Abstract
Administration of therapeutic proteins by methods other than injection is limited, in part, by inefficient penetration of epithelial barriers. Therefore, unique approaches to breaching these barriers are needed. The neonatal constant region fragment (Fc) receptor (FcRn), which is responsible for IgG transport across the intestinal epithelium in newborn rodents, is expressed in epithelial cells in adult humans and non-human primates. Here we show that FcRn-mediated transport is functional in the lung of non-human primates and that this transport system can be used to deliver erythropoietin (Epo) when it is conjugated to the Fc domain of IgG1. FcRn-dependent absorption was more efficient when the EpoFc fusion protein was deposited predominantly in the upper and central airways of the lung, where epithelial expression of FcRn was most prominently detected. To optimize fusion protein absorption in the lung, we created a recombinant “monomeric-Epo” Fc fusion protein comprised of a single molecule of Epo conjugated to a dimeric Fc. This fusion protein exhibited enhanced pharmacokinetic and pharmacodynamic properties. The bioavailability of the EpoFc monomer when delivered through the lung was approximately equal to that reported for unconjugated Epo delivered s.c. in humans. These studies show that FcRn can be harnessed to noninvasively deliver bioactive proteins into the systemic circulation in therapeutic quantities.
The neonatal constant region fragment (Fc) receptor (FcRn), first isolated from neonatal rodent intestine (1), transports maternal Ig (IgG) from milk into the bloodstream to provide immunity to newborn animals in the first 2-3 weeks of life (2). Transport occurs because of interactions between well defined contact sites within the Fc fragment of IgG and FcRn (3, 4). After weaning, FcRn expression in the rodent gut epithelium decreases precipitously and remains low in epithelial tissues throughout adult life (5). In contrast to rodents, FcRn is expressed in adult humans, in the placenta where it serves to transport IgG from mother to fetus (6), and in several absorptive epithelial tissues, including the lung, kidney, and intestine (7-9). The expression of FcRn in absorptive epithelia, coupled with the recent description of FcRn transport of IgG through human intestinal epithelial cells in vitro (10), suggested to us that FcRn could potentially be exploited for the delivery of therapeutic proteins by conjugation of the proteins to an FcRn-binding ligand such as the Fc fragment of IgG1. This would allow their transport across the epithelium.
Numerous effector molecules such as soluble cytokine receptors and growth factors have been coupled to the Fc domain of IgG1, with retention of their biological activities (11). These Fc-fusion molecules are known to have increased circulating half-lives, due to the ability of Fc to bind to FcRn, which serves a critical function in IgG homeostasis, protecting molecules bound to it from catabolism (12). Therefore, we created an Fc-fusion protein that could be used to demonstrate FcRn-mediated transport across an epithelial surface in adult non-human primates.
Erythropoietin (Epo) is a glycoprotein hormone drug that stimulates red blood cell production (13). Epo currently requires chronic therapy with either i.v. or s.c. injection, and thus an alternative noninvasive method of delivery would be a significant advancement. The lung was chosen as the portal for delivery due to evidence for expression of FcRn in the epithelium at this site (9) and that delivery through this organ has led to some success with proteins of modest molecular weight (e.g., insulin, Mr = 5,700) (14). However, pulmonary delivery of major biotherapeutic products having larger molecular weights has not been successful. This is not unexpected, because many studies have demonstrated an inverse correlation between molecular size and the rate of clearance from the lung, i.e., absorption into the bloodstream (15, 16). Our approach to delivery of large proteins such as Epo through the epithelial barrier in the lung was, therefore, to exploit an active carrier system, the FcRn pathway. Our data with EpoFc indicate a functional FcRn-dependent transport pathway in the lung that can be used for the delivery of therapeutic proteins.
Materials and Methods
Drugs. Epogen (epoetin alfa, Amgen) was obtained from a hospital pharmacy.
Immunohistochemistry. Cynomolgus monkey lung tissue was embedded with OCT compound (Tissue-Tek, Sakura Finetek, Torrance, CA) and frozen at -80°C. Frozen sections were cut into 6-μm sections with a cryostat. Sections of each specimen were stained with hematoxylin/eosin. The primary rabbit anti-human FcRn antibodies were raised against a peptide from a conserved region of FcRn (17). Slides were then incubated with goat anti-rabbit IgG (Sternberger Monoclonals, Lutherville, MD) and a rabbit peroxidase antiperoxidase (Sternberger Monoclonals) and finally FcRn detection was carried out by using a diaminobenzidine (Vector Laboratories) chromogen that resulted in a positive brown staining. A negative control was provided by incubating slides with normal rabbit serum instead of the anti-FcRn antibodies before the other exposures.
Cloning, Expression, and Purification of EpoFc. All molecular biology procedures were performed following standard techniques (18). The coding sequences for human Epo and the Fc region of human IgG1 (hinge and CH2 and CH3 domains) were obtained by PCR and cloned into the mammalian expression vector pED.dC (Genetics Institute, Cambridge, MA). The construct was designated pED.dC.natEpo-Fc. An EpoFc fusion protein that does not bind FcRn was generated by introducing three amino acid substitutions (I253A/H310A/H435A) into the Fc domain (3, 4). This fusion construct was designated pcDNA6/hEpo-Fc/IHH. The coding sequence for a FLAG peptide tag was fused to the 5′ terminus of the coding sequence of the Fc region of IgG1 and cloned into the plasmid pcDNA3.1 (Invitrogen) for expression of FLAG-Fc. This expression plasmid was designated pcDNA3.1/FLAGFc.
Chinese hamster ovary DG44 cells (19) were transfected with the plasmids pED.dC.natEpoFc or pcDNA6/hEpoFc/IHH, and the secreted fusion proteins were purified from culture medium by using protein A (protein G for the EpoFc/IHH mutant) affinity chromatography. Proteins were applied to Protein A Sepharose in PBS, the column washed with the same buffer, and then the proteins were eluted with 0.1 M glycine (pH 2.7), and each fraction was neutralized with 1 M Tris·HCl (pH 8.0). After dialysis and concentration, the proteins were stored at -80°C.
The EpoFc monomer was produced by transfecting Chinese hamster ovary DG44 cells with pEDdC.natEpoFc and pcDNA3.1/FLAGFc (a vector containing the DNA sequence for Fc modified at its N terminus with the 8-aa FLAG affinity tag), and cells expressing the protein products of both plasmids were selected by using methotrexate and G418. Three protein products were produced by the cells: the EpoFc/EpoFc homodimer (EpoFc “dimer”), the EpoFc/FLAG-Fc heterodimer (EpoFc “monomer”), and the FLAG-Fc:FLAG-Fc homodimer. The EpoFc monomer was purified by using a combination of protein A chromatography as above, size exclusion chromatography, and, finally, immunoaffinity chromatography on an anti-FLAG-Sepharose column. The purified protein was stored at -80°C.
Binding of Fusion Proteins to Soluble FcRn. Chinese hamster ovary DG44 cells were transfected with DNA sequences for both the human FcRn (hFcRn) (pcDNA6:shFcRn) and human β2-microglobulin (pEDdC:β2m). Soluble hFcRn:β2m (shFcRn) was purified by using affinity chromatography on IgG-Sepharose (Amersham Pharmacia Biotech) followed by ion exchange chromatography on a Uno-Q (Bio-Rad) column. Kinetic constants for the interaction of Fc-fusion proteins with shFcRn were measured by using a Biacore 3000 (Biacore, Piscataway, NJ) for surface plasmon resonance, as described (20). Briefly, shFcRn was coupled to a carboxy-methyl dextran-coated (CM5) sensor chip via primary amines at a density sufficient to produce 3,500-5,000 response units, and fusion proteins were passed over the shFcRn-coated surface. All protein samples were diluted into 50 mM phosphate buffer (pH 6.0), containing 100 mM NaCl and 0.005% Tween 20 before injection into the Biacore cell. Kinetic constants were derived from the resulting sensorgrams with biaevaluation 3.1 software (Biacore) using a heterogeneous ligand model, which assumes that there are two populations of FcRn on the chip for binding at equilibrium.
TF-1 Cell Proliferation and Receptor-Binding Assays. TF-1 human erythroleukemia cells (American Type Culture Collection, no. CRL-2003) were maintained, cell proliferation measured, and Epo receptor-binding affinity determined essentially as described (21, 22).
Pulmonary Delivery in Non-Human Primates. All studies on cynomolgus monkeys (2-8 kg) were conducted by using approved protocols, following all National Institutes of Health guidelines for the care and use of research animals. A standard method was developed to favor central airway deposition. Animals were anesthetized with a combination of valium and ketamine, and aerosols were administered directly into the lungs through an endotracheal tube. An Aeroneb Pro nebulizer (AeroGen, Mountain View, CA) (mass median aerodynamic diameter of 4.1 μm and geometric standard deviation of 3.1 μm, as measured with an Andersen Cascade Impactor, ThermoAndersen, Smyrna, GA) was used in-line with a Bird Mark (Bird Products, Palm Springs, CA) 7A respirator, to administer aerosols to monkeys. Aerosols were generated until ≈2 ml of the drug solution per animal had exited the nebulizer (≈5 min). For shallow breathing, animals were regulated at 25-32 breaths per min (pressure setting of 12-15 cm/H2O on the respirator) and for deep breathing, 20 breaths per min with a 3-sec breath hold (pressure setting = 25-28 cm H2O). Additionally, a nebulizer that produced smaller aerosol particles (Bird Micronebulizer, mass median aerodynamic diameter of 2.5 μm) was used for deep-breathing animals, to direct deposition deeper into the lung.
γ scintigraphy (23) was used to characterize the delivery of the EpoFc aerosol in the lungs. The fraction of the nebulizer dose that was deposited in the lung and the pattern of distribution (central vs. peripheral) of EpoFc in the lung was determined by using our standard method of administration. EpoFc was derivatized with a chelating agent, mercaptoacetyltriglycine (MAG3), by using a standard technique (24), to allow labeling with 99mTc. The final EpoFc-MAG3-99mTc complex contained <5% free 99mTc and was administered to monkeys within 1 hr of labeling. Immediately after the aerosol administration, γ camera computer images were acquired (with the assistance of Robert Beihn, Scintiprox, Indianapolis) for the posterior and anterior thoracic regions. Based on tissue attenuation factors for each of four test monkeys, previously determined by using radioactive γ standards, a deposited radioactive dose of 2.9 ± 1.0 mCi EpoFc/monkey was calculated. The deposition fraction (percentage of the nebulizer dose deposited in the lung) was determined to be 12.8 ± 2.2% (n = 4), and this value was used to calculate the lung-deposited doses in each subsequent experiment. Using the acquired scintigraphic images, regions of interest, i.e., peripheral and central regions, were defined for the lungs of each monkey, and the percentage of radioactivity deposited in each region was determined. A peripheral to central ratio of 0.4 ± 0.2 (n = 4) was observed, suggesting that deposition of EpoFc was greater in the central regions of the lungs and less in the lung periphery.
In all pharmacokinetic experiments with EpoFc and EpoFc/IHH, serum concentrations were measured by using a specific ELISA kit (Quantikine Human Epo Immunoassay, R & D Systems). Pharmacokinetic parameters were derived by using noncompartmental analysis with winnonlin software (Version 4.1, Professional, Pharsight, Mountain View, CA). Bioavailability was defined as the area under the serum concentration vs. time curve (AUC) for a lung-deposited dose of EpoFc divided by the AUC obtained after i.v. injection.
Results
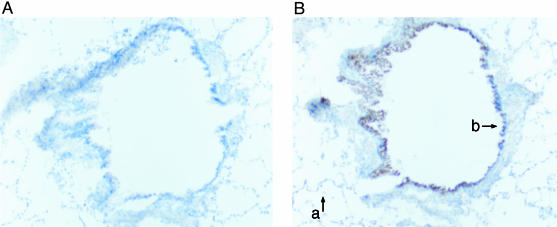
Characterization of FcRn Expression in Cynomolgus Monkey Lungs. Initial studies showed that FcRn was expressed in epithelial cells lining the small and large airways through examination of paraffin sections from both human and monkey lungs (9). Using freshly frozen tissue from monkey lung, we confirmed that FcRn expression appears higher in epithelial cells in the conducting airways than in the alveolar epithelial cells (Fig. 1). Although FcRn may be expressed in alveolar epithelial cells, the apparent higher level of expression in the bronchi suggested that FcRn-mediated transcytosis might be more active in the conducting airways than in the alveoli.
Fig. 1.
Expression of FcRn in non-human primate lung. Immunohistochemical localization of FcRn in cynomolgus monkey bronchus (b) and alveoli (a) was performed by using immunoperoxidase staining after exposure of the tissue sections to nonspecific rabbit serum (A) or a rabbit serum containing a specific anti-FcRn antibody (B). The dark-brown deposits (on the right) indicate the presence of FcRn. Many similar sections of lung were examined from one male and one female monkey with similar staining patterns being observed. The section shown here is from a female monkey.
Detection of an FcRn-Mediated Transport Pathway in the Lungs of Non-Human Primates. To test whether FcRn is functional in the lungs of cynomolgus monkeys, we first constructed a recombinant fusion protein with human Epo conjugated to the Fc region of human IgG1. The fusion protein, EpoFc (≈112,000 Da), retained the biological activity of native Epo when tested in vitro (Table 1), binding to the Epo receptor with an affinity not significantly different from that for Epogen. EpoFc also stimulated TF-1 cell proliferation. However, the affinity of EpoFc for FcRn was less than for IgG1 binding to FcRn (Fig. 2). EpoFc stimulated TF-1 cell proliferation but was slightly less potent than Epogen. We also created a mutant with substitutions in three amino acid residues (I253A, H310A, and H435A) within the Fc domain known to be critical for FcRn binding (3, 4). As predicted, EpoFc/IHH did not bind FcRn in surface plasmon resonance assays (Fig. 2).
Table 1. Biological activity of the EpoFc dimer, EpoFc monomer, and Epo in vitro.
| Drug molecule | Epo receptor binding,*Kd, nM | TF-1 cell proliferation,† ED50, nM |
|---|---|---|
| EpoFc | 0.28 ± 0.13 (n = 8) | 0.07 ± 0.02 (n = 29) |
| EpoFc monomer | 0.3 ± 0.10 (n = 3) | 0.09 ± 0.02 (n = 3) |
| Epo | 0.19 ± 0.06 (n = 3) | 0.03 ± 0.01 (n = 13) |
Experiments were performed as described in Materials and Methods. Numerical values represent the mean ± SD.
Epo receptor binding was determined in TF-1 cells by competition for binding of 125-l-epogen.
Growth of TF-1 cells was monitored over 48 hr. The values represent the concentrations of drugs that stimulate growth by 50% of maximum (ED50).
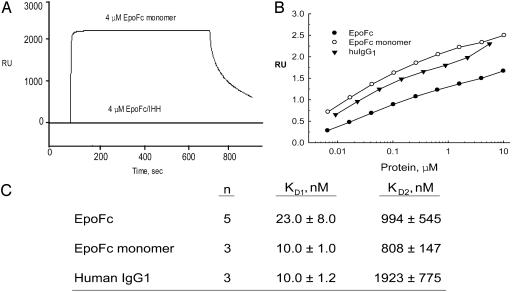
Fig. 2.
Surface plasmon resonance analysis of the interaction of human FcRn with EpoFc and human IgG1. Different concentrations of IgG1 or EpoFc fusion proteins (0.004-10 μM) were injected over a flow cell coupled with human FcRn (3,500-5,000 response units) at a flow rate of 10 μl/min. (A) Representative sensorgrams obtained with 4 μM EpoFc monomer and 4 μM EpoFc/IHH are shown. All sensorgrams were zero-adjusted and reference cell data subtracted. (B) Response units at equilibrium are plotted as a function of the log of IgG1 or EpoFc fusion protein concentrations. (C) Binding affinities were derived from the data in B by using biaevaluation software.
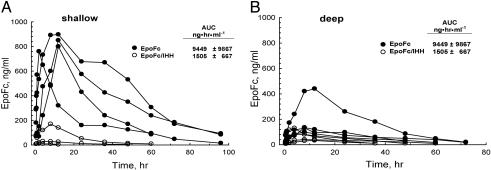
Aerosolized EpoFc was administered directly into the lungs of cynomolgus monkeys that had their breathing controlled by a respirator, and concentrations of the fusion molecule in serum were measured at various time points. Because FcRn expression appeared highest in the central airways, we used both shallow breathing maneuvers designed to target the fusion proteins predominantly to the central airways and deep-breathing maneuvers to target deposition in the alveoli. Peak serum concentrations (Cmax) of EpoFc were observed to be 800-900 ng/ml in four animals that received EpoFc at a deposited dose of 300 μg/kg during shallow breathing (Fig. 3). Delivery of EpoFc at the same deposited dose but using deep breathing maneuvers resulted in significantly less absorption based on an analysis of peak concentrations of EpoFc in the serum and AUC. However, one monkey of the four treated with EpoFc using deep breathing had a peak serum concentration >400 ng/ml and an AUC of 24,120 ng·hr·ml-1, values significantly higher than the other three monkeys in this treatment group. If this animal is excluded from the analysis, the mean AUC ± SD for the remaining three monkeys was 4,529 ± 900 ng·hr·ml-1. Although it is not clear why this monkey absorbed more EpoFc during deep breathing, it can be speculated that more of the fusion protein was deposited in the central airways rather than in the deep lung. As predicted, absorption of EpoFc/IHH was poor when delivery was by either shallow or deep breathing (Fig. 3), suggesting that the uptake of these large fusion proteins depends on FcRn-mediated transport and does not effectively cross the epithelial barrier in the lung by nonspecific processes. After pulmonary administration, EpoFc/IHH had a serum t1/2 = 13.8 ± 2.8 h (n = 5) compared to a serum t1/2 = 17.7 ± 6.7 h (n = 8) for EpoFc. It is unlikely that the difference in t1/2 can account for the striking differences in the uptake of the two molecules.
Fig. 3.
Effects of breathing pattern on absorption of EpoFc from the lungs of non-human primates. Approximately 0.3 mg/kg EpoFc or mutant EpoFc/IHH was deposited into the lungs of monkeys that were shallow breathing (EpoFc, n = 4; EpoFc/IHH, n = 3) or deep breathing (EpoFc, n = 4; EpoFc/IHH, n = 4). EpoFc and EpoFc/IHH in serum from each animal were measured over time by using a specific Epo ELISA. The AUC were calculated for each fusion protein, and the values shown are means ± SD.
Although uptake through the lung was reproducible, the bioavailability of EpoFc was only ≈5% at deposited doses of 10-20 μg/kg, i.e., doses necessary to achieve a minimum level of increase in circulating reticulocytes (see Table 3). We felt that uptake through the FcRn pathway was not optimum with the initial EpoFc fusion protein. Therefore, some effort was made to improve the EpoFc molecule to achieve better FcRn-mediated uptake.
Table 3. Biological effects of the EpoFc dimer and EpoFc monomer after pulmonary delivery in cynomolgus monkeys.
| Dose,* μg/kg
|
Reticulocytes
|
|||
|---|---|---|---|---|
| Drug/route | Pretreatment, % | Posttreatment, % | n | |
| EpoFc monomer Pulmonary | 5 | 0.53 ± 0.35 | 2.47 ± 0.38 | 3 |
| 20 | 0.53 ± 0.12 | 3.40 ± 1.24 | 3 | |
| i.v. | 10 | 0.7 | 2.65 | 2 |
| EpoFc dimer Pulmonary | 10 | 0.60 | 1.75 | 2 |
| 20 | 0.55 | 1.75 | 2 | |
| Epogen Pulmonary | 1.8† | 0.93 ± 0.41 | 1.60 ± 0.25 | 3 |
Monkeys received single doses of the EpoFc monomer either by pulmonary administration or i.v. injection. The EpoFc dimer and Epogen were given once by pulmonary administration. Values represent either the mean of two monkeys or mean ± SD where three or more monkeys were used.
Lung-deposited dose for pulmonary delivery.
Using a conversion factor of 10 ng/unit; equimolar to 5 μg/kg EpoFc monomer.
Development and Characterization of an EpoFc Monomer. Ig fusion proteins are most commonly constructed as homodimers with an effector molecule attached to each chain of an Fc dimer (11), but heterodimers in which two distinct effector molecules are attached to each chain of Fc have also been described (25). We constructed a Fc-fusion protein with a single Epo molecule attached to the Fc dimer, a molecule we call EpoFc monomer. It was proposed that better transport could be achieved by decreasing both the molecular weight and the overall glycosylation of the Fc fusion protein as well as potentially decreasing steric hindrance within the Fc fusion protein. As described below, the resultant EpoFc monomer exhibited higher affinity for FcRn and improved pulmonary uptake and efficacy.
The biological activity of the EpoFc monomer was similar to that for EpoFc with regard to Epo receptor binding and stimulation of TF-1 cell proliferation in vitro (Table 1). However, the EpoFc monomer bound to FcRn with 2-fold better affinity than EpoFc and with the same affinity as that determined for human IgG1 (Fig. 2). A representative sensorgram is shown only for the EpoFc monomer. The sensorgrams for all the different concentrations for the EpoFc monomer, EpoFc dimer, and IgG1 were of the same shape but of different heights (response units) at equilibrium. The response units at equilibrium increased linearly with increases in the protein ligand concentration as shown in Fig. 2B. The values in the graph of protein concentration vs. response units were used by the biaevaluation software to derive the kinetic constants KD1 and KD2 for EpoFc, the EpoFc monomer, and IgG1 (Fig. 2C).
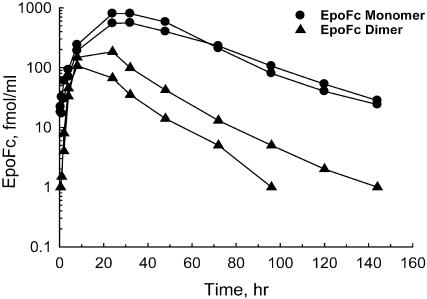
When delivered to the lungs of cynomolgus monkeys, the EpoFc monomer was absorbed significantly better than EpoFc (Fig. 4). At equivalent deposited doses (by mass), the average Cmax (EpoFc monomer = 680 fmol/ml, EpoFc = 146 fmol/ml) and average AUC (EpoFc monomer = 38,352 fmol·hr·ml-1, EpoFc = 4,606 fmol·hr·ml-1) were higher with the EpoFc monomer. When normalized for dose (D), the total systemic exposure to the monomer (AUC/D) was ≈6-fold higher than with EpoFc. The circulating t1/2 of EpoFc monomer (25 h) also appeared slightly longer when compared to the t1/2 for EpoFc (16 h). Therefore, a more complete analysis of the EpoFc monomer was performed.
Fig. 4.
Pulmonary delivery of EpoFc and EpoFc monomer. EpoFc and EpoFc monomer were aerosolized with an Aeroneb Pro nebulizer, and the aerosol was delivered directly into the lungs of two monkeys each via shallow breathing through an endotracheal tube. Blood was drawn at selected times after administration, and the serum concentrations of the EpoFc monomer and EpoFc were measured with an Epo ELISA.
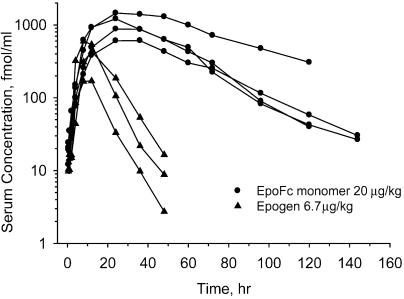
We next compared the pulmonary uptake of the EpoFc monomer (20 μg/kg) to that for an equimolar dose of recombinant human Epo (Epogen, 6.7 μg/kg) (Fig. 5). Administration of the EpoFc monomer resulted in a much higher systemic exposure to Epo than that obtained with Epogen. The pharmacokinetic parameters derived from the time course of absorption for both EpoFc and Epogen (Fig. 5) are shown in Table 2. There was a dose-dependent increase in Cmax and AUC with the EpoFc monomer, with some indication of nonlinearity of each parameter with increasing dose. The bioavailability of the EpoFc monomer at 20 μg/kg was 35% compared to 15% for Epogen at the same molar dose. As expected, the circulating half-life of the EpoFc monomer (25 ± 8 h, n = 4) was significantly longer than that calculated for Epogen (6.5 ± 0.8 h, n = 3). The half-life for the EpoFc monomer was the same whether the drug was administered via the lung or i.v., indicating that the longer terminal half-life was not simply due to slower absorption through the lung.
Fig. 5.
Comparison of pulmonary absorption of Epogen and the EpoFc monomer in cynomolgus monkey lung. Equal molar doses of the EpoFc monomer and Epogen were aerosolized with an Aeroneb Pro nebulizer, and the aerosol was delivered directly into the lungs of three monkeys each. Serum concentrations of the two molecules were determined by using an Epospecific ELISA.
Table 2. Pharmacokinetic parameters for the EpoFc dimer, EpoFc monomer, and epogen in cynomolgus monkeys.
| Route | Dose, μg/kg | Dose,* nmol/kg | Cmax,† ng/ml | AUC, ng·hr·ml−1 | t½,‡ hr | Bioavailability,§ % | n | |
|---|---|---|---|---|---|---|---|---|
| EpoFc | Pulmonary | 20 | 0.18 | 17.5 | 557 | 16 | 5.8 | 2 |
| EpoFc monomer | Pulmonary | 1 | 0.01 | 1.9 | 159 | 54 | 21 | 2 |
| 5 | 0.05 | 12.5 | 719 | 30 | 19 | 2 | ||
| 20 | 0.2 | 86 ± 31 | 5,279 ± 2,909 | 25 ± 8 | 35 ± 19 | 4 | ||
| i.v. | 25 | 0.3 | 622 ± 110 | 18,913 ± 3,022 | 23 ± 1 | 3 | ||
| Epogen | Pulmonary | 6.7 | 0.22 | 12 ± 7 | 206 ± 104 | 6.5 ± 0.8 | 15 ± 8 | 3 |
| i.v. | 20 | 0.66 | 514 ± 172 | 3,936 ± 636 | 6.3 ± 0.6 | 3 |
Experiments were conducted as described in Materials and Methods. Values represent either the mean of two monkeys or mean ± SD where three or more monkeys were used.
Lung-deposited dose or injected dose.
Maximum concentration measured in serum.
Half-life in serum.
Bioavailability is the AUC per dose for a lung-deposited dose of EpoFc divided by the AUC per dose obtained after i.v. injection.
Biological Response to the EpoFc Monomer. Finally, we also compared the biological activity of the EpoFc monomer to EpoFc and Epogen after pulmonary delivery (Table 3). Single pulmonary or i.v. doses of the EpoFc monomer increased reticulocytes in the peripheral circulation 5-7 days after treatment. EpoFc and Epogen also increased reticulocytes but were not as effective as the EpoFc monomer.
Discussion
The results of these studies show that bioactive Fc fusion proteins of large molecular weight can be delivered to the systemic circulation by coopting the endogenous FcRn-IgG transport pathway normally expressed in the monkey lung. Transport depended on the presence of a functional FcRn-binding site within the Fc domain of the fusion protein, as shown by diminished absorption of a mutant EpoFc disabled in its ability to bind to FcRn. Consistent with an FcRn-dependent pathway of transcytosis, which diverts immunoglobulins away from degradation in the lysosomes, EpoFc crossed the epithelial barrier intact and fully functional. This is shown by the retention of biological activity of EpoFc after transit through lung epithelial cells, i.e., circulating reticulocytes increased after a single pulmonary administration of EpoFc. In addition, when EpoFc was measured in the serum by using a sandwich ELISA that detected both components of the fusion molecule, the concentrations of EpoFc were identical to those measured with the standard assay kit that detects only the Epo moiety (data not shown).
Our data also show that FcRn-mediated transport in the primate lung is apparently associated with the central, conducting airways, where FcRn is more highly expressed. For these studies, we used a shallow breathing pattern, associated with deposition of aerosol particles in the central airways. The aerosol particle size used in most of our experiments was relatively large (mass median aerodynamic diameter = 4.1 μm). Large particles tend to be deposited in the upper and central airways, enhancing the effect of the shallow breathing maneuvers. Utilization of a physiological receptor transport pathway that appears to be more highly expressed in the airways than in the alveoli differentiates this method of delivery for therapeutic proteins from other pulmonary protein drug delivery technologies that use novel formulations and/or devices to target proteins to the deep lung (26).
Such efficient transport of Fc fusion proteins in the upper and central respiratory tract is remarkable, because the barriers to absorption of macromolecules in the central airways, i.e., thick epithelium, relatively small surface area, and highly active mucociliary clearance mechanisms, are formidable (27). Peptides and proteins, especially those of high molecular weight, are rarely absorbed systemically from the upper and central airways (28, 29).
Ig fusion proteins have routinely been constructed as dimeric molecules with respect to both the Fc component and the effector molecule attached to the Fc (11). EpoFc is an example of such a fusion protein. Although EpoFc bound to FcRn with affinity only slightly lower than that of native IgG1 and was delivered through the epithelial cells lining the non-human primate lung, its bioavailability was relatively low. Limitations on EpoFc moving through epithelial cells could have been related to its size (112,000 Da), steric hindrance between the Epo and Fc moieties, or charge and other physicochemical properties, including interaction of the sugar moieties on the molecule with the mucus that lines the airways. One approach taken by us to improve the uptake characteristics of EpoFc was to create a “monomeric” Fc fusion protein comprised of a single Epo conjoined to a dimeric Fc subunit. This construct was transported into the circulation significantly better than both EpoFc and Epogen when delivered to the epithelial surfaces of the lung, and it exhibited enhanced efficacy in vivo. The uptake of the EpoFc monomer is of the same order of efficiency as a s.c. injection of Epogen in humans, ≈30-35% (13).
Because of the apparent similarities in FcRn expression in the lungs of adult humans and non-human primates (9), the results presented herein are expected to translate well into clinical practice. We propose that pulmonary delivery of such therapeutic Fc fusion proteins represents a clinically feasible and potentially important alternative to drug delivery by painful injections.
Acknowledgments
W.I.L. and R.S.B. were supported by National Institutes of Health Grant DK53056 and the Harvard Digestive Diseases Center. R.S.B. was supported by National Institutes of Health Grant DK44319.
Abbreviations: Fc, constant region fragment; FcRn, neonatal Fc receptor; Epo, erythropoietin; EpoFc, EpoFc fusion protein; AUC, area under the serum concentration vs. time curve.
Pamela J. Bjorkman has served as a consultant for Syntonix Pharmaceuticals.
References
- 1.Simister, N. E. & Rees, A. R. (1985) Eur. J. Immunol. 15, 733-738. [DOI] [PubMed] [Google Scholar]
- 2.Israel, E. J., Patel, V. K., Taylor, S. F., Marshak-Rothstein, A. & Simister, N. E. (1995) J. Immunol. 154, 6246-6251. [PubMed] [Google Scholar]
- 3.Martin, W. L., West, A. P., Jr., Gan, L. & Bjorkman, P. J. (2001) Mol. Cell 7, 867-877. [DOI] [PubMed] [Google Scholar]
- 4.Kim, J. K., Firan, M., Radu, C. G., Kim, C. H., Ghetie, V. & Ward, E. S. (1999) Eur. J. Immunol. 29, 2819-2825. [DOI] [PubMed] [Google Scholar]
- 5.Berryman, M. & Rodewald, R. (1995) J. Cell Sci. 108, 2347-2360. [DOI] [PubMed] [Google Scholar]
- 6.Story, C. M., Mikulska, J. E. & Simister, N. E. (1994) J. Exp. Med. 180, 2377-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel, E. J., Taylor, S., Wu, Z., Mizoguchi, E., Blumberg, R. S., Bhan, A. & Simister, N. E. (1997) Immunology 92, 69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haymann, J. P., Levraud, J. P., Bouet, S., Kappes, V., Hagege, J., Nguyen, G., Xu, Y., Rondeau, E. & Sraer, J. D. (2000) J. Am. Soc. Nephrol. 11, 632-639. [DOI] [PubMed] [Google Scholar]
- 9.Spiekermann, G. M., Finn, P. W., Ward, E. S., Dumont, J., Dickinson, B. L. Blumberg, R. S. & Lencer, W. I. (2002) J. Exp. Med. 196, 303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson, B. L., Badizadegan, K. Z., Wu, Z., Ahouse, J. C., Zhu, Z., Simister, N. E., Blumberg, R. S. & Lencer, W. I. (1994) J. Clin. Invest. 104, 903-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashkenazi, A., Capon, D. J. & Ward, R. H. R. (1993) Int. Rev. Immunol. 10, 219-227. [DOI] [PubMed] [Google Scholar]
- 12.Junghans, R. P. & Anderson, C. L. (1996) Proc. Natl. Acad. Sci. USA 93, 5512-5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaharty, K. K. (1990) Pharmacotherapy 10, 9S-14S [PubMed] [Google Scholar]
- 14.Cefalu, W. T. (2001) Ann. Med. 33, 579-586. [PubMed] [Google Scholar]
- 15.Patton, J. S., Trinchero, P. & Platz, R. M. (1994) J. Contr. Rel. 28, 79-85. [Google Scholar]
- 16.Komada, F., Iwakawa, S., Yamamoto, N., Sakakibara, H. & Okumura, K. (1994) J. Pharmacol. Sci. 83, 863-867. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy, K. M., Lam, M., Subramanian, L.,Shakya, R., Wu, Z., Newton, E. E. & Simister, N. E. (2001) J. Cell Sci. 114, 1591-1598. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd Ed.
- 19.Urlaub, G., Mitchell, P. J., Kas, E., Chasin, L. A., Funanage V. L., Myoda, T. T. & Hamlin, J. (1986) Som. Cell Mol. Genet. 12, 555-566. [DOI] [PubMed] [Google Scholar]
- 20.West, A. P., Jr., & Bjorkman, P. J. (2000) Biochemistry 39, 9698-9708. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura, T., Tange, T., Terasawa, T., Chiba, S., Kuwaki, T., Miyagawa, K., Piao, Y. F., Miyazono, K., Urabe, A. & Takaku, F. (1989) Cell. Physiol. 140, 323-334. [DOI] [PubMed] [Google Scholar]
- 22.Hammerling, U., Kroon, R., Wilhelmsen, T. & Sjodin, L. (1996) J. Pharmacol. Biomed. Anal. 14, 1455-1469. [DOI] [PubMed] [Google Scholar]
- 23.Newman, S. P. (1993) Crit. Rev. Ther. Drug Carr. Syst. 10, 65-109. [PubMed] [Google Scholar]
- 24.Lei, K., Rusckowski, M., Chang, F., Qu, T., Mardirossian, G. & Hnatowich, D. J. (1996) Nucleic Med. Biol. 23, 917-922. [DOI] [PubMed] [Google Scholar]
- 25.Economides, A. N., Carpenter, L. R., Rudge, J. S., Wong, V., Koehler-Stec, E. M., Hartnett, C., Pyles, E. A., Xu, X., Daly, T. J., Young, M. R., et al. (2003) Nat. Med. 9, 47-52. [DOI] [PubMed] [Google Scholar]
- 26.Suarez, S. & Hickey, A. J. (2000) Respir. Care 45, 652-666. [PubMed] [Google Scholar]
- 27.Patton, J. S. (1996) Drug Del. Rev. 19, 3-36. [Google Scholar]
- 28.Byron, P. R. & Patton, J. S. (1994) J. Aerosol. Med. 7, 49-75. [DOI] [PubMed] [Google Scholar]
- 29.Gupta, P. K. & Adjei, A. L., eds. (1997) Lung Biology Health and Disease (Dekker, New York), pp. 89-131.