Abstract
Association between tumor necrosis factor-α (TNF-α) G-308A (rs1800629) polymorphism and susceptibility to aggressive periodontitis (AgP) were inconsistent, hence we performed this meta-analysis to clarify the association between them using Comprehensive Meta-Analysis v2.2 software. 16 case-control studies were searched from the PubMed, Embase and CNKI databases up to February 2, 2015. The meta-analysis showed a significantly increased risk in A vs. G (OR = 1.23, 95%CI = 1.04–1.44), AA vs. GG (OR = 2.07, 95%CI = 1.11–3.87), and AA vs. AG+GG genetic models (OR = 2.09, 95%CI = 1.13–3.86); however, the non-significantly increased risk was shown in AG vs. GG (OR = 1.06, 95%CI = 0.85–1.32) and AA+AG vs. GG genetic models (OR = 1.06, 95%CI = 0.85–1.31). Cumulative analysis showed that the association changed from non-significant to significant with new studies accumulated and the CIs became more and more narrow, sensitivity analysis indicated results were statistically robust. Stratified analyses of confirmed of HWE, Asians, Caucasians, and population-based controls obtained results similar to that of overall analysis. There was no evidence of publication bias. In summary, current evidence demonstrates that TNF-a G-308A polymorphism might be associated with AgP susceptibility, especially in Asians and Caucasians.
Aggressive periodontitis (AgP) is featured of the rapid rate of disease progression, onset in healthy individuals without large accumulation of plaque and/or calculus, and with genetic familial trait1,2,3. In 1999, the new term “aggressive periodontitis” have been proposed to replace the former nomenclature “early-onset periodontitis”, which contained “juvenile periodontitis”, “rapidly progressive periodontitis”, and “prepubertal periodontitis”4. Concerning the serious hazards of periodontal tissues of patients with AgP in adolescents, it has widely involved more and more scholars and etiological researches referred to the molecular biology, genetics, microbiology, cell biology and other fields for its complex etiology. Surely, some pathogens are the external initiating factor in the pathogenesis of AgP, and the degrees of harm risk are inconsistent, suggesting that host heterogeneity might be a decisive factor in the pathogenesis of AgP5. Hence, AgP can be considered as a complex genetic disease and the influence of genes and environmental factors determine the individual phenotype corporately6.
With the deepen research of human gene, the more extensive evidence of pathogenesis of periodontitis has been provided7. Researches indicated nearly half of the clinical differences of periodontal disease rooted from gene polymorphism8. Therefore, many gene polymorphisms have been investigated9,10,11,12,13. Tumor necrosis factor (TNF) - α is one of the most potent proinflammatory cytokines and play a role in tissue injury and induced bone resorption in the immune response system, and its coding gene has been mapped to chromosome 6. The G-308A (rs1800629) is a polymorphism causing a substitution from the guanine (G) to adenine (A) and leads to two- to three fold higher transcriptional activity of TNF-α upon stimulation with bacterial lipopolysaccharide14,15. Carriage of the rare -308 A allele is associated with significantly greater TNF-α production and transcription. In addition, the A allele has been associated with increased risk for various non-related infectious and inflammatory diseases, including periodontitis.
In 2013, Song et al. performed a meta-analysis and indicated that TNF-a -308 A allele was associated with periodontitis10; However, their meta-analysis pooled chronic periodontitis (CP) and AgP together and included only 6 case-control studies for AgP. Nowadays, there were 17 studies that explored the association between TNF-α G-308A polymorphism and AgP have been published. The results of these published studies remain inconsistent. Therefore, this meta-analysis was conducted to provide an updated approach on the overall relationship between TNF-α G-308A polymorphism and AgP. Subgroup analyses were also performed on smoking and non-smoking status to investigate smoking-specific effects and cumulative analysis was used to investigate the trend of association.
Methods
We followed the recommended Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement16 to report this meta-analysis, and the ethnic review was not required.
Eligibility criteria
According to the “PICOS” principle16, this meta-analysis included studies which met the following criteria: (i) evaluating the association between TNF-α G-308A polymorphism and AgP, or all periodontitis but the data for AgP could be extracted; (ii) the study design was a case-control or cohort; (iii) the publication language was Chinese or English, and the full-text can be obtained; (iv) the relevant data was detailed enough to be used in the calculation, or the odds ratio (OR) with its 95% confidence interval (CI) was reported; (v) when more than one publications covered the same study population, we only included the study with higher quality and more comprehensive information. Animal experiments, conference abstracts, and comments were excluded. Studies with AgP patients who had any systematic diseases, such as diabetes mellitus, coronary heart disease were also excluded.
Search strategy
A systematical search to retrieve published literatures from PubMed, Embase, and CNKI (China Knowledge Resource Integrated) databases was conducted up to February 2, 2015. The following key words and subject terms were used: “tumor necrosis factor alpha”, “TNF-α”, “TNF-alpha”, “periodontitis”, “periodontal disease”, “polymorphism”, “mutation”, and “variant”. Hand-searching of listed references of included studies, previous meta-analysis, and recent reviews was also performed to identify additional studies.
Data extraction
The process of the data extraction was independently conducted and cross checked by two authors, and a third author participated in the discussion in case of divergence. We extracted the information as follows: the first author and year of publication, study area, ethnicity, smoking status, genotyping methods, the source of the controls, Hardy-Weinberg equilibrium (HWE) of control, and numbers of eligible genotyped cases and controls with the exhaustive data of each genotype distribution or the OR with its 95%CI.
Statistical analysis
The OR with its 95% CI in the allelic contrast (A vs. G), recessive model (AA vs. AG+GG), dominant model (AA+AG vs. GG), and co-dominant model (AA vs. GG and AG vs. GG) were calculated to assess the association between TNF-α G-308A polymorphism and AgP risk. First, heterogeneity among included studies was detected using I2 and Cochran Q statistics, and the value of I2 ≤50% and p > 0.1 indicates low heterogeneity and using fixed-effects model, otherwise the random-effects model is used17,18,19,20. We conducted stratification analyses on the bias of control conform to HWE, ethnicity, source of control, and smoking status. In order to investigate the robustness of overall analysis, we conducted sensitivity analysis by deleting each included study in turn21,22,23. The cumulative analysis was also carried out to explore the trend of association24,25. Moreover, we performed meta-regression to figure the influence of smoking status for TNF-α G-308A polymorphism and the susceptibility of AgP. Publication bias was analyzed by funnel plot and Egger’s test26. All the above mentioned statistical analyses were undertaken using the Comprehensive Meta-Analysis v2.2 software21,22,23.
Results
Characteristics of included studies
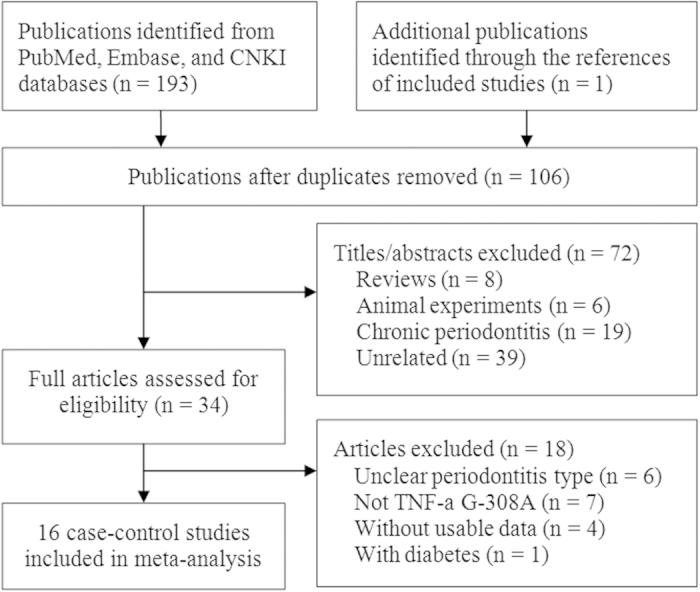
A total of 194 citations was yielded and finally 16 case-control studies involving 905 cases and 1270 controls were included27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. One was excluded due to the AgP patients with type 1 diabetes mellitus43. Figure 1 showed the process of study selection.
Figure 1. Study selection flow diagram.
Seven studies both included smokers and non-smokers30,31,33,34,35,37,41, six only included non-smokers29,32,36,38,40,42, and three did not report smoking status27,28,39. One study only reported the OR and 95%CI of Allele comparison39, the others all reported the genotyping distribution. The controls of two studies out of HWE30,42. Detailed information of characteristics of included studies is available in Table 1.
Table 1. Characteristics of studies included in the meta-analysis.
| References | Country (ethnicity) | Smoking status | Genotyping method | Source of control | Case/ Control |
HWE | ||
|---|---|---|---|---|---|---|---|---|
| Sample | G | A | ||||||
| Endo 2001 | Japan (Asian) | Unknown | PCR-SSOP | PB | 46/104 | 90/204 | 2/4 | Yes |
| Shapira 2001 | Israel (Caucasian) | Unknown | PCR-TaqMan | PB | 16/27 | 29/47 | 3/7 | Yes |
| Pérez 2004 | Chile (Chileans) | Non-smokers | PCR-RFLP | PB | 27/30 | 49/55 | 5/5 | Yes |
| Brett 2005 | UK (Caucasian) | Mixed | PCR-SSP | PB | 50/97 | 76/155 | 24/39 | No |
| Sakellari 2006 | Greek (Caucasian) | Mixed | PCR | PB | 46/90 | 78/156 | 14/24 | Yes |
| Zhu 2007 | China (Asian) | Mixed | PCR-RFLP | HB | 64/78 | 111/144 | 17/12 | Yes |
| de Freitas 2007 | Brazil (Caucasian) | Non-smokers | PCR-RFLP | PB | 30/70 | 46/102 | 14/38 | Yes |
| Guzeldemir 2008 | Turkey (Caucasian) | Non-smokers | PCR | PB | 31/31 | 46/55 | 16/7 | Yes |
| Schulz 2008 | Germany (Caucasian) | Mixed | PCR | PB | 69/52 | 109/86 | 29/18 | Yes |
| Menezes 2008 | Brazil (Brazilian) | Mixed | PCR-RFLP | HB | 38/51 | 61/73 | 15/29 | Yes |
| Moreira 2009 | Brazil (Brazilian) | Mixed | PCR-RFLP | HB | 55/43 | 95/72 | 15/14 | Yes |
| Erciyas 2010 | Turkey (Caucasian) | Non-smokers | PCR-SSP | PB | 35/85 | 55/154 | 15/16 | Yes |
| Scapoli 2011 | Italy (Caucasian) | Unknown | PCR-MassARRAY | PB | 122/246 | 1.19(0.81–1.74)* | Yes | |
| Ebadian 2013 | Iranian (Caucasian) | Non-smokers | PCR-RFLP | PB | 58/60 | 74/88 | 42/32 | Yes |
| Yang 2013 | China (Asian) | Mixed | PCR-RFLP | HB | 180/180 | 275/292 | 85/68 | Yes |
| Özer Yücel 2015 | Turkey (Caucasian) | Non-smokers | PCR-RFLP | HB | 38/26 | 56/35 | 20/17 | No |
PB, population-based; HB, hospital-based; HWE, Hardy-Weinberg equilibrium; Mixed, smokers and non-smokers; *odds ratio with its 95% confidence interval.
Overall, cumulative, and sensitivity analyses
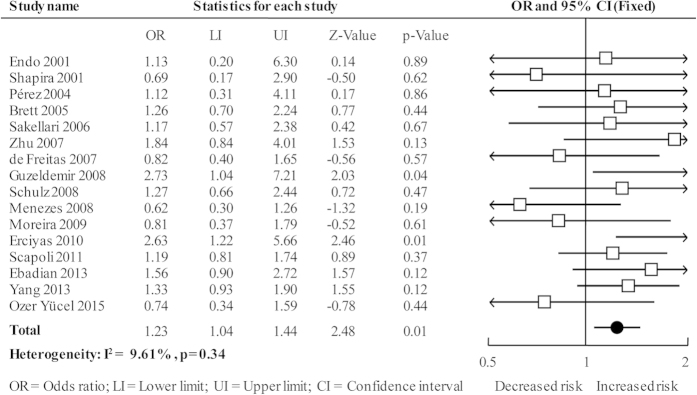
The overall analysis showed a significantly increased risk in A vs. G (OR = 1.23, 95%CI = 1.04–1.44; Fig. 2), AA vs. GG (OR = 2.07, 95%CI = 1.11–3.87; see Supplementary Fig. S1 online), and AA vs. AG+GG genetic models (OR = 2.09, 95%CI = 1.13–3.86; see Supplementary Fig. S2 online); however, the non-significant increased risk was shown in AG vs. GG (OR = 1.06, 95%CI = 0.85–1.32; see Supplementary Fig. S3 online) and AA+AG vs. GG genetic models (OR = 1.06, 95%CI = 0.85–1.31; see Supplementary Fig. S4 online). The heterogeneity of all the five genetic models were acceptable and the results were pooled using fixed-effects model (Table 2).
Figure 2. Forest plot of overall analysis in allele comparison.
Table 2. Results of overall and subgroups analyses.
| Overall and subgroups | Number of Studies | Heterogeneity |
Model | Meta-analysis |
||
|---|---|---|---|---|---|---|
| p | I2(%) | OR(95%CI) | p for OR | |||
| A vs. G | 16 | 0.34 | 9.61 | Fixed | 1.23(1.04–1.44) | 0.01 |
| HWE (Yes) | 14 | 0.32 | 12.35 | Fixed | 1.26(1.06–1.49) | 0.01 |
| HWE (No) | 2 | 0.28 | 15.29 | Fixed | 1.04(0.65–1.65) | 0.88 |
| Population-based | 11 | 0.56 | 0 | Fixed | 1.31(1.07–1.61) | 0.01 |
| Hospital-based | 5 | 0.14 | 41.6 | Fixed | 1.10(0.85–1.43) | 0.47 |
| Smoking (Mixed) | 7 | 0.45 | 0 | Fixed | 1.19(0.95–1.48) | 0.13 |
| Smoking (No) | 6 | 0.09 | 47.68 | Random | 1.39(0.88–2.17) | 0.15 |
| Asian | 3 | 0.74 | 0 | Fixed | 1.39(1.01–1.92) | 0.04 |
| Brazilian | 2 | 0.62 | 0 | Fixed | 0.70(0.40–1.18) | 0.18 |
| Caucasian | 9 | 0.3 | 15.37 | Fixed | 1.27(1.04–1.55) | 0.02 |
| Chileans | 1 | / | / | / | 1.12(0.31–4.11) | 0.86 |
| AA vs. GG | 15 | 0.14 | 36.5 | Fixed | 2.07(1.11–3.87) | 0.02 |
| HWE (Yes) | 13 | 0.14 | 36.5 | Fixed | 2.07(1.11–3.87) | 0.02 |
| HWE (No) | 2 | – | – | – | – | – |
| Population-based | 10 | 0.27 | 22.38 | Fixed | 2.73(1.01–7.35) | 0.05 |
| Hospital-based | 5 | 0.04 | 75.6 | Random | 1.15(0.17–7.87) | 0.89 |
| Smoking (Mixed) | 7 | 0.11 | 50.97 | Random | 2.18(0.56–8.44) | 0.26 |
| Smoking (No) | 6 | 0.18 | 38.63 | Fixed | 1.95(0.64–6.00) | 0.24 |
| Asian | 3 | 1 | 0 | Fixed | 2.71(1.09–6.73) | 0.03 |
| Brazilian | 2 | 1 | 0 | Fixed | 0.37(0.07–2.02) | 0.25 |
| Caucasian | 9 | 0.27 | 22.38 | Fixed | 2.73(1.01–7.35) | 0.05 |
| Chileans | 1 | / | / | / | – | – |
| AG vs. GG | 15 | 0.22 | 20.56 | Fixed | 1.06(0.85–1.32) | 0.62 |
| HWE (Yes) | 13 | 0.2 | 24.15 | Fixed | 1.06(0.83–1.34) | 0.64 |
| HWE (No) | 2 | 0.18 | 44.51 | Fixed | 1.06(0.60–1.87) | 0.85 |
| Population-based | 10 | 0.19 | 28.16 | Fixed | 1.15(0.85–1.55) | 0.35 |
| Hospital-based | 5 | 0.35 | 9.53 | Fixed | 0.96(0.69–1.32) | 0.79 |
| Smoking (Mixed) | 7 | 0.56 | 0 | Fixed | 1.01(0.77–1.32) | 0.96 |
| Smoking (No) | 6 | 0.04 | 57.69 | Random | 1.21(0.81–1.82) | 0.34 |
| Asian | 3 | 0.32 | 12.67 | Fixed | 1.14(0.77–1.69) | 0.52 |
| Brazilian | 2 | 0.92 | 0 | Fixed | 0.75(0.40–1.42) | 0.38 |
| Caucasian | 9 | 0.08 | 42.99 | Random | 1.06(0.70–1.59) | 0.79 |
| Chileans | 1 | / | / | / | 1.14(0.29–4.45) | 0.85 |
| AA vs. AG+GG | 15 | 0.13 | 37.95 | Fixed | 2.09(1.13–3.86) | 0.02 |
| HWE (Yes) | 13 | 0.13 | 37.95 | Fixed | 2.09(1.13–3.86) | 0.02 |
| HWE (No) | 2 | – | – | – | – | – |
| Population-based | 10 | 0.22 | 29.33 | Fixed | 2.65(0.99–7.04) | 0.05 |
| Hospital-based | 5 | 0.05 | 73.93 | Random | 1.22(0.20–7.62) | 0.83 |
| Smoking (Mixed) | 7 | 0.11 | 49.57 | Fixed | 2.20(1.05–4.61) | 0.04 |
| Smoking (No) | 6 | 0.15 | 43.12 | Fixed | 1.86(0.62–5.63) | 0.27 |
| Asian | 3 | 1 | 0 | Fixed | 2.75(1.12–6.75) | 0.03 |
| Brazilian | 2 | 1 | 0 | Fixed | 0.42(0.08–2.19) | 0.3 |
| Caucasian | 9 | 0.22 | 29.33 | Fixed | 2.65(0.99–7.04) | 0.05 |
| Chileans | 1 | / | / | / | – | – |
| AA+AG vs. GG | 15 | 0.91 | 0 | Fixed | 1.06(0.85–1.31) | 0.61 |
| HWE (Yes) | 13 | 0.93 | 0 | Fixed | 1.06(0.84–1.33) | 0.64 |
| HWE (No) | 2 | 0.18 | 44.51 | Fixed | 1.06(0.60–1.87) | 0.85 |
| Population-based | 10 | 0.99 | 0 | Fixed | 1.08(0.80–1.45) | 0.62 |
| Hospital-based | 5 | 0.24 | 26.65 | Fixed | 1.03(0.76–1.41) | 0.84 |
| Smoking (Mixed) | 7 | 0.58 | 0 | Fixed | 1.10(0.85–1.43) | 0.45 |
| Smoking (No) | 6 | 0.83 | 0 | Fixed | 0.98(0.65–1.46) | 0.91 |
| Asian | 3 | 0.52 | 0 | Fixed | 1.29(0.89–1.88) | 0.18 |
| Brazilian | 2 | 0.74 | 0 | Fixed | 0.70(0.38–1.30) | 0.26 |
| Caucasian | 9 | 0.92 | 0 | Fixed | 1.02(0.76–1.37) | 0.89 |
| Chileans | 1 | / | / | Fixed | 1.14(0.29–4.45) | 0.85 |
OR, odds ratio; CI, confidence interval; HWE, Hardy-Weinberg equilibrium; –, the AA frequency = 0; /, only one study included.
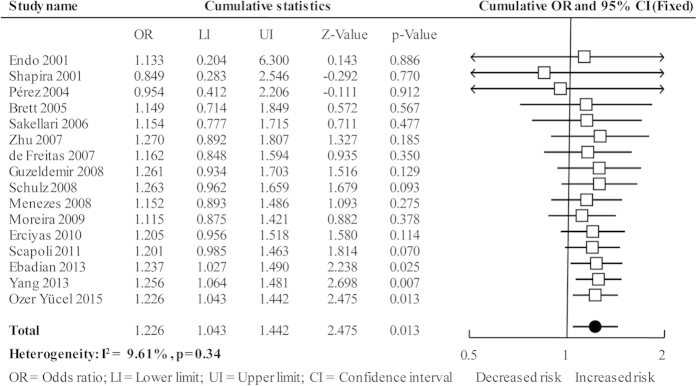
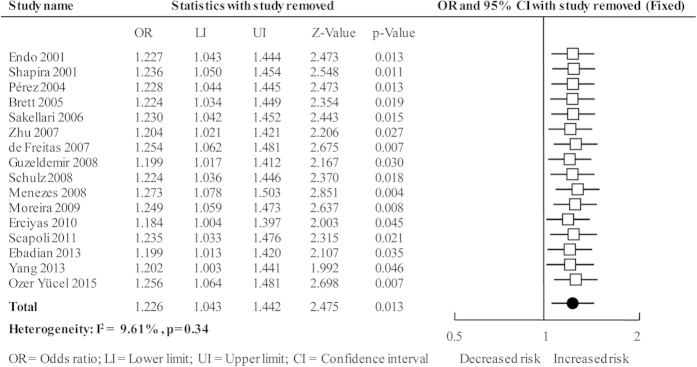
The cumulative analysis was performed by accumulating included studies sequentially with increasing publication year, and the result showed that the association changed from non-significant to significant with new studies accumulated and the CIs became more and more narrow (Fig. 3). The sensitivity analysis was performed by deleting every included studies of this meta-analysis one by one, the results were not materially altered that indicating our results were statistically robust (Fig. 4).
Figure 3. Cumulative analysis plot of overall analysis in allele comparison.
Figure 4. Sensitivity analysis plot of overall analysis in allele comparison.
Subgroups, meta-regression, and publication bias analyses
Table 2 showed the results of all performed subgroup analyses. Stratified analyses of confirmed of HWE, Asians, Caucasians, and population-based controls obtained results similar to that of the overall analysis; however, the results of out of HWE studies, hospital-based controls, mixed smoking status, non-smokers, Brazilians, and Chileans all revealed non-significant association between TNF-α G-308A polymorphism and AgP. The results of meta-regression analysis showed that the smoking status can’t affect the association between TNF-α G-308A polymorphism and the susceptibility to AgP (see Supplementary Fig. S5 online).
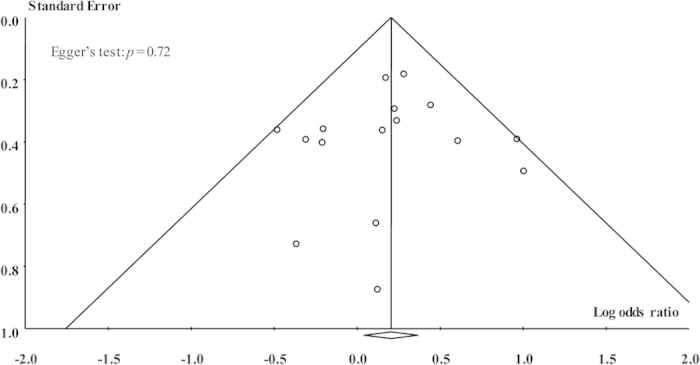
Dependably, no evidence of publication bias was found in this meta-analysis for any genetic models, which was supported by Egger’s test (A vs. G: p = 0.72; AA vs. GG: p = 0.99; AG vs. GG: p = 0.66; AA vs. AG+GG: p = 0.97; and AA+AG vs. GG: p = 0.25) and symmetric funnel plots (Fig. 5).
Figure 5. Funnel plot of overall analysis in allele comparison.
Discussion
Main findings
AgP, a distinguished subgroup of CP with the characteristics of familial aggregates, rapid disease progression and high incidence among period of adolescents, has a strong influence on the health of periodontal tissue. Epidemiologic studies showed that the incidence of AgP differed widely among different areas, different countries and different races. Unsatisfactorily, related reported dates were relative limited, ranging from 0.1% to 15% among overseas44. Now there are numerous studies on gene polymorphisms of inflammatory factor but haven’t come to an accordant conclusion45. As the local inflammation of the body, inflammatory factor TNF-α has an important stimulation and has a wide biological effect on leukocytes, vascular endothelium cells and different cells in connective tissue. TNF-α can cause the destruction of connective tissue and strengthen the formation and activity of osteoclast, thereby limiting the repair of periodontal tissue46. Our meta-analysis with 16 case-control studies quantitatively explores the relationship between TNF-α G-308A polymorphism and the susceptibility to AgP, demonstrating that the TNF-α G-308A polymorphism confers a weak susceptibility to AgP, and sensitivity analyses showed the results were robust. Subgroups analyses showed that TNF-a G-308A polymorphism might be associated with Caucasian and Asians, but not associated with Brazilians and Chileans. The results of cumulative analysis showed that the association changed from non-significant to significant with new studies accumulated, and this might indicate that statistical significance could be detected when sample size became enough.
Considering smoking is responsible for periodontitis to some certain degree47,48, we conducted a subgroup analysis based on smoking status. The results showed that the association between TNF-α G-308A polymorphism and the susceptibility to AgP was not affected by the smoking status in all contrast gene type, thus smoking status maybe not be a factors of special importance in TNF-α G-308A polymorphism, and this was also confirmed by meta-regression analysis. However, the results of HWE and source of controls indicated that studies out of HWE and studies with controls from hospital might bias the result.
Strengths of study
The previous meta-analysis by Song et al.10 in 2013 investigated the TNF-α G-308A polymorphism and CP and AgP at the same time. This meta-analysis included 6 case-control studies for AgP and yielded negative association. Compared with CP, AgP refers to uncommon forms of bacterially-induced periodontitis and is considered as a genetically inherited disease6,22,23,49. Hence, our study is the first meta-analysis focusing on the association between TNF-α G-308A polymorphism and AgP risk. We also considered the influence of smoking status and stratified analysis according to the smoking status, which could provide more valuable information on this topic. Additionally, our meta-analysis included 16 case-control studies and the results are more precise, and the cumulative analyses also proved this. Moreover, we conducted four subgroups meta-analysis based on characteristics of studies and explained these roundly, while their just for race. Obviously, compared with this meta-analysis, our study carried out cumulative meta-analysis, smoking status based analysis and meta-regression analysis. Of course, our meta-analysis provided more advisory for periodontitis prevention, diagnosis, and treatment.
Our meta-analysis, conducted following a canonical systematically processes, for which some specific points need to be interpreted here. As we all know, the nomenclature of AgP has evolved with further understanding about the disease, but some researchers failed to capture the new terms in time, precisely, we not only took the present name “aggressive periodontitis, AgP” but also the former name “early-onset periodontitis, EOP” into account to ensure comprehensively covering eligible studies. The studies of Zhu et al. in 200733 compared the differences of gene polymorphisms between male and female and reached the conclusion that TNF-α G-308A might be associated with the susceptibility to AgP for male individuals in China, while other included studies did not implement analysis by gender, accordingly the factor of genders cannot be analyzed here, but may be of value.
Limitations of study
Meta-analysis is a secondary research, and the result of its researches largely depended on primary studies50, so there inevitably existed some limitations. First, 9 included studies were conducted based on Caucasian but for other races only 1 to 3 studies could be acquired, indicating limitations from the insufficient numbers of primary researches for some subgroups based on ethnicity; moreover, the sample sizes in some included studies, overall and subgroups analyses were relatively small. The limited sample sizes might bias the result. The cumulative analysis also indicated that the association became significant when the thirteen study accumulated. According to this, we must treat all results of subgroup analyses with caution. Second, it is hard for researchers to ensure strict consistency when they conduct research under the control of related factors criteria, such as age range, physical condition and lifestyle of the included populations, the diagnose index of AgP, the genotyping methods of included studies, which will affect the result of research to some degree. Of course, these are also the shortcomings of case-control study. Our meta-analysis based on case-control studies and did not escape from the influence of these factors. Third, publication bias test showed no evidence of publication bias existed, but this research conducted a comprehensive retrieval with language limited to Chinese and English, thus it may lead to the corresponding language bias, although we know this was limited by the accessibility to databases and ability of language. Fourth, the defect of a single gene may be a weak factor to promote the cause of disease directly for the normal function of proteins will not easily change that kind of greatly, so the combined polygenic factors are worth considering strengthening the evidence. Additionally, the adjusted data could not be extracted from included studies and we can only carry our analyses based on crude data. All in all, we should take the conclusion of this study seriously.
Implications for practice and research
From the foregoing, as for the association between TNF-α G-308A polymorphism and AgP susceptibility, large-scale and high-quality researches can be implemented in point. Owing to the complex causes of AgP alone with a wide range of risk of factors, such as individual immunity, special microbial infection, heredity, certain diseases (e.g. diabetes), gender, habits (e.g. smoking, diet), stress, certain genetic polymorphisms, etc, future researches should try to strictly control various confounding factors to ensure the reliability of the results with explicit and strict inclusion criteria of cases. Thus, there are some points we recommend for further practices and researches. As we know, smoking is a risk factor and influence the treatment effect of periodontitis48,51, moreover, subgroup analysis and meta-regression analysis both indicated that this polymorphism was not influenced by smoking status (Table 2 and see Supplementary Fig. S5 online). This might mean that we could not know whether TNF-α G-308A polymorphism is a marker of smoking rather or a risk factor of AgP susceptibility. Hence, what the role of TNF-α G-308A polymorphism in the development of AgP is necessary to research either in smokers or non-smokers. Large-scale studies combining multiple loci might be of significance; then, further investigations based on the difference between population characterizes, such as gender, age, family history, can be developed; furthermore, combining with other type diseases or risk factors facilitated to AgP to explore the susceptibility between gene polymorphisms and AgP with multiple dangerous risk factors may be an additional direction of research. Moreover, from the implications of subgroups analysis and cumulative analysis, more relevant studies should be performed due to the small sample size currently. We also suggest that further studies should provide adjusted data, use population-based design, use uniformed AgP diagnosis criteria, and clearly reported the smoking status and ethnicity.
Additional Information
How to cite this article: Wei, X.-M. et al. Tumor necrosis factor-a G-308A (rs1800629) polymorphism and aggressive periodontitis susceptibility: a meta-analysis of 16 case-control studies. Sci. Rep. 6, 19099; doi: 10.1038/srep19099 (2016).
Supplementary Material
Footnotes
Author Contributions X.M.W. and X.T.Z. designed this study; Y.J.C. and L.J.C. searched databases and collected full-text papers; L.W. and D.W.H. extracted and analyzed data; X.M.W., Y.J.C. and L.W. wrote the manuscript, X.T.Z. reviewed the manuscript.
References
- Taba M. Jr., Kinney J., Kim A. S. & Giannobile W. V. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am 49, 551–571, vi (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente M. A. & Griffiths G. S. Periodontal status among relatives of aggressive periodontitis patients and reliability of family history report. J Clin Periodontol 33, 121–125 (2006). [DOI] [PubMed] [Google Scholar]
- Javed F., Al-Hezaimi K., Salameh Z., Almas K. & Romanos G. E. Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine 53, 8–12 (2011). [DOI] [PubMed] [Google Scholar]
- Armitage G. C. Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4, 1–6 (1999). [DOI] [PubMed] [Google Scholar]
- Meng H., Xu L., Li Q., Han J. & Zhao Y. Determinants of host susceptibility in aggressive periodontitis. Periodontol 2000 43, 133–159 (2007). [DOI] [PubMed] [Google Scholar]
- Hart T. C. et al. Evidence of genetic heterogeneity for hereditary gingival fibromatosis. J Dent Res 79, 1758–1764 (2000). [DOI] [PubMed] [Google Scholar]
- McFarlane C. G., Reynolds J. J. & Meikle M. C. The release of interleukin-1 beta, tumor necrosis factor-alpha and interferon-gamma by cultured peripheral blood mononuclear cells from patients with periodontitis. J Periodontal Res 25, 207–214 (1990). [DOI] [PubMed] [Google Scholar]
- Michalowicz B. S. et al. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol 71, 1699–1707 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Associations between CD14 -159 C>T polymorphism and chronic/aggressive periodontitis susceptibility. Oral Dis 19, 805–811 (2013). [DOI] [PubMed] [Google Scholar]
- Song G. G., Choi S. J., Ji J. D. & Lee Y. H. Association between tumor necrosis factor-alpha promoter -308 A/G, -238 A/G, interleukin-6 -174 G/C and -572 G/C polymorphisms and periodontal disease: a meta-analysis. Mol Biol Rep 40, 5191–5203 (2013). [DOI] [PubMed] [Google Scholar]
- Mao M. et al. Interleukin-1alpha -899 (+4845) C−>T polymorphism increases the risk of chronic periodontitis: evidence from a meta-analysis of 23 case-control studies. Gene 532, 114–119 (2013). [DOI] [PubMed] [Google Scholar]
- Zheng J. et al. Association between TLR4 polymorphism and periodontitis susceptibility: a meta-analysis. Crit Rev Eukaryot Gene Expr 23, 257–264 (2013). [DOI] [PubMed] [Google Scholar]
- Prakash G. et al. COX-2 gene polymorphisms and risk of chronic periodontitis: a case-control study and meta-analysis. Oral Dis (2013). [DOI] [PubMed] [Google Scholar]
- Laine M. L., Moustakis V., Koumakis L., Potamias G. & Loos B. G. Modeling susceptibility to periodontitis. J Dent Res 92, 45–50 (2013). [DOI] [PubMed] [Google Scholar]
- Laine M. L., Crielaard W. & Loos B. G. Genetic susceptibility to periodontitis. Periodontol 2000 58, 37–68 (2012). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G. & Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng P., Liao Y., Ruan Z. & Liang H. Increased risk of cutaneous melanoma associated with p53 Arg72Pro polymorphism. PLoS One 10, e0118112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng P. et al. Genetic association between PER3 genetic polymorphisms and cancer susceptibility: a meta-analysis. Medicine (Baltimore) 94, e568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. T. et al. Meta-analysis on the association between toothbrushing and head and neck cancer. Oral Oncol 51, 446–451 (2015). [DOI] [PubMed] [Google Scholar]
- Yan Y., Weng H., Shen Z. H., Wu L. & Zeng X. T. Association between interleukin-4 gene -590 c/t, -33 c/t, and 70-base-pair polymorphisms and periodontitis susceptibility: a meta-analysis. J Periodontol 85, e354–362 (2014). [DOI] [PubMed] [Google Scholar]
- Chen Y. J. et al. Interleukin-1beta rs1143634 polymorphism and aggressive periodontitis susceptibility: a meta-analysis. Int J Clin Exp Med 8, 2308–2316 (2015). [PMC free article] [PubMed] [Google Scholar]
- Zeng X. T. et al. Meta-Analysis of Association Between Interleukin-1beta C-511T Polymorphism and Chronic Periodontitis Susceptibility. J Periodontol 86, 812–819 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang J., Zeng X. T., Lei J. R., Tang Y. J. & Yang J. No association between XRCC1 gene Arg194Trp polymorphism and risk of lung cancer: evidence based on an updated cumulative meta-analysis. Tumour Biol 35, 5629–5635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng W. D., Zeng X. T., Kwong J. S. W. & Hua X. P. Periodontal disease and risk of coronary heart disease: an updated meta-analysis of prospective cohort studies. Int J Cardiol 201, 469–472 (2015). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. et al. Analysis of single nucleotide polymorphisms in the 5′-flanking region of tumor necrosis factor-alpha gene in Japanese patients with early-onset periodontitis. J Periodontol 72, 1554–1559 (2001). [DOI] [PubMed] [Google Scholar]
- Shapira L., Stabholz A., Rieckmann P. & Kruse N. Genetic polymorphism of the tumor necrosis factor (TNF)-alpha promoter region in families with localized early-onset periodontitis. J Periodontal Res 36, 183–186 (2001). [DOI] [PubMed] [Google Scholar]
- Perez C. et al. The -308 polymorphism in the promoter region of the tumor necrosis factor-alpha (TNF-alpha) gene and ex vivo lipopolysaccharide-induced TNF-alpha expression in patients with aggressive periodontitis and/or type 1 diabetes mellitus. Eur Cytokine Netw 15, 364–370 (2004). [PubMed] [Google Scholar]
- Brett P. M. et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res 84, 1149–1153 (2005). [DOI] [PubMed] [Google Scholar]
- Sakellari D. et al. No correlation of five gene polymorphisms with periodontal conditions in a Greek population. J Clin Periodontol 33, 765–770 (2006). [DOI] [PubMed] [Google Scholar]
- Maria de Freitas N. et al. Analysis of IL-1A(-889) and TNFA(-308) gene polymorphism in Brazilian patients with generalized aggressive periodontitis. Eur Cytokine Netw 18, 142–147 (2007). [DOI] [PubMed] [Google Scholar]
- Zhu X. L. et al. [Relationship between tumor necrosis factor A-308 gene polymorphism and aggressive periodontitis]. Zhonghua Kou Qiang Yi Xue Za Zhi 42, 268–271 (2007). [PubMed] [Google Scholar]
- Guzeldemir E., Gunhan M., Ozcelik O. & Tastan H. Interleukin-1 and tumor necrosis factor-alpha gene polymorphisms in Turkish patients with localized aggressive periodontitis. J Oral Sci 50, 151–159 (2008). [DOI] [PubMed] [Google Scholar]
- Menezes N. G. & Colombo A. P. Lack of association between the TNF-alpha -308 (G/A) genetic polymorphism and periodontal disease in Brazilians. Braz Oral Res 22, 322–327 (2008). [DOI] [PubMed] [Google Scholar]
- Schulz S. et al. Genetic markers of tumour necrosis factor alpha in aggressive and chronic periodontitis. J Clin Periodontol 35, 493–500 (2008). [DOI] [PubMed] [Google Scholar]
- Moreira P. R., Costa J. E., Gomez R. S., Gollob K. J. & Dutra W. O. TNFA and IL10 gene polymorphisms are not associated with periodontitis in Brazilians. Open Dent J 3, 184–190 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erciyas K. et al. Association between TNF-alpha, TGF-beta1, IL-10, IL-6 and IFN-gamma gene polymorphisms and generalized aggressive periodontitis. Clin Invest Med 33, E85 (2010). [DOI] [PubMed] [Google Scholar]
- Scapoli C. et al. Gene–gene interaction among cytokine polymorphisms influence susceptibility to aggressive periodontitis. Genes Immun 12, 473–480 (2011). [DOI] [PubMed] [Google Scholar]
- Ebadian A. R. et al. Gene Polymorphisms of TNF-alpha and IL-1beta Are Not Associated with Generalized Aggressive Periodontitis in an Iranian Subpopulation. Iran J Allergy Asthma Immunol 12, 345–351 (2013). [PubMed] [Google Scholar]
- Yang W., Jia Y. & Wu H. Four tumor necrosis factor alpha genes polymorphisms and periodontitis risk in a Chinese population. Hum Immunol 74, 1684–1687 (2013). [DOI] [PubMed] [Google Scholar]
- Ozer Yucel O. et al. Analysis of TNF-alpha (−308) polymorphism and gingival crevicular fluid TNF-alpha levels in aggressive and chronic periodontitis: A preliminary report. Cytokine 72, 173–177 (2015). [DOI] [PubMed] [Google Scholar]
- Settin A., Ismail A., El-Magd M. A., El-Baz R. & Kazamel A. Gene polymorphisms of TNF-alpha-308 (G/A), IL-10(-1082) (G/A), IL-6(-174) (G/C) and IL-1Ra (VNTR) in Egyptian cases with type 1 diabetes mellitus. Autoimmunity 42, 50–55 (2009). [DOI] [PubMed] [Google Scholar]
- Eres G., Saribay A. & Akkaya M. Periodontal treatment needs and prevalence of localized aggressive periodontitis in a young Turkish population. J Periodontol 80, 940–944 (2009). [DOI] [PubMed] [Google Scholar]
- Kinane D. F., Shiba H. & Hart T. C. The genetic basis of periodontitis. Periodontol 2000 39, 91–117 (2005). [DOI] [PubMed] [Google Scholar]
- Graves D. T. et al. Tumor necrosis factor modulates fibroblast apoptosis, PMN recruitment, and osteoclast formation in response to P. gingivalis infection. J Dent Res 80, 1875–1879 (2001). [DOI] [PubMed] [Google Scholar]
- Gelskey S. C. Cigarette smoking and periodontitis: methodology to assess the strength of evidence in support of a causal association. Community Dent Oral Epidemiol 27, 16–24 (1999). [DOI] [PubMed] [Google Scholar]
- Kotsakis G. A., Javed F., Hinrichs J. E., Karoussis I. K. & Romanos G. E. Impact of cigarette smoking on clinical outcomes of periodontal flap surgical procedures: a systematic review and meta-analysis. J Periodontol 86, 254–263 (2015). [DOI] [PubMed] [Google Scholar]
- Wang W. F., Shi J., Chen S. J., Niu Y. M. & Zeng X. T. Interleukin-1alpha -899 (+4845) C−>T polymorphism is not associated with aggressive periodontitis susceptibility: A meta-analysis based on 19 case-control studies. Biomed Rep 2, 378–383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8, 2–10 (2015). [DOI] [PubMed] [Google Scholar]
- Fiorini T., Musskopf M. L., Oppermann R. V. & Susin C. Is there a positive effect of smoking cessation on periodontal health? A systematic review. J Periodontol 85, 83–91 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.