Abstract
The retinoblastoma protein (pRB) is a critical regulator of cell proliferation and differentiation and an important tumor suppressor. In the G1 phase of the cell cycle, pRB localizes to perinucleolar sites associated with lamin A/C intranuclear foci. Here, we examine pRB function in cells lacking lamin A/C, finding that pRB levels are dramatically decreased and that the remaining pRB is mislocalized. We demonstrate that A-type lamins protect pRB from proteasomal degradation. Both pRB levels and localization are restored upon reintroduction of lamin A. Lmna-/- cells resemble Rb-/- cells, exhibiting altered cell-cycle properties and reduced capacity to undergo cell-cycle arrest in response to DNA damage. These findings establish a functional link between a core nuclear structural component and an important cell-cycle regulator. They further raise the possibility that altered pRB function may be a contributing factor in dystrophic syndromes arising from LMNA mutation.
The RB gene is a common target for inactivating mutations in human retinoblastomas, as well as a variety of other human tumors (1). Consistently, mice heterozygous for Rb develop tumors (2, 3). Rb-/- mice die in mid-gestation, with defects in neurogenesis, fetal liver erythropoiesis, and lens development (3, 4). Recent evidence, however, suggests that many of these phenotypes derive in part from loss of retinoblastoma protein (pRB) in extraembryonic tissue (5). When complemented by a wild-type placenta, Rb-/- embryos survive until birth but die shortly after, exhibiting severe defects in lens and skeletal muscle tissues (6). Indeed, there is extensive evidence to suggest that pRB function is required for normal muscle and fat cell differentiation (7–11). These findings and others have led to the hypothesis that an important role of pRB is to promote terminal differentiation.
pRB is tethered to the nuclear matrix when hypophosphorylated during the G1 phase of the cell cycle (12–14). The finding by several groups that A-type nuclear lamins can interact with pRB in vitro suggested a mechanism of nuclear tethering (12, 15, 16). Lamin A and C, alternatively spliced products of the LMNA locus, are the predominant A-type lamins. A third member of the A-type lamin family, lamin C2, is restricted in expression to germ cells (17). Indeed, the lamin A/C binding protein, LAP2α, interacts with pRB and is important for nuclear tethering of the tumor suppressor (18). It remains unknown whether nuclear tethering of pRB is important for its functions in cell-cycle control and tumor suppression.
In addition to the nuclear periphery, A-type lamins concentrate in discrete intranuclear foci in primary human cells (19). We have reported that, in primary cells, pRB localizes to a limited number of perinucleolar foci that overlap strongly with intranuclear sites of lamin A/C (20). E2F family members and transcriptional corepressors that are recruited by pRB also localize to these foci, suggesting that they represent sites of pRB cell-cycle control. We reasoned that A-type lamins might assist pRB targeting to perinucleolar foci. In this report, we describe an analysis of pRB levels, localization, and activity in cells derived from Lmna-/- mice. From the results of this analysis, we conclude that A-type lamins are required for the stability, localization, and hence activity of the pRB.
Materials and Methods
Cell Culture. Rb-/- mouse embryo fibroblasts and 3T3 cell lines, as well as litter-matched controls, have been described (21). Lmna-/- murine embryonic fibroblast (MEF) litter-matched controls have been described (22). Standard methods were used to culture cells (20). For transfections, the following constructs were used: C1-GFP (Clontech), CMV-pRB (gift of W. Kaelin, Harvard Medical School, Boston), and GFP-lamin A (gift of C. Hutchison, University of Durham, Durham, U.K.), in which GFP is fused to the N terminus of full-length lamin A.
Flow Cytometry. Protein and DNA content of cell lysates was determined as described (21). For flow cytometric analysis, cells were labeled with BrdUrd cell label (Amersham Pharmacia) for 30 min, harvested, and analyzed according to manufacturer's instructions. To measure the relative size in asynchrony and at confluence, single cells were gated for the G1 population and forward-scatter histograms (FSC-H) were plotted. The mean FSC-H was calculated by using cellquest software. At least three independent pairs of Lmna-/- cells and littermate controls were assayed to verify size differential.
Immunofluorescence. Indirect immunofluorescence was performed as described (20), except that Alexa secondary antibodies (Molecular Probes) were used in some cases. The following antibodies were used: G3-245 mouse monoclonal antibody against pRB at 1:1,500 (Pharmingen); SD15 mouse monoclonal supernatant against p107 at 1:3; mouse monoclonal antibody (636) at 1:1,500 (Santa Cruz) or 5881 (gift of L. Gerace, University of California, San Diego) against lamin A/C; E2F-1-rabbit polyclonal antibody as suggested (Santa Cruz Biotechnology); and fibrillarin (human autoantigen) at 1:20,000 (gift of M. Pollard, The Salk Institute, San Diego).
Some images were generated by using the Deltavision charge-coupled device (CCD)/deconvolution system. Images were collected with the CCD camera but were not deconvolved. Images comparing pRB or p107 levels in Lmna+/+ and Lmna-/- cells were collected by using equal exposure time and processed identically.
For DNA damage experiments, early-passage MEFs were irradiated with γ rays from a 137Cesium source at a dose of 5.66 Gy/min. Cells were then incubated with BrdUrd, and anti-BrdUrd antibodies were used as described (20).
Protein Analysis and Immunoprecipitation. Protein extracts from total cells were harvested in RIPA buffer (0.15 M NaCl/0.05 mM Tris·HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS). The following antibodies were used: G3-245 to detect pRB (Boehringer); a mixture of antibodies to detect p107 (SD2, -6, -9, and -15); p130 (Transduction Laboratories, Lexington, KY); emerin (NovoCastra, Newcastle, U.K.); and p27KIP1 (Boehringer). Spectroscopy and Coomassie staining were performed on all extracts to equilibrate protein levels for Western blot analysis. Immunoprecipitations for p107 were conducted in RIPA buffer by using a mixture of antibodies (SD2, -6, -9, and -15) bound to protein A beads.
Real-Time RT-PCR Analysis. Total RNA was isolated from contact-inhibited MEFs lacking lamin A/C and their litter mate controls using RNeasy mini kit (Qiagen, Valencia, CA). The following primers were used: for HPRT, tggaaagaatgtcttgattgttgaa and agcttgcaaccttaaccattttg; for p107, gccatcagtggagatgcaga and atcaggcaaagggtgacgtt; and for pRB, tgccgtgtgtttgtttcaca and tcaaagcatgtgttgggtga. cDNA (20 ng/μl) was generated by reverse transcription using the protocols established with DNase I (Invitrogen) and M-MLV Reverse Transcriptase (Promega). In control samples, M-MLV Reverse Transcriptase was omitted.
The PCR amplifications were performed by using Opticon 1 (MJ Research, Cambridge, MA). The reverse transcriptase reaction mixture contained 1× SYBR Green PCR Master Mix buffer (Applied Biosystems) and 8.0-pmol primers. To monitor the formation of primer dimers and to exclude other possible forms of contamination, a melting curve analysis was conducted to all finished PCR products. The relative gene expression was quantified using the 2-ΔΔCT method (Table 2, which is published as supporting information on the PNAS web site) (23).
Small Interfering RNA (siRNA) Transfections. U2OS cells were transfected with 70 pmol (≈1 μg) of siRNA specific to lamin A/C (24) by using Lipofectamine and Plus reagents. After 3 h, the media mixture was refed with fresh serum containing media. Cells were fixed at 60 h posttransfection, and immunofluorescence was performed by using standard methods (20). Isotype-specific secondary antibodies were used to examine lamin A/C and pRB in the same cells (Molecular Probes).
Results
pRB and p107 Levels in Lmna-/- Fibroblasts. Given that pRB family members localize to intranuclear sites enriched for A-type lamins (20) and that lamin A/C had been reported to interact with pRB in vitro (12, 15, 16), we reasoned that this nuclear structural component might mediate subnuclear localization of pRB family members. Therefore, we examined pRB levels, localization, and activity in either MEFs or immortalized mouse fibroblasts derived from Lmna-/- mice, which lack all A-type lamins (22). In all experiments, Lmna-/- cells were compared with cells derived from litter-matched controls. Multiple sets of independently derived, age-matched Lmna-/- MEFs and littermate controls were examined to confirm any potential differences in pRB levels or localization.
Initially, we measured pRB levels in early-passage Lmna-/- MEFs. Surprisingly, we found that pRB levels were reduced ≈5-fold relative to litter-matched controls (Fig. 1A). This result was somewhat unexpected because pRB levels generally remain constant in cells. Instead, regulation of pRB activity is primarily exerted at the level of phosphorylation. To confirm and extend these findings, we examined pRB levels in immortalized fibroblasts generated from Lmna-/- MEFs. As expected, these immortalized fibroblasts do not express lamin A/C (see Fig. 4C). Again, pRB levels were reduced ≈5-fold relative to control in proliferating cells (Fig. 1B). The reduction in pRB levels was apparent but less severe under conditions of serum depletion or contact inhibition.
Fig. 1.
Levels of pRB family members in Lmna-/- cells. In all blots, -/- indicates the Lmna-/- genotype and +/+ indicates the litter-matched Lmna+/+ genotype. Other genotypes are as described. (A) pRB levels in mouse embryo fibroblasts. Antibodies to Rho GDP were used as loading control. (B) pRB levels in immortalized fibroblasts. Antibodies to Rho GDP were used as loading control. (C) Equilibrated extracts from immortalized fibroblasts of indicated genotype were immunoprecipitated with antibodies to p107. Immunoprecipitated proteins were analyzed by Western blots with p107-specific antibodies. The lower band is the heavy chain of the p107 antibodies used for immunoprecipitation. (D) p130 levels in immortalized fibroblasts. Flanking bands are nonspecific. (E) p27KIP1 levels in immortalized fibroblasts. (F) Emerin levels in immortalized fibroblasts.
Fig. 4.
A-type lamins regulate pRB stability. (A) Real-time RT-PCR analysis of the ratios of Rb and p107 mRNA in Lmna-/- to Lmna+/+ fibroblasts. Rb and p107 mRNA levels were normalized to HPRT RNA levels in each cell type under each condition. A variety of cDNA concentrations were used to ensure consistency of read-outs. See Table 2 for quantitation. (B) The proteasome inhibitor MG132 (25 μM for 6 h) restores pRB stability in immortalized Lmna-/- fibroblasts. Actin levels were determined for loading control. For actin blot, extracts were diluted 1/10 relative to blot probed for pRB. -/-, Lmna-/- fibroblasts. +/+, Lmna+/+ fibroblasts. (C) GFP-lamin A was introduced by transient transfection into Lmna-/- immortalized fibroblasts, and protein levels of pRB were determined by immunoprecipitation and Western analysis. (D) GFP-Lamin A was introduced by transient transfection into Lmna-/- immortalized fibroblasts, and immunofluorescence was performed to identify transfected cells and determine pRB levels and localization. In the images, one transfected cell and two untransfected cells are displayed for comparison. Images were collected by CCD microscopy
Because pRB levels are reduced in cells lacking lamin A/C, we examined the levels of the two other pRB family members, p107 and p130. Similarly to pRB, p107 levels were ≈3-fold reduced relative to litter-matched controls in Lmna-/- cells (Fig. 1C). In contrast to pRB and p107, the levels of p130 were not altered in cells lacking lamin A/C (Fig. 1D). It is unclear why p130 behaves differently from pRB and p107.
As a control, we tested whether the levels of another cell-cycle regulatory factor, p27KIP1, was regulated by lamin A/C status (Fig. 1E). The levels of p27KIP1, as well as cdk1 (not shown), were found to be identical in both cell lines. Also, consistent with previous reports, we observed that the level of emerin, a known lamin A/C binding protein, was not altered in cells lacking lamin A/C (22, 25, 26) (Fig. 1F). Together, these findings suggested that lamin A/C is required for normal levels of pRB and p107 in mouse fibroblasts.
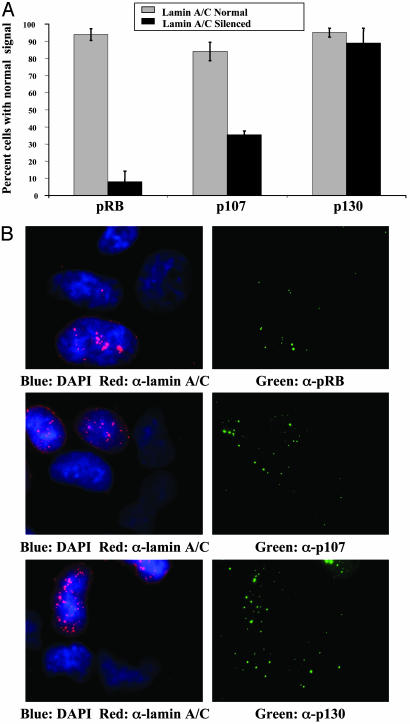
Are A-type lamins required for pRB stability in other cell types? To test this, we generated siRNA oligonucleotides directed against LMNA and transfected duplex RNA into a human osteosarcoma cell line (U2OS) to determine whether pRB, p107, and p130 levels would be affected by forced reduction of lamin A/C. These oligonucleotides have been shown to be effective for reducing expression of lamin A/C (24).
Indirect immunofluorescence was performed to compare pRB, p107, or p130 levels in siRNA-transfected and untransfected cells. Data were tabulated from three independent experiments (Fig. 2A). To generate the data, cells were first scored for normal or reduced lamin A/C levels and then examined for pRB, p107, or p130. Representative images (Fig. 2B) were collected by CCD microscopy. More than 90% of untransfected cells had easily detectable pRB levels. By contrast, <10% of transfected cells with reduced lamin A/C levels displayed normal pRB levels. Similar results were obtained for p107, but, in the case of p130, we could detect no alteration in fluorescence intensity associated with reduction of lamin A/C. These findings suggested a lamin A/C requirement to establish proper pRB and p107 levels and/or localization in human cells and mouse cells, and that the dependence is not restricted to fibroblasts.
Fig. 2.
Lamin A/C is required for pRB and p107 stability in human osteosarcoma cells. U2OS cells were transfected with siRNA oligos to reduce lamin A/C expression. pRB, p107, or p130 expression was determined by indirect immunofluorescence by using an antibody specific for lamin A/C and an antibody specific for each of the pRB family members. (A) Cells were scored for normal or reduced lamin A/C expression and then for pRB, p107, or p130. Gray bars represent the percentage of cells with normal pRB, p107, or p130 levels within the population of cells with normal lamin A/C expression. Black bars represent the percentage of cells with normal pRB, p107, or p130 levels within the population of cells with reduced lamin A/C expression. Each immunofluorescence experiment was performed in triplicate. (B) Images depict representative cells from the siRNA experiment. Lamin A/C signals are shown in red and pRB family members in green. Images were collected by CCD microscopy. Each field includes both lamin A-down-regulated and unaffected cells.
pRB and p107 Localization in Fibroblasts Lacking Lamin A/C. Previously, we observed that pRB family members localize to perinucleolar sites that overlap with intranuclear lamin A/C foci. Although pRB levels are reduced in Lmna-/- cells, we performed indirect immunofluorescence in an attempt to determine whether the remaining protein was correctly localized to perinucleolar foci. The antibodies used to recognize pRB and p107 are specific for each protein (11). We examined immortalized cells arrested in G1 phase by contact inhibition because pRB localization to perinucleolar foci is most prominent in this phase of the cell cycle (Fig. 3A). We find that, unlike wild-type cells where levels of pRB appear uniform, there are clear disparities in pRB and p107 staining among Lmna-/- cells. A majority of cells have little or no immunofluorescence signal when exposed to pRB or p107 antibodies. Other cells display clear staining although still reduced relative to Lmna+/+ cells.
Fig. 3.
pRB and p107 localization in Lmna-/- fibroblasts. CCD imaging was used to generate images. -/-, Immortalized Lmna-/- fibroblasts. +/+, Litter-matched immortalized Lmna+/+ fibroblasts. Cells were arrested by contact inhibition before indirect immunofluorescence. (A) Exposure times were equilibrated with each fluorophore for images depicting pRB staining in Lmna+/+ and Lmna-/- cells and for images depicting p107 staining in Lmna+/+ and Lmna-/- cells. All further processing of images was performed identically. (B) Localization of E2F-1 and pRB in Lmna+/+ and Lmna-/- fibroblasts. Merging of red and green signals appears yellow.
Importantly, in Lmna-/- cells that have low but detectable levels of pRB, the proportion of pRB signal localizing to perinucleolar foci was reduced. Although pRB remains nuclear, the foci no longer concentrate in regions around the nucleolus (Fig. 3A, compare pRB in green to the nucleolar protein fibrillarin in red both in wild-type and Lmna-/- cells). p107 localization is similarly disrupted, exhibiting little or no overlap with perinucleolar regions.
Having shown that pRB and E2F-1 overlap in perinucleolar foci (27), we next questioned whether colocalization of these two proteins was disrupted in Lmna-/- cells (Fig. 3B). Cells lacking lamin A/C displayed reduced overlap between pRB and E2F-1, leading us to infer that pRB control of E2F-1 activity may be deregulated. Together, these findings indicate that lamin A/C, in addition to controlling levels of pRB and p107, is required for proper targeting of these proteins to perinucleolar foci.
A-Type Nuclear Lamins Mediate pRB Stability. We considered two potential mechanisms by which A-type lamins regulate levels of pRB and p107. The first, that lamin A/C might regulate transcription of RB, is supported by the recent reports that A-type lamins are required to sustain normal transcription levels in general (28) and by speculation that these proteins might regulate gene-specific transcription (29). To test this possibility, we performed Northern analysis, comparing proliferating Lmna-/- immortalized fibroblasts and litter-mate controls (not shown). Northern analysis indicates that Lmna-/-cells contain the same levels of Rb mRNA as control cells. Given that these two cells do not have identical cell-cycle profiles (see below), we also compared mRNA levels in cells arrested by contact inhibition, this time using real-time RT-PCR (Fig. 4A and Table 2). Again, our results indicate that Rb mRNA levels are nearly identical under these conditions when normalized to control mRNA (HPRT). Similar results were seen for p107 mRNA levels under these conditions. Therefore, we conclude that lamin A/C control of pRB and p107 levels is not exerted at the level of mRNA production or stability.
A second possibility is that lamin A/C mediates pRB stability. If true, lamin A/C interaction might protect pRB from proteasome-mediated degradation. Evidence for pRB degradation through the proteasomal pathway comes from studies of the human proteasomal protein E7, which binds to pRB family members and stimulates their degradation (30–32). We examined whether the presence of MG132, a proteasome inhibitor, was sufficient to stabilize endogenous pRB in the absence of lamin A/C (Fig. 4B). In Lmna-/- cells, we find pRB levels are rescued to that of Lmna+/+ cells after short-term incubation with MG132, consistent with the hypothesis that proteasomal degradation of pRB is increased in cells lacking lamin A/C. We cannot, however, rule out the possibility that MG132 stabilizes another direct target of lamin A/C that, in turn, regulates the rate of translation of the Rb message. Interestingly, in cells treated with MG132, the increased levels of pRB are not accompanied by relocalization of pRB to the perinucleolar region, again arguing that lamin A is required for correct localization as well as maintaining correct pRB levels (data not shown).
Reintroduction of Lamin A into Lmna-/- Cells. We have established that both Lmna-/- MEFs and immortalized cells exhibit reduced levels of pRB and p107 relative to controls. However litter-matched Lmna+/+ and Lmna-/- immortalized cells likely have other genetic changes. To attribute changes in pRB stability and function directly to loss of lamin A/C, we reintroduced human GFP-tagged lamin A into Lmna-/- cells by transient transfection to determine whether pRB levels and cell-cycle phenotypes were rescued under these conditions. Western analysis was performed on extracts immunoprecipitated with pRB-specific antibodies to determine pRB levels (Fig. 4C). We find that reintroduction of GFP-lamin A results in a significant increase in pRB levels, although not to the extent of Lmna+/+ cells. This result can be explained by the observation that only 25% of the cells were transfected with GFP-lamin A. Levels of pRB would not be increased in untransfected cells. An alternative possibility is that expression of only lamin A fails to completely rescue due to the continued absence of lamin C.
pRB levels were also determined by indirect immunofluorescence in GFP-lamin A-transfected cells as a means to test the interdependence of these two proteins in individual cells. We consistently find that transfected cells had higher levels of pRB as compared with equally exposed, untransfected neighboring cells (Fig. 4D). Perinucleolar localization of pRB is also restored (Fig. 5B and data not shown).
Fig. 5.
Lmna-/- fibroblasts have phenotypes resembling Rb-/- fibroblasts. (A) Fluorescence-activated cell sorting analysis was performed on proliferating Lmna+/+ and Lmna-/- immortalized fibroblasts. See Table 1 for quantification. (B) Lmna-/- fibroblasts are smaller than their litter-matched counterparts. Images depict cells maintained at confluence for 2 days. Forward scatter histograms were conducted to determine mean cell size. (C) Lmna-/- fibroblasts enter S phase prematurely after arrest in G1. To achieve cell-cycle arrest, cultures were maintained at confluence for a period of 48 h. At this time, >90% of cells have 2N DNA content. (D) Lmna-/- fibroblasts have a defective cell-cycle response to DNA damage. Representative data are shown from one of three experiments conducted. Proliferating cells of each genotype were exposed to γ irradiation of the amount indicated. Eight hours after exposure, cells were pulsed with BrdUrd for 15 min, and immunofluorescence was performed to determine the percentage of cells undergoing DNA replication.
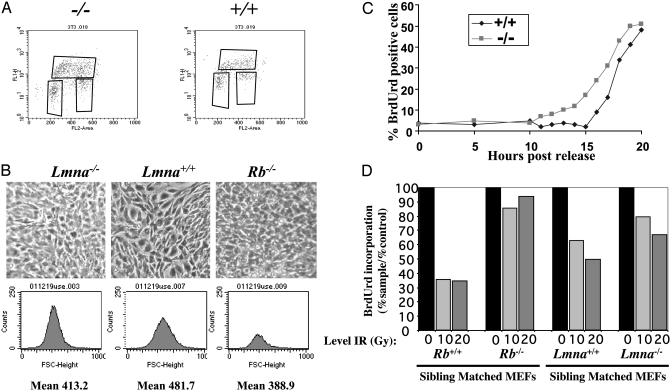
Lmna-/- Cells Are Phenotypically Similar to Rb-/- Cells. Does the reduction in pRB levels in Lmna-/- cells have meaningful consequences in changing the function of pRB? Cells lacking pRB display a number of phenotypes in cell culture, including an increased frequency of cells undergoing DNA replication during rapid proliferation, premature S-phase entry after release from G1 arrest, higher cell density, and smaller cell size (21, 33). Rb-/- cells also fail to undergo cell-cycle G1 exit in response to γ irradiation-induced DNA damage (34, 35). Given that pRB stability is reduced in cells lacking lamin A/C, we reasoned that these cells might have cell-cycle phenotypes in common with Rb-/- fibroblasts. Moreover, lamin A/C and their binding partner LAP2α were reported to be required for pRB nuclear tethering (18). We therefore chose to directly test the hypothesis that pRB tethering is relevant to pRB function.
Fluorescence-activated cell sorting analysis was performed on proliferating cultures of Lmna-/- immortalized fibroblasts and litter-matched controls (Fig. 5A and Table 1). Similar to Rb-/- or Rb-/-/p107-/- 3T3 cells (21), we find that a larger proportion of proliferating Lmna-/-cells are in S phase of the cell cycle. As with cells lacking pRB, overall cell-cycle time is not significantly altered in Lmna-/-cells. Rather, there is a commensurate decrease in percentage of G1- and G2/M-phase cells. Notably, the increase in S-phase cells is not as dramatic as that observed in Rb-/- cells (Table 1). We have observed that the magnitude of this cell-cycle phenotype, as well as the others discussed below, is not as dramatic in cells lacking lamin A/C relative to those lacking pRB. This finding may be explained by the fact that pRB levels are diminished but not absent in Lmna-/- cells.
Table 1. Lmna-/- cells have altered cell cycle phenotypes.
| % Cells
|
|||||
|---|---|---|---|---|---|
| Genotype | Doubling time | G1 | S | G2/M | Saturation density, ×106 |
| +/+ | 21 h | 34 | 33 | 33 | 1.9 |
| -/- | 21 h | 31 | 42 | 27 | 3.9 |
| +/+ | - | 41 | 39 | 20 | 1.1 |
| Rb-/- | 21 h | 29 | 64 | 8 | 2.7 |
| Rb-/-/p107-/- | 20 h | 35 | 59 | 5 | 3.8 |
Saturation densities and cell-cycle analysis of Rb-/-, Rb-/-/p107-/- and +/+ litter-mate controls have been reported (21) and are provided for comparison.
Cell density of Lmna-/- and control cells was monitored after G1 arrest by contact inhibition. Cells lacking lamin A/C reach substantially higher density under these conditions but still arrest with over 95% of cells containing 2N DNA content (Table 1). Again, this phenotype bears strong resemblance to that observed for Rb-/- and Rb-/-/p107-/- 3T3 cells (21). We then used fluorescence-activated cell sorting analysis on single-gated cells and plotted forward scatter histograms to determine mean relative cell size (Fig. 5B). In this assay, Lmna-/- cells were routinely observed to be smaller than their litter-matched counterparts. Therefore, with regard to cell-cycle arrest by contact inhibition, Lmna-/- cells behave very similarly to Rb-/- and Rb-/-/p107-/- cells, suggesting that loss of A-type lamins is a phenocopy of the loss of pRB and p107.
After release from G1 arrest, 3T3 cells lacking pRB or pRB and p107 prematurely initiate DNA synthesis. We tested whether immortalized Lmna-/- cells behaved similarly. Cells were synchronized in G1 by contact inhibition and then permitted to reenter the cell cycle by replating at low density. Timing of S-phase entry was determined by pulsing cells at various time points with the nucleotide analog BrdUrd, followed by indirect immunofluorescence with BrdUrd-specific antibodies. We find that Lmna-/- cells initiated DNA synthesis ≈2 h before their Lmna+/+ counterparts (Fig. 5C). This finding, coupled with the observation that G1 phase is underrepresented in asynchronously proliferating cells, indicates that cells lacking lamin A/C spend less time in G1, similar to Rb-/- and Rb-/-/p107-/- cells.
In addition to mediating cell-cycle arrest before differentiation, pRB is required for cell-cycle exit after DNA damage induced by γ irradiation. We examined early-passage MEFs generated from mice lacking lamin A/C, as well as their litter-matched counterparts (Fig. 5D). Asynchronously dividing cells were exposed to irradiation, and the percentage of cells undergoing DNA synthesis was determined 8 h after treatment by monitoring BrdUrd incorporation. Similar results were seen at 2 and 4 h after DNA damage (not shown). Cell-cycle arrest was diminished in Lmna-/- cells relative to Lmna+/+ counterparts, although not to the same extent as Rb-/- MEFs relative to litter-matched Rb+/+ controls. Together, this set of experiments indicates that Lmna-/- cells have altered cell-cycle properties that closely resemble those of cells lacking pRB or pRB and p107, implying a functional link between A-type lamins and pRB family members.
Discussion
In addition to their role in nuclear assembly, nuclear structural proteins are known to interact with a number of proteins that serve key regulatory roles, including transcription factors (36, 37). However, the functional significance of these interactions has been hard to ascertain. Using fibroblasts generated from Lmna-/-, we have addressed the role of lamin A/C in regulating the pRB, demonstrating that lamin A/C controls the stability, localization, and therefore activity of pRB. Lamin A/C may exert its affects directly through interaction with pRB or indirectly, for instance, by regulating the activity of LAP2α. Regardless, these findings establish a clear functional link between lamin A/C, a nuclear structure component, and an important tumor suppressor protein, pointing to the significance of nuclear organization in cell proliferation control.
Recently, mutations in LMNA have been discovered in a number of human disease syndromes, including forms of muscular dystrophy and lipodystrophy (laminopathies) (38). Many of these syndromes are dystrophic in nature, with tissue seemingly developing normally, but then degenerating over time. Sullivan et al. (22) have established the causal affects of these mutations, showing that mice lacking lamin A/C seem normal at birth but rapidly develop muscular dystrophy.
How does lamin A/C act to maintain healthy differentiated tissue? One model is that this core nuclear constituent acts to regulate tissue-specific transcription factors required for differentiation and/or maintenance of the differentiated state. One possible transcription factor is sterol regulatory element-binding protein (SREBP), which is known to interact with lamin A/C and be involved in adipogenesis (37). In addition, a transcriptional repressor, germ cell-less, has been reported to interact with emerin-lamin A/C complexes (39). Given our findings that lamin A/C is required for pRB stability and function, and the well-established importance of pRB in muscle and fat differentiation, we raise the possibility that dystrophic syndromes arising from LMNA mutation may result at least in part from deregulated pRB function.
Supplementary Material
Acknowledgments
We thank L. Gerace, C. Hutchison, W. Kaelin, and M. Pollard for supplying reagents. We thank M. Classon, F. Dick, S. Hauschka, and S. Kaestner for technical assistance and E. Smith for comments on the manuscript. Microscopy was performed in part at the Keck Imaging Center at the University of Washington. D.A.B. was supported by a Howard Hughes Medical Research Training Fellowship. E.H. is an American Cancer Society Research Professor. B.K.K. is a Searle Scholar and has received support from the Wendy Will Case Cancer Fund.
Abbreviations: pRB, retinoblastoma protein; MEF, mouse embryonic fibroblast; CCD, charge-coupled device; siRNA, small interfering RNA.
References
- 1.Yamasaki, L. (2003) Cancer Treat. Res. 115, 209-239. [DOI] [PubMed] [Google Scholar]
- 2.Clarke, A., Maandag, E., van Roon, M., van der Lugt, N., van der Valk, M., Hooper, M., Berns, A. & te Riele, H. (1992) Nature 359, 328-330. [DOI] [PubMed] [Google Scholar]
- 3.Jacks, T., Fazeli, A., Schmitt, E., Bronson, R., Goodell, M. & Weinberg, R. (1992) Nature 359, 295-300. [DOI] [PubMed] [Google Scholar]
- 4.Lee, E. Y.-H. P., Chang, C.-Y., Hu, N., Wang, Y.-C. J., Lai, C.-C., Herrup, K., Lee, W.-H. & Bradley, A. (1992) Nature 359, 288-294. [DOI] [PubMed] [Google Scholar]
- 5.Wu, L., de Bruin, A., Saavedra, H. I., Starovic, M., Trimboli, A., Yang, Y., Opavska, J., Wilson, P., Thompson, J. C., Ostrowski, M. C., et al. (2003) Nature 421, 942-947. [DOI] [PubMed] [Google Scholar]
- 6.de Bruin, A., Wu, L., Saavedra, H. I., Wilson, P., Yang, Y., Rosol, T. J., Weinstein, M., Robinson, M. L. & Leone, G. (2003) Proc. Natl. Acad. Sci. USA 100, 6546-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riley, D., Liu, C. & Lee, W. (1997) Mol. Cell. Biol. 17, 7342-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zacksenhaus, E., Jiang, Z., Chung, D., Marth, J. D., Phillips, R. A. & Gallie, B. L. (1996) Genes Dev. 10, 3051-3064. [DOI] [PubMed] [Google Scholar]
- 9.Lasorella, A., Noseda, M., Beyna, M. & Iavarone, A. (2000) Nature 407, 592-598. [DOI] [PubMed] [Google Scholar]
- 10.Chen, P. L., Riley, D. J., Chen-Kiang, S. & Lee, W. H. (1996) Proc. Natl. Acad. Sci. USA 93, 465-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Classon, M., Kennedy, B. K., Mulloy, R. & Harlow, E. (2000) Proc. Natl. Acad. Sci. USA 97, 10826-10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancini, M. A., Shan, B., Nickerson, J. A., Penman, S. & Lee, W.-H. (1994) Proc. Natl. Acad. Sci. USA 91, 418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Templeton, D. J., Park, S.H., Lanier, L. & Weinberg, R.A. (1991) Proc. Natl. Acad. Sci. USA 88, 3033-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mittnacht, S. & Weinberg, R. A. (1991) Cell 65, 381-393. [DOI] [PubMed] [Google Scholar]
- 15.Shan, B., Zhu, X., Chen, P.-L., Durfee, T., Yang, Y., Sharp, D. & Lee, W.-H. (1992) Mol. Cell. Biol. 12, 5620-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki, T., Saijo, M., Murakami, K., Enomoto, H., Taya, Y. & Sakiyama, S. (1994) Oncogene 9, 2649-2653. [PubMed] [Google Scholar]
- 17.Furukawa, K., Inagaki, H. & Hotta, Y. (1994) Exp. Cell Res. 212, 426-430. [DOI] [PubMed] [Google Scholar]
- 18.Markiewicz, E., Dechat, T., Foisner, R., Quinlan, R. A. & Hutchison, C. J. (2002) Mol. Biol. Cell 13, 4401-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridger, J. M., Kill, I. R., O'Farrell, M. & Hutchison, C. J. (1993) J. Cell Sci. 104, 297-306. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, B. K., Barbie, D. A., Classon, M., Dyson, N. & Harlow, E. (2000) Genes Dev. 14, 2855-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classon, M., Salama, S., Gorka, C., Mulloy, R., Braun, P. & Harlow, E. (2000) Proc. Natl. Acad. Sci. USA 97, 10820-10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan, T., Escalante-Alcalde, D., Bhatt, H., Anver, M., Bhat, N., Nagashima, K., Stewart, C. L. & Burke, B. (1999) J. Cell Biol. 147, 913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak, K. J. & Schmittgen, T. D. (2001) Methods 25, 402-408. [DOI] [PubMed] [Google Scholar]
- 24.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 25.Clements, L., Manilal, S., Love, D. R. & Morris, G. E. (2000) Biochem. Biophys. Res. Commun. 267, 709-714. [DOI] [PubMed] [Google Scholar]
- 26.Fairley, E. A., Kendrick-Jones, J. & Ellis, J. A. (1999) J. Cell Sci. 112, 2571-2582. [DOI] [PubMed] [Google Scholar]
- 27.Lai, A., Kennedy, B. K., Barbie, D. A., Bertos, N. R., Yang, X. J., Theberge, M. C., Tsai, S. C., Seto, E., Zhang, Y., Kuzmichev, A., et al. (2001) Mol. Cell. Biol. 21, 2918-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spann, T. P., Goldman, A. E., Wang, C., Huang, S. & Goldman, R. D. (2002) J. Cell Biol. 156, 603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson, K. L. (2000) Trends Cell Biol. 10, 125-129. [DOI] [PubMed] [Google Scholar]
- 30.Munger, K., Werness, B. A., Dyson, N., Phelps, W. C., Harlow, E. & Howley, P. M. (1989) EMBO J. 8, 4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer, S., Wazer, D. & Band, V. (1996) Cancer Res. 56, 4620-4624. [PubMed] [Google Scholar]
- 32.Jones, D., Thompson, D. & Munger, K. (1997) Virology 239, 97-107. [DOI] [PubMed] [Google Scholar]
- 33.Hurford, R., Cobrinik, D., Lee, M.-H. & Dyson, N. (1997) Genes Dev. 11, 1447-1463. [DOI] [PubMed] [Google Scholar]
- 34.Brugarolas, J., Moberg, K., Boyd, S., Taya, Y., Jacks, T. & Lees, J. (1999) Proc. Natl. Acad. Sci. USA 96, 1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington, L. J., Bruce, J., Harlow, E. & Dyson, N. (1998) Proc. Natl. Acad. Sci. USA 95, 11945-11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dreuillet, C., Tillit, J., Kress, M. & Ernoult-Lange, M. (2002) Nucleic Acids Res. 30, 4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd, D. J., Trembath, R. C. & Shackleton, S. (2002) Hum. Mol. Genet. 11, 769-777. [DOI] [PubMed] [Google Scholar]
- 38.Burke, B. & Stewart, C. L. (2002) Nat. Rev. Mol. Cell Biol. 3, 575-585. [DOI] [PubMed] [Google Scholar]
- 39.Holaska, J. M., Lee, K. K., Kowalski, A. K. & Wilson, K. L. (2003) J. Biol. Chem. 278, 6969-6975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.