Quantifiable neurite outgrowth within controls and at lower cadmium concentrations was evinced at both days 7 and 14. Within control cultures, neurite outgrowth increased from day 1 to day 14, with significant increase in extension at every time point compared to earlier time points (Fig. 4B). On day 1, neurite outgrowth was not significantly different between controls and Cd-receiving cultures (Fig. 4B), at all the concentrations (10 nM - 10 μM). While 10 pM cadmium did not affect neurite outgrowth over the entire 14 day culture compared to controls, 100 pM inhibited neurite length only at day 4. However, at each time point, 1 nM cadmium significantly suppressed neurite outgrowth from day 4 to day 14, compared to controls or lower cadmium concentrations.
Figure 4.
(A) Representative immunofluorescence images of neurite outgrowth within NSC cultures, at 1, 7 and 14 day time points. Quantification of neurite outgrowth at each dosage of cadmium (B), mercury (C), and lead (D), performed at regular intervals over a 14-day culture. Cadmium chloride, mercury chloride and lead acetate were each added at 10 pM to 1 nM dosage. Metal-free cultures served as controls. Scale bar: 50 μm. * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
Similar to that noted within Cd-supplemented cultures, higher neurite outgrowth could be seen within cultures at lower mercury concentrations over the 14 days (Fig. 4C). On day 1, neurite outgrowth was significantly lower within 100 pM – 10 μM mercury-supplemented cultures compared to controls. A significant decrease in neurite outgrowth was recorded on days 1 and 4, when 100 pM mercury was added (p < 0.001 vs. 10 pM). In the presence of 1 nM mercury, neurite outgrowth was significantly suppressed on all the days, when compared to controls or lower concentrations. No measurable neurite outgrowth was observed within 10 nM – 10 μM mercury-treated cell cultures on day 4 and beyond. The average neurite outgrowth increased by 2.4 to 5.2 fold between days 1 and 14, within 10 pM – 1 nM Hg-supplemented cultures.
Interesting trends in neurite outgrowth were noted within lead-supplemented cultures, over the 14-day culture period (Fig. 4D). When 10 pM lead was added, neurite outgrowth significantly reduced by day 1 compared to controls, then almost tripled by day 4 (p < 0.01 vs. day 1), with no significant changes thereafter. When NSC cultures were exposed to 100 pM lead, neurite outgrowth significantly decreased by day 1 relative to controls, then increased 2.5-fold by day 4 (p < 0.01 vs. day 1), but significantly decreased between days 10 and 14 (p < 0.01 vs. day 4 within Pb-added cultures). In the presence of 1 nM lead, neurite outgrowth was significantly suppressed on all the days, when compared to controls or lower concentrations. The average neurite outgrowth within 10 nM – 10 μM lead supplemented cultures was between 8 – 12 μm. Similar to that noted within Cd- or Hg- additive cultures, no measurable neurite outgrowth was observed within 10 nM - 10 μM lead-treated cell cultures on day 4 and beyond. Nevertheless, the average neurite outgrowth increased by 1.7 to 3.4 fold between days 1 and 14, within 10 pM to 1 nM lead-additive cultures. In general, neurite outgrowth within 10 pM - 1 nM lead- and mercury-supplemented cultures on day 14 was significantly lower than that within Cd-exposed cultures.
3.4. Differentiation into motor neurons
NSCs were cultured for 7 or 14 days in the presence or absence of heavy metal ions, and stained for HB9 marker, which specifically identifies the presence of motor neurons in the neural cell population. In general, lower than 3% of the cells stained for HB9 at day 7 within controls, while 7% or fewer cells stained by day 14. On day 7, compared to controls, mercury and lead inhibited motor neuron formation even at 10 pM concentration (Fig. 5B, C), while cadmium had no significant effect (Fig. 5A). NSC differentiation into motor neuron lineage significantly decreased to less than 1% at 100 pM concentration by day 7, for all the three metals studied (p < 0.03 vs. 10 pM). Within 1 nM additive cultures for all the three metal ions, no HB9 positive cells were detected by day 7. On the other hand, 10 pM concentration of cadmium or mercury had no significant effect on motor neuron differentiation at day 14, compared to respective controls. However, exposure to lead modestly inhibited motor neuron differentiation even at concentrations as low as 10 pM (Fig. 5C). At 100 pM to 1 nM levels, all the three metal ions significantly suppressed NSC differentiation into motor neuron lineage by day 14, relative to controls. It should be noted that motor neuron formation by day 14 is significantly higher than that by day 7 (p < 0.01), for these three metals at all the concentrations tested. Taken together, results show that NSC differentiation to motor neuron lineage could be affected upon exposure to heavy metal ions, even at as low as 1 nM concentration.
Figure 5.
NSC differentiation into motor neuron lineage (HB9 staining), at day 7 and day 14, in the presence of cadmium chloride (A), mercury chloride (B), or lead acetate (C), at 10 pM to 1 nM dosages. Metal-free cultures served as controls at each time point. * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
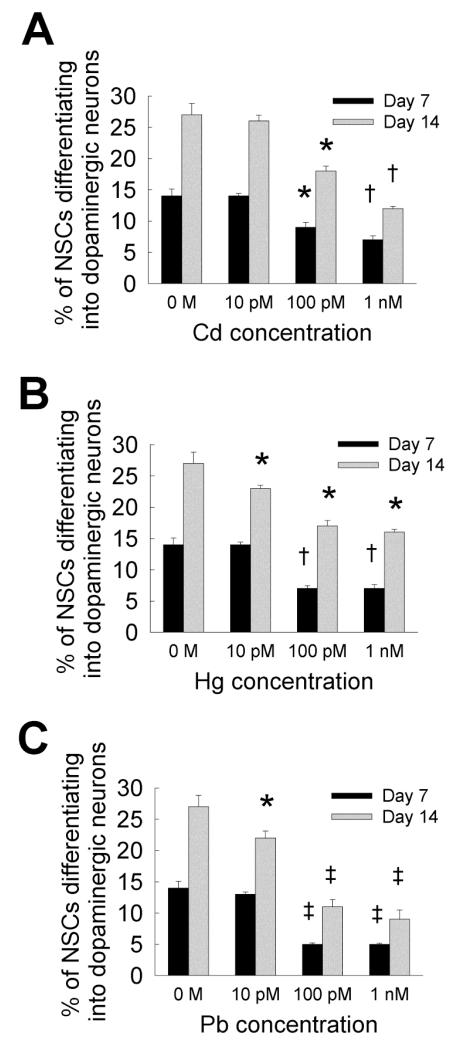
3.5. Differentiation into dopaminergic neurons
NSCs were cultured for 7 or 14 days in the presence or absence of metal ions, and stained for tyrosine hydroxylase (TH) marker, specific for identifying the presence of dopaminergic neurons in the neural cell population. NSC differentiation into dopaminergic neurons increased from day 7 to day 14 (Fig. 6), for the three metals at all the concentrations. While dopaminergic neuron formation was not affected at 10 pM dose, significant decrease at 100 pM and 1 nM concentrations of all the three metals was noted by day 7 compared to control. Exposure to lead significantly inhibited dopaminergic neuron formation compared to cadmium or mercury, at all concentrations. By day 14, mercury and lead suppressed dopaminergic neuron formation even at 10 pM concentration, and all the three metals significantly inhibited dopaminergic neuron formation at higher concentrations (≥ 100 pM), compared to controls as well as 10 pM concentration within respective cases. It is worth noting that the percentage of NSCs differentiating into dopaminergic neurons is significantly higher than that committing to motor neurons, within each metal type (Cd, Hg, and Pb), at all concentrations tested (≤ 1 nM).
Figure 6.
(A) NSC differentiation into dopaminergic neuron lineage (TH staining), at day 7 and day 14, in the presence of cadmium chloride (A), mercury chloride (B), or lead acetate (C), at 10 pM to 1 nM. Metal-free cultures served as controls at each time point. * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
3.6. Mechanisms of action
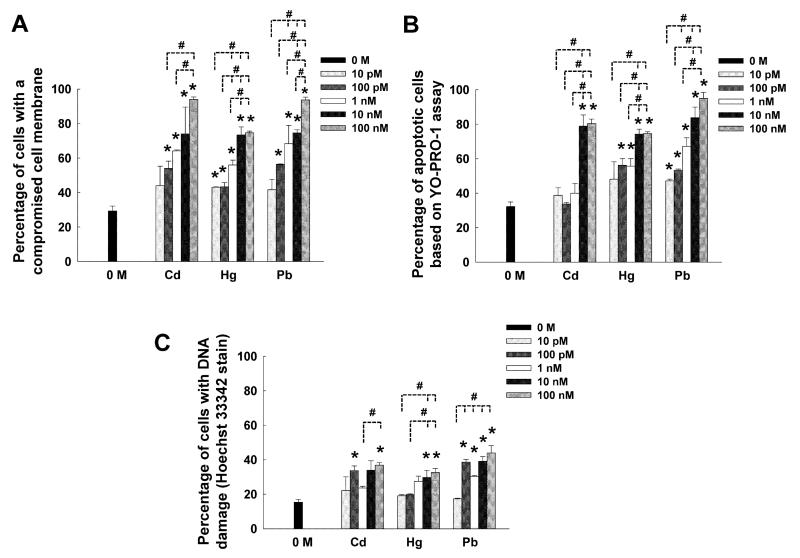
Results so far attest to the deleterious effects of heavy metal ions (highly-dependent on the type and concentration) on NSC phenotype and functionality, and how such effects could be studied using 3D culture models. To elucidate the underlying sub-cellular mechanisms as to how these changes are manifested, we used qualitative and quantitative assays. Figure 7A shows representative immunofluorescence images of these cultures on day 14, when processed using JC-1 mitochondrial membrane potential assay to assess mitochondrial function. While the presence of intact mitochondrial membranes (red florescence) was pronounced within control cultures, progressive transformation to green florescence (disrupted mitochondria) was evident with increasing concentration of each metal from 100 pM to 10 nM. Furthermore, compared to cadmium and mercury, exposure to lead appeared to promote significant compromise in mitochondrial membrane, even at as low as 100 pM concentration. These results were quantified based on co-localization of DAPI and red/green florescence (Fig. 7B). Compared to controls, cell density with compromised mitochondrial function significantly increased upon exposure to mercury and lead at 100 pM concentration, and all the three metals at 1 nM or 10 nM concentrations (p < 0.001 for 10 nM vs. 1 nM or 100 pM).
Figure 7.
(A) Representative immunofluorescence images of NSC cultures processed with JC-1 mitochondrial membrane potential assay kit to identify cells (green) with disrupted mitochondria. Cell cultures were treated with lead, mercury or cadmium at 100 pM to 10 nM for 14 days. High density of healthy cells (red channel) could be seen within metal-free controls. Scale bar: 200 μm. (B) Quantification of cells with disrupted membranes within these cultures. * indicates p < 0.05, † indicates p < 0.01, ‡ indicates p < 0.001, and no notation for p > 0.05, compared to controls.
Results from SYTOX® Green assay suggests that exposure to cadmium, mercury or lead at concentrations higher than 10 pM resulted in a compromise in cell membrane, compared to that noted in controls (significance denoted by *, Fig. 8A). Higher concentrations significantly increased the number of cells with disrupted membrane compared to lower concentrations (significance denoted by #, Fig. 8A), irrespective of the metal type. At 100 nM concentration, almost all the cells within cadmium or lead exposed cultures exhibited compromised cell membrane, significantly higher than that noted under mercury exposure. Similar results were noted from the YO-PRO-1 assay, which identifies apoptotic cells (Fig. 8B). Evidently, at concentrations beyond 10 nM for cadmium, 100 pM for mercury, and 10 pM for lead, significantly higher apoptotic cell density was observed compared to controls. Irrespective of the metal type tested, at least 80% of the cells were undergoing apoptosis-induced cell death at concentrations beyond 10 nM. Finally, cells with damaged DNA were quantified using Hoechst 33342 stain (Fig. 8C). While lower concentrations (≤ 1 nM) of Cd and Hg did not significantly damage cellular DNA (except 100 pM Cd) relative to controls, dosages higher than 10 nM appeared detrimental to maintenance of healthy DNA. However, lead seemed to affect DNA even at as low as 100 pM concentration. In all the three metal cases, the concentration-dependent affects seem to be attenuated beyond 10 pM.
Figure 8.
Quantification of mechanisms by which various concentrations (10 pM – 100 nM) of heavy metal ions were influencing NSC cultures, over the 14-day culture period. (A) The percentage of cells with a compromised membrane was quantified using SYTOX® Green assay. (B) The percentage of cells committed to an apoptotic pathway was quantified using a YOPRO® assay. (C) Hoechst 33342 staining was used to quantify the cells with damaged DNA. Outcomes from each assay were compared to that noted within controls (0 M). * indicates p < 0.05 vs. controls. Differences in data between various concentrations within the same metal type was deemed statistically significant for p < 0.05 and denoted by #.
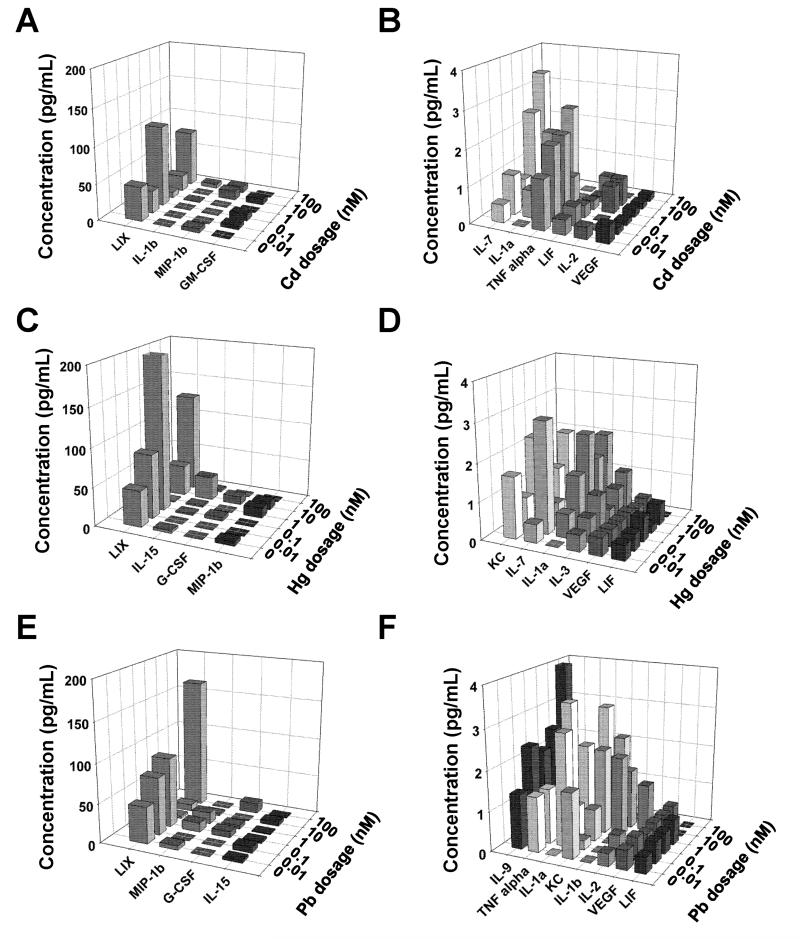
3.7. Release of cytokines/ chemokines
Figure 9 shows the cytokines and chemokines released by cells in the presence of various concentrations of cadmium (panels A, B), mercury (panels C, D) and lead (panels E, F). Panels A, C and E depict the analytes detected at relatively higher levels (≥ 5 pg/mL), while panels B, D and F show markers released at ≤ 5 pg/mL. Analytes that did not produce any significant signal were not shown here. It could be seen that the types and amounts of analytes released varied from metal to metal, and highly-dependent on the concentration of each metal. It was noted that TNF-α was released only in the presence of Cd and Pb but not Hg, and LIX (i.e., CXCL5) seems to have been released in significantly higher amounts than all the other analytes. VEGF release remained unaffected even in the presence of metal ions, and lead seemed to be stimulating the release of larger number and higher amounts of analytes compared to the other two metals.
Figure 9.
Amounts of various cytokines/ chemokines within NSC cultures exposed to cadmium, mercury or lead were quantified using a multiplexing laser bead assay (Mouse Cytokine/Chemokine Array 31-Plex). Respective supernatants (100 μL/metal/ concentrations) pooled over the entire 14-day culture duration was analyzed and compared to metal-free controls. Metal dosage was varied between 0.01 – 100 nM. Panels A, C and E depict the analytes detected at relatively higher levels (≥ 5 pg/mL), while panels B, D and F show markers released at ≤ 5 pg/mL.
4. Discussion
Post-embryogenesis, residual NSCs in the CNS (e.g., sub-ventricular zone, hippocampus, spinal cord) not only help in further development of nervous system after birth and during childhood, but also aid in limited repair post-injury or disease in the CNS. Thus, protecting the developmental cues that modulate survival and functionality of NSCs from environmental toxicants is crucial to maintain homeostasis and continued normal functioning of CNS. Although this study was designed to specifically investigate the effect of heavy metal ions on NSCs during differentiation process, our future studies will elucidate how these metal ions influence NSC behavior, pre- and post- differentiation. Results in the current study on embryonic murine NSC cultures within 3D collagen gels revealed that cell survival was quickly affected (within 1 - 4 days) at metal concentrations ≥ 10 nM, in agreement with that reported in prior studies. Thus, NSC exposure to inorganic metal complexes at lower concentrations (≤ 1 nM) induces a more delayed effect on NSC survival in vitro, usually by day 7 and beyond, for all the three metals tested.
Due to the complexities associated with delineating toxicological mechanistic pathways in vivo, simple 2D cultures have long been utilized to categorize the specific nature and effect of environmental toxicants (Järup, 2003; Järup and Akesson, 2009; Peraza et al., 1998). However, recent studies suggest that cells cultured as conventional 2D monolayers differ markedly in their morphology, physiology, protein/gene expression, and metabolism from cells cultured in a 3D configuration (Haycock, 2011; Lee et al., 2008; Pampaloni et al., 2007; Shamir and Ewald, 2014). Such 2D cultures inhibit formation of tissue-like structures by limiting intercellular contacts and integrin-mediated interaction with substrates, leading to loss of their phenotypic properties. To overcome some of these limitations and provide more physiological resemblance to neural cells in neurotoxicity studies, 3D cell cultures in hydrogels, polymeric scaffolds, and in 3D hanging droplets have been gaining traction.
Similar metal-induced cytotoxic effects have been reported by Culbreth et al. (Culbreth et al., 2012), who observed human NPC viability to reduce by 50% in the presence of cadmium, and mouse NSC viability to decrease by 50% with exposure to cadmium or CH3Hg (1 μM - 100 mM). Gulisano et al. noted that 10 μM CdCl2 stimulated human fetal olfactory neuroepithelium-derived neuroblast growth, while 100 μM CdCl2 reduced cell viability and induced apoptosis (Gulisano et al., 2009). Such Cd-induced neuronal apoptosis has been shown to proceed via the activation of mitogen-activated protein kinases (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways, inhibition of which rescued cells from Cd-induced death (Chen et al., 2008). CdCl2 and CH3HgCl has been shown to inhibit human umbilical cord blood derived NSC survival at as low as 10 nM concentration, with CdCl2 being relatively more toxic (Buzanska et al., 2009). On the other hand, Xu et al. observed that the survival of rat embryonic cerebral cortex derived NPCs remained unaffected within 2.5 - 50 nM concentration of CH3Hg, but started to drastically decrease beyond 500 nM dosage (Xu et al., 2010). At lower concentrations (2.5 nM or 5 nM), CH3Hg did not influence rat embryonic cortical NSC viability but reduced cell proliferation (Bose et al., 2012). Furthermore, primary embryonic rat cortical NSCs were more susceptible to toxic effects of CH3Hg (0.1 - 0.5 μM) compared to a murine-derived multipotent NSC cell line (Tamm et al., 2006). In similar studies, 0.01 - 100 μM lead has been shown to inhibit embryonic rat brain derived NSC proliferation, but not their viability (Huang and Schneider, 2004). Moreover, such effects were pronounced on striatum- or ventral mesencephalon- derived NSCs, but not on cortex-derived NSCs.
Epidemiological studies have shown that 0.01 – 0.22 μM levels of cadmium in blood induces neurotoxicity whereas 5.3 nM is non-toxic, and 2–11 μM levels of methylmercury chloride in blood is neurotoxic whereas ≤ 0.4 μM is non-toxic (Buzanska et al., 2009). Thus, it could be deduced that NSC (or NPC) viability is highly-dependent on the metal form (organic vs. inorganic) and types and concentrations, duration of exposure, species of origin, and region of the brain from which they are isolated.
Our study shows that dose-dependent Cd-induced inhibition of NSC differentiation into neural and glial lineages proceeds slowly over time (> 7 days), with relatively higher adverse impact on neural lineage formation. Unfortunately, there is a paucity of literature on the effects of cadmium on NSC differentiation in vitro. In one recent study, Gulisano et al. have shown that CdCl2 (10 or 100 μM) enhanced mRNA and protein expression of GFAP and TUJ1 within human fetal olfactory neuroepithelial neuroblast cultures, and promoted differentiation into neuron and astrocyte lineages, in a dose-dependent manner (Gulisano et al., 2009). Interestingly, cell proliferation decreased dramatically with increasing concentration, and some of these cells simultaneously expressed both neuronal and astrocyte markers when treated with CdCl2.
Depending on the type of bio-functional substrate on which they were cultured, human umbilical cord blood derived NSCs have shown a considerable susceptibility in their differentiation to neural and glial lineages when exposed to CH3HgCl (0.0625 - 1 μM; 48 h) (Zychowicz et al., 2014). Similarly, 1 μM CH3Hg was shown to inhibit retinoic acid induced neural differentiation within human NSCs over a 48 h culture period, via induction of caspase-dependent apoptosis (Chang et al., 2013). Single doses of CH3Hg (2.5 or 5 nM) drastically inhibited neuronal differentiation of primary embryonic rat cortical NSCs (bFGF omission, 120 h culture) (Tamm et al., 2006), compared to controls under similar conditions. Huang et al. have shown that 0.01 - 10 μM lead significantly inhibited neuronal lineage formation within NSCs originating from striatum and ventral mesencephalon regions of embryonic rat brain, but not cortex (Huang and Schneider, 2004). Interestingly, the authors found that oligodendrocyte differentiation decreased whereas astrocyte formation increased regardless of the NSC origin, with increasing lead concentration. Our current study shows that mercury and lead, at as low as 10 pM - 1 nM concentrations, significantly suppressed neuronal differentiation from rat embryonic NSCs within 7 days of culture. Such neurogenesis inhibition further increased by day 14, with additional suppression of glial cell differentiation, compared to controls.
Quantification of neurite (axons and dendrites) outgrowth is one of the widely used endpoints to assess the role of toxicants on neurodevelopment in vitro (Carmeliet, 2003). In our study, no measurable neurite outgrowth was observed within 10 nM - 10 μM Cd-exposed cell cultures from day 4 and beyond. This suggests that at higher concentrations, heavy metal ions not only contribute to cell death and compromise neuronal differentiation, but also inhibit neurite outgrowth of the surviving cells. However, the molecular mechanism by which this neurite outgrowth inhibition is modulated within NSCs needs further investigation. The average neurite outgrowth increased by 2.9 to 3.4 –fold between days 1 and 14, within 10 pM to 1 nM Cd-supplemented cultures, suggesting that NSCs may be undergoing some intracellular compensatory mechanism to overcome the toxic effects over a period of time (Johansson et al., 2007; Pramanik et al., 2001; Xu et al., 2010), although this speculation needs further investigation.
Although 10 pM cadmium and mercury did not significantly affect neurite growth, 100 pM concentration of these two metals decreased neurite growth initially within the first four days, after which neurite outgrowth was restored to the values noted in controls. However, all the three metals appeared to influence the process of neurite outgrowth at 1 nM concentration and beyond. Thus, in cell cultures with heavy metal ions added in media, the degree of toxicity is in the order: lead > mercury > cadmium.
While the influence of cadmium, mercury or lead on neurite outgrowth within NSC cultures remains categorically unexplored, numerous earlier studies investigated their effect in mature neuronal cultures (Audesirk et al., 1989; Cline et al., 1996; Davidovics and DiCicco-Bloom, 2005; Pramanik et al., 2001; Radio et al., 2008; Radio and Mundy, 2008; Schneider et al., 2003; Spoerri et al., 1990; Sugawara et al., 1983) and assessed the role of these environmental toxicants on neuritogenesis in vitro under a broad range of culture conditions. In the current study, we note that neurite outgrowth was completely inhibited beyond day 1 within NSC cultures at higher metal concentrations (≥ 10 nM), irrespective of the metal type (Pb, Cd, Hg), and neurite outgrowth steadily increased over time at lower Cd or Hg concentrations (≤ 100 pM). However, neurite outgrowth remained arrested beyond day 4 in the presence of low concentrations of lead.
Exposure to cadmium, mercury or lead in utero has been linked to poor motor functions in children (Bonithon-Kopp et al., 1986; Ceccatelli et al., 2010; Grandjean and Landrigan, 2006; Schmidt, 1999) and in animal models (Chow et al., 2008). Numerous studies attest to the unique capability of mature motor neurons to uptake and retain mercury in their cell bodies in vivo (Arvidson, 1990; Pamphlett and Waley, 1996). Exposure to mercury, even at moderate levels, has been shown to impair motor skills in adult humans. However, it remained unexplored as to how these metal ions specifically influence NSC differentiation into motor neuron lineage. We here show that exposing NSCs to these three metal ions, even at as low as 100 pM, induced a significant suppression of their ability to differentiate into motor neuron lineage by as early as day 7.
The effects of mercury ions on neurodevelopment in vitro and in vivo have been well documented. Specifically, low concentrations of CH3HgCl has been shown to induce changes in dopamine release, degenerate dopaminergic neurons, subdue expression of neurotrophic factors necessary for dopaminergic neuron formation and maturation, suppress dopamine transport activity, induce cell death, suppress neurite outgrowth, induce cytoskeletal alteration and cell shrinkage, and promote formation of nuclei with chromatin condensation (Dare et al., 2003; Gimenez-Llort et al., 2001; Götz et al., 2002; Zimmer et al., 2011). On the other hand, survival of rat E15 primary ventral mesencephalic dopaminergic neurons in vitro was significantly affected only at higher concentrations of lead acetate (1 or 10 μM), although their neurite outgrowth was compromised even at very low concentrations (1 nM) (Schneider et al., 2003). Nevertheless, the role of these heavy metal ions on dopaminergic neuron differentiation from NSCs received less attention.
Our results show that NSC survival, differentiation and neurite outgrowth are strongly modulated by their exposure to heavy metals, over a wide range of concentrations. This led us to wonder which sub-cellular organelles were affected and compromised in the presence of metal ions, leading to the manifestation of these outcomes. Literature suggests that disruption of active mitochondria and changes in mitochondrial membrane potential, DNA damage, and compromise in cell membrane are some factors that could influence cellular phenotype, and might act as indicators of the early stages of apoptosis. Results from our assays conclusively suggest that 10 pM – 1 nM concentrations of these metals begin to mildly affect cellular organelles, and higher concentrations (≥ 1 nM) are significantly more detrimental and lead to cell death, possibly via apoptotic pathway. These outcomes also explain the compromise in neurite outgrowth and neuronal/glial differentiation noted within NSC cultures, at these metal concentrations. It is possible that multiple cellular organelles could have been affected simultaneously, upon exposure to metal ions, leading to the observed changes in cellular phenotype. Further studies are needed to investigate the genetic level changes within these cells, upon exposure to a larger variety and dosage of heavy metal ion concentrations.
Methylmercury chloride has been shown to induce apoptosis within human umbilical cord blood derived NSCs at concentrations greater than 0.05 μM (Buzanska et al., 2009). On the other hand, methylmercury induced caspase-dependent apoptosis and autophagy in human NSCs within 48 h exposure at 1 μM dosage, via inhibition of Akt1/mTOR signaling (Chang et al., 2013). Although cadmium, methylmercury or lead did not activate caspase 3 or p53 (indicators of apoptosis) in human fetal cortex neuroprogenitor cells, exposure to cadmium or methylmercury doubled apoptotic cells within embryonic mouse cortical NSCs (Culbreth et al., 2012). Tamm et al. have shown that at least 0.25 μM of methylmercury was required to induce significant apoptosis (~15%) within murine NSCs, while much lower dosages (≥ 0.025 μM) were sufficient to induce similar levels of apoptosis within embryonic rat cortical NSCs (Tamm et al., 2006). Our results are in agreement with these studies, and suggest that metal ion concentrations as low as 10 pM are sufficient to induce significant alterations in embryonic NSC phenotype and mechanistic pathways.
Although how NPCs respond to various cytokines and chemokines is now known, the release of such molecules by these cells has received less attention. Human NPCs have been shown to express a variety of cytokines, although not all of them are released by murine NPCs (Klassen et al., 2003). Here, we quantified the various types of cytokines and chemokines released by murine NSCs, either stand-alone or in the presence of various concentrations of heavy metal ions. Interestingly, it was noted that among the cytokines and chemokines expressed in high levels by these cells in the presence of metal ions, LIX and MIP-1β are chemotactic chemokines, and GM-CSF is a major chemo-attractant cytokine. These markers were released in higher amounts in the presence of all the three metal ions tested. In contrast to our expectations that heavy metals might induce a large inflammatory response as part of neurotoxicity, the expression levels of inflammatory cytokines such as TNF-α, ILs and IFN-γ are quite low, albeit higher than that in controls. It is possible that some of these analytes such as LIX (CXCL5) might have been released as a result of downstream signaling or secondary effects induced by select cytokines and chemokines (e.g., ILs, TNF-α) primarily released in response to metal ion exposure (Chandrasekar et al., 2003). We hypothesize that the release of such markers could be dependent on the developmental stages, and might be predominant at more advanced stages of CNS maturation (Camarillo et al., 2007).
5. Conclusions
Investigating the influence of environmental pollutants on NSCs during developmental stages is necessary to formulate guidelines for regulating systemic exposure levels of such toxicants in pregnant women, neonates, and children, and to assess their effects on pediatric neurodevelopmental disorders. This could be partially accomplished by developing scalable NSC-based in vitro assays to overcome the financially-prohibitive and time-consuming testing of environmental toxicants on developmental neurotoxicity in vivo. Although our study is not comprehensive, such results in the long run would allow us to (a) associate observations on time- and dose- dependent cellular responses to a wide-range of toxicant types and exposure levels, (b) decipher the mechanistic effects on key signal transduction pathways, (c) correlate data from in vitro studies to in vivo and epidemiological studies from literature, (d) propose limits on exposure levels of these toxicants, (e) test neurotoxicity of selective high-risk pollutants in vivo, and finally (f) help formulate effective prevention strategies.
Supplementary Material
Highlights.
Toxic effects of Cd, Hg and Pb on embryonic rat NSCs investigated.
NSCs exposed to metals within 3D collagen gels, continuously for 14 days.
Exposure duration and metal dose influenced NSC survival, differentiation and neurite outgrowth.
Observed degree of toxicity is in the order: lead > mercury > cadmium.
Mechanisms by which metal ions affect cell phenotype investigated.
Acknowledgements
Financial support from Cleveland State University Startup and the 2012 - 2013 Faculty Research Development Award to Chandra Kothapalli is appreciated. Research reported in this study was partially supported by National Institute of Environmental Health Sciences of the National Institutes of Health under award number R01ES025779 to Drs. Lee and Kothapalli. Kurt Farrell is grateful for funding from the Cellular and Molecular Medicine Specialization Fellowship as well as the Doctoral Dissertation Research award from CSU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No competing financial interests exist.
References
- Annau Z, Eccles CU. Prenatal exposure. Johns Hopkins University Press; Baltimore, MD: 1986. [Google Scholar]
- Arvidson B. Accumulation of mercury in brainstem nuclei of mice after retrograde axonal transport. Acta Neurol Scand. 1990;82:234–237. doi: 10.1111/j.1600-0404.1990.tb01612.x. [DOI] [PubMed] [Google Scholar]
- Audesirk G, Shugarts D, Nelson G, Przekwas J. Organic and inorganic lead inhibit neurite growth in vertebrate and invertebrate neurons in culture. In Vitro Cell Dev Biol. 1989;25:1121–1128. doi: 10.1007/BF02621263. [DOI] [PubMed] [Google Scholar]
- Bonithon-Kopp C, Huel G, Moreau T, Wendling R. Prenatal exposure to lead and cadmium and psychomotor development of the child at 6 years. Neurobehav Toxicol Teratol. 1986;8:307–310. [PubMed] [Google Scholar]
- Bose R, Onishchenko N, Edoff K, Janson Lang AM, Ceccatelli S. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol Sci. 2012;130:383–390. doi: 10.1093/toxsci/kfs257. [DOI] [PubMed] [Google Scholar]
- Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J, Coecke S. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27:2591–2601. doi: 10.1002/stem.179. [DOI] [PubMed] [Google Scholar]
- Camarillo C, Kumar LS, Bake S, Sohrabji F, Miranda RC. Ethanol regulates angiogenic cytokines during neural development: evidence from an in vitro model of mitogen-withdrawal-induced cerebral cortical neuroepithelial differentiation. Alcohol Clin Exp Res. 2007;31:324–335. doi: 10.1111/j.1530-0277.2006.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Daré E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact. 2010;188:301–308. doi: 10.1016/j.cbi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Cedrola S, Guzzi G, Ferrari D, Gritti A, Vescovi AL, Pendergrass JC, La Porta CA. Inorganic mercury changes the fate of murine CNS stem cells. FASEB J. 2003;17:869–871. doi: 10.1096/fj.02-0491fje. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Sarau HM, Raveendran M, Perla RP, Marelli-Berg FM, Dulin NO, Singh IS. Chemokine-cytokine cross-talk. The ELR+ CXC chemokine LIX (CXCL5) amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase-NF-kappa B pathway. J Biol Chem. 2003;278:4675–4686. doi: 10.1074/jbc.M207006200. [DOI] [PubMed] [Google Scholar]
- Chang SH, Lee HJ, Kang B, Yu KN, Minai-Tehrani A, Lee S, Kim SU, Cho MH. Methylmercury induces caspase-dependent apoptosis and autophagy in human neural stem cells. J Toxicol Sci. 2013;38:823–831. doi: 10.2131/jts.38.823. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J Neurochem. 2008;105:251–261. doi: 10.1111/j.1471-4159.2007.05133.x. [DOI] [PubMed] [Google Scholar]
- Chow ES, Hui MN, Lin CC, Cheng SH. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat Toxicol. 2008;87:157–169. doi: 10.1016/j.aquatox.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H, Lendahl U, Frisén J. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Cline HT, Witte S, Jones KW. Low lead levels stunt neuronal growth in a reversible manner. Proc Natl Acad Sci U S A. 1996;93:9915–9920. doi: 10.1073/pnas.93.18.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth ME, Harrill JA, Freudenrich TM, Mundy WR, Shafer TJ. Comparison of chemical-induced changes in proliferation and apoptosis in human and mouse neuroprogenitor cells. Neurotoxicology. 2012;33:1499–1510. doi: 10.1016/j.neuro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Dare E, Fetissov S, Hokfelt T, Hall H, Ogren SO, Ceccatelli S. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:500–508. doi: 10.1007/s00210-003-0716-5. [DOI] [PubMed] [Google Scholar]
- Davidovics Z, DiCicco-Bloom E. Moderate lead exposure elicits neurotrophic effects in cerebral cortical precursor cells in culture. J Neurosci Res. 2005;80:817–825. doi: 10.1002/jnr.20539. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Ahlbom E, Dare E, Vahter M, Ogren S, Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol. 2001;9:61–70. doi: 10.1016/s1382-6689(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Gishto A, Farrell K, Kothapalli CR. Tuning composition and architecture of biomimetic scaffolds for enhanced homing and matrix synthesis by cardiomyocytes. J Biomed Mater Res Part A. 2014;103:693–708. doi: 10.1002/jbm.a.35217. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. The sychobiological approach to developmental issues. John Wiley and Sons; New York, NY: 1983. [Google Scholar]
- Götz ME, Koutsilieri E, Riederer P, Ceccatelli S, Daré E. Methylmercury induces neurite degeneration in primary culture of mouse dopaminergic mesencephalic cells. J Neural Transm. 2002;109:597–605. doi: 10.1007/s007020200049. [DOI] [PubMed] [Google Scholar]
- Grandjean P. Only one chance. How environmental pollution impairs brain development – and how to protect the brains of the next generation. Oxford University Press; New York: 2013. [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulisano M, Pacini S, Punzi T, Morucci G, Quagliata S, Delfino G, Sarchielli E, Marini M, Vannelli GB. Cadmium modulates proliferation and differentiation of human neuroblasts. J Neurosci Res. 2009;87:228–237. doi: 10.1002/jnr.21830. [DOI] [PubMed] [Google Scholar]
- Guo BQ, Yan CH, Cai SZ, Yuan XB, Shen XM. Low level prenatal exposure to methylmercury disrupts neuronal migration in the developing rat cerebral cortex. Toxicology. 2013;304:57–68. doi: 10.1016/j.tox.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Haycock JW. 3D cell culture: a review of current approaches and techniques. Methods Mol Biol. 2011;695:1–15. doi: 10.1007/978-1-60761-984-0_1. [DOI] [PubMed] [Google Scholar]
- Huang F, Schneider JS. Effects of lead exposure on proliferation and differentiation of neural stem cells derived from different regions of embryonic rat brain. Neurotoxicology. 2004;25:1001–1012. doi: 10.1016/j.neuro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Johansson C, Castoldi AF, Onishchenko N, Manzo L, Vahter M, Ceccatelli S. Neurobehavioural and molecular changes induced by methylmercury exposure during development. Neurotox Res. 2007;11:241–260. doi: 10.1007/BF03033570. [DOI] [PubMed] [Google Scholar]
- Kalyani AJ, Piper D, Mujtaba T, Lucero MT, Rao MS. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermani S, Karbalaie K, Madani SH, Jahangirnejad AA, Eslaminejad MB, Nasr-Esfahani MH, Baharvand H. Effect of lead on proliferation and neural differentiation of mouse bone marrow-mesenchymal stem cells. Toxicol In Vitro. 2008;22:995–1001. doi: 10.1016/j.tiv.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10:255–265. doi: 10.1016/0896-6273(93)90316-j. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Imfeld KL, Kirov II, Tai L, Gage FH, Young MJ, Berman MA. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22:101–106. doi: 10.1016/s1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Honarmandi P. Theoretical and experimental quantification of the role of diffusive chemogradients on neuritogenesis within three-dimensional collagen scaffolds. Acta Biomater. 2014;10:3664–3674. doi: 10.1016/j.actbio.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Kamm RD. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials. 2013;34:5995–6007. doi: 10.1016/j.biomaterials.2013.04.042. [DOI] [PubMed] [Google Scholar]
- Kothapalli CR, Van Veen JE, de Valence S, Chung S, Zervantonakis IK, Gertler FB, Kamm RD. A high-throughput microfluidic assay to study axonal response to growth factor gradients. Lab Chip. 2011;11:497–507. doi: 10.1039/c0lc00240b. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Goldman LR. Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011;30:842–850. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- Lewis M, Worobey J, Ramsay DS, McCormack MK. Prenatal exposure to heavy metals: effect on childhood cognitive skills and health status. Pediatrics. 1992;89:1010–1015. [PubMed] [Google Scholar]
- Matés JM, Segura JA, Alonso FJ, Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. 2010;49:1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E. Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect. 2009;117:1131–1138. doi: 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DGJ, Sjödin A, Turner WE, Weihe P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000;84:20–35. doi: 10.1006/enrs.2000.4069. [DOI] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Waley P. Motor neuron uptake of low dose inorganic mercury. J Neurol Sci. 1996;135:63–67. doi: 10.1016/0022-510x(95)00258-4. [DOI] [PubMed] [Google Scholar]
- Peraza MA, Ayala-Fierro F, Barber DS, Casarez E, Rael LT. Effects of micronutrients on metal toxicity. Environ Health Perspect. 1998;106:203–216. doi: 10.1289/ehp.98106s1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Rao MS. The stem-cell menagerie. TINS. 2003;26:351–359. doi: 10.1016/S0166-2236(03)00169-3. [DOI] [PubMed] [Google Scholar]
- Pramanik R, Ishido M, Kunimoto M. Effects of cadmium chloride on neurite outgrowth and gene expression in human neuroblastoma NB-1 cells. J Health Sci. 2001;47:478–482. [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Radio NM, Breier JM, Shafer TJ, Mundy WR. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol Sci. 2008;105:106–118. doi: 10.1093/toxsci/kfn114. [DOI] [PubMed] [Google Scholar]
- Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–376. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Rana SV. Metals and apoptosis: recent developments. J Trace Elem Med Biol. 2008;22:262–284. doi: 10.1016/j.jtemb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone SJ. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW. Poisoning young minds. Environ Health Perspectives. 1999;107:A302–A307. doi: 10.1289/ehp.99107a302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Huang FN, Vemuri MC. Effects of low-level lead exposure on cell survival and neurite length in primary mesencephalic cultures. Neurotoxicol Teratol. 2003;25:555–559. doi: 10.1016/s0892-0362(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Shamir ER, Ewald AJ. Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol. 2014;15:647–664. doi: 10.1038/nrm3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerri PE, Dozier AK, Roisen FJ. Calcium regulation of neuronal differentiation: the role of calcium in GM1-mediated neuritogenesis. Brain Res Dev Brain Res. 1990;56:177–188. doi: 10.1016/0165-3806(90)90080-i. [DOI] [PubMed] [Google Scholar]
- Sugawara N, Aoshima K, Kasuya M. Effect of cadmium chloride and Cdmetallothionein on the nervous tissue culture. Toxicol Lett. 1983;16:95–101. doi: 10.1016/0378-4274(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Mollnow E. John Wiley and sons; New York, NY: 1987. Behvaioral teratogensis: long-term influences on behavior from early exposure to environmental agents. [Google Scholar]
- Watanabe J, Nakamachi T, Ohtaki H, Naganuma A, Shioda S, Nakajo S. Low dose of methylmercury (MeHg) exposure induces caspase mediated-apoptosis in cultured neural progenitor cells. J Toxicol Sci. 2013;38:931–935. doi: 10.2131/jts.38.931. [DOI] [PubMed] [Google Scholar]
- White PM, Morrison SJ, Orimoto K, Kubu CJ, Verdi JM, Anderson DJ. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 2001;29:57–71. doi: 10.1016/s0896-6273(01)00180-5. [DOI] [PubMed] [Google Scholar]
- Xu M, Yan C, Tian Y, Yuan X, Shen X. Effects of low level of methylmercury on proliferation of cortical progenitor cells. Brain Res. 2010;1359:272–280. doi: 10.1016/j.brainres.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Schildknecht S, Kuegler PB, Tanavde V, Kadereit S, Leist M. Sensitivity of dopaminergic neuron differentiation from stem cells to chronic low-dose methylmercury exposure. Toxicol Sci. 2011;121:357–367. doi: 10.1093/toxsci/kfr054. [DOI] [PubMed] [Google Scholar]
- Zychowicz M, Dziedzicka D, Mehn D, Kozlowska H, Kinsner-Ovaskainen A, Stȩpień PP, Rossi F, Buzanska L. Developmental stage dependent neural stem cells sensitivity to methylmercury chloride on different biofunctional surfaces. Toxicol In Vitro. 2014;28:76–87. doi: 10.1016/j.tiv.2013.06.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.