Abstract
The isocortex of several primates and rodents shows a systematic increase in the number of neurons per unit of cortical surface area from its rostro-lateral to caudo-medial border. The steepness of the gradient in neuronal number and density is positively correlated with cortical volume. The relative duration of neurogenesis along the same rostro-caudal gradient predicts a substantial fraction of this variation in neuron number and laminar position, which is produced principally from layers II–IV neurons. Virtually all of our quantitative knowledge about total and laminar variation in cortical neuron numbers and neurogenesis comes from rodents and primates, however, leaving whole taxonomic groups and many intermediate-sized brains unexplored. Thus, the ubiquity in mammals of the covariation of longer cortical neurogenesis and increased cortical neuron number deriving from cortical layers II–IV is undetermined. To begin to address this gap, we examined the isocortex of the manatee using the optical dissector method in sectioned tissue, and also assembled partial data from published reports of the domestic cat brain. The manatee isocortex has relatively fewer neurons per total volume, and fewer II–IV neurons than primates with equivalently sized brains. The gradient in number of neurons from the rostral to the caudal pole is intermediate between primates and rodents, and like those species observed only in the upper cortical layers. The cat isocortex (Felis domesticus) shows a similar structure. Key species for further tests of the origin, ubiquity, and significance of this organizational feature are discussed.
Graphical abstract
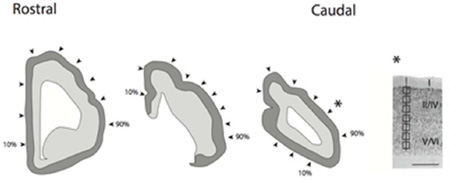
Illustration of a coronally-sectioned manatee brain used for neuron number estimates. In coronal planes, we systematically sampled sites (i.e., every 10th percentile) along the medial to lateral axis of the isocortex. The most medially located region is designated as the 10th percentage and the most laterally selected region is designated as the 90th percentile. Arrow-heads point to the approximate sites selected for neuron number estimates. We counted neuron numbers (layers II–VI neurons) under a unit of cortical surface area. Neuron number estimates were made through the depth of the cortex. Counting frames were selected at equidistant sites through the depth of the cortex. Sites were sampled orthogonal to the axis of the cortical surface. Scale bar is 1mm.
Introduction
In primate brains (e.g., baboons, macaques, capuchins) a pronounced variation in neuron numbers per unit of cortical surface area (neurons/unit column) aligns roughly with the original rostral-to-caudal axis of the embryonic isocortex, with as much as three times more neurons/unit column in posterior visual cortex than in prefrontal cortex (Collins et al., 2010; Cahalane et al., 2012). In smaller primate brains (e.g., owl monkey, tamarin) and large rodent brains (i.e., paca, agouti), a reduced version of this gradient is evident (Charvet et al., 2015). In all the brains described, more neurons/unit column are produced by more neurons in Layers IV to II. In the small rodent brains (e.g., hamster and mouse), no gradient in neurons/unit column is in evidence, although a significant, but slight, cross-cortex reversal in the number of neurons in the upper versus the lower cortical layers can be seen (Charvet et al., 2015).
Correspondingly, in neural development, a gradient in duration of neurogenesis (or related aspects of neural maturation) has been reported in all mammals studied closely for this feature, shorter at variously situated rostral points and longest at the caudal pole (e.g., mouse, Suter et al., 2007; Caviness et al., 2009; rat, Bayer and Altman, 1987, 1991; possum, Sanderson and Weller, 1990; ferret, McSherry et al., 1984, Kroenke et al., 2009; macaque, Rakic, 1974, 2002; Smart et al., 2002; human, Miller et al., 2014). Species with longer neurodevelopment (e.g., macaque, human) produce greater relative discrepancies in neurons/unit column between the rostral to caudal pole than species with shorter development (Workman et al., 2013; Charvet and Finlay, 2014). One systematic deviation from this pattern can be seen in timing of maturation in primary sensory areas (Kroenke et al., 2009) and reduced neuron loss in the same locations (Finlay and Slattery, 1983), which is echoed in increased neurons/unit column in the mature primary sensory areas superimposed upon the overall-rostral-caudal gradient (Cahalane et al., 2012). These two effects of course do not exhaust all possible developmental sources of variation in neuron numbers and organization. The rostral to caudal gradient in neurogenesis duration can be viewed as the primary scaffold on which further variations may be situated. Using available information on progenitor numbers, cell cycle parameters, duration of neurogenesis and neuron death, and adult cortical numbers and disposition, we have recently produced an empirically-constrained model, producing across-species and within-cortex differences in total cortical neuron numbers, cortical area, and laminar distribution of neurons within cortical regions as arising from a common suite of mechanisms operating over variable durations (Cahalane et al., 2014).
The collection of species represented in the studies described does not begin to exhaust or even explore the taxonomic divisions of mammals, however, and so the idea that this model might be mammalian-general must remain provisional until more species are represented. Finding any basic description of the scaling of the fundamental cortical scaffold would be an immense benefit to understanding its variations. While studies of niche adaptations (e.g., nocturnal/diurnal, Kaskan et al., 2005; Krubitzer et al., 2011), species-specific sensorimotor adaptations (e.g. whiskers and star noses, hands and hooves, Van der Loos and Welker, 1985; Catania and Kaas, 1995; Catania, 2004; Nudo and Masterton, 1990) or learned variation in cortex (e.g. ocular dominance organization, musical expertise; Abraham and Bear, 1996, Herholz and Zatorre, 2012) are numerous, only a minority of these studies, like those cited, have attempted to place such specializations in the range of whole cortex variation. Valuable wholesale characterizations of basic cortical organization across taxonomic groups have been offered (Valverde, 1990; Kaas, 2011, Krubitzer and Seelke, 2012). These characterizations must necessarily undergo continual revision, as sampling the entire range of mammalian variation is such an immense task. For example, the claim of a relative lack of differentiation of the cetacean cortex (Morgane et al., 1985) underwent major revision as more species and methods of describing cytoarchitecture became available (Hof et al., 2005). The evo-devo approach allows predictable scaling to be dissociated from species-specific adaptations, an intrinsically difficult problem as taxonomic groups are typically associated with particular brain size ranges and rates of development (Darlington et al., 1999; Workman et al., 2013).
This study examines neuron numbers across the isocortex of manatees (Trichechus manatus), a species that provides a maximal contrast from the species examined so far on multiple dimensions. Manatees belong to the order Siriena, which includes dugongs, and the superorder Afrotheria, which includes hyraxes and elephants. Manatees are distantly related to primates and rodents (Bininda-Emonds et al., 2007). Although large-brained compared to most rodents studied, manatees’ “encephalization” (the ratio or population deviation of brain to body mass) is low (O’Shea and Reep, 1990). Although diurnal, they are not visual specialists like primates. In a further effort to gather any available data from additional taxonomic groups, particularly from brains of intermediate size between the rodents and primates gathered to date, we re-present data gathered by Beaulieu and Colonnier, 1989 on cortical organization in cats reorganized on the rostral-to-caudal dimension of interest
Materials and Methods
Two adult manatee brains were used in this study. These specimens were obtained from a fresh carcass salvage program (Reep et al., 1989; Marshall and Reep, 1995). No manatees were killed for this study. One brain was sectioned coronally and the other brain was sectioned sagittally on a freezing microtome as described previously (Reep et al., 1989; Marshall and Reep, 1995). The sections were stained with cresyl violet. Specimen ID and histological information are listed in Table 1. Brain sections were examined with a Leitz Diaplan microscope and a Neurolucida imaging system with a mechanical stage and estimates of neuron numbers were made with a lucivid (Microbrightfield, Inc., Colchester, VT, USA). Coronal and sagittal sections were selected to cover the rostral-caudal axis and medial-lateral axes of the manatee isocortex.
Table 1.
Information about the two specimens used in the analyses.
| Manatee | Manatee | |
|---|---|---|
| ID | 84-49 | 85-8 |
| Brain weight (grams) | 317 g | 372 g |
| Body weight (kilograms) | 485 kg | 596 kg |
| Plane of section | Coronal | Sagittal |
| Section thickness | 30 µm | 30 µm |
| Guard zone | 5 µm | 5 µm |
| Sampled sites | 27 | 36 |
| Total number of layer II–VI neurons sampled | 715 | 805 |
In each selected coronal or sagittal section, we measured the length of the cortical surface. We then selected 9 equidistant sites along the cortical surface area of each selected section. We then overlaid a grid on the magnified image. The axes of a grid were aligned orthogonal to the isocortical surface and counting boxes (41 µm × 41 µm) were placed at 100 µm intervals between the border between layer I and layer II and the border between layer VI and the underlying white matter (Fig. 1). In total, we selected 36 ‘cortical columns’ for the sagittally-sectioned hemisphere and 27 ‘cortical columns’ for the coronally-sectioned hemisphere.
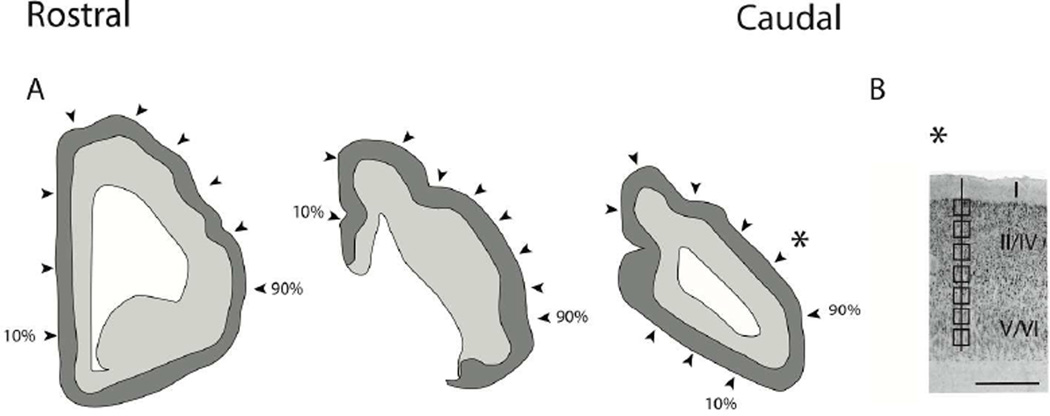
Figure 1.
(A) Illustration of a coronally-sectioned manatee brain used for neuron number estimates. In coronal planes, we systematically sampled sites (i.e., every 10th percentile) along the medial to lateral axis of the isocortex. The most medially located region is designated as the 10th percentage and the most laterally selected region is designated as the 90th percentile. Arrow-heads point to the approximate sites selected for neuron number estimates. We counted neuron numbers (layers II–VI neurons) under a unit of cortical surface area. (B) Neuron number estimates were made through the depth of the cortex. Counting frames were selected at equidistant sites through the depth of the cortex. Sites were sampled orthogonal to the axis of the cortical surface. A star shows the approximate location of (B). Scale bar is 1mm.
As in prior studies, we used the optical dissector method to estimate neuron numbers contained in each box's volume (Williams and Rakic, 1988; Williams et al., 2003). A 5 µm thick guard zone was applied, whereby cells that lay on the 3 exclusion planes (x, y, and z planes) were excluded. Measurements of layers were made orthogonal to the cortical surface area. We quantified layers II–IV, layers V–VI neurons and layers II–VI neurons across the isocortex in a manner similar to that described by Charvet et al., 2015 in order to facilitate comparison in cortical neuron numbers between manatees, primates and rodents. Layer IV as defined by an aggregation of small cells, is evident although small in the manatee. It is evident across many different cortical areas although it is elusive in the rostral regions of the isocortex (Fig. 2; Reep et al., 1989; Marshall and Reep, 1995). We followed the work of Reep et al., 1989 and Marshall and Reep, 1995 in defining cortical layers for the manatee.
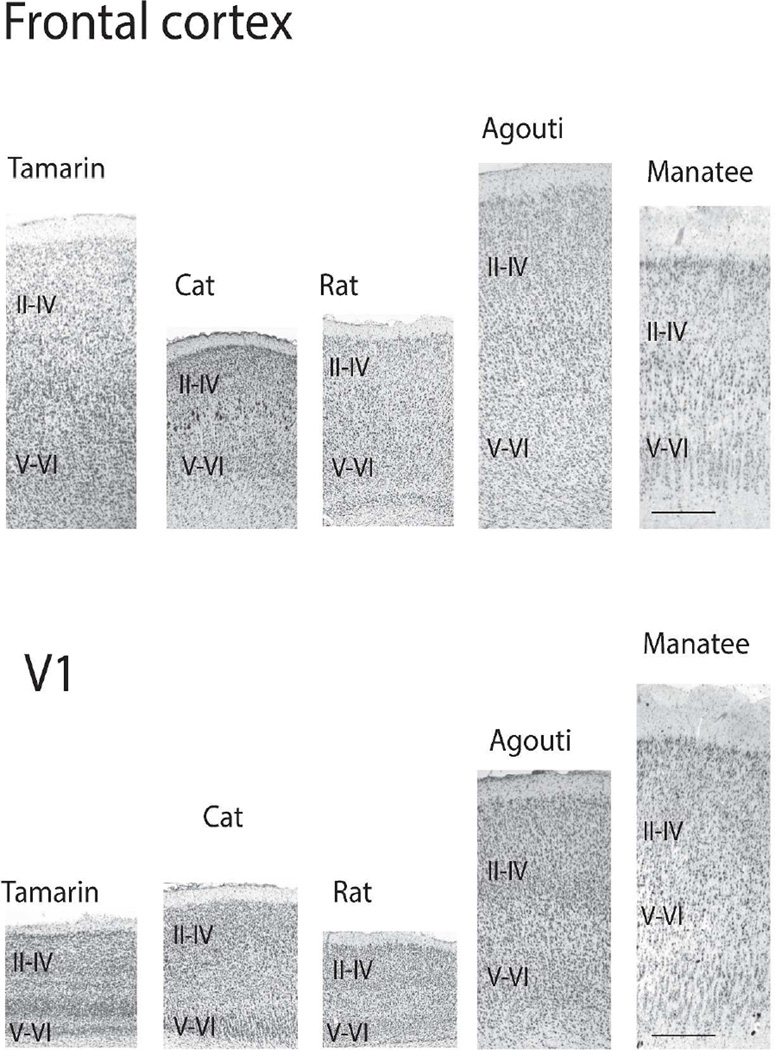
Figure 2.
Nissl-stained sections through the primary visual cortex and frontal cortex of a tamarin, agouti and a manatee. Labels refer to layers II–IV and layers V–VI combined. A well-defined granular layer is evident in the tamarin and to a lesser extent the agouti. A small granular layer is evident in the primary visual cortex of the manatee although layer IV is less evident in the frontal cortex of the manatee. Scale bar: 0.5mm.
We estimated neuron numbers under a unit of cortical surface area according to the following equation:
B is the base area of the counting frame, D is the distance between counting boxes, H is the height of the optical dissector, and T is the average tissue thickness after histological processing.
Correction for Curvature of the isocortex
Although the manatee cortex is lissencephalic (Reep and O'Shea, 1990), its isocortex curves along its rostro-caudal and medio-lateral axes. Estimating neuron numbers under a unit of cortical surface area in sections may be compromised if the plane of section is not orthogonal to the cortical surface. Attempts to avoid regions of curvature eliminate large, essential regions of the rostral and caudal cortex, and judgments about curvature without reconstruction are likely to be in error. To correct for curvature, we used a previously reconstructed manatee brain from the comparative mammalian brain collection (http://brainmuseum.org/). We located the samples of interest on the reconstructed manatee brain sections and we estimated the length of the cortical column that was orthogonal to the cortical surface. First, we selected sites in which there was minimal variation in the curvature of the cortex when possible. We also estimated the length of the cortical column for the plane in which neuron number estimates were made. The correction factor was estimated by dividing the length of the cortical column that was orthogonal to the cortical surface area by the length of the cortical column that was used for neuron estimates.
We reconstructed the distance of the sampled sites across the rostral to caudal and medial to lateral axes of the cortex. To that end, we located sampled sites on the reconstructed manatee cortex sections. We used the reconstructed manatee cortex to estimate the length of the cortical surface area across the rostral to caudal and medial to lateral axes (Fig. 1).
Statistical Analysis
For cross-species comparisons, data on neurons under a unit of cortical surface area of 3 primate species (i.e., owl monkey, tamarin, capuchin) and 3 rodent species (i.e., hamster, agout, paca) were obtained from a previous publication (Charvet et al., 2015). Neuron estimates for these species were obtained with the optical disector method.
We used phylogeny generalized least squares regressions to correct for the fact that species are not independent data points. These statistics are highly similar to what we had done previously (Finlay et al., 2014). Briefly, we used phylogeny generalized least squares statistics (PGLS) to obtain phylogenetically-controlled regressions in R (version 2.15.0; caper package). The phylogeny, which includes branch lengths, for the three rodent species (i.e., hamster, agouti, paca), three primate species (i.e., tamarin, owl monkey, capuchin) and manatee was taken from Bininda-Emonds et al., 2007. Agouti paca was used in place of Cuniculus paca because Cuniculus paca was not listed in Bininda-Emonds et al.,’s 2007 phylogeny. The two manatee specimens were averaged for cross-species comparisons. Regression parameters were found by maximum likelihood estimates.
We performed a natural-log regression of neuron numbers, upper and lower layer neurons versus the rostral to caudal axis of the cortical surface area in millimeters for each manatee to determine whether neuron numbers vary significantly across the rostral to caudal axis of the isocortex. Statistical analyses were performed in JMPPro11.
Results
Cortical neuron numbers
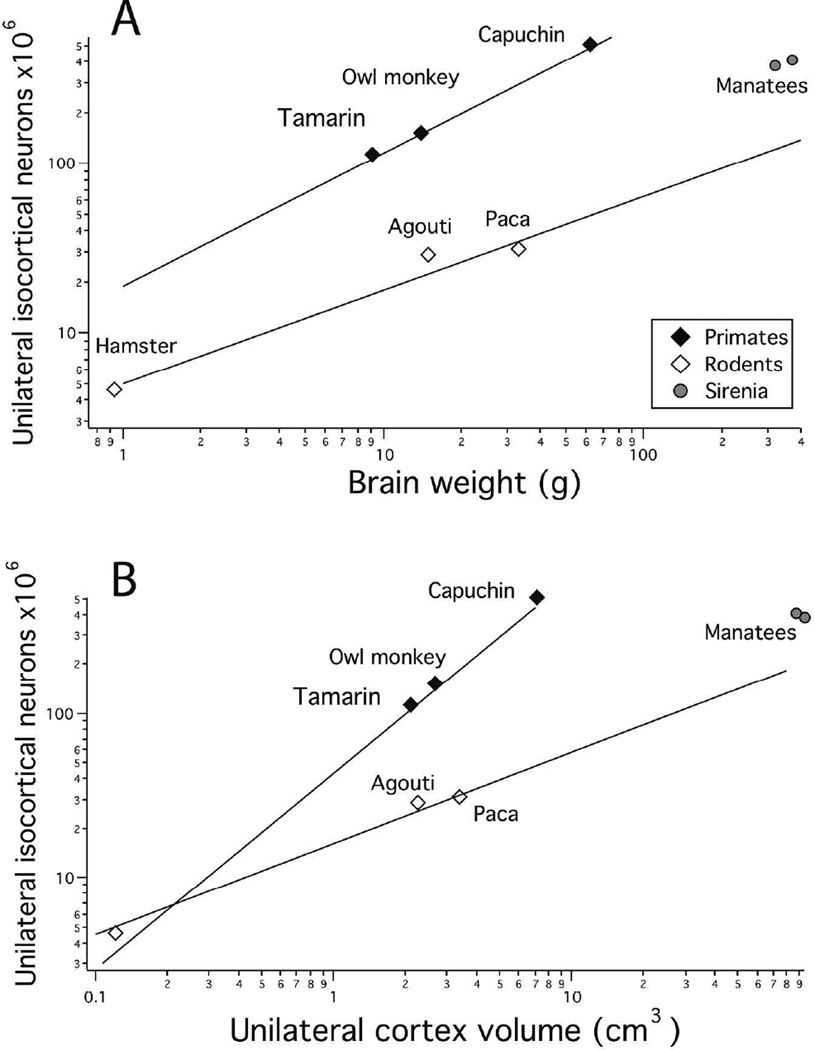
The two manatee brains we measured yielded very similar estimates of 381–409 million neurons per unilateral cortex. In Figure 3, we compare the numbers of neurons in manatee cortex to the same estimations in rodents and primates we described previously (Charvet et al., 2015), in this case representing both with respect to whole brain volume. Manatees fall closer to the slopes of cortical neuron numbers versus brain weight for rodents than for primates (Fig. 3A). Figure 3B compares neuron numbers in the cortex with respect to cortex volume, and we can see that with increasing cortex volume, the relative number of neurons decreases in rodents compared to primates, and the manatees close to the rodent line. Thus, both reduced allocation of tissue mass to cortex and reduced density of cortical neurons characterize the manatee brain, as they do in rodents, although no comparisons of allometry can be made without additional sirenian species.
Figure 3.
A: Total unilateral isocortical neuron numbers are plotted against brain weight in primates, rodents and manatees. Isocortical neuron numbers of manatees and rodents are lower than would be predicted for by an equivalently-sized primates brain. B: Total unilateral isocortical neuron numbers regressed against cortex volume in primates, rodents and manatees paints a similar picture. That is, neuron numbers are fewer than would be predicted for by an equivalently-sized primate brain. Natural-logged regressions were fit to primates and rodents separately. These regressions show that manatees resemble rodents more than primates in the scaling of neuron numbers versus brain weight or unilateral cortex volume.
The allometry of neurons by layer
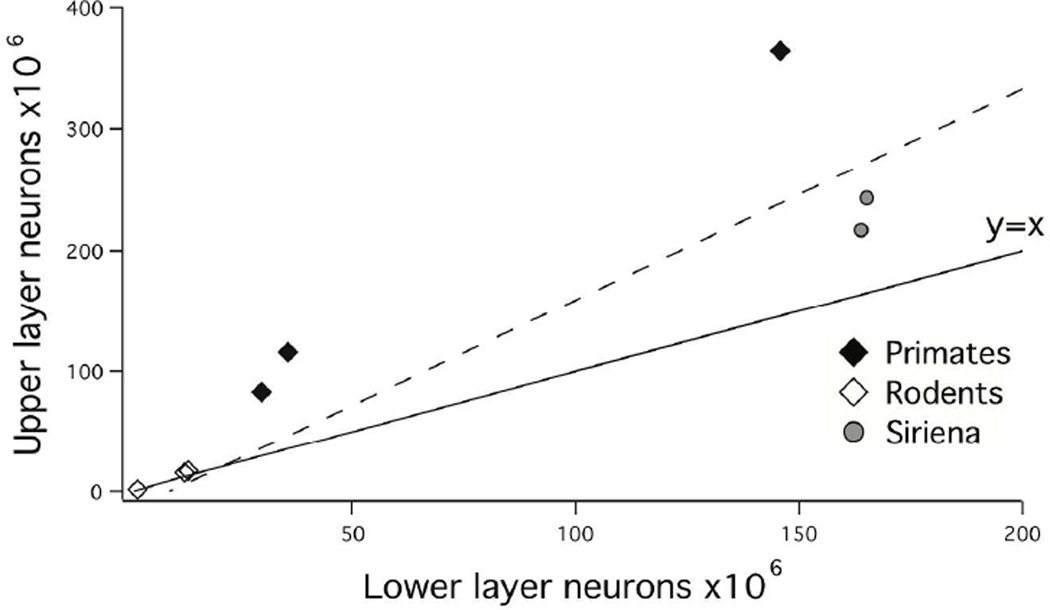
Independent of the relative density of neurons in the cortex, the number of layer II–IV neurons relative to layer V–VI neurons increases regularly and disproportionately with total cortical neuron number, and manatees appear to conform to this trend (Fig. 4). A linear regression of layer II–IV neurons versus layer V–VI neurons demonstrates that layer II–IV neurons increase with a slope greater than 1 when regressed against layer VVI neurons in these seven species (y=2.09x−23.82; adj R2 =0.925; F=75.05; p<0.05; λ=1). That is, layer II–IV neurons increase with a positive allometry relative to layer V–VI neurons in this sample.
Figure 4.
Upper layer neurons are plotted against lower layer neurons in primates, rodents and manatees. Primates exhibit proportionally more upper layer neurons than manatees and rodents do. Across all three orders, upper layer neurons expand disproportionately relative to lower layer neurons.
Rostro-caudal variation in neurons/unit column in the manatee
In the manatee cortex, neurons/unit column vary significantly across the rostral to caudal axis in both specimens (Table 2) with relatively fewer neurons in the rostral cortex compared with caudal regions of the cortex. Considering individual manatees, manatee #85-8 exhibits, neurons per unit of cortical surface area that range between 52,600 neurons per mm2 of cortical surface area in the most rostral regions and 88,700 neurons in the most caudal regions. That is, neurons vary by a factor of 1.68 between the 4 most rostrally (i.e., frontal cortex) and the 4 most caudally selected regions (n=4; caudal pole; Reep et al., 1989; Marshall and Reep, 1995), In the second manatee (84-49, coronally-sectioned), neurons ranged between 74,500 in the most rostrally and 100,900 neurons per mm2 caudally (see Figure 1 for selected locations). That is, neurons vary by a factor of 1.35 between the 9 most rostrally and 9 most caudally selected cortical regions. Taken together, these findings demonstrate that neurons per unit of cortical surface area are higher in the caudal cortex relative to the rostral cortex in the manatee.
Table 2.
Summary statistics of natural-log regression of total neurons (layers II–VI), upper (layer II–IV) neurons or lower layer (layers V–VI) neurons per mm2 of cortical surface area regressed against the rostro-caudal axis of the cortex for each individual manatee.
| Manatee ID #84-49 | Manatee ID #85-8 | |||||||
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | R2 | F ratio | Slope | Intercept | Adj R2 | F ratio | |
| Total neurons | 0.30* | 10.00* | 0.42 | 26.5* | 0.27* | 10.12* | 0.22 | 8.36* |
| Upper layers | 0.25* | 9.61* | 0.22 | 11.19* | 0.31* | 9.43* | 0.14 | 5.31* |
| Lower layers | 0.35* | 8.88* | 0.23 | 11.95* | 0.26 | 9.23* | 0.06 | 2.78 |
indicates that the p value is less than 0.05.
Variation between specimens #84-49 and #85-8 with regards to neuron counts may be related to shrinkage differences. For brain 84-49, linear shrinkage was calculated to be 28.5%, corresponding to volumetric shrinkage of 63.4% (Reep et al., 2007). Brain #85-8 has linear shrinkage of 31.1%, corresponding to volumetric shrinkage of 67.3%. The greater shrinkage seen in specimen #85-8 may have contributed to variation in neuron density between the two specimens.
Rostro-caudal variation in upper and lower layer neurons/unit column in the manatee
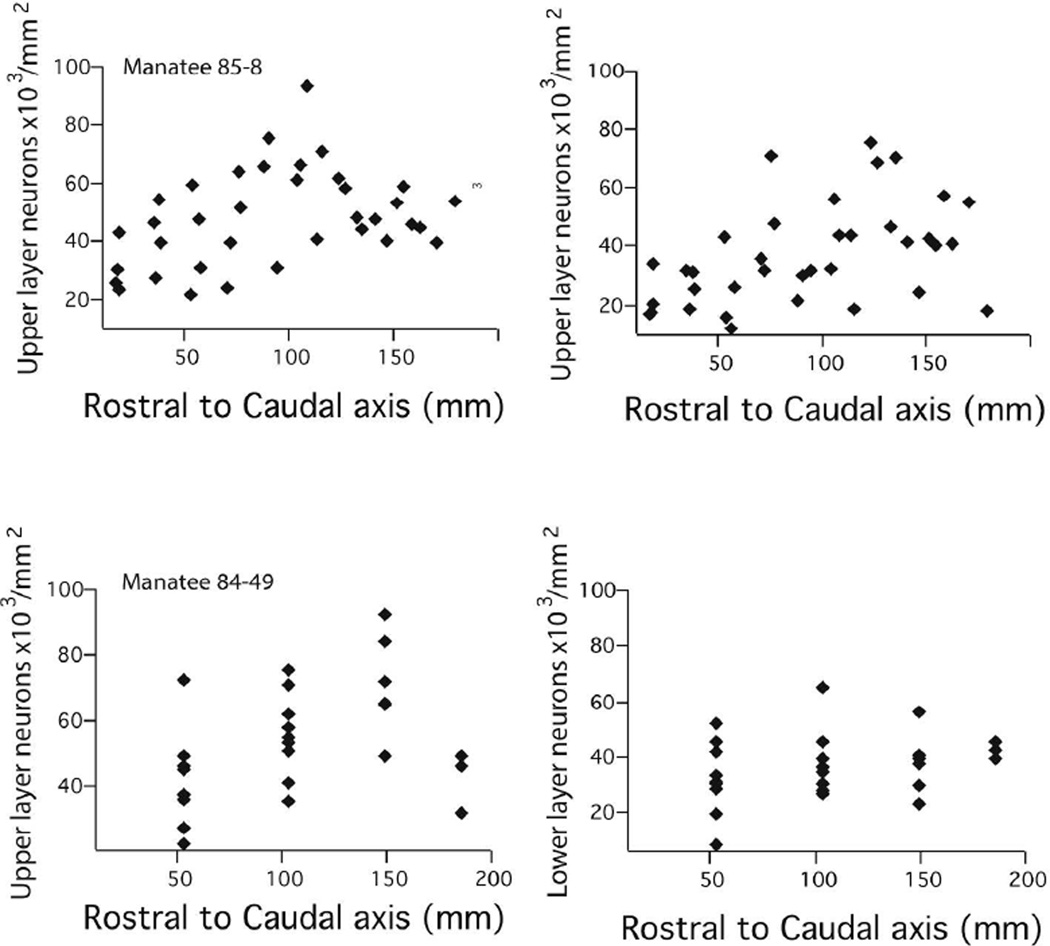
Upper layer (i.e., layer II–IV) and lower layer neurons per unit of cortical surface area generally increase across the rostral to caudal axis of the isocortex (Fig. 5; Table 2). Some of the variation in total neurons per unit/column is accounted for by variation in upper layer neurons. This is evident from the observation that layer II–IV neurons per unit of cortical surface area vary by a factor of 1.45–1.49, ranging between 30,500 to 42,300 neurons per mm2 of cortical surface area in rostral regions to 45,600 to 61,600 neurons in the caudal regions in both manatees (Fig. 5). Layer V–VI vary by a factor of 1.22–1.95 between the most rostrally and most caudally selected regions, ranging between 22,100–32,300 and 43,000–39,300 neurons per mm2 of cortical surface area in both specimens.
Figure 5.
Layer II–IV and V–VI neurons per mm2 of cortical surface area are plotted against the rostro-caudal axes of the cortex of each manatee. Manatee #85-8 is shown for the two upper panels and manatee 84-49 is displayed on the lower two panels.
Cross-species statistical comparison in cortical neuron numbers
Finally, we investigated whether the disparity in neurons per unit of cortical surface area increases as a function of total isocortical neurons across the three orders (i.e, Siriena, primates, rodents), comparing the percentage variation in neuron numbers per mm2 of cortical surface area between the rostral and caudal poles in each of the selected species (three primate species, three rodent species, a manatee). A phylogenetically-controlled regression shows that total isocortical neuron numbers predicts the magnitude of disparity between the rostral and caudal pole across three mammalian orders. More specifically, the percentage variation in neurons per unit of cortical surface area between the rostral and caudal pole increases in species with greater isocortical neuron numbers (y=0.001x+1.28; adj R2=0.71; F=15.81; p<0.05; λ=1).
Discussion
The new data compiled from the manatee show the increase in neuron number/unit column from rostral to caudal cortex. The density of neurons in the manatee cortex is more like the prior rodent sample than the primate sample. The allometry of rostral to caudal increase in neuron numbers appears to vary between orders and depends on absolute neuron number in the cortex, not neuron density or resultant cortical volume, and is most marked in the upper cortical layers.
Rostral-to caudal variation in neuron numbers in the cat
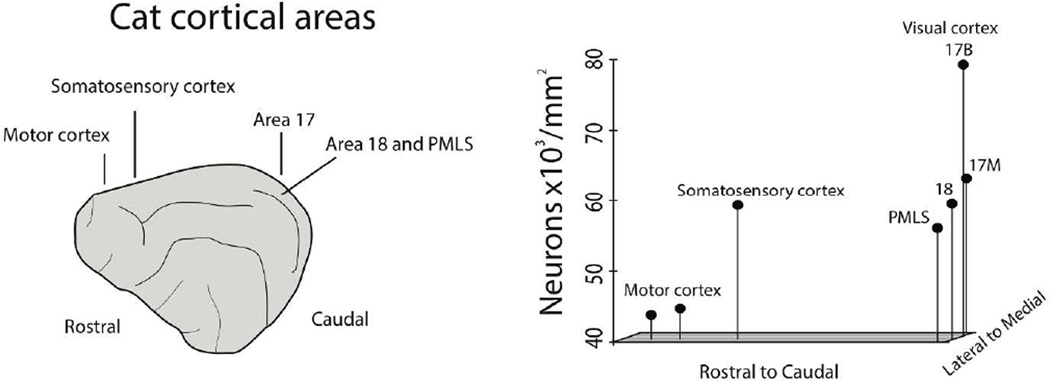
We overview the variation in neurons per unit of cortical surface area with respect to the rostro-caudal axis across the cat isocortex to broaden our cross-species comparative analyses. Estimates of neurons under 1mm2 of cortical surface area for cats (n=6) were obtained with the size-frequency distribution method (Beaulieu and Colonnier, 1989). Neurons per mm2 of cortical surface area vary systematically across the rostral to caudal axis of the cat cortex (Fig. 6). In particular, rostrally-selected regions (i.e., motor cortex) exhibit approximately 40,000 neurons per mm2 of cortical surface area whereas more caudally selected regions exhibit up to twice as many neurons, ranging between 60,000–80,000 neurons per mm2 of cortical surface area. One noticeable feature is that the increase in neurons across the cat cortex favors the caudo-medial pole. This is in contrast to what is observed in primates where variation in neuron numbers favors the entire caudal pole of the cortex (Collins et al., 2010, Cahalane et al., 2012). Both the new data compiled for manatees and cats demonstrate that the variation in neurons per unit of cortical surface area is intermediate between the rodents and primate samples though they resemble more rodents than primates.
Figure 6.
A: Beaulieu and Colonnier, 1989 estimated neuron numbers per mm2 of cortical surface area in various cortical areas in the cat. The relative position of neuron numbers per mm2 of cortical surface area are represented across the rostral to caudal and medial to lateral axis of the cat cortex. Neuron numbers are highest in the caudo-medial region (i.e., primary visual cortex) and lowest in rostral regions (i.e., motor cortex).
Methodological assessments and counter claims
Our studies have been designed to obtain neuron counts across the isocortex, identified for laminar position and corrected for section curvature and stereology, to relate evolutionary patterns of cytoarchitecture in the cortex within and between species to the developmental mechanisms that might produce them. Of particular interest is the gradient of neurogenesis duration and other maturational features across the dorsal cortex and its possible linkage to the rostral to caudal increase in neuron numbers/unit column, that most amplified in the upper cortical layers (Cahalane et al, 2014). Cingulate, entorhinal, olfactory and all other allocortex do not participate in either the developmental maturational gradient, or the gradient in neural numbers in adulthood (note the falloff in neuron number per column occasionally observed in the extreme caudo-medial pole (Fig. 5 and Charvet et al, 2015) when it has been unclear which class of cortex the region might be assigned to.
Recent studies using isotropic fractionator in the elephant (Herculano-Houzel et al, 2014) and the pig, kudu, springbok and giraffe (Artiodactyla; Kazu et al., 2014) have concluded that neuron numbers do not vary significantly across the isocortex in these mammals (Kazu et al., 2014). The assumption that NeuN, which is used to label neurons would label the same populations of neurons equivalently across a broad range of mammals has not been validated. Moreover, the methodology of these studies prohibits any conclusion about the neuron number gradients of the isocortical area in question – the coronally-amassed samples do not contain isocortex alone but contain major varying components of various allocortex (olfactory and subicular cortices as well as some basal forebrain cell groups), only omitting hippocampus. The relative contribution of allocortex to cortex neuron numbers varies with coronal position. The collated coronal section counts are likely misaligned to variable degrees with the developmental rostrolateral- to-caudomedial gradient of interest depending on brain size. Laminar contributions cannot be distinguished, further diluting the signal from the superficial layers. Remarkably, even with these handicaps, the rostral-to-caudal gradient in neuron number can be seen for four of the six species, with the greatest densities of neurons in the posterior samples (Fig. 10, Kazu et al., 2014), but the methodological limitations of these studies prohibit their use as positive or negative evidence.
The lissencephaly of manatees
The manatee cortex is peculiar in that it is large and lissencephalic (O'Shea and Reep, 1990; Sun and Hevner, 2014). This is unlike the cortices of many big-brained mammals, which are generally gyrencephalic (Van Essen, 1997; Martinez-Cerdeno et al., 2006; Kriegstein et al., 2006; Lewitus et al., 2013; Zilles et al., 2013). Our findings demonstrate that gyrencephaly coincides with the relative expansion of upper layer neurons relative to lower layer neurons (Fig. 4). That is, New World monkeys (e.g., capuchin; which are gyrencephalic) exhibit proportionately more upper layer neurons than the lissencephalic manatees (Fig. 4; Charvet et al., 2015).
In gyrencephaly, the outer cortical surface area is generally expanded relative to the inner cortical surface, which should accommodate relatively more upper layer neurons relative to lower layer neurons. The finding of a relative expansion of upper layer neurons in gyrencephalic primates relative to the lissencephalic manatee cortex supports the notion that gyrencephaly may coincide with an expansion of upper layer neurons relative to upper layer neurons (Fig. 4). Whether gyrencephaly coincides with an expansion of upper layer neurons across a broad range of mammalian species remains to be investigated.
The seemingly disparate hypotheses concerning the evolution of gyrencephaly may be intrinsically linked. That is, alterations in neurogenetic schedules coincide with evolutionary changes in the relative proportion of different progenitor cells (e.g., SVZ) and the relative expansion of late generated neurons (i.e., upper layer neurons; Finlay and Darlington, 1995; Cahalane et al., 2014; Charvet and Finlay, 2014). Heterochronic changes in neurogenesis duration might entail changes in progenitor cell populations in development as well as the disproportionate increase in upper layer neurons and concomitant changes in connectivity patterns and gyrencephaly in adulthood.
Evolutionary changes in gradients in neuron numbers
Our analysis suggests that the manatee and the cat follow the hypothesized mammalian pattern derived from rodents and primates, with higher neurons per unit of cortical surface area in caudal than rostral cortex (Fig. 5). The gradient of neuron number per unit of cortical surface area appears to be steeper in primates than manatees, but the limited sample of species and individuals prevents much speculation on the mechanistic source of the difference.
The “grade shift” in cortical numbers with respect to whole brain volume in manatees and rodents has at least two possible sources. Primates and carnivores, including several marine mammals, allocate relatively more neural mass to isocortex and less to multiple limbic and olfactory structures (Finlay and Darlington 1995; Reep et al., 2007) by delaying and extending cortical neurogenesis with respect to other mammalian taxa (Workman et al., 2013). Additionally and likely independently, the density of neurons in the primate cortex is higher than in rodents and other groups, and the allometry of change in density in primates with cortical volume is close to one, while the density in larger rodent brains is reduced compared to smaller ones (Herculano-Houzel et al., 2011; Charvet et al., 2015).
Useful species for further quantification of the detail of isocortical neuron number topography may be relatively easy to locate, as large domesticated mammals (goats, sheep, pigs, cows and horses) would provide a range of brain sizes, some quite large. These species provide a further benefit, as details of their gestation and relative rate of growth have been reasonably well studied. The alterations in rate and relative timing of neural development they exhibit should provide a further window into the theme and variations of the mammalian cortex.
Acknowledgments
Supported by: WR Kenan Jr. Chair Research Support to BLF and NIH fellowship F32HD067011 to CJC
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Author Contributions
All authors contributed substantially to the manuscript. Christine Charvet formulated ideas, gathered and analyzed data, wrote the paper and prepared figures; Roger Reep prepared the histological material, provided expertise on manatee anatomy and edited the paper; Barbara Finlay helped formulate ideas, organized the basic plan, wrote sections and edited the paper.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Directions in neurogenetic gradients and patterns of anatomical connections in the telencephalon. Prog Neurobiol. 1987;29:57–106. doi: 10.1016/0301-0082(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical development. New York: Raven Press; 1991. [Google Scholar]
- Beaulieu C, Colonnier M. Number of neurons in individual laminae of areas 3B:4 gamma, and 6a alpha of the cat cerebral cortex: a comparison with major visual areas. J Comp Neurol. 1989;279:228–234. doi: 10.1002/cne.902790206. [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- Cahalane DJ, Charvet CJ, Finlay BL. Systematic, balancing gradients in neuron density and number across the primate isocortex. Front Neuroanat. 2012;6:28. doi: 10.3389/fnana.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalane DJ, Charvet CJ, Finlay BL. Modeling local and cross-species neuron number variations in the cerebral cortex as arising from a common mechanism. Proc Natl Acad Sci U S A. 2014;111:17642–17647. doi: 10.1073/pnas.1409271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC. Correlates and possible mechanisms of neocortical enlargment and diversification in mammals. Int J Comp Psychol. 2004;17:71–91. [Google Scholar]
- Catania KC, Kaas JH. Organization of the somatosensory cortex of the star-nosed mole. J Comp Neurol. 1995;351:549–567. doi: 10.1002/cne.903510406. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Nowakowski RS, Bhide PG. Neocortical neurogenesis: morphogenetic gradients and beyond. Trends Neurosci. 2009;32:443–450. doi: 10.1016/j.tins.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Cahalane DJ, Finlay BL. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex. 2015;25:147–160. doi: 10.1093/cercor/bht214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvet CJ, Finlay BL. Evo-devo and the primate isocortex: The central organizing role of intrinsic gradients of neurogenesis. Brain Behav Evol. 2014;84:81–92. doi: 10.1159/000365181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci USA. 2010;107:15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington RB, Dunlop SA, Finlay BL. Neural development in metatherian and eutherian mammals: variation and constraint. J Comp Neurol. 1999;411:359–368. [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Slattery M. Local differences in the amount of early cell death in neocortex predict adult local specializations. Science. 1983;219:1349–1351. doi: 10.1126/science.6828866. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Charvet CJ, Bastille I, Cheung DT, Muniz JA, de Lima Silveira LC. Scaling the primate lateral geniculate nucleus: niche and neurodevelopment in the regulation of magnocellular and parvocellular cell number and nucleus volume. J Comp Neurol. 2014;522:1839–1857. doi: 10.1002/cne.23505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Ribeiro P, Campos L, Valotta da Silva A, Torres LB, Catania KC, Kaas JH. Updated neuronal scaling rules for the brains of Glires (rodents/lagomorphs) Brain Behav Evol. 2011;78:302–314. doi: 10.1159/000330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz SC, Zatorre RJ. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron. 2012;76:486–502. doi: 10.1016/j.neuron.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Hof PR, Chanis R, Marino L. Cortical complexity in cetacean brains. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1142–1152. doi: 10.1002/ar.a.20258. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Neocortex in early mammals and its subsequent variations. Ann NY Acad Sci. 2011;1225:28–36. doi: 10.1111/j.1749-6632.2011.05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaskan PM, Franco EC, Yamada ES, Silveira LC, Darlington RB, Finlay BL. Peripheral variability and central constancy in mammalian visual system evolution. Proc Biol Sci. 2005;272:91–100. doi: 10.1098/rspb.2004.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazu RS, Maldonado J, Mota B, Manger PR, Herculano-Houzel S. Cellular scaling rules for the brain of Artiodactyla include a highly folded cortex with few neurons. Front Neuroanat. 2014;12:128. doi: 10.3389/fnana.2014.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Taber EN, Leigland LA, Knutsen AK, Bayly PV. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb Cortex. 2009;19:2916–2929. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L, Campi KL, Cooke DF. All rodents are not the same: A modern synthesis of cortical organization. Brain Behav Evol. 2011;78:51–93. doi: 10.1159/000327320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Seelke AMH. Cortical evolution in mammals: The bane and beauty of phenotypic variability. Proc Natl Acad Sci USA. 2012;109:10647–10654. doi: 10.1073/pnas.1201891109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus E, Kelava I, Huttner WB. Conical expansion of the outer subventricular zone and the role of neocortical folding in evolution and development. Front Hum Neurosci. 2013;7:424. doi: 10.3389/fnhum.2013.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CD, Reep RL. Manatee cerebral cortex: cytoarchitecture of the caudal region in Trichechus manatus latirostris. Brain Behav Evol. 1995;45:1–18. doi: 10.1159/000113381. [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeño V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–i161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- McSherry GM. Mapping of cortical histogenesis in the ferret. J Embryol Exp Morphol. 1984;81:239–252. [PubMed] [Google Scholar]
- Miller JA, Ding S-L, Sunkin SM, Smith KA, Ng L, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Jacobs MS, Galaburda A. Conservative features of neocortical evolution in the dolphin brain. Brain, Behavior and Evolution. 1985;26:176–1184. doi: 10.1159/000118774. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masteron RB. Descending pathways to the spinal cord, IV: Some factors related to the amount of cortex devoted to the corticospinal tract. J Comp Neurol. 1990;296:584–597. doi: 10.1002/cne.902960406. [DOI] [PubMed] [Google Scholar]
- O'Shea TJ, Reep RL. Encephalization quotients and life-history traits in the Sirenia. J Mammal. 1990;71:534–543. [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Pre- and post-developmental neurogenesis in primates. Clin Neurosci Res. 2002;2:29–39. [Google Scholar]
- Reep RL, Finlay BL, Darlington RB. The limbic system in Mammalian brain evolution. Brain Behav Evol. 2007;70:57–70. doi: 10.1159/000101491. [DOI] [PubMed] [Google Scholar]
- Reep RL, Johnson JI, Switzer RC, Welker WI. Manatee cerebral cortex: cytoarchitecture of the frontal region in Trichechus manatus latirostris. Brain Behav Evol. 1989;34:365–386. doi: 10.1159/000116523. [DOI] [PubMed] [Google Scholar]
- Reep RL, O'Shea TJ. Regional brain morphometry and lissencephaly in the Sirenia. Brain Behav Evol. 1990;35:185–194. doi: 10.1159/000115866. [DOI] [PubMed] [Google Scholar]
- Sanderson KJ, Weller WL. Gradients of neurogenesis in possum neocortex. Dev Brain Res. 1990;55:269–274. doi: 10.1016/0165-3806(90)90208-g. [DOI] [PubMed] [Google Scholar]
- Suter B, Nowakowski RS, Bhide PG, Caviness VS. Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. J Neurosci. 2007;27:10777–10784. doi: 10.1523/JNEUROSCI.3091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Hevner RT. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci. 2014;15:217–232. doi: 10.1038/nrn3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Aspects of phylogenetic variability in neocortical intrinsic organization. In: Finlay BL, Innocenti C, Scheich H, editors. The Neocortex: Ontogeny and Phylogeny. New York: Plenum; 1990. pp. 87–102. [Google Scholar]
- Van der Loos H, Welker E. Development and plasticity of somatosensory brain maps. In: Rowe M, Willis JWD, editors. Development, Organization, and Processing in Somatosensory Pathways; Neurology and Neurobiology. New York: Alan R. Liss; 1985. pp. 53–67. [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Williams RW, von Bartheld CS, Rosen GD. Counting cells in sectioned material: a suite of techniques, tools, and tips. Curr Protoc Neurosci. 2003;Chapter 1(Unit 1.11) doi: 10.1002/0471142301.ns0111s24. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36:275–284. doi: 10.1016/j.tins.2013.01.006. [DOI] [PubMed] [Google Scholar]