Abstract
Erythropoietin (EPO) and insulin-like growth factor I (IGF-I) are cytokines that inhibit neuronal apoptosis. However, their maximal antiapoptotic effect, even at high concentrations, is observed only when neurons are pretreated for several hours before insult. Here we show that simultaneous administration of EPO and IGF-I (EPO+IGF-I) eliminates the preincubation period required to prevent N-methyl-d-aspartate (NMDA)-induced apoptosis in cultured rat cerebrocortical neurons. The synergistic effect of EPO+IGF-I was mediated, at least in part, by activation of phosphatidylinositol 3-kinase (PI3-K). EPO+IGF-I synergistically activated Akt (protein kinase B), a downstream target of PI3-K, and prevented dephosphorylation of Akt. Overexpression of a dominant interfering form of Akt (dnAkt) abrogated EPO+IGF-I-mediated neuroprotection. EPO+IGF-I treatment did not prevent initial NMDA-induced caspase-3 activation, which was observed within 6 h of insult; however, EPO+IGF-I-treated neurons survived at least 2 days after NMDA insult. These cytokines prevented neuronal apoptosis downstream of caspase activation by facilitating association between X-linked inhibitor of apoptosis protein, an inhibitor of caspase proteolytic activity, and activated caspase-3. These results imply that EPO+IGF-I exert cooperative actions that afford acute neuroprotection via activation of the PI3-K-Akt pathway.
Keywords: apoptosis, phosphatidylinositol 3-kinase, Akt (protein kinase B), X-linked inhibitor of apoptosis protein, caspase-3
Apoptosis contributes to neuronal destruction in a variety of neurological disorders. In the search for potential neuroprotective agents against apoptosis, the cytokines erythropoietin (EPO) and insulin-like growth factor I (IGF-I) were individually found to be effective (1, 2). However, despite potential crosstalk between their signaling pathways, synergistic neuroprotective effects of EPO and IGF-I have not been previously investigated.
EPO and its receptor (EPO-R) are expressed in the mammalian CNS (3-5). Exogenous or endogenous EPO is neuroprotective in animal models of cerebral hypoxia/ischemia (stroke), spinal cord and retinal injury (6, 7), and neurodegenerative diseases (8-10). In fact, EPO diminished stroke damage in a recent well designed human phase 2 clinical trial (11). EPO activates phosphatidylinositol 3-kinase (PI3-K) (12, 13) and inhibits apoptosis through an Akt-dependent pathway (14). Akt is a serine-threonine kinase that, when activated by EPO, counteracts neuronal apoptosis by up-regulating the X-linked inhibitor of apoptosis protein (XIAP) and Bcl-2 (15-17), resulting in subsequent inhibition of caspase activity (18).
IGF-I and its receptor (IGF-IR) are expressed in the CNS (19, 20) and are essential for neuronal survival, differentiation, and neurogenesis (21-24). Its biological action can be regulated by binding proteins (IGF-BP's), several of which are known to bind free IGF-I with high affinity and either enhance or inhibit its activation of IGF-IRs (25). IGF-I induces EPO expression in astrocytes (26). In neurons, IGF-I/IGF-IR interaction promotes activation of PI3-K and Akt (27, 28), which in turn ameliorates effects of brain injury (29) and glutamate-induced oxidative/nitrosative stress (30). IGF-I inhibits neuronal caspase-3 activity by phosphorylating Bad via the PI3-K-Akt pathway (28, 29, 31).
Akt inhibition abrogates neuroprotection by IGF-I, whereas constitutively active Akt promotes neuronal survival (32). Furthermore, IGF-I cooperates with EPO to promote erythroid cell maturation and survival (33).
In the present study, we hypothesized that EPO and IGF-I act together to produce acute and prolonged neuroprotection, and we investigated the mechanisms underlying these effects. We exposed cerebrocortical neurons to the glutamate analogue N-methyl-D-aspartate (NMDA), which activates caspase-9/-3, resulting in neuronal apoptosis (34). EPO+IGF-I prevented neurodegeneration in vitro when added concurrently with NMDA or as long as 5 h after the NMDA insult. EPO+IGF-I lowered the minimum neuroprotective concentration of each cytokine by synergistically activating PI3-K-Akt and preventing the dephosphorylation of Akt for up to 9 h. Our results suggest that synergistic activation of the PI3-K pathway by EPO+IGF-I converges on Akt and acts downstream of caspase-3 activation, extending the window of therapeutic intervention.
Materials and Methods
Neuronal Cell Cultures. Mixed neuronal and glial cerebrocortical cultures were prepared as described in detail (35). In brief, cortical cells from embryonic day 16 Sprague-Dawley rats were plated on culture dishes in serum-containing medium and incubated at 37°C in a humidified environment of 5% CO2/balance air for at least 17 days to permit full expression of NMDA receptors (35). Additionally, relatively pure neuronal cultures were prepared from rat cortices; to enhance neuronal content, the culture medium was replaced on the second day after plating with Neurobasal medium containing B27 supplement (Life Technologies). These cultures were then maintained for an additional 15-16 days and were found to contain >95% neurons by specific Ab staining (36).
NMDA, EPO, and IGF-I Incubations. Cerebrocortical cultures were exposed to NMDA (200 μM, 20 min) in nominally Mg2+-free Earle's balanced salt solution (EBSS) containing 1.8 μM CaCl2 and 5 μM glycine. These conditions are known to induce predominantly apoptotic-like neuronal cell death (17, 37). After NMDA exposure, cultures were washed with EBSS, returned to their original preconditioned tissue culture medium after filtering, and placed back into the incubator. Human recombinant EPO (Amgen, Epoietin α, 2,000 units/ml) and/or IGF-I (Invitrogen) were directly added to the cell culture medium as indicated.
Preparation of Total Cell Lysates. Cultured cells were washed briefly in PBS and lysed in ice-cold cell lysis buffer (Cell Signaling Technology, Beverly, MA). After vortexing (15 s) and centrifugation (10,000 × g, 20 min), supernatants were used to determine protein concentration (BCA-Protein assay kit, Pierce).
Immunoblotting and Immunoprecipitations. Equivalent amounts of protein (15-30 μg) were resolved on Bis-Tris SDS gels (10%, Invitrogen) under reducing conditions, and then electroblotted onto a nitrocellulose membrane (Amersham Pharmacia). After nonspecific binding was blocked with ChemiBlock (Chemicon) for 1 h, the blots were subsequently incubated overnight at 4°C with the following primary antibodies: anti-EPO-R (1:200, R & D Systems), anti-p85 PI3-K (1:400, Upstate Biotechnology), anti-phospho-Akt (1:2,000, Cell Signaling Technology), anti-Akt (1:2,000, Cell Signaling Technology), and anti-XIAP (1:500, Trevigen, Gaithersburg, MD). After washing, membranes were incubated with secondary Ab conjugated to horseradish peroxidase (HRP) (1:20,000, Vector Laboratories) for 1 h and developed with an enhanced chemiluminescence kit (Amersham Pharmacia). For evaluation of Western blots with an infrared fluorescence detection system (Odyssey, Licor Biosciences), secondary antibodies conjugated with fluorophores were used following the manufacturer's protocol. Color images were converted into grayscale. For immunoprecipitations from whole cell lysates, a commercial kit (Catch and Release, Upstate Biotechnology) and the following antibodies were used: anti-EPO-R Ab (2 μg, R & D Systems), anti-phospho-Akt (2 μg, Cell Signaling Technology), or anti-caspase-3 (activated form, 2 μg, Cell Signaling Technology). Immunoprecipitates were separated on SDS gels, transferred to membranes, and probed with antibodies for the Odyssey system. For loading controls, blots were stripped and reprobed with appropriate primary and secondary antibodies.
Immunofluorescence and Detection of Apoptotic Neurons. Sixteen hours after NMDA exposure, cerebrocortical cells were fixed in 4% paraformaldehyde for 10 min and permeabilized with acetone. Apoptotic neurons were identified by morphological appearance and terminal-deoxynucleotidyl-transferase-mediated dUTP nick-end labeling (TUNEL) (Apoptosis Detection System, Promega) of cells colabeled with the neuron-specific marker microtubule associated protein-2 (MAP-2, Sigma) (17). When long-term (>1 day) survival was assessed after NMDA insult, we counted the total number of MAP-2-positive cells rather than scoring neuronal apoptosis, because at these time points secondary necrosis can occur after initial apoptosis, thereby obfuscating the number of apoptotic cells (38).
PI3-K and Akt Inhibition. PI3-K activity was inhibited pharmacologically with the antagonist LY294002 (Calbiochem), which was dissolved in DMSO (Sigma) and added 30 min before EPO+IGF-I. Akt activation was inhibited by adenoviral-mediated expression of a hemagglutinin (HA)-tagged, nonphosphorylatable, dominant-negative mutant of Akt (dnAkt) (39). HA-tagged wild-type Akt (wtAkt) was used as a control. After a 4-h exposure to the adenoviral construct, primary neurons were incubated in filtered, preconditioned medium for 24-36 h before experimental use. Expression of the viral Akt constructs was confirmed by immunofluorescence labeling with a monoclonal anti-HA Ab (1:100, Roche Diagnostics, data not shown).
Caspase Assays. Caspase-3 (Asp-Glu-Val-Asp, DEVD) cleavage assays were performed as described (37). Cerebrocortical cultures were lysed in cold buffer [10% sucrose/0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate/100 mM Hepes/10 mM DTT, pH 7.5]. Cytoplasmic protein extracts (200 μg) were incubated for 30 min at 37°C with the colorimetric caspase-3 substrate DEVD-7-amino-4-trifluoromethylcoumarin (DEVD-AFC, 80 μM; Enzyme Systems Products). Free AFC released by caspase-3 activity was measured on a FluoroMax2 fluorometer at 400-nm excitation and 505-nm emission wavelengths and was reported in nanomoles per minute per microgram of protein against standard.
EPO ELISA. An ELISA to monitor EPO levels was performed following the manufacturer's guidelines (Quantikine IVD, R & D Systems).
Statistical Analysis. All data reported were analyzed and plotted as means ± SE and represent a minimum of three separate experiments for each treatment. An ANOVA and post hoc comparison was performed for each treatment paradigm, and P < 0.05 was considered a statistically significant effect.
Results
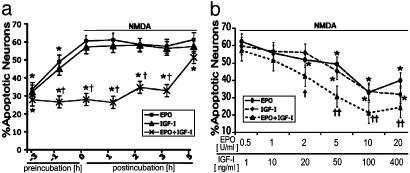
NMDA-Induced Neurotoxicity Is Ameliorated by Coincubation with EPO and IGF-I. To first determine whether EPO and IGF-I act synergistically to prevent neuronal apoptosis after an excitotoxic insult, rat primary cerebrocortical cultures were exposed to NMDA for 20 min. Neurons, but not glia, died in response to NMDA in these mixed neuronal-glial cultures (38). NMDA produced an apoptotic morphology and TUNEL reactivity in 64 ± 3% of MAP-2-labeled neurons. Preincubation for 3 h with either EPO (10 units/ml) or IGF-I (100 ng/ml) significantly attenuated neuronal apoptosis (P < 0.05). These represent maximally effective concentrations of these factors, as determined previously from dose-response curves (17, 30). When neurons were simultaneously treated with NMDA and either EPO or IGF-I, cell death was not significantly reduced. However, simultaneous treatment of NMDA with EPO+IGF-I was more effective in preventing apoptosis than a 3-h preincubation in either factor alone. Moreover, significant neuroprotection was observed when EPO+IGF-I were added up to 5 h after NMDA exposure (Fig. 1a). The time course of neuroprotection provided by EPO+IGF-I suggests that the signal transduction pathways activated by EPO+IGF-I promote more rapid neuroprotection than either factor can initiate alone. Control incubations with EPO and/or IGF-I in the absence of NMDA exposure did not affect neuronal viability. An ELISA was used to measure the levels of endogenous EPO and its possible up-regulation after cytokine incubation. Baseline levels of EPO were undetectable in these cultures. Incubation with IGF-I did not produce a detectable up-regulation of endogenous levels of EPO (see Fig. 6, which is published as supporting information on the PNAS web site). Next, we investigated the dose-response of EPO+IGF-I-mediated neuroprotection. To achieve synergistic protection from an NMDA insult, the minimum required effective concentration was 2 units/ml EPO and 20 ng/ml IGF-I; maximal protective effects were observed at 10 units/ml EPO and 100 ng/ml IGF-I (Fig. 1b). When compared to the minimal effective concentration of each cytokine alone, coapplication of EPO+IGF1 reduces the minimum concentration of each cytokine required for protection from NMDA-induced apoptosis.
Fig. 1.
Simultaneous coadministration of EPO and IGF-I ameliorates NMDA-induced neuronal apoptosis. (a) Rat cerebrocortical cultures were incubated with NMDA (200 μM) for 20 min. EPO (10 units/ml), IGF-I (100 ng/ml), or the combination was applied before, simultaneously with, or up to 5 h after NMDA exposure. *, P < 0.05 by ANOVA vs. NMDA alone; †, P < 0.02 vs. EPO or IGF-I. (b) Rat cerebrocortical cultures were incubated with varying amounts of EPO (0.5-20 units/ml) and/or IGF-I (1-400 ng/ml). Cytokine incubation began simultaneously with NMDA exposure. *, P < 0.05 vs. NMDA alone; †, P < 0.05, or ††, P < 0.01 vs. EPO or IGF-I. Apoptotic neurons are represented by the percentage of MAP-2-positive pyknotic cells that colabeled with TUNEL 16 h after NMDA exposure. In this and subsequent figures, results are means ± SE (n = 3-5).
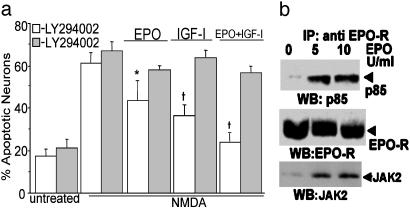
PI3-K Is Required for Neuroprotection by EPO and IGF-I. Previous studies have shown that PI3-K is involved in EPO and IGF-I signaling in both neural and nonneural tissues (12, 28). To begin to elucidate the possible role of PI3-K in the neuroprotective effects of EPO and IGF-I, rat cerebrocortical neurons were preincubated for 3 h with EPO, IGF-I, or EPO+IGF-I in the presence or absence of the PI3-K inhibitor LY294002 (10 μM). We found that preincubation in EPO, IGF-I, or EPO+IGF-I decreased NMDA-induced neuronal apoptosis, and LY294002 largely abrogated this neuroprotective effect (Fig. 2a). These findings suggested that PI3-K activity is required for maximal EPO- and IGF-I-mediated neuroprotection. As a control, LY294002 alone did not increase neuronal apoptosis in cerebrocortical cultures or affect the degree of NMDA-induced cell death. Note that when EPO was present, some degree of increased survival persisted even after LY294002 incubation compared to control, consistent with our previous report that EPO also offers neuroprotection via a separate, NF-κB-mediated pathway (17).
Fig. 2.
EPO and IGF-I signaling via PI3-K. (a) Inhibition of PI3-K abrogates the antiapoptotic effect of EPO+IGF-I. Cerebrocortical cultures were exposed to 10 μM LY294002 (gray bars) for 30 min before incubation with EPO, IGF-I, and/or NMDA. Neuronal apoptosis was assessed 16 h later by determining the percentage of MAP-2-positive pyknotic cells that were TUNEL stained. LY294002 decreased the neuroprotective effect of EPO, IGF-I, and EPO+IGF-I. *, P < 0.05 or †, P < 0.01 vs. same treatment plus LY294002. (b) EPO signaling through PI3-K. EPO treatment induced association of the p85 subunit of PI3-K with the EPO-R. Cerebrocortical cells were stimulated with 5 or 10 units/ml EPO for 30 min. EPO-R protein was immunoprecipitated from total cell lysates and separated on an SDS/polyacrylamide gel. The blot was then probed with anti-p85 Ab, stripped, and reblotted with anti-Jak2 Ab and subsequently anti-EPO-R Ab. This experiment was replicated three times.
In DA-3 cells, a murine interleukin-3-dependent cell line, EPO binding to its receptor results in direct association of PI3-K with the EPO-R (40, 41). Previously, we had shown that, in our mixed neuronal/glial cerebrocortical cultures, EPO-Rs are located exclusively on neurons (17). Therefore, to determine whether EPO+EPO-R binding promotes PI3-K/EPO-R interaction in primary neurons, the EPO-R complex was immunoprecipitated from lysates of cerebrocortical cultures that had been treated with EPO (5 or 10 units/ml) for 30 min and subjected to Western blotting. Western blot for the p85 subunit of PI3-K (Fig. 2b) demonstrated that EPO induces association between the EPO-R and p85 subunit of PI-3-K. To confirm the previously reported association of Janus kinase 2 (Jak2) with the EPO-R complex in cortical neurons (17), the same blot was reprobed with anti-Jak2 antibodies. Equal protein loading was confirmed by probing blots with anti-EPO-R Ab. These findings indicate that ligand binding to the neuronal EPO-R promotes the association of p85 and Jak2 with the EPO-R. Association of Jak2 and the EPO-R may lead to phosphorylation and activation of PI3-K (42, 43). Neither EPO, IGF-I, nor EPO+IGF-I induced signal transducer and activator of transcription 5 phosphorylation on this time scale (data not shown).
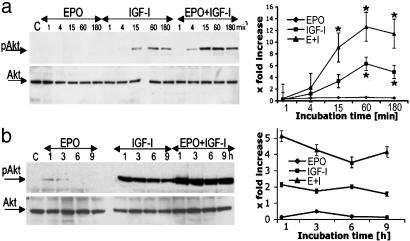
EPO and IGF-I Cooperate Synergistically to Activate Akt. Akt kinase is activated downstream of PI3-K-mediated production of 3′ phospholipids (44). The production of phosphotidylinositol-3,4,5-trisphosphate promotes phosphorylation of Akt at two critical sites: serine-473 and threonine-308 (27). Results presented here thus far identify PI3-K as a key player in the EPO+IGF-I-signaling pathway. Therefore, we next investigated the activation of Akt by EPO (10 units/ml) and IGF-I (100 ng/ml). Exposing cerebrocortical cultures to EPO or IGF-I for 1 to 180 min resulted in modest activation of Akt, as detected with a specific Ab against phosphoserine-473 Akt. EPO+IGF-I incubation resulted in a much larger increase in phosphoserine-473 Akt than either EPO or IGF-1 alone (Fig. 3a). The total amount of Akt protein, however, was unchanged. Next, we studied whether the EPO+IGF-I combination prevented dephosphorylation and inactivation of Akt. To magnify the minor degree of Akt phosphorylation produced by EPO alone, we doubled the amount of protein loaded onto our blots (Fig. 3b). Under these conditions, we found that EPO induced modest phosphorylation of Akt at 1-3 h, but within6hof incubation, Akt was dephosphorylated. In neuronal cultures incubated with IGF-I alone, dephosphorylation of Akt was less prominent but began to occur within 6-9 h. In contrast, coincubation with EPO+IGF-I largely prevented reduction in phospho-Akt even 9 h after cytokine exposure. Control incubations revealed that total Akt levels were not significantly altered by either or both cytokines. Taken together, these results show that treatment with EPO+IGF-I synergistically induced Akt phosphorylation at serine-473. Moreover, EPO+IGF-I prolonged the duration of Akt phosphorylation, thereby possibly contributing to the observed extended window of neuroprotection.
Fig. 3.
EPO+IGF-I treatment induces prolonged Akt phosphorylation. (a) Rat cerebrocortical cultures were treated for 1-180 min with EPO (10 units/ml), IGF-I (100 ng/ml), or both EPO+IGF-I. (Left) Whole-cell lysates were subjected to immunoblot analysis with anti-phospho-Akt Ab (pAkt, each lane loaded with 15 μg of total protein). The blots were then stripped and reprobed with an anti-total Akt Ab. (Right) Densitometry revealed a significant increase in phospho-Akt after incubation with EPO+IGF-I (*, P < 0.05 EPO+IGF-I vs. IGF-I alone, or IGF-I vs. EPO). (b) Cerebrocortical cultures were incubated with EPO (10 units/ml), IGF-I (100 ng/ml), or both for 1-6 h. (Left) Whole-cell lysates were subjected to immunoblot analysis as described above (except each lane was loaded with 30 μg of total protein). (Right) Densitometry of blots. The level of phospho-Akt was corrected for total Akt in each lane (control lane served as the baseline). Because more protein was loaded in b than in a, the baseline was higher in b, creating somewhat different apparent fold-increases at the same time point between the two blots (e.g., at 1 h). Each experiment was repeated at least three times with similar results.
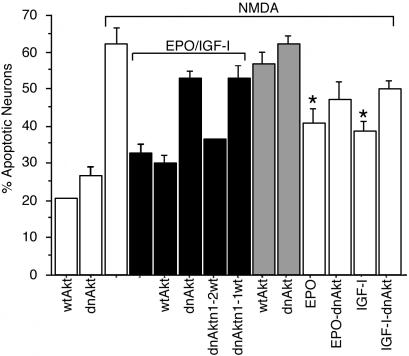
Inhibition of Akt Phosphorylation Abrogates the Neuroprotective Effects of EPO+IGF-I. To characterize the role of Akt in the EPO+IGF-I signaling pathway, Akt activation was prevented by infection with an adenoviral vector coding for dnAkt (39). This dnAkt cannot be activated because the critical phosphorylation site has been mutated. We infected cerebrocortical cultures with dnAkt and assayed the effects of NMDA and/or EPO+IGF-I on neurons expressing the dominant negative construct. As a control, sibling cultures were infected with an adenoviral vector encoding wtAkt. Within 36 h of infection, neither dnAkt nor wtAkt manifested a statistically significant effect on neuronal viability. Overexpression of wtAkt did not significantly inhibit NMDA-induced apoptosis and did not alter EPO+IGF-I-mediated neuroprotection from NMDA (Fig. 4). Additionally, dnAkt expression did not increase neuronal apoptosis in the presence of NMDA. However, dnAkt abrogated the neuroprotective action of EPO+IGF-I. Overexpression of wtAkt prevented the effect of dnAkt expression when coinfected at a molar excess of 2:1 (wtAkt/dnAkt) but not at a 1:1 ratio. These findings suggest that the increased neuronal apoptosis was caused by the specific expression of dnAkt rather than a nonspecific effect of protein overexpression. In conjunction with the findings presented above, these results are consistent with the notion that Akt phosphorylation/activation plays a key role in EPO+IGFI-mediated neuroprotection.
Fig. 4.
Akt contributes to EPO+IGF-I-mediated neuroprotection. Cerebrocortical cultures were infected with an adenoviral vector encoding wild-type (wt) or dominant negative (dn) forms of Akt. Sibling cultures were coinfected with wt and dn constructs in a molar ratio of 1:1 or 2:1 (wt/dn). After 36 h, cells were incubated with EPO (10 units/ml), IGF-I (100 ng/ml), or EPO+IGF-I with or without simultaneous exposure to NMDA (200 μM). After an additional 16 h, pyknotic cells that stained positive for TUNEL and MAP-2 were scored as apoptotic neurons. In the presence of NMDA, exposure to EPO or IGF-I produced significantly less neuroprotection than the combination of EPO+IGF-I (*, P < 0.05). Addition of dnAkt decreased the neuroprotective effect of EPO+IGF-I from NMDA, and this inhibition was abrogated by excess wtAkt (†, P < 0.01).
EPO+IGF-I Protect Neurons Downstream of Caspase-3 Cleavage. Caspases, a family of cysteine-aspartyl proteases, play an important role in the signal transduction and execution of neuronal apoptosis through activation of proteolytic cascades (45). We have previously shown that, after brief NMDA insult, mitochondria in our cultured neurons release cytochrome c, contributing to activation of the intrinsic caspase pathway (caspase-9, -3) (37). Akt is an important regulatory factor in the activation of the intrinsic caspase cascade (28). Because constitutively active Akt prevents the activation of caspases-9 and -3 downstream of cytochrome c release (46), we asked whether EPO+IGF-I incubation could affect caspase activation in cerebrocortical cells after NMDA insult.
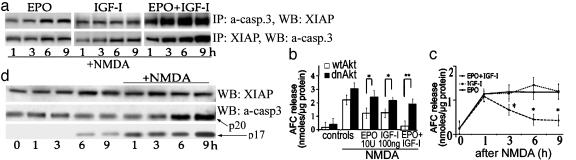
If EPO+IGF-I act downstream of caspase-3 activation to prolong neuronal survival, one possibility is that these cytokines prevent the proteolytic activity of active caspase-3 by promoting its interaction with endogenous caspase inhibitors, such as XIAP. To test this hypothesis, the active form of caspase-3 was immunoprecipitated from cultures previously incubated with EPO+IGF-I and NMDA. The immunoprecipitate was probed for XIAP on immunoblots (Fig. 5a Upper). In reverse order, immunoprecipitations with the XIAP Ab confirmed the association with active caspase-3 (Fig. 5a Lower). We demonstrated that active caspase-3 was bound to XIAP and that EPO+IGF-I treatment increased the relative amount of XIAP associated with active caspase-3 (Fig. 5a). Interestingly, NMDA exposure alone also appeared to produce a modest increase in the amount of XIAP associated with active caspase-3 (Fig. 5d).
Fig. 5.
EPO+IGF-I treatment promotes association of XIAP with activated caspase-3 and decreases NMDA-induced caspase activity in cerebrocortical neurons. (a) Treatment with EPO somewhat increased the degree of association of XIAP and cleaved caspase-3 in cells exposed to NMDA. IGF-I treatment alone did not alter XIAP expression, but also resulted in an increase in cleaved capsase-3 coimmunoprecipitated with XIAP. However, EPO+IGF-I exposure led to a large, significant increase in the amount of XIAP associated with active caspase-3. (Upper) Blots representing immunoprecipitates with anti-active caspase-3 Ab that were probed with XIAP Ab. (Lower) Blots from immunoprecipitates of the same lysates made with XIAP Ab and probed with anti-active caspase-3 Ab (a-casp. 3), which recognizes both the p17 and p20 subunits. The blots in a and b were repeated in three separate experiments with similar results. (b) EPO+IGF-I, but neither cytokine alone, reduced caspase-3 activity for at least 9 h after NMDA insult (*, P < 0.05). Caspase activity in cell lysates was assessed 0, 1, 3, 6, and 9 h after drug exposure and is shown as relative DEVDase activity, expressed as nanomoles of free 7-amino-4-trifluoromethylcoumarin (AFC) per microgram of protein. NMDA, EPO, and IGF-I were all added concurrently. (c) Transduction with dnAkt largely prevented the inhibitory effect of EPO+IGF-I on NMDA-induced caspase-3 activity. Caspase activity was assessed 6 h after drug exposure in this experiment (*, P < 0.05; **, P < 0.01). (d) NMDA exposure resulted in cleavage of caspase-3 in the absence of significant changes in XIAP at 6 h. Controls manifest no significant increase in caspase-3 cleavage or XIAP expression.
The presence of a caspase-3/XIAP complex in neurons suggests that caspase-3 may be activated by proteolytic cleavage but remains functionally inactive because of bound XIAP. To study the effect of the combination of EPO+IGF-I on the proteolytic activity of caspase-3, protein lysates were obtained from cerebrocortical cultures after NMDA exposure. Then, caspase-3 activity was assessed by cleavage of the fluorescent substrate DEVD-7-amino-4-trifluoromethyl-coumarin (Fig. 5b) (37). NMDA exposure increased DEVDase activity, whereas simultaneous application of EPO+IGF-I decreased NMDA-induced caspase-3-like activity for at least 9 h after insult. This finding is consistent with the notion that the caspase-3/XIAP complex formed in response to EPO+IGF-I prevented DEVDase activity.
Next, we determined whether activation of Akt signaling was responsible for the decrease in caspase-3 activity observed after treatment with EPO+IGF-I in the face of an NMDA insult (Fig. 5c). We found that transduction with dnAkt largely abrogated the inhibitory effect of EPO+IGF-I on caspase-3 activity, suggesting that Akt was indeed involved in this pathway. Taken together, these findings suggest that EPO+IGF-I can promote neuronal survival downstream of caspase activation via a neuronal signal transduction pathway involving XIAP binding to active caspase-3 to prevent its proteolytic, proapoptotic function.
In summary, the experiments presented here identify a synergistic effect of EPO and IGF-I in preventing NMDA-induced neuronal apoptosis. Because of this synergy, lower concentrations of each cytokine can be used to effect neuroprotection. Additionally, the synergy between these two factors also allows neuroprotection to be initiated later than treatment with either factor alone. Furthermore, we demonstrate that PI3-K and Akt kinase are signaling components of the EPO+IGF-I-induced neuroprotective pathway. EPO+IGF-I treatment prevents NMDA-induced neuronal apoptosis despite the finding that the active form of caspase-3 is present, possibly through facilitation of an inhibitory interaction between XIAP and caspase-3. It remains possible that additional effects of XIAP or other factors, including up-regulation of NF-κB activity, also contribute to neuroprotection as we have reported (17, 47).
Discussion
Currently, the mechanisms underlying EPO- and IGF-I-mediated neuroprotection are under intense investigation. When added before an oxidative insult, IGF-I has been reported to protect neurons from apoptosis (30, 48). This neuroprotective effect of IGF-I was predominantly mediated by activation of PI3-K and Akt. However, IGF-I has also been reported to increase the sensitivity of cerebellar granule cells to NMDA-induced excitotoxicity (49). In contrast to granule cells, we demonstrate here that preincubation with IGF-I provides modest neuroprotection from NMDA-induced neuronal apoptosis in cerebrocortical cultures. We found that the neuroprotective effect of IGF-I was abrogated by the PI3-K inhibitor LY294002 and diminished by expression of dnAkt. Endogenous levels of EPO were unaffected by IGF-I incubation. Consequently, we can exclude the contribution of endogenous EPO to the neuroprotective effect of IGF-I described here.
As with IGF-I, we found that EPO also led to activation of PI3-K, and that LY294002 or dnAkt inhibited EPO-mediated protection from NMDA-induced neuronal apoptosis. In agreement with our prior study (17), we observed that neuroprotection by EPO alone required preincubation before the neurotoxic insult. We previously reported that EPO stimulated Jak2 activity in neurons, resulting in NF-κB activation, and thereby promoting synthesis of antiapoptotic proteins such as bcl-XL, c-IAP2, and XIAP (17). These findings suggested that preincubation with EPO may prevent neuronal apoptosis via NF-κB-mediated gene transcription as well as by Akt activation.
Here we report that the neuroprotective effects of EPO and IGF-I are synergistic. EPO+IGF-I provided neuroprotection at lower concentrations than either cytokine alone. In fact, concentrations of EPO or IGF-I that were totally ineffective when administered individually afforded significant neuroprotection when combined. Furthermore, the combination of EPO and IGF-I prevented neuronal apoptosis when added up to 5 h after NMDA insult, whereas either cytokine alone required preincubation to be effective. These results support the hypothesis that this combination of factors may activate a previously undescribed form of cooperation in the neuroprotective signal transduction pathway. Along these lines, we show here that both factors synergistically activate the PI3-K/Akt pathway in neurons.
Previous reports had demonstrated that phosphorylation/activation of Akt is crucial for EPO- or IGF-I-mediated signaling in a variety of cell types (9, 28). In the present study, we show that incubation with EPO+IGF-I resulted in phosphorylation of Akt in neurons. Intriguingly, we observed much more robust activation of Akt after costimulation with EPO+IGF-I compared to preincubation with EPO or IGF-I alone. Moreover, the combined application of EPO and IGF-I also appeared to delay dephosphorylation of Akt, thus keeping it active for a longer period. Expression of dnAkt prevented EPO+IGF-I-mediated protection from NMDA-induced neuronal apoptosis. However, transduction with excess wtAkt partially reversed the proapoptotic effect of dnAkt. These findings are consistent with the notion that the synergism between the EPO and IGF-I neuroprotective pathways converge at the point of Akt activation.
One possible mechanism for EPO and IGF-I interaction in the Akt pathway involves PI3-K activity. The inactive form of PI3-K is a complex between the catalytic portion of the enzyme and the p85 regulatory subunit (43, 50). The catalytic portion of PI3-K is liberated when the p85 subunit is phosphorylated, and then the catalytic portion associates with a variety of growth factor receptors or receptor-associated kinases (33, 40). Previous studies have shown that IGF-I leads to p85 phosphorylation in neurons (51), which is likely mediated by the IGF-I receptor (52). Similarly, we show here in neurons that EPO/EPO-R binding promotes association of the EPO-R with p85 and Jak2. We had previously shown that EPO activates Jak2 in neurons (17), and it was previously known in nonneuronal cells that active Jak2 can interact with the p85 subunit of PI3-K, leading to Akt phosphorylation/activation (53, 54). Thus, our results suggest that simultaneous exposure to EPO+IGF-I promotes increased PI3-K activity in neurons by stimulating multiple pathways for p85 phosphorylation. Our finding of synergistic activation of Akt by EPO+IGF-I may therefore be mediated by their cooperative activation of PI3-K. The fact that we also observed neuroprotective synergy between EPO and IGF-I in pure neuronal cultures suggests that signal transduction mechanisms intrinsic to neurons are sufficient to mediate cooperative neuroprotection by these two factors. It may be of interest to investigate the role of IGF binding proteins (BPs) in this model of neuronal death and in the therapeutic approach proposed herein, because IGF-BP dysregulation has been noted in pathophysiological conditions such as amyotrophic lateral sclerosis (55) and fetal hypoxia. Specifically, excess IGF-BP5 has been shown to decrease the survival of certain populations of neurons by inhibiting IGF-I receptor activation and the Akt caspase-3 signaling pathway downstream of it (23, 56).
Caspase activation plays an important role in neuronal apoptosis (45). We previously reported that the NMDA insult used in these experiments leads to activation of the intrinsic (mitochondrial) caspase pathway via release of mitochondrial cytochrome c and subsequent activation of the downstream executioner caspase-3 (37). We show here that EPO+IGF-I treatment can prevent NMDA-induced neuronal apoptosis downstream of caspase-3 processing, promoting long-term neuronal survival. Previously, we demonstrated that EPO leads to increased expression of antiapoptotic proteins, including endogenous caspase inhibitors such as XIAP (17). Using coimmunoprecipitation, we demonstrate here that EPO+IGF-I increase the amount of XIAP associated with the active form of caspase-3. Because the binding of XIAP can inhibit caspase proteolytic activity, this mechanism may represent a primary neuroprotective action of EPO+IGF-I downstream of caspase activation. We also found that a dominant interfering form of Akt largely prevented the inhibition of caspase-3 activity by EPO+IGF-I. This finding suggests that the Akt pathway may, at least in part, mediate this neuroprotective effect of EPO-IGF-I. Additionally, there is precedent for other neuroprotective actions of Akt that may be important in this paradigm, including phosphorylation of GSK-3β, BAD, JNK, and forkhead transcription factor (57).
In summary, we describe a cooperative neuroprotective effect of EPO and IGF-I that is mediated by a signal transduction pathway involving PI3-K and Akt. Our previous studies had revealed that EPO-mediated neuroprotection also involved NF-κB-activated transcriptional events. Our current findings suggest that, in addition, EPO can exert a more immediate neuroprotective action when administered in concert with a second neurotrophic factor, such as IGF-I, capable of synergistic activation of the PI3-K-Akt pathway. Additionally, our results suggest that the coadministration of synergistic neuroprotective agents rather than a single agent might provide greater benefit to patients suffering from acute NMDA receptor-mediated insults such as cerebral ischemia (stroke), head or spinal cord trauma, and epilepsy. EPO is already a clinically useful agent for the treatment of anemia and exhibits few side effects. IGF-I has a proven safety record in phase 1 and 2 clinical trials. Thus, treatment with EPO+IGF-I could be a future therapeutic strategy for a variety of acute neurological events.
Supplementary Material
Acknowledgments
We thank Dr. K. Walsh (St. Elizabeth's Medical Center, Boston) for generously providing adenoviral constructs. This work was supported in part by National Institutes of Health Grants P01 HD29587, R01 EY05477, R01 EY09024, and R01 NS41207 and by a Grant-in-Aid from the American Heart Association.
Abbreviations: EPO, erythropoietin; Jak2, Janus kinase 2; EPO-R, EPO receptor; IGF-I, insulin-like growth factor I; IGF-IR, IGF-I receptor; PI3-K, phosphatidylinositol 3-kinase; XIAP, X-linked inhibitor of apoptosis protein; NMDA, N-methyl-D-aspartate; TUNEL, terminal deoxynucleotidyl-transferase-mediated dUTP nick-end labeling; MAP-2, microtubule-associated protein 2; dnAKT, dominant-negative Akt; wtAkt, wild-type Akt; DEVD, Asp-Glu-Val-Asp.
References
- 1.Sakanaka, M., Wen, T. C., Matsuda, S., Masuda, S., Morishita, E., Nagao, M. & Sasaki, R. (1998) Proc. Natl. Acad. Sci. USA 95, 4635-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Ercole, A. J., Ye, P., Calikoglu, A. S. & Gutierrez-Ospina, G. (1996) Mol. Neurobiol. 13, 227-255. [DOI] [PubMed] [Google Scholar]
- 3.Digicaylioglu, M., Bichet, S., Marti, H. H., Wenger, R. H., Rivas, L. A., Bauer, C. & Gassmann, M. (1995) Proc. Natl. Acad. Sci. USA 92, 3717-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin, K., Yu, X., Beleslin-Cokic, B., Liu, C., Shen, K., Mohrenweiser, H. W. & Noguchi, C. T. (2000) Brain Res. Mol. Brain Res. 81, 29-42. [DOI] [PubMed] [Google Scholar]
- 5.Ruscher, K., Freyer, D., Karsch, M., Isaev, N., Megow, D., Sawitzki, B., Priller, J., Dirnagl, U. & Meisel, A. (2002) J. Neurosci. 22, 10291-10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celik, M., Gokmen, N., Erbayraktar, S., Akhisaroglu, M., Konakc, S., Ulukus, C., Genc, S., Genc, K., Sagiroglu, E., Cerami, A. & Brines, M. (2002) Proc. Natl. Acad. Sci. USA 99, 2258-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junk, A. K., Mammis, A., Savitz, S. I., Singh, M., Roth, S., Malhotra, S., Rosenbaum, P. S., Cerami, A., Brines, M. & Rosenbaum, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 10659-10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brines, M. L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N. C., Cerami, C., Itri, L. M. & Cerami, A. (2000) Proc. Natl. Acad. Sci. USA 97, 10526-10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siren, A. L., Fratelli, M., Brines, M., Goemans, C., Casagrande, S., Lewczuk, P., Keenan, S., Gleiter, C., Pasquali, C., Capobianco, A., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 4044-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumral, A., Ozer, E., Yilmaz, O., Akhisaroglu, M., Gokmen, N., Duman, N., Ulukus, C., Genc, S. & Ozkan, H. (2003) Biol. Neonate 83, 224-228. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenreich, H., Hasselblatt, M., Dembowski, C., Cepek, L., Lewczuk, P., Stiefel, M., Rustenbeck, H. H., Breiter, N., Jacob, S., Knerlich, F., et al. (2002) Mol. Med. 8, 495-505. [PMC free article] [PubMed] [Google Scholar]
- 12.Mayeux, P., Dusanter-Fourt, I., Muller, O., Mauduit, P., Sabbah, M., Druker, B., Vainchenker, W., Fischer, S., Lacombe, C. & Gisselbrecht, S. (1993) Eur. J. Biochem. 216, 821-828. [DOI] [PubMed] [Google Scholar]
- 13.Damen, J. E., Mui, A. L., Puil, L., Pawson, T. & Krystal, G. (1993) Blood 81, 3204-3210. [PubMed] [Google Scholar]
- 14.Chong, Z. Z., Lin, S. H., Kang, J. Q. & Maiese, K. (2003) J. Neurosci. Res. 71, 659-669. [DOI] [PubMed] [Google Scholar]
- 15.Ito, T., Deng, X., Carr, B. & May, W. S. (1997) J. Biol. Chem. 272, 11671-11673. [DOI] [PubMed] [Google Scholar]
- 16.Holcik, M. & Korneluk, R. G. (2001) Nat. Rev. Mol. Cell Biol. 2, 550-556. [DOI] [PubMed] [Google Scholar]
- 17.Digicaylioglu, M. & Lipton, S. A. (2001) Nature 412, 641-647. [DOI] [PubMed] [Google Scholar]
- 18.Chong, Z. Z., Kang, J. Q. & Maiese, K. (2002) J. Cereb. Blood Flow Metab. 22, 503-514. [DOI] [PubMed] [Google Scholar]
- 19.LeRoith, D. & Roberts, C. T., Jr. (1993) Ann. N.Y. Acad. Sci. 692, 1-9. [DOI] [PubMed] [Google Scholar]
- 20.Kar, S., Chabot, J. G. & Quirion, R. (1993) J. Comp. Neurol. 333, 375-397. [DOI] [PubMed] [Google Scholar]
- 21.Dore, S., Kar, S. & Quirion, R. (1997) Trends Neurosci. 20, 326-331. [DOI] [PubMed] [Google Scholar]
- 22.Aberg, M. A., Aberg, N. D., Hedbacker, H., Oscarsson, J. & Eriksson, P. S. (2000) J. Neurosci. 20, 2896-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong, J., Deng, J., Ghetti, B. & Lee, W. H. (2002) J. Neurosci. Res. 70, 36-45. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Martin, M., Cifuentes, M., Grondona, J. M., Bermudez-Silva, F. J., Arrabal, P. M., Perez-Figares, J. M., Jimenez, A. J., Garcia-Segura, L. M. & Fernandez-Llebrez, P. (2003) Eur. J. Neurosci. 17, 205-211. [DOI] [PubMed] [Google Scholar]
- 25.Clemmons, D. R. (1997) Cytokine Growth Factor Rev. 8, 45-62. [DOI] [PubMed] [Google Scholar]
- 26.Masuda, S., Chikuma, M. & Sasaki, R. (1997) Brain Res. 746, 63-70. [DOI] [PubMed] [Google Scholar]
- 27.Russell, J. W., Windebank, A. J., Schenone, A. & Feldman, E. L. (1998) J. Neurobiol. 36, 455-467. [DOI] [PubMed] [Google Scholar]
- 28.Kermer, P., Klocker, N., Labes, M. & Bahr, M. (2000) J. Neurosci. 20, 2-8. [PubMed] [Google Scholar]
- 29.Noshita, N., Lewen, A., Sugawara, T. & Chan, P. H. (2002) Neurobiol. Dis. 9, 294-304. [DOI] [PubMed] [Google Scholar]
- 30.Heck, S., Lezoualc'h, F., Engert, S. & Behl, C. (1999) J. Biol. Chem. 274, 9828-9835. [DOI] [PubMed] [Google Scholar]
- 31.Barber, A. J., Nakamura, M., Wolpert, E. B., Reiter, C. E., Seigel, G. M., Antonetti, D. A. & Gardner, T. W. (2001) J. Biol. Chem. 276, 32814-32821. [DOI] [PubMed] [Google Scholar]
- 32.Dudek, H., Datta, S. R., Franke, T. F., Birnbaum, M. J., Yao, R., Cooper, G. M., Segal, R. A., Kaplan, D. R. & Greenberg, M. E. (1997) Science 275, 661-665. [DOI] [PubMed] [Google Scholar]
- 33.Merchav, S. (1998) J. Pediatr. Endocrinol. Metab. 11, 677-685. [DOI] [PubMed] [Google Scholar]
- 34.Tenneti, L., D'Emilia, D. M., Troy, C. M. & Lipton, S. A. (1998) J. Neurochem. 71, 946-959. [DOI] [PubMed] [Google Scholar]
- 35.Lipton, S. A., Choi, Y. B., Pan, Z. H., Lei, S. Z., Chen, H. S., Sucher, N. J., Loscalzo, J., Singel, D. J. & Stamler, J. S. (1993) Nature 364, 626-632. [DOI] [PubMed] [Google Scholar]
- 36.Johnson, M. D., Kinoshita, Y., Xiang, H., Ghatan, S. & Morrison, R. S. (1999) J. Neurosci. 19, 2996-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garden, G. A., Budd, S. L., Tsai, E., Hanson, L., Kaul, M., D'Emilia, D. M., Friedlander, R. M., Yuan, J., Masliah, E. & Lipton, S. A. (2002) J. Neurosci. 22, 4015-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P. & Lipton, S. (1995) Proc. Natl. Acad. Sci. USA 92, 7162-7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujio, Y. & Walsh, K. (1999) J. Biol. Chem. 274, 16349-16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He, T. C., Zhuang, H., Jiang, N., Waterfield, M. D. & Wojchowski, D. M. (1993) Blood 82, 3530-3538. [PubMed] [Google Scholar]
- 41.Damen, J. E., Cutler, R. L., Jiao, H., Yi, T. & Krystal, G. (1995) J. Biol. Chem. 270, 23402-23408. [DOI] [PubMed] [Google Scholar]
- 42.Scheid, M. P. & Woodgett, J. R. (2001) Nat. Rev. Mol. Cell Biol. 2, 760-768. [DOI] [PubMed] [Google Scholar]
- 43.Pleiman, C. M., Hertz, W. M. & Cambier, J. C. (1994) Science 263, 1609-1612. [DOI] [PubMed] [Google Scholar]
- 44.Brazil, D. P., Park, J. & Hemmings, B. A. (2002) Cell 111, 293-303. [DOI] [PubMed] [Google Scholar]
- 45.Yuan, J. & Yankner, B. A. (2000) Nature 407, 802-809. [DOI] [PubMed] [Google Scholar]
- 46.Zhou, H., Li, X. M., Meinkoth, J. & Pittman, R. N. (2000) J. Cell. Biol. 151, 483-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvesen, G. S. & Duckett, C. S. (2002) Nat. Rev. Mol. Cell Biol. 3, 401-410. [DOI] [PubMed] [Google Scholar]
- 48.Yamada, M., Tanabe, K., Wada, K., Shimoke, K., Ishikawa, Y., Ikeuchi, T., Koizumi, S. & Hatanaka, H. (2001) J. Neurochem. 78, 940-951. [DOI] [PubMed] [Google Scholar]
- 49.Calissano, P., Ciotti, M. T., Battistini, L., Zona, C., Angelini, A., Merlo, D. & Mercanti, D. (1993) Proc. Natl. Acad. Sci. USA 90, 8752-8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamothe, B., Bucchini, D., Jami, J. & Joshi, R. L. (1995) FEBS Lett. 373, 51-55. [DOI] [PubMed] [Google Scholar]
- 51.Kimpinski, K. & Mearow, K. (2001) J. Neurosci. Res. 63, 486-499. [DOI] [PubMed] [Google Scholar]
- 52.Kim, B., Cheng, H. L., Margolis, B. & Feldman, E. L. (1998) J. Biol. Chem. 273, 34543-34550. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen, M. H., Ho, J. M., Beattie, B. K. & Barber, D. L. (2001) J. Biol. Chem. 276, 32704-32713. [DOI] [PubMed] [Google Scholar]
- 54.Park, J., Hill, M. M., Hess, D., Brazil, D. P., Hofsteenge, J. & Hemmings, B. A. (2001) J. Biol. Chem. 276, 37459-37471. [DOI] [PubMed] [Google Scholar]
- 55.Wilczak, N., de Vos, R. A. & De Keyser, J. (2003) Lancet 361, 1007-1011. [DOI] [PubMed] [Google Scholar]
- 56.D'Ercole, A. J., Ye, P. & O'Kusky, J. R. (2002) Neuropeptides 36, 209-220. [DOI] [PubMed] [Google Scholar]
- 57.Alvarez-Tejado, M., Naranjo-Suarez, S., Jimenez, C., Carrera, A. C., Landazuri, M. O. & del Peso, L. (2001) J. Biol. Chem. 276, 22368-22374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.