Abstract
Caspases are implicated in neuronal death in neurodegenerative and other Central Nervous System (CNS) diseases. In a rat model of HIV-1 associated neurocognitive disorders (HAND), we previously characterized HIV-1 envelope gp120-induced neuronal apoptosis by TUNEL assay. In this model, neuronal apoptosis occurred probably via gp120-induced reactive oxygen species (ROS). Antioxidant gene delivery blunted gp120-related apoptosis. Here, we studied the effect of gp120 on different caspases (3, 6, 8, 9) expression. Caspases production increased in the rat caudate-putamen (CP) 6h after gp120 injection into the same structure. The expression of caspases peaked by 24h. Caspases colocalized mainly with neurons. Prior gene delivery of the antioxidant enzymes Cu/Zn superoxide dismutase (SOD1) or glutathione peroxidase (GPx1) into the CP before injecting gp120 there reduced levels of gp120-induced caspases, recapitulating the effect of antioxidant enzymes on gp120-induced apoptosis observed by TUNEL. Thus, HIV-1 gp120 increased caspases expression in the CP. Prior antioxidant enzyme treatment mitigated production of these caspases, probably by reducing ROS levels.
Keywords: HIV-1, gp120, caspases, apoptosis, antioxidant enzymes, gene therapy
1. Introduction
The caspases family of proteases is conserved from nematodes through mammals. They are central to apoptotic death and are expressed as inactive zymogens that become cleaved during apoptosis (Ribe et al., 2008). Initiator caspases autoactivate and self-process upon recruitment to adaptor proteins. Then, they proceed to cleave and thereby activate the executioner/effector caspases. Activated executioner/effector caspases proceed to process key structural and nuclear proteins and thereby cause the disassembly and death of the cell (Madden and Cotter, 2008). Two major caspases pathways have been described: the intrinsic pathway is initiated by cytochrome c release from the mitochondrion while the extrinsic pathway is initiated by the binding of ligands to plasma-membrane death receptors (Sims and Muyderman, 2010).
Intrinsic apoptosis pathway is required for fetal and postnatal brain development, but is downregulated through the suppression of the expression of one of its key mediator, caspase-3 (Madden and Cotter, 2008). During stroke and neurodegenerative diseases, some caspases are upregulated in the brain (Ribe et al., 2008). Cerebral ischemia triggers both the intrinsic and extrinsic pathways of apoptosis (Broughton et al., 2009; Sims and Muyderman, 2010). Mounting evidence suggests the involvement of caspases in the disease process associated with neurodegenerative diseases such as Alzheimer's disease (AD) (Rohn, 2010) and amyotrophic lateral sclerosis (ALS) (Madden and Cotter, 2008). Caspase activation has also been documented in the brains of patients with HIV-1 associated dementia (Petito and Roberts, 1995; Kaul et al., 2001).
Under physiologic conditions, reactive oxygen species (ROS), which include superoxide (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH-), are generated at low levels and play important roles in signaling and metabolic pathways (Broughton et al., 2009). ROS levels are controlled by endogenous antioxidant such as superoxide dismutases (SOD), glutathione peroxidase (GPx1), glutathione and catalase. Increased levels of ROS are a major cause of tissue injury after cerebral ischemia, in neurodegenerative diseases, as well as in HIV-1 associated neurocognitive disorders (HAND) (Mattson et al., 2005; Antinori et al., 2007; Broughton et al., 2009). Interaction of ROS with other tissue components produces a variety of other radicals: following activation of inducible nitric oxide synthase (iNOS), nitric oxide (NO) can bind superoxide anion to form the highly reactive peroxynitrite (Bonfoco et al., 1995). The latter may attack lipids, proteins and DNA, to enhance oxidant-related injury. Mitochondria are the primary source of ROS involved in many brain tissue injuries (i.e., hypoxia, excitotoxicity). Once generated, mitochondrial ROS influence the release of cytochrome c and other apoptotic proteins from the mitochondria into the neuronal cytosol, which leads to apoptosis (Broughton et al., 2009). For example, once released into the cytosol, cytochrome c forms a complex referred to as an apoptosome with procaspase-9, apoptotic protease activating factor 1 (APAF-1) and dATP. The formation of the apoptosome activates caspase-9 which then cleaves other procaspases. The activation of caspase-3 by this process, among other effectors, has multiple effects including proteolysis of an inhibitor of the caspase-activated DNase (Sims and Muyderman, 2010). Thus, a link between oxidative stress and activation of some caspases seems highly probable.
We previously reported that injection of HIV-1 envelope gp120 into the caudate-putamen (CP) induces neuronal apoptosis, as well as oxidative stress (Agrawal et al. 2006; Louboutin et al., 2007a). We examined here the expression of different caspases, both initiators and effectors, following intra-CP gp120 injection. Finally, we tested if prior gene delivery of the antioxidant enzymes Cu/Zn superoxide dismutase (SOD1) or glutathione peroxidase (GPx1) into the CP before injecting gp120 reduces caspases expression.
2. Experimental Procedures
2.1 Animals
Female Sprague-Dawley rats (300-350g) were purchased from Charles River Laboratories (Wilmington, MA). Protocols for injecting and euthanizing animals were approved by the Thomas Jefferson University Institutional Animal Care and Use Committee (IACUC), and are consistent with Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) standards. Experiments were done in female rats at similar points of their estrous cycle determined by vaginal smears. Animals were preferably injected during the diestrus stage of the estrous cycle. Oestrogens are typically low during this stage. In any case, animals were not injected during the estrus stage of the cycle when oestrogens levels are elevated. The diet that the animals received was a standard commercial, regular powdered rodent diet without any component that might cause oxidative stress (e.q., such as high fat diet, or high manganese) and was not folate/methyl or iron deficient. Animals had free access to water and diet. Numbers of animals used in experiments are indicated in the “Experimental Design” section.
2.2 Antibodies
Diverse primary antibodies were used: rabbit anti-caspase-3 (IgG; 1: 100), goat anti-caspase-6 (IgG; 1: 100), rabbit anti-caspase-8 (IgG; 1: 100), mouse anti-caspase-9 (IgG2a; 1: 100) (Santa Cruz, Santa Cruz, CA), rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1) (IgG; 1:100), a marker of quiescent and active microglia (Waco Chemicals, Osaka, Japan), mouse anti-glial fibrillary acidic protein (GFAP) (IgG2b; 1: 100) (BD Pharmingen Franklin Lakes, NJ), mouse anti-NeuN (IgG1; 1: 100) (Chemicon International, Temecula, CA). Secondary antibodies were used at 1:100 dilution: Fluorescein IsoThioCyanate (FITC) and Tetramethyl Rhodamine IsoThioCyanate (TRITC)-conjugated goat anti-mouse IgG ( -chain specific and against whole molecule respectively), TRITC-conjugated goat anti-rabbit IgG (whole molecule), FITC-conjugated sheep anti-rabbit IgG (whole molecule), FITC-conjugated rabbit anti-goat IgG (whole molecule), Cy3-conjugated rabbit anti-goat IgG (whole molecule) (Sigma, Saint-Louis, MO), FITC and TRITC-conjugated donkey anti-mouse IgG (whole molecule), Cy3-conjugated donkey anti-rabbit IgG (whole molecule) and anti-goat IgG (whole molecule) (Jackson ImmunoResearch Laboratories, Inc, WestGrove, PA).
2.3 Vector production
The general principles for making recombinant, Tag-deleted, replication-defective SV40 viral vectors have been previously reported (Strayer, 1999; McKee and Strayer, 2002). Cu/Zn superoxide dismutase (SOD1) or glutathione peroxidase (GPx1) transgenes were subcloned into pT7[RSVLTR], in which transgene expression is driven by the Rous Sarcoma Virus long terminal repeat (RSV-LTR). The cloned rSV40 genome was excised from its carrier plasmid, gel-purified and recircularized, then transfected into COS-7 cells. These cells supply large T-antigen (Tag) and SV40 capsid proteins in trans, which are needed to produce recombinant replication-defective SV40 viral vectors (Strayer et al., 1997). Crude virus stocks were prepared as cell lysates, then band-purified by discontinuous sucrose density gradient ultracentrifugation and titered by quantitative (Q)-PCR (Strayer et al., 2001). SV(human bilirubin-uridine 5′-diphosphate-glucuronosyl-transferase) (BUGT), which was used here as negative control vector, with a non-toxic byproduct, has been reported (Sauter et al., 2000).
2.4 Experimental design
2.4.1 Gp120 injection
In order to study gp120-induced abnormalities, 1 l saline containing 100 ng, 250 ng, or 500 ng gp120 was injected stereotaxically into the caudate-putamen (CP) of rats whose brains were harvested at 6, 24 and 48 hours after the injection with 5 rats at each time point; total: n = 45 rats). Controls (n = 4 at each time point; total: 12) received saline instead of gp120 in the CP. In order to test the specificity of the effects of gp120, 1 l saline containing 500 ng rat IgG (Sigma) was injected into the CP as a control unrelated protein (n = 4 at each time point; total: 12). The contralateral side of the unilaterally injected brains was also used as control. Recombinant HIV-1 BaL gp120 was obtained through the NIH AIDS Research & Reference Reagent Program, Division of AIDS, NIAID, NIH, Germantown, MD.
2.4.2 Challenge with gp120 after administration of SV(GPx1)/SV(SOD1
To study possible protection by rSV40-mediated overexpression of SOD1 and GPx1 from gp120-related injury, we first injected the CP of rats with SV(SOD1) (n = 5) and SV(GPx1) (n = 5). One month later, the CP in which SV(SOD1) or SV(GPx1) has been administered was injected with 500 ng gp120. Brains were harvested one day after injection of gp120 into the CP. They were studied for caspases immunoreactivity and TUNEL. In all cases, controls received SV(BUGT) in the CP instead of SV(SOD1) and SV(GPx1) (n = 5).
2.5 In vivo transduction and injection of gp120
Rats were anesthetized with isofluorane UPS (BaxterHealthcare Corp., Deerfield, IL) (1.0 unit isofluorane/1.5 l O2 per min) and placed in a stereotaxic apparatus (Stoelting Corp., Wood Dale, IL) for cranial surgery. Body temperature was maintained at 37°C by using a feedback-controlled heater (Harvard Apparatus, Boston, MA). Glass micropipettes (1.2 mm outer diameter; World Precisions Instruments, Inc., Sarasota, FL) with tip diameters of 15 m were backfilled with either 5 l of SV(BUGT), SV(SOD1) or SV(GPx1) viral vector, which contains approximately 107 particles. The vector-filled micropipettes were placed in the CP using coordinates obtained from the rat brain atlas of Paxinos and Watson (1986). For injection into the CP, a burr hole was placed +0.48 mm anterior to bregma and −3.0 mm lateral to the sagittal suture. Once centered, the micropipette was placed 6.0 mm ventral from the top of the brain. Same coordinates were used for injecting 500 ng gp120 in 1 l saline, as well as for injecting saline and 1 l saline containing 500 ng rat IgG. Gp120 or the vector were given by a Picospritzer II (General Valve Corp., Fairfield, NJ) pulse of compressed N2 duration 10 ms at 20 psi until the fluid was completely ejected from the pipette. Following surgery, animals were housed individually with free access to water and food.
2.6 Procedure for harvesting the tissue
After a variable survival period, rats were anesthetized by intraperitoneal injection of sodium pentobarbital (Abbott Laboratories, North Chicago, IL) at 60 mg/kg and perfused transcardially though the ascending aorta with 10 ml heparinized saline followed by ice cold 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1M phosphate buffer (pH 7.4). Immediately following perfusion-fixation, the rat brains were removed, placed in 4% paraformaldehyde for 24 h, then in a 30% sucrose solution for 24 h, then frozen in methyl butane cooled in liquid nitrogen. Samples were cut on a cryostat (10 m sections).
2.7 Immunocytochemistry
For immunofluorescence, coronal cryostat sections (10 m thick) were processed for indirect immunofluorescence. Blocking was performed by incubating 60 min with 10% goat, or 10% donkey, serum in phosphate buffer saline (PBS; pH 7.4). Then, sections were incubated with antibodies diluted according to manufacturer's recommendations: 1h with primary antibody, then 1 h with secondary antibody diluted 1: 100, all at room temperature. Double immunofluorescence was performed as previously described (Rouger et al., 2001). All incubations were followed by extensive washing with PBS. To stain nuclei, we used mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). Specimens were finally examined under a Leica DMRBE microscope (Leica Microsystems, Wetzlar, Germany) under 4X, 10X and 20X objectives with appropriate filters. Negative controls performed each time immunostaining was done consisted of preincubation with PBS, substitution of non-immune isotype-matched control antibodies for the primary antibody, and/or omission of the primary antibody. Data presented are representative of at least 3 independent experiments.
2.8 TUNEL staining
Apoptotic cells were assessed by TUNEL assay as previously described (Agrawal et al., 2006).
2.9 Staining of neurons using NeuroTrace
Neurotrace (NT) staining has been used as a neuronal marker in studies focusing on the characterization of neurons (Morinville et al., 2004; Nikonov et al., 2005) and NT staining has been performed as previously reported (Agrawal et al., 2006; Louboutin et al., 2006, 2007a,b). After rehydration in 0.1 M PBS, pH 7.4, sections were treated with PBS plus 0.1 % Triton X-100 10 min, washed twice for 5 min in PBS then stained by NT (Molecular Probes, Inc., Eugene, OR) (1: 100), for 20 min at room temperature. Sections were washed in PBS plus 0.1 % Triton X-100 then × 2 with PBS, then let stand for 2 h at room temperature in PBS before being counterstained with DAPI. Combination NT + antibody staining was performed using primary and secondary antibodies staining first (see above), followed by staining with the NT fluorescent Nissl stain. All experiments were repeated 3 times and test and control slides were stained the same day.
2.10 Morphometry
Caspases- and TUNEL-positive cells were enumerated manually on the injected and uninjected sides in the whole CP (and not randomly chosen field) of animals injected with gp120, or saline or rat IgG in saline, in at least 5 consecutive sections using a computerized imaging system (Image-Pro Plus, MediaCybernetics, Bethesda, MD) as previously described (Louboutin et al., 2007a, 2010a). In all cases, the final number was an average of results measured in the different sections. This procedure already described for assessment of numbers of transgene-positive cells in the brain (Mandel et al., 1998; Louboutin et al., 2007b) allows quantitative and relative comparisons among different time points, although it does not reflect the total number of transduced cells in vivo.
2.11 Statistical analysis
Comparison of medians between 2 groups was achieved by using the Mann-Whitney test (with a two-tail p value). Comparison of medians between more than 2 groups was done by using the Kruskall-Wallis test. The difference between the groups was considered significant when P < 0.05. On graphs, values are represented as means +/−s.e.m.
3. Results
3.1 Direct administration of gp120 into the CP induces expression of caspases
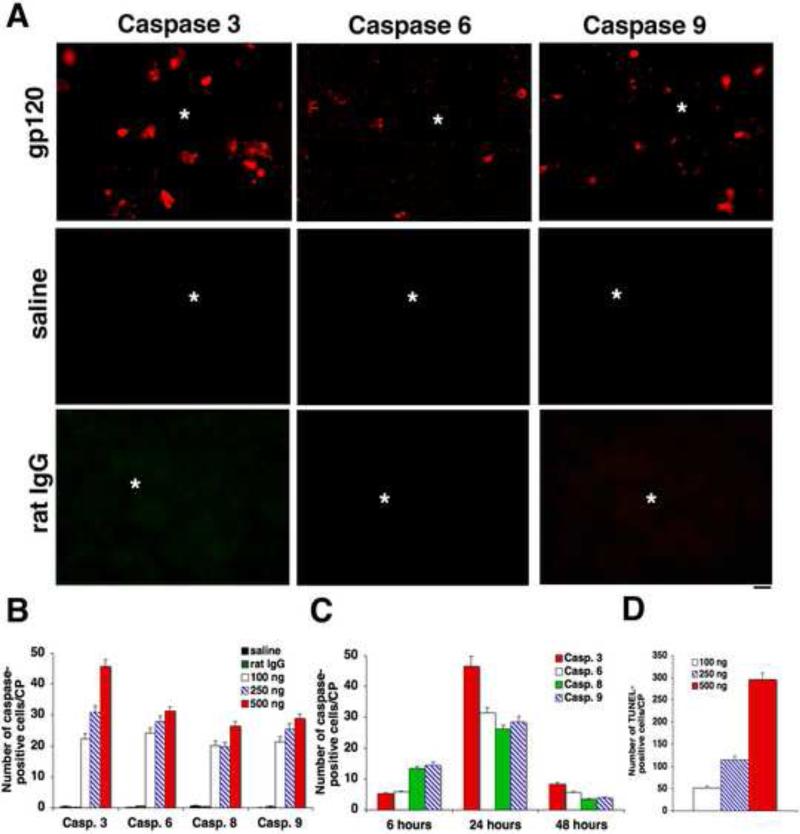
After injection of different concentrations of gp120 (100, 250 and 500 ng l−1) into the CP, immunocytochemistry for caspases was performed from 6 to 48 hours. Caspases-positive cells were counted in the whole CP, in at least 5 different sections for each animal. Caspases-positive cells peaked 1 day after gp120 injection (Fig. 1A). Extremely rare caspases-positive cells were seen when CP was injected with saline or saline containing rat IgG (negative control). We then enumerated the number of caspases-positive cells on the whole CP (not randomly chosen fields) in at least 5 different sections for each sample. Morphometric data for each caspase for each concentration at day 1 and for each caspase at different time points for 500 ng gp120 are shown on Fig. 1B and 1C respectively. Numbers of caspases-positive cells were higher for 500 ng gp120 than for other doses. Caspase-3-positive cells were more numerous than cells positive for other caspases (Fig. 1B). Numbers of caspase-8- and caspase-9-positive cells were higher 6 hours after injection of 500 ng gp120 compared to numbers of caspase-3- and caspase-6-positive cells at the same time (Fig. 1C). Thus, cells positive for markers of initiator caspases were more numerous at 6 hours compared to cells positive for markers of effector caspases. Compared to the results at day 1, less caspases-positive cells were seen at 6 and 48 hours for the different caspases studied as well as for the different concentrations considered (Fig. 1C). As previously reported, TUNEL-positive cells, measured as previously described, peaked 1 day after gp120 injection (Fig. 1D). No TUNEL-positive cells were seen after injection of saline or saline containing rat IgG.
Figure 1.
Intra-CP injection of gp120 induces overexpression of caspases and apoptosis. Immunostaining for different caspases and TUNEL assay were performed from 6 h to 48 h after injection of different concentrations of gp120 into the CP to detect apoptosis. Caspases- and TUNEL-positive cells were counted in the whole CP, at least in 5 different sections for each animal. A. Extremely rare caspases-positive cells were detected when the CP was injected with saline or rat IgG (negative controls). The site of injection is indicated by *. B. The number of caspases-positive cells increased with the doses of gp120. C. Caspases-positive cells were more numerous 24 hours after 500 ng gp120 injection, compared to 6 and 48 hours. D. The number of TUNEL-positive cells peaked 1 day after gp120 injection. Bar: A: 40 m.
3.2 Caspases-positive cells are mainly neurons
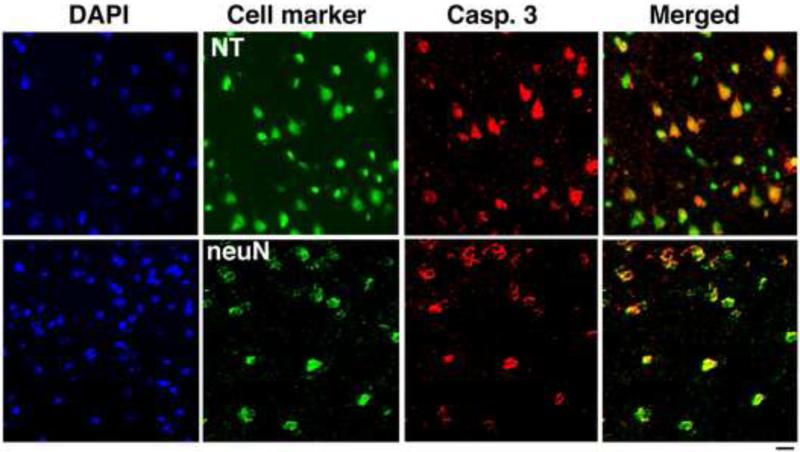
Sections of the CP obtained 1 day after injection of gp120 were double stained for NeuN and Neurotrace (NT), both neuronal markers, and caspase 3. After injection of 500 ng gp120, numerous caspase-3-positive cells were seen, the vast majority of which were also immunopositive for NT and NeuN, identifying them as neurons (Fig. 2). Caspases colocalized mainly with neurons (97, 96, 95 and 97% for caspases 3, 6, 8, and 9 respectively). By contrast, caspase-3-positive cells that were also Iba1-positive, and so were consistent with microglia, were extremely rare (not shown). Astrocytes, which we visualized by immunostaining for GFAP, were negative for caspase-3 (not shown).
Figure 2.
The majority of caspases-positive cells following intra-CP injection of gp120 are neurons. Sections from the CP obtained 1 day after gp120 injection were double stained for caspase-3, NeuN and NT, both markers of neurons, or Iba1, a marker of microglial cells. The majority of caspase-3-positive cells were also immunoreactive for NeuN and NT identifying them as neurons. By contrast, exceptional caspase-3-positive cells were also Iba1-positive, and so were consistent with microglia (not shown). Bar: 40 m.
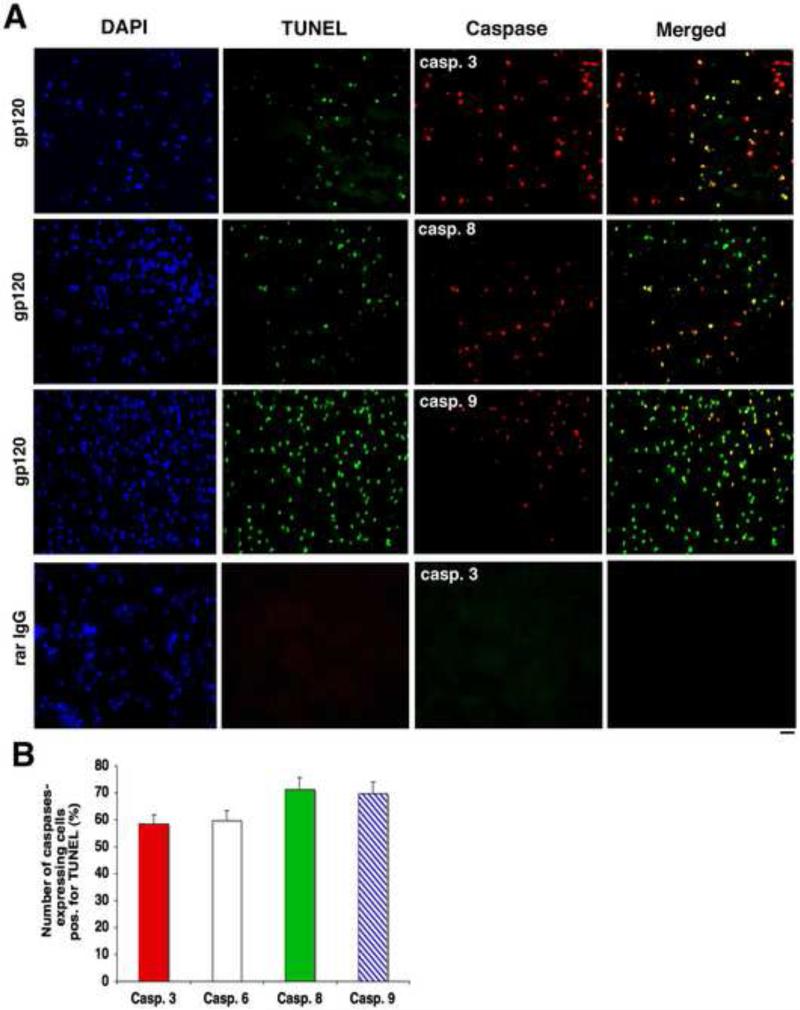
3.3 Numerous TUNEL-positive cells are caspases-positive
We then double-stained sections of gp120-injected CPs for TUNEL and caspases. Several TUNEL-positive cells were caspases-positive. TUNEL-positive cells were positive for the different caspases. However, not all caspases-expressing cells were TUNEL-positive, and not all TUNEL-positive cells were caspases-positive (Fig. 3A). We measured the number of caspases-positive cells on several serial cryostat sections (the whole section being examined, not random fields, with at least five sections for each sample). Numbers of caspases-expressing cells positive for TUNEL (expressed as percentages per CP) are shown on Fig. 3B.
Figure 3.
Numerous TUNEL-positive cells are caspases-positive. A. Sections of 500 ng gp120-injected CPs were double-stained for TUNEL and caspases. Numerous TUNEL-positive cells were caspases-positive, particularly for caspase-3. However, not all caspases-expressing cells were TUNEL-positive, and not all TUNEL-positive cells were caspases-positive. Bar: 60 m. B. Graph showing the numbers of caspases-expressing cells positive for TUNEL (expressed as percentages per CP).
3.4 rSV40-delivery of SOD1 and GPx1 in the CP reduces the number of caspases-positive cells after gp120 injection
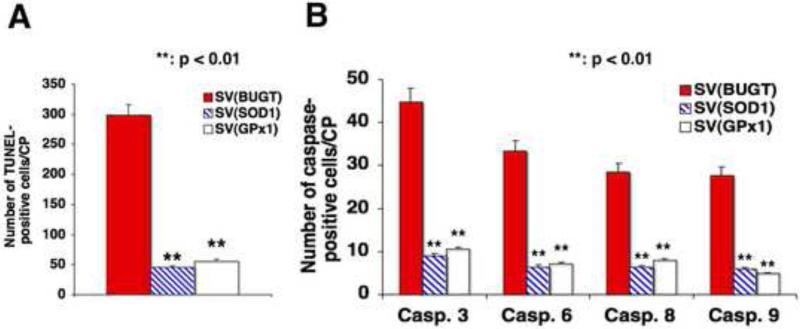
Four weeks after injection of SV(SOD1), SV(GPx1) or a control vector, SV(BUGT), into the CP, we injected an oxidant neurotoxic challenge intra-CP, 500 ng l−1 gp120. Apoptotic and caspases-positive cells were enumerated by TUNEL and immunocytochemistry one day later as previously described. Prior injection of SV(SOD1) and SV(GPx1) into the CP reduced the number of TUNEL-positive cells in the same structure after gp120 challenge by approximately 81% [SV(GPx1)] to 85% [(SVSOD1)], P < 0.01 for both, compared to the control vector SV(BUGT) (Fig. 4A). The numbers of caspases-positive cells were reduced between 82 to 85% for SV(SOD1) and between 75 to 83% for SV(GPx1), P < 0.01 for both compared to SV(BUGT) (Fig. 4B).
Figure 4.
Gene delivery of antioxidant enzymes reduces the number of caspases-positive cells after gp120 injection. Four weeks after injection of SV(SOD1), SV(GPx1) or SV(BUGT) into the CP, we injected an oxidant neurotoxic challenge intra-CP, 500 ng ml−1 gp120. Apoptotic and caspases-positive cells were enumerated by TUNEL and immunocytochemistry one day later. A. The number of TUNEL-positive cells in the CP after gp120 challenge was reduced by prior injection of SV(SOD1) and SV(GPx1) into the same structure by approximately 81% [SV(GPx1)] to 85% [(SVSOD1)], P < 0.01 for both, compared to the control vector SV(BUGT). B. The numbers of caspases-positive cells were reduced between 82 to 85% for SV(SOD1) and between 75 to 83% for SV(GPx1), P < 0.01 for both compared to SV(BUGT).
4. Discussion
We show here that injection of HIV-1 envelope gp120 into the CP elicits the expression of different caspases. Moreover, gene delivery of antioxidant enzymes reduces gp120-induced apoptosis and caspases expression.
Although many of the key apoptotic proteins have been identified, our understanding of the complex underlying mechanisms remains poor. However, recent advances have helped broaden our knowledge of apoptosis after different brain insults. Further to the simplistic concept that apoptosis occurs predominantly in neurons and is caspase-dependent, accumulating evidence now indicates that apoptosis can occur in nonneuronal cells and that caspase-independent mechanisms also play a key role (Broughton et al., 2009).
The involvement of caspases in HIV-1 neurotoxicity has been documented in vitro and in vivo. Higher levels of caspase-3 and caspase-6 have been shown in the brains of patients with HAD (Petito and Roberts, 1995; James et al., 1999; Noorbakhsh et al., 2010). Both HIV-1 neurotoxins gp120 and Tat significantly increase caspase-3 activation in striatal neurons in vitro. However, gp120 acts in large part through the activation of caspase(s), while Tat-induced neurotoxicity is also accompanied by activating an alternative pathway involving endonuclease G (Singh et al., 2004). Tat can induce both caspases 3/7 and 9 in hippocampal cell cultures (Askenov et al., 2009). Increased expression of caspase-3 has been shown in neurons following exposure to Tat (Bonavia et al., 2001; Kruman and Mattson, 1999; Kruman et al., 1998; Singh et al., 2004) and to gp120 (Nosheny et al., 2006, 2007; Bachis et al., 2006; Ahmed et al., 2009). In HIV-1 transgenic mice, Tat induction increased the percentage of neurons expressing caspase-3 (Bruce-Keller et al., 2008). Caspase-3-positive cells were also observed in a model of protracted exposure to gp120, SV(gp120) (Louboutin et al., 2009a).
However, so far, there was no study focused on the expression of different caspases following gp120 injection. We show here that different caspases were expressed after gp120 administration into the CP and that there was a relationship with the concentration of gp120 injected. Both initiator (caspases 8 and 9) and effector/executioner (caspases 3 and 6) were increased after gp120 injection.
We show here that about 70% of caspase-8- and 9-positive cells were TUNEL-positive while about 60% of caspase-3- and 6-positive cells were TUNEL-positive one day after intra-CP injection of gp120. These results suggest that not all caspases-positive cells undergo apoptosis, at least as assessed by the methods used here and/or at the time points we considered. It is also possible that apoptosis will occur in the remaining caspases-positive cells at later time points. Gp120-induced caspase-3 activity may also be causing nonlethal neuron injury. As previously noted (Bruce-Keller et al., 2008), if cell death in response to caspase-3 depends on total enzyme activity within a cell, the caspase-3 activity detected may be below the threshold required to initiate neuron death. This is difficult to determine based on immunocytochemistry. It has also been shown that activated caspase-3 rapidly degrades itself (Cribbs et al., 2004).
Caspases are a family of at least 12 cysteine aspartate proteases and besides caspases 3, 6, 8 and 9, we did not check the expression of all of these proteases in the present work, neither did we establish the relationship of all of these proteases with apoptosis. The role of other caspases cannot thus be excluded. It must also be emphasized that there is some redundancy in the activities of the various caspases (Cribbs et al., 2004).
Numerous data suggest the role of caspases in animal models of neurodegenerative disorders. In vitro data suggest that caspase-3 activation precedes and is not a consequence of apoptotic cell death in Parkinson's disease (PD) (Hartmann et al., 2000). In an animal model of PD, it has been shown that expression of caspase-3 and caspase-8 activation was an early event but that pycnotic neurons persist until day 7 post injury (Turmel et al., 2001; Hartmann et al., 2001).
One way to establish the role of caspases in apoptosis would be to show that caspase inhibition can be neuroprotective. This strategy has been used in different animal models of neurodegenerative diseases using pharmacological tools or transgenic animals. Administration of a pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp fluoromethyl-ketone (Z-VAD-fmk) to treat transgenic mice expressing mutant human SOD1, a model of ALS delayed disease onset and mortality in ALS mice (Li et al., 2000). A role for caspases in ALS was supported by studies in which transgenic ALS mice were crossed with Bcl-2 overexpressing (OE) mice (Martinou et al., 1994). Overexpression of Bcl-2 attenuated neurodegeneration, delayed the activation of caspases, and prevented the cleavage of beta-actin (Vukosavic et al., 2000). A similar approach demonstrated that intracerebroventricular administration of a caspase inhibitor delayed disease progression and death in a mouse model of Huntington's disease (Ona et al., 1999). Caspase inhibition reduced apoptosis, increased the survival of dopaminergic neurons and improved functional recovery in a rat model of PD (Schierle et al., 1999). To test the involvement of caspases in AD disease progression, Rohn and Head generated 3×Tg-AD mice that overexpress the antiapoptotic protein Bcl-2, named 3×Tg-AD/Bcl-2 OE mice. Overexpression of Bcl-2 in the neurons of 3×Tg-AD mice blocked caspase activation (caspase-9 and caspase-3) and the cleavage of tau leading to its accumulation within neurons (Rohn and Head, 2009). Despite the high protein levels of tau, there was little evidence for fibrillary tangle formation in 3×TgAD mice overexpressing Bcl-2, suggesting that the caspase-cleavage of tau is a critical step leading to NFT formation. In 3×Tg-AD/Bcl-2 OE mice, it appeared as though APP was not being turned over properly and accumulated in the apical dendritic compartments of cortical neurons (Rohn and Head, 2009). Other studies have found significant protection and neurological improvement following caspase inhibition in animal models of acute neurologic diseases including traumatic injury or ischemia (Friedlander et al., 2003). These studies raised expectations that therapeutics would soon follow. Unfortunately, these promising studies employing caspase inhibitors have not yielded practical therapeutics that inhibits apoptosis in the clinical setting. The reasons for this are unclear but may represent potential toxic side effects caused by the fluoromethyl-ketone residue and poor tissue penetration of Z-VAD-fmk (Braun et al., 2007). Moreover, cotreatment of 1-methyl-4-phenylpyridinium (MPP+)-intoxicated primary dopaminergic cultures with broad-spectrum and specific caspase-8 inhibitors did not result in neuroprotection but seemed to trigger a switch from apoptosis to necrosis, probably related to ATP depletion (Hartmann et al., 2001). Concerning HAND, Tat induced caspase activation and apoptosis in cultured embryonic rat hippocampal neurons, and the caspase inhibitor zVAD-fmk prevented Tat-induced neuronal death (Kruman et al., 1998). The cell permeant caspase inhibitor z-DEVD-fmk significantly attenuated gp120-induced, but not Tat-induced, neuronal death of mouse striatal neurons, suggesting that gp120 acts in large part through the activation of caspase(s), whereas Tat-induced neurotoxicity was accompanied by activating an alternative pathway involving endo G (Singh et al., 2004). These reports suggest that treatment of brain injury by manipulating apoptotic pathways remains a daunting task.
Oxidative stress has been strongly implicated as an important factor in neuronal death in stroke, neurodegenerative diseases, as well as in HAND (Beal et al., 1995; Smith et al., 1995; Bruce-Keller et al., 1998; Cao et al., 1998; Mollace et al., 2001; Askenov et al., 2001, 2003; Turchan et al., 2003). Oxidative stress in HAND has been documented by analyses of brain tissue, including increased levels of lipid peroxidation product [i.e., malondialdehyde (MDA) and hydroxynonenal (HNE)] and the presence of oxidized proteins. Membrane-associated oxidative stress correlates with HIV-1 dementia pathogenesis and cognitive impairment (Mattson et al., 2005). Mitochondria are a major site of production of superoxide in normal cells and probably contribute to increased oxidative stress in these diseases. Overexpression of mitochondrial Mn2+-superoxide dismutase results in moderate reductions in infarction in temporary ischemia. Glutathione, the major water-soluble antioxidant, is localized in both the cytosol and the mitochondria. Mice overexpressing the cytosolic enzyme Cu2+Zn2+-superoxide dismutase develop smaller infarcts than wild-type ones, with a decrease in multiple events associated with mitochondrially mediated apoptosis, including the release of cytochrome c (Sims and Muyderman, 2010; Fujimura et al., 2000). It is thus possible that cytosolic overexpression of antioxidant enzymes delivered by SV40-derived vectors can mitigate the apoptotic events linked to mitochondria.
A potential therapeutic strategy for treatment of HAND would be to limit oxidative stress-related neurotoxicity. Gene transfer of antioxidant enzymes has been achieved in a gene therapy perspective in numerous models of neurological disorders by using diverse viral vectors: lentiviruses (Ridet et al., 2006), herpes simplex (Hoehn et al., 2003), adenovirus (Watanabe et al., 2003). We previously demonstrated that SV40-derived vectors deliver long-term transgene expression to brain neurons and microglia, when given by several different routes of administration (Louboutin et al., 2007b, 2010a, 2011). Intracerebral injection of rSV40s carrying antioxidant enzymes, Cu/Zn superoxide dismutase (SOD1) or glutathione peroxidase (GPx1), SV(SOD1) or SV(GPx1), into the CP, significantly protects neurons from apoptosis caused by injection of recombinant HIV-1 envelope glycoprotein, gp120 or Tat at the same location (Agrawal et al., 2006, 2007, 2011; Louboutin et al., 2007a). Moreover, intra-CP SV40-mediated gene delivery of antioxidant enzymes protects against several deleterious consequences following gp120 injection into the CP (Louboutin et al., 2009b, 2010b,c,d). Vector administration into the lateral ventricle (LV) or the cisterna magna, particularly if preceded by mannitol i.p., protects from intra-CP gp120-induced neurotoxicity comparably to intra-CP vector administration (Louboutin et al., 2007a; Louboutin et al., in press). rSV40s were employed in the current study because they transduce cells in G0 with high efficiency; they infect a wide range of cell types from humans and other mammals and deliver genes to nondividing cells efficiently and achieve long-term transgene expression in vitro and in vivo (Strayer, 1999). These vectors can transduce > 95% of cultured human NT2-derived neurons, primary human neurons (Cordelier et al., 2003a,b) and microglia (Cordelier and Strayer, 2006) without detectable toxicity. We show here that such treatment decreases expression of different caspases, both effectors and initiators. These results also suggest the influence of oxidative stress in the expression of caspases.
Thus, detoxification of ROS by SV(SOD1) and SV(GPx1) protects from HIV-1 gp120-induced apoptosis and expression of caspases and may serve as a potential gene therapy strategy for HAND. As survival improves with chronic HIV-1 infection improves, the number of people harboring the virus in their CNS, where it is largely impervious to highly active anti-retroviral therapeutic drugs (HAART), increases (Nath and Sacktor, 2006). Thus, the prevalence of HIV-associated neurocognitive disorder (HAND) encephalopathy continues to rise, and less fulminant forms of HAND such as minor neurocognitive/motor disorder (MCMD) have become more common than their more fulminant predecessors, and their presence remains a significant independent risk factor for AIDS mortality (McArthur et al., 2005; Ances et al., 2007; Antinori et al., 2007).
5. Conclusions
We showed here that administration of HIV-1 gp120 into the rat CP increased caspases expression. Caspases colocalized mainly with neurons. Prior gene delivery of the antioxidant enzymes Cu/Zn superoxide dismutase (SOD1) or glutathione peroxidase (GPx1) into the CP before injecting gp120 in the same structure reduced levels of gp120-induced caspases, recapitulating the effect of antioxidant enzymes on gp120-induced apoptosis observed by TUNEL. Thus, prior antioxidant enzyme treatment mitigated production of these caspases, probably by reducing ROS levels. While the present study strongly implicates caspases 3, 6, 8 and 9, additional studies are needed to determine the relative contribution of the various caspases to neuronal demise in HAND.
We studied the effect of HIV-1 gp120 on different caspases (3, 6, 8, 9) expression.
Gp120 was injected into the rat caudate-putamen.
Caspases expression peaked by 24h and they colocalized mainly with neurons.
We then delivered antioxidant enzymes SOD1or GPx1 into the CP before injecting gp120.
Antioxidant enzymes delivery reduced production of caspases, by reducing ROS levels.
6. Acknowledgements
This work was supported by NIH grants MH70287, MH69122 and AH48244 to DS.
Abbreviations
- AD
Alzheimer's disease
- AIDS
Acquired Immune Deficiency Syndrome
- ALS
amyotrophic lateral sclerosis
- APAF-1
apoptotic protease activating factor 1
- BUGT
human bilirubin-uridine 5′-diphosphate-glucuronosyl-transferase
- CNS
Central Nervous System
- CP
caudate-putamen
- DAPI
4′,6-diamidino-2-phenylindole
- FITC
Fluorescein Isothiocyanate
- GFAP
glial fibrillary acidic protein
- GPx1
glutathione peroxidase
- HAART
highly active anti-retroviral therapeutic drugs
- HAD
HIV-1-Associated Dementia
- HAND
HIV-1 associated neurocognitive disorders
- H2O2
hydrogen peroxide
- HIV-1
Human Immunodeficiency Virus type 1
- HNE
hydroxynonenal
- Iba1
ionized calcium binding adaptor molecule 1
- iNOS
inducible nitric oxide synthase
- LV
lateral ventricle
- MCMD
minor neurocognitive/motor disorder
- MDA
malondialdehyde
- NO
nitric oxide
- NT
Neurotrace
- OH-
hydroxyl radical
- PD
Parkinson's disease
- PBS
Phosphate buffer saline
- ROS
reactive oxygen species
- RSV-LTR
Rous Sarcoma Virus long terminal repeat
- SOD1
Cu/Zn superoxide dismutase
- SV40
Simian Virus 40
- TRITC
Tetramethyl Rhodamine Iso-Thiocyanate
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal L, Louboutin JP, Reyes BAS, van Bockstaele EJ, Strayer DS. Antioxidant enzyme gene delivery to protect from HIV-1 gp120-induced neuronal apoptosis. Gene Ther. 2006;13:1645–1656. doi: 10.1038/sj.gt.3302821. [DOI] [PubMed] [Google Scholar]
- Agrawal L, Louboutin JP, Strayer DS. Preventing HIV-1 Tat-induced neuronal apoptosis using antioxidant enzymes: mechanistic and therapeutic implications. Virology. 2007;363:462–472. doi: 10.1016/j.virol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Agrawal L, Louboutin JP, Reyes BAS, van Bockstaele EJ, Strayer DS. HIV-1 Tat neurotoxicity: a model of acute and chronic exposure, and neuroprotection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.10.005. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Ahmed F, McArthur L, De Bernardi M, Mocchetti I. Retrograde and anterograde transport of HIV protein gp120 in the nervous system. Brain Behav Immun. 2009;23:355–364. doi: 10.1016/j.bbi.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, et al. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Askenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, et al. Temporal relationship between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Askenov MY, Askenova MV, Mactutus CF, Booze RM. Attenuated neurotoxicity of the transactivation-defective HIV-Tat protein in hippocampal cell cultures. Exp Neurol. 2009;219:586–590. doi: 10.1016/j.expneurol.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Aden SA, Nosheny RL, Andrews PM, Mocchetti I. Axonal transport of human immunodeficiency virus type 1 envelope protein glycoprotein 120 is found in association with neuronal apoptosis. J Neurosci. 2006;26:6771–6780. doi: 10.1523/JNEUROSCI.1054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptosis death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol. 2007;206:183–191. doi: 10.1016/j.expneurol.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Broughton BRS, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Li YJ, Lovell MA, Kraemer PJ, Gary DS, Brown RR, et al. 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J Neuropathol Exp Neurol. 1998;57:257–267. doi: 10.1097/00005072-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Carney JM, Duchon A, Floyd RA, Chevion M. Oxygen free radicals involvement in ischemia and reperfusion of the brain injury to brain. Neurosci Lett. 1998;88:233–238. doi: 10.1016/0304-3940(88)90132-2. [DOI] [PubMed] [Google Scholar]
- Cordelier P, Calarota SA, Pomerantz RJ, Xiaoshan J, Strayer DS. Inhibition of HIV-1 in the central nervous system by IFN-alpha2 delivered by an SV40 vector. J Interferon Cytokine Res. 2003a;23:477–488. doi: 10.1089/10799900360708605. [DOI] [PubMed] [Google Scholar]
- Cordelier P, Van Bockstaele E, Calarota SA, Strayer DS. Inhibiting AIDS in the central nervous system: gene delivery to protect neurons from HIV. Mol Ther. 2003b;7:801–810. doi: 10.1016/s1525-0016(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Cordelier P, Strayer D S. Using gene delivery to protect HIV-susceptible CNS cells: inhibiting HIV replication in microglia. Virus Res. 2006;118:87–97. doi: 10.1016/j.virusres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer's disease. Am J Pathol. 2004;165:353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N Engl J Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, et al. Caspase-3: a vulnerability factor and a final effector in the apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Troadec J-D, Hunot S, Kikly K, Faucheux BA, Mouatt-Prigent A, et al. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease, but pathway inhibition results in neuronal necrosis. J Neurosci. 2001;21:2247–2255. doi: 10.1523/JNEUROSCI.21-07-02247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn B, Yenari MA, Sapolsky RM, Steinberg GK. Glutathione peroxidase overexpression inhibits cytochrome C release and proapoptotic mediators to protect neurons from experimental stroke. Stroke. 2003;34:2489–2494. doi: 10.1161/01.STR.0000091268.25816.19. [DOI] [PubMed] [Google Scholar]
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GW, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;19:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kruman LL, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kruman II, Mattson MP. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J Neurochem. 1999;72:529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Liu B, Reyes BAS, Van Bockstaele EJ, Strayer DS. Rat bone marrow progenitor cells transduced in situ by rSV40 vectors differentiate into multiple CNS cell lineages. Stem Cells. 2006;24:2801–2809. doi: 10.1634/stemcells.2006-0124. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BAS, Van Bockstaele EJ, Strayer DS. Protecting neurons from HIV-1 gp120-induced oxidant stress using both localized intracerebral and generalized intraventricular administration of antioxidant enzymes delivered by SV40-derived vectors. Gene Ther. 2007a;14:1650–1661. doi: 10.1038/sj.gt.3303030. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Reyes BAS, Agrawal L, Van Bockstaele EJ, Strayer DS. Strategies for CNS-directed gene delivery: in vivo gene transfer to the brain using SV40-derived vectors. Gene Ther. 2007b;14:939–949. doi: 10.1038/sj.gt.3302939. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BAS, Van Bockstaele EJ, Strayer DS. A rat model of human immunodeficiency virus 1 encephalopathy using envelope glycoprotein gp120 expression delivered by SV40 vectors. J Neuropathol Exp Neurol. 2009a;68:456–473. doi: 10.1097/NEN.0b013e3181a10f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BAS, Van Bockstaele EJ, Strayer DS. HIV-1 gp120 neurotoxicity proximally and at a distance from the point of exposure: protection by rSV40 delivery of antioxidant enzymes. Neurobiol Dis. 2009b;34:462–476. doi: 10.1016/j.nbd.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Chekmasova AA, Marusich E, Chowdhury JR, Strayer DS. Efficient CNS gene delivery by intravenous injection. Nature Meth. 2010a;7:905–907. doi: 10.1038/nmeth.1518. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Agrawal L, Reyes BAS, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol. 2010b;69:801–816. doi: 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louboutin JP, Reyes BAS, Agrawal L, Van Bockstaele EJ, Strayer DS. Blood-brain barrier abnormalities caused by exposure to HIV-1 gp120- Protection by gene delivery of antioxidant enzymes. Neurobiol Dis. 2010c;38:313–325. doi: 10.1016/j.nbd.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Reyes BAS, Agrawal L, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced neuroinflammation: relationship to neuron loss and protection by rSV40-delivered antioxidant enzymes. Exp Neurol. 2010d;221:231–45. doi: 10.1016/j.expneurol.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Marusich E, Fisher-Perkins J, Dufour J, Bunnell BA, Strayer DS. Gene transfer to the Rhesus monkey brain using SV40-derived vectors is durable and safe. Gene Ther. 2011;8:682–691. doi: 10.1038/gt.2011.13. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Reyes BAS, Agrawal L, Van Bockstaele EJ, Strayer DS. Intracisternal rSV40 administration provides effective pan-CNS transgene expression. Gene Ther. doi: 10.1038/gt.2011.75. in press. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- McKee HJ, Strayer DS. Immune responses against SIV envelope glycoprotein, using recombinant SV40 as a vaccine delivery vector. Vaccine. 2002;20:3613–3625. doi: 10.1016/s0264-410x(02)00243-8. [DOI] [PubMed] [Google Scholar]
- Madden SD, Cotter TG. Cell death in brain development and degeneration: control of caspase expression may be key! Mol Neurobiol. 2008;37:1–6. doi: 10.1007/s12035-008-8021-4. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Rendahl KG, Spratt SK, Snyder RO, Cohen LK, Leff SE. Characterization of intrastriatal recombinant adeno-associated virus-mediated gene transfer of human tyrosine hydroxylase and human GTP-cyclohydrolase I in a rat model of Parkinson's disease. J Neurosci. 1998;18:4271–4284. doi: 10.1523/JNEUROSCI.18-11-04271.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou J-C, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, et al. Overexpression of bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Diff. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mollace V, Nottet HS, Clayette P, Turco MC, Muscoli C, Salvemini D, et al. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–416. doi: 10.1016/s0166-2236(00)01819-1. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, et al. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Sacktor N. Influence of highly active antiretroviral therapy on persistence of HIV in the central nervous system. Curr Opin Neurol. 2006;19:358–361. doi: 10.1097/01.wco.0000236614.51592.ca. [DOI] [PubMed] [Google Scholar]
- Nikonov AA, Finger TE, Caprio J. Beyond the olfactory bulb: an odotopic map in the forebrain. Proc Natl Acad Sci USA. 2005;102:18688–18693. doi: 10.1073/pnas.0505241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Ramachandran R, Barsby N, Ellestad KK, LeBlanc A, Dickie P, et al. MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. FASEB J. 2010;24:1799–1812. doi: 10.1096/fj.09-147819. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Bachis A, Aden SA, De Bernardi MA, Mocchetti I. Intrastriatal administration of human immunodeficiency virus-1 glycoprotein 120 reduces glial cell-line derived neurotrophic factor levels and causes apoptosis in the substantia nigra. J Neurobiol. 2006;66:1311–1321. doi: 10.1002/neu.20288. [DOI] [PubMed] [Google Scholar]
- Nosheny RL, Ahmed F, Yakoviev A, Meyer EM, Ren K, Tessarollo L, et al. Brain-derived neurotrophic factor prevents the nigrostriatal degeneration induced by human immunodeficiency virus-1 glycoprotein 120 in vivo. Eur J Neurosci. 2007;25:2275–2284. doi: 10.1111/j.1460-9568.2007.05506.x. [DOI] [PubMed] [Google Scholar]
- Ona VO, Li M, Vonsattel JP, Andrews LJ, Khan SQ, Chung WM, et al. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; New York, NY: 1986. [Google Scholar]
- Petito CK, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Ribe EM, Serrano-Saiz E, Akpan N, Troy CM. Mechanisms of neuronal death in disease: defining the models and the players. Biochem J. 2008;415:165–182. doi: 10.1042/BJ20081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Bensadoun JC, Deglon N, Aebischer P, Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer's disease: is it time to ‘cut’ the chase? Int J Clin Exp Pathol. 2009;2:108–118. [PMC free article] [PubMed] [Google Scholar]
- Rohn TT. The role of caspases in Alzheimer's disease: potential novel therapeutic opportunities. Apoptosis. 2010;15:1403–1409. doi: 10.1007/s10495-010-0463-2. [DOI] [PubMed] [Google Scholar]
- Rouger K, Louboutin JP, Villanova M, Cherel Y, Fardeau M. X-linked vacuolated myopathy: TNF-alpha and IFN-gamma expression in muscle fibers with MHC class I on sarcolemma. Am J Pathol. 2001;158:355–359. doi: 10.1016/s0002-9440(10)63976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter BV, Parashar B, Chowdhury NR, Kadakol A, Ilan Y, Singh H, et al. A replication-deficient rSV40 mediates liver-directed gene transfer and a long-term amelioration of jaundice in gunn rats. Gastroenterology. 2000;119:1348–1357. doi: 10.1053/gast.2000.19577. [DOI] [PubMed] [Google Scholar]
- Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med. 1999;5:97–100. doi: 10.1038/4785. [DOI] [PubMed] [Google Scholar]
- Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophysis Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, et al. Apoptotic cell death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: Differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Monnier VM, Perry G. Radical ageing in Alzheimer's disease. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- Strayer DS, Kondo R, Milano J, Duan LX. Use of SV40-based vectors to transduce foreign genes to normal human peripheral blood mononuclear cells. Gene Ther. 1997;4:219–225. doi: 10.1038/sj.gt.3300368. [DOI] [PubMed] [Google Scholar]
- Strayer DS. Gene therapy using SV40-derived vectors: what does the future hold? J Cell Physiol. 1999;181:375–384. doi: 10.1002/(SICI)1097-4652(199912)181:3<375::AID-JCP1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Strayer DS, Lamothe M, Wei D, Milano J, Kondo R. Generation of recombinant SV40 vectors for gene transfer. SV40 protocols. In: Raptis L, editor. Methods in Molecular biology. Vol. 165. Humana Press; Totowa, NJ: 2001. pp. 103–117. 2001. [DOI] [PubMed] [Google Scholar]
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, et al. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60:307–314. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, et al. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Exp Neurol. 2001;16:185–189. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- Vukosavic S, Stefanis L, Jackson-Lewis V, Guegan C, Romero N, Chen C, et al. Delaying caspase activation by Bcl-2. A clue to disease retardation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2000;20:9119–9125. doi: 10.1523/JNEUROSCI.20-24-09119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Chu Y, Andresen JJ, Nakane H, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase reduces cerebral vasospasm after subarachnoid hemorrhage. Stroke. 2003;34:434–440. doi: 10.1161/01.str.0000051586.96022.37. [DOI] [PubMed] [Google Scholar]