Abstract
Posttraumatic stress disorder (PTSD) is considered a disorder of recovery where individuals fail to learn and retain extinction of the traumatic fear response. In maltreated youth, PTSD is common, chronic, and associated with comorbidity. Studies of extinction-related structural volumes (amygdala, hippocampus, anterior cingulate cortex (ACC), and ventral medial prefrontal cortex (vmPFC)) and this stress diathesis, in maltreated youth were not previously investigated. In this cross-sectional study, neuroanatomical volumes associated with extinction in maltreated youth with PTSD (N=31), without PTSD (N=32), and in non-maltreated healthy volunteers (n=57) were examined using magnetic resonance imaging. Groups were sociodemographically similar. Participants underwent extensive assessments for strict inclusion/exclusion criteria and DSM-IV disorders. Maltreated youth with PTSD demonstrated decreased right vmPFC volumes compared with both maltreated youth without PTSD and non-maltreated controls. Maltreated youth without PTSD demonstrated larger left amygdala and right hippocampal volumes compared with maltreated youth with PTSD and non-maltreated control youth. PTSD symptoms inversely correlated with right and left hippocampal and left amygdala volumes. Confirmatory masked voxel base morphometry analyses demonstrated greater medial orbitofrontal cortex gray matter intensity in controls than maltreated youth with PTSD. Volumetric results were not influenced by psychopathology or maltreatment variables. We identified volumetric differences in extinction-related structures between maltreated youth with PTSD from those without PTSD. Alterations of the vmPFC may be one mechanism that mediates the pathway from PTSD to comorbidity. Further longitudinal work is needed to determine neurobiological factors related to chronic and persistent PTSD, and to PTSD resilience despite maltreatment.
Introduction
Child maltreatment is a serious public health problem that leads to psychopathology (De Bellis and Zisk, 2014). Abuse and neglect are developmental traumas that are interpersonal, enduring, co-occurring, and associated with high rates of pediatric and adult posttraumatic stress disorder (PTSD). Although many cases of youth with maltreatment-related PTSD recover with time, the limited published longitudinal data suggest that chronic PTSD is seen in 20–30% of maltreated youth identified by Child Protective Services (CPS) (Famularo et al, 1993, 1996, McLeer et al, 1998; McLeer and Ruggiero, 1999).
Children who suffer from PTSD generalize fear to traumatic stimuli, experience difficulty extinguishing fear associations, and exhibit anxiety-related behaviors based on traumatic reminders (De Bellis and Zisk, 2014). The influence of early life trauma on the brain structures implicated in extinction learning (the ability to inhibit the fear response in relationship to traumatic reminders) and extinction recall (the ability to maintain this new learning after a time period) in maltreated youth who suffer from PTSD, compared with those without PTSD, may elucidate neuro-mechanisms of the stress diathesis that leads to chronic PTSD vs those without PTSD.
In adults, PTSD is considered a disorder of recovery, where individuals who suffer from trauma fail to learn extinction and extinction recall of the fear response elicited by traumatic reminders, an active process of acquiring and maintaining new learning (Yehuda and LeDoux, 2007; Milad and Quirk, 2012; Pitman et al, 2012). The key brain structures involved in these processes are the amygdala, hippocampus, anterior cingulate cortex (ACC), and ventral medial prefrontal cortex (vmPFC). These brain regions are rich in glucocorticoid receptors, are stress-sensitive (Lupien et al, 2009), and the connections between these cortical and limbic regions are tightly regulated in humans and primates (Beckmann et al, 2009). Effective regulation of the amygdala leads to successful extinction learning and extinction recall in the context of trauma stimuli (Milad and Quirk, 2012); and may be markers of resilience to PTSD following maltreatment.
Although neuroanatomical studies of maltreatment are limited, adults with PTSD secondary to maltreatment have smaller hippocampal (Thomaes et al, 2010), ACC (Kitayama et al, 2006; Thomaes et al, 2010), and vmPFC volumes (Thomaes et al, 2010) compared with adults without maltreatment. Healthy adults with maltreatment histories also demonstrated smaller hippocampal (Dannlowski et al, 2012; Teicher et al, 2012), ACC (Cohen et al, 2006; Dannlowski et al, 2012; van Harmelen et al, 2010), and vmPFC volumes (Dannlowski et al, 2012). The inverted U-shaped model predicts that, although acute stress might be adaptive through acute adrenergic and cortisol effects that promote increased dendritic and apical spines along with vigilance and learning, chronic stress leads to neural damage and smaller brain structures (Lupien et al, 2009). The adult data support structural abnormalities in some extinction-related structures of adults with PTSD and those resilient to adult PTSD from maltreatment. Thus, investigations of structural brain differences in maltreated youth may elucidate these developmental mechanisms.
Unlike findings in adult PTSD, maltreated youth with PTSD show no anatomical differences in stress-sensitive hippocampal or amygdala structures, either cross-sectionally (Carrion et al, 2001; De Bellis et al, 2002) or longitudinally (De Bellis et al, 2001). However, the majority of studies show higher basal cortisol levels in maltreated youth compared with non-maltreated youth (De Bellis and Zisk, 2014), which may lead to damage in these structures in some individuals later in life. Smaller prefrontal cortex (PFC) volumes were not seen in maltreated children with PTSD, after controlling for smaller cerebral volumes (De Bellis et al, 1999), but attenuation of frontal lobe asymmetry (Carrion et al, 2001), larger (Richert et al, 2006; Carrion et al, 2009) and smaller gray matter volumes in ventral PFC were reported in youth with PTSD (Keding and Herringa, 2014) and maltreatment (Hanson et al, 2010) compared with non-maltreated controls. In these studies, it was not known whether brain differences were due to PTSD or maltreatment. Additionally, peer-reared juvenile monkeys showed larger dorsomedial prefrontal and dorsal ACC and no differences in CSF cortisol levels or hippocampal volumes compared with mother-reared monkeys, indicating that stress-sensitive brain structures can increase during atypical primate development (Spinelli et al, 2009). Nevertheless, it is not known whether these differences in brain structures are compensatory adaptive responses or markers of increased risk.
Therefore, this study examined the brain structures associated with extinction in maltreated youth with and without PTSD, noting that these structures are also involved in other functions (emotional regulation, decision-making, and reward processes) that are impaired in PTSD (Schoenbaum and Shaham, 2008; Pitman et al, 2012). Identifying the neuro-mechanisms that lead to PTSD resilience despite the severe allostatic load from childhood maltreatment may lead to previously unidentified targets for intervention designed to diminish the enduring effects of maltreatment. We examined key brain structural volumes involved in extinction (amygdala, hippocampus, ACC, and vmPFC) in three groups of medically healthy youth: maltreated youth with PTSD, maltreated youth without PTSD, and healthy non-maltreated volunteers. We hypothesized that maltreated youth with PTSD would show smaller vmPFC and ACC volumes compared with healthy volunteers and maltreated youth without PTSD; while maltreated youth without PTSD youth would show larger amygdala, hippocampus, ACC, and vmPFC compared with both healthy volunteers and PTSD youth. Planned comparisons were undertaken to determine the relationship between these brain structures, PTSD variables, and trauma load.
Materials and Methods
Subjects
Originally, the sample consisted of maltreated youth with chronic PTSD (N=38), without PTSD (N=35), and non-maltreated participants (n=59), who underwent studies of cerebral and cerebeller volumes, and diffusion tensor imaging measures of the corpus callosum using semi-automatic and hand-tracing approaches (De Bellis et al, 2015). Of these subjects, 120 had image quality data that were suitable for analyses with FreeSurfer to obtain data on these specific regions of interest (ROIs). Subjects consisted of 57 non-maltreated healthy youth and 63 maltreated youth assessed with DSM-IV-TR and DSM-5, resulting in a maltreated without PTSD (n=32) and a PTSD group (n=31). The maltreated groups were defined by a positive forensic investigation conducted by CPS that indicated physical and sexual abuse and/or neglect. Maltreated participants were recruited through statewide advertisements targeted at CPS agencies. To reduce bias, participants who lived more than 75 miles from the research program were given overnight accommodations. Non-maltreated healthy volunteers, with no history of DSM Axis I disorders or Type A traumas, recruited from schools and community settings, had a negative maltreatment screen on initial telephone interview. Comprehensive research interviews that indicated any positive history of participant or participant sibling maltreatment, or positive review of pediatric and birth medical records that met or would have met state CPS maltreatment criteria, excluded a potential healthy volunteer. Healthy volunteers were recruited to be of similar age, gender, handedness, race, and socioeconomic status (SES), and their IQ for inclusion was limited to within 1 standard error of measure (~3 IQ points)(Wechsler, 1991) of the lowest and highest scores of the maltreated youth. Because lower IQ (Perez and Widom, 1994; De Bellis et al, 2009; De Bellis et al, 2013) is an inherent confound in maltreatment studies, this procedure was used as an attempt to control for this confound. After complete description of the study was given to the legal guardians and participants, written informed consent/assent were obtained to undertake this IRB-approved study.
Exclusion criteria were: IQ<70; chronic medical illness; daily prescription medication; head injury with loss of consciousness; traumatic brain injury; neurological disorder; schizophrenia; anorexia nervosa; pervasive developmental disorder; obsessive compulsive disorder; bipolar I disorder or mania; birth weight under 5 lbs.; or severe prenatal (eg, fetal alcohol and/or drug exposure) or perinatal complications (eg, NICU stay); current or lifetime nicotine dependence/alcohol/substance use disorder; contraindications for safe MRI scan; and Axis I disorder or report of maltreatment that warranted CPS investigation in non-maltreated controls. Recruitment was challenging given that prenatal substance exposure, low SES, alcohol and substance dependence, use of psychotropic medications, and medical illnesses are overrepresented in maltreated youth (Smith et al, 2007) and can independently negatively influence brain maturation. Initial recruitment and scanning begin in 2003 and ended in 2011.
Measures
To examine psychiatric diagnoses and maltreatment characteristics, the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL) (Kaufman et al, 1997) was administered to all caregivers and youth. Because multiple sources of information are needed to gather accurate maltreatment history and related symptoms, we requested and reviewed archival records (eg, pediatric records, school attendance records, birth records, forensics records) as sources of mental health, birth history, trauma history, and pediatric health data (Kaufman et al, 1994). If information from these data sources produced evidence meeting exclusionary criteria, the participant was excluded. KSADS-PL interviewer training and modifications were previously described (De Bellis et al, 2009). Child maltreatment was defined as witnessing domestic violence, experiencing physical, sexual, or emotional abuse, and/or neglect that resulted in eight maltreatment categories (failure to supervise, failure to provide, physical abuse, witnessing intimate partner violence, emotional abuse, sexual abuse, witnessing or victim of other interpersonal violence, and corporal punishment).
The Child Behavior Checklist (CBCL) measured total behavior problems reported by the child's caregiver. Children Global Assessment Scale score (Shaffer et al, 1983) was scored by the interviewer after assessment of all clinical data to provide a continuous measure of child function. Participants were administered the 2-subtest short-form (Vocabulary and Block Design) of the Wechsler Intelligence Scale for Children-III to obtain an IQ score (Wechsler, 1991).
Demographic and Behavioral Characteristics of the Groups
Demographic, clinical, and maltreatment information are reported by group in Tables 1 and 2. The groups were similar in age, gender, race, handedness, and SES. Post hoc pairwise group differences revealed that IQ scores were similar in both maltreated groups and significantly lower than controls. The relationship between IQ and PTSD symptoms (p=0.67), and IQ scores and trauma load (p=0.18) were not significant. The PTSD group showed the most emotional and behavioral symptoms and lowest levels of global function than the maltreated youth without PTSD, whose symptoms were also significantly different from the non-maltreated group.
Table 1. Demographic and Clinical Characteristics of the Study Participants.
|
Healthy controls (Group 1)
N=57 |
Maltreated (Group 2)
N=32 |
Maltreated with PTSD (Group 3)
N=31 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Statistic | p | Pairwise group difference |
| Age (years) (age range) | 10.82 (6.4–16.1) | 2.5 | 10.0 (6.3–16.2) | 2.7 | 9.9 (6.2–15.7) | 2.5 | F(1,119)=1.79 | 0.17 | |
| Female/Male | 32/25 | 17/15 | 16/15 | X2=0.18 | 0.91 | ||||
| Right/Left Handed | 51/6 | 30/2 | 28/3 | X2=0.50 | 0.78 | ||||
| Race (Caus/AA/other) | 23/26/8 | 13/14/5 | 14/15/2 | X2=1.63 | 0.80 | ||||
| SES (SES range) | 41.9 (14–65) | 12.9 | 38.5 (14–61) | 15.8 | 37.5 (14–60.4) | 13.8 | F(1,119)=1.2 | 0.30 | |
| FSIQ (FSIQ range) | 102.2 (74–118) | 11.6 | 93.0 (71–115) | 12.6 | 92.6 (71–115) | 12.1 | F(1,119)=9.27 | 0.0002 | Groups: 1>2,3a |
| CBCL Total Score (CBCL range) | 40.39 (24–58) | 8.83 | 57.16 (40–83) | 11.68 | 64.55 (43–78) | 9.73 | F(1,119)=68.2 | <0.0001 | Groups: 1<2<3a |
| CGAS (CGAS range) | 89.2 (70–98) | 5.8 | 67.8 (50–90) | 9.1 | 55.0 (40–70) | 7.4 | F(1,119)=242.4 | <0.0001 | Groups: 1>2>3a |
Abbreviations: AA, African American; Caus, Caucasian; CBCL, Child Behavior Checklist total score; CGAS, Children's Global Assessment Scale score; FSIQ, Full Scale IQ estimated from 2-factors; Other, Multi-racial; SES, socio-economic status measured by the Hollingshead Four factor index.
Comparisons tests for all pairs using Tukey-Kramer honestly significant difference (HSD) q=2.37, p<0.05.
Table 2. Maltreatment, PTSD Symptoms, and Diagnostic Clinical Characteristics of Maltreated Youth and Maltreated Youth with PTSD.
|
Maltreated
N=32 |
Maltreated with PTSD
N=31 |
|||||
|---|---|---|---|---|---|---|
| History of maltreatment types | Mean | SD | Mean | SD | Statistic | p |
| Witnessing Intimate partner violence (Yes/No) | 26/6 | 26/5 | FET | 1.0 | ||
| Physical abuse (Yes/No) | 20/12 | 26/5 | FET | 0.09 | ||
| Sexual abuse (Yes/No) | 6/26 | 14/17 | FET | 0.03 | ||
| Neglect-failure to supervise (Yes/No) | 30/2 | 28/3 | FET | 0.67 | ||
| Neglect-failure to provide (Yes/No) | 15/17 | 17/14 | FET | 0.62 | ||
| Emotional abuse (Yes/No) | 13/19 | 23/8 | FET | 0.01 | ||
| Witnessing or victim of other personal violence (violent crime) (Yes/No) | 18/14 | 16/15 | FET | 0.80 | ||
| Corporal punishment (Yes/No) | 30/2 | 30/1 | FET | 1.0 | ||
| Mean Number of maltreatment types (8 total) Mean±SD (range) | 5.22 (2–8) | 1.34 | 6.19 (3–8) | 1.40 | F(1,62)=7.98 | 0.006 |
| DSM-IV TR Diagnoses | ||||||
| PTSD chronic | — | — | 31 | |||
| Age of onset PTSD years (Mean±SD) (range) | — | — | 6.88 (3.0–12.5) | 2.26 | ||
| Duration of PTSD years (Mean±SD) (range) | — | — | 2.89 (0.25–8.33) | 2.31 | ||
| Total # of PTSD symptoms (Mean±SD) (range) | 3.57 (0–8) | 2.5 (7–15) | 11.51 | 2.3 | F(1,62)=162.1 | <0.0001 |
| Mean number of Internalizing disorders | 0.22 | 0.42 | 2.1 | 0.94 | F(1,62)=105.3 | <0.0001 |
| Major depression (Yes/No) | 3/29 | 15/16 | FET | 0.0008 | ||
| Dysthymia (Yes/No) | 0/32 | 6/25 | FET | 0.01 | ||
| Major depressive disorder NOS (Yes/No) | 1/31 | 0/31 | FET | 1.0 | ||
| Generalized anxiety disorder (Yes/No) | 4/28 | 8/23 | FET | 0.21 | ||
| Separation anxiety disorder (Yes/No) | 0/32 | 5/26 | FET | 0.02 | ||
| Mean number of disruptive disorders | 0.78 | 0.83 | 1.48 | 1.03 | F(1,62)=8.91 | 0.004 |
| ADHD (any) (Yes/No) | 17/15 | 23/8 | FET | 0.12 | ||
| ADHD-combined type (Yes/No) | 6/26 | 15/16 | FET | <0.02 | ||
| ADHD-inattentive type (Yes/No) | 8/24 | 4/27 | FET | 0.34 | ||
| ADHD-hyperactive type (Yes/No) | 1/31 | 1/30 | FET | 1.0 | ||
| ADHD NOS (Yes/No) | 2/30 | 3/28 | FET | 0.67 | ||
| Oppositional defiant disorder (Yes/No) | 5/27 | 15/16 | FET | 0.007 | ||
| Conduct disorder (Yes/No) | 1/31 | 6/25 | FET | 0.05 | ||
| Disruptive behavioral disorder NOS (Yes/No) | 2/30 | 2/29 | FET | 1.0 | ||
| Mean number of above Axis I disorders | 1.00 | 0.98 | 3.58 | 1.61 | F(1,62)=59.5 | <0.0001 |
| Adjustment disorder with depressed mood (Yes/No) | 1/31 | 0/31 | FET | 1.0 | ||
| Adjustment disorder with mixed anxiety and depressed mood (Yes/No) | 4/28 | 0/31 | FET | 0.11 | ||
| Adjustment disorder with anxiety (Yes/No) | 7/25 | 0/31 | FET | 0.01 | ||
| Mean total number of Adjustment Disorders | 0.38 | 0.49 | 0 | 0 | F(1,62)=18.0 | <0.0001 |
Abbreviations: ADHD, Attention-Deficit/Hyperactivity Disorder; FET, Fisher's Exact Test; NOS, not otherwise specified.
Maltreated youth with PTSD were more likely to experience sexual and emotional abuse, greater trauma load, and have more total Axis I disorders than the maltreated youth without PTSD. Their PTSD was both chronic and persistent with a mean duration of 2.89 years. However, both groups of maltreated youth experienced multiple types of severe and continuing maltreatments. Although the maltreated youth without PTSD group did not have PTSD, they had lesser degrees of clinical impairment including adjustment disorders with anxiety. Only five participants in maltreated youth without PTSD youth had no current Axis I diagnosis, while only two subjects in the PTSD group had chronic PTSD as the sole diagnosis. Four of the maltreated youth without PTSD had met DSM-IV criteria for PTSD in the past, but were in complete remission for 12 months prior to this study.
Magnetic Resonance Imaging and Structural Image Analysis
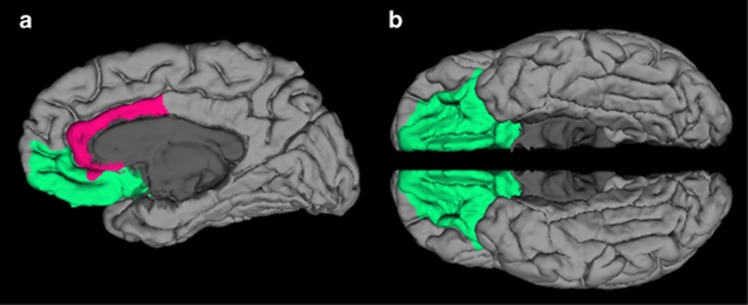
All anatomical images were acquired using the same Siemens Trio 3.0 Tesla MRI system (Trio, Siemens Medical Systems) scanner (3D, GRE (MPRAGE), axial, TR=1750 ms, T1=1100 ms, 25.6 cm FOV, 1.0 mm slice thickness, flip=20°, Bandwidth: (220 Hz/pixel), 256 (phase) × 256 (frequency), number of excitations=1). All T1 images were visually inspected to assure appropriate quality. Automated segmentation and labeling of the amygdala, hippocampus, ACC, and vmPFC, and estimation of total intracranial volume from participants' T1 images were performed using the FreeSurfer image analysis suite (version 5.1.0; http://surfer.nmr.mgh.harvard.edu/) and its library tool recon-all. Details of these FreeSurfer parcellations were previously described (Fischl et al, 2004; Desikan et al, 2006; Destrieux et al, 2010). The use of FreeSurfer segmentation has been validated in youth as young as age 3, with results published in high-quality journals (Østby et al, 2009; Fjell et al, 2012). Automated segmentation of the amygdala and hippocampus compared with manual tracing with FreeSurfer was previously validated (Morey et al, 2009). Spatial normalization by affine registration to Talairach space and skull stripping were performed on the T1 images. FreeSurfer registration, segmentation, and labeling of structures were previously described (Morey et al, 2012). Registration and segmentation of the ROI were overlaid on original T1 images and visually inspected (CCH, RAM) slice-by-slice for correct location and shape. All scans were reviewed by a neuroradiologist who ruled out clinically significant abnormalities. Total vmPFC was composed of two regions defined by FreeSurfer terminology, the sum of the right and left medial orbitofrontal and lateral orbitofrontal cortexes (Desikan et al, 2006). This includes the ventral sector of the medial PFC, the orbitofrontal cortex medial to the straight gyrus, and the orbital sulcus laterally, and is consistent with other approaches (Boes et al, 2009). Total ACC volumes were summed from the following FreeSurfer parcellations: rostral and caudal ACC divisions; ACC sulcus and gyri; and subcallosal gyrus (Desikan et al, 2006; Destrieux et al, 2010) (Figure 1).
Figure 1.
(a) Ventromedial prefrontal cortex (vmPFC) and anterior cingulate cortex (ACC) regions-of-interest as defined by FreeSurfer on a medial view of the right hemisphere (vmPFC in green and ACC in fuchsia). (b) This image of the vmPFC is from the ventral view in both hemispheres. The vmPFC, defined by FreeSurfer, is the sum of the right and left medial orbitofrontal and lateral orbitofrontal cortexes.
To confirm any of the main results from the present ROI approach study, we performed an optimized masked (ie, orbitofrontal cortex, amygdala, hippocampus, and the ACC) voxel-based morphometry (VBM) protocol using FSL-VBM tools (Smith et al, 2004; Douaud et al, 2009) www.fmrib.ox.ac.uk/fsl; Version 1.1) to assess significant differences between groups. For these between-group comparisons, a left-right symmetric study-specific gray matter template was built using the scans from the group with fewer subjects and an equal number of subjects from the larger group; 31 maltreated youth with PTSD and 31 randomly selected maltreated youth without PTSD contributed to gray matter-segmented native images. Equal numbers of images were selected from each of the groups to minimize the chance of a particular group introducing a bias into the template. These 62 gray matter images underwent non-linear registration with FNIRT to the ICBM-152 gray matter template (http://www.fmrib.ox.ac.uk/fsl/fnirt) and then flipped along the x-axis and averaged to create a contrast-specific template for comparing the maltreated group with the PTSD group. Subsequently, the complete set of 63 gray matter images were non-linearly registered to this contrast-specific template. Likewise, the comparison of the maltreated group with the control group and the comparison of the PTSD group with the control group followed the same approach for creating contrast-specific templates. The optimized protocol introduces a compensation (modulation) for the non-linear contraction/enlargement component of the transformation. For the modulation, each voxel of each registered gray matter image was divided by the Jacobian of the warp field. Finally, all 120 modulated registered gray matter volume images were smoothed with an isotropic Gaussian kernel with a sigma of 3 mm (~7-mm FWHM). To achieve accurate inference between-group comparisons, we used permutation-based nonparametric inference within the framework of the general linear model based on a study sample-specific distribution obtained from 5000 permutations of group assignment (Nichols and Holmes, 2002). Our statistical design involved the following contrasts: control>maltreated, maltreated>control, control>PTSD, PTSD>control, maltreated>PTSD, and PTSD>maltreated. The masked images were analyzed with nonparametric permutation-based inferential statistics that included voxelwise regressors for age and sex as adjustment for ICV and are part of the VBM analyses. Results in gray matter were calculated for significant voxels at p<0.05 (corrected) based on the threshold-free cluster enhancement method (Smith and Nichols, 2009).
Statistical Analysis
Outcome measures were the a priori four extinction structures segmented by FreeSurfer. We used general linear modeling to examine group effects on amygdala, hippocampus, ACC, and vmPFC volumes. The general linear modeling included the following covariates that are known to be associated with brain structural volumes in this age range: intracranial volume (Carrion et al, 2001; De Bellis et al, 1999; De Bellis and Kuchibhatla, 2006; Giedd and Rapoport, 2010; Satterthwaite et al, 2014); age (Giedd and Rapoport, 2010); gender (Giedd and Rapoport, 2010; Satterthwaite et al, 2014); SES (De Bellis et al, 1999); IQ (Andreasen et al, 1993; Reiss et al, 1996; Lange et al, 2010); and their interactions with group. If covariates demonstrated p⩾0.2, they were dropped from the model. We examined the relationship between ROIs and PTSD symptoms, trauma load (defined as the number of maltreatment types experienced), and comorbidity (defined as the current number of Axis I disorders) on ROI. Because clinical data were not normally distributed, we used Spearman's rho correlations. Alpha was 0.05 (two-tailed) given our a priori hypotheses of volume differences in these ROI. Analyses were undertaken using JMP Pro 11 software (SAS).
Results
Volumetry Results
Group means, standard deviations, and the general linear modeling results are summarized in Table 3. Pairwise planned comparisons between groups revealed that the PTSD group had smaller right vmPFC volumes than the maltreated youth without PTSD and non-maltreated control group. There was a trend for smaller total vmPFC volumes in the PTSD group. Right vmPFC volumes remained smaller when controlling for trauma load (p=0.02) and comorbidity (p<0.005). We saw no differences between groups in the ACC or the subregions that made up the ROI. Post hoc pairwise group differences revealed that the maltreated youth without PTSD had greater left amygdala and right hippocampal volumes than non-maltreated control and PTSD groups. Post hoc pairwise group differences revealed that maltreated youth without PTSD showed greater total amygdala and hippocampal volumes compared with PTSD youth and a trend for larger volumes than controls in the amygdala (p<0.08) and hippocampus (p<0.09). Greater left amygdala and right hippocampal volumes remained significant when controlling for trauma load (p=0.02; p<0.03) and comorbidity (p=0.01; p<0.05), respectively.
Table 3. Brain Volumes in Healthy Non-Maltreated Youth, Maltreated Youth, and Maltreated Youth with PTSD.
|
Healthy controls (Group 1)
N=57 |
Maltreated (Group 2)
N=32 |
Maltreated with PTSD (Group 3)
N=31 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Structure mm3(mean±SD adjusted for ICV) | Mean | SD | Mean | SD | Mean | SD | Statistic | p | Pairwise group difference |
| Amygdala | |||||||||
| Total | 3055.66 | 286.08 | 3182.13 | 328.05 | 3017.43 | 365.91 | F(2,109)=4.30 | <0.02 | 2>3a |
| Left | 1490.69 | 156.51 | 1569.85 | 193.55 | 1438.46 | 208.78 | F(2,109)=7.39 | 0.001 | 2>1,3a |
| Right | 1564.96 | 163.62 | 1612.28 | 160.63 | 1578.97 | 184.48 | F(2,111)=0.81 | 0.45 | |
| Hippocampus | |||||||||
| Total | 7758.10 | 672.51 | 7916.36 | 673.25 | 7504.48 | 801.39 | F(2,105)=4.16 | <0.02 | 2>3a |
| Left | 3823.55 | 367.61 | 3881.97 | 361.17 | 3698.24 | 410.13 | F(2,105)=3.28 | 0.04 | 2>3a |
| Right | 3934.55 | 337.14 | 4034.39 | 345.40 | 3806.24 | 428.40 | F(2,102)=4.43 | <0.02 | 2>1,3a |
| Anterior cingulate cortex | |||||||||
| Total | 22853.4 | 2458.57 | 22847.0 | 2343.4 | 22395.4 | 2075.75 | F(2,115)=0.84 | 0.43 | |
| Left | 11851.5 | 1441.97 | 11901.8 | 1479.06 | 11616.0 | 1158.51 | F(2,115)=0.66 | 0.52 | |
| Right | 11743.5 | 1290.59 | 11744.1 | 1162.74 | 11579.8 | 1100.01 | F(2,115)=0.46 | 0.63 | |
| vmPFC | |||||||||
| Total | 28367.8 | 2149.24 | 28584.2 | 2304.44 | 27504.3 | 2530.00 | F(2,115)=2.86 | 0.06 | |
| Left | 14212.7 | 1055.76 | 14224.0 | 1255.41 | 13867.9 | 1614.37 | F(2,115)=1.41 | 0.25 | |
| Right | 14155.1 | 1270.88 | 14360.2 | 1357.75 | 13636.3 | 1071.40 | F(2,115)=3.62 | <0.03 | 1,2>3a |
Abbreviations: ICV, intracranial volume; vmPFC, ventral medial prefrontal cortex.
Least Means Differences Dunnett Test, Q=2.24, p⩽0.05.
In maltreated youth, PTSD symptoms significantly and negatively correlated with right (Spearman's rho=−0.37, p<0.008), left (Spearman's rho=−0.32, p<0.03), total hippocampal (Spearman's rho=−0.36, p<0.02), and left amygdala volumes (Spearman's rho=−0.32, p<0.03). No other significant relationships were seen between PTSD variables, internalizing or externalizing symptoms, and trauma load, and ROIs. Please see Supplementary Tables 1 and 2 for Spearman's rho correlations of variables for the non-maltreated and maltreated groups, respectively. Intracranial volume was significantly correlated with all ROIs (all p-values<0.01) in the non-maltreated and maltreated groups, respectively. Controls showed significantly greater correlations than maltreated youth between ICV and (i) total and left amygdala volumes; (ii) age; (iii) gender; and (iv) SES. Controls showed significantly greater correlations than maltreated youth between SES and (i) left ACC; and (ii) total, left, and right vmPFC. See Supplementary Table 3.
Confirmatory Analyses with VBM
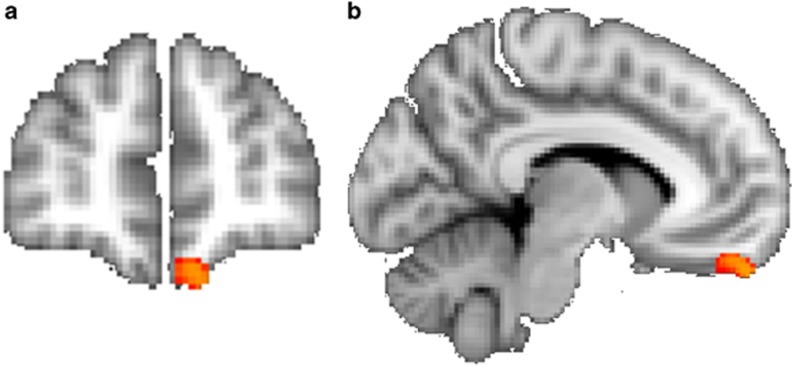
The masked VBM analysis only showed greater gray matter intensity in controls than PTSD (p<0.05; corrected) in the left medial orbitofrontal cortex (x=−10, y=52, z=−26; t=4.33; 114 voxels) (Figure 2). We also performed a correlation between mean gray matter intensity of the orbitofrontal cluster obtained from the VBM and the number of PTSD symptoms and maltreatment types. The correlations were non-significant. We performed an additional exploratory whole-brain VBM analyses to examine whether there were any other brain regions which significantly differed between groups. This analysis showed no significant group differences at the corrected threshold.
Figure 2.
(a and b) The confirmatory masked VBM analysis showing greater gray matter intensity in controls than maltreated youth with PTSD (p<0.05; corrected) in the left medial orbitofrontal cortex (MNI: x=−10, y=52, z=−26; t=4.33; 114 voxels). The mask, obtained from the FreeSurfer, included voxels within the orbitofrontal cortex, amygdala, hippocampus, and the anterior cingulate cortex. The masked images were analyzed with nonparametric permutation-based inferential statistics that included voxelwise regressors for age and sex.
Discussion
To the best of our knowledge, this is the first study to examine brain differences in extinction-related structures in maltreated youth with and without chronic PTSD. Maltreated youth with PTSD were neurobiologically different from maltreated youth without PTSD and non-maltreated controls by demonstrating smaller right vmPFC volumes, a complex brain structure with many functions including emotional regulation, decision-making, assignment of value to context, and the mediation of extinction learning and retention (Schoenbaum and Shaham, 2008). VBM analyses also confirmed less gray matter in the left orbital frontal cortex in the PTSD group compared with controls. This finding extends and agrees with a study of adults who have child maltreatment-related PTSD (Thomaes et al, 2010) and a youth study of PTSD that was secondary to heterogeneous trauma types compared with healthy youth (Keding and Herringa, 2014) both of which also demonstrated smaller vmPFC volumes. Trauma studies in adults showed smaller ACC (Cohen et al, 2006; Kitayama et al, 2006; Thomaes et al, 2010) and hippocampal (Dannlowski et al, 2012; Teicher et al, 2012) volumes, and no differences (Woon and Hedges, 2009), smaller (Morey et al, 2012) and larger amygdala volumes (Kuo et al, 2012). By contrast, maltreated youth without PTSD demonstrated larger left amygdala and right hippocampal volumes compared with maltreated youth with PTSD and non-maltreated controls. PTSD symptoms inversely correlated with hippocampal and left amygdala volumes. As in our study, one of the adult cross-sectional studies also found significant correlational interactions between smaller amygdala volumes and trauma before age 13, and higher degree of combat exposure (Kuo et al, 2012). The results of this study also agreed with an investigation finding larger amygdala volumes in adults without PTSD who experienced adversity at ages 10–11 years (Pechtel et al, 2014). Taken together, the results from our cross-sectional study suggest dynamic changes in the amygdala associated with sensitive developmental periods, higher degrees of trauma, and PTSD symptoms that need to be confirmed in longitudinal investigations. No differences were seen in ACC volumes between maltreated youth and controls. Although maltreated youth with PTSD had greater numbers of Axis I disorders and trauma load, both maltreatment groups suffered from significant traumas. The group differences remained significant when controlling for trauma load and comorbidity.
Individuals who suffer from PTSD exhibit anxiety-related behaviors (ie, conditioned responses) to traumatic reminders based on previous trauma. The vmPFC tracks predictions of stimuli associated with safety and danger (Schiller et al, 2008). This tracking is adaptive to allow the individual to easily shift fear responses from one stimulus to another based on the environment. Thus, dysregulation of the vmPFC can lead to PTSD resulting from failing to learn extinction of traumatic reminders. In healthy adults, investigators found positive correlations between vmPFC cortical thickness and successful extinction retention (Hartley et al, 2011). Thus, the structural compromise in vmPFC in pediatric maltreatment-related PTSD may represent neurobiological factors that predate the trauma, or alternatively a PTSD-related process secondary to maltreatment exposure. Although our cross-sectional study cannot determine this, a twin study of combat-related PTSD suggests that the deficit of extinction recall is acquired as a result of trauma leading to PTSD, because extinction retention (assessed by skin conductance response) was greater in the twins with PTSD compared with their co-twins and non-PTSD combat veterans (Milad et al, 2008). Additionally, another investigation demonstrated that fear extinction is impaired in adult PTSD (Milad et al, 2009). It is possible that the effects of maltreatment and having PTSD on the vmPFC, a structure rich in glucocorticoid receptors (Lupien et al, 2009), leads to volume differences over time along with decreased ability to learn extinction of traumatic reminders, and inability to respond appropriately to the current environment.
The vmPFC is a complex structure with complex function. Deficits in this structure may be a developmentally shared mechanism that leads to disruptions in emotional regulation, decision-making, cognitive functions, fear learning, extinction retention, and fear generalization, and may contribute to the high prevalence of comorbid disorders common to youth PTSD. In our sample, the PTSD group suffered from numerous Axis I comorbidities, including depressive and behavioral disorders that put the PTSD group at a higher risk for adolescent onset SUD than the maltreated youth without PTSD. Depression in adulthood, which has significant symptom overlap and comorbidity with PTSD throughout the lifespan, is associated with smaller vmPFC volumes (Wagner et al, 2008). SUD are neurobiological disorders of impulsiveness or failure to postpone short-term desires, with serious long-term costs. Although our subjects did not have a history of SUD, smaller right vmPFC volumes in youth are associated with impulsivity (Boes et al, 2009), a common symptom of PTSD and SUD. Adults with SUD have smaller vmPFC volumes (Tanabe et al, 2009). Hypoactivation of the vmPFC during response inhibition predicts early initiation of substance use (Cheetham et al, 2012). Thus, studies of childhood maltreatment should consider focusing on altered vmPFC structure and function as an early neurodevelopmental cortical pathway to PTSD that can lead to adolescent and young adult depression and SUD. The vmPFC is a relatively large and complex structure and one study showed a smaller vmPFC volume cluster in healthy adults with maltreatment histories (Dannlowski et al, 2012), suggesting that future studies of maltreatment-related PTSD and its resilience should focus on specific vmPFC subregion connectivity to limbic areas. In fact, a recent meta-analysis demonstrated that maltreated individuals with a variety of disorders and studied across the lifespan exhibited significantly smaller gray matter volumes in the right orbitofrontal/superior temporal gyrus extending to the amygdala, insula, and parahippocampal and middle temporal gyri, and in the left inferior frontal and postcentral gyri; which suggests deficits in structures that mediate emotional regulation, cognitive control, social cognition, and extinction processes (Lim et al, 2014). Our study did not find widespread voxel-based differences perhaps owing to the younger age of sample, as the later study (Lim et al, 2014) consisted mainly of adults with maltreatment histories.
Our data are consistent with previous studies showing no amygdala and hippocampus volume differences in maltreated youth with PTSD compared with non-maltreated controls (Carrion et al, 2001; De Bellis et al, 2002). Instead, we found that maltreated youth without PTSD demonstrated larger left amygdala and right hippocampal volumes compared with maltreated youth with PTSD and non-maltreated controls. In rodents, chronic stress leads to increased dendrites and apical spines in the basolateral nucleus of the amygdala, whose function is to activate the central nucleus of the amygdala, the subregion responsible for the fear response. This inverted U-shaped model predicts that exposure to stress might be adaptive through acute adrenergic and cortisol effects to promote vigilance and learning processes (Lupien et al, 2009). This modulation of noradrenergic function by cortisol may explain the enhanced memory for emotional events experienced under stress (Lupien et al, 2009; Roozendaal et al, 2009). Peer-reared juvenile monkeys showed larger dorsomedial prefrontal and dorsal ACC compared with mother-reared monkeys, indicating that stress-sensitive brain structures can increase during atypical primate development (Spinelli et al, 2009). Maltreatment stress might lead to increased hippocampal and amygdala volume until a critical threshold of exposure to maltreatment and/or persistent PTSD symptoms are reached, which is subsequently followed by atrophy of the hippocampus and amygdala. Such an explanation would be consistent with our results of inverse PTSD symptom correlations with hippocampal and left amygdala volumes. A longitudinal study demonstrated that maltreated adolescents had larger left hippocampal volumes at baseline; but, if the youth had psychopathology, the hippocampus grew more slowly over an approximately 4-year follow-up period (Whittle et al, 2013). Another study suggested that hippocampal atrophy in youth with impairing PTSD symptoms may be a latent developmental effect (Carrion et al, 2007). The one study showing greater right hippocampal volume in youth with maltreatment-related PTSD compared with controls (Tupler and De Bellis, 2005) enrolled youth who were in active PTSD treatment, leading to speculation that neurobiological markers may show neuroplasticity that resembles the maltreated youth without PTSD group when patients are given treatment. It is possible that neurobiological markers change with maturation and treatment, prior to alleviation of the PTSD symptoms, and this begs the question of including such markers as outcomes in treatment studies of pediatric PTSD. Consequently, these markers may have clinical significance as the current evidence-based treatment, trauma focused cognitive behavioral therapy, shows small-to-medium effects sizes (MacMillan et al, 2009) indicating that this treatment is not successful for all youth with PTSD. However, future longitudinal studies must determine whether maltreatment stress might lead to increased hippocampal and amygdala volume until a critical threshold of exposure to maltreatment and/or persistent PTSD symptoms are reached, which is subsequently followed by atrophy of the hippocampus and amygdala.
There are a number of limitations of this study. The maltreated youth differed from the control group in IQ, which may contribute to results independently from maltreatment. However, the two maltreatment groups showed very similar means and distribution of IQ scores. This limitation is inherent in child maltreatment studies where both cross-sectional (De Bellis et al, 2013) and longitudinal studies demonstrate lower IQ in victims of maltreatment (Perez and Widom, 1994). Because higher IQ participants demonstrate a linear relationship with neural efficiency compared with lower IQ participants (Neubauer and Fink, 2009) and cortical thickness in this study's age range and IQ range (average to high average) show linear associations (Shaw et al, 2006), we believe IQ group differences were appropriately addressed using general linear modeling statistical methods; however, the lower IQ in the maltreated groups compared with the non-maltreated group remains a limitation when interpreting maltreatment imaging studies in youth. Additionally, although having a lower IQ is considered a risk factor for combat PTSD (Macklin et al, 1998; Gilbertson et al, 2006), lower IQ was not associated with PTSD in the maltreated youth studied here. We were not able to examine age of maltreatment onset or duration because maltreated youth had multiple episodes and types of maltreatment experiences. Thus, determining the age of maltreatment was not a simple construct and was not feasible in our study. We note that the masked VBM analyses only confirmed vmPFC differences between controls and maltreated youth with PTSD. Although we attempted to control for developmental effects by using group-specific templates, it is possible these types of analyses are too conservative for pediatric studies where individuals show high variance in brain maturation measures (Ridgway et al, 2008). Further, this study used a cross-sectional design, which limits inferences about the causal relationships between maltreatment, PTSD status, and brain structures.
We studied the extremes of psychopathology (PTSD) and the volumes of brain structures associated with extinction in a group of youth with an extraordinary level of trauma exposure. Both maltreatment groups suffered a mean of at least four maltreatment types, putting both groups at extremely high risk for adolescent and adult psychopathology and health risk behaviors associated with the leading causes of death in adulthood (Felitti et al, 1998). We recruited a reasonably large sample of maltreated youth who were medically healthy and non-medicated. This was challenging given that prenatal substance exposure, low SES, alcohol and substance dependence, use of psychotropic medications, and medical illnesses are overrepresented in maltreated youth (Smith et al, 2007) and can cause study confounds by negatively influencing brain maturation. Within the constraints of our stringent inclusion/exclusion criteria, we enrolled a sufficient sample size for this MRI study to have confidence in our findings.
This study demonstrated volumetric differences in brain structures associated with extinction between maltreated youth with chronic and persistent PTSD and those without PTSD, using automated ROI methods. This advances the field by providing evidence that cortical differences are seen in childhood PTSD, early in the development of this illness. These brain structures are also associated with the successful attainment of age-appropriate emotional regulation and decision-making. Longitudinal research is needed to determine whether the neurobiology associated with PTSD is a shared mechanism for disorders that reflect impaired emotional regulation and decision-making such as depression and SUD in adolescents and adulthood, and if the neurobiology of resilience to PTSD following severe maltreatment is a marker for healthier adulthood adaptations to childhood trauma.
Funding and Disclosure
The authors declare no conflict of interest.
Acknowledgments
We would like to thank the staff of the Healthy Childhood Brain Development Research Program, and the individuals who participated in this study. We acknowledge the following support for this research: Supported in parts by K24MH71434 & K24 DA028773 (MDDB), R01 MH63407 (MDDB), R01 AA12479 (MDDB), R01 MH61744 (MDDB) and VHA VISN 6 MIRECC (RAM), VHA CSR&D 5I01CX000120-03 (RAM), VHA CSR&D 5I01CX000748-03(RAM), and NINDS 5R01NS086885-02 (RAM).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Andreasen NC, Flaum M, Swayze V, O'Leary DS, Alliger R, Cohen G et al (1993). Intelligence and brain structure in normal individuals. Am J Psychiatry 150: 130–134. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P (2009). Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD et al (2001). Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 50: 943–951. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL (2007). Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics 119: 509–516. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL (2009). Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res 172: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI (2012). Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry 71: 684–692. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D et al (2006). Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 59: 975–982. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al (2012). Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71: 286-293. [DOI] [PubMed] [Google Scholar]
- De Bellis M, Kuchibhatla M (2006). Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 60: 697–703. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G (2001). A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 50: 305–309. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper S, Spratt EG, Woolley DW (2009). Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc 15: 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR, Chen SD, Provenzale JM, Boyd BD, Glessner CE et al (2015). Posterior structural brain volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Development and Psychopathology (in press). [DOI] [PMC free article] [PubMed]
- De Bellis MD, Keshavan M, Clark DB, Casey BJ, Giedd J, Boring AM et al (1999). A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry 45: 1271–1284. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan M, Shifflett H, Iyengar S, Beers SR, Hall J et al (2002). Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 52: 1066–1078. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Woolley DP, Hooper SR (2013). Neuropsychological findings in pediatric maltreatment: relationship of PTSD, dissociative symptoms, and abuse/neglect indices to neurocognitive outcomes. Child Maltreat 18: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A (2014). . The biological effects of childhood trauma. In: Cozza SJ, Cohen JA, Dougherty JG (eds). Child and Adolescent Psychiatric Clinics of North America: Disaster and Trauma, Vol. 23. Elsevier, pp 185–222. [DOI] [PMC free article] [PubMed]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Mackay C, Andersson J, James S, Quested D, Ray MK et al (2009). Schizophrenia delays and alters maturation of the brain in adolescence. Brain 132: 2437–2448. [DOI] [PubMed] [Google Scholar]
- Famularo R, Fenton T, Augustyn M, Zuckerman B (1996). Persistence of pediatric post traumatic stress disorder after 2 years. Child Abuse Negl 20: 1245–1248. [DOI] [PubMed] [Google Scholar]
- Famularo R, Fenton T, Kinscherff R (1993). Child maltreatment and the development of post traumatic stress disorder. Am J Dis Child 147: 755–760. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V et al (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes if death in adults. Am J Prev Med 14: 245–258. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH et al (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex 14: 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Brown TT, Kuperman JM, Chung Y, Hagler DJ Jr et al (2012). Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci USA 109: 19620–19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL (2010). Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Paulus MW, Williston SK, Gurvits TV, Lasko NB, Pitman RK et al (2006). Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol 115: 484–495. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ et al (2010). Early stress is associated with alterations in the orbitofrontal cortex: a tensor based morphometry investigation of brain structure and behavioral risk. J Neurosci 30: 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA (2011). Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cereb Cortex 21: 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Jones B, Stieglitz E, Vitulano L, Mannarino A (1994). The use of multiple informants to assess children's maltreatment experiences. J Fam Violence 9: 227–248. [Google Scholar]
- Keding TJ, Herringa RJ (2014). Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40: 537–545 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama N, Quinn S, Bremner JD (2006). Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord 90: 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JR, Kaloupek DG, Woodward SH (2012). Amygdala volume in combat-exposed veterans with and without posttraumatic stress disorder: a cross-sectional study. Arch Gen Psychiatry 69: 1080–1086. [DOI] [PubMed] [Google Scholar]
- Lange N, Froimowitz MP, Bigler ED, Lainhart JE, and Brain Development Cooperative Group (2010). Associations between IQ, total and regional brain volumes, and demography in a large normative sample of healthy children and adolescents. Dev Neuropsychol 35: 296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Radua J, Rubia K (2014). Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry 171: 854–863. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10: 434–445. [DOI] [PubMed] [Google Scholar]
- Macklin ML, Metzger LJ, Litz BT, McNally RJ, Lasko NB, Orr SP et al (1998). Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 66: 323–326. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Wathen CN, Barlow J, Fergusson DM, Leventhal JM, Taussig HN (2009). Interventions to prevent child maltreatment and associated impairment. Lancet 373: 250–266. [DOI] [PubMed] [Google Scholar]
- McLeer SV, Dixon JF, Henry D, Ruggiero K, Escovitz K, Niedda T et al (1998). Psychopathology in non-clinically referred sexually abused children. J Am Acad Child Adoles Psychiatry 37: 1326–1333. [DOI] [PubMed] [Google Scholar]
- McLeer SV, Ruggiero K (1999). Diagnostic stability following termination of child sexual abuse. Scientific Proceedings of the Annual Meeting of the American Academy of Child & Adolescent Psychiatry XV: 105. [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK (2008). Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 62: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC et al Mid-Atlantic MIRECC Workgroup. (2012). Amygdala volume changes with posttraumatic stress disorder in a large case-controlled veteran group. Arch Gen Psychiatry 69: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV et al (2009). A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 45: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AC, Fink A (2009). Intelligence and neural efficiency. Neurosci Biobehav Rev 33: 1004–1023. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB (2009). Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 29: 11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MT (2014). Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage 97: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez C, Widom CS (1994). Childhood victimization and long-term intellectual and academic outcomes. Child Abuse Negl 18: 617–633. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW et al (2012). Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MA (1996). Brain development, gender and IQ in children: a volumetric imaging study. Brain 119: 1763–1774. [DOI] [PubMed] [Google Scholar]
- Richert KA, Carrion VG, Karchemskiy A, Reiss AL (2006). Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depress Anxiety 23: 17–25. [DOI] [PubMed] [Google Scholar]
- Ridgway GR, Henley SMD, Rohrer JD, Scahill RI, Warren JD, Fox NC (2008). Ten simple rules for reporting voxel-based morphometry studies. NeuroImage 40: 1429–1435. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S (2009). Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C et al (2014). Sex differences in the effect of puberty on hippocampal morphology. J Am Acad Child Adolesc Psychiatry 53: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA (2008). From fear to safety and back: reversal of fear in the human brain. J Neurosci 28: 11517–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y (2008). The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry 63: 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H et al (1983). A children's global assessment scale. Arch Gen Psychiatry 40: 1228–1231. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N et al (2006). Intellectual ability and cortical development in children and adolescents. Nat Lett 440: 676–679. [DOI] [PubMed] [Google Scholar]
- Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS (2007). Child maltreatment and foster care: unpacking the effects of prenatal and postnatal parental substance use. Child Maltreat 12: 150. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44: 83–98. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E (2009). Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry 66: 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T et al (2009). Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 65: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109: E563–E572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH et al (2010). Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 71: 1636–1644. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD (2005). Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry 59: 523–529. [DOI] [PubMed] [Google Scholar]
- van Harmelen A-L, van Tol M-J, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P et al (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry 68: 832–838. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlösser RGM (2008). Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci 33: 199–208. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1991) Wechsler Intelligence Scale for Children. 3rd edn. The Psychological Corporation: San Antonio. [Google Scholar]
- Whittle S, Dennison M, Vijayakumar N, Simmons JG, Yucel M, Lubman DI et al (2013). Childhood maltreatment and psychopathology affect brain development during adolescence. J Am Acad Child Adolesc Psychiatry 52: 940–952. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW (2009). Amygdala volume in adults with posttraumatic stress disorder: a meta-analysis. J Neuropsychiatry Clin Neurosci 21: 5–12. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux JE (2007). Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56: 19–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.