Abstract
Lichens are symbiotic organisms that produce various unique chemicals that can be used for pharmaceutical purposes. With the aim of screening new anti-cancer agents that inhibit cancer cell motility, we tested the inhibitory activity of seven lichen species collected from the Romanian Carpathian Mountains against migration and invasion of human lung cancer cells and further investigated the molecular mechanisms underlying their anti-metastatic activity. Among them, Alectoria samentosa, Flavocetraria nivalis, Alectoria ochroleuca, and Usnea florida showed significant inhibitory activity against motility of human lung cancer cells. HPLC results showed that usnic acid is the main compound in these lichens, and (+)-usnic acid showed similar inhibitory activity that crude extract have. Mechanistically, β-catenin-mediated TOPFLASH activity and KITENIN-mediated AP-1 activity were decreased by (+)-usnic acid treatment in a dose-dependent manner. The quantitative real-time PCR data showed that (+)-usnic acid decreased the mRNA level of CD44, Cyclin D1 and c-myc, which are the downstream target genes of both β-catenin/LEF and c-jun/AP-1. Also, Rac1 and RhoA activities were decreased by treatment with (+)-usnic acid. Interestingly, higher inhibitory activity for cell invasion was observed when cells were treated with (+)-usnic acid and cetuximab. These results implied that (+)-usnic acid might have potential activity in inhibition of cancer cell metastasis, and (+)-usnic acid could be used for anti-cancer therapy with a distinct mechanisms of action.
Introduction
Lung cancer is the leading cause of cancer death in developed countries. Due to the lack of efficient treatment for advanced disease, the prognosis of lung cancer is still poor, with less than 15% surviving 5 years after diagnosis [1]. Adjacent invasion and distant metastasis are the major causes of cancer-related death [2]. Therefore a search for inhibitors for cancer cell invasion and migration ability could reveal a new therapy for cancer treatment. Although herbs have been employed in the treatment of cancers for thousands of years, they remain a very important source of biologically active products. The aim of this study was to identify potential therapeutic agents to improve the survival of patients with lung cancer metastasis.
Lichens are symbiotic organisms that produce a large number of bioactive substances over 800 [3], comprising many classes of compounds: amino acid derivatives, sugar alcohols, aliphatic acids, γ-, δ- and macrocyclic lactones, monocyclic aromatic compounds, quinones, chromones, xanthones, dibenzofuranes, depsides, depsidones, depsones, terpenoids, steroids, carotenoids [4] and diphenyl ethers [5, 6]. Slowly growing organisms in low-resource habitats produce higher levels of defense chemicals [7]. Therefore, lichens are a source of unique chemical agents of which some have already been proved to be effective against various cancer in vitro models [8]. Here, the current study examined the inhibitory activity of seven lichen species collected from the Romanian Carpathian Mountains against migration and invasion ability of human lung cancer cells and further investigated the possible molecular mechanisms underlying their anti-metastatic activity to identify potential compounds for novel anti-metastasis agents.
Material and Methods
Preparation of lichen extracts
Lichen specimens used in this study, collected from Romania in 2011, were identified at the Korean Lichen Research Institute (KoLRI), Sunchon National University, Korea. Briefly, thalli of lichen were collected from Romania in 2011 during the field trip in the National Park Călimani (47°07'28.6"N, 25°13'34.8"E) and the Natural Park Bucegi (45°20'21.7"N, 25°27'41.4"E) organized by Dr. Crişan at Babeş-Bolyai University, Cluj-Napoca, Romania [9]. The permit to collect lichen specimens from those locations was issued by the Administration of the National Park Călimani and the Administration of the Natural Park Bucegi, with the approval of the Commission for Protection of Natural Monuments (Romanian Academy). The field studies did not involve any endangered or protected species. The duplicates were deposited into the Korean Lichen and Allied Bioresource Center (KOLABIC) in the Korean Lichen Research Institute (KoLRI), Sunchon National University, Korea. The dried thalli of the lichens were extracted with acetone at room temperature for 48 h. The acetone extracts were then filtered and dried in rotary vacuum evaporator at 45°C. The dry extracts were dissolved in dimethylsulfoxide (DMSO) as 5 mg/ml concentration (1000×) for all experiments. Seven Romanian lichen species and their voucher specimen numbers used in this study were listed in Table 1.
Table 1. Seven Romanian lichen species used in this study.
| Collection No. | Family | Lichen species | Known lichen substances | Reference |
|---|---|---|---|---|
| RO11025 | Parmeliaceae | Alectoria samentosa | (-)-Usnic acid, Physodic acid, 8’-O-ethyl-P-alectoronic acid, Alectosarmentin | [13] |
| RO11045 | Parmeliaceae | Flavocetraria nivalis | (±)- Usnic acid, Isousnic acid, Divaricatic acid, p-Hydroxybenzoic acid, Vanillic acid | [14–17] |
| RO11084 | Parmeliaceae | Alectoria ochroleuca | Diffractaic acid, (-)-Usnic acid, Isousnic acid, Friedelin, Barbatic acid | [16, 18] |
| RO11111 | Parmeliaceae | Bryoria capillaris | Alectorialic acid, Barbatolic acid | [19] |
| RO11166 | Parmeliaceae | Hypogymnia physodes | Atronorin, Physodalic acid, Protocetraric acid, Physodic acid | [18, 19] |
| RO11176 | Parmeliaceae | Usnea florida | (+)-Usnic acid, Barbatic acid, Salazinic acid, Norstic acid, β-Orcinol depsidones, (±)-Thamnolic acid, Stictic acid | [18, 20] |
| RO11209 | Parmeliaceae | Evernia divaricata | Divaricatic acid | [19] |
High performance liquid chromatography (HPLC) analysis of lichen material
Acetone extract of lichen thalli at a concentration of 5 mg/ml were subjected to high performance liquid chromatography (HPLC) analyses (LC-20A; Shimadzu, Kyoto, Japan) on a YMC-Pack ODS-A (150 × 3.9 mm I.D.) reversed-phase column containing fully end-capped C18 material (particle size, 5 μm; pore size, 12 nm). Elution was performed at a flow rate of 1 ml/min under the following conditions before subsequent injection: column temperature, 40°C; solvent system, methanol: water: phosphoric acid (80: 20: 1, v/v/v). Analyses were monitored by a photodiode array detector (SPD-M20A; Shimadzu) with a range of 190~800 nm throughout the HPLC run. Observed peaks were scanned between 190 and 400 nm. The standard used for salazinic acid (tR = 2.27 ± 0.2 min) was isolated from lichen Lobaria pulmonaria. Usnic acid used in our study was purchased from Sigma-Aldrich (St. Louis, USA) (329967-5G). Voucher specimens were deposited in the herbarium of the Lichen & Allied Bioresource Centre at the Korean Lichen Research Institute, Sunchon National University, South Korea.
Liquid chromatography-mass spectrometry (LC-MS) and optical rotation analaysis of lichen material
LC—MS spectra were recorded on a spectrometer with an electrospray ionization source using Agilent 6460 triple Quadrupole LC/MS. The values of optical rotation were measured at 25°C using Jasco P-1010 polarimeter with a sodium lamp, and described as follows: [α]D, T (c (g/100 mL), solvent). Specific rotation of pure (+)-usnic acid (Sigma-Aldrich, St. Louis, USA) is a physical property of at a given wavelength and temperature and can be looked up in literature.
Cell culture
The human lung cancer cells including A549, H460, H1650, and H1975 were cultured in RPMI 1640 culture medium supplemented with 10% fetal bovine serum, 1% Penicillin-Streptomycin solution under a humidified 5% CO2 atmosphere at 37°C.
Wound healing assay
A549 cells were plated at a density of 2.5 × 105 cells/well on 6-well tissue culture plates (Corning, New York, USA) and grown overnight to confluence. Monolayer cells were scratched with a pipette tip to create a wound. The cells were then washed twice with serum-free RPMI 1640 to remove floating cells and incubated in RPMI1640 culture medium supplemented with 2% FBS with 5 μg/ml of the lichen extract or 5 μM usnic acid. Photographs of cells were taken at 0, 24, 48, and 72 h after wounding to measure the width of the wound. For each sample, an average of five wound assays was taken to determine the average rate of migration at a given concentration of acetone extract or usnic acid. Experiments were repeated at least three times.
Invasion assay
Invasion assays were performed in transwell chambers (Corning, New York, USA) with 8μm pore size polycarbonate membrane coated with 1% gelatin. Cells were plated at 2 × 105 cells/well in RPMI1640 containing 0.2% bovine serum albumin in the upper compartment of the chamber with or without 5 μg/ml crude lichen extracts. Then RPMI1640 medium with 10 μg/ml fibronectin was added to the lower chamber to serve as a chemotactic agent. After 48-h incubation, the cells in the upper chamber were fixed with Diff Quik kit (Sysmex, Kobe, Japan). Then the cells inside the chamber were mechanically removed from the membrane with a cotton swab, and the cells adhering to the under-side of the membrane were stained and counted under light microscope (5 fields per chamber). Each invasion assay was repeated in three independent experiments. The results are expressed as the mean number of cells migrating per high-power field.
Reporter assay
HEK293T cells were plated into 24-well plates 12 h before transfection. After transfection of the TOPFLASH or AP-1 reporter plasmid with the respective activator, β-catenin or KITENIN, cells were treated with usnic acid for 48 h and then analyzed using a Dual-Luciferase® reporter assay system (Promega, Madison, WI, USA). The Renilla luciferase reporter plasmid (pRL-TK) was used as the internal control for the transfection efficiency. The experiments were performed in triplicate, and at least three results from independent experiments were included in the analysis. Fold changes were calculated using values normalized to Renilla luciferase activity.
Quantitative real-time PCR
The quantitative real-time PCR was performed as described previously [9]. Briefly, total RNA was isolated from human lung cancer cells by using RNAiso Plus (TaKaRa, Otsu, Shiga 520–2193, Japan) according to the manufacturer’s instructions. Total RNA (1 μg) from each group of treated cells was converted to cDNA using a M-MLV reverse Transcriptase kit (Invitrogen, Carlsbad, USA) and SYBR green (Enzynomics, Seoul, Korea). The primers used for real-time PCR were Cyclin D1 (forward) 5’-ccgtccatgcggaagatc-3’ and (reverse) 5’-gaagacctcctcctcgcact-3’; c-myc (forward) 5’-aatgaaaaggcccccaaggtagttatcc-3’ and (reverse) 5’-gtcgtttccgcaacaagtcctcttc-3’; CD44 (forward) 5’-tgccgctttgcaggtgtat-3’ and (reverse) 5’-ggcctccgtccgagaga-3’; GAPDH (forward) 5’-atcaccatcttccaggagcga-3’ and (reverse) 5’-agttgtcatggatgaccttggc-3’. Real-time PCR reaction and analysis were performed using CFX (Bio-Rad, Hercules, USA).
Affinity Precipitation of Cellular GTPases
The cellular Rac1 and Cdc42 activities were determined using GST-RBD/PBD as previously described [10, 11]. Briefly, the cells were lysed in lysis buffer (50 mM Tris, pH 7.4, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, and protease inhibitor mixture). The lysates were incubated with GST- RBD/PBD beads at RT for 1 h. The beads were then washed four times with washing buffer (50 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, and protease inhibitor mixture). The bound Rac1 and Cdc42 proteins were detected by immunoblotting using a monoclonal antibody against Rac1 (MILLIPORE 05–389) and Cdc42 (SANTA CRUZ SC-87). The relative activity of each GTPase was determined by quantifying each band of GTP-bound GTPase and the total amount of GTPase using Multi-Gauge 3.0, and the values of the GTP-bound bands were normalized to the value of the total amount. All results were determined using three different exposures from at least three independent experiments.
Results
Inhibition of A549 cell motility by lichen extracts
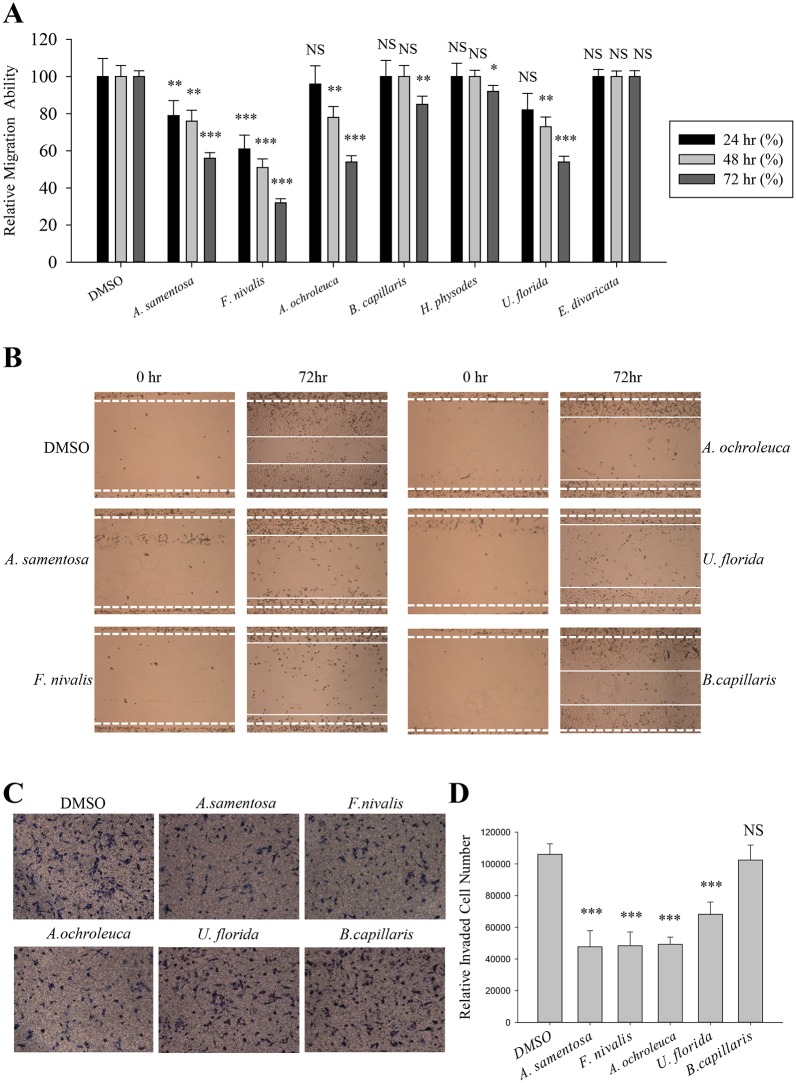
Cell migration plays a crucial role during cancer metastasis. To find the anti-migratory lichen secondary metabolite on human lung cancer cells, wound healing assay was performed among seven acetone extracts of Romanian lichens listed in Table 1. Lichens produce unique polyketide secondary metabolites including depsides, depsidones, dibenzofurans, and depsones; and these hydrophobic compounds were normally extracted with acetone [12]. As shown in Fig 1A, Alectoria samentosa, Flavocetraria nivalis, Alectoria ochroleuca, and Usnea florida inhibited A549 cell migration at a concentration of 5 μg/ml. The length between the edges of the wound at 72 h with these candidates were significantly wider than that in the DMSO-treated group or the non-active sample (Bryoria capillaris). In particular, F. nivalis showed more than 60% inhibitory activity compared to control (Fig 1A and 1B).
Fig 1. Inhibition of A549 cell motility by acetone extracts of lichens.
(A–B) Quantitative analysis of migration assay of A549 cells treated with 5 μg/ml of acetone extracts of Alectoria samentosa, Flavocetraria nivalis, Alectoria ochroleuca, Bryoria capillaris, Hypogymnia physodes, Usnea florida and Evernia divaricata (A), and representative images of migration assay of A549 cells treated with the extracts of A. samentosa, F. nivalis, A. ochroleuca, U. florida and B. capillaris (B). (C-D) Invasion assay of A549 cells treated with 5 μg/ml of acetone extracts of A. samentosa, F. nivalis, A. ochroleuca, U. florida and B. capillaris (C), and quantitative analysis of invaded cell numbers in each group (D). Representative images were shown from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). ***p<0.001; NS, no significant difference compared to 0.01% DMSO-treated A549 cells.
The effects of acetone extracts of A. samentosa, F. nivalis, A. ochroleuca, and U. florida on A549 cell invasion were then determined using transwell chamber invasion assay. As a result, the lichen extracts significantly decreased the invaded cell numbers by as much as 50% compared with DMSO or B. capillaries (negative control) (Fig 1C and 1D). These findings demonstrated that acetone extracts of A. samentosa, F. nivalis, A. ochroleuca, and U. florida inhibited both migration and invasion ability of A549 lung cancer cells.
Usnic acid is the active inhibitor from the lichen species
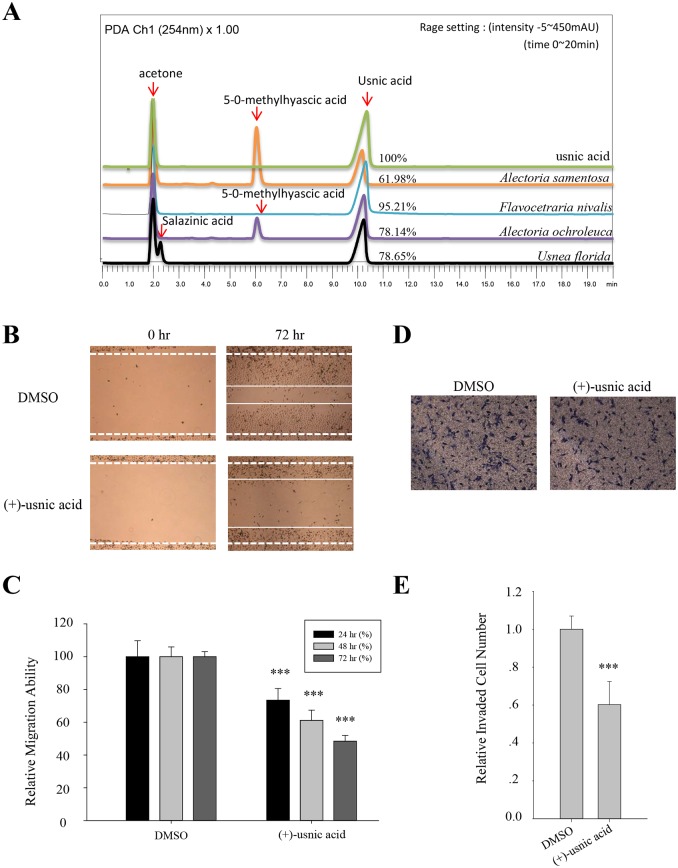
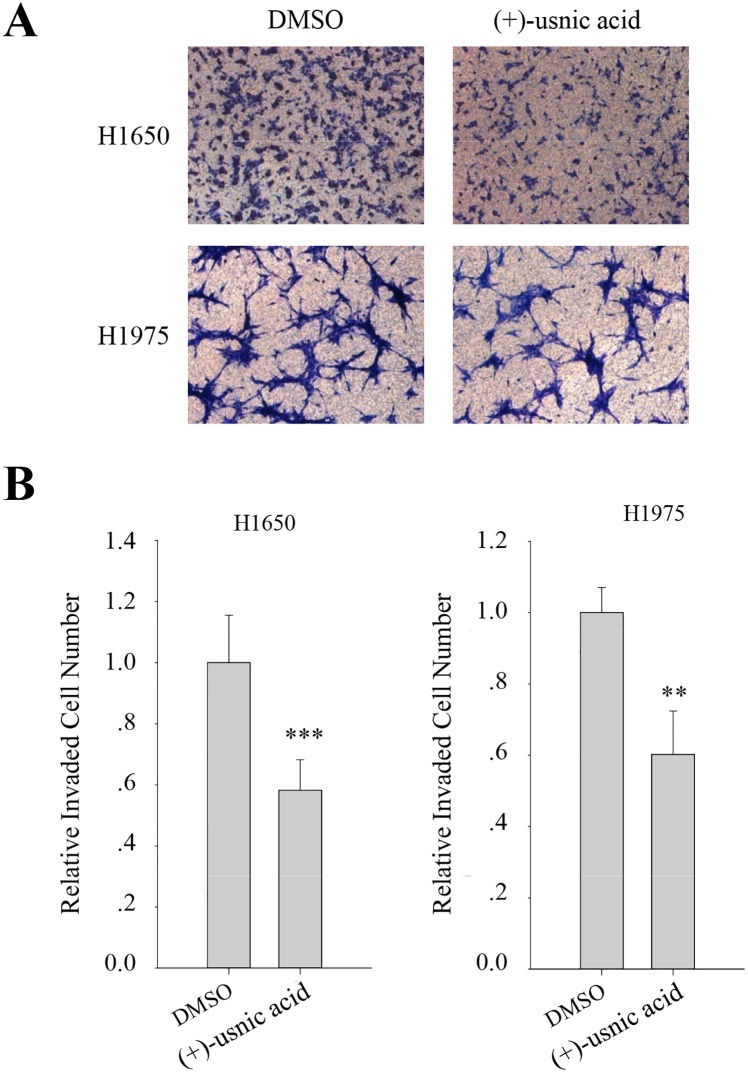
To identify the components of the acetone extract of lichen, A. samentosa, F. nivalis, A. ochroleuca, and U. florida extracts were run on HPLC. As shown in Fig 2A, usnic acid was identified as the main compound in all four of these candidates after comparison with the internal standard of purified (+)-usnic acid (Sigma-Aldrich, St. Louis, USA), and these were consistent with previous report (Table 1) [13–20]. Identity of usnic acid and their optical status were analyzed by LC-MS analysis and optical activity analysis, respectively (S1 File). The %intensity of peak for the usnic acid in the candidate lichens at a concentration of 5 mg/ml was obtained by comparing to that of peak for pure 5 mg/ml usnic acid. It is worth noting that usnic acid content is highest in F. nivalis extract, which may explain its potent inhibitory effect on cell migration (Fig 2A). It was speculated that (-)-usnic acid has similar or more potent inhibitory activity on cell motility as acetone extract of A. samentosa and A. ochroleuca which are known to have (-)-usnic acid as their subcomponent [13, 16, 18] showed similar or more potent inhibitory activity on migration and invasion, respectively, than (+)-usnic acid containing U. florida (Fig 1). In our previous report, we showed that acetone extract of lichen F. cucullata and its component, usnic acid, inhibited tumorigenicity and motility of cancer cells [9]. In accordance with this, (+)-usnic acid at concentration of 5 μM significantly inhibited the migration and invasion of A549 cells (Fig 2). At this concentration, usnic acid did not show cytotoxicity and/or inhibit cell proliferation (IC50 value of usnic acid on A549 cells = 65.3 ± 0.65 μM) [9]. As shown in Fig 2B–2E, inhibitory activity of (+)-usnic acid at 5 μM is as high as 50% at 72 h treatment for migration and is around 40% at 48 h treatment for invasion. To further examine the inhibitory activity of (+)-usnic acid on the other lung cancer cells, invasion assay was performed using H1650, and H1975 cells. As a result, (+)-usnic acid treatment significantly decreased invaded cell number of H1650 and H1975 cells at a concentration of 5 μM (Fig 3A). The quantitative analysis revealed that inhibition was as high as 40% in both cells compared with vehicle-treated cells (Fig 3B). Together, the results demonstrated that (+)-usnic acid has inhibitory activity against cell motility of human lung cancer cells.
Fig 2. Identification of lichen secondary metabolite from candidate lichens.
(A) High performance liquid chromatography (HPLC) analysis of lichen acetone extracts. The %intensity of peak for the usnic acid in the extract at a concentration of 5 mg/ml was obtained by comparing to that of peak for pure 5 mg/ml usnic acid. (B–C) Migration assay of A549 cells treated with 5 μM of (+)-usnic acid (B), and quantitative analysis of wound length (C). (D–E) Invasion assay of A549 cells treated with 5 μM of (+)-usnic acid (D), and quantitative analysis of invaded cell numbers in each group (E). Representative images are shown from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). ***p<0.001; NS, no significant difference compared to 0.01% DMSO-treated A549 cells.
Fig 3. (+)-Usnic acid inhibits invasion of H1650 and H1975 human lung cancer cell.
(A-B) Invasion assay of H1650, and H1975 cells treated with 5 μM of (+)-usnic acid (A), and quantitative analysis of invaded cell numbers in each cell line (B). Representative images are shown from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). **p<0.01; ***p<0.001; NS, no significant difference compared to 0.01% DMSO-treated A549 cells.
(+)-Usnic acid decreases β-catenin-mediated TOPFLASH activity and KITENIN-mediated AP-1 activity
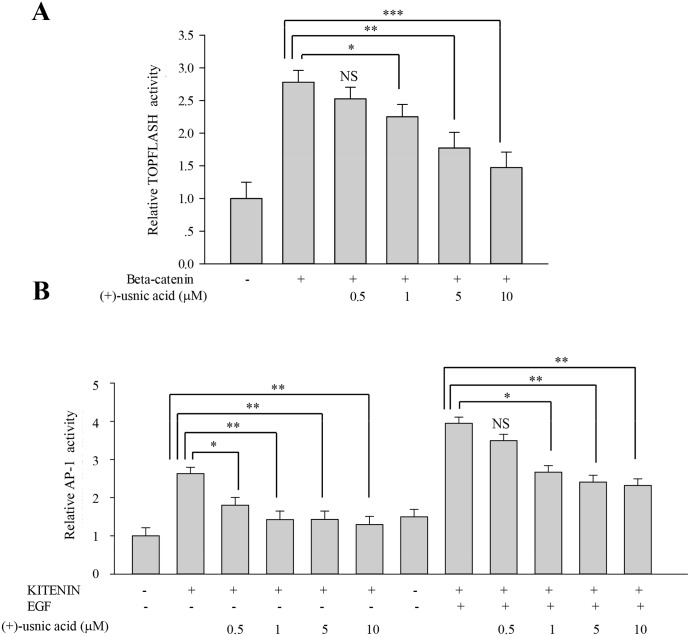
To investigate underlying mechanisms for the inhibitory activity of (+)-usnic acid, we performed TOPFLASH and AP-1 reporter assays to assess whether (+)-usnic acid can modulate β-catenin-mediated and/or KITENIN-mediated signaling activity. As shown in Fig 4A, (+)-usnic acid significantly decreased TOPFLASH activity by 18% at 1 μM and 37% at 10 μM. As for AP-1 activity, dose-dependent decreases were observed from 0.5 μM, and the decrease was significant, as much as 50% at 10 μM. Moreover, (+)-usnic acid also significantly decreased EGF-activated KITENIN-mediated AP-1 activity by 32% at 1 μM and 41% at 10 μM (Fig 4B). To check whether the level of downstream target genes of β-catenin/LEF and c-jun/AP-1 were affected by (+)-usnic acid treatment, quantitative real-time PCR analysis was performed. As shown in Fig 5, relative expression levels of CD44, cyclin D1, and c-myc were significantly decreased by (+)-usnic acid treatment in lung cancer cells to different extents (Fig 5A–5D). These results suggest that (+)-usnic acid shows inhibitory activity against cell motility through the modulation of β-catenin-mediated and KITENIN-mediated signaling activity in lung cancer cells.
Fig 4. (+)-Usnic acid decreases β-catenin-mediated TOPFLASH activity and KITENIN-mediated AP-1 activity.
(A) β-Catenin-mediated transcriptional activity of TOPFLASH promoter was decreased by (+)-usnic acid treatment. HEK 293T cells were transfected with β-catenin and TOPFLASH reporter plasmid. After 12 h transfection, cells were treated with (+)-usnic acid for 48h. (B) KITENIN-mediated transcriptional activity of AP1 promoter was decreased by (+)-usnic acid treatment. The HEK 293T cells were transfected with KITENIN and AP-1 reporter plasmid. After 12 h transfection, cells were treated with (+)-usnic acid for 48h with or without EGF. Experiments were performed in at least three independent cultures, n = 3. Data represent mean ± S.E.M. (standard error of the mean). *p<0.05; **p<0.01; ***p<0.001; NS, no significant difference compared to 0.01% DMSO-treated HEK 293T cells.
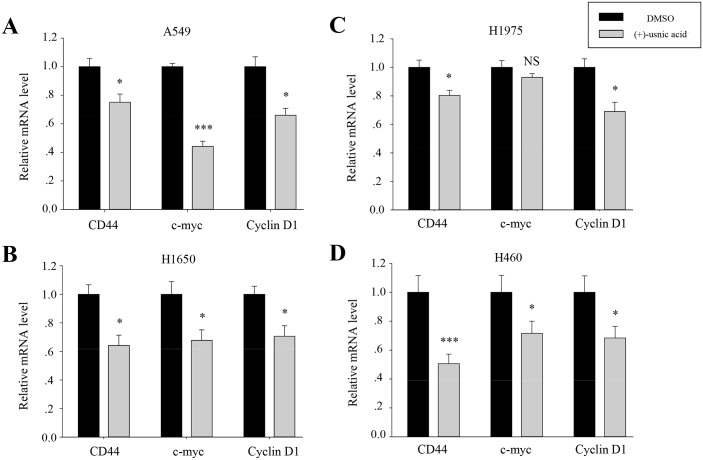
Fig 5. (+)-Usnic acid decreases mRNA level of downstream target genes of β-catenin/LEF and c-jun/AP-1.
(A-D) Quantitative analysis of the mRNA level of CD44, c-myc, and Cyclin D1 in A549 (A), H1650 (B), H1975 (C), and H460 (D) cells treated with 5 μM of (+)-usnic acid. Data represent mean ± S.E.M. (standard error of the mean), n = 3. *p<0.05; ***p<0.001; NS, no significant difference when compared to the 0.01% DMSO-treated group in each cell line.
(+)-Usnic acid decreases GTP-Rac1 and -RhoA level
The activities of Rac1 and Cdc42 are involved in mesenchymal mode of migration [21, 22]. To determine whether (+)-usnic acid can affect the activities of these proteins in A549 cells, GST pull-down assays were performed using GST-PBD (p21-binding domain). As shown in Fig 6, (+)-usnic acid treatment significantly decreased the level of GTP-Rac1 by 22% compared to vehicle-treated cells (Fig 6A). However, no significant change in the level of GTP-Cdc42 was observed by (+)-usnic acid treatment (Fig 6B). RhoA promotes junctional formation, apical constriction, and reduces adhesion and cell spreading [23, 24]. To determine whether (+)-usnic acid can affect the activity of RhoA in A549 cells, GST pull-down assays were performed using GST-RBD (Rho-binding domain). As shown in Fig 6C, (+)-usnic acid treatment significantly decreased the level of GTP-RhoA by 40% compared to vehicle-treated cells. Taken together, these results suggest that (+)-usnic acid inhibits cell motility through the regulation of Rho GTPases.
Fig 6. Regulation of RhoGTPases activity by (+)-usnic acid.
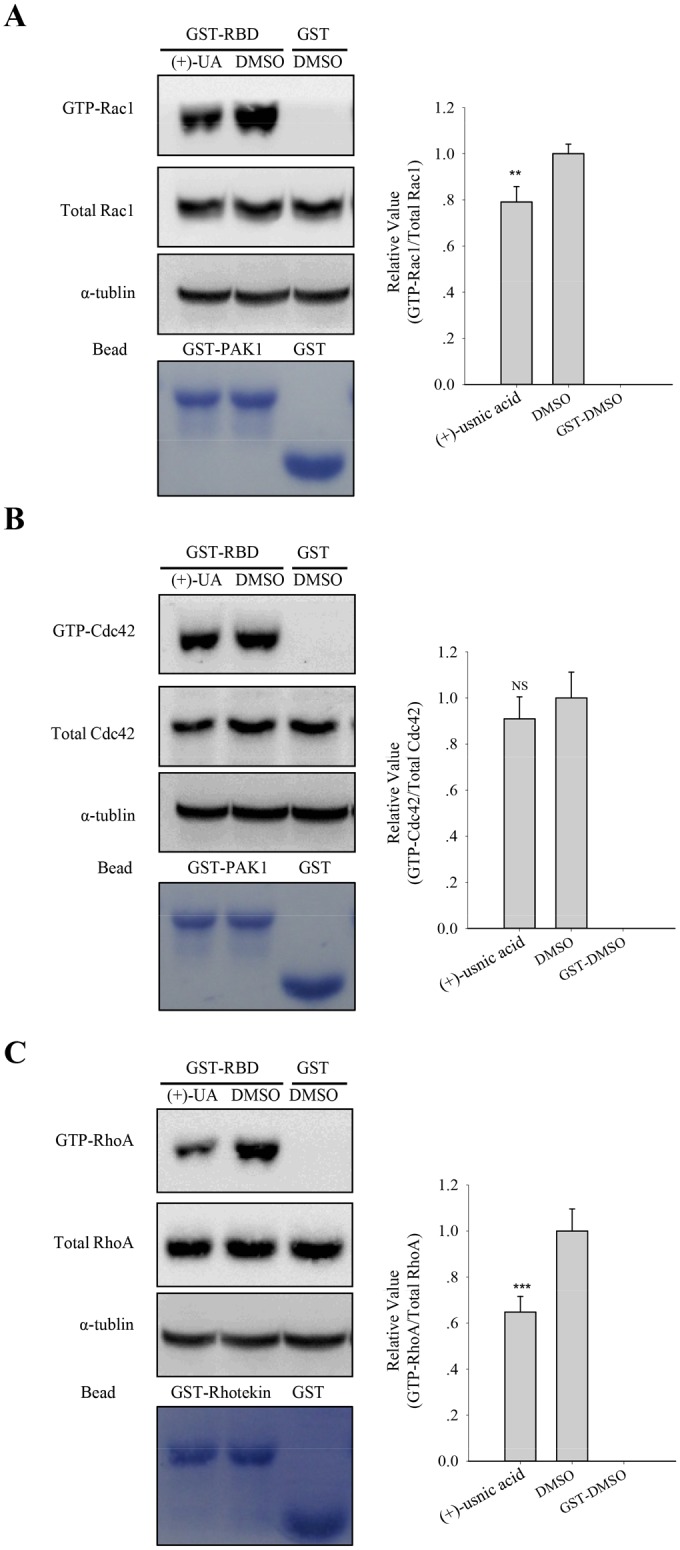
(A-C) The levels of GTP-bound Rac1, Cdc42 and RhoA were measured in A549 cells treated with 5 μM of (+)-usnic acid. GTP-Rac1 and -Cdc42 were measured using GST-PBD, and GTP-RhoA was measured using GST-RBD. The total amounts of RhoA, Rac1, and Cdc42 were also shown. The relative activities of Rac1 (A), Cdc42 (B), and RhoA (C) were determined as described in Materials and Methods. The data represent the mean ± SEM (standard error of the mean), n = 3. **p<0.01; ***p<0.001; NS, no significant difference compared to 0.01% DMSO-treated A549 cells.
(+)-Usnic acid shows additive inhibitory activity with cetuximab
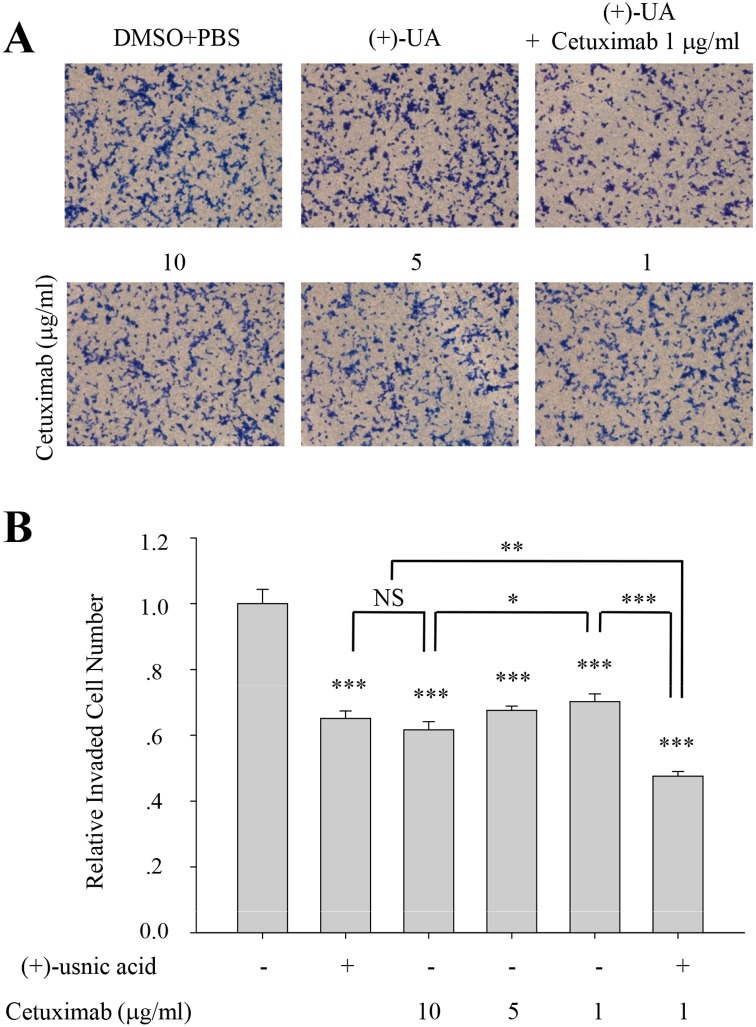
Cetuximab (Erbitux, C225), monoclonal antibody to epidermal growth factor receptor (EGFR) is used as one of anti-EGFR agents for the treatment of metastatic colon and lung cancer. To examine whether (+)-usnic acid has therapeutic relevancy with cetuximab, invasion assay was performed with various concentration of cetuximab and/or (+)-usnic acid. As shown in Fig 7, inhibitory activity of cetuximab was ~30% at 1 μg/ml and ~40% at 10 μg/ml on A549 cells, and treatment of 5 μM (+)-usnic acid showed similar inhibitory activity with 10 μg/ml of cetuximab (~40% inhibition) on these cells. Interestingly, higher inhibitory activity for cell invasion was observed when the cells were treated with 1 μg/ml of cetuximab together with 5 μM of (+)-usnic acid (Fig 7). These results suggest that (+)-usnic acid not only can inhibit lung cancer cell motility by alone but also can potentiate the therapeutic activity of cetuximab. As usnic acid decreased KITENIN-mediated AP-1 activity (Figs 4 and 5) and KITENIN/ErbB4-mediated downstream signal of EGF plays one of the molecular basis for conferring resistance to anti-EGFR agents [25], these results suggest that usnic acid may have potential beneficial activity in overcoming the limited clinical efficacy of anti-EGFR therapy.
Fig 7. (+)-Usnic acid shows additive inhibitory activity with cetuximab.
(A-B) Invasion assay of A549 cells treated with 5 μM of (+)-usnic acid and/or various concentration of cetuximab (A), and quantitative analysis of invaded cell numbers in each group (B). Representative images are shown from three independent experiments, n = 3. Data represent mean ± S.E.M. (standard error of the mean). *p<0.05; **p<0.01; ***p<0.001; NS, no significant difference between indicated group.
Discussion
Cancer cell acquires biological capabilities including resisting cell death, sustaining proliferative signaling, evading growth suppressors, activating invasion and metastasis, and so forth in developing from early to late stages [26, 27], and targeting either of these acquirements can be grouped as ‘anticancer’. In this regard, our observations that (+)-usnic acid inhibits migration and invasion ability in lung cancer cells are novel in anticancer activity of (+)-usnic acid. In addition, our results demonstrated that (+)-usnic acid have specific mechanisms of action for their anticancer activity, and these are quite different from those of previous literature showing cytotoxicity, one of mechanism of actions for the anticancer activity of (+)-usnic acid in various cancer cells.
Hepatotoxicity of usnic acid may restrict their potential medicinal use in cancer therapeutics [28]. However, most of hepatotoxicity in human was observed when high dose of usnic acid was orally administrated as a dietary supplement for the purpose of weight loss [29–31]. In cancer therapeutics, hepatotoxicity can be avoided by adjusting dosage, formulation and/or route of medication. For example, da Silva Santos et al. showed that nano-encapsulation of usnic acid enable to maintain and improve antitumor activity and considerably reduce the hepatotoxicity [32]. Furthermore, it has been shown that supplementation of anti-oxidant, i.e. vitamin E, together with usnic acid could greatly reduce the usnic acid-induced hepatotoxicity in primary cultured mouse hepatocytes [33]. It should also be noted that some of the papers showing hepatotoxicity were carried on HepG2 cells, originated from hepatocellular carcinoma tissue of 15 years male adolescent [34, 35]. In our previous paper [9], we demonstrated that usnic acid somehow has selective cytotoxicity in cancer cells when compared to normal cells. In alternative point of views, severe cytotoxicity of usnic acid on HepG2 cells reflects selective and specific anticancer activity of usnic acid on liver cancer in tested concentrations.
In our previous report, it was shown that the cytotoxicity of usnic acid is specific to cancer cells such as HT29 (colorectal cancer cells; IC50 = 95.2 ± 0.85 μM), AGS (gastric cancer cell; IC50 = 15.01 ± 0.52 μM), A549 (lung cancer cell; IC50 = 65.3 ± 0.65 μM), and CWR22Rv-1 (prostate cancer cells; IC50 = 24.1 ± 0.63 μM), while non-cancer cells such as MDCK (Mardin-Darby canine kidney; IC50 = 133.04 ± 3.5 μM), RIE (rat intestinal epithelial cells; IC50 = 126 ± 4.25 μM), NIH 3T3 (mouse embryonic fibroblast; IC50 = 164.2 ± 3.7 μM), and HaCaT (human keratinocyte; IC50 = 185.7 ± 4.8 μM) cells, were not severely damaged [9]. Given that the average circulating blood volume for mice is 72 mL/kg [36] and the molecular weight of usnic acid is 344, LD50 value of usnic acid (mouse-oral; 838 mg/kg) in MSDS sheet of usnic acid can be calculated to 33.8 mM. As inhibitory activity of usnic acid in inhibiting lung cancer cell motility is observed at concentration of 5 μM, our results indicated that usnic acid would be used for anti-metastasis agent with little toxicity at working concentrations.
Usnic acid has a low degree of aqueous solubility, which is an obstacle in the drug development as it is likely to result in poor bioavailability. To avoid this problem, various approaches can be made for the enhancement of solubility with its own compound by particle size reduction, crystal engineering, salt formation, solid dispersion, use of surfactant, complexation, and so forth [37]. The selection of the enhancement method should be determined to each drug in required dosage form characteristics. For usnic acid, recent article reported that potassium salt of usnic acid (potassium usnate) has 100% solubility without losing its biological activity at 10 μg/ml (approximately 26 μM) [38]. Together with the facts that (+) and (-) enantiomeric forms of usnic acid showed moderate to strong biological activities [39, 40], further study is required to evaluate the potential medicinal usage of usnic acid in anticancer therapy in various cancers.
Supporting Information
(PDF)
Abbreviations
- AP-1
activator protein-1
- EGF
epidermal growth factor
- DMSO
dimethylsulfoxide
- HPLC
high performance liquid chromatography
- KITENIN
KAI1 COOH-terminal interacting tetraspanin
- LEF
lymphoid enhancing factor
- PBD
p21-binding domain
- PCR
polymerase chain reaction
- RBD
Rho-binding domain
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF-2013R1A1A2004677, NRF-2013R1A2A2A07067609, NRF-2015R1A4A1041219 and MRC-2011-0030132) and by the Korea National Research Resource Center Program (NRF-2014M3A9B8002115). This study also received support from a research grant funded by the Sunchon Research Center for Natural Medicine.
References
- 1.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: a cancer journal for clinicians. 2000;50(1):7–33. Epub 2000/03/29. . [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nature reviews Cancer. 2003;3(1):55–63. Epub 2003/01/02. 10.1038/nrc967 . [DOI] [PubMed] [Google Scholar]
- 3.Huneck S. The significance of lichens and their metabolites. Die Naturwissenschaften. 1999;86(12):559–70. Epub 2000/01/22. . [DOI] [PubMed] [Google Scholar]
- 4.Huneck S, Yoshimura I. Identification of lichen substances: Springer Berlin Heidelberg; 1996. [Google Scholar]
- 5.Elix JA, Whitton AA, Sargent MV. Recent progress in the chemistry of lichen substances: Springer-Vienna; 1984. [Google Scholar]
- 6.Fiedler P, Gambaro V, Garbarino JA, Quilhot W. Epiphorellic acids 1 and 2, two diaryl ethers from the lichen cornicularia epiphorella. Phytochemistry. 1986;25(2):461–5. [Google Scholar]
- 7.Coley PD. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense: Springer-Verlag; 1988. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Kim KK, Hur JS. Anticancer activity of lichen metabolites and their mechanisms at the molecular level In: Upreti DK, Divakar PK, Shukla V, Bajpai R, editors. Modern methods and approaches in lichen systematics and culture techniques. 2: Springer; India; 2015. p. 201–8. [Google Scholar]
- 9.Nguyen TT, Yoon S, Yang Y, Lee HB, Oh S, Jeong MH, et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PloS one. 2014;9(10):e111575 Epub 2014/11/02. 10.1371/journal.pone.0111575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Han JR, Park J, Oh M, James SE, Chang S, et al. Delta-catenin-induced dendritic morphogenesis. An essential role of p190RhoGEF interaction through Akt1-mediated phosphorylation. The Journal of biological chemistry. 2008;283(2):977–87. Epub 2007/11/13. 10.1074/jbc.M707158200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Oh M, Lu Q, Kim K. E-Cadherin negatively modulates delta-catenin-induced morphological changes and RhoA activity reduction by competing with p190RhoGEF for delta-catenin. Biochemical and biophysical research communications. 2008;377(2):636–41. Epub 2008/10/22. 10.1016/j.bbrc.2008.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orange A, James PW, White FJ. Microchemical Methods for the Identification of Lichens: British Lichen Society; 2001. p. 101. [Google Scholar]
- 13.Gollapudi SR, Telikepalli H, Jampani HB, Mirhom YW, Drake SD, Bhattiprolu KR, et al. Alectosarmentin, a new antimicrobial dibenzofuranoid lactol from the lichen, Alectoria sarmentosa. Journal of natural products. 1994;57(7):934–8. Epub 1994/07/01. . [DOI] [PubMed] [Google Scholar]
- 14.Bjerke JW, Elvebakk A. Distribution of the lichen genus Flavocetraria (Parmeliaceae, Ascomycota) in the Southern Hemisphere. New Zealand Journal of Botany. 2004;42(4):647–56. [Google Scholar]
- 15.Bjerke JW, Lerfall K, Elvebakk A. Effects of ultraviolet radiation and PAR on the content of usnic and divaricatic acids in two arctic-alpine lichens. Photochemical & Photobiological Sciences. 2002;1(9):678–85. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita Y, Yamamoto Y, Yoshimura I. Distribution of optical isomers of usnic and isousnic acids analyzed by high performance liquid chromatography. Journal of the Hattori Botanical Laboratory. 1997;(83):173–8. [Google Scholar]
- 17.Zagoskina N, Nikolaeva T, Lapshin P, Zavarzin A, Zavarzina A. Water-soluble phenolic compounds in lichens. Microbiology. 2013;82(4):445–52. [PubMed] [Google Scholar]
- 18.Culberson CF. Chemical and Botanical Guide to Lichen Products: University of North Carolina Press; 1969. [Google Scholar]
- 19.Brodo IM, Sharnoff SD, Sharnoff S. Lichens of north America: Yale University Press; 2001. [Google Scholar]
- 20.Fiscus SA. A survey of the chemistry of the Usnea florida group in North America. Bryologist. 1972:299–304. [Google Scholar]
- 21.Murali A, Rajalingam K. Small Rho GTPases in the control of cell shape and mobility. Cellular and molecular life sciences: CMLS. 2014;71(9):1703–21. Epub 2013/11/28. 10.1007/s00018-013-1519-6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yui Y, Itoh K, Yoshioka K, Naka N, Watanabe M, Hiraumi Y, et al. Mesenchymal mode of migration participates in pulmonary metastasis of mouse osteosarcoma LM8. Clinical & experimental metastasis. 2010;27(8):619–30. Epub 2010/09/28. 10.1007/s10585-010-9352-x . [DOI] [PubMed] [Google Scholar]
- 23.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda). 2010;25(1):16–26. Epub 2010/02/06. 10.1152/physiol.00034.2009 . [DOI] [PubMed] [Google Scholar]
- 24.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–35. Epub 2002/12/13. 10.1038/nature01148 . [DOI] [PubMed] [Google Scholar]
- 25.Bae JA, Yoon S, Park SY, Lee JH, Hwang JE, Kim H, et al. An unconventional KITENIN/ErbB4-mediated downstream signal of EGF upregulates c-Jun and the invasiveness of colorectal cancer cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(15):4115–28. Epub 2014/06/05. 10.1158/1078-0432.CCR-13-2863 . [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. Epub 2000/01/27. . [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. Epub 2011/03/08. 10.1016/j.cell.2011.02.013 . [DOI] [PubMed] [Google Scholar]
- 28.Araujo AA, de Melo MG, Rabelo TK, Nunes PS, Santos SL, Serafini MR, et al. Review of the biological properties and toxicity of usnic acid. Natural product research. 2015:1–14. Epub 2015/02/25. 10.1080/14786419.2015.1007455 . [DOI] [PubMed] [Google Scholar]
- 29.Favreau JT, Ryu ML, Braunstein G, Orshansky G, Park SS, Coody GL, et al. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Annals of internal medicine. 2002;136(8):590–5. Epub 2002/04/17. . [DOI] [PubMed] [Google Scholar]
- 30.Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. Journal of hepatology. 2004;41(6):1062–4. Epub 2004/12/08. 10.1016/j.jhep.2004.06.028 . [DOI] [PubMed] [Google Scholar]
- 31.Durazo FA, Lassman C, Han SH, Saab S, Lee NP, Kawano M, et al. Fulminant liver failure due to usnic acid for weight loss. The American journal of gastroenterology. 2004;99(5):950–2. Epub 2004/05/07. 10.1111/j.1572-0241.2004.04165.x . [DOI] [PubMed] [Google Scholar]
- 32.da Silva Santos NP, Nascimento SC, Wanderley MS, Pontes-Filho NT, da Silva JF, de Castro CM, et al. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2006;64(2):154–60. Epub 2006/08/11. 10.1016/j.ejpb.2006.05.018 . [DOI] [PubMed] [Google Scholar]
- 33.Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochemical pharmacology. 2004;67(3):439–51. Epub 2004/03/24. 10.1016/j.bcp.2003.09.032 . [DOI] [PubMed] [Google Scholar]
- 34.Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282(5739):615–6. Epub 1979/12/06. . [DOI] [PubMed] [Google Scholar]
- 35.Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209(4455):497–9. Epub 1980/07/25. . [DOI] [PubMed] [Google Scholar]
- 36.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of applied toxicology: JAT. 2001;21(1):15–23. Epub 2001/02/17. . [DOI] [PubMed] [Google Scholar]
- 37.Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN pharmaceutics. 2012;2012:195727 Epub 2012/07/26. 10.5402/2012/195727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins MC, Silva MC, Silva LR, Lima VL, Pereira EC, Falcao EP, et al. Usnic acid potassium salt: an alternative for the control of Biomphalaria glabrata (Say, 1818). PloS one. 2014;9(11):e111102 Epub 2014/11/07. 10.1371/journal.pone.0111102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingolfsdottir K. Usnic acid. Phytochemistry. 2002;61(7):729–36. Epub 2002/11/28. . [DOI] [PubMed] [Google Scholar]
- 40.Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Die Naturwissenschaften. 2002;89(4):137–46. Epub 2002/06/14. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.