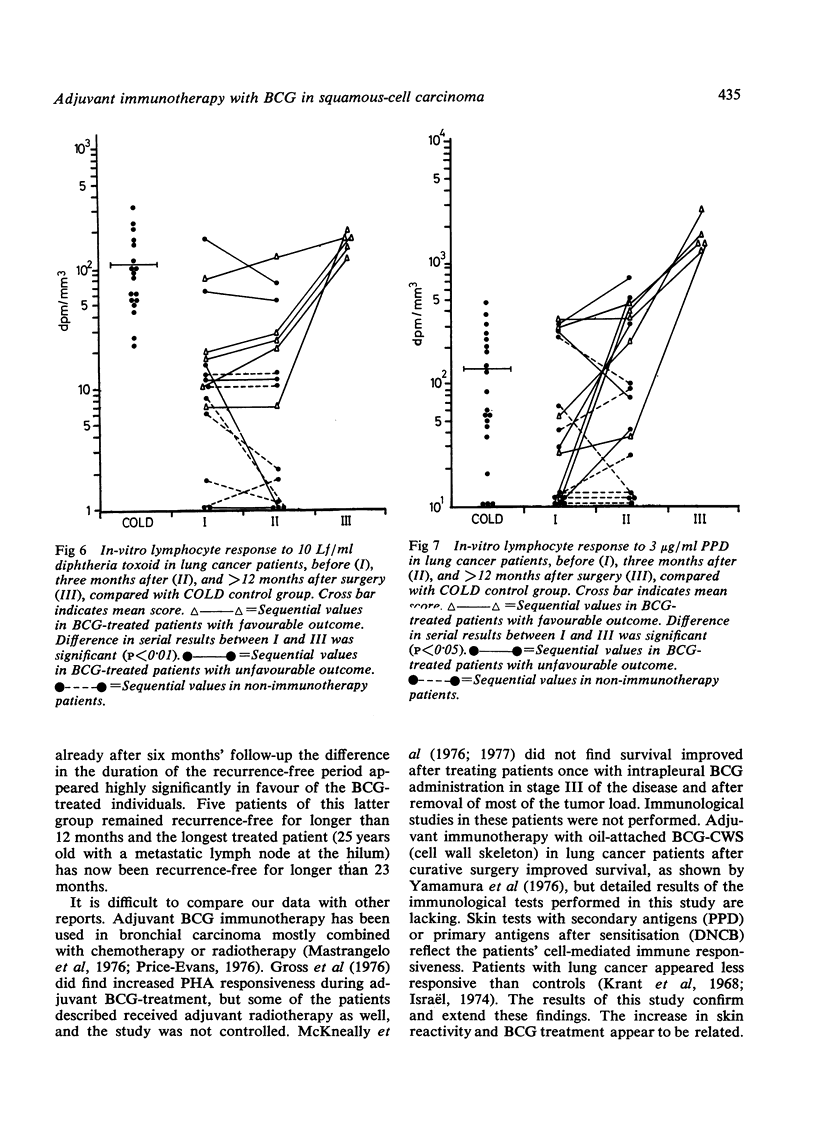
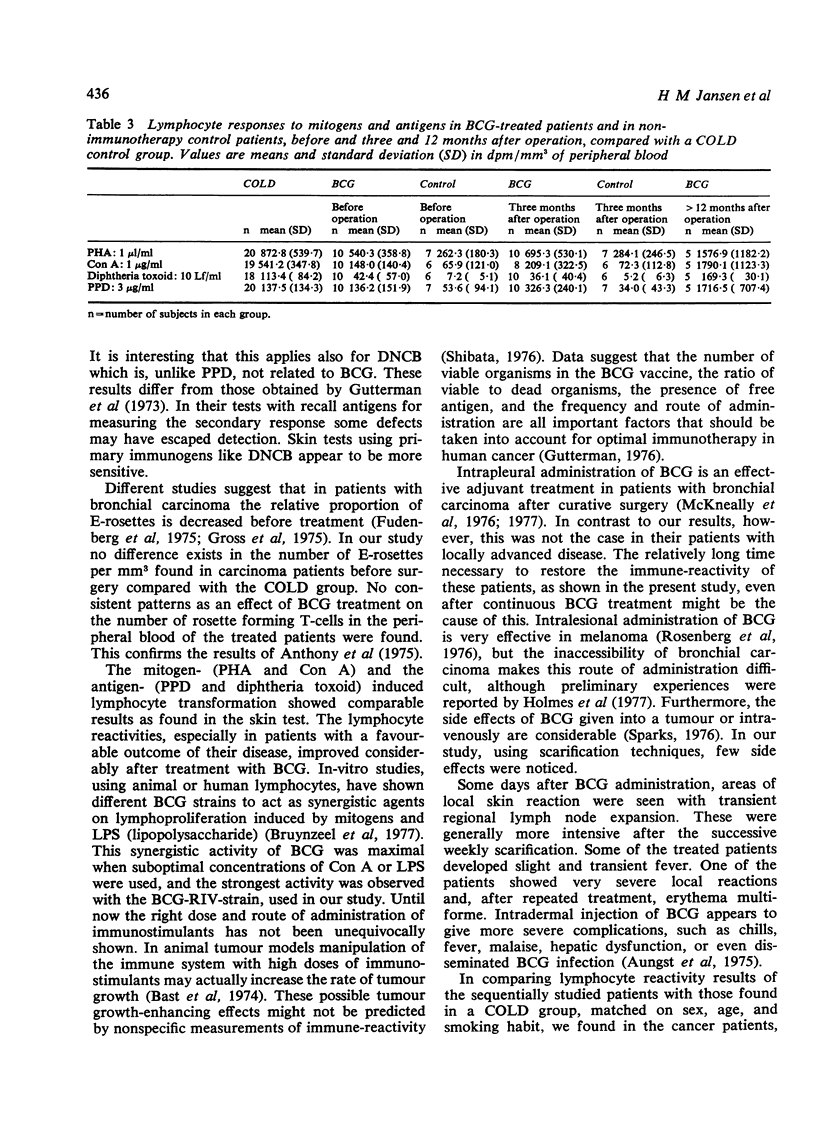
Abstract
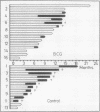
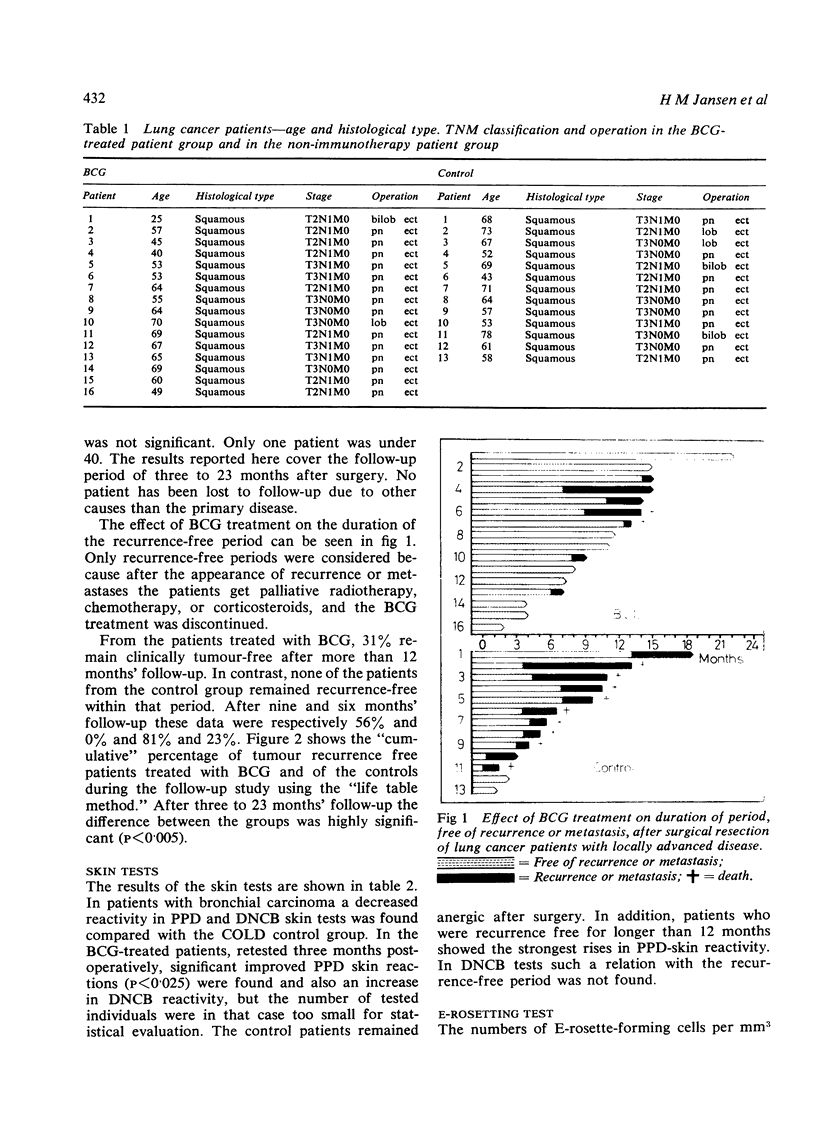
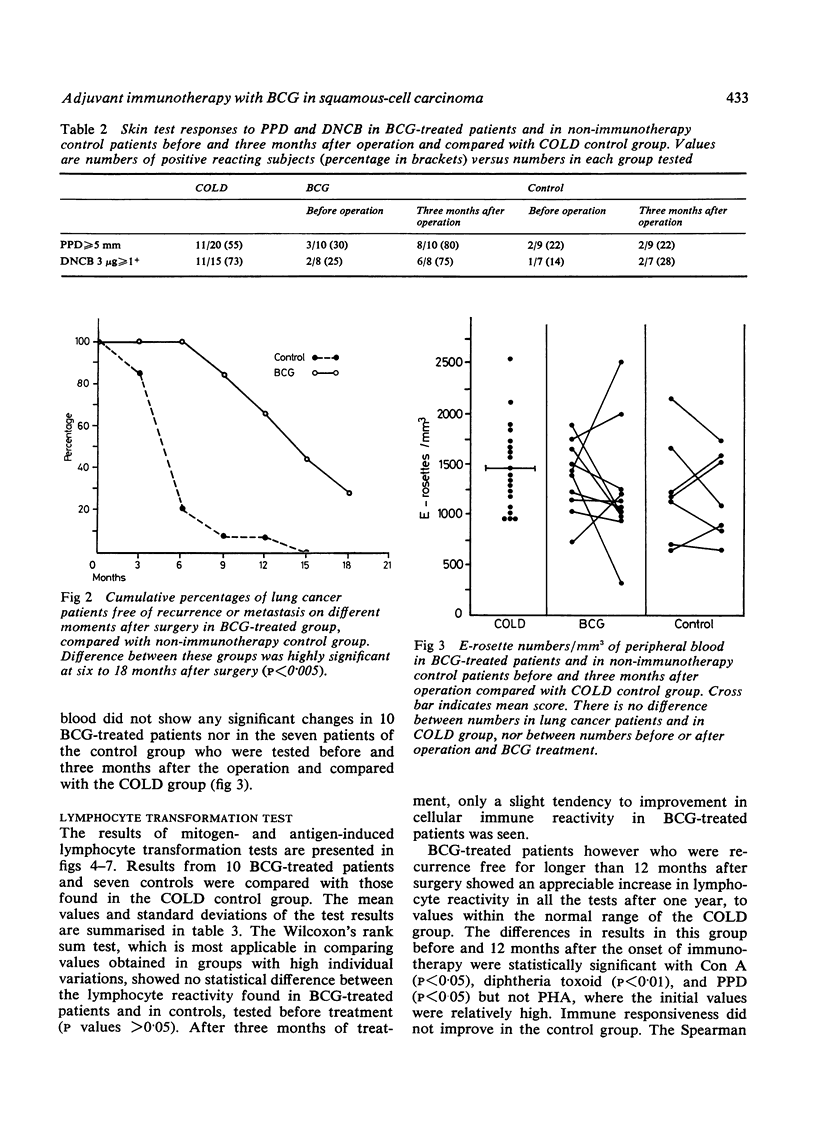
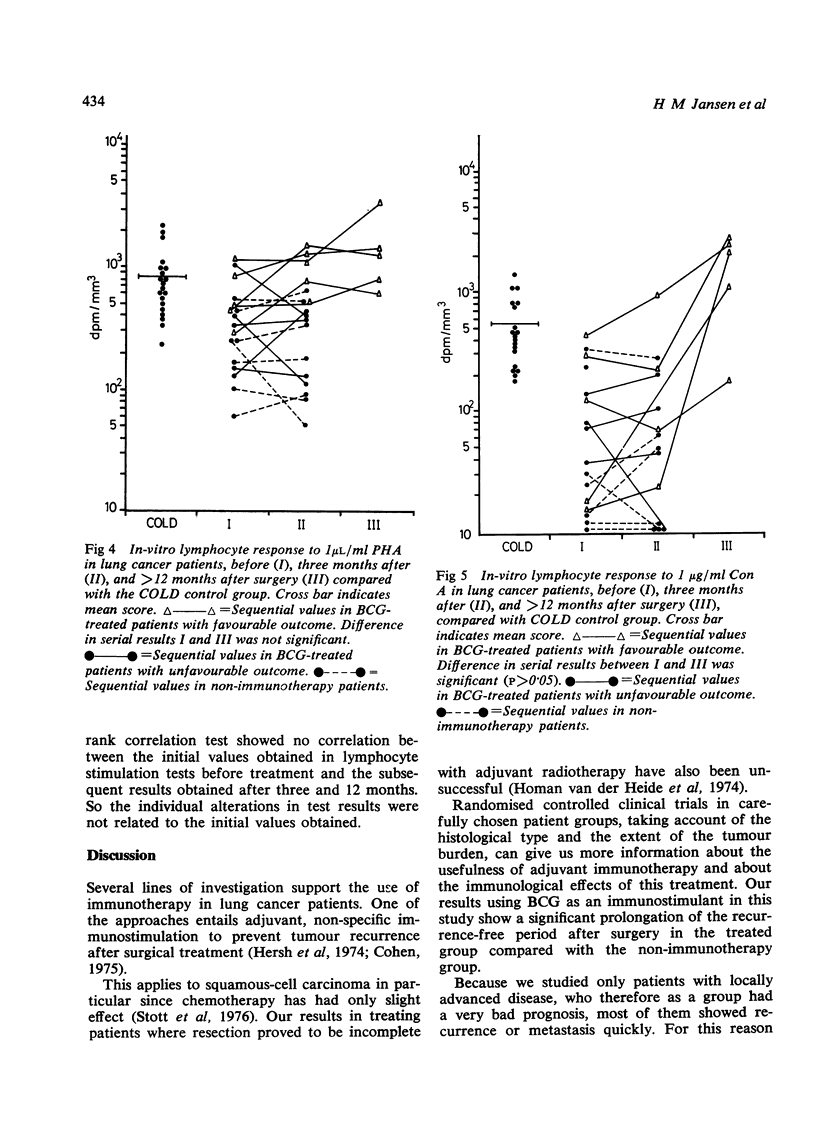
Jansen, H M, The, T H, de Gast, G C, Esselink, M T, van der Wal, A M, and Orie, N G M (1978).Thorax, 33, 429-438. Adjuvant immunotherapy with BCG in squamous-cell bronchial carcinoma. Immune-reactivity in relation to immunostimulation (preliminary results in a controlled trial). Twenty-nine patients with, at operation, evidence of locally advanced primary squamous-cell bronchial carcinoma (stage II, UICC, Geneva, 1974) had lung resection to remove all the visible tumour. Postoperatively a randomly chosen group of 16 patients received adjuvant BCG immunostimulation by scarifications, while the control group received no adjuvant treatment. Follow-up studies were done from three to 23 months. Immune-reactivity in vivo with PPD and DNCB skin tests, and in vitro with E-rosetting tests and lymphocyte transformation tests with PHA, Con A, diphtheria toxoid, and PPD was monitored in 10 treated and in seven untreated patients. Recurrence rates decreased appreciably in the BCG-stimulated group after a six to 23 months' follow-up (p<0·005). A pronounced increase in both in-vivo and in-vitro immune-reactivity went in parallel with a more favourable clinical outcome in the BCG-treated group. In these cases there was a significant increase in skin reactivity to PPD three months after surgery (p<0·025) and a statistically significant rise in lymphocyte reactivity to Con A (p<0·05), diphtheria toxoid (p<0·01), and PPD (p<0·05) but not to PHA 12 months after surgery. DNCB skin reactivity increased as well in the BCG-treated group, but the number of individuals was too small for statistical evaluation. Increase in immune responsiveness did not occur in the control group and appeared to be independent of the initial immune state of the patients. No differences were found in the numbers of E-rosetting lymphocytes in relation to immunotherapy. It is concluded that adjuvant BCG immunotherapy used in patients with minimal residual bronchial carcinoma improves the prognosis and a favourable clinical outcome is mirrored by an increase in cellular immune reactivity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Activated macrophages and the antitumor action of BCG. Natl Cancer Inst Monogr. 1973 Dec;39:127–133. [PubMed] [Google Scholar]
- Anthony H. M., Kirk J. A., Madsen K. E., Mason M. K., Templeman G. H. E and EAC rosetting lymphocytes in patients with carcinoma of bronchus. II. A sequential study of thirty patients: effect of BCG. Clin Exp Immunol. 1975 Apr;20(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Aungst C. W., Sokal J. E., Jager B. V. Complications of BCG vaccination in neoplastic disease. Ann Intern Med. 1975 May;82(5):666–669. doi: 10.7326/0003-4819-82-5-666. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Bast B. S. Critical review of previously reported animal studies of tumor immunotherapy with nonspecific immunostimulants. Ann N Y Acad Sci. 1976;277(00):60–93. doi: 10.1111/j.1749-6632.1976.tb41692.x. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Zbar B., Borsos T., Rapp H. J. BCG and cancer. N Engl J Med. 1974 Jun 27;290(26):1458–1469. doi: 10.1056/NEJM197406272902605. [DOI] [PubMed] [Google Scholar]
- Bleumink E., Nater J. P., Schraffordt Koops H., The T. H. A standrard method for DNCB sensitization testing in patients with neoplasms. Cancer. 1974 Apr;33(4):911–915. doi: 10.1002/1097-0142(197404)33:4<911::aid-cncr2820330404>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Cleveland R. P., Meltzer M. S., Zbar B. Tumor cytotoxicity in vitro by macrophages from mice infected with mycobacterium bovis strain BCG. J Natl Cancer Inst. 1974 Jun;52(6):1887–1895. doi: 10.1093/jnci/52.6.1887. [DOI] [PubMed] [Google Scholar]
- Cohen M. H. Guest editorial: Lung cancer: a status report. J Natl Cancer Inst. 1975 Sep;55(3):505–511. doi: 10.1093/jnci/55.3.505. [DOI] [PubMed] [Google Scholar]
- Evans D. A. Immunology of bronchial carcinoma. Thorax. 1976 Oct;31(5):493–506. doi: 10.1136/thx.31.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudenberg H. H., Wybran J., Robbins D. Editorial: T-rosette forming cells, cellular immunity and cancer. N Engl J Med. 1975 Feb 27;292(9):475–476. doi: 10.1056/NEJM197502272920910. [DOI] [PubMed] [Google Scholar]
- GEHAN E. A. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965 Jun;52:203–223. [PubMed] [Google Scholar]
- Gross N. J., Eddie-Quartey A. C. Monitoring of immunologic status of patients receiving BCG therapy for malignant disease. Cancer. 1976 May;37(5):2183–2193. doi: 10.1002/1097-0142(197605)37:5<2183::aid-cncr2820370505>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gutterman J. U., Mavligit G. M., Hersh E. M. Chemoimmunotherapy of human solid tumors. Med Clin North Am. 1976 May;60(3):441–462. doi: 10.1016/s0025-7125(16)31891-0. [DOI] [PubMed] [Google Scholar]
- Gutterman J. U., Mavligit G., McBride C., Frei E., 3rd, Freireich E. J., Hersh E. M. Active immunotherapy with B.C.G. for recurrent malignant melanoma. Lancet. 1973 Jun 2;1(7814):1208–1212. doi: 10.1016/s0140-6736(73)90526-6. [DOI] [PubMed] [Google Scholar]
- Han T. Specific effect of neuraminidase on blastogenic response of sensitized lymphocytes. Immunology. 1975 Feb;28(2):283–286. [PMC free article] [PubMed] [Google Scholar]
- Hawrylko E. BCG immunopotentiation of an antitumor response: evidence for a cell-mediated mechanism. J Natl Cancer Inst. 1975 Aug;55(2):413–423. doi: 10.1093/jnci/55.2.413. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Campbell D. A., Jr, Oldham R. K., Bonnard G. D., Ting C. C., Holden H. T., Glaser M., Djeu J., Oehler R. Immunogenicity of tumor antigens. Ann N Y Acad Sci. 1976;276:26–44. doi: 10.1111/j.1749-6632.1976.tb41634.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B. Cell-mediated immunity to tumor cells. Adv Cancer Res. 1974;19(0):207–263. doi: 10.1016/s0065-230x(08)60055-x. [DOI] [PubMed] [Google Scholar]
- Hersh E. M., Mavligit G. M., Gutterman J. U. Immunotherapy as related to lung cancer: a review. Semin Oncol. 1974 Sep;1(3):273–278. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Ramming K. P., Mink J., Coulson W. F., Morton D. L. New method of immunotherapy for lung cancer. Lancet. 1977 Sep 17;2(8038):586–587. doi: 10.1016/s0140-6736(77)91431-3. [DOI] [PubMed] [Google Scholar]
- Homan van der Heide J. N., Stam H. C., Van der Wal A. M. The results of a combined attack on bronchial carcinoma by radiotherapy and surgery. Bronches. 1974 Mar-Apr;24(2):70–78. [PubMed] [Google Scholar]
- Israel L. Non-specific immunostimulation in bronchogenic cancer. Scand J Respir Dis Suppl. 1974;89:95–105. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krant M. J., Manskopf G., Brandrup C. S., Madoff M. A. Immunologic alterations in bronchogenic cancer. Sequential study. Cancer. 1968 Apr;21(4):623–631. doi: 10.1002/1097-0142(196804)21:4<623::aid-cncr2820210414>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Mastrangelo M. J., Berd D., Bellet R. E. Critical review of previously reported clinical trials of cancer immunotherapy with nonspecific immunostimulants. Ann N Y Acad Sci. 1976;277(00):94–123. doi: 10.1111/j.1749-6632.1976.tb41693.x. [DOI] [PubMed] [Google Scholar]
- Mathé G. Active immunotherapy. Adv Cancer Res. 1971;14:1–36. doi: 10.1016/s0065-230x(08)60517-5. [DOI] [PubMed] [Google Scholar]
- McKneally M. F., Maver C. M., Kausel H. W. Intrapleural B.C.G. immunostimulation in lung cancer. Lancet. 1977 Mar 12;1(8011):593–593. doi: 10.1016/s0140-6736(77)92015-3. [DOI] [PubMed] [Google Scholar]
- McKneally M. F., Maver C., Kausel H. W. Regional immunotherapy of lung cancer with intrapleural B.C.G. Lancet. 1976 Feb 21;1(7956):377–379. doi: 10.1016/s0140-6736(76)90212-9. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Eilber F. R., Holmes E. C., Sparks F. C., Ramming K. BCG immunotherapy as a systemic adjunct to surgery in malignant melanoma. Med Clin North Am. 1976 May;60(3):431–439. doi: 10.1016/s0025-7125(16)31890-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Rapp H. J. Intralesional immunotherapy of melanoma with BCG. Med Clin North Am. 1976 May;60(3):419–430. doi: 10.1016/s0025-7125(16)31889-2. [DOI] [PubMed] [Google Scholar]
- Rümke C. L., Bezemer P. D., Kuik D. J. Normal values and least significant differences for differential leukocyte counts. J Chronic Dis. 1975 Dec;28(11-12):661–668. doi: 10.1016/0021-9681(75)90077-6. [DOI] [PubMed] [Google Scholar]
- Scott M. T., Bomford R. Comparison of the potentiation of specific tumor immunity in mice by Corynebacterium parvum or BCG. J Natl Cancer Inst. 1976 Sep;57(3):555–559. doi: 10.1093/jnci/57.3.555. [DOI] [PubMed] [Google Scholar]
- Shibata H. R., Jerry L. M., Lewis M. G., Mansell P. W., Capek A., Marquis G. Immunotherapy of human malignant melanoma with irradiated tumor cells, oral Bacillus Calmette-Guérin, and levamisole. Ann N Y Acad Sci. 1976;277(00):355–366. doi: 10.1111/j.1749-6632.1976.tb41714.x. [DOI] [PubMed] [Google Scholar]
- Sjögren H. O., Hellström I., Bansal S. C., Warner G. A., Hellström K. E. Elution of "blocking factors" from human tumors, capable of abrogating tumor-cell destruction by specifically immune lymphocytes. Int J Cancer. 1972 Mar 15;9(2):274–283. doi: 10.1002/ijc.2910090205. [DOI] [PubMed] [Google Scholar]
- Sparks F. C. Hazards and complications of BCG immunotherapy. Med Clin North Am. 1976 May;60(3):499–509. doi: 10.1016/s0025-7125(16)31894-6. [DOI] [PubMed] [Google Scholar]
- Stott H., Stephens R. J., Fox W., Roy D. C. 5-year follow-up of cytotoxic chemotherapy as an adjuvant to surgery in carcinoma of the bronchus. Br J Cancer. 1976 Aug;34(2):167–173. doi: 10.1038/bjc.1976.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y., Azuma I., Taniyama T., Sugimura K., Hirao F., Tokuzen R., Okabe M., Nakahara W., Yasumoto K., Ohta M. Immunotherapy of cancer with cell wall skeleton of Myocabacterium bovis-Bacillus Calmette-Guérin: experimental and clinical results. Ann N Y Acad Sci. 1976;277(00):209–227. doi: 10.1111/j.1749-6632.1976.tb41699.x. [DOI] [PubMed] [Google Scholar]