Abstract
To investigate essential components mediating stress signaling in plants, we initiated a large-scale stress response screen using Arabidopsis plants carrying the firefly luciferase reporter gene under the control of the stress-responsive RD29A promoter. Here we report the identification and characterization of a mutant, hos9-1 (for high expression of osmotically responsive genes), in which the reporter construct was hyperactivated by low temperature, but not by abscisic acid or salinity stress. The mutants grow more slowly, and flower later, than do wild-type plants and are more sensitive to freezing, both before and after acclimation, than the wild-type plants. The HOS9 gene encodes a putative homeodomain transcription factor that is localized to the nucleus. HOS9 is constitutively expressed and not further induced by cold stress. Cold treatment increased the level of transcripts of the endogenous RD29A, and some other stress-responsive genes, to a higher level in hos9-1 than in wild-type plants. However, the C repeat/dehydration responsive element-binding factor (CBF) transcription factor genes that mediate a part of cold acclimation in Arabidopsis did not have their response to cold altered by the hos9-1 mutation. Correspondingly, microarray analysis showed that none of the genes affected by the hos9-1 mutation are controlled by the CBF family. Together, these results suggest that HOS9 is important for plant growth and development, and for a part of freezing tolerance, by affecting the activity of genes independent of the CBF pathway.
After a period of low-temperature exposure, plants are able to better tolerate freezing temperatures (sustain less injury) (1, 2). This phenomenon is referred to as cold acclimation (1, 3). Part of the acclimation process is known to involve the accumulation of gene transcripts via cold perception and signal transduction leading to promoter activation on target genes (4). One class of these target genes includes those encoding late-embryo abundant transcripts that contain in their promoters a C repeat (CRT)/dehydration responsive element (DRE). This element confers responsiveness to cold, desiccation, and salinity (5). The hormone abscisic acid (ABA) is also able to activate some genes responsive to cold that are in this class, such as the RD29A (COR78/LT178) gene. This gene contains both CRT/DRE and the ABA responsive element (6). Activation of RD29A can occur through the binding of transcription factors from the ethylene responsive element-binding protein/Apetala2 family (7, 8). Specifically, the CRT/DRE-binding factor (CBF)1-4 transcription factors recognize the CRT/DRE and participate in adaptive (acclimating) responses to either cold (CBF1-3) or desiccation (CBF4) (9-11). In fact, ectopic overexpression of some CBF genes results in both activation of target genes and enhanced freezing, salt, or desiccation tolerance of transgenic plants (7, 8, 11, 12). CBF transcription factor genes themselves are activated by stress (9), and it has been reported recently that a MYC-type transcription factor binds to and controls the activity of the CBF3 promoter in response to stress (13).
A number of lines of evidence indicate that other signal pathways in addition to those mediated by CBF transcription factors are also involved in stress-adaptive responses, including cold acclimation (14, 15). For instance, the eskimo1 mutant is constitutively freezing tolerant and therefore does not require signaling through the cold-activated CBF factors (16). Also microarray transcript analysis experiments have shown directly that not all cold stress-responsive target genes contain CRT/DRE or are under the control of the CBF family (14, 17). In addition, constitutive expression of the normally cold-induced CBF genes does not lead to full cold acclimation of Arabidopsis plants (7, 8, 11, 12).
It is possible that other upstream signal components bypass CBF activators. Alternatively, one or more additional transcription factor families might activate stress adaptive genes independently of CBF. Signal components that mediate cold tolerance and have little or no effect on CBF gene expression would be expected to act through such an alternative pathway or might modify CBF activity itself (18). Although there are examples of stress-mediated signal components that appear to act independently of CBF transcription, such as SFR6 (18, 19), their sequence identities are yet to be determined.
Here we report the isolation of an Arabidopsis mutant hos9-1 (high expression of osmotically genes), which displays several altered phenotypic features, including increased sensitivity to freezing stress. The hos9-1 mutation occurs in a homeodomain transcription factor gene that affects gene expression and freezing tolerance without changing the expression of CBF genes. In addition, mutation of HOS9 also alters several developmental characteristics including growth rate, flowering time, and trichome density. Our results suggest that HOS9 controls freezing tolerance mainly through a constitutive pathway separate from the CBF regulon.
Materials and Methods
Plant Materials. Arabidopsis thaliana plants (ecotype C24) expressing the homozygous transgene RD29A::LUC (referred to as wild type) were mutagenized with an Agrobacterium tumefaciens-mediated (strain GV3101) T-DNA transformation (20, 21). Seeds from T2 plants were used for screening mutants that exhibited altered expression of RD29A::LUC in response to cold, ABA, and/or osmotic stress by luminescence imaging by using a charge-coupled device camera, as described (20).
Freezing Tolerance. Three-week-old hos9-1 and wild-type plants were grown in soil at room temperature or at 4°C under a long-day photoperiod (16 h light/8 h dark) for 1 week. Fully developed rosette leaves were used for electrolyte leakage measurements, as described (22-24).
Whole-plant freezing tests were as described (25) with modifications. Wild-type and hos9-1 plants were grown in soil in a growth chamber (23 ± 2°C), under a long-day photoperiod (16 h light/8 h dark) or short-day photoperiod (8 h light/16 h dark) for 3 weeks and then incubated at 4°C for 1 week for cold acclimation under either long- or short-day photoperiods. The plants were then placed in a temperature chamber (model Tenney-JR, Tenney Engineering, Garland, TX) with the following freezing temperature regimen: from 4°C to -2°C in 30 min, then hold at -2°C for 1 h; then an identical timing sequence (30 min to reach the next temperature, hold there for 1 h) for successive 2°C decreases until -12°C was reached. Plants damage was scored 7 d later (25).
Northern Blot and Microarray Analyses. Wild-type and hos9-1 seedlings were grown on separate halves of the same Murashige and Skoog (26) agar plates for 14 d and then left untreated or treated with low temperature, ABA, or NaCl. Total RNA was extracted from whole seedlings, and RNA analysis was conducted as described (27). Gene specific probes for RD29A, Actin, FLC, CBF1, CBF2, CBF3, and β-tubulin were as described (27-29).
Total RNA (20 μl) extracted with the RNeasy Plant Mini Kit (Qiagen, Valencia, CA) from 21-d-old wild-type and hos9-1 seedlings after cold treatment (24 h at 0°C) was used to make biotin-labeled cRNA targets. Microarray analysis (Affymetrix GeneChip array) was performed as described (13).
Cloning of the HOS9 Gene. The genomic DNA fragment flanking the left border of the inserted T-DNA in hos9-1 plants was isolated by thermal asymmetric interlaced PCR, as described (21, 30). The following primer pair was designed to perform the T-DNA diagnosis PCR: forward, 5′-TACTTCTGAGGTACTTTATTAGGTGAC-3′; reverse, 5′-TCAACGTGGACATACCATTTAAAG-3′ (21). To estimate the functional T-DNA copy number in the hos9-1 mutant genome, the following primers, for the basta gene were used, 5′-AAACCCACGTCATGCCAGTTC-3′ and 5′-CCATCGTCAACCACTACATCGAGAC-3′.
A genomic fragment that includes the putative HOS9 gene including 1,457 bp upstream of the initiation codon and 556 bp downstream of the stop codon from bacterial artificial chromosome (BAC) clone F2I9 was amplified by PCR. The PCR fragment was cloned into the binary vector pCAMBIA1200 (CAMBIA, Black Mountain, Australia) between the KpnI and PstI sites and the identity of the clone insert was confirmed by sequencing. The construct was introduced into hos9-1 mutant plants through an A. tumefaciens-mediated (strain GV3101) T-DNA transformation. Primary transformants were isolated on Murashige and Skoog medium containing 50 mg/l hygromycin (Invitrogen) and transferred to soil to grow to maturity. Progenies of these transformants were examined for RD29A::LUC expression with the charge-coupled device camera and for freezing tolerance in the temperature chamber as described above.
RT-PCR Analysis. Total RNA (3 μg) was extracted from plant tissues by using the RNeasy Plant Mini Kit and used for first-strand cDNA synthesis using thermoscript RT-PCR system (Invitrogen). PCR amplifications for HOS9 were performed by using PfuTurbo DNA polymerase (Stratagene) following the manufacturer's instructions. The gene specific primers for HOS9 were as follows: forward primer, 5′-ATGGGCTACATCTCCAACAACAAC-3′; and reverse primer, 5′-TCAGTTCTTCAGAGGCATGAACTC-3′. Gene-specific primers for RD29A, Tubulin and Actin were as described (27, 28). The RT-PCR product amplified from nontreated wild-type plants was subcloned into the pGEM-T Easy Vector with (Promega). The resulting 9-22 clone and the sequence of the insert were confirmed by sequencing. Gene-specific primers used to confirm the microarray results were as follow: At2g46400-F, 5′-TGAATGCAAAGATGATGG-3′ and At2g46400-R, 5′-TTGCCCATATTTTCTCCAGCAG-3′ for At2g46400; At2g32210-F, 5′-TCCTACGCCGCCAGTGTCTAC-3′ and At2g32210-R, 5′-GTCCACGT TGACTA ACCGGT-3′ for At2g32210; At5g44420-F, 5′-TGGCTAAGTTTGCTTCCATCATC-3′ and At5g44420-R, 5′-CAACGGGAAAATAAACATTAAAAC-3′ for At5g44420.
Analysis of HOS9 Promoter::GUS Expression. A genomic fragment including 1,457 bp upstream of the initiation codon from BAC clone F2I9 that was used for the gene complementation test was amplified by PCR and cloned into the binary vector pCAMBIA1381Z (CAMBIA) between the BamHI and HindIII sites. The identity of the cloned insert was confirmed by sequencing. A. tumefaciens strain GV3101 containing this construct was used to transform Arabidopsis Columbia wild-type plants. To measure β-glucuronidase (GUS) activity, tissues from transgenic plants were incubated overnight at 37°C in the dark, in 1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (Rose Scientific, Cincinnati) and 0.1 M potassium phosphate buffer (pH 7.5) with 0.1% Triton X-100 (31). Chlorophyll was removed by washing several times with 70% ethanol.
GFP-HOS9 Fusion Protein Construct. The HOS9 coding region was amplified by PCR and cloned in-frame into pGEAD vector between the EcoRI and BamHI sites and the entire insert and the conjunction regions were sequenced. This construct was then introduced into Arabidopsis wild-type plants (ecotype Columbia) by using floral dip transformation with Agrobacterium strain GV3101.
Cytosolic Free Ca2+ Measurement. Lines expressing 35S::Aequorin (32) were crossed with the hos9-1 mutant. F2 plants from the cross homozygous for the hos9-1 mutation and for the Aequorin gene were selected by PCR analysis. The 35S::Aequorin expressing line was also crossed with HOS9 wild-type plants (C24 with RD29A::LUC). F2 plants homozygous for the Aequorin transgene were selected by PCR analysis and used for controls. To determine the cytosolic free Ca2+, Aequorin-dependent luminescence from 8-day-old seedlings grown on agar plates was measured with a charge-coupled device camera, as described (18, 20).
Results
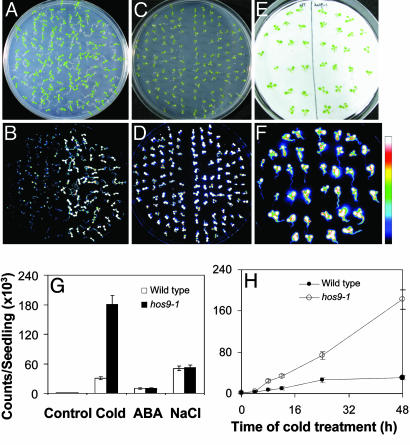
Isolation of the hos9-1 Mutant. To identify the genetic loci that affect cold perception and signaling leading to target gene induction, we used A. tumefaciens-mediated T-DNA insertion/activation mutagenesis (pSKI015 vector) of Arabidopsis plants that were expressing a homozygous firefly luciferase reporter gene driven by the stress-responsive RD29A promoter (6, 20, 21). T2 plants were used to screen for mutants that exhibited altered expression of RD29A::LUC in response to low temperature, ABA, or osmotic treatment. One mutant, hos9-1, was isolated, which displays hyperexpression of the RD29A::LUC marker specifically in response to low-temperature treatment (Fig. 1 A and B) and not to ABA or NaCl treatment (Fig. 1 C-F) compared to wild-type seedlings. Quantification of RD29A::LUC expression indicated that cold-activated RD29A::LUC expression in hos9-1 mutant seedlings was >3-fold higher than that in wild type (Fig. 1G) and remained substantially higher in hos9-1 plants for 3 h or longer (Fig. 1H).
Fig. 1.
The RD29A::LUC expression was hyperinduced in hos9-1 mutant plants in response to low temperature. RD29A::LUC expression was quantitatively measured as luminescence intensity (counts/seedling). The scale bar at the right shows the luminescence intensity from dark blue (lowest) to white (highest). (A) Wild-type (on the left) and hos9-1 (on the right) seedlings grown on an agar plate. (B) Luminescence of A after low-temperature treatment at 0°C for 24 h. (C) Wild-type (on the left) and hos9-1 (on the right) seedlings grown on an agar plate. (D) Luminescence of C after treatment with 100 μM ABA for 3 h. (E) Wild-type (on the left) and hos9-1 (on the right) seedlings on filter paper saturated with 300 mM NaCl. (F) Luminescence of E after treatment with 300 mM NaCl for 4 h. (G) Quantification of the luminescence intensity in B (Cold), D (ABA), and F (NaCl). Also shown are data for untreated plants (Control). (H) Time course of RD29A::LUC expression in hos9-1 and wild-type plants in response to low temperature (0°C). [Bars (G and H) represent standard deviation (n = 20).]
hos9-1 mutant plants were backcrossed with wild type, and all of the resulting F1 plants exhibited a wild-type phenotype and were resistant to bialaphos (conferred by the basta gene on the pSKI015 vector) when transferred to soil. The F2 progeny of the selfed F1 plants segregated in an ≈3:1 ratio of wild type: mutant phenotype indicating that hos9-1 is a recessive mutation in a single nuclear gene. Analysis of the bialaphos resistance and the presence of the basta gene by PCR of the F1 and F2 plants also revealed the presence of a single functional T-DNA that is inserted in the genome of the hos9-1 mutant (ref. 21 and data not shown).
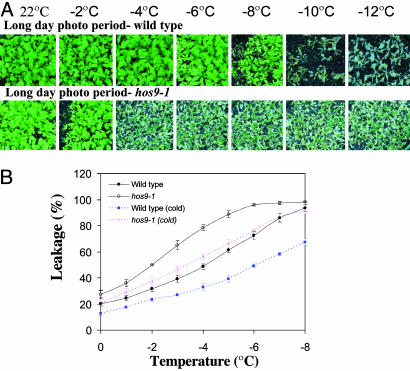
hos9-1 Plants Are Hypersensitive to Freezing Treatment. To evaluate the effect of the hos9-1 mutation on plant freezing tolerance, we performed the whole-plant-freezing test (Fig. 2A) (25). Without cold acclimation neither wild-type nor hos9-1 plants can tolerate freezing treatment at -4°C or lower (data not shown). When grown and then acclimated at 4°C for 1 week under a long-day photoperiod, the majority of the wild-type plants survived freezing treatment as low as -8°C, but none of the hos9-1 mutant plants survived freezing treatments at -4°C or lower temperatures (Fig. 2 A). Similar results were obtained after growing and acclimating the plants under a short-day photoperiod (data not shown). An electrolyte leakage assay was also performed to quantify the freezing tolerance of hos9-1 (Fig. 2B). Without cold acclimation, hos9-1 plants have considerably reduced tolerance to freezing treatments compared to wild-type plants. In addition, after a 4°C treatment for 1 week hos9-1 plants were unable to fully acclimate, compared to wild-type plants (Fig. 2B). Taken together, all of these results clearly indicate that the hos9-1 mutation both decreases constitutive cold tolerance and impairs cold acclimation ability.
Fig. 2.
Freezing tolerance of hos9-1 plants. (A) Tolerance of hos9-1 plants at different temperatures below freezing under long-day photoperiod. The photographs were taken 10 d after freezing treatments. (B) Leakage of electrolytes in hos9-1 and wild-type plants when treated at temperatures below freezing. Wild-type (cold) and hos9-1 (cold), cold-acclimated wild-type, and hos9-1 plants, respectively. [Bars are standard deviation (n = 8).]
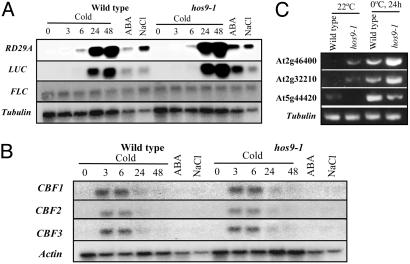
Regulation of Gene Expression in hos9-1 Plants. To investigate whether the hos9-1 mutation alters expression of the endogenous RD29A gene, Northern hybridization was performed by using total RNA extracted from hos9-1 and wild-type seedlings, which were exposed or not exposed to low temperature, ABA, or NaCl (Fig. 3). Cold induction of the luciferase transcript and the endogenous RD29A gene was higher in the hos9-1 mutant than in the wild-type plants at 24- and 48-h time points (Fig. 3A). However, no significantly higher induction of luciferase and the endogenous RD29A transcripts than in wild type was observed in hos9-1 plants in response to NaCl treatment (Fig. 3A). Unlike the luciferase image, the transcript levels of luciferase and RD29A appear higher in hos9-1 in response to ABA treatments (Fig. 3A). The expression levels of the cold-specific transcription factors that activate the expression of cold-regulated genes and mediate cold acclimation were not altered in hos9-1 mutant plants compared to wild-type plants (Fig. 3B).
Fig. 3.
Gene regulation in hos9-1 and wild-type plants. (A) Expression of stress-responsive gene in hos9-1 and wild-type plants. Plants were subjected to low temperature (0°C) for the indicated time periods (h). ABA, 100 μM, for 3 h; NaCl, 300 mM, for 4 h. Tubulin gene was used as loading control. (B) Steady-state transcript levels of CBF genes in hos9-1 and wild-type plants. The plants were subjected to the same treatments as stated in A. Actin gene was used as loading control. (C) RT-PCR analysis of three of the genes that were tested in the microarray analysis. The two cold-stimulated genes with higher expression in the hos9-1 than wild type in the microarray analysis encode a WRKY family transcription factor (At2g46400) and an expressed protein (At2g32210). The hos9-1 target gene with lower expression than wild type in the microarray analysis encodes a plant defensin protein (At5g44420). The tubulin gene was used as loading control.
In addition to the Northern analysis, we also examined changes in global gene expression in the hos9-1 mutant under cold treatment by using the Affymetrix near-full genome GeneChip array (13). Analyses of the microarray data indicated that, compared to wild-type plants, the expression of 140 genes was higher in the hos9-1 mutant by at least 3-fold (Table 1, which is published as supporting information on the PNAS web site) and that of 35 genes was lower by at least 2-fold in the hos9-1 mutant (Table 2, which is published as supporting information on the PNAS web site). We examined the steady-state RNA levels of three of the genes by RT-PCR to confirm the microarray results. The expression of the At2g46400 and At2g32210 genes was higher in the hos9-1 mutant and that of At5g44420 was lower in the mutant, consistent with results obtained from the microarrays (Fig. 3C).
Of the 140 genes that were more expressed in the hos9-1 mutant, 41 appeared as cold-induced in a microarray analysis of cold induction using the Affymetrix array (Table 3, which is published as supporting information on the PNAS web site; refs. 13 and 14). The HOS9 gene is apparently important for both constitutive expression and cold-induced expression of genes that may be required for full tolerance to freezing stress.
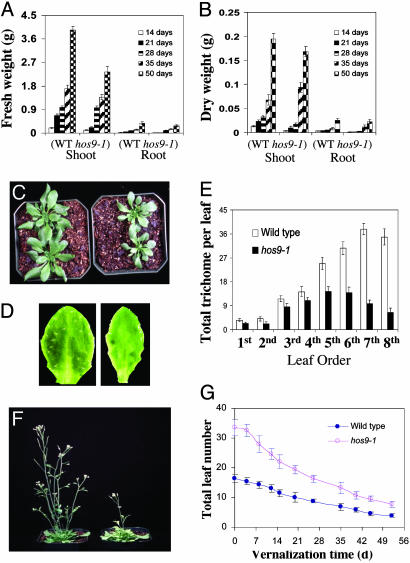
Effects of hos9-1 Mutation on Plant Growth and Development. Plants that carry the hos9-1 mutation grow more slowly than wild-type plants under greenhouse conditions (Fig. 4). The final size of hos9-1 plants after flowering is smaller (Fig. 4 C and F) and leaves of hos9-1 are narrower and smaller (Fig. 4 C and D). Both shoots and roots of hos9-1 plants exhibited slower growth rates when measured as either fresh (Fig. 4A) or dry (Fig. 4B) weight gains. We also observed that there are fewer trichomes formed on the hos9-1 leaves at different developmental stages (Fig. 4 D and E). Under our experimental conditions, hos9-1 plants also flower >10 d later than wild-type plants (Fig. 4F and data not shown). Because in Arabidopsis, flowering time can be influenced by exposing plants to low nonfreezing temperatures (i.e., vernalization), we tested the vernalization responses of hos9-1 and wild-type plants. Even though hos9-1 plants cannot be induced to flower as early as wild-type plants by cold treatment for as long as 50 d, they do respond to vernalization treatment (Fig. 4G) and display normal basal transcript level of flowering time locus C (FLC) gene (Fig. 3A), the major mediator of vernalization.
Fig. 4.
Growth defects of hos9-1 plants. (A and B) The growth as indicated by shoot and root fresh and dry weights. Shoots or roots were separated from wild-type and hos9-1 plants grown in soil under long-day photoperiod at indicated developmental stages. The fresh weight of shoot or root was determined, and the samples were then dried in an oven at 65°C for 48 h and dry weight was measured. [Bars represent standard deviation (n = 15).] (C) Three-week-old of wild-type (Left) and hos9-1 (Right) plants grown under long-day photoperiod. (D) Trichomes on the fifth leaf of the wild-type (Left) and the hos9-1 (Right) plants. (E) Quantification of trichome numbers on rosette leaves of wild-type and hos9-1 plants. The number of trichome on different leaves was counted on a Nikon Optiphot microscope (Nikon). [Bars represent standard deviation (n = 20).] (F) hos9-1 plant (Right) flowers later than wild-type plant (Left) under normal conditions (16 h light/8 h dark). (G) Vernalization responses of wild-type and hos9-1 plants (as indicated by flowering time/total leaf number at flowering) under long-day photoperiod. [Bars represent standard deviation (n = 20).]
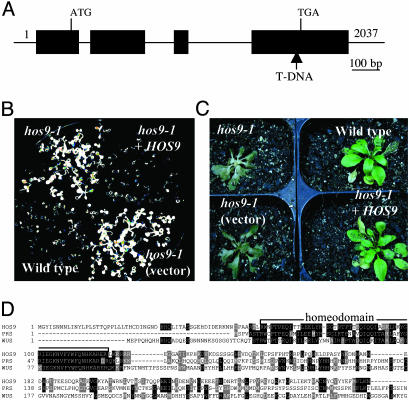
Identification of the HOS9 Locus. The genomic fragment flanking the left border of the T-DNA insert of hos9-1 mutant plants was isolated by thermal asymmetric interlaced PCR. Database searches revealed that the T-DNA tag was inserted at nucleotide 51,349 in BAC F2I9 on Arabidopsis chromosome 2, a position 319 bp upstream of the predicted TGA stop codon of gene At2g01500 (Fig. 5A). We then designed two pairs of primers to determine whether this T-DNA insertion cosegregates with the hos9-1 mutant phenotype. We found that the T-DNA insert was homozygous in all plants (48 of 48) that were homozygous for the hos9-1 mutant phenotype.
Fig. 5.
HOS9 encodes a putative homeobox protein. (A) The structure of HOS9 gene and the position of the T-DNA insert in hos9-1 mutant genome. Positions are relative to the transcription start site. Filled boxes represent exons and lines between filled boxes represent introns. (B) Luminescence image after low-temperature treatment (0°C for 48 h) of wild-type, hos9-1, and hos9-1 transformed with pCAMBIA1200 empty vector [hos9-1 (vector)] and hos9-1 transformed with pCAMBIA1200 containing the HOS9 genomic fragment (hos9-1+HOS9). The plants weregrown on an agar plate. (C) Freezing tolerance of 1-mo-old plants shown in B. The treatment was done at -5°C for 5 h, and the photograph was taken 7 d after treatment. (D) Comparison of HOS9 with its homologs. Compared proteins are: HOS9 (AAC67326) from Arabidopsis; PRS (PRESSED FLOWER, BAB79446) from Arabidopsis; WUS (WUCHEL, CAA09986) from Arabidopsis.
Using a genomic fragment containing the putative HOS9 gene, we complemented the hos9-1 mutant plants. When the wild-type copy of the HOS9 locus was introduced into hos9-1 plants, RD29A::LUC expression returned to the wild-type level (Fig. 5B). The freezing tolerance (Fig. 5C), flowering time and trichome formation phenotypes (data not shown) of hos9-1 plants also reverted to nearly wild type in the complemented mutant plants.
HOS9 Encodes a Putative Homeobox Protein. There is a full-length cDNA clone (Ceres_18820) available for the HOS9 gene, and we further confirmed this sequence information by RT-PCR analysis. HOS9 is composed of four exons and three introns (Fig. 5A) and it encodes a 271-aa polypeptide with a predicted molecular mass of 31,435 Da. Comparison of the predicted HOS9 amino acid sequence with those of other gene products revealed that HOS9 shares greatest similarity, within the homeodomain motif, with the Arabidopsis proteins WUSCHEL (WUS), and PRESSED FLOWER (PRS) (Fig. 5D) (33, 34). The homeodomain encoded by a 180-bp consensus DNA sequence, called a homeobox, is present in a number of proteins involved in developmental processes (35). Homeobox-containing proteins act as transcription factors, regulating the expression of target genes via sequence-specific recognition.
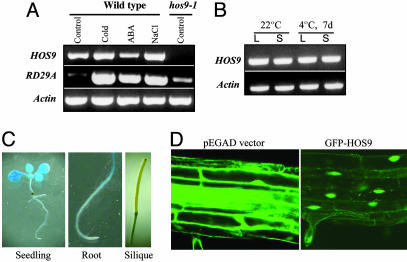
HOS9 Gene Expression and HOS9 Protein Subcellular Localization. HOS9 is constitutively expressed, and perhaps its expression is decreased somewhat after ABA treatment of seedlings (Fig. 6A). Its expression is also not regulated by different day lengths (Fig. 6B). To determine the tissue and cell type expression pattern of HOS9, the HOS9 promoter was fused to the GUS reporter gene, and GUS activities were examined in transgenic Arabidopsis plants. GUS expression was observed in all tissues of young seedlings but was relatively stronger in the vascular tissues and weaker in root tips (Fig. 6C).
Fig. 6.
Expression of the HOS9 gene and subcellular localization of HOS9 protein. (A) The HOS9 expression level was determined by RT-PCR. Actin gene was used as loading control. Control, no treatment; Cold, 0°C for 48 h; ABA, 100 μM for 3 h; NaCl, 300 mM for 5 h. (B) Expression of HOS9 under different photoperiods at room temperature (22°C) or low temperature (4°C, 7 d) by RT-PCR. Three micrograms of total RNA was used to synthesize the first-strand cDNA. Actin gene was used as loading control. L, long-day photoperiod; S, short-day photoperiod. (C) Histochemical staining of transgenic plants expressing HOS9 promoter::GUS.(D) The GFP-HOS9 fusion protein is localized in the nucleus. Confocal image of root cell in plants transformed with empty vector only (Left) and confocal image of root cell in GFP-HOS9 transgenic plants (Right).
To examine the subcellular localization of the HOS9 protein, HOS9 was fused in-frame to the C terminus of the GFP and expressed under the control of the CaMV 35S promoter (36). Confocal imaging of GFP fluorescence in the transgenic plants revealed that the GFP-HOS9 fusion protein is present in the nucleus (Fig. 6D).
Discussion
The hos9-1 mutation was identified in a screen for altered activity of the stress-controlled promoter of the RD29A gene. The hos9-1 mutant displays specific enhancement of cold induction of the RD29A::LUC reporter (Figs. 1 and 3). Northern blot analysis confirmed the hyperinduction by cold but also revealed enhanced induction by ABA of both the luciferase and native RD29A transcripts. Discrepancies between the luminescence intensity and luciferase steady-state transcript level, as well as differences in expression between the RD29A promoter::LUC fusion gene and the native RD29A gene have been observed with other mutants and possible explanations have been discussed (27, 29).
The effect of the hos9-1 mutation on constitutive freezing tolerance is quite clear, resulting in a decrease of maximum freezing tolerance by 3-6 degrees. This gene also appears to have a similar, perhaps somewhat less effect on the ability of the plants to cold acclimate (Fig. 2). This suggests that HOS9 mainly controls basal or constitutive freezing tolerance in Arabidopsis. In addition, it is possible that the role of HOS9 in cold acclimation is redundant with other regulatory factors.
The expression of the HOS9 gene is disrupted by the T-DNA insertion, and a HOS9 transcript was not detected (Fig. 6A). The HOS9 gene is constitutively expressed in wild-type plants and is not induced by cold or NaCl stress (Fig. 6A). This is in sharp contrast to the CBF gene family that is not expressed except after a short period after stress (12). HOS9 is the first Arabidopsis transcription factor to be identified that has been reported to control cold tolerance but not to affect the expression of CBF genes (37). The SFR6 locus has been shown to affect cold tolerance also, either by a possible nontranscriptional control of CBF activity or by a mechanism independent of CBF (18). However, cloning of the SFR6 gene has not been reported.
Because the CBF genes are induced normally by cold treatment in hos9-1 mutant plants, the cold sensitivity of hos9-1 must be largely the result of disruption of expression or function of genes other than those targeted by CBF, or the hos9-1 mutation has posttranscriptional effects on the function (activity) of CBF without disrupting accumulation of its transcript. Microarray analysis of hos9-1 mutant plants did not reveal the disruption of cold induction of any genes reported to be controlled by CBF (Table 1), indicating that HOS9 controls the expression of genes that are important for cold tolerance but are not part of the CBF regulon. However, because the microarray analyses of CBF target genes (14) included only 31.4% (8,000 of 25,500) of a full genome array, it is still possible that some genes targeted by CBF are among those whose expression is controlled also by HOS9 (14, 38). The HOS9 gene apparently does moderately affect the expression of some gene targets of CBF as seen from the enhancement of cold induction of RD29A, one of the targets of CBF. In view of this, it appears that HOS9 may also be a negative regulator of some CBF target genes, as is true of HOS1 (28). In any case, HOS9 must permit cold tolerance by mediating the constitutive activity of some genes or gene products that are essential for this trait. The loss of this activity in hos9-1 mutant plants may then result in increased expression of cold-induced genes by a compensating response to the increased cold sensitivity.
One of the earliest and most universal signal responses to cold exposure is the elevation of cytosolic Ca2+ (39). To see whether HOS9 function includes creation of this Ca2+ flux essential for cold tolerance (40-42), we examined the effect of hos9-1 mutation on cytosolic Ca2+ levels with F2 progeny of crosses between hos9-1 and aequorin expressing plants (18). This technique showed that the relative cytosolic Ca2+ levels in hos9-1 and wild-type plants are identical (data not shown) (18, 20). Thus, HOS9 gene functions occur after the stress-induced increase in cytosolic calcium.
Supplementary Material
Acknowledgments
We are grateful to Zhizhong Gong and Günsu Inan for their excellent technical assistance. We also thank the Arabidopsis Biological Resource Center for providing the BAC clone F2I9 and the pEGAD vector. This work was supported by National Science Foundation Grants DBI9813360, IBN-0212346, and MCB-0241450 and by U.S. Department of Agriculture National Research Initiative Grant 2003-00751.
Abbreviations: hos, high expression of osmotically genes; ABA, abscisic acid; CRT, C repeat; DRE, dehydration resopnsive element; CBF, CRT/DRE-binding factor; BAC, bacterial artificial chromosome; GUS, β-glucuronidase.
References
- 1.Guy, C. L. (1990) Annu. Rev. Plant Physiol. Plant Mol. Biol. 41, 187-223. [Google Scholar]
- 2.Hughes, M. A. & Dunn, M. A. (1996) J. Exp. Bot. 47, 291-305. [Google Scholar]
- 3.Thomashow, M. F. (1998) Plant Physiol. 118, 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomashow, M. F. (1999) Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571-599. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi-Shinozaki, K. & Shinozaki, K. (1994) Plant Cell 6, 251-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi-Shinozaki, K. & Shinozaki, K. (1993) Mol. Gen. Genet. 236, 331-340. [DOI] [PubMed] [Google Scholar]
- 7.Jaglo-Ottosen, K. R., Gilmour, S. J., Zarka, D. G., Schabenberger, O. & Thomashow, M. F. (1998) Science 280, 104-106. [DOI] [PubMed] [Google Scholar]
- 8.Kasuga, M., Liu, Q., Miura, S., Yamaguchi-Shinozaki, K. & Shinozaki, K. (1999) Nat. Biotechnol. 17, 287-291. [DOI] [PubMed] [Google Scholar]
- 9.Gilmour, S. J., Zarka, D. G., Stockinger, E. J., Salazar, M. P., Houghton, J. M. & Thomashow, M. F. (1998) Plant J. 16, 433-442. [DOI] [PubMed] [Google Scholar]
- 10.Medina, J., Bargues, M., Terol, J., Perez-Alonso, M. & Salinas, J. (1999) Plant Physiol. 119, 463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake, V., Cook, D., Riechmann, J. L., Pineda, O., Thomashow, M. F. & Zhang, J. Z. (2002) Plant Physiol. 130, 639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, Q., Kasuga, M., Sakuma, Y., Abe, H., Miura, S., Yamaguchi-Shinozaki, K. & Shinozaki, K. (1998) Plant Cell 10, 1391-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinnusamy, V., Ohta, M., Kanrar, S., Lee, B.-H., Hong, X., Agarwal, M. & Zhu, J.-K. (2003) Genes Dev. 17, 1043-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler, S. & Thomashow, M. F. (2002) Plant Cell 14, 1675-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreps, J. A., Wu, Y, Chang, H. S., Zhu, T., Wang, X., Harper, J. F. (2002) Plant Physiol. 130, 2129-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin, Z. & Browse, J. (1998) Proc. Natl. Acad. Sci. USA 95, 7799-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki, M., Narusaka, M., Ishida, J., Nanjo, T., Fujita, M., Oono, Y., Kamiya, A., Nakajima, M., Enju, A., Sakurai, T., et al. (2002) Plant J. 31, 279-292. [DOI] [PubMed] [Google Scholar]
- 18.Knight, H., Veale, E. L., Warren, G. J. & Knight, M. R. (1999) Plant Cell 11, 875-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J. C., Lee, S. H., Cheong, Y. H., Yoo, C.-M., Lee, S. I., Chun, H. J., Yun, D.-J., Hong, J. C., Lee, S. Y., Lim, C. O., et al. (2001) Plant J. 25, 247-259. [DOI] [PubMed] [Google Scholar]
- 20.Ishitani, M., Xiong, L., Stevenson, B. & Zhu, J.-K. (1997) Plant Cell 9, 1935-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu, J., Gong, Z., Zhang, C., Song, C.-P., Damsz, B., Inan, G., Koiwa, H., Zhu, J.-K., Hasegawa, P. M. & Bressan, R. A. (2002) Plant Cell 14, 3009-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sukumaran, N. P. & Weiser, C. J. (1972) Hort. Sci. 7, 467-468. [Google Scholar]
- 23.Ristic, Z. & Ashworth, E. N. (1993) Protoplasma 172, 111-123. [Google Scholar]
- 24.Guo, Y., Xiong, L., Ishitani, M. & Zhu, J.-K. (2002) Proc. Natl. Acad. Sci. USA 99, 7786-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong, L., Ishitani, M., Lee, H. & Zhu, J.-K. (2001) Plant Cell 13, 2063-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murashige, T. & Skoog, F. (1962) Physiol. Plant 15, 473-497. [Google Scholar]
- 27.Ishitani, M., Xiong, L., Lee, H., Stevenson, B. & Zhu, J.-K. (1998) Plant Cell 10, 1151-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee, H., Xiong, L., Gong, Z., Ishitani, M., Stevenson, B. & Zhu, J.-K. (2001) Genes Dev. 15, 912-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, B.-H., Lee, H., Xiong, L. & Zhu, J.-K. (2002) Plant Cell 14, 1235-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y. G., Mitsukawa, N., Oosumi, T. & Whittier, R. F. (1995) Plant J. 8, 457-463. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. (1987) EMBO J. 6, 3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight, M. R., Campbell, A. K., Smith, S. M. & Trewavas, A. J. (1991) Nature 352, 524-526. [DOI] [PubMed] [Google Scholar]
- 33.Mayer, K. F., Schoof, H., Haecker, A., Lenhard, M., Jurgens, G. & Laux, T. (1998) Cell 95, 805-815. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto, N. & Okada, K. (2001) Genes Dev. 15, 3355-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehring, W. J., Affolter, M. & Bülleter, T. (1994) Annu. Rev. Biochem. 63, 487-526. [DOI] [PubMed] [Google Scholar]
- 36.Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. (2000) Proc. Natl. Acad. Sci. USA 97, 3718-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong, L., Schumaker, K. S. & Zhu, J.-K. (2002) Plant Cell 14, S165-S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Arabidopsis Genome Initiative (2000) Nature 408, 796-815. [DOI] [PubMed] [Google Scholar]
- 39.Knight, H., Trewavas, A. J. & Knight, M. R. (1996) Plant Cell 8, 489-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tähtiharju, S., Sangwan, V., Monroy, A. F., Dhindsa, R. S. & Borg, M. (1997) Planta 203, 442-447. [DOI] [PubMed] [Google Scholar]
- 41.Sangwan, V., Foulds, I., Singh, J. & Dhindsa, R. S. (2001) Plant J. 27, 1-12. [DOI] [PubMed] [Google Scholar]
- 42.Knight, M. R. (2002) Philos. Trans. R. Soc. London B 357, 871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.