The University of North Carolina at Chapel Hill has created a clinic to provide medical and radiation oncology, neurosurgical, and supportive services to patients with breast cancer brain metastases. Of the 65 patients seen between January 2012 and January 2015, 78% returned for a follow-up visit and 32% were enrolled in a clinical trial. The clinic is a model that can be adapted at other centers.
Keywords: Breast cancer, Brain metastases, Multidisciplinary, Clinic
Abstract
Background.
Breast cancer brain metastasis (BCBM) confers a poor prognosis and is unusual in requiring multidisciplinary care in the metastatic setting. The University of North Carolina at Chapel Hill (UNC-CH) has created a BCBM clinic to provide medical and radiation oncology, neurosurgical, and supportive services to this complex patient population. We describe organization and design of the clinic as well as characteristics, treatments, and outcomes of the patients seen in its first 3 years.
Methods.
Clinical and demographic data were collected from patients in a prospectively maintained database. Descriptive statistics are reported as percentages and means. The Kaplan-Meier method was used to estimate time-to-event outcomes.
Results.
Sixty-five patients were seen between January 2012 and January 2015. At the time of presentation to the BCBM clinic, most patients (74%) had multiple (≥2) brain metastases and had received prior systemic (77%) and whole-brain radiation therapy and/or central nervous system stereotactic radiosurgery (65%) in the metastatic setting. Seventy-eight percent returned for a follow-up visit; 32% were enrolled in a clinical trial. Median time from diagnosis of brain metastasis to death was 2.11 years (95% confidence interval [CI] 1.31–2.47) for all patients, 1.15 years (95% CI 0.4–2.43) for triple-negative breast cancer, 1.31 years (95% CI 0.51–2.52) for hormone receptor-positive/HER2− breast cancer, and 3.03 years (95% CI lower limit 1.94, upper limit not estimable) for HER2+ breast cancer (p = .0037).
Conclusion.
Patients with BCBM have unique and complex needs that require input from several oncologic disciplines. The development of the UNC-CH multidisciplinary BCBM clinic is a model that can be adapted at other centers to provide coordinated care for patients with a challenging and complex disease.
Implications for Practice:
Patients with breast cancer brain metastases often require unique multidisciplinary care to meet the numerous and uncommon challenges associated with their conditions. Here, the development and characteristics of a clinic designed specifically to provide for the multidisciplinary needs of patients with breast cancer brain metastases are described. This clinic may serve as a model for other institutions interested in creating specialty clinics with similar objectives.
Abstract
摘要
背景. 乳腺癌脑转移 (BCBM) 预后不佳, 且因为转移性的性质而不常寻求多学科治疗。北卡罗来纳大学教堂山分校 (UNC-CH) 创立了一家 BCBM 诊所, 为这一复杂的患者群体提供肿瘤内科及肿瘤放疗科、神经外科和支持治疗。本文介绍了这家诊所的机构和设计, 以及最初3年收治患者的特征、治疗和转归。
方法. 从前瞻性保存的数据库中获取患者的临床和人口统计学数据。描述性统计数据以百分比和均值形式报告。使用 Kaplan-Meier 方法估算至事件发生时间的转归。
结果. 2012 年 1 月至 2015 年 1 月期间共有 65 例患者就诊。多数患者 (74%) 在 BCBM 诊所就诊时已经有多处 (≥2 处) 脑转移且既往曾接受过针对转移性病灶的系统性治疗 (77%) 或全脑放射治疗和/或中枢神经系统立体定向放射外科手术 (65%)。78%的患者回到诊所接受随访, 32%的患者进入临床试验。所有患者诊断为脑转移至死亡的中位时间为 2.11 年[95%可信区间 (CI) 1.31∼2.47], 三阴性乳腺癌患者为 1.15 年 (95%CI 0.4∼2.43), 激素受体+/HER2-乳腺癌患者为 1.31 年 (95%CI 0.51∼2.52), HER2+患者为 3.03 年 (95%CI 下限 1.94, 上限不可估计) (P = 0.0037)。
结论. BCBM 患者的需求独特且复杂, 需要多个肿瘤学科的投入。UNC-CH 多学科 BCBM 诊所的建立带来了难治性和综合性疾病患者的协作治疗经验, 为在其他中心建立类似诊所提供了一个范本。The Oncologist 2016;21:16–20
对临床实践的提示: 乳腺癌脑转移患者常需要多学科治疗以满足这类疾病众多的非常规需求。本文介绍了一家专为乳腺癌脑转移患者设计的诊所的建立和患者特征。这家诊所可能会成为其他有志于为类似患者建立专科诊所的中心的范本。
Introduction
Approximately 15% of women with newly diagnosed metastatic breast cancer will develop metastases to the brain [1]; however, autopsy studies show that the incidence may be as high as 30% [2]. Although all patients with breast cancer are at risk for central nervous system (CNS) recurrence, those with advanced human epidermal growth receptor 2-positive (HER2+) and triple-negative breast cancer are at highest risk, with approximately 30% and 50% developing brain metastases, respectively [3, 4].
Survival from the diagnosis of breast cancer brain metastasis (BCBM) varies by study, but historically ranges from 2 to 16 months [2, 5]. Survival after brain recurrence depends on many factors, including the extent of extracranial disease, performance status, and receipt of local or systemic therapy [5, 6]. Moreover, retrospective studies have illustrated profound differences in survival after a diagnosis of brain metastases arising from breast cancer by receptor status: approximately 15 months for luminal subtype, up to 12–18 months for HER2+, and only 4 months for triple-negative breast cancer [7–10]. Differences in outcome for patients with breast cancer brain metastases based on these many factors highlight the need for a tailored approach and coordinated care of this patient population.
At present, local therapy remains the mainstay for the majority of patients with brain metastases arising from breast cancer. Neurosurgical resection is generally reserved for situations in which the diagnosis is uncertain or there is a solitary, large, or symptomatic lesion in the brain [11]. Whole-brain radiation therapy (WBRT), typically reserved for patients with multiple brain metastases, has shown documented response in more than 60% of patients in randomized controlled studies [12]. Survival after WBRT is highly dependent on performance status, control of extracranial disease, breast cancer subtype, and patient age [13, 14]. Stereotactic radiation surgery (SRS) is generally reserved for fewer intracranial lesions (≤3) of smaller size, generally <3 cm [15]. Central nervous system recurrence rate after SRS in the salvage setting is approximately 6 months, and overall survival is 10 months [16]. Moreover, SRS can be recommended alone or with WBRT. Although neurocognitive decline is less apparent after SRS compared with SRS plus WBRT, intracranial recurrence rates are higher and more frequent [17]. Receipt of systemic endocrine therapy, chemotherapy, or biologic therapy prolongs survival in patients with hormone receptor-positive (HR+) and HER2+ breast cancer brain metastases [7]. Given the need for careful sequencing of therapeutic options as well as prompt decision-making in complex health care situations, open communication between neurosurgery, medical, and radiation oncology is essential to develop the most efficient and effective plan of care for patients with BCBM.
We have designed and implemented a weekly multidisciplinary BCBM clinic at the University of North Carolina at Chapel Hill (UNC-CH) and Lineberger Comprehensive Cancer Center. Our goal is to improve outcome, enhance quality of life, and offer novel and coordinated therapeutic strategies for patients with BCBM. To our knowledge, this is the first clinic of its kind devoted specifically to the multidisciplinary care of patients with breast cancer brain metastases. Herein, we describe the logistics of this unique clinic, patient demographics and clinical characteristics, and treatment approaches for patients seen during the first 36 months of the clinic’s operation as a mechanism to disseminate our experiences to the broader oncology community, with the goal of fostering the development of similar clinics at cancer centers both within and outside of the United States.
Materials and Methods
Clinic Organization
The University of North Carolina Chapel Hill Breast Cancer Brain Metastases Clinic was established on January 1, 2012. It is a weekly multidisciplinary clinic designed to meet the needs of patients with central nervous system metastases from breast cancer. Patients are often referred to us by their primary oncologist within or outside of the UNC system. During their initial visit to the clinic, patients are seen by radiation oncology, medical oncology, and neurosurgery (as indicated), and an initial treatment plan is recommended. Patients are offered available clinical trials, both local and systemic, as appropriate. The need for evaluation by additional supportive services such as palliative care, physical therapy, psychiatry, or nutrition is assessed, and referrals or same-day consultations are arranged as necessary. Team members include a medical oncologist specializing in breast oncology, a radiation oncologist, a neurosurgeon, a nurse practitioner, and a nurse navigator. Recent imaging studies, including brain magnetic resonance imaging (MRI) and or computed tomography, are reviewed by team members before and during each appointment. Some patients are seen for a one-time consultation; however, some patients return to the clinic for continued care with medical oncology, radiation oncology, or neurosurgery. If patients are managed by an outside medical oncologist, they return to their local medical oncologist to carry out recommended treatments. If recommended therapies are not available closer to home (i.e., in a clinical trial only), patients will have the option to transition their ongoing care during a clinical trial, for instance, to their UNC brain metastases clinic provider. Once the trial activities are complete, they have the option to return to their local medical oncologist. Patients then typically return to UNC for routine brain MRI screening every 12 weeks for continued recommendations based on their routine surveillance scans. If a patient has a UNC provider, the brain metastases consulting medical oncologist is frequently asked to meet with patients at time of progression or for clinical trial screening. As with patients from outside the UNC system, patients are able to return to their UNC provider, even if on a clinical trial, as our providers are all listed as coinvestigators on the BCBM clinical trials.
Data Collection
A protocol was designed and approved by the institutional review board to retrospectively collect and analyze basic demographic data and clinical information of patients seen at the UNC-CH Breast Cancer Brain Metastases Clinic. Patients were identified through query of the UNC Breast Cancer Metastatic Database, a prospectively maintained internal RedCap clinical database containing information pertaining to clinical factors and outcomes of metastatic breast cancer patients seen at the UNC Cancer Hospital. All information within the database was manually extracted from UNC’s electronic medical records (EPIC). Additional information was obtained from Mosaiq, the radiation oncology intake system used at UNC, and WebCis, an older electronic medical record previously used at UNC. The following information was obtained for all new patients seen in this clinic during the specified time period: interval between first clinic date and first treatment, information on referral date and return appointments, number of brain metastases, immunohistochemistry subtype (based on tissue available from craniotomy, primary breast resection, or any non-CNS metastatic site), stage at diagnosis, enrollment in clinical trials, time from diagnosis of breast cancer to brain metastases, and time from brain metastases to death. Survival status was primarily obtained from electronic medical records, but if a patient was no longer regularly seen at UNC, survival status was obtained from obituaries as well as reports from the social security death index and North Carolina Vital Statistics.
Statistical Analysis
Patients were classified into subtypes based on immunohistochemistry testing of brain tissue when available (n = 13) or by primary tumor (n = 52). Clinical breast cancer was subdivided into three categories by status of HR (estrogen receptor [ER], progesterone receptor [PR], or both) and HER2: HR+/HER2−, HR+/HER2+, and ER−, PR−, and HER2− (triple-negative breast cancer [TNBC]). Descriptive statistics are reported as percentages and means. The Kaplan-Meier method was used to estimate time-to-event outcomes, with median times reported.
Results
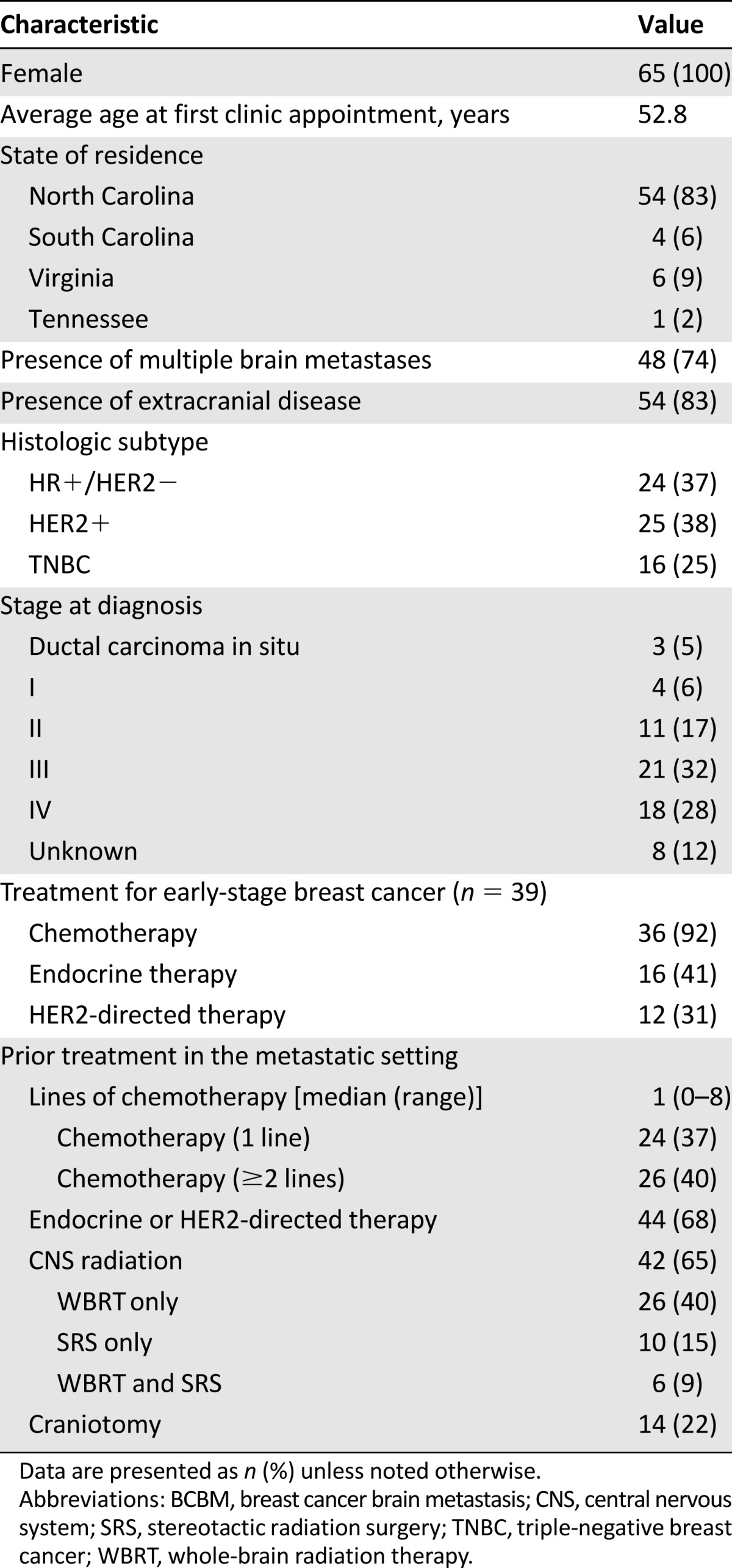
Sixty-five patients with breast cancer and brain metastases were seen in the UNC Breast Cancer Brain Metastases Clinic from January 1, 2012, to January 1, 2015. Baseline clinical characteristics at the time of presentation are summarized in Table 1. In brief, all patients were female, and average age at first clinic appointment was 52.8 years. The majority of referrals were from within North Carolina (83%), but patients from South Carolina, Tennessee, and Virginia were also seen. More than half of the patients were stage III or IV at initial breast cancer diagnosis. Breast cancer receptor status was as follows: 37% HR+, 38% HER2+, and 25% TNBC; 83% had extracranial disease. A significant proportion of patients (92%) received chemotherapy for treatment of early-stage breast cancer (premetastatic), and the majority (77%) in the metastatic setting (40% had ≥2 lines of chemotherapy in the metastatic setting). Prior adjuvant treatment with endocrine therapy (41%) and HER2-directed therapy (31%) was observed in a significant minority of patients; 68% of patients received at least one of these treatments in the metastatic setting. Nearly two thirds (65%) of patients had received CNS radiation before entering the clinic (26 of 65 [40%] received WBRT only, 10 of 65 [15%] received SRS only, and 6 of 65 [9%] received both WBRT and SRS). With regard to neurosurgery, 22% had undergone a prior craniotomy. The majority (74%) had multiple (≥2) brain metastases on presentation to the clinic.
Table 1.
Demographic and clinical characteristics of patients (n = 65) at presentation to the multidisciplinary BCBM clinic
The average time from request for a consult to first appointment was 4.5 days (median 2 days); 89% of patients were seen within 1 week. Seventy-eight percent of patients returned for a follow-up visit, and 32% of clinic patients received an investigational systemic therapy through a clinical trial. Twenty-five patients went on to receive CNS radiation treatment during the time period examined: 8 of 25 (32%) WBRT, 15 of 25 (60%) SRS, and 2 of 25 (8%) both. Seven (11%) patients underwent craniotomy after consultation at the BCBM clinic during the time period examined. Of these 7 patients, 5 (71%) had SRS and 2 (29%) had no additional radiation (none had WBRT or SRS plus WBRT). Almost all patients received systemic therapy recommendations at the initial visit, and 35 clinic patients received systemic therapy at UNC after their clinic consult: 31 (89%) received chemotherapy and 22 (63%) received hormone- or HER2-targeted therapy.
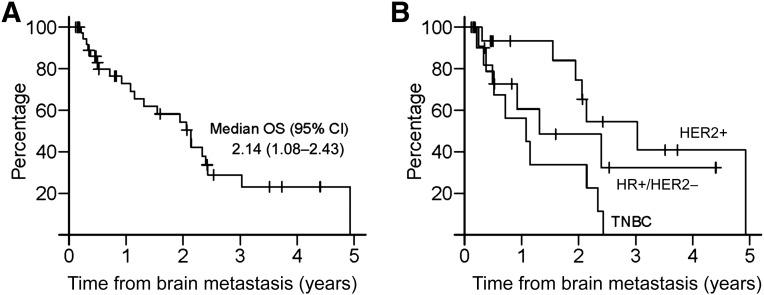
Median follow-up for patients in this cohort was 10.3 months. At the time of this analysis, 37 patients had died. The median time from diagnosis of primary breast cancer to diagnosis of brain metastases was 3.11 years (95% confidence interval [CI] 2.22–3.88) for all patients, 2.91 years (95% CI 1.19–3.46) for TNBC, 5.57 years (95% CI 2.26–8.67) for HR+/HER2−, and 1.67 years (95% CI 1.23–5.1) for HER2+ (p = .0025). The median time from diagnosis of brain metastases to death was 2.11 years (95% CI 1.31–2.47) for all patients, 1.15 years (95% CI 0.4–2.43) for TNBC, 1.31 years (95% CI 0.51–2.52) for HR+/HER2−, and 3.03 years (95% CI lower limit 1.94, upper limit not estimable) for HER2+ (p = .0037) (Fig. 1). The median time from first clinic visit to death was 1 year (95% CI 0.70–1.85) for all patients, 0.38 years (95% CI 0.3–0.72) for TNBC, 0.89 years (95% CI 0.49–1.95) for HR+/HER2−, and 1.85 years (95% CI 0.93 to not estimable) for HER2+ (p = .0016).
Figure 1.
Overall survival after diagnosis of brain metastases in all patients (A) and by subtype (B).
Abbreviations: CI, confidence interval; HR+, hormone receptor-positive; HER2+, human epidermal growth receptor 2-positive; OS, overall survival; TNBC, triple negative breast cancer.
Discussion
The mission of the UNC-CH Breast Cancer Brain Metastases Clinic is to improve outcomes, enhance quality of life, and offer new therapeutic strategies for patients with BCBM. We report on the first 36 months of our clinic, during which time 65 patients with BCBM were seen, evaluated, and treated in this unique clinic. The patient population was heterogeneous, comprising patients with newly diagnosed and untreated brain metastases as well as patients previously treated elsewhere. Most patients seen in the clinic received systemic therapy recommendations from a breast medical oncologist with particular experience treating brain metastases; more than half of patients received that systemic therapy at UNC, often on a clinical trial. More than a third of patients received radiation therapy through a radiation oncology team specializing in treatment of patients with CNS metastases. Those who required neurosurgical intervention had timely access to appropriate care, whether the intervention was performed after their initial visit or later in their disease course. Most patients returned for follow-up care, and one third were enrolled in available clinical trials appropriate for patients with CNS metastases.
To our knowledge, the UNC-CH Breast Cancer Brain Metastases Clinic is the first specialty clinic dedicated to the care of patients with brain metastases exclusively from breast cancer. A 2010 article published in Supportive Care in Cancer [18] described a pilot brain metastases clinic that was established in Alberta, Canada. It reported factors such as referral patterns, time from referral date to clinic date, basic demographic and clinical data of the patients, performance status, what services the patients required and used, and results of patient satisfaction surveys. Multiple tumor types were included in the report, but patients with lung cancer represented the majority of patients [18]. Given that patients with breast cancer brain metastases represent a distinct population with different needs from patients with non-CNS metastatic breast cancer, and whose prognosis differs from patients with brain metastases of other primary types, we believe the development of our clinic and the subsequent report of our first 3 years of experience illustrate the feasibility of effectively addressing the needs of many patients with BCBM in a specialized, multidisciplinary setting. Moreover, and given the rapid rate of clinical decline when they are left untreated, we feel that it is imperative that patients with BCBM be seen as expeditiously as possible and receive coordinated care during what can often be a narrow window of clinical stability.
One of the strengths of a dedicated BCBM clinic is the ability to offer unique opportunities for patients through clinical trial participation in relevant areas such as drug development, novel radiation techniques, neuroimaging, cancer genomics, and quality of life studies. For example, the current clinical trial portfolio at UNC includes several therapeutic clinical trials evaluating novel drug combinations and/or novel therapeutic delivery platforms for patients with breast cancer brain metastases (ClinicalTrials.gov identifiers NCT01305941 and NCT02048059), novel imaging techniques to evaluate radiation necrosis/pseudoprogression versus true tumor progression using positron emission tomography/MRI techniques, and behavioral studies including evaluation of neurocognitive outcomes after whole-brain radiation therapy in patients with newly diagnosed BCBM. Furthermore, tissue-based correlative studies are particularly important in this population. Studying the kinetics of anticancer therapy in CNS is difficult and requires multidisciplinary coordination. Studies such as the phase I dose escalation study examining plasma pharmacokinetics and cerebrospinal fluid concentration of erlotinib in patients with high-grade gliomas [19] and the recently published work by Morikawa et al. on the penetrance of lapatinib and capecitabine into breast cancer brain metastases when given preoperatively [20] could be designed and implemented seamlessly in a dedicated BCBM clinic.
Historically, survival time after a diagnosis of BCBM was 2 to 16 months [2, 5]. Evidence from our clinic and recent studies suggests that this survival is now more than 14 months to 2 years from CNS metastatic diagnosis [21, 22], including a median survival of approximately 18 months to 3 years for HER2+ disease [22]. This improvement may be due to newer therapeutic strategies and better supportive care, but also argues for a focus on coordinated and specialized care including neurosurgical, radiation oncology, and medical oncology disciplines dedicated to prolonging survival while maintaining optimal quality of life.
Although intriguing, this report of the initial experience in this clinic has several limitations. First, we evaluated a relatively small cohort of 65 patients seen over a period of 3 years, which inherently limits conclusions that can be drawn from the results. Second, to date, we have only evaluated BCBM using this model; however, we believe that this template could also be applicable to other institutions interested in developing brain tumor clinics to treat other solid tumor histologies (e.g., lung cancer, melanoma). Finally, the referral information for supportive services such as palliative care and the comprehensive cancer support program were difficult to track, because many referrals were made verbally and not captured in the medical record, and so we cannot provide accurate data on the percentage of patients who benefited from these programs. As a future direction, we are actively designing a prospective pilot study collecting symptom burden and quality of life for patients seen in the BCBM clinic. We believe this valuable information will lead to targeted interventions to best address the needs of our patients.
Conclusion
In summary, the UNC-CH Breast Cancer Brain Metastases Clinic has been successful at providing vital multidisciplinary care for a patient population facing a challenging diagnosis. In addition, this model has provided a cohesive clinical paradigm for the spectrum of providers involved in BCBM clinical care and research. As we turn toward the future, we envision expanding the scope of our services by adding a pharmacist to the team, improving coordination with our cancer support program so that we may understand how our patients benefit from available supportive services at UNC-CH, increasing our trial portfolio to include studies focused on tissue-based research, and expanding our specialized services to patients with brain metastases arising from other solid tumor types (e.g., melanoma, lung cancer) to reach a wider patient population. We believe that our experience with a dedicated, multidisciplinary clinic to treat patients with breast cancer brain metastases has been successful in providing our patients with the most comprehensive care including medical oncology, radiation oncology, and neurosurgical services in an efficient and coordinated manner. We hope that this description of the development and logistics of our clinic will be useful to other institutions who may be interested in undertaking a similar endeavor to provide such care to patients with BCBM and other solid-tumor CNS metastases.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We acknowledge our UNC Medical Oncology team (Hy Muss, Frances Collichio, Tim Brotherton, and Trevor Jolly) as well as Larry Marks, Shelley Earp, and Patricia Saponaro. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number K23CA157728. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Carey K. Anders is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-64-12). Further financial support was provided via Breast SPORE Career Development funding 2-P50-CA058223-20 for Carey K. Anders, and database support was provided in part through Komen Foundation Grant SAC110044 for E. Claire Dees.
Author Contributions
Conception/Design: Megan J. McKee, Amy L. Garrett, Matthew G. Ewend, Carey K. Anders, Timothy M. Zagar
Provision of study material or patients: Megan J. McKee, E. Claire Dees, Lisa A. Carey, Matthew G. Ewend, Carey K. Anders, Timothy M. Zagar
Collection and/or assembly of data: Megan J. McKee, Kevin Keith, Allison M. Deal, Amy L. Garrett, Amy A. Wheless, Rebecca L. Green, Julie M. Benbow, Carey K. Anders, Timothy M. Zagar
Data analysis and interpretation: Megan J. McKee, Kevin Keith, Allison M. Deal, Amy L. Garrett, Julie M. Benbow, E. Claire Dees, Lisa A. Carey, Matthew G. Ewend, Carey K. Anders, Timothy M. Zagar
Manuscript writing: Megan J. McKee, Kevin Keith, Allison M. Deal, Lisa A. Carey, Matthew G. Ewend, Carey K. Anders, Timothy M. Zagar
Final approval of manuscript: Megan J. McKee, Kevin Keith, Allison M. Deal, Amy L. Garrett, Amy A. Wheless, Rebecca L. Green, Julie M. Benbow, E. Claire Dees, Lisa A. Carey, Matthew G. Ewend, Carey K. Anders, Timothy M. Zagar
Disclosures
E. Claire Dees: Novartis, Pfizer, Merck, Eli Lilly, Cerulean, Bayer (RF); Carey K. Anders: Novartis, Sanofi, BBB Therapeutics, Geron, AngioChem, Merrimack, Eli Lilly, Genentech (C/A), Novartis, Sanofi, BBB Therapeutics, AngioChem, Merrimack, Bristol-Myers Squibb, Eli Lilly, and Puma (RF).The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Kirsch DG, Loeffler JS. Brain metastases in patients with breast cancer: New horizons. Clin Breast Cancer. 2005;6:115–124. doi: 10.3816/CBC.2005.n.013. [DOI] [PubMed] [Google Scholar]
- 2.Boogerd W, Vos VW, Hart AA, et al. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol. 1993;15:165–174. doi: 10.1007/BF01053937. [DOI] [PubMed] [Google Scholar]
- 3.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 4.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weil RJ, Palmieri DC, Bronder JL, et al. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwińska A, Murawska M, Pogoda K. Breast cancer brain metastases: Differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT) Ann Oncol. 2010;21:942–948. doi: 10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch R, Rottenfusser A, Wenzel C, et al. Trastuzumab prolongs overall survival in patients with brain metastases from HER2 positive breast cancer. J Neurooncol. 2007;85:311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- 9.Okita Y, Narita Y, Suzuki T, et al. Extended trastuzumab therapy improves the survival of HER2-positive breast cancer patients following surgery and radiotherapy for brain metastases. Mol Clin Oncol. 2013;1:995–1001. doi: 10.3892/mco.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Chen J, Yu X, et al. Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer. 2015 doi: 10.1007/s12282-015-0631-x. [DOI] [PubMed] [Google Scholar]
- 11.Ewend MG, Morris DE, Carey LA, et al. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Canc Netw. 2008;6:505–513; quiz 514. doi: 10.6004/jnccn.2008.0038. [DOI] [PubMed] [Google Scholar]
- 12.Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 13.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 14.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly PJ, Lin NU, Claus EB, et al. Salvage stereotactic radiosurgery for breast cancer brain metastases: Outcomes and prognostic factors. Cancer. 2012;118:2014–2020. doi: 10.1002/cncr.26343. [DOI] [PubMed] [Google Scholar]
- 17.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 18.Danielson B, Fairchild A. Beyond palliative radiotherapy: A pilot multidisciplinary brain metastases clinic. Support Care Cancer. 2012;20:773–781. doi: 10.1007/s00520-011-1149-1. [DOI] [PubMed] [Google Scholar]
- 19.Buie LWLC, Shih T, Ewend M, et al. Plasma pharmacokinetics and cerebrospinal fluid concentrations of erlotinib in high-grade gliomas: A novel, phase I, dose escalation study. J Clin Oncol 2007;25(18S):2054. [Google Scholar]
- 20.Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro-oncol. 2015;17:289–295. doi: 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leone JP, Lee AV, Brufsky AM. Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Med. 2015;4:989–994. doi: 10.1002/cam4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Q, Sahin AA, Hess KR, et al. Breast cancer with brain metastases: Clinicopathologic features, survival, and paired biomarker analysis. The Oncologist. 2015;20:466–473. doi: 10.1634/theoncologist.2014-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]