Abstract
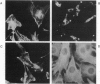
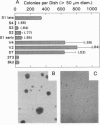
Synthesis of certain members of the tropomyosin family of microfilament-associated proteins is suppressed in fibroblasts neoplastically transformed by a number of retroviral oncogenes, by transforming growth factor alpha, and by chemical mutagens. To test whether tropomyosin suppression is a required event in neoplastic transformation, expression of one of two suppressed tropomyosins in NIH 3T3 mouse cells transformed by the ras oncogene was restored by retrovirally mediated cDNA transfer. Cells expressing the inserted cDNA showed partial restoration of microfilament bundle formation (which is typically deranged in transformed cells) together with increased cytoplasmic spreading. More importantly, they lost anchorage-independent growth capability, and the onset of tumor growth in athymic mice was delayed. When tumors arose they no longer expressed the inserted cDNA. These observations support the conclusion that tropomyosin suppression is a necessary event for the expression of components of the transformed phenotype, particularly with respect to anchorage-independent growth and tumorigenesis, which correlate closely with neoplastic potential. This potentially reversible requirement may link different initial events produced by a variety of oncogenic modalities to a common pathway leading to neoplastic growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auersperg N., Roskelley C. Retroviral oncogenes: interrelationships between neoplastic transformation and cell differentiation. Crit Rev Oncog. 1991;2(2):125–160. [PubMed] [Google Scholar]
- Bhattacharya B., Ciardiello F., Salomon D. S., Cooper H. L. Disordered metabolism of microfilament proteins, tropomyosin and actin, in mouse mammary epithelial cells expressing the Ha-ras oncogene. Oncogene Res. 1988;3(1):51–65. [PubMed] [Google Scholar]
- Bhattacharya B., Prasad G. L., Valverius E. M., Salomon D. S., Cooper H. L. Tropomyosins of human mammary epithelial cells: consistent defects of expression in mammary carcinoma cell lines. Cancer Res. 1990 Apr 1;50(7):2105–2112. [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Cohen I., Cohen C. A tropomyosin-like protein from human platelets. J Mol Biol. 1972 Jul 21;68(2):383–387. doi: 10.1016/0022-2836(72)90220-3. [DOI] [PubMed] [Google Scholar]
- Cooper H. L., Bhattacharya B., Bassin R. H., Salomon D. S. Suppression of synthesis and utilization of tropomyosin in mouse and rat fibroblasts by transforming growth factor alpha: a pathway in oncogene action. Cancer Res. 1987 Aug 15;47(16):4493–4500. [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. L., Park M. H., Folk J. E. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell. 1982 Jul;29(3):791–797. doi: 10.1016/0092-8674(82)90441-x. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factors-alpha and -beta and their potential roles in neoplastic transformation. Cancer Treat Res. 1989;47:177–195. doi: 10.1007/978-1-4613-1599-5_8. [DOI] [PubMed] [Google Scholar]
- Field J. K., Spandidos D. A. The role of ras and myc oncogenes in human solid tumours and their relevance in diagnosis and prognosis (review). Anticancer Res. 1990 Jan-Feb;10(1):1–22. [PubMed] [Google Scholar]
- Glück U., Kwiatkowski D. J., Ben-Ze'ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with alpha-actinin cDNA. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafström R. C. Carcinogenesis studies in human epithelial tissues and cells in vitro: emphasis on serum-free culture conditions and transformation studies. Acta Physiol Scand Suppl. 1990;592:93–133. [PubMed] [Google Scholar]
- Helfman D. M., Cheley S., Kuismanen E., Finn L. A., Yamawaki-Kataoka Y. Nonmuscle and muscle tropomyosin isoforms are expressed from a single gene by alternative RNA splicing and polyadenylation. Mol Cell Biol. 1986 Nov;6(11):3582–3595. doi: 10.1128/mcb.6.11.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Hunter T. The functions of oncogene products. Prog Clin Biol Res. 1989;288:25–34. [PubMed] [Google Scholar]
- Jancsó A., Graceffa P. Smooth muscle tropomyosin coiled-coil dimers. Subunit composition, assembly, and end-to-end interaction. J Biol Chem. 1991 Mar 25;266(9):5891–5897. [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoobyarian N., Marczynska B. Transformation of primate cells by chemicals and oncogenes: requirements for multiple factors. Anticancer Res. 1989 Sep-Oct;9(5):1367–1376. [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Latter G., Lutomski L., Goldstein D., Burbeck S. Tropomyosin isoform switching in tumorigenic human fibroblasts. Mol Cell Biol. 1986 Jul;6(7):2721–2726. doi: 10.1128/mcb.6.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller J. P., Helfman D. M. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991 Sep;13(9):429–437. doi: 10.1002/bies.950130902. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Stafford W. F., 3rd Preferential assembly of the tropomyosin heterodimer: equilibrium studies. Biochemistry. 1991 Jun 11;30(23):5682–5688. doi: 10.1021/bi00237a007. [DOI] [PubMed] [Google Scholar]
- Leonardi C. L., Warren R. H., Rubin R. W. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. doi: 10.1016/0167-4889(82)90007-6. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. The bacterial cell cycle. Curr Opin Cell Biol. 1990 Apr;2(2):241–245. doi: 10.1016/0955-0674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Narayanan R., Lawlor K. G., Schaapveld R. Q., Cho K. R., Vogelstein B., Bui-Vinh Tran P., Osborne M. P., Telang N. T. Antisense RNA to the putative tumor-suppressor gene DCC transforms Rat-1 fibroblasts. Oncogene. 1992 Mar;7(3):553–561. [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R. Retrovirus-mediated gene expression in mammalian cells. Curr Opin Biotechnol. 1991 Oct;2(5):708–712. doi: 10.1016/0958-1669(91)90039-8. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
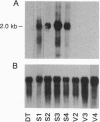
- Prasad G. L., Meissner S., Sheer D. G., Cooper H. L. A cDNA encoding a muscle-type tropomyosin cloned from a human epithelial cell line: identity with human fibroblast tropomyosin TM1. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1068–1075. doi: 10.1016/0006-291x(91)90647-p. [DOI] [PubMed] [Google Scholar]
- Raz A., Geiger B. Altered organization of cell-substrate contacts and membrane-associated cytoskeleton in tumor cell variants exhibiting different metastatic capabilities. Cancer Res. 1982 Dec;42(12):5183–5190. [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Robbins P. F., Kantor J. A., Salgaller M., Hand P. H., Fernsten P. D., Schlom J. Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res. 1991 Jul 15;51(14):3657–3662. [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Sabanay I., Zöller M., Ben-Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992 Oct;119(2):427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo Y., Perlaky L., Valdez B. C., Busch R. K., Henning D., Zhang W. W., Busch H. The effect of antisense p120 construct on p120 expression and cell proliferation in human breast cancer MCF-7 cells. Cancer Lett. 1993 Feb;68(2-3):95–104. doi: 10.1016/0304-3835(93)90134-u. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Perroteau I. Growth factors in cancer and their relationship to oncogenes. Cancer Invest. 1986;4(1):43–60. doi: 10.3109/07357908609039826. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Strauss M., Hering S., Lieber A., Herrmann G., Griffin B. E., Arnold W. Stimulation of cell division and fibroblast focus formation by antisense repression of retinoblastoma protein synthesis. Oncogene. 1992 Apr;7(4):769–773. [PubMed] [Google Scholar]
- Vandekerckhove J., Bauw G., Vancompernolle K., Honoré B., Celis J. Comparative two-dimensional gel analysis and microsequencing identifies gelsolin as one of the most prominent downregulated markers of transformed human fibroblast and epithelial cells. J Cell Biol. 1990 Jul;111(1):95–102. doi: 10.1083/jcb.111.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti G. P., Olson D., Labow M., Levine A. J. A mutant p53 protein is required for maintenance of the transformed phenotype in cells transformed with p53 plus ras cDNAs. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3952–3956. doi: 10.1073/pnas.89.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]