Abstract
Background
Tyrosine kinase inhibitors (TKI) significantly improve survival in patients with chronic myeloid leukemia in chronic phase (CML-CP). Conditional probability provides survival information in patients who have already survived for a specific period of time after treatment.
Methods
Cumulative response and survival data in six consecutive front-line TKI clinical trials were analyzed. Conditional probability was calculated for failure-free (FFS), transformation-free (TFS), event-free (EFS), and overall survival (OS) according to depth of response within 1 year of start of TKI, including: complete cytogenetic response (CCyR), major molecular response, molecular response with 4 or 4.5-log reduction (MR4 and MR4.5).
Results
A total of 483 patients with a median follow-up of 99.4 months from start of TKI were analyzed. Conditional probabilities of FFS, TFS, EFS, and OS for one additional year for patients alive after 12 months of therapy ranged from 92.0% to 99.1%, 98.5% to 100%, 96.2% to 99.6%, and 96.8% to 99.7%, respectively. Conditional FFS for one additional year did not improve with deeper response each year. Conditional probabilities of TFS, EFS and OS for one additional year maintained more than 95% during the period.
Conclusion
In the era of TKIs, patients with CML-CP who survived for certain years maintained excellent clinical outcomes in each age group.
Keywords: Chronic myeloid leukemia, tyrosine kinase inhibitors, conditional survival
INTRODUCTION
Tyrosine kinase inhibitors (TKIs) improve the survival in patients with chronic phase chronic myeloid leukemia (CML-CP),1–7 such that their survival is nearly identical to that of the general population.7 A common clinical scenario is for a patient who has survived for a certain period of time with a given response to TKI to try to understand their prognosis with continued therapy. Literature has documented the long-term probability of survival endpoints from start of therapy. However, once a patient has initiated therapy and achieved a given response, their survival is modulated by the time they have already been on therapy and the response they have achieved. These long-term survival endpoint probabilities after specific time periods and how this is modulated by the response status at that time has been seldom addressed in the literature.8, 9 Conditional survival probability represents the probability of being alive as a function of the time already lived. The effect of the response achieved can be incorporated into the evaluation of these probabilities providing relevant information of the expectations based on outcome already achieved.10 In this analysis we aimed to analyze the conditional survival probabilities of failure-free (FFS), transformation-free (TFS), event-free (EFS), and overall survival (OS) in patients with CML-CP receiving front-line TKI therapy and as a function of the depth of response at different time points.
METHODS
Patients with newly diagnosed CML-CP who enrolled in six consecutive or parallel prospective clinical trials of imatinib (starting dose 400 mg or 800 mg daily, with or without pegylated interferon), nilotinib (400 mg twice daily) or dasatinib (50 mg twice daily, or 100 mg daily) at a single institution were analyzed.4–6, 11, 12 These trials were registered at www.clinicaltrial.gov (NCT00038649, NCT00048672, NCT00333840, NCT00050531, NCT00254423, and NCT00129740). All protocols were approved by the institutional review board and informed consent was obtained in accordance with the Declaration of Helsinki. The inclusion criteria were similar for all trials including age ≥15 years, adequate organ function, and Eastern Cooperative Oncology Group performance status 0–2. The following response categories were analyzed using standard definitions 13: complete cytogenetic response (CCyR); major molecular response (MMR); and molecular response with 4- and 4.5-log (international scale) reduction (MR4 and MR4.5).
OS was dated from the start of therapy until death from any cause at any time. EFS was calculated from the start of therapy to loss of complete hematologic response, loss of major cytogenetic response, transformation to accelerated (AP) or blast phase (BP), or death from any cause during study therapy. TFS was calculated from the start of therapy to transformation to AP or BP, or death during study therapy. FFS was calculated from the start of TKI to an event (as defined above), discontinuation of therapy for any reason, or death. Patients who were alive at the end of the study period were censored at the date of last follow-up. Survival status of patients who lost to follow-up was monitored with telephone, letter, or the social security death index.
The probability of survival by response was estimated by the Kaplan-Meier method.14 Conditional survival was calculated as the probability of surviving an additional y years, given that the patients has already survived x years.15 Landmark analysis was performed to calculate conditional survival for each additional year. P values were two-tailed and P values of less than 0.05 were considered statistically significant. Cox proportional hazard model with a time-dependent variable was performed for univariate and multivariate analysis.16 Variables with a P value <0.10 were included in a multivariate analysis. All statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
Patients
From May 2000 to January 2012, 483 patients with CML-CP were enrolled in the clinical trials analyzed (70 patients, imatinib 400mg/day; 43, imatinib 800mg/day; 158, imatinib 800mg/day ± pegylated interferon; 105, nilotinib; 107, dasatinib).4, 6, 11, 12 The median age at diagnosis was 48.3 years (range; 15.1–84.8). (Table 1) The overall median follow-up was 99.4 months. Median follow-up was significantly longer for patients with imatinib (126.3 months), compared to patients with nilotinib (47.8 months) or dasatinib (50.4 months) (P<0.001). Within 1 year of start of therapy 425 (88%) patients achieved CCyR, 349 (72%) MMR, 202 (42%) MR4, and 162 (34%) MR4.5. FFS, TFS, EFS, and OS by response within 1 year are presented in figure 1. Overall, 16 patients (3%) lost to follow-up; 13 patients, imatinib; 2, dasatinib; and 1, nilotinib. Fifty-three patients (11%) died in our study: 12 (23%) died of progressive disease; 5 (9%), complications of stem cell transplantation; 9 (17%), secondary malignancy; 9 (17%), cardiovascular events; 2 (4%), sepsis; 2 (4%), dementia; 2 (4%), surgical complications; 2 (4%), car accident; 1 (2%), gastrointestinal bleeding; 1 (2%), hepatitis C cirrhosis; 1 (2%), Parkinson disease; 1 (2%), suicide; 1 (2%), bowel obstruction; and 5 (9%), unknown.
Table 1.
Patient characteristics
| All [n=483] | CCyR* [n=425] | MMR* [n=349] | MR4* [n=202] | MR4.5* [n=162] | |
|---|---|---|---|---|---|
| Median age, y (range) | 48.3 (15.1–84.8) | 49.1 (15.1–84.8) | 49.0 (15.1–84.8) | 49.8 (15.1–84.8) | 49.7 (18.5–84.8) |
| Median follow-up, months (range) | 99.4 (1.8–154.3) | 99.3 (3.0–154.3) | 98.4 (3.0–147.5) | 102.5 (8.0–147.5) | 90.1 (8.0–147.5) |
| Sokal risk score, No. (%) | |||||
| Low | 335 (69) | 299 (70) | 245 (70) | 139 (69) | 108 (67) |
| Intermediate | 116 (24) | 103 (24) | 83 (24) | 54 (27) | 47 (29) |
| High | 32 (7) | 23 (5) | 21 (6) | 9 (5) | 7 (4) |
| Initial TKI, No. (%) | |||||
| Imatinib | 271 (56) | 229 (54) | 184 (53) | 110 (54) | 81 (50) |
| Nilotinib | 105 (22) | 97 (23) | 86 (25) | 46 (23) | 43 (27) |
| Dasatinib | 107 (22) | 99 (23) | 79 (23) | 46 (23) | 38 (24) |
| Clonal evolution at diagnosis, No. (%) | 20 (4) | 19 (5) | 17 (5) | 12 (6) | 9 (6) |
| Days from diagnosis to treatment, median (range) | 26.0 (0–377) | 26.0 (0–377) | 25.0 (0–377) | 26.5 (0–233) | 26.5 (0–233) |
| Best cumulative response, No. (%) | |||||
| CCyR* | 437 (91) | 425 (100) | 349 (100) | 202 (100) | 162 (100) |
| MMR* | 387 (80) | 375 (88) | 349 (100) | 202 (100) | 162 (100) |
| MR4* | 348 (72) | 341 (80) | 310 (89) | 202 (100) | 162 (100) |
| MR4.5* | 332 (69) | 326 (77) | 299 (86) | 196 (97) | 162 (100) |
: CCyR includes CCyR or better; MMR includes MMR or better; MR4 includes MR4 or better; MR4.5 includes MR4.5 or better.
Abbreviations: TKI, tyrosine kinase inhibitor; CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale; OS, overall survival.
Figure 1.
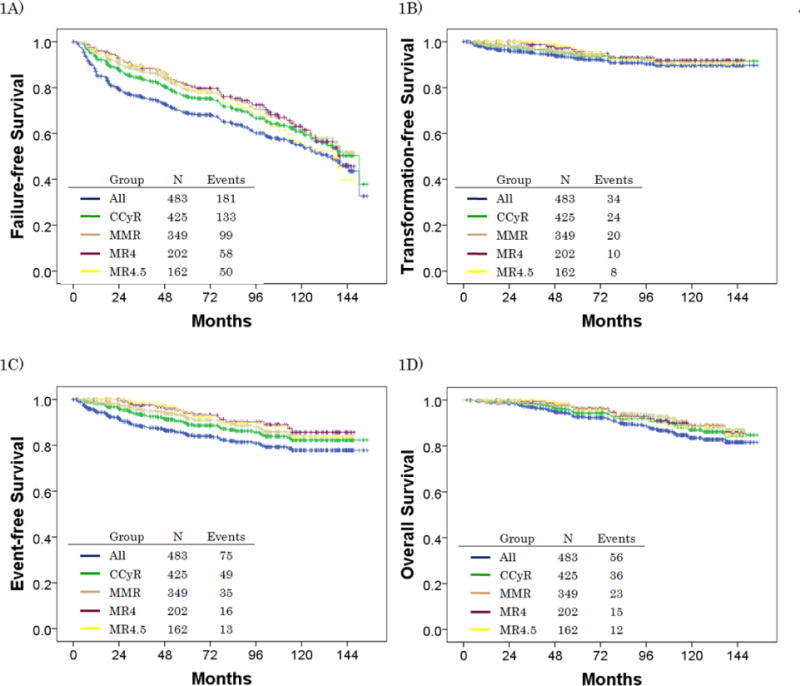
Clinical outcomes by response within 1 year*: 1A) failure-free survival, 1B) transformation-free survival, 1C) event-free survival, 1D) overall survival
*: CCyR includes CCyR or better; MMR includes MMR or better; MR4.5 includes MR4.5 or better.
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale.
Conditional Survival
Conditional probability of FFS for one additional year for all patients (i.e., regardless of response beyond CCyR) ranged from 92% to 99% (table 2). The conditional probability of living free from failure one additional year after having lived any number of years from the start of therapy did not improve for patients who had survived for up to 9 years from diagnosis. For example, after 4 years of therapy, the conditional FFS for one additional year was 94.4%, 94.3%, 93.8%, 93.8%, and 92.9% for all patients, or those who had achieved CCyR, MMR, MR4, or MR4.5, respectively. Similarly, at 8 years from the start, the conditional FFS for one additional year was 95.5%, 95.3%, 94.5%, 92.5%, and 89.6%, respectively.
Table 2.
Conditional one-year failure-free survival for each additional year up to 9 years after surviving the first year of therapy by response
| Time, (month) | Survival Estimates | Patient at Risk | Conditional Probability of Survival by Time Point, (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | |||
| % | N* | % | % | % | % | % | % | % | % | % | |
| All | |||||||||||
| 12 | 86.3 | 400 | 92.0 | 88.1 | 84.4 | 79.7 | 78.9 | 74.3 | 69.7 | 66.5 | 63.8 |
| 24 | 79.4 | 339 | – | 95.7 | 91.7 | 86.6 | 85.8 | 80.8 | 75.7 | 72.3 | 69.4 |
| 36 | 76.0 | 293 | – | – | 95.8 | 90.5 | 89.6 | 84.4 | 79.1 | 75.5 | 72.5 |
| 48 | 72.8 | 263 | – | – | – | 94.4 | 93.6 | 88.1 | 82.6 | 78.8 | 75.7 |
| 60 | 68.7 | 227 | – | – | – | – | 99.1 | 93.3 | 87.5 | 83.5 | 80.1 |
| 72 | 68.1 | 196 | – | – | – | – | – | 94.2 | 88.3 | 84.3 | 80.9 |
| 84 | 64.1 | 171 | – | – | – | – | – | – | 93.7 | 89.5 | 85.9 |
| 96 | 60.1 | 146 | – | – | – | – | – | – | – | 95.5 | 91.6 |
| 108 | 57.4 | 111 | – | – | – | – | – | – | – | – | 96.0 |
| CCyR within 12 months | |||||||||||
| 12 | 92.9 | 384 | 94.6 | 90.5 | 86.6 | 81.7 | 81.0 | 76.2 | 71.7 | 68.4 | 65.6 |
| 24 | 87.9 | 334 | – | 95.7 | 91.6 | 86.4 | 85.6 | 80.5 | 75.8 | 72.3 | 69.3 |
| 36 | 84.1 | 291 | – | – | 95.7 | 90.3 | 89.5 | 84.1 | 79.3 | 75.6 | 72.4 |
| 48 | 80.5 | 259 | – | – | – | 94.3 | 93.5 | 87.9 | 82.8 | 79.0 | 75.7 |
| 60 | 75.9 | 224 | – | – | – | – | 99.1 | 93.2 | 87.8 | 83.7 | 80.2 |
| 72 | 75.2 | 192 | – | – | – | – | – | 94.0 | 88.6 | 84.5 | 81.0 |
| 84 | 70.7 | 167 | – | – | – | – | – | – | 94.2 | 89.8 | 86.1 |
| 96 | 66.6 | 143 | – | – | – | – | – | – | – | 95.3 | 91.4 |
| 108 | 63.5 | 108 | – | – | – | – | – | – | – | – | 95.8 |
| MMR within 12 months | |||||||||||
| 12 | 95.1 | 324 | 94.6 | 91.5 | 88.0 | 82.6 | 81.6 | 78.5 | 73.8 | 69.7 | 66.2 |
| 24 | 89.9 | 284 | – | 96.7 | 93.1 | 87.3 | 86.3 | 83.0 | 78.0 | 73.7 | 70.0 |
| 36 | 87.0 | 247 | – | – | 96.2 | 90.3 | 89.3 | 85.8 | 80.7 | 76.2 | 72.4 |
| 48 | 83.7 | 221 | – | – | – | 93.8 | 92.7 | 89.2 | 83.8 | 79.2 | 75.2 |
| 60 | 78.5 | 186 | – | – | – | – | 98.9 | 95.1 | 89.4 | 84.5 | 80.2 |
| 72 | 77.6 | 161 | – | – | – | – | – | 96.1 | 90.4 | 85.4 | 81.1 |
| 84 | 74.6 | 143 | – | – | – | – | – | – | 94.0 | 88.8 | 84.3 |
| 96 | 70.2 | 123 | – | – | – | – | – | – | – | 94.5 | 89.7 |
| 108 | 66.3 | 88 | – | – | – | – | – | – | – | – | 94.9 |
| MR4 within 12 months | |||||||||||
| 12 | 96.5 | 191 | 96.2 | 92.7 | 89.5 | 84.0 | 82.4 | 78.0 | 75.1 | 69.4 | 65.3 |
| 24 | 92.8 | 170 | – | 96.4 | 93.1 | 87.3 | 85.7 | 81.1 | 78.0 | 72.2 | 67.9 |
| 36 | 89.5 | 148 | – | – | 96.5 | 90.5 | 88.9 | 84.1 | 81.0 | 74.9 | 70.5 |
| 48 | 86.4 | 133 | – | – | – | 93.8 | 92.1 | 87.1 | 83.8 | 77.6 | 73.0 |
| 60 | 81.0 | 112 | – | – | – | – | 98.2 | 92.9 | 89.4 | 82.7 | 77.8 |
| 72 | 79.6 | 97 | – | – | – | – | – | 94.6 | 91.0 | 84.2 | 79.3 |
| 84 | 75.3 | 85 | – | – | – | – | – | – | 96.2 | 89.0 | 83.8 |
| 96 | 72.4 | 75 | – | – | – | – | – | – | – | 92.5 | 87.0 |
| 108 | 67.0 | 54 | – | – | – | – | – | – | – | – | 94.1 |
| MR4.5 within 12 months | |||||||||||
| 12 | 96.3 | 152 | 95.9 | 93.0 | 89.7 | 83.3 | 81.3 | 75.3 | 71.2 | 63.8 | 58.6 |
| 24 | 92.3 | 135 | – | 97.0 | 93.5 | 86.9 | 84.8 | 78.5 | 74.3 | 66.5 | 61.1 |
| 36 | 89.5 | 116 | – | – | 96.5 | 89.6 | 87.4 | 80.9 | 76.6 | 68.6 | 63.0 |
| 48 | 86.4 | 103 | – | – | – | 92.9 | 90.6 | 83.9 | 79.4 | 71.1 | 65.3 |
| 60 | 80.2 | 84 | – | – | – | – | 97.6 | 90.3 | 85.5 | 76.6 | 70.3 |
| 72 | 78.3 | 69 | – | – | – | – | – | 92.6 | 87.6 | 78.5 | 72.1 |
| 84 | 72.5 | 60 | –– | – | – | – | – | – | 94.6 | 84.7 | 77.9 |
| 96 | 68.6 | 52 | – | – | – | – | – | – | – | 89.6 | 82.3 |
| 108 | 61.4 | 39 | – | – | – | – | – | – | – | – | 91.9 |
Indicates number still alive and not censored
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale.
The conditional TFS for one additional year ranged was stable at approximately 99% (table 3). There was a trend of improvement, albeit modest, with deeper response such that for the overall population, those alive and on therapy at 12 months had a TFS conditional probability at 24 months of 98.7%; the conditional probability was 99.2% for those with CCyR, 99.3% if they had achieved MMR; 100% if MR4; and 100% if MR4.5.
Table 3.
Conditional one-year transformation-free survival for each additional year up to 9 years after surviving the first year of therapy by response
| Time, (month) | Survival Estimates | Patient at Risk | Conditional Probability of Survival by Time Point, (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | |||
| % | N* | % | % | % | % | % | % | % | % | % | |
| All | |||||||||||
| 12 | 97.4 | 418 | 98.7 | 97.9 | 96.6 | 95.1 | 94.7 | 93.3 | 92.7 | 92.1 | 92.1 |
| 24 | 96.1 | 362 | – | 99.1 | 97.8 | 96.4 | 95.9 | 94.5 | 93.9 | 93.3 | 93.3 |
| 36 | 95.3 | 316 | – | – | 98.7 | 97.2 | 96.8 | 95.4 | 94.8 | 94.1 | 94.1 |
| 48 | 94.0 | 287 | – | – | – | 98.5 | 98.1 | 96.6 | 96.0 | 95.4 | 95.4 |
| 60 | 92.6 | 248 | – | – | – | – | 99.6 | 98.1 | 97.5 | 96.8 | 96.8 |
| 72 | 92.2 | 210 | – | – | – | – | – | 98.5 | 97.9 | 97.2 | 97.2 |
| 84 | 90.9 | 182 | – | – | – | – | – | – | 99.4 | 98.7 | 98.7 |
| 96 | 90.3 | 158 | – | – | – | – | – | – | – | 99.3 | 99.3 |
| 108 | 89.7 | 122 | – | – | – | – | – | – | – | – | 100 |
| CCyR within 12 months | |||||||||||
| 12 | 98.8 | 393 | 99.2 | 98.3 | 96.9 | 95.4 | 95.0 | 94.0 | 93.4 | 92.7 | 92.7 |
| 24 | 98.0 | 346 | – | 99.1 | 97.7 | 96.2 | 95.8 | 94.8 | 94.2 | 93.5 | 93.5 |
| 36 | 97.1 | 302 | – | – | 98.6 | 97.1 | 96.7 | 95.7 | 95.0 | 94.4 | 94.4 |
| 48 | 95.8 | 275 | – | – | – | 98.4 | 98.0 | 97.0 | 96.3 | 95.7 | 95.7 |
| 60 | 94.3 | 237 | – | – | – | – | 99.6 | 98.5 | 97.9 | 97.2 | 97.2 |
| 72 | 93.8 | 200 | – | – | – | – | – | 99.0 | 98.3 | 97.6 | 97.6 |
| 84 | 92.9 | 175 | – | – | – | – | – | – | 99.4 | 98.7 | 98.7 |
| 96 | 92.3 | 152 | – | – | – | – | – | – | – | 99.3 | 99.3 |
| 108 | 91.6 | 116 | – | – | – | – | – | – | – | – | 100 |
| MMR within 12 months | |||||||||||
| 12 | 98.5 | 326 | 99.3 | 98.6 | 97.4 | 96.0 | 95.5 | 94.4 | 93.6 | 92.9 | 92.9 |
| 24 | 97.9 | 292 | – | 99.3 | 98.1 | 96.7 | 96.2 | 95.0 | 94.3 | 93.5 | 93.5 |
| 36 | 97.2 | 257 | – | – | 98.8 | 97.4 | 96.9 | 95.7 | 95.0 | 94.2 | 94.2 |
| 48 | 96.0 | 232 | – | – | – | 98.6 | 98.1 | 96.9 | 96.1 | 95.3 | 95.3 |
| 60 | 94.6 | 197 | – | – | – | – | 99.5 | 98.2 | 97.5 | 96.7 | 96.7 |
| 72 | 94.1 | 167 | – | – | – | – | – | 98.8 | 98.0 | 97.2 | 97.2 |
| 84 | 93.0 | 151 | – | – | – | – | – | – | 99.2 | 98.4 | 98.4 |
| 96 | 92.3 | 132 | – | – | – | – | – | – | – | 99.2 | 99.2 |
| 108 | 91.5 | 96 | – | – | – | – | – | – | – | – | 100 |
| MR4 within 12 months | |||||||||||
| 12 | 100 | 192 | 100 | 98.8 | 98.1 | 95.8 | 95.0 | 93.0 | 93.0 | 91.8 | 91.8 |
| 24 | 100 | 174 | – | 98.8 | 98.1 | 95.8 | 95.0 | 93.0 | 93.0 | 91.8 | 91.8 |
| 36 | 98.8 | 153 | – | – | 99.3 | 97.0 | 96.2 | 94.2 | 94.2 | 92.9 | 92.9 |
| 48 | 98.1 | 138 | – | – | – | 97.7 | 96.8 | 94.8 | 94.8 | 93.5 | 93.5 |
| 60 | 95.8 | 117 | – | – | – | – | 99.1 | 97.1 | 97.1 | 95.7 | 95.7 |
| 72 | 95.0 | 100 | – | – | – | – | – | 97.9 | 97.9 | 96.6 | 96.6 |
| 84 | 93.0 | 89 | – | – | – | – | – | – | 100 | 98.6 | 98.6 |
| 96 | 93.0 | 78 | – | – | – | – | – | – | – | 98.6 | 98.6 |
| 108 | 91.8 | 58 | – | – | – | – | – | – | – | – | 100 |
| MR4.5 within 12 months | |||||||||||
| 12 | 100 | 153 | 100 | 100 | 99.2 | 96.1 | 95.0 | 92.3 | 92.3 | 90.5 | 90.5 |
| 24 | 100 | 138 | – | 100 | 99.2 | 96.1 | 95.0 | 92.3 | 92.3 | 90.5 | 90.5 |
| 36 | 100 | 120 | – | – | 99.2 | 96.1 | 95.0 | 92.3 | 92.3 | 90.5 | 90.5 |
| 48 | 99.2 | 107 | – | – | – | 96.9 | 95.8 | 93.1 | 93.1 | 91.3 | 91.3 |
| 60 | 96.1 | 88 | – | – | – | – | 98.8 | 96.0 | 96.0 | 94.2 | 94.2 |
| 72 | 95.0 | 71 | – | – | – | – | – | 97.1 | 97.1 | 95.3 | 95.3 |
| 84 | 92.3 | 63 | – | – | – | – | – | – | 100 | 98.1 | 98.1 |
| 96 | 92.3 | 55 | – | – | – | – | – | – | – | 98.1 | 98.1 |
| 108 | 90.5 | 43 | – | – | – | – | – | – | – | – | 100 |
Indicates number still alive and not censored
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale.
The 10-year projected probability of EFS for those alive and free from events at 12 months was 95%. Their conditional EFS for one additional year ranged from 97% to 100% (supplemental table 1) and improved minimally with deeper response. The projected probability at 24 months was 96.9% for all patients and improved to 97.9% for those in CCyR at 12 month; 98.7% for patients in MMR; 100% if MR4; and 100% in MR4.5.
Conditional OS for one additional year ranged from 97% to 100% regardless of response and time on therapy (table 4). Overall, all conditional probabilities of FFS, TFS, EFS, OS for one additional year was constant over 95% regardless of depth of response beyond CCyR (figure 2).
Table 4.
Conditional one-year overall survival for each additional year up to 9 years after surviving the first year of therapy by response
| Time, (month) | Survival Estimates | Patient at Risk | Conditional Probability of Survival by Time Point, (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 | 120 | |||
| % | N* | % | % | % | % | % | % | % | % | % | |
| All | |||||||||||
| 12 | 99.2 | 465 | 99.6 | 97.9 | 95.8 | 93.5 | 93.1 | 90.3 | 89.5 | 87.0 | 84.2 |
| 24 | 98.7 | 429 | – | 98.3 | 96.2 | 93.9 | 93.6 | 90.7 | 89.9 | 87.4 | 84.6 |
| 36 | 97.0 | 387 | – | – | 97.9 | 95.5 | 95.2 | 92.3 | 91.5 | 88.9 | 86.1 |
| 48 | 95.0 | 353 | – | – | – | 97.6 | 97.3 | 94.3 | 93.5 | 90.8 | 88.0 |
| 60 | 92.7 | 312 | – | – | – | – | 99.7 | 96.7 | 95.8 | 93.1 | 90.1 |
| 72 | 92.4 | 279 | – | – | – | – | – | 97.0 | 96.1 | 93.4 | 90.5 |
| 84 | 89.6 | 246 | – | – | – | – | – | – | 99.1 | 96.3 | 93.3 |
| 96 | 88.8 | 221 | – | – | – | – | – | – | – | 97.2 | 94.1 |
| 108 | 86.3 | 179 | – | – | – | – | – | – | – | – | 96.8 |
| CCyR within 12 months | |||||||||||
| 12 | 99.3 | 413 | 100 | 99.2 | 97.4 | 95.2 | 95.2 | 92.8 | 92.8 | 90.9 | 87.7 |
| 24 | 99.3 | 384 | – | 99.2 | 97.4 | 95.2 | 95.2 | 92.8 | 92.8 | 90.9 | 87.7 |
| 36 | 98.5 | 348 | – | – | 98.2 | 95.9 | 95.9 | 93.6 | 93.6 | 91.6 | 88.4 |
| 48 | 96.7 | 321 | – | – | – | 97.7 | 97.7 | 95.3 | 95.3 | 93.3 | 90.0 |
| 60 | 94.5 | 284 | – | – | – | – | 100 | 97.5 | 97.5 | 95.5 | 92.2 |
| 72 | 94.5 | 252 | – | – | – | – | – | 97.5 | 97.5 | 95.5 | 92.2 |
| 84 | 92.1 | 204 | – | – | – | – | – | – | 100 | 97.9 | 94.5 |
| 96 | 92.1 | 201 | – | – | – | – | – | – | – | 97.9 | 94.5 |
| 108 | 90.2 | 164 | – | – | – | – | – | – | – | – | 96.5 |
| MMR within 12 months | |||||||||||
| 12 | 99.1 | 338 | 100 | 99.7 | 98.9 | 97.3 | 97.3 | 94.9 | 94.9 | 93.1 | 89.9 |
| 24 | 99.1 | 315 | – | 99.7 | 98.9 | 97.3 | 97.3 | 94.9 | 94.9 | 93.1 | 89.9 |
| 36 | 98.8 | 286 | – | – | 99.3 | 97.7 | 97.7 | 95.2 | 95.2 | 93.4 | 90.2 |
| 48 | 98.1 | 265 | – | – | – | 98.4 | 98.4 | 95.9 | 95.9 | 94.1 | 90.8 |
| 60 | 96.5 | 234 | – | – | – | – | 100 | 97.5 | 97.5 | 95.6 | 92.3 |
| 72 | 96.5 | 234 | – | – | – | – | – | 97.5 | 97.5 | 95.6 | 92.3 |
| 84 | 94.1 | 186 | – | – | – | – | – | – | 100 | 98.1 | 94.7 |
| 96 | 94.1 | 168 | – | – | – | – | – | – | – | 98.1 | 94.7 |
| 108 | 92.3 | 136 | – | – | – | – | – | – | – | – | 96.5 |
| MR4 within 12 months | |||||||||||
| 12 | 100 | 199 | 100 | 99.4 | 98.8 | 96.2 | 96.2 | 92.9 | 92.9 | 89.9 | 88.6 |
| 24 | 100 | 185 | – | 99.4 | 98.8 | 96.2 | 96.2 | 92.9 | 92.9 | 89.9 | 88.6 |
| 36 | 99.4 | 169 | – | – | 99.4 | 96.7 | 96.7 | 93.5 | 93.5 | 90.4 | 89.1 |
| 48 | 98.8 | 158 | – | – | – | 97.3 | 97.3 | 94.0 | 94.0 | 91.0 | 89.6 |
| 60 | 96.2 | 138 | – | – | – | – | 100 | 96.6 | 96.6 | 93.5 | 92.1 |
| 72 | 96.2 | 124 | – | – | – | – | – | 96.6 | 96.6 | 93.5 | 92.1 |
| 84 | 92.9 | 111 | – | – | – | – | – | – | 100 | 96.8 | 95.3 |
| 96 | 92.9 | 99 | – | – | – | – | – | – | – | 96.8 | 95.3 |
| 108 | 89.9 | 82 | – | – | – | – | – | – | – | – | 98.5 |
| MR4.5 within 12 months | |||||||||||
| 12 | 100 | 159 | 100 | 100 | 99.2 | 95.8 | 95.8 | 92.5 | 92.5 | 88.5 | 88.5 |
| 24 | 100 | 148 | – | 100 | 99.2 | 95.8 | 95.8 | 92.5 | 92.5 | 88.5 | 88.5 |
| 36 | 100 | 134 | – | – | 99.2 | 95.8 | 95.8 | 92.5 | 92.5 | 88.5 | 88.5 |
| 48 | 99.2 | 124 | – | – | – | 96.5 | 96.5 | 93.2 | 93.2 | 89.1 | 89.1 |
| 60 | 95.8 | 105 | – | – | – | – | 100 | 96.6 | 96.6 | 92.4 | 92.4 |
| 72 | 95.8 | 91 | – | – | – | – | – | 96.6 | 96.6 | 92.4 | 92.4 |
| 84 | 92.5 | 81 | – | – | – | – | – | – | 100 | 95.7 | 95.7 |
| 96 | 92.5 | 73 | – | – | – | – | – | – | – | 95.7 | 95.7 |
| 108 | 88.5 | 62 | – | – | – | – | – | – | – | – | 100 |
Indicates number still alive and not censored
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale.
Figure 2.
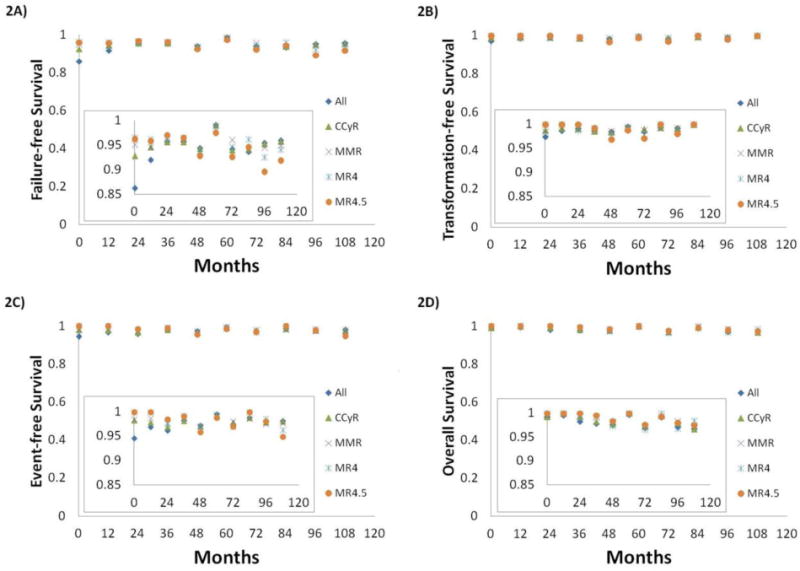
Conditional probabilities for one additional year by response within 1 year*: 2A) failure-free survival, 2B) transformation-free survival, 2C) event-free survival, 2D) overall survival
*: CCyR includes CCyR or better; MMR includes MMR or better; MR4.5 includes MR4.5 or better.
Abbreviations: CCyR, complete cytogenetic response; MMR, major molecular response; MR4, molecular response with 4 log reduction by international scale; MR4.5, molecular response with 4.5 log reduction by international scale.
Multivariate Analysis
We then performed a multivariate Cox proportional hazard analysis to investigate adverse prognostic factors for clinical outcome. This analysis demonstrated best cumulative response status at any time as a prognostic factor for FFS, TFS, EFS, and OS, and older age and presence of clonal evolution at diagnosis as adverse factors for OS (supplementary table 2). The lack of CCyR had the highest hazard ratio for all survival endpoints except for OS where it was equal to that for MMR (CCyR HR 6.250; MMR HR 6.579).
DISCUSSION
To our knowledge, this is the first report of conditional survival of FFS, TFS, EFS and OS with long-term follow-up in patients with newly diagnosed CML-CP receiving TKI as initial therapy. Our data suggests that in patients with CML-CP the conditional survival probabilities of FFS, TFS, EFS, and OS for each additional year up to 9 years are approaching 100%. There is small constant decrement of conditional FFS regardless of response status and duration of TKI. This is expected as FFS has the most inclusive definition for what is considered failure, including conditions that are not directly affected by the efficacy of TKI including toxicity or lack of availability because of insurance. Other long-term endpoints almost exclusively reflect the efficacy of TKI which is known to be excellent.17 Only deaths during therapy (for EFS or TFS) that may be unrelated to CML would be measured by such endpoints but these are known to represent a minority of events. Thus, the relatively stable EFS with worsening FFS over the years indicates a somewhat constant rate of discontinuation of TKI over time. Still, with a median follow-up of 99.4 months, this cumulative incidence of TKI discontinuation apparently did not affect OS. After failure to frontline TKI, salvage therapy with other TKIs are frequently effective contributing to a stable OS. Patients without a deep response to therapy may have an inferior EFS but the effect on OS may not be seen for many years. It might be possible that longer follow-up demonstrates worsening OS due to the eventual occurrence of events with more direct impact on survival such as transformation to blast phase.
Conditional TFS for one additional year was consistently nearly 100%. This finding suggests patients who can tolerate the front-line TKI without evidence of losing response or progression at 12 months or later are not likely to progress with an incidence of progression ≤1% for each subsequent year. Multivariate Cox proportional hazards with a time dependent variable showed the presence of CCyR within one year affected TFS the most. Of 425 patients who achieved CCyR within 1 year 11 patients (3%) progressed to advanced phase; 7 patients, on imatinib (3%); 2, on nilotinib (2%); and 2, on dasatinib (2%).
Overall, conditional OS for one additional year ranged from 97% to 100%. As expected, older age at diagnosis was associated with shorter OS (supplemental table 1). We recently reported survival in patients with newly diagnosed CML-CP with access to TKI was similar to that of general population.7 The decrement of survival in older patients was associated with other medical conditions such as cardiovascular disease and secondary malignancy, particularly among patients that had achieved at least a CCyR within 1 year of TKI. Conditional survival of general population for one additional year was 99.9256% at age of 20, 99.8971% at age of 30, 99.7337% at 40, 99.5823% at 50, 99.1233% at 60, 98.0452% at 70, and 94.0854% at 80, respectively.18
There are limitations in our study. First, no patients >85 were enrolled. However, patients >85 are likely to have other medical comorbidities which might obscure the true conditional clinical outcomes related to CML-CP. Second, the follow-up of second generation TKIs was significantly shorter than that of imatinib. Conditional survival after five years of treatment might significantly improve with wide availability of nilotinib and dasatinib. Third, longer follow-up is needed to see the stability of survival as there is a small but consistent decrement of FFS each year. Finally, there might be additional benefits of achievement of deeper responses that are not measurable by tis analysis, notably the possibility of elective treatment discontinuation.
In conclusion, patients with CML-CP who survived for certain years maintained excellent clinical outcomes in the era of TKI. However, over time patients may discontinue therapy for a variety of reasons which over time may affect overall survival. Importantly, this excellent expected conditional long-term outcomes reflect close continued monitoring of patients for CML and other co-morbidities and adverse events associated with TKI use such as is performed in clinical trials. It cannot be assumed that this can be extrapolated to circumstances in which patients are less stringently managed.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Osamu Miura at Tokyo Medical and Dental University for useful feedback.
Funding: This study was partly supported by the MD Anderson Cancer Center support grant CA016672 and the National Cancer Institute (award number P01 CA049639).
Footnotes
Conflicts of interests: J.C. received research support from Ariad, BMS, Novartis, Pfizer, and Teva, and is a consultant for Ariad, BMS, Novartis and Pfizer. N.P. is a consultant for Novartis. F.R. received research funding from Novartis and BMS. E.J. received consultancy for Ariad, BMS, TEVA, and Pfizer. H.K. received research grants from Novartis, BMS, Pfizer, Ariad. Other authors have nothing to disclose.
Authorship contributions: K.S. treated the patients, collected data, designed the study, analyzed the data, and wrote the manuscript. J.C. treated the patients, designed the study, analyzed the data and edited the manuscript. S.P. managed the data. P.J. treated the patient and collected the data. S.O., E.J., F.R., M.K., K.T., G.B., N.P., N.D., H.K. treated the patients. All authors provided significant intellectual input, and reviewed and approved the final version of the manuscript.
References
- 1.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115:4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 4.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. The Lancet Haematology. 2015;2:e118–e128. doi: 10.1016/S2352-3026(15)00021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitorera: analysis of patient data from six prospective clinical trials. The Lancet Haematology. 2:e186–e193. doi: 10.1016/S2352-3026(15)00048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintas-Cardama A, Choi S, Kantarjian H, Jabbour E, Huang X, Cortes J. Predicting outcomes in patients with chronic myeloid leukemia at any time during tyrosine kinase inhibitor therapy. Clin Lymphoma Myeloma Leuk. 2014;14:327–334.e328. doi: 10.1016/j.clml.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner H, Gondos A, Pulte D. Recent trends in long-term survival of patients with chronic myelocytic leukemia: disclosing the impact of advances in therapy on the population level. Haematologica. 2008;93:1544–1549. doi: 10.3324/haematol.13045. [DOI] [PubMed] [Google Scholar]
- 10.Janssen-Heijnen ML, Gondos A, Bray F, et al. Clinical relevance of conditional survival of cancer patients in europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010;28:2520–2528. doi: 10.1200/JCO.2009.25.9697. [DOI] [PubMed] [Google Scholar]
- 11.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113:6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan E, M P. Nonparanietric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 15.Skuladottir H, Olsen JH. Conditional Survival of Patients With the Four Major Histologic Subgroups of Lung Cancer in Denmark. Journal of Clinical Oncology. 2003;21:3035–3040. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 16.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman & Hall; 1984. [Google Scholar]
- 17.Kantarjian HM, Cortes J, Guilhot F, Hochhaus A, Baccarani M, Lokey L. Diagnosis and management of chronic myeloid leukemia. Cancer. 2007;109:1365–1375. doi: 10.1002/cncr.22523. [DOI] [PubMed] [Google Scholar]
- 18.Arias E. United States life tables, 2010. Natl Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


