Abstract
Interleukin-1 beta (IL-1β) is a pro-inflammatory cytokine important for local and systemic immunity. However, aberrant production of this cytokine is implicated in pathogenic mechanisms of a number of inflammatory diseases including Behçet’s disease and age-related macular degeneration. We report here the increased secretion of IL-1β in the retina by neutrophils, macrophages, and dendritic cells during ocular inflammation and show that loss of IL-1R signaling confers protection from experimental autoimmune uveitis (EAU). Moreover, the amelioration of EAU in Il1r-deficient mice was associated with reduced infiltration of inflammatory cells into the retina and decreased numbers of uveitogenic Th17 cells that mediate uveitis. These findings indicate the possible utility of IL-1R blocking agents for the treatment of ocular inflammatory diseases.
Introduction
Uveitis is a diverse group of potentially sight-threatening intraocular inflammatory diseases that includes Behçet’s disease, birdshot retinochoroidopathy, Vogt-Koyanagi-Harada disease, sympathetic ophthalmia, and ocular sarcoidosis. The etiology of uveitis is not fully understood but may be linked to autoimmunity (1). Cytokine signaling plays critical role(s) in the pathology of uveitis. IL-17 is substantially elevated in freshly-isolated PBMC from patients with active uveitis, as well as in PBMC and lymph nodes of mice with experimental autoimmune uveitis (EAU), a model of human uveitis. The increase in IL-17 up-regulates tumor necrosis factor (TNF)-α in the retina, suggesting a mechanism by which Th17 cells contribute to ocular pathology during uveitis (1). In addition, IL-17 blocking antibodies ameliorate EAU while IL-27 and IL-35 protect mice from EAU by inhibiting the expansion of Th17 cells, underscoring the pathogenic role of IL-17 and TNF-α in intraocular inflammatory diseases (1, 2). These findings suggest that manipulation of cytokine signaling has therapeutic potential in the treatment of uveitis.
IL-1β is mainly produced by monocytes, macrophages, dendritic cells (DCs), and neutrophils and serves a role in host defense to pathogens (3). It triggers inflammatory responses by multiple mechanisms, including the induction of cyclooxygenase-2 and inducible nitric oxide synthase, and recruitment of inflammatory cells (4). More recently, IL-1 signaling has been shown to synergize with IL-6 and IL-23 to promote Th17 cell differentiation via induction of critical regulators IRF4 and RORγt (5), and IL-1β combined with IL-23 induces production of IL-17 in γδ T cells (6). Dysregulation of IL-1β expression leads to the development of autoimmune diseases, such as rheumatoid arthritis, type 2 diabetes, and gout, as well as autoinflammatory diseases, such as Familial Mediterranean Fever and cryopyrin-associated periodic syndromes (4, 7, 8). For this reason, IL-1 receptor antagonist (IL-1RA; Anakinra), soluble decoy IL-1 receptor (Rilonacept), and IL-1β neutralizing antibody (Canakinumab) have been approved for treating these diseases, with therapeutic benefit (9).
In the eye, increased IL-1β secretion has been implicated in Behçet’s disease and age-related macular degeneration (AMD) (10, 11) and intraperitoneal administration of IL-1β during priming phase of EAU increases disease severity, suggesting that IL-1β might play a pathogenic role in the eye (12). However, the exact role of IL-1β in ocular inflammatory diseases is controversial and not well defined. Here we report that myeloid cells produce IL-1β in the retina of mice during EAU, and analysis of Il1r-deficient mice provides direct evidence that IL-1 signaling is required for the development of uveitis. We further show that the reduced severity of intraocular inflammation in Il1r-deficient mice correlates with impaired Th17 cell differentiation and decreased recruitment of inflammatory cells into the retina. Our data thus indicate that agents blocking IL-1 signaling pathway may have considerable potential for treating uveitis.
Materials and Methods
Mice and antibodies
WT and Il1r−/− mice on the C57BL/6J background were from the Jackson Laboratory. All protocols were approved by the NHLBI and NEI Animal Care and Use Committees and followed NIH guidelines for using animals in intramural research. Anti-pro-IL-1β-APC and the corresponding isotype control (rat IgG1κ) were from eBioscience; other antibodies for flow cytometric analysis were from Biolegend.
Induction and evaluation of EAU
EAU induction was performed as previously described (2). Mice were immunized s.c. with 150 μg bovine IRBP and 300 μg human IRBP peptide (amino acids 1–20) in 0.2 ml 1:1 vol/vol emulsion with complete Freund’s adjuvant containing 2.5 mg/ml Mycobacterium tuberculosis strain H37RA. Mice also received i.p. 0.3 μg Bordetella pertussis toxin at the time of IRBP immunization. Disease severity was evaluated by fundoscopy at days 14 and 20 and by histological analysis at day 21, as previously described (13).
Immune cell identification and cytokine expression in the retina
At day 21 of EAU induction, mouse retinae were isolated, cut into pieces, and incubated with 1 mg/ml collagenase D and 500 μg/ml DNase I (Roche) at 37°C for 30 min. Single cell suspension was subjected to flow cytometry analysis (FACSCanto II). To determine intracellular pro-IL-1β expression, cells were stained with surface markers, fixed and permeabilized (BD Biosciences), and stained with anti-pro-IL-1β. To assess intracellular IL-17 and IFN-γ expression, cells were seeded into a 96-well plate and re-stimulated with 50 ng/ml PMA and 1 μM ionomycin for 4 h; intracellular cytokine expression was then determined.
Cytokine analysis in the retina and lymph nodes
Mouse retinae were isolated and incubated with RIPA buffer containing protease inhibitor cocktail (100 μl/retina) at 4°C for 20 min. IL-1β levels in the retina were quantified by an ELISA kit (BD Biosciences) that uses an IL-1β-specific antibody that does not cross-react with pro-IL-1β (14). For analysis of cytokine secretion by peripheral T cells, cervical and draining lymph nodes cells were isolated and stimulated (5 × 106 cells) with IRBP1–20 peptide (10 μg/ml) for 3 d. IL-17, IFN-γ, and IL-1β levels in culture supernatant was determined by ELISA (BD Biosciences).
Statistical analysis
Statistical comparison between samples was done by unpaired t test; P < 0.05 was considered as statistically significant.
Results and Discussion
Myeloid cells produce IL-1β in the retina during EAU
Chronic uveitis in humans is characterized by repeated cycles of remission and recurrent inflammation and is often accompanied by vascular, fibrotic, and neurodegenerative changes (15). A recent report showed that intraperitoneal injection of recombinant IL-1β protein at the priming phase (days 0–6), but not in the effector phase, enhanced the severity of acute uveitis in mice (12), consistent with a possible pathogenic role for this cytokine in uveitis. In this study, we induced uveitis in C57BL/6J WT mice and show here that compared to retina from un-immunized mice, IL-1β secretion was increased in the retina during EAU (Fig. 1A). Our detection of more than 100 pg IL-1β in the retina of mice with EAU raises the possibility that in situ secretion of IL-1β in the retina may promote the development of severe uveitis. We then investigated which cell populations in the retina potentially produced the IL-1β, as assessed by intracellular cytokine staining and flow cytometric analysis. Because microglial cells, resident macrophage-like cells that reside in the retina, play essential roles in regulating ocular inflammation, we examined whether IL-1β was produced by myeloid cells in the retina during EAU. We used cell-surface expression of CD11b and Ly6G to identify macrophages/DCs (CD11bhi Ly6Glow, R1) and CD11bhi Ly6Ghi for neutrophils (CD11bhi Ly6Ghi, R2) (Fig. 1B, upper panel). Macrophages and DCs in the retina were identified as CD11c+ MHC IIlow (R3) and CD11chi MHC IIhi (R4) subpopulations, respectively, of the R1 population (Fig. 1B, lower panel). Neutrophils, macrophages, and DCs (R2, R3, and R4) all expressed pro-IL-1β, with most neutrophils (R2) and the CD11chi MHC IIhi DCs (R4) expressing the greatest amount of this protein (Fig. 1C, 1D). These results indicate that the secretion of IL-1β in the retina during EAU was by myeloid cells.
FIGURE 1.
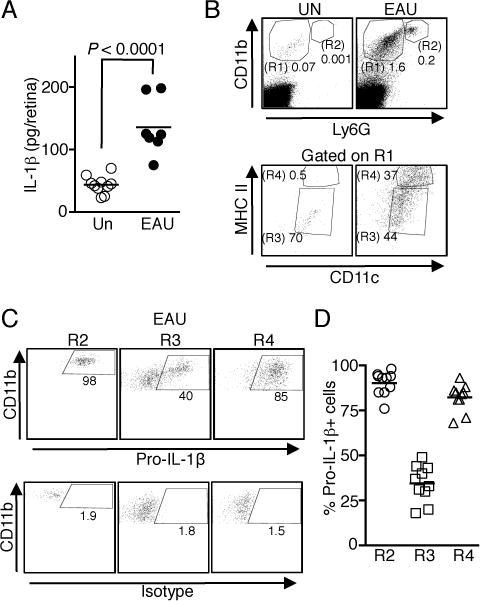
IL-1β is expressed in the retina during EAU. Retinae of WT mice without immunization (Un) and with EAU were isolated on day 21 post-immunization, digested with collagenase and analyzed by ELISA (A) or flow cytometry (B–D). (A) Analysis of IL-1β secretion by ELISA. Data are from 2 independent experiments with a total of 7–10 mice. (B) Macrophages/DCs (CD11b+ Ly6G−, R1) and neutrophils (CD11b+ Ly6G+, R2) that had infiltrated into the retina were first identified by CD11b and Ly6G staining. Macrophages and DCs were defined as CD11c+ MHC IIlow macrophages (R3) and CD11chi MHC IIhi DCs (R4) (C and D) Expression of intracellular pro-IL-1β in the immune cells identified in Fig. 1B after EAU induction. Shown are representative flow cytometric plots (C) and as well as data from 10 individual mice (D).
Il1r-deficient mice develop less severe EAU
We next used mice that cannot respond to IL-1β to directly examine whether IL-1β is indeed necessary for the development of severe uveitis. We induced EAU in C57BL/6J or Il1r−/− mice and monitored disease progression by fundoscopy. Fundus images show that WT mice developed severe inflammation characterized by blurred optic disc margins and enlarged juxtapapillary area, retinal vasculitis with moderate cuffing, vitreitis, choroiditis and yellow-whitish retinal and choroidal infiltrates (Fig. 2A). In contrast, disease in the Il1r−/− mice was significantly suppressed, with an average clinical score of approximately 1.0 compared to 3.0 for WT eyes (Fig. 2B). Histological analysis further revealed cardinal pathological features of severe uveitis, including the infiltration of inflammatory cells into the retina, development of granulomas, and retinal folds in WT mice, whereas no evidence of retina folding or the other features of severe uveitis were observed in the eyes of Il1r−/− mice (Fig. 2C). The histological score for Il1r−/− mice was markedly decreased compared to WT mice (Fig. 2D). Furthermore, Il1r−/− mice had fewer neutrophils (Fig. 3A, B), macrophages and DCs (Fig. 3A, C), and lymphocytes (CD4+ and CD8+) (Fig. 3D–3F), suggesting that IL-1R signaling might be critical for immune cell infiltration into the eyes during EAU.
FIGURE 2.
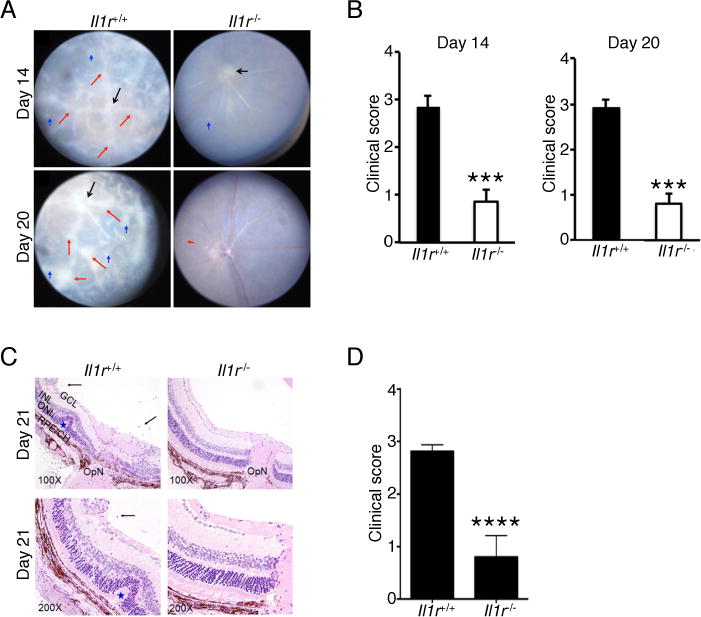
Il1r-deficient mice develop less severe EAU. EAU was induced in WT and Il1r−/− mice. (A) Fundus images of the eyes at days 14 and 20 after EAU induction. Compared to the Il1r−/− mice, the retinae of WT mice reveals obvious inflammation with blurred optic disc margins and enlarged juxtapapillary area (black arrows), retinal vasculitis with moderate or severe cuffing (red arrows), and yellow-whitish retinal and choroidal infiltrates (blue arrows). (B) Clinical score and assessment of disease severity were based on changes at the optic nerve disc or retinal vessels and retinal and choroidal infiltrates as described in the text. ***, P < 0.001. (C) Histological analysis of the retina at day 21 after EAU induction shows increased numbers of inflammatory cells in the vitreous (black arrows), retinal folds (blue asterisk) in retina of WT compared to Il1r−/− mice. (D) Significant reduction of histological score in Il1r−/− mice compared to WT mice. Data are representative of 2 independent experiments (total of 10 mice/group). ****, P < 0.0001. Sections were stained with H&E staining. V, vitreous, black arrow, infiltrated for inflammatory cells, Blue asterisk, retinal folds; OpN, optic nerve; GCL, ganglion cell layer; INL, inner nuclear layer; ONL outer nuclear layer; RPE/CH retinal pigment epithelial cell layer; and choroid.
FIGURE 3.
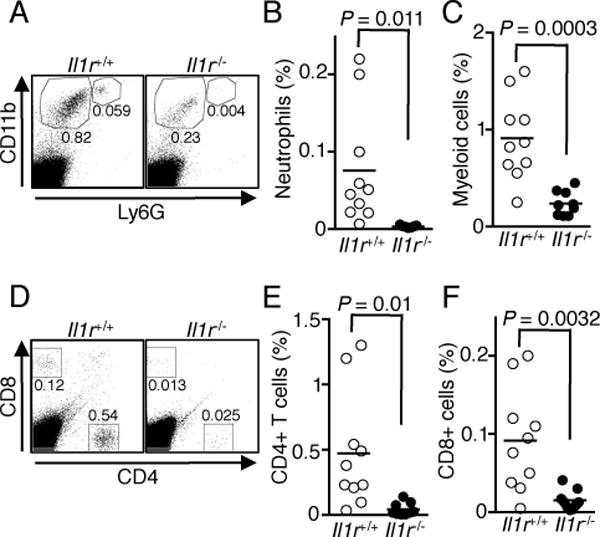
Il1r−/− mice have fewer immune cells infiltrating into the retina during EAU. EAU was induced in WT and Il1r−/− mice, retinae were isolated at day 21 post-immunization, digested with collagenase and analyzed by flow cytometry. (A) Shown are representative blots of CD11b versus Ly6G staining. (B and C) Percentages of neutrophils (B) and macrophages/DCs (C) from individual mice. (D) Shown are representative blots of CD4 and CD8 staining. (E and F) Percentages of CD4+ (E) and CD8+ (F) T cells. Data shown are from 2 independent experiments with a total of 10 mice/group.
Loss of IL-1R signaling inhibits expansion of Th17 cells and the secretion of IL-17
IL-1β together with IL-6 and IL-23 promotes the differentiation of IL-17-producing Th17 cells in the absence of TGF-β signaling (16), and IL-17 has been implicated in the pathogenesis of uveitis in humans and mice (1). Consistent with published reports (1, 17), we found a significant increase in IL-17-producing Th17 cells in the retina during EAU (Fig. 4A, 4B). The much lower level of IL-17-producing cells in the retina of the Il1r−/− mice (Fig. 4A, 4B) correlated with reduced severity of EAU, suggesting that IL-1R-dependent signaling plays a crucial role in driving IL-17 production during EAU. Interestingly, loss of IL-1β signaling had no significant effect on the levels of IFN-γ-producing Th1 cells in the retina during EAU (Fig. 4C), consistent with reports that EAU pathology is primarily mediated by Th17 cells (1, 17). This is also consistent with a report that Th17-induced ocular inflammation is driven by recruitment of neutrophils into the retina, whereas Th1 cells in the retina mainly induce higher proportions of CD8 cells (18). Thus, the marked reduction in neutrophils in the retina during EAU (Fig. 3B) is in line with reduction of Th17 cells in the retina. However, in contrast to the retina, the loss of IL-1R signaling in peripheral lymphoid tissues was associated with a significant reduction in both Th1 and Th17 cells, underscoring the requirement of IL-1R signaling in promoting inflammatory responses (Fig. 4D, 4E). These results also suggest that additional cytokine signal(s) may be involved in inducing IFN-γ expression in retinal CD4+ T cells during EAU. Finally, unlike the production of IL-17 and IFN-γ, pro-IL-1β and IL-1β were minimally produced in the cervical and draining lymph nodes during EAU, and the levels were not altered in Il1r−/− mice (Fig. 4F and Supplemental Fig. 1), and correspondingly, IL-1β was not detected in the serum during EAU (Supplemental Fig. 2).
FIGURE 4.
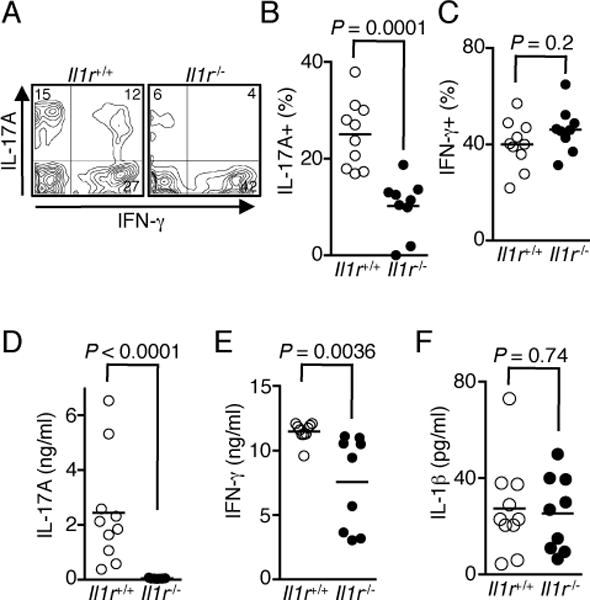
IL-1R-dependent signaling is required for Th17 cell differentiation. (A–C) EAU was induced in WT and Il1r−/− mice, retinae were isolated on day 21 post-immunization and analyzed by the intracellular cytokine staining assay. Shown are representative flow cytometric plots (A) and percentages of CD4+ T cells expressing IL-17 (B) and IFN-γ (C) from individual mice. (D and E) Cervical and draining lymph node cells were re-stimulated with IRBP1–20 peptide for 3 d. Amounts of IL-17 (D), IFN-γ (E), and IL-1β (F) in the culture were determined by ELISA. Data shown are from 2 independent experiments with a total of 10 mice/group.
In summary, our studies reveal that IL-1β is produced in the retinae during EAU by myeloid cells and indicate a potential pathogenic role for IL-1R signaling in uveitis. Analysis of the retinae of Il1r−/− mice with EAU further suggests that IL-1-dependent recruitment of uveitogenic Th17 cells into the retinae is critical for developing severe EAU. Our studies thus indicate that IL-1/IL-1R-blocking agents might be useful for treating uveitis as well as potentially other ocular inflammatory diseases.
Supplementary Material
Acknowledgments
We thank Dr. Cheng-Rong Yu for technical assistance, the Pathology Core of NEI for histological analysis, and Dr. Rosanne Spolski for critical comments.
Financial support: This work was supported by the Division of Intramural Research National Heart, Lung, and Blood Institute, and National Eye Institute.
Contributor Information
Charles E. Egwuagu, Email: egwuaguc@nei.nih.gov.
Warren J. Leonard, Email: wjl@helix.nih.gov.
References
- 1.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 2.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature medicine. 2014;20:633–641. doi: 10.1038/nm.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends in immunology. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nature reviews Rheumatology. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 5.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Science translational medicine. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nature reviews. Drug discovery. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EH, Park MJ, Park S, Lee ES. Increased expression of the NLRP3 inflammasome components in patients with Behcet’s disease. Journal of inflammation. 2015;12:41. doi: 10.1186/s12950-015-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O’Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nature medicine. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R, Zhou H, Zhang J, Liu X, Su SB. Interleukin-1beta promotes the induction of retinal autoimmune disease. International immunopharmacology. 2014;22:285–292. doi: 10.1016/j.intimp.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Yu CR, Kim HP, Liao W, Telford WG, Egwuagu CE, Leonard WJ. Key role for IL-21 in experimental autoimmune uveitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9542–9547. doi: 10.1073/pnas.1018182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan CK, Li P, Spolski R, Oh J, Andraski AB, Du N, Yu ZX, Dillon CP, Green DR, Leonard WJ. IL-21-mediated non-canonical pathway for IL-1beta production in conventional dendritic cells. Nature communications. 2015;6:7988. doi: 10.1038/ncomms8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh HM, Yu CR, Lee Y, Chan CC, Maminishkis A, Egwuagu CE. Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. J Immunol. 2011;187:3338–3346. doi: 10.4049/jimmunol.1004019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Lee YS, Yu CR, Egwuagu CE. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J Immunol. 2008;180:6070–6076. doi: 10.4049/jimmunol.180.9.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, Siegel RM, Gery I. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


