Abstract
Competition for resources is thought to play a critical role in both the origins and maintenance of biodiversity. Although numerous laboratory evolution experiments have confirmed that competition can be a key driver of adaptive diversification, few have demonstrated its role in the maintenance of the resulting diversity. We investigate the conditions that favour the origin and maintenance of alternative generalist and specialist resource-use phenotypes within the same population. Previously, we confirmed that competition for hosts among φ6 bacteriophage in a mixed novel (non-permissive) and ancestral (permissive) host microcosm triggered the evolution of a generalist phenotype capable of infecting both hosts. However, because the newly evolved generalists tended to competitively exclude the ancestral specialists, coexistence between the two phenotypes was rare. Here, we show that reducing the relative abundance of the novel host slowed the increase in frequency of the generalist phenotype, allowing sufficient time for the specialist to further adapt to the ancestral host. This adaptation resulted in ‘evolutionary rescue’ of the specialists, preventing their competitive exclusion by the generalists. Thus, our results suggest that competition promotes both the origin and maintenance of biodiversity when it is strong enough to favour a novel resource-use phenotype, but weak enough to allow adaptation of both the novel and ancestral phenotypes to their respective niches.
Keywords: bacteriophage, host range, intraspecific competition, resource polymorphism, resource ratio, evolutionary rescue
1. Background
Resource competition has long been viewed as an important agent of diversifying selection [1,2]. When competition for a preferred resource is strong, competitively mediated selection will tend to favour individuals that can exploit underused resources, even if these resources are novel, poor or toxic [3]. Consequently, competition drives phenotypes apart in niche space, thereby decreasing competition between them [1–6]. If the diverging phenotypes coexist, then the end result is a resource polymorphism [6]. Resource polymorphisms have long fascinated evolutionary biologists, primarily because their evolution is thought by some to represent a critical early stage in the origin of novel resource-use traits and possibly even new species [2,6,7]. Thus, identifying the conditions under which resource polymorphism evolves is critical for understanding the origins and maintenance of biodiversity.
A growing number of laboratory experiments have demonstrated that competition can indeed promote the evolution of resource polymorphism. For example, competition for food drives toxic cadmium tolerance in Drosophila melanogaster [3], competition for glucose favours evolution of glucose–acetate generalists in Escherichia coli [8–11], competition for oxygen promotes the evolution of different strategies for colonizing the air–liquid interface in Pseudomonas fluorescens [12], and competition for bacterial hosts drives the evolution of host generalists in the bacteriophage φ6 [13]. Yet only some of these studies demonstrated stable maintenance of the evolved resource polymorphism. Among this subset, all demonstrated a role of antagonistic pleiotropy, in which adaptation to one resource resulted in a cost on the alternative resource [14–17].
Here, we investigate whether stable maintenance of evolved resource polymorphisms is possible even when antagonistic pleiotropy is absent. We build on our previous experiment [13] in which competition for hosts in the bacteriophage φ6 readily promoted the evolution of a novel host generalist but rarely resulted in stable coexistence of the new generalist and the ancestral specialist. The absence of antagonistic pleiotropy in the generalist phage [13] probably contributed to this outcome. However, we hypothesize that the strength of competition and the consequent speed of competitive exclusion also played a major role. Our reasoning is that trade-offs, arising from a variety of mechanisms including (but not limited to) antagonistic pleiotropy, are expected to emerge as alternative resource-use phenotypes adapt to their respective niches [18,19]. This niche-specific adaptation should stabilize the evolved resource polymorphism, but only if it transpires quickly relative to competitive exclusion [20,21].
The prediction that adaptation can enable persistence in the face of environmental change, recently termed ‘evolutionary rescue’, is well grounded in theory [20,22–25] and experiment [26–31]. However, this prior work has tended to focus on abiotic change (but see [25]). By contrast, our focus is on the appearance of a biotic change—the evolution of a novel host generalist—that threatens the persistence of an ancestral host specialist. Additionally, our use of the term ‘evolutionary rescue’ to describe rescue of a phenotype from competitive exclusion differs from its conventional use to describe rescue of a population from extinction. Nonetheless, the same principles should apply. Extending the principles of evolutionary rescue to our experimental system, we predict that for a host-use polymorphism to arise and persist, competition should be strong enough to selectively favour the new host generalist phenotype, but not so strong that it promotes competitive exclusion of the ancestral host specialist phenotype before it evolves adaptations that permit coexistence [21]. Essentially, competition should be sufficiently weak to allow time for evolutionary rescue to occur. In this way, evolutionary rescue might play a critical role in the origin and maintenance of resource polymorphism.
Here, we test this prediction by building on a previous experiment in which competition for hosts in experimental populations of the bacteriophage φ6 readily promoted the evolution of a novel host generalist that rapidly competitively excluded the ancestral host specialist. In this experiment, we increased the ratio of standard to novel hosts from 1 : 1 to 9 : 1 to reduce the selective advantage of the generalist and slow the competitive exclusion of the specialist. We tested the prediction that these slower dynamics would increase the opportunity for evolutionary rescue of the specialist by monitoring the evolution of φ6 populations in liquid culture containing a 9 : 1 ratio of standard : novel hosts. Host generalists again evolved in response to competition but, in contrast to the previous experiment, the ancestral specialist coexisted stably with the new generalists for the duration of the experiment, approximately 300 phage generations. We used a series of competition assays in different environments to confirm that the long-term stability of this polymorphism depended critically on adaptation of the specialists to the ancestral host. Our results confirmed our a priori expectations regarding the conditions that favour the origin and maintenance of resource polymorphism, namely that competition should be strong enough to selectively favour a new resource-use phenotype but weak enough to allow sufficient time for evolutionary rescue to occur.
2. Methods
(a). Strains
The RNA bacteriophage φ6 used in this study is a laboratory strain descended from the original isolate [32]. The ‘wild-type’ phage used to found all experimental populations readily infects the standard laboratory host bacterium Pseudomonas syringae pathovar phaseolicola (hereafter Ps phaseolicola) strain HB10Y [33,34], but cannot infect the novel host bacterium P. pseudoalcaligenes pathovar ERA [33] (hereafter Pp ERA).
(b). Culture conditions
All phage and bacteria were grown in LC medium (5 g l−1 yeast extract, 10 g l−1 bactotryptone, 5 g l−1 NaCl) at 25°C. Plate media contained 1.5% (bottom) or 0.5% (top) agar. Evolved phage was archived for later analysis at −20°C in LC medium plus 20% v/v glycerol.
We monitored the evolution of generalist phenotypes by plating phage population samples on bacterial lawns composed of 200 µl of an overnight culture of the standard host Ps phaseolicola and 2 µl of the novel host Pp ERA. Generalist phage that gained the ability to use the novel host produced clear plaques, whereas specialist phage produced turbid plaques.
(c). Experimental evolution of host-use polymorphism
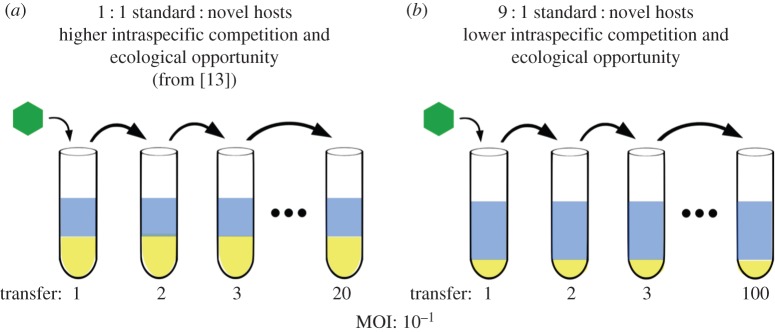
Phage populations were evolved using serial transfer into fresh bacterial cultures containing a mixture of the standard laboratory host and a novel (non-permissive) host at a total density of 2 × 108 cells ml−1 (diagrammed in figure 1). These evolution experiments were performed in the exact same manner as the populations under strong competition described in our earlier study [13] with one exception: the host ratio was changed from a 1 : 1 ratio of standard to novel hosts to a 9 : 1 ratio (figure 1). By increasing the density of standard hosts and decreasing the density of novel hosts, we reduced both intraspecific competition for the standard host and the ecological opportunity (i.e. the underused resource [35]) represented by the novel host. Prior to the start of each serial transfer, fresh bacterial hosts were grown to an OD600 corresponding to a density of 2 × 108 cells ml−1 (Ps phaseolicola OD600 = 0.33 and Pp ERA OD600 = 0.12) and mixed together at a ratio of 9 : 1 Ps phaseolicola : Pp ERA. Individual populations were propagated by initiating each transfer at a multiplicity of infection (MOI or ratio of phage to hosts) of 0.1. As in our earlier study [13], we adjusted total culture volume to 10, 100 or 1000 µl to achieve a constant initial host density and MOI across phage transfer population size treatments of n = 105, 106 or 107 phage, respectively. We used these population sizes in the previous experiment [13] to test the effect of mutation supply on generalist evolution. Although we detected no significant effect of population size in that experiment [13], we used the same population sizes here to replicate the experimental design as closely as possible. Cultures were incubated shaking at 25°C for 6 h (approx. three phage generations) before filtering to remove host cells. A sample of 105, 106 or 107 of the resulting phage was used to initiate the next transfer cycle, and the remainder were archived at −20°C for later analysis. We measured the total number of phage and the frequency of generalists at the end of every transfer. This protocol was repeated for 20 transfers in six replicate populations. Three of the six replicates were extended for a total of 100 transfers.
Figure 1.
Experimental design. Our experiment monitored the evolution and maintenance of host-use polymorphism during serial transfer into fresh cultures containing different ratios of the standard and novel hosts. (a) Our previous experiment [13] imposed a 1 : 1 ratio of standard (blue) to novel (yellow) hosts. (b) The current experiment imposes a 9 : 1 host ratio. Although the phage population bottleneck at the start of each transfer varied from N = 105 to 107, total culture volume was adjusted accordingly so that all transfers were initiated at MOI = 10−1. Populations were evolved for 20 transfers under the 1 : 1 host ratio or 100 transfers under the 9 : 1 host ratio.
(d). Competition assays
We used competition experiments to assess the relative fitness of generalists and specialists in mixed and pure host environments. Generalist and specialist phage clones were isolated from populations of interest by plating a sample of the population on a mixed lawn of the standard and novel hosts, and randomly harvesting a single generalist (clear plaque) and specialist (turbid plaque). Generalist and specialist clones were then combined to achieve mixtures containing 10%, 50% or 90% generalists, depending on the experiment. These mixtures were incubated with either the standard host Ps phaseolicola only or both hosts under conditions that exactly mimicked the evolution experiments. In particular, initial total host density was held at 2 × 108 cells ml−1 and initial total phage density at 108 phage ml−1, and the resulting culture was incubated shaking for 6 h at 25°C. The frequency of the generalist and specialist phage was determined by plating on mixed lawns at the beginning (t = 0 h) and end (t = 6 h) of each incubation. The fitness of the generalist relative to the specialist phage was calculated as W = R6/R0, where Rt is the ratio of generalists to specialists at time t hours.
(e). Estimating fitness
In the populations evolved in the 1 : 1 host ratio in which generalists reached fixation (achieved a frequency fgen = 1.0) by transfer 20, we could not measure the fitness of generalists relative to specialists using competition assays. Instead, we used logistic regression to estimate fitness from the counts of generalists and specialists we obtained by sampling the evolving population. To ensure we obtained estimates of generalist fitness when common, we used counts only from transfers in which the generalist was at a high frequency but not yet fixed: 0.8 < fgen < 0.97. Logistic regression models are of the form
| 2.1 |
where β1 is the expectation of lnW. We estimated lnW = β1 using the generalized linear model function (glm) and obtained standard errors of the estimate using the confidence interval function (confint) in the R v. 3.1.2 statistical package. This method differs from the competition assays only in that replication was achieved by using multiple transfers in series (logistic regression) or in parallel (competition assays), and therefore the initial frequency of generalists was slightly more variable in the logistic regression method. The experimental conditions were identical, and the estimated fitness metrics (W) were equivalent.
(f). Adsorption rate assays
About 2000 phage in 1 ml of LC were mixed with 1 ml of approximately 2 × 108 exponentially growing host cells and incubated shaking at 25°C. Initially and after 40 min, 500 µl of this mixture was centrifuged for more than 1 min at 6600 r.p.m. to pellet the cells, and 200 µl of supernatant was plated on a lawn of the standard host Ps phaseolicola to obtain a count of the free phage. The adsorption rate constant was calculated as k = −ln(P40/P0)/N, where N is the concentration of host cells and Pt is the concentration of free phage at time t, determined by colony and plaque assays, respectively.
(g). Statistical analysis
Statistical analyses were conducted in R v. 3.1.2. We conducted tests for experimental treatment effects by estimating linear-mixed models using the lme function from the nlme package. Experimental lineage was treated as a random effect in all models. Host ratio, population size, transfer number and initial generalist frequency were treated as fixed effects. We also used the lme function to test whether ln relative fitness (lnW) differed significantly from zero. In cases where we a priori decided to test if lnW < 0 (e.g. when we tested for the presence of trade-offs), we used one-tailed tests.
3. Results and discussion
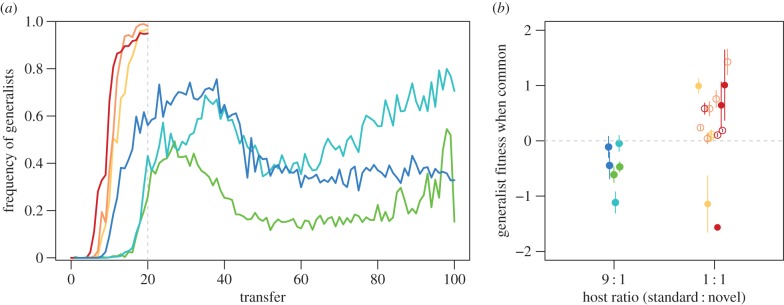
Using the bacteriophage φ6, we sought to experimentally evaluate the conditions that favour the origin and maintenance of alternative generalist and specialist resource-use phenotypes within the same population. We specifically predicted that reducing the relative abundance of a novel host would slow the increase in frequency of the generalist phenotype, allowing sufficient time for the specialist to further adapt to the ancestral host (i.e. to undergo evolutionary rescue), thereby enabling coexistence of the two phenotypes. We found that experimental φ6 populations subjected to weaker competition in a high 9 : 1 ratio of standard : novel hosts nonetheless evolved a new generalist phenotype capable of infecting the novel host (figure 2a, cool colours). As predicted, generalists increased in frequency more slowly in the 9 : 1 host ratio than in the lower 1 : 1 host ratio (figure 2a, warm colours) [13]. In addition, the ultimate outcome of evolution differed dramatically between the host ratio treatments. Whereas generalists tended to competitively exclude specialists in the 1 : 1 host ratio within 20 transfers, the two phenotypes coexisted for the 100-transfer duration of our 9 : 1 host ratio experiment in all replicate lineages. Moreover, the trajectory of generalist frequency over time exhibited the (inverted) U shape (figure 2a; analysis in electronic supplementary material, figure S1) that is expected [20] if evolutionary rescue of the specialists enabled their persistence in the 9 : 1 host ratio treatment. Because the population recovery indicated by the U shape is also consistent with other evolutionary and ecological processes, we provide more rigorous tests of evolutionary rescue below.
Figure 2.
The origin and maintenance of resource polymorphism. In both panels colour distinguishes populations evolved under 9 : 1 host ratio (cool colours; transfer population sizes N = 105: green, 106: light blue, 107: blue) or 1 : 1 host ratio (warm colours; transfer population sizes N = 105: yellow, 106: orange, 107: red). (a) Frequency of generalists over time, averaged across replicate populations for each host ratio by population size treatment combination. Each population size treatment was conducted with fivefold replication under the 1 : 1 host ratio and with twofold replication under the 9 : 1 host ratio through transfer 20. After transfer 20, each 9 : 1 host ratio line corresponds to a single replicate population. (b) Mean ln(fitness) ± s.e.m. of generalists relative to specialists for each replicate population at transfer 20, assayed at a high initial generalist frequency of approximately 0.9 in the host ratio in which they were evolved. Open and closed circles represent populations in which specialists were absent (i.e. had gone extinct) or present at transfer 20, respectively.
We examined the stability of coexistence between generalists and specialists in the 9 : 1 host ratio populations by testing for negative frequency-dependent selection. As generalists were able to invade, and therefore had an advantage when rare, we measured only the relative fitness of generalist phage when the generalist was common (figure 2b). Coexistence was confirmed only if generalists exhibited a fitness disadvantage (lnW < 0) when assayed at a high initial frequency (fgen = 0.9). As expected from the persistence of the polymorphism in the 9 : 1 host ratio experiment, generalists exhibited significant average disadvantages when common at transfer 20 (figure 2b) and for the remainder of the experiment (electronic supplementary material, figure S2b; mean lnW ≤ −0.470 at each of transfers 20, 40, 60, 80 and 100; p < 0.05 for each of transfers 20, 60, 80 and 100 by a linear-mixed-effect model specifying replicate population as a random factor). These data confirm that the resource polymorphism was stable in the 9 : 1 host ratio experiment as early as transfer 20. Moreover, these results stand in sharp contrast to the average fitness advantages exhibited by generalists evolved in our previous 1 : 1 host ratio experiment (figure 2b; mean lnW ± s.e.m. = 0.269 ± 0.201, d.f. = 15, t = 1.338, p = 0.2007).
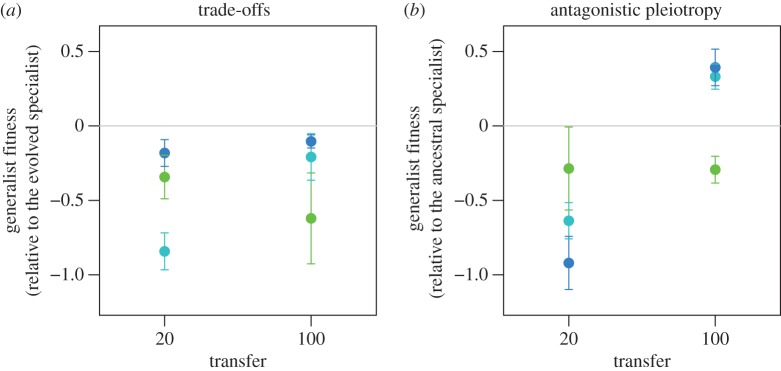
The higher stability of the resource polymorphism in the 9 : 1 host ratio experiment (figure 2), combined with the apparent absence of trade-offs observed previously in the 1 : 1 host ratio experiment [13], led us to predict that the coexistence of generalists and specialists in the 9 : 1 host ratio experiment must be underpinned by a trade-off that evolved over the course of the experiment. In the 9 : 1 host ratio experiment, the only reasonable explanation for the reduced relative fitness of the generalists when common is that the rare specialist phage have a competitive advantage on the standard host. To confirm this explanation, we used competition assays to compare the fitness of generalists relative to specialists when grown on the standard host only. On average, generalists exhibited a fitness disadvantage (lnW < 0) relative to evolved specialists (their contemporaries) at both transfers 20 (figure 3a; mean lnW ± s.e.m. = −0.456 ± 0.199, d.f. = 12, t = 2.292, p = 0.0204) and 100 (figure 3a; mean lnW ± s.e.m. = −0.311 ± 0.158, d.f. = 12, t = 1.970, p = 0.0362), confirming the presence of a trade-off in performance on the standard host at both early and late transfers.
Figure 3.
Trade-offs on the standard host. The fitness of evolved generalist clones was determined from competition assays on the standard host only. (a) We first tested for a trade-off in the fitness of the generalist relative to a contemporary evolved specialist. (b) We then tested if that trade-off could be attributed to antagonistic pleiotropy by testing if generalists performed more poorly on the standard host relative to the ancestral specialist. Data are mean ln(fitness) ± s.e.m. of generalists relative to specialists obtained from five replicate competition assays initiated at initial generalists frequencies of fgen = 0.5.
Next, we asked whether the trade-off resulted from an evolutionary response in the generalist phage, the specialist phage or both. To determine whether antagonistic pleiotropy in the generalists contributed to the observed trade-offs, we measured the fitness of evolved generalists relative to the ancestral specialists (from transfer 0), again on the standard host only. On average, evolved generalists at transfer 20 did exhibit a fitness disadvantage relative to the ancestral specialist (figure 3b; mean lnW ± s.e.m. = −0.615 ± 0.183, d.f. = 12, t = 3.349, p = 0.0029), confirming that generalist adaptation at this early transfer was characterized by antagonistic pleiotropy (i.e. mutations that increased fitness on the novel host also decreased fitness on the standard host). However, by transfer 100, the generalists' average disadvantage relative to the ancestral specialist disappeared (mean lnW ± s.e.m. = 0.143 ± 0.219, d.f. = 12, t = 0.6541, p = 0.2627), and antagonistic pleiotropy was entirely ameliorated in two out of three populations (figure 3b). Consistent with this observation, between transfers 20 and 100 generalists evolved higher adsorption (binding) rates to the standard host (electronic supplementary material, figure S3; F1,7 = 9.726, p = 0.0169), but not to the novel host (electronic supplementary material, figure S3; F1,7 = 2.97326, p = 0.1283).
To determine whether adaptation of the specialists contributed to the observed trade-offs, we compared the relative fitness of evolved and ancestral specialist phage when grown on the standard host only, using an evolved generalist as a common competitor. We repeated this comparison for evolved phages isolated from transfer 20 and transfer 100. We found that evolved specialists exhibited higher fitness than the ancestral specialist at transfer 100 (figure 4; mean ± s.e.d. of lnW100 − lnW0 = 0.454 ± 0.125, t = 3.628, d.f. = 26, p = 0.0012), confirming adaptation of the specialists over the course of the experiment. Such adaptation was not apparent at transfer 20 (figure 4; mean ± s.e.d. of lnW100 − lnW0 = −0.159 ± 0.155, t = −1.025, d.f. = 26, p = 0.3147). In sum, antagonistic pleiotropy may have been sufficient to explain the coexistence of generalists and specialists during the early transfers, but adaptation of the specialists was required to explain the persistence of the trade-off, and therefore of coexistence for the duration of the experiment.
Figure 4.
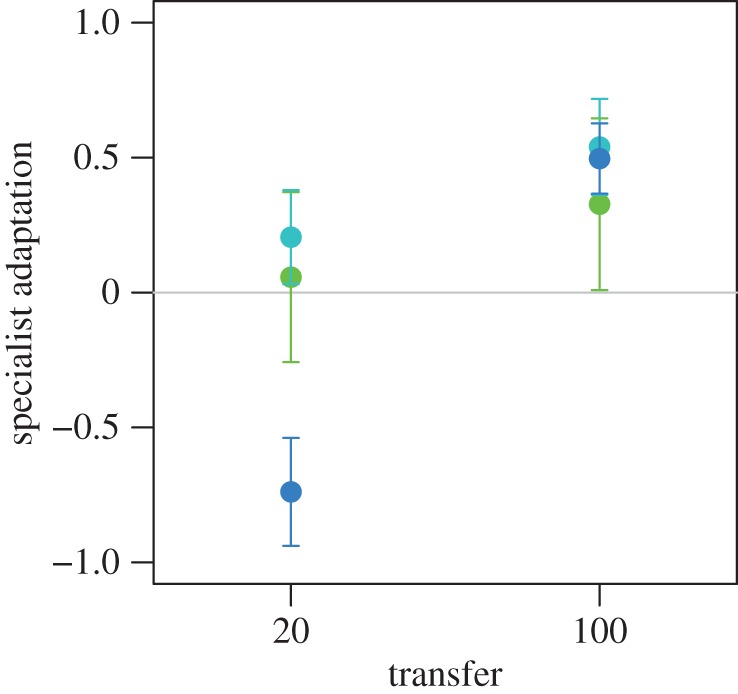
Adaptation to the standard host. The fitness of evolved and ancestral specialist clones was determined from competition assays on the standard host only, using an evolved generalist clone as a common competitor. Pairs of evolved generalist and specialist clones were isolated randomly from each transfer 20 and transfer 100 population. Data are 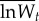 –
–
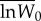 , where
, where 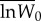 and
and 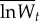 are the means of five log fitness measures of specialists isolated from transfer 0 and transfer t, respectively, relative to generalists isolated from transfer t. Error bars correspond to standard errors of the difference.
are the means of five log fitness measures of specialists isolated from transfer 0 and transfer t, respectively, relative to generalists isolated from transfer t. Error bars correspond to standard errors of the difference.
These results, together with those from our earlier study [13], indicate that competition readily drives the origin of resource polymorphism. They also highlight the critical role that the nature and strength of trade-offs play in the stability of that polymorphism. The contribution of antagonistic pleiotropy to trade-offs in our experiments was modest: we detected significant antagonistic pleiotropy only in the 9 : 1 host ratio experiment and only early in that experiment. This modest contribution of antagonistic pleiotropy probably explains why competition favoured the initial emergence of generalists even when novel hosts were at a low density in the environment (in the 9 : 1 host ratio), and why generalists competitively excluded specialists when novel hosts were at a high density (in the 1 : 1 host ratio). Both these outcomes emerge from resource ratio theory [36–38] when trade-offs are weak. Moreover, the transient nature of the antagonistic pleiotropy we did observe suggests that antagonistic pleiotropy could not, by itself, ensure the long-term stability of the resource polymorphism at either host ratio. Instead, stable maintenance of the resource polymorphism depended critically on whether trade-offs could be generated by adaptation of the specialists before the specialists were competitively excluded. Because antagonistic pleiotropy is often weak or non-existent [18,39,40], our data illustrate that the evolutionary rescue framework may often be relevant for predicting whether ancestral phenotypes win the evolutionary race against competitive exclusion following the emergence of a resource polymorphism.
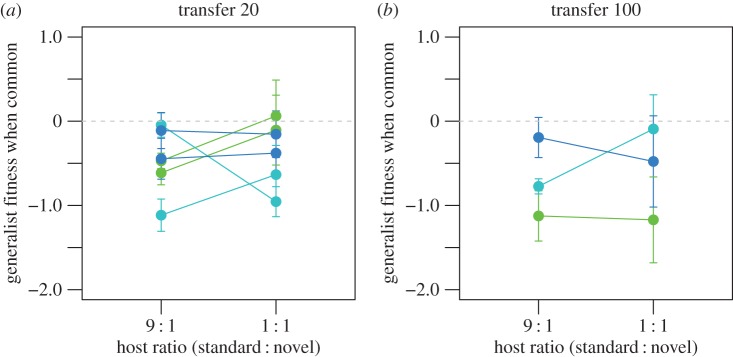
One unanticipated consequence of achieving a stable resource polymorphism through evolutionary rescue was that the stringency of the ecological requirements for maintaining the polymorphism was relaxed once stability was achieved. We tested whether the polymorphisms that evolved in the 9 : 1 ratio of standard : novel hosts were stable if we shifted the ratio to 1 : 1. We measured fitness when common of generalists evolved in the 9 : 1 ratio of standard : novel hosts, this time altering the host ratio used in the competition assay to 1 : 1. We found that, unlike generalists evolved in the 1 : 1 host ratio (figure 2b), generalists evolved in the 9 : 1 host ratio exhibited disadvantages when common in the 1 : 1 host ratio (figure 5; transfer 20: grand mean lnW = −0.361, t = −2.34, d.f. = 5, p = 0.0333; transfer 100: grand mean lnW −0.58, t = −1.84, d.f. = 2, p = 0.104 by one-tailed t-tests conducted on the population means). Thus, although stable resource polymorphisms were unlikely to evolve in the 1 : 1 host ratio environment, they could be maintained in that environment after evolutionary rescue established a stable polymorphism in the 9 : 1 host ratio environment. This result suggests an optimistic perspective on the likelihood of coexistence between generalists and specialists. Whereas theory [41–44] and some previous experiments [13,15] indicate that the ecological requirements for coexistence are stringent, our results indicate that evolution can eventually ensure that the ecological requirements are met by increasing the strength of the performance trade-off among resources. This relaxation in the stringency of ecological requirements for coexistence over time seems likely to characterize any population in which both alternative resource-use phenotypes are able to adapt on a time scale relevant to their changing environment.
Figure 5.
Relative fitness of evolved generalists when common at different host ratios. Pairs of generalist and specialist phage clones isolated from (a) transfer 20 and (b) transfer 100 populations were subjected to competition assays both in the 9 : 1 host ratio to which they were adapted (repeated from figure 2b and electronic supplementary material, figure S2b for comparison) and in the 1 : 1 host ratio. Data are mean ln(fitness) ± s.e.m. of generalists relative to specialists obtained from five replicate competition assays initiated at generalist frequencies of fgen = 0.9.
Supplementary Material
Acknowledgements
We thank two anonymous referees for their helpful comments. We also thank M. Brokob, E. Fisher and K. Vang for technical assistance, J. Kingsolver, R Gomulkiewicz, M. Travisano, K. Koelle and J. Umbanhowar for conceptual and statistical advice, K. Peck and A. Monroy for writing advice, and the K. and D. Pfennig, C. Burch, C. Jones and D. Matute laboratory groups for feedback at various stages of the project.
Data accessibility
Data deposited in Dryad: http://dx.doi.org/10.5061/dryad.p6d88.
Authors' contributions
L.M.B. carried out laboratory work and data analysis, participated in the design of the study, produced the figures and drafted the manuscript; C.L.G. carried out laboratory work, and participated in the design of the study and drafting the manuscript; C.L.B. and D.W.P. conceived of, designed and coordinated the study, and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by National Science Foundation grant no. DEB-0922111 to C.L.B. and D.W.P. L.M.B. was supported by the UNC Molecular Biology of Viral Diseases Predoctoral Training Grant and a UNC Dissertation Completion Fellowship.
References
- 1.Schluter D. 1994. Experimental evidence that competition promotes divergence in adaptive radiation. Science 266, 798–801. ( 10.1126/science.266.5186.798) [DOI] [PubMed] [Google Scholar]
- 2.Pfennig DW, Pfennig KS. 2012. Evolution’s wedge: competition and the origins of diversity. Berkeley, CA: University of California Press. [Google Scholar]
- 3.Bolnick DI. 2001. Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature 410, 463–466. ( 10.1038/35068555) [DOI] [PubMed] [Google Scholar]
- 4.Maynard Smith J. 1962. Disruptive selection, polymorphism and sympatric speciation. Nature 195, 60–62. ( 10.1038/195060a0) [DOI] [Google Scholar]
- 5.Rosenzweig M. 1978. Competitive speciation. Biol. J. Linn. Soc. 10, 275–289. ( 10.1111/j.1095-8312.1978.tb00016.x) [DOI] [Google Scholar]
- 6.Smith TB, Skulason S. 1996. Evolutionary significance of resource polymorphisms in fishes, amphibians, and birds. Annu. Rev. Ecol. Syst. 27, 111–133. ( 10.1146/annurev.ecolsys.27.1.111) [DOI] [Google Scholar]
- 7.West-Eberhard MJ. 2003. Developmental plasticity and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 8.Helling RB, Vargas CN, Adams J. 1987. Evolution of Escherichia coli during growth in a constant environment. Genetics 116, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friesen ML, Saxer G, Travisano M, Doebeli M. 2004. Experimental evidence for sympatric ecological diversification due to frequency-dependent competition in Escherichia coli. Evolution 58, 245–260. ( 10.1554/03-369) [DOI] [PubMed] [Google Scholar]
- 10.Rozen DE, Lenski RE. 2000. Long-term experimental evolution in Escherichia coli. VIII. Dynamics of a balanced polymorphism. Am. Nat. 155, 24–35. ( 10.1086/303299) [DOI] [PubMed] [Google Scholar]
- 11.Tyerman JG, Bertrand M, Spencer CC, Doebeli M. 2008. Experimental demonstration of ecological character displacement. BMC Evol. Biol. 8, 34 ( 10.1186/1471-2148-8-34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson GC, Bertels F, Rainey PB. 2013. Adaptive divergence in experimental populations of Pseudomonas fluorescens. V. Insight into the niche specialist fuzzy spreader compels revision of the model Pseudomonas radiation. Genetics 195, 1319–1335. ( 10.1534/genetics.113.154948) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bono LM, Gensel CL, Pfennig DW, Burch CL. 2013. Competition and the origins of novelty: experimental evolution of niche-width expansion in a virus. Biol. Lett. 9, 20120616 ( 10.1098/rsbl.2012.0616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner PE, Souza V, Lenski RE. 1996. Tests of ecological mechanisms promoting the stable coexistence of two bacterial genotypes. Ecology 77, 2119–2129. ( 10.2307/2265706) [DOI] [Google Scholar]
- 15.Rainey PB, Buckling A, Kassen R, Travisano M. 2000. The emergence and maintenance of diversity: insights from experimental bacterial populations. Trends Ecol. Evol. 15, 243–247. ( 10.1016/S0169-5347(00)01871-1) [DOI] [PubMed] [Google Scholar]
- 16.Treves DS, Manning S, Adams J. 1998. Repeated evolution of an acetate-crossfeeding polymorphism in long-term populations of Escherichia coli. Mol. Biol. Evol. 15, 789–797. ( 10.1093/oxfordjournals.molbev.a025984) [DOI] [PubMed] [Google Scholar]
- 17.Spiers AJ, Kahn SG, Bohannon J, Travisano M, Rainey PB. 2002. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics 161, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry JD. 1996. The evolution of host specialization: are trade-offs overrated? Am. Nat. 148, S84–S107. ( 10.1086/285904) [DOI] [Google Scholar]
- 19.Whitlock MC. 1996. The red queen beats the jack-of-all-trades: the limitations on the evolution of phenotypic plasticity and niche breadth. Am. Nat. 148, S65 ( 10.1086/285902) [DOI] [Google Scholar]
- 20.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction? Evolution 49, 201 ( 10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 21.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 22.Lynch M, Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva PM, Kingsolver JG, Huey RB), pp. 234–250. Sunderland, MA: Sinauer. [Google Scholar]
- 23.Holt RD, Gomulkiewicz R. 2004. Conservation implication of niche conservatism and evolution in heterogeneous environments. In Evolutionary conservation biology (eds Ferrière R, Dieckmann U, Couvet D), pp. 244–264. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Orr HA, Unckless RL. 2008. Population extinction and the genetics of adaptation. Am. Nat. 172, 160–169. ( 10.1086/589460) [DOI] [PubMed] [Google Scholar]
- 25.Fussmann GF, Gonzalez A. 2013. Evolutionary rescue can maintain an oscillating community undergoing environmental change. Interface Focus 3, 20130036 ( 10.1098/rsfs.2013.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agashe D. 2009. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am. Nat. 174, 255–267. ( 10.1086/600085) [DOI] [PubMed] [Google Scholar]
- 27.Agashe D, Falk JJ, Bolnick DI. 2011. Effects of founding genetic variation on adaptation to a novel resource. Evolution 65, 2481–2491. ( 10.1111/j.1558-5646.2011.01307.x) [DOI] [PubMed] [Google Scholar]
- 28.Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948. ( 10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 29.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330. ( 10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 30.Samani P, Bell G. 2010. Adaptation of experimental yeast populations to stressful conditions in relation to population size. J. Evol. Biol. 23, 791–796. ( 10.1111/j.1420-9101.2010.01945.x) [DOI] [PubMed] [Google Scholar]
- 31.Ramsayer J, Kaltz O, Hochberg ME. 2013. Evolutionary rescue in populations of Pseudomonas fluorescens across an antibiotic gradient. Evol. Appl. 6, 608–616. ( 10.1111/eva.12046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidaver AK, Koski RK, Van Etten JL. 1973. Bacteriophage φ6: a lipid-containing virus of Pseudomonas phaseolicola. J. Virol. 11, 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferris MT, Joyce P, Burch CL. 2007. High frequency of mutations that expand the host range of an RNA virus. Genetics 176, 1013–1022. ( 10.1534/genetics.106.064634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duffy S, Burch CL, Turner PE. 2007. Evolution of host specificity drives reproductive isolation among RNA viruses. Evolution 61, 2614–2622. ( 10.1111/j.1558-5646.2007.00226.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 36.Tilman D. 1980. Resources a graphical mechanistic approach to competition and predation. Am. Nat. 116, 362–393. ( 10.1086/283633) [DOI] [Google Scholar]
- 37.Tilman D. 1982. Resource competition and community structure. Monogr. Popul. Biol. 17, 1–296. [PubMed] [Google Scholar]
- 38.Miller TE, Burns JH, Munguia P, Walters EL, Kneitel JM, Richards PM, Mouquet N, Buckley HL. 2005. A critical review of twenty years’ use of the resource-ratio theory. Am. Nat. 165, 439–448. ( 10.1086/428681) [DOI] [PubMed] [Google Scholar]
- 39.Futuyma DJ, Moreno G. 1988. The evolution of ecological specialization. Annu. Rev. Ecol. Syst. 19, 207–233. ( 10.1146/annurev.es.19.110188.001231) [DOI] [Google Scholar]
- 40.Leiby N, Marx CJ. 2014. Metabolic erosion primarily through mutation accumulation, and not tradeoffs, drives limited evolution of substrate specificity in Escherichia coli. PLoS Biol. 12, e1001789 ( 10.1371/journal.pbio.1001789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dempster ER. 1955. Maintenance of genetic heterogeneity. Quant. Biol. 20, 25–32. ( 10.1101/SQB.1955.020.01.005) [DOI] [PubMed] [Google Scholar]
- 42.Felsenstein J. 1976. The theoretical population genetics of variable selection and migration. Annu. Rev. Genet. 10, 253–280. ( 10.1146/annurev.ge.10.120176.001345) [DOI] [PubMed] [Google Scholar]
- 43.Maynard Smith J, Hoekstra R. 1980. Polymorphism in a varied environment: how robust are the models? Genet. Res. 35, 45–57. ( 10.1017/S0016672300013926) [DOI] [PubMed] [Google Scholar]
- 44.Van Tienderen PH. 1997. Generalists, specialists, and the evolution of phenotypic plasticity in sympatric populations of distinct species. Evolution 51, 1372–1380. ( 10.2307/2411189) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data deposited in Dryad: http://dx.doi.org/10.5061/dryad.p6d88.