Summary
In the last five years, childhood tuberculosis (TB) has received increasing attention from international organisations, national tuberculosis programmes, and academics. For the first time, a number of different groups are developing techniques to estimate the burden of childhood TB. We review the challenges in diagnosing TB in children and the reasons cases in children can go unreported. We discuss the importance of an accurate understanding of burden for identifying problems in programme delivery, targeting interventions, monitoring trends, setting targets, allocating resources appropriately and providing strong advocacy. We briefly review the estimates produced by new analytical methods, outline the reasons for recent improvements in our understanding, and potential future directions. We conclude that while innovation, collaboration and better data have improved our understanding of childhood TB burden, it remains substantially incomplete.
Keywords: Estimation, Burden, TB, Paediatric
Introduction
Childhood tuberculosis (TB) was neglected for many years by the international community. This included a lack of interest from international agencies, national TB programmes (NTPs), clinicians, academics, advocates and funders. In March 2011 a meeting was convened in Stockholm, Sweden, to discuss childhood TB.1 Over 110 participants attended representing a wide variety of stakeholders and the group discussed the challenges in addressing childhood TB, as well as identifying key advocacy areas for development. The meeting resulted in a ‘Call to Action for Childhood TB’, which was endorsed by over 800 individuals and organisations in nearly 100 countries. Since then, interest in childhood TB has increased, resulting in greater visibility, funding, research and advocacy. In 2012 the World Health Organization (WHO) published their first estimate of the number of children that develop TB each year;2 estimates are now reported annually, and the methodology used continues to evolve. In 2013, the WHO, in collaboration with other organisations such as The International Union Against Tuberculosis and Lung Disease (The Union) and United Nations Children’s Fund (UNICEF), published the International Roadmap for Childhood Tuberculosis.3 As a critical first step in moving forward, the Roadmap highlighted the need to “know your epidemic”. Also in 2013, the WHO and TB Alliance (TBA) organised a global consultation to define and prioritise data gaps and analytical methods relevant to our understanding of childhood TB burden. This consultation shaped collaborations between relevant stakeholders and spurred the development of complementary analytical methods.4 This article discusses some of the challenges in estimating the burden of childhood TB, describes the importance of robust estimates, considers the varied techniques used to arrive at estimates and discusses future directions.
Challenges to estimating the burden of childhood tuberculosis
In many settings, and particularly where TB is common, very few TB cases in children are bacteriologically confirmed for a number of reasons. It can be challenging to obtain samples from young children for laboratory diagnosis; the paucibacillary nature of disease in many children means that the yield from bacteriological techniques such as smear microscopy is often low,5,6 and laboratory diagnosis with culture or Xpert is usually not available in facilities where children present. Diagnosis therefore often relies upon clinical assessment supported by diagnostic tools (e.g. chest X-ray) that have significant acknowledged limitations of specificity and sensitivity.7,8 In addition to the diagnostic uncertainties, a major challenge for estimating burden is under-reporting. Until recently, NTPs of most TB endemic countries were required to only report sputum smear-positive cases and would report children in a broad age category of 0–14 years. This led to the perception (or misperception) that the burden in children was low. NTPs are now requested to report all TB cases and by two age bands for children (0–4 years, 5–14 years). However, the NTP can only report data for those children that are registered with the NTP at the time of diagnosis. Unfortunately, a large but unknown number of children are treated for TB but are not registered with the NTP.9,10
The challenges of confirming diagnosis are greatest in infants and young children (<5 years age); importantly, this age group also has an increased risk of severe disease and TB-related mortality. Although uncomplicated lymph node disease is common in children, a substantial proportion also develop severe forms of disseminated TB, such as miliary TB or TB meningitis,11 that are associated with significant morbidity and mortality,12,13 or present with concomitant severe pneumonia or malnutrition.14 Finally, from a public health viewpoint, it is important to recognise that children can transmit TB to contacts, especially older children and adolescents who often develop adult-type or cavitary TB that is highly infectious.15–19
What is meant by disease burden?
The term disease burden describes the number and the associated rate of individuals in a community with a particular condition and its consequences for morbidity, disability and mortality. Traditionally, in the field of TB, incidence, prevalence and mortality have all been estimated and reported as measures of disease burden. The three measures are related but each require a different estimation approach and tell us different things about the epidemic. Incidence refers to the number of individuals who develop TB each year; prevalence the number at a given time point who have TB; and mortality the number who die each year with TB thought to be the primary cause. To take into account the size of the population in reference, and to compare across communities and with other diseases, the corresponding incidence, prevalence and mortality rates are also calculated.
Since 2012 WHO has estimated the number of HIV-negative children dying from TB each year and the methodology used is described in detail elsewhere.20,21 They estimated that 80,000 HIV-negative children (range 64,000–97,000) died of TB in 2013, equivalent to about 7% of the total number of TB deaths in HIV-negative individuals globally.21 The prevalence of childhood TB has not yet been estimated. In this article, we will describe the ongoing work being done to estimate paediatric TB incidence as a case study for how a successful collaboration between institutions and academic groups can catalyse improvement in analytical methods and estimates. Within the article we consider children as those aged less than fifteen years.
Importance of estimates
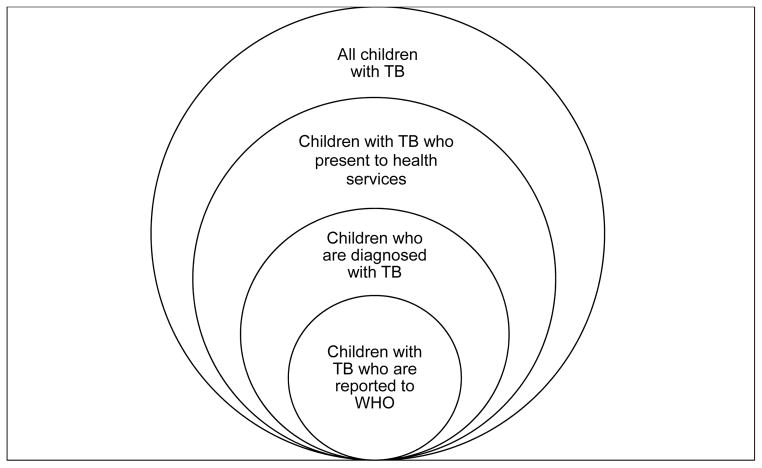
Accurate and reliable childhood TB incidence estimates, when compared with the number of reported and treated cases from national surveillance systems, quantify the degree to which children with TB are not being found, diagnosed or treated. This may help to identify the weak links in the cascade from symptoms to presentation to diagnosis to treatment to official notification (Figure 1). Investigation of these links may then suggest actions to improve case detection and reporting. Discrepancies in notifications or quality of detection and reporting among epidemiologically similar settings may alert programmes to existing problems and provide new insights into how these problems may be resolved. Specific programmatic indices may also give a crude indication of overall childhood TB management (Table 1).
Figure 1.
The cascade from symptoms to reporting in children with tuberculosis
Table 1.
Programmatic indicators that may give an indication of how well childhood tuberculosis is being diagnosed and reported
| Indicator | Approximate expected value1 | Likely interpretation if: | |
|---|---|---|---|
| Too high | Too low | ||
| Proportion of overall burden found in children | 5%–20%, increasing with overall TB incidence | Over-diagnosis of childhood TB | Under-diagnosis of childhood TB |
| Proportion of treated paediatric cases with a confirmed diagnosis | 20–30%, increasing with age and resources | Not enough children treated on clinical grounds | Not enough effort made to confirm the diagnosis |
| Proportion of paediatric cases that are sputum smear-positive2 | 10% in 0–14 age group as a whole | Not enough children treated on clinical grounds | Not enough effort made to confirm the diagnosis |
| Proportion paediatric cases that are under-5 years | Slightly over 50% | Too many young children being treated clinically | Only older children with ‘classic’ symptoms being treated or only children with confirmed disease treated |
| Proportion of paediatric cases that are EPTB | 10% in 0–14 age group as a whole; 25% in 0–4 age group | Children with various clinical characteristics (such as cervical lymphadenopathy) being diagnosed with TB when many do not have TB | Only confirmed cases (which are frequently PTB) classified as TB |
TB: tuberculosis; EPTB: extrapulmonary tuberculosis
These expected values provide a rule-of-thumb or guide only. Enormous variability in these parameters has been described in studies across different settings
Since 2013, cases are now reported to WHO according to whether bacteriologically confirmed, which includes confirmation by smear microscopy, culture and GeneXpert
As children can only have been infected in the few years since birth, and as most progression is within 12 months,22 TB in children represents recent transmission. Childhood TB therefore also provides insight into which strains of M. tuberculosis are currently circulating in a community (including drug-resistant strains). TB incidence in children reflects local transmission rates, and therefore is a potential indicator for TB control more generally.23 Accurate baseline numbers and trends over time allow appropriate national and global targets to be set, and assessment of whether they are met.
Robust estimates help inform the service planning, resource allocation, and the targeting of interventions to where they are needed most. In addition they permit an appropriate assessment of the potential market for new diagnostics, vaccines and drugs. Industry, academic funding organisations, development agencies, non-governmental organisations and NTPs, all want to make rational investment decisions, and burden quantification is therefore an essential component in engaging with them. Further, for purposes of advocacy, knowing the burden of disease is a tool to raise the profile of these vulnerable children and motivate better diagnostics, treatments, funding, rights, support or recognition. The importance of accurate estimates is summarised in Table 2.
Table 2.
Reasons for the importance of more accurate estimates of the burden of childhood tuberculosis
| Needs for better estimates | Rationale for better estimates |
|---|---|
| Political engagement and political will | Accurate data of the burden of tuberculosis in children are required to engage the leadership and support of the tuberculosis control sector, the child health sector, government health ministries, advocacy and the wider community. |
| Inform situational analysis and identify gaps | It is critical to “know your epidemic” in order to identify current gaps and challenges as well as priorities for implementation to address child tuberculosis. |
| Child TB is an indicator for surveillance of recent transmission | Accurate data of tuberculosis in young children monitored over time could be an important tuberculosis control indicator as a sensitive indicator of recent transmission and an early indicator of transmission “hot-spots.” |
| Resource allocation for health systems and NTP | The numbers of children with drug-sensitive and drug-resistant tuberculosis will inform health service and human resource requirements to ensure effective programmatic management. |
| Procurement needs of diagnostics and therapeutics | The numbers of children with drug-sensitive and drug-resistant tuberculosis will inform the needs and sufficient procurement of diagnostic tools and anti-tuberculosis medication, including medication suitable for young children. |
| Engage the Maternal and Child Health sector | Data that show the importance of tuberculosis in the context of child morbidity and mortality are required to engage the leadership and support of the maternal and child health sector and government, especially as most countries include child health as a major national priority. |
| Advocacy and engagement of civil society | Accurate data of the burden of tuberculosis with direct and indirect consequences on child health are extremely valuable for advocacy groups, national champions and civil society to highlight the need for action. |
| Monitoring and evaluation tool | Accurate baseline data are required to monitor and evaluate implementation of activities aiming to improve the detection, prevention and management of child tuberculosis. |
| Identification of needs and improves quality of research | Accurate data would greatly strengthen the many opportunities for operational research in children as well as the quality of clinical trials that evaluate novel diagnostics or therapeutic regimens. |
| Potential for investment in novel diagnostics and therapeutics | The potential “size of the market” is one important factor that informs investment in research and development of novel diagnostics and therapeutics. |
TB: tuberculosis; NTP: National TB Programme
Methodology for estimation of childhood tuberculosis incidence
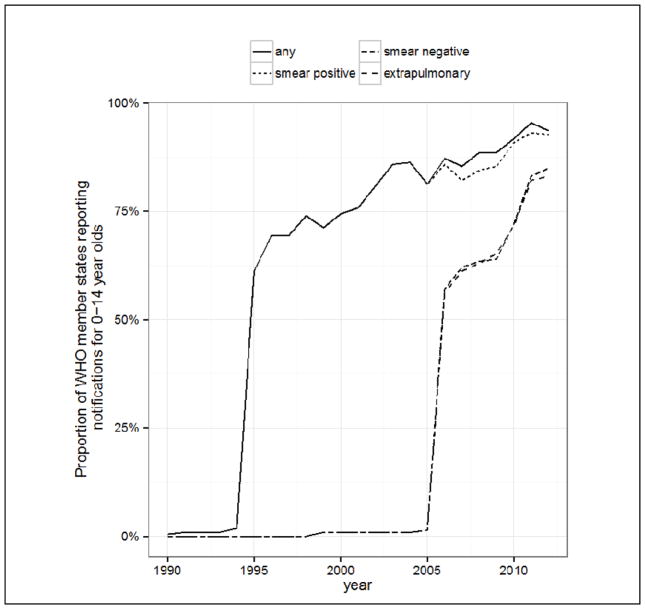
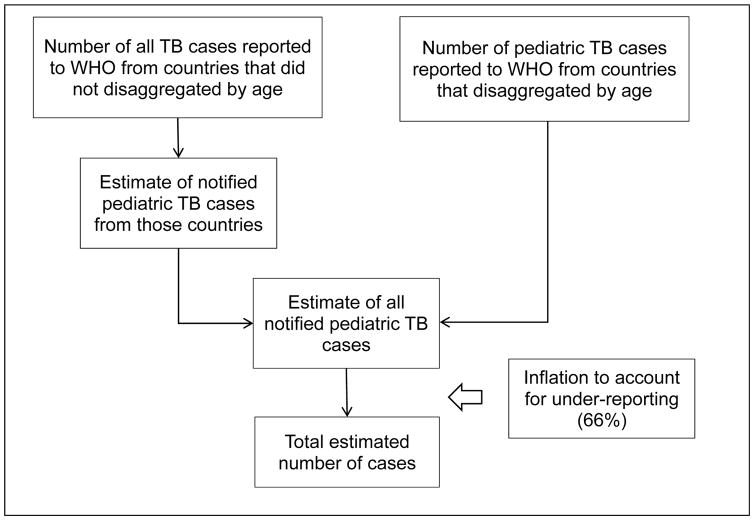
Until recently, the WHO did not publish separate childhood TB estimates, partly due to difficulties in interpreting notification data for children, and partly because many countries did not then disaggregate notifications by age. Over the last ten years the number of countries reporting disaggregated data has sharply increased (Figure 2). The WHO published its first official estimate in 2012.2 As a starting point, they followed a two-step procedure (Figure 3): first estimating paediatric notifications for countries that did not disaggregate by age, and secondly estimating the underlying incidence through dividing notifications by a case detection ratio (CDR). This procedure gave a global childhood TB incidence estimate of 490,000 (range 470,000 – 510,000), equivalent to about 6% of the total number of 8.7 million incident cases in 2011.2 Acknowledged limitations included the assumption that the paediatric CDR was the same as the CDR for adults (66%, range 64–69%); the assumption of no misclassification of TB in the paediatric notifications; and the assumption that the proportion of TB burden among children was the same whether countries disaggregated notifications by age or not. Commentators were concerned that the assumption of an equal CDR for adults and children was at odds with observational evidence of under-reporting and under-diagnosis,9,10 and would lead to an underestimated paediatric incidence estimate.
Figure 2.
Improvements in age-disaggregated case reporting between 1990 and 2012
Figure 3. Methodology employed by the World Health Organization to estimate the incidence of tuberculosis in children.
TB: tuberculosis; WHO: World Health Organization
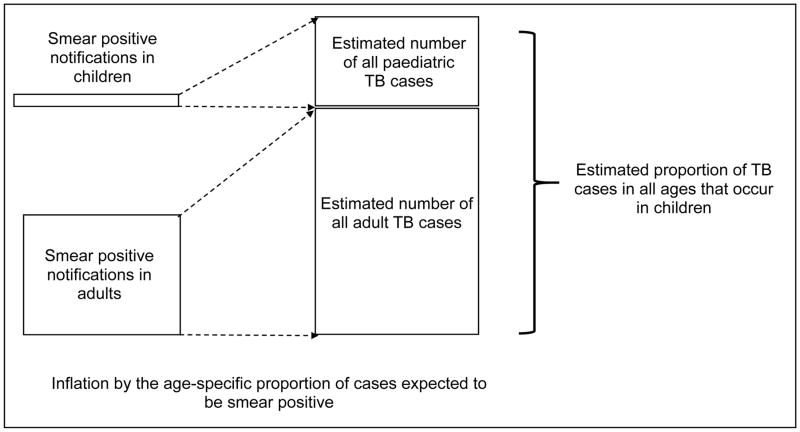
More recently, other groups have used complementary methods to estimate the TB burden in children. Jenkins and colleagues24 followed a different procedure based on using the expected proportion of smear positive cases in each age group25 to obtain an adjusted proportion of TB incidence among children (Figure 4). A regression of the proportion of TB in children against total incidence26 was then used to predict this proportion in countries not disaggregating notifications by age. Finally, these country-level proportions were multiplied by the WHO total country TB estimates and aggregated to predict that 999,792 (95% confidence interval: 937,877–1,055,414) children developed TB in 2010. Limitations of this approach include the shortcomings of notification data and challenges in estimating TB incidence, which represent sources of error and uncertainty that are not captured in the confidence interval of this paediatric TB estimate. Furthermore, the assumption that the age-specific proportions of TB cases that are smear-positive from previous studies25 are representative of the present day proportions across all countries requires further review; an effort that is current in progress. Going forward, as use of smear microscopy decreases and countries scale-up use of other diagnostic tools, this estimation method, which is reliant on smear positive notifications, will require adaptation.
Figure 4. Methodology employed by Jenkins et al. in the estimation of tuberculosis in children.
TB: tuberculosis
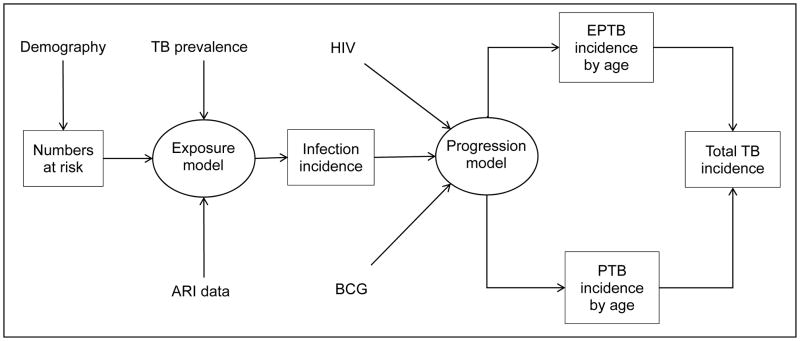
Dodd and colleagues used a mathematical modelling approach to produce an estimate independent of paediatric notifications,27 initially focussing on the twenty-two high-burden countries in 2010. Demographic data and WHO TB prevalence estimates were used to predict the incidence of TB infection in children. An age-dependent model of progression to extra-pulmonary TB and pulmonary TB was then used to estimate the incidence of disease, taking into account country-level BCG vaccination coverage and HIV prevalence (Figure 5). This resulted in the following global estimates for 2013: incidence of TB infection 9.0 (inter-quartile range [IQR]: 6.9–11.7) million; prevalence of latent infection 62.9 (IQR: 48.9–80.6) million; and incidence of TB disease of median 827,000 (IQR: 549,000–1,245,000). Limitations include shortcomings in adult TB prevalence estimates, uncertainty around the impact of BCG and HIV, and the applicability of data from the literature to present-day risk of disease progression.
Figure 5. Methodology employed by Dodd et al. in the estimation of tuberculosis in children.
TB: tuberculosis; ARI: annual risk of infection; HIV: human immunodeficiency virus; BCG: Bacillus Calmette–Guérin; EPTB: extra-pulmonary tuberculosis; PTB: pulmonary tuberculosis
The Institute of Health Metrics and Evaluation (IHME) also produce estimates for childhood TB,28 as part of a larger Bayesian approach addressing multiple conditions. The group estimated that in 2013, 150,000 children developed TB.
In 2014 WHO used an ensemble approach to estimate paediatric TB incidence,21 producing a weighted average of their notification-based estimate and the estimate derived from the mathematical model by Dodd et al.27 The resulting estimate of global TB incidence among children in 2013 was 550,000 (range 470,000–640,000), equivalent to about 6% of the total number of 9.0 million incident cases.
Jenkins and colleagues also estimated the burden of multidrug-resistant (MDR) TB in children. Their systematic review evaluated a linear association between the proportion of MDR-TB in children and treatment naïve adults. Combined with their estimates of childhood TB incidence, this implied 31,948 (IQR: 25,594–38,663) children developed MDR-TB in 2010.24 In a subsequent study, Yuen and colleagues undertook a systematic review of the proportion of paediatric cases that were isoniazid-resistant in 2010.29 The group estimated that 12.1% (95%CI: 9.8–14.8%) of all children with TB have isoniazid-resistant disease, resulting in 120,872 (95%CI: 96,628–149,059) incident cases in 2010.30
The changing landscape of disease burden estimation
Estimates for childhood TB burden are improving for several reasons. First, a number of different, complementary approaches have been taken. The existence of these disparate methods, and the collaboration between the groups that have developed them, provide an opportunity to scrutinize and understand differences in estimates in order to refine and improve methods. Second, increased training, education and policy changes mean more paediatric cases are being identified, registered and reported; non-bacteriologically confirmed cases are increasingly being entered into registers. Third, the number of countries that disaggregate data by age has increased. Fourth, many countries have developed paediatric TB committees or sub-groups within the NTP and age-specific indicators have been promoted in a number of settings. Fifth, inventory (or capture-recapture) studies to determine the discrepancy between treated cases and reported cases are being conducted in several countries, and will give valuable data in countries with a large private heath-provider sector. Finally, children who died of TB in hospital were frequently not registered with NTPs; this is improving.
Scientific developments in diagnostics may increase the number of children who are diagnosed, treated and reported to NTPs. Recently, Xpert MTB/RIF was evaluated in children and was found to be more sensitive than sputum smear microscopy.6 An RNA gene expression study has identified a unique ‘signature’ in the immune response that, if converted into a point-of-care test, could dramatically improve our ability to diagnose TB in children.31
In 2013, TBA was awarded USD16.7 million from UNITAID to develop child-friendly formulations for TB drugs for children.32 Part of this project is to quantify the potential market for first- and second-line TB drugs for children, in order to engage with pharmaceutical companies. This funding, as well as providing estimates of market, has funded additional work into estimating and describing the burden of TB in children.
NTP reviews have been one of the motivating factors used to drive through change in national TB policy to identify, treat and report childhood TB. In many countries, funding from the Global Fund is contingent on demonstrating responses to suggestions made in NTP reviews. Increasingly there is a paediatric TB specialist on the team that conducts these reviews and evaluates paediatric-specific indicators. The specialist then provides suggestions and targets specifically for childhood TB.
Future Perspectives
Increased use of modelling and better data on which to build models will improve the accuracy of new estimates. Comparison and synthesis of modelling methodology will also help. Assessing these estimates over time also allows an appreciation of changing trends. Ideally, further disaggregation of reported data would take place so that children are reported in five-year age-bands (0–4 years, 5–9 years, and 10–14 years). In addition, the inclusion of children into appropriately designed prevalence surveys would allow a better grasp of primary data, and lead to better-validated models. Children have not been included in prevalence surveys due to a number of logistical, financial and ethical challenges.20,33 However, it may be possible to include children, using a modified protocol, in certain sentinel sites. As estimates become more accurate and modelling becomes more sophisticated, it will be possible to model the impact of interventions on the burden of childhood TB. Sound estimates of both the cost and cost-effectiveness of these interventions will provide information and powerful motivation to policy-makers and politicians.
Conclusion
Collaboration among the WHO, the Union, the Child Health Epidemiology Reference Group (CHERG), IHME, TBA and different academic groups has greatly improved our understanding of the burden of childhood TB in the last couple of years. New and innovative methods are being used to estimate burden and improvements in reporting are being seen. There has been increased investment and significant progresses in scientific research. However, we are still some way from a complete understanding of which children get TB and how best to find them.
Acknowledgments
HEJ was funded by US National Institutes of Health US NIH K01AI102944. The content of the article is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Allergy and Infectious Disease or the Office of the Director, NIH. The funder had no role in the decision to publish this manuscript.
References
- 1.Sandgren A, Cuevas LE, Dara M, et al. Childhood tuberculosis: progress requires advocacy strategy now. Eur Respir J. 2012 doi: 10.1183/09031936.00187711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Tuberculosis Report. World Health Organization; Geneva, Switzerland: 2012. [accessed 20 April 2015]. WHO/HTM/TB/2012.6 Available at: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf. [Google Scholar]
- 3.Roadmap for Childhood Tuberculosis. World Health Organization; Geneva, Switzerland: [accessed October 2013]. 2013. Available at: http://apps.who.int/iris/bitstream/10665/89506/1/9789241506137_eng.pdf. [Google Scholar]
- 4.TB Alliance. [accessed 25 May 2015];Global Consultation on Paediatric Tuberculosis: Disease Burden Estimation and Quantification of its Drug Market. 2013 Available at: http://www.tballiance.org/downloads/children/response/Global-Consult-on-Ped-TB-Meeting-Summary-12NOV13%20FINAL.pdf.
- 5.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365(9454):130–134. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 6.Detjen AK, DiNardo AR, Leyden J, et al. Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in children: a systematic review and meta-analysis. The lancet. Respiratory medicine. 2015 doi: 10.1016/S2213-2600(15)00095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6(12):1038–1045. [PubMed] [Google Scholar]
- 8.Guidance for national tuberculosis programme on the management of tuberculosis in children. 2. World Health Organization; Geneva, Switzerland: [accessed 29 May 2014]. 2014. Available at: http://apps.who.int/iris/bitstream/10665/112360/1/9789241548748_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 9.Lestari T, Probandari A, Hurtig AK, Utarini A. High caseload of childhood tuberculosis in hospitals on Java Island, Indonesia: a cross sectional study. BMC Public Health. 2011;11:784. doi: 10.1186/1471-2458-11-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Preez K, Schaaf HS, Dunbar R, et al. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action. 2011;1(1):19–24. doi: 10.5588/pha.11.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis. 2006;10(7):732–738. [PubMed] [Google Scholar]
- 12.Chaing SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infec Dis. 2014 doi: 10.1016/S1473-3099(14)70852-7. Published online August 7. [DOI] [PubMed] [Google Scholar]
- 13.Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Detjen AK, Black RE. Importance of tuberculosis control to address child survival. Lancet. 2014;383(9928):1605–1607. doi: 10.1016/S0140-6736(14)60420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliwa JN, Karumbi JM, Marais BJ, Madhi SA, Graham SM. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. The Lancet Respiratory Medicine. 2015;3(3):235–243. doi: 10.1016/S2213-2600(15)00028-4. [DOI] [PubMed] [Google Scholar]
- 15.Cruz AT, Starke JR. A current review of infection control for childhood tuberculosis. Tuberculosis (Edinb) 2011;91(Suppl 1):S11–15. doi: 10.1016/j.tube.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Rabalais G, Adams G, Stover B. PPD skin test conversion in health-care workers after exposure to Mycobacterium tuberculosis infection in infants. Lancet. 1991;338(8770):826. doi: 10.1016/0140-6736(91)90719-6. [DOI] [PubMed] [Google Scholar]
- 17.Cardona M, Bek MD, Mills K, Isaacs D, Alperstein G. Transmission of tuberculosis from a seven-year-old child in a Sydney school. J Paediatr Child Health. 1999;35(4):375–378. doi: 10.1046/j.1440-1754.1999.00385.x. [DOI] [PubMed] [Google Scholar]
- 18.Curtis AB, Ridzon R, Vogel R, et al. Extensive transmission of Mycobacterium tuberculosis from a child. N Engl J Med. 1999;341(20):1491–1495. doi: 10.1056/NEJM199911113412002. [DOI] [PubMed] [Google Scholar]
- 19.Starke JR. Transmission of Mycobacterium tuberculosis to and from children and adolescents. Semin Pediatr Infect Dis. 2001;12(2):115–123. [Google Scholar]
- 20.Sismanidis C, Glaziou P, Grzemska M, Floyd K, Raviglione M. Global Epidemiology of Childhood Tuberculosis. In: Starke JR, Donald PR, editors. Book chapter in Tuberculosis in Children and Adolescents. 2015. [Google Scholar]
- 21.Global Tuberculosis Report. World Health Organization; Geneva, Switzerland: 2014. [Google Scholar]
- 22.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 23.Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infect Dis. 2003;3(10):624–632. doi: 10.1016/s1473-3099(03)00771-0. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins HE, Tolman AW, Yuen CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383(9928):1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis. 1990;65(1):6–24. [PubMed] [Google Scholar]
- 26.Donald PR. Childhood tuberculosis: out of control? Curr Opin Pulm Med. 2002;8(3):178–182. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. The Lancet Global Health. 2014;2(8):e453–459. doi: 10.1016/S2214-109X(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 28.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen CM, Tolman AW, Cohen T, Parr JB, Keshavjee S, Becerra MC. Isoniazid-resistant tuberculosis in children: a systematic review. Pediatr Infect Dis J. 2013;32(5):e217–226. doi: 10.1097/INF.0b013e3182865409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen CM, Jenkins HE, Rodriguez CA, Keshavjee S, Becerra MC. Global and regional burden of isoniazid-resistant tuberculosis. Pediatrics. 2015 doi: 10.1542/peds.2015-0172. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson ST, Kaforou M, Brent AJ, et al. Diagnosis of childhood tuberculosis and host RNA expression in Africa. N Engl J Med. 2014;370(18):1712–1723. doi: 10.1056/NEJMoa1303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TB Alliance. [accessed October 2013];TB Alliance Receives Grant from UNITAID to Develop Pediatric TB Drugs. 2012 Available at: http://www.tballiance.org/newscenter/view-brief.php?id=1058#sthash.xLlC3Bn0.dpufhttp://www.tballiance.org/newscenter/view-brief.php?id=1058.
- 33.Tuberculosis prevalence surveys: a handbook. World Health Organization; Geneva, Switzerland: [accessed 12 May 2015]. 2010. WHO/HTM/TB/2010.17. Available at: http://whqlibdoc.who.int/publications/2011/9789241548168_eng.pdf?ua=1. [Google Scholar]