Abstract
Dengue fever (DF) is a vector-borne disease caused by dengue viruses (DENVs). Epidemic dengue occurs intermittently in Taiwan. In 2014, Taiwan experienced its largest DF outbreak. There were 15,732 DF cases reported. There were a total of 136 dengue hemorrhagic fever (DHF) cases, of which 20 resulted in death. Most DF cases were reported in southern Taiwan. A total of 15,043 (96%) cases were from Kaohsiung, a modern city in southern Taiwan. This report reviews DF epidemics in Taiwan during 2005–2014. The correlation between DF and DHF along with temperature and precipitation were conjointly examined. We conclude that most dengue epidemics in Taiwan resulted from imported DF cases. Results indicate three main factors that may have been associated with this DF outbreak in Kaohsiung: an underground pipeline explosion combined with subsequent rainfall and higher temperature. These factors may have enhanced mosquito breeding activity, facilitating DENV transmission.
Dengue fever (DF) is caused by dengue viruses (DENVs) infection. It is a mosquito-borne viral disease in tropical and subtropical regions of the world.1 In recent decades, the impact of dengue has increased geographically and in intensity.1 DENV belongs to the genus Flavivirus in the family of Flaviviridae. DENV contains four serotypes, which are further identified into different genotypes.2,3 In 2007, a fifth dengue serotype was detected in Malaysia,4 which may increase the complexity of diagnosis and disease development of DENV infection. In recent years, the number of countries with dengue endemic or epidemic has increased drastically. This may be due to spread of DENVs through frequent global traveling and global warming, growing numbers of susceptible human hosts, and expanding habitat of the dengue vectors Aedes mosquitoes.1,2
Taiwan is located in southeastern coast of mainland China in the western Pacific Ocean with a warm tropical and subtropical climate. Since the late nineteenth century, dengue epidemic has been circulating in southern Taiwan, especially in Kaohsiung City, which has a population of 1.5 million.5,6 Dengue vectors Aedes albopictus is found throughout Taiwan, whereas Aedes aegypti is mainly found in the south.2,5
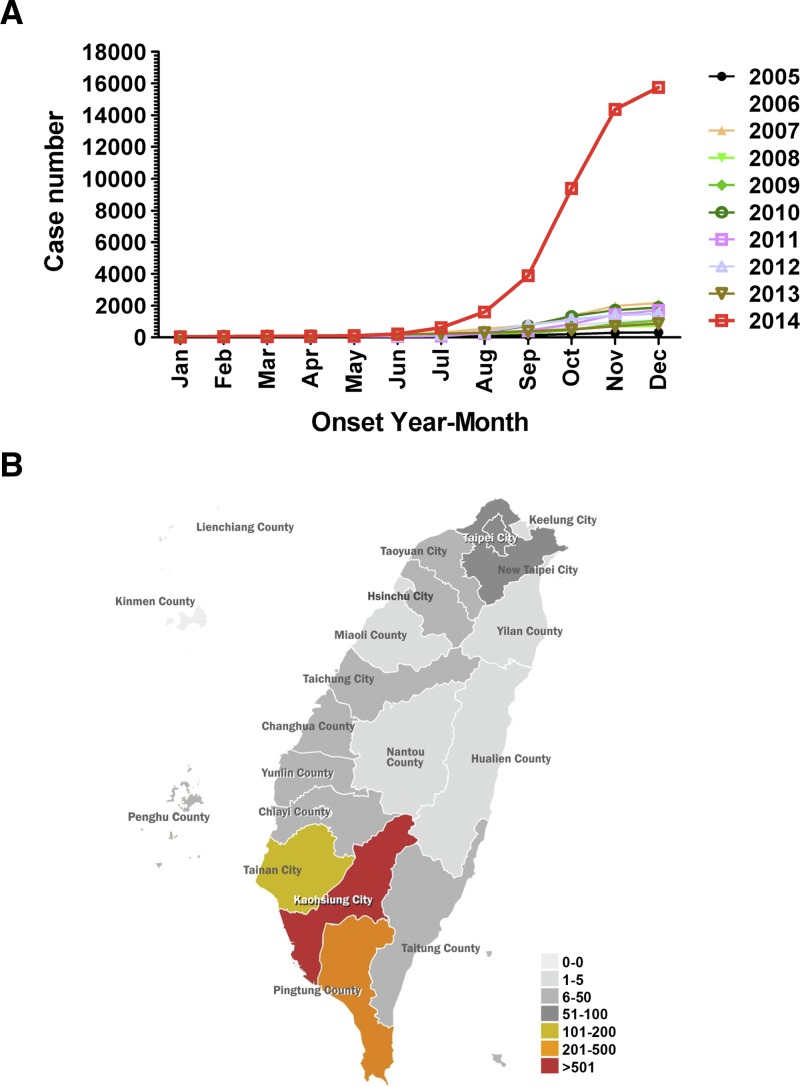
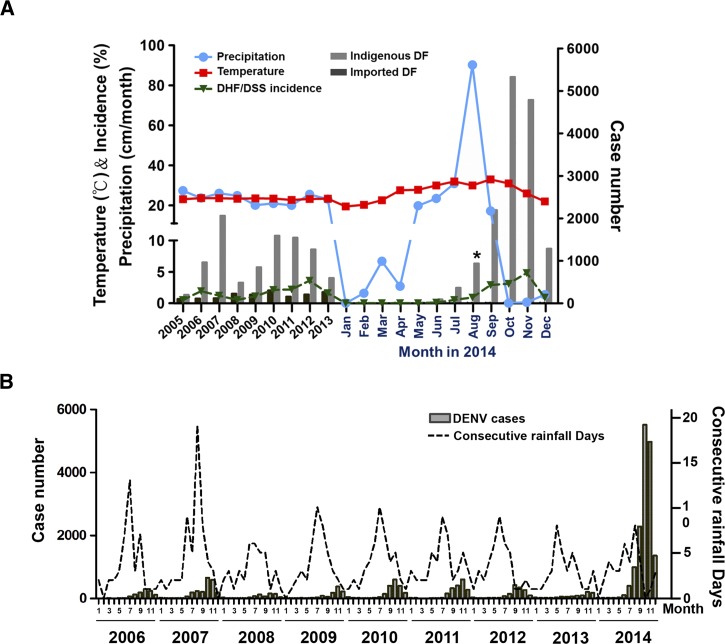
There has been the largest outbreak of DF in Taiwan in 2014 (Figure 1A ). According to the reports from the Center for Disease Control, Taiwan (Taiwan CDC),7 there were 15,732 reported cases of DF in 2014, including 15,492 indigenous cases and 240 imported cases. The dramatic increase of DF cases were reported in Kaohsiung City (15,043 cases; 95.6%)7 (Figures 1B and 2A). Results from Figure 2A illustrate the cumulative reported cases of DF in association with average precipitation and temperature from 2005 to 2013 in Taiwan (left panel) and the dramatic increase in reported DF cases in 2014 in Kaohsiung (right panel). The data indicate that DF cases in Kaohsiung were around 300–2,000 cases annually during 2005–2013. In 2014, the case numbers surged in July and August then reached its highest peak in October (5,335 cases) (Figure 2A). The cause of this dengue outbreak in Kaohsiung is still unknown.
Figure 1.
Dengue fever (DF) outbreaks in Taiwan during 2014. (A) Accumulated DF case numbers reported annually in Taiwan during 2005–2014. (B) The geographic map of the DF cases distributed in Taiwan in 2014.
Figure 2.
Dengue fever (DF) outbreaks in Taiwan. (A) Cumulative imported and indigenous DF cases reported annually in Taiwan during 2005–2013 (left) and cases reported monthly in Kaohsiung City in 2014 (right). The correlation between DF case numbers and the incidence of dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS) with average temperature and precipitation is illustrated. DHF and DSS reported case numbers and the incidence rate of DHF/DSS in Kaohsiung City and Taiwan were offered by the Department of Health, Kaohsiung City Government, and Taiwan Centers for Disease Control and Prevention. The asterisk indicates the month of pipeline explosion in Kaohsiung City. (B) The correlation between consecutive raining days and number of dengue reported cases in Kaohsiung during 2006–2014 is shown.
Kaohsiung is the center of petrochemical industry in Taiwan and many pipelines run underground Kaohsiung's streets. There was an accidental serial explosions of underground pipeline in Kaohsiung at midnight of July 31, 2014.8 The cause of this explosion is suspected to be the leakage of propylene. As a result, there were 26 deaths and 269 injured persons.8 Most people living nearby the gas explosion areas were forced to evacuate and leave their homes. There was continuous rain for several days in early August in Kaohsiung. The evacuation may have led to an increase of stagnant waters in unattended containers (such as water storage containers, flower pots, buckets, and tin cans) in the balconies, gardens and yards of the houses, as these could not be checked regularly to remove stagnant water. This may have led to an increase in habitation and potential breeding sites for mosquitoes. We found the percentage of Breteau index (BI) to be above 5–9% (level II) for the measure of mosquito density increasing from 36% to 48% during July–September 2014. DENV cases surfaced during June–August and decreased during the winter season (after December) (Figure 1A). We noted that the DENV cases increased during June and July 2014, which was similar with previous years but DENV cases dramatically increased after August 2014. These facts implicated that certain factors triggered or enhanced this outbreak.
Climate changes have a significant impact on the transmission and incidence of DF.9,10 It is essential to examine the association between climatic variables and DF epidemic. Our data show that precipitation peaked in August and the highest temperature was measured during the months of July–September in Kaohsiung. The DF cases also increased dramatically in 1–2 months after the gas explosion (Figure 2A). Taiwan CDC reported that major DF cases (10,828; 71.9%) were recorded in the districts in or nearby the gas explosion areas.7 The highest and earliest annual peak incidence of DF cases that were reported correlate with climate. We found that the average temperatures in Kaohsiung from June to September in 2014 were 0.4°C–1.5°C higher than that of previous years (Figure 2A). The factors leading to the dengue outbreak are very complicated. We analyzed the rainfall and temperature before and after pipeline explosion with DENV case numbers. We found rainfall and higher temperature significantly correlate with 2014 dengue outbreak (Spearman correlation analysis; P < 0.0001; r = 0.87 and 0.78, respectively). We therefore conclude that rainfall combined with higher temperatures enhanced the breeding and activity of mosquitoes. The underground gas explosion may be one of the factors to initiate or indirectly contribute to this dengue outbreak.
The life span of a mosquito from egg to adult lasts about 8–10 days. Consecutive raining days may play an important role contributing to dengue epidemic11 by maintaining a wet environment. This provides mosquitoes with adequate conditions for eggs to hatch and larva survival. We correlated DF occurrence with consecutive raining days and found that DF cases increased in the following 1–2 months after the long consecutive raining days (Figure 2B). For example, in 2007, after 19 continuous raining days, there were 2,179 DF cases occurring. In contrast, in 2008, after short consecutive raining days (6 days), there were only 714 DF cases occurring (Figure 2B). We noted that, in 2014, the consecutive raining days were around 5–8 days during June–August, which should not have facilitated dengue outbreak. Therefore, we suggest that other factors, such as underground pipeline explosion, higher temperature, and higher precipitation may have synergistically contributed to this dengue outbreak in 2014. Detailed temporal and geographic analyses of DENVs in Kaohsiung need to be conducted to discern the correlation between this incident and the dengue outbreak in Kaohsiung.
DENV infection was defined according to the following criteria: virus isolation, positive result of real-time polymerase chain reaction (PCR), positive seroconversion (≥ 4-fold rise in dengue-specific IgM or IgG antibody from the acute phase compared with convalescent phase) and positive result of high-titer dengue-specific IgM and IgG antibody in each serum specimen, in which cross-reaction with Japanese encephalitis had been excluded and those tested positive by nonstructural protein (NS1) antigen testing.2,12 All these 15,732 DENV cases were laboratory confirmed by Taiwan CDC using the assays mentioned above. We collected around 4,400 serum samples from laboratory-confirmed dengue cases from Kaohsiung Municipal Siaogang Hospital, Municipal Ta-Tung Hospital, and Kaohsiung Medical University Hospital. These samples were randomly selected for viral isolation. Serotypes and genotype of DENV were further confirmed by real-time reverse transcription (RT) PCR and phylogenetic analysis, respectively.13 Our results showed that all outbreak-associated DENV isolates were genotype I and serotype 1 (Table 1).
Table 1.
DF in Taiwan during 2000–2014
| Year | Imported cases | Epidemic DENVs | Phylogenetic related source | ||
|---|---|---|---|---|---|
| Imported | Indigenous | Serotype | Genotype | ||
| 2000 | 26 | 113 | DENV-4* | Genotype II | Thailand |
| 2001 | 54 | 227 | DENV-2* | Cosmopolitan | Philippines |
| 2002 | 52 | 5,336 | DENV-1 | Genotype II | Indonesia |
| DENV-2* | Cosmopolitan | Philippines | |||
| 2003 | 59 | 86 | DENV-2* | Cosmopolitan | Philippines |
| 2004 | 91 | 336 | DENV-1* | Genotype II | Philippines |
| DENV-4 | Genotype II | Vietnam | |||
| 2005 | 104 | 202 | DENV-2 | Asian/America | Vietnam |
| DENV-3* | Genotype I | Philippines | |||
| DENV-3 | Genotype II | Vietnam | |||
| 2006 | 109 | 956 | DENV-2 | Asian I | Vietnam |
| DENV-3* | Genotype II | Cambodia | |||
| 2007 | 176 | 2,000 | DENV-1* | Genotype I | Thailand |
| DENV-2 | Asian I | Vietnam | |||
| 2008 | 226 | 448 | DENV-1* | Genotype I | Vietnam/Thailand |
| DENV-2 | Asian I | Cambodia | |||
| 2009 | 205 | 857 | DENV-1 | Genotype I | Thailand |
| DENV-2 | Asian I | Vietnam | |||
| DENV-3* | Genotype I | Philippines | |||
| 2010 | 303 | 1,585 | DENV-1 | Genotype I | Vietnam/Cambodia |
| DENV-2 | Cosmopolitan | Philippines | |||
| DENV-3* | Genotype I | Philippines | |||
| DENV-4 | Genotype II | Indonesia | |||
| 2011 | 157 | 1,543 | DENV-1 | Genotype I | Dominican |
| DENV-2* | Cosmopolitan | Philippines | |||
| DENV-3* | Genotype I | Indonesia | |||
| 2012 | 207 | 1,270 | DENV-1 | Genotype I | Dominican |
| DENV-2* | Cosmopolitan | Indonesia/Thailand | |||
| DENV-3 | Genotype I | Indonesia | |||
| DENV-4 | N/A | N/A | |||
| 2013 | 261 | 596 | DENV-1 | Genotype I | Dominican/Malaysia |
| DENV-2* | Cosmopolitan | Indonesia | |||
| DENV-3 | Genotype I | Indonesia | |||
| 2014 | 245 | 15,509 | DENV-1* | Genotype I | Indonesia/Philippines |
DENVs * dengue viruses; DF * dengue fever; N/A * no available data.
The genotype and serotype were determined using phylogenetic analysis and real-time reverse transcriptase polymerase chain reaction described previously.2,13,14 The data are partially from Taiwan Centers for Disease Control and Prevention7 and the references.2,3,5
The dominant strain in that year. All these DENVs were laboratory confirmed.
Epidemiologic and immunologic studies have revealed that dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) occur when individuals have secondary infections with heterotypic DENVs.1 In this outbreak, there were 136 cases of DHF, of which 20 resulted in death. Regarding the clinical symptoms of DENV infection in this outbreak, fever, rash, lethargy and joint pain were the most common presentations of DENV-infected patients. These symptoms were similar with previous records of DENV-infected patients. Our data show incidence of DHF/DSS to be around 0.5–3.6% annually in Taiwan during 2005–2013 and 0.1–4.8% monthly in Kaohsiung during 2014 (Figure 2A). This suggests that these severe dengue-infected patients may have been previously infected with heterotypic DENVs. However, the annual incidence of DHF/DSS in 2014 in Kaohsiung was 0.88%, which is lower than the incidence in 2011–2013 (1.6–3.6%). Regarding DENV incidence in age distribution, we found DENV cases to be mainly distributed in elderly people (83.7 and 88.9 per 100,000 inhabitants of 51–60 and > 60 years old, respectively) (Supplemental Figure 1). This distribution pattern is seldom found in children (< 10 years), but the infection risk of DF increases in elder persons. The prevalence of DNEV infection is mainly prevalent in individuals above 40 years old, similar with previous DENV infections during 2005–2013 in Taiwan (Supplemental Figure 1). A study of clinical dengue incidence by Egger and others14 in communities of Brazil in 1997 indicated that the risk for clinical disease after primary dengue infection is relatively low throughout childhood and increases rapidly through adolescence and early adulthood. Recently, Thai et al.15 indicated that both primary and secondary infections, DENV infection at higher age showed to result in higher risk of severe clinical condition. They suggested age to be an important modulator of clinical dengue infection especially for the developing countries in southeast Asia.
DF cases were identified as imported or indigenous cases according to the corresponding case definition. The imported case of DF is defined as the case that 1) came from dengue endemic or epidemic regions or countries outside Taiwan with a history of being bitten by mosquito within 15 days before the onset of illness and has no history of being bitten by mosquitoes in domestic regions, 2) the viral gene sequence (such as envelope (E) gene) from DENV patients is highly homologous with gene sequence from dengue endemic or epidemic countries where the patients had traveled. Indigenous case is defined according to the absence of evidence for the case being imported. Results from Table 1 showed the serotypes and genotypes of dengue epidemics in Taiwan during 2000–2014. We found that almost four types of DENVs were detected in Taiwan during 2010–2013. The data from Taiwan CDC and partially our laboratory-confirmed DF cases (4,478 cases) by phylogenetic analysis2,3 and real-time RT-PCR13 showed that all these outbreak-associated DENVs to be of genotype I of serotype 1 (Table 1). The suspected source of this outbreak was from Indonesia or Philippines. Result in Table 1 also shows that in 2012 and 2013, the endemic dominant strain is DENV-2. This serotype changes in 2014, which may due to a new imported dengue strain from Indonesia or Philippines (Table 1). Although DENV-1 was detected in 2012–2013, the subgenotype may be different. A recent study indicates that 2014 outbreak-associated strains in Taiwan belonged to subgenotype E4, which is different from the previous endemic serotype 1 strain.16 The viral factors that potentially triggered this outbreak in Taiwan need further investigation.
Previous studies have indicated that dengue epidemic or outbreak in Taiwan often originates from imported DF cases.2,3,5 These studies also reveal the constant importation of DENVs from the neighboring southeast Asian countries. We found that earlier trends of imported DF occurrences significantly caused the later indigenous epidemics or outbreaks (Spearman correlation analysis; P < 0.0001) (Supplemental Figure 2). We noted that most epidemic DENVs in Taiwan sourced from our neighboring countries (Table 1). At present, most imported DENV cases are found through airport fever screening set up by Taiwan CDC.7,17 DF has been classified as a reportable infectious disease, and suspected cases must be reported within 24 hours of clinical diagnosis in Taiwan.17 For effective surveillance, both passive (hospital-based reporting system) and active (such as health statements from inbound passengers, self-report, expanded screening of contacts of confirmed cases, patients with fever of unknown origin) surveillance systems were established in central and local health departments. We therefore suggest that active surveillance and retrospective studies are needed to ascertain the reasons and origin of dengue epidemics or outbreaks.
In 2014, Taiwan experienced its most severe DF outbreak and most cases were reported in Kaohsiung. During the past 2 years (2014–2015) outbreaks of DF have also been reported in our neighboring countries such as China,12 Japan,18 Malaysia,19 and Singapore.19 This reminds us that we ought to pay more attention to this infectious disease as it is becoming an issue in southeastern Asian countries. We suggest that active surveillance systems, mosquito vector monitoring, and development of fast diagnostic assays and dengue vaccine should be reinforced for effective control of DENV infection and dissemination.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff from Kaohsiung Medical University Hospital and Taiwan CDC for their assistance in dengue virus identification and providing essential and meaningful data and Esmeralda Merari Erazo and Brookanna Griffth for their assistance in editing. We also thank Pei-Chi Lu at Center for Infectious Disease and Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan, for her assistance in statistical analysis.
Footnotes
Financial support: This work was supported by grants from Center for Infectious Disease and Cancer Research, Kaohsiung Medical University (KMUTP104E04 and KMUTP104E00) and Center for Disease Control, Taiwan (grant MOHW104-CDC-C-114-114901). It was also partially supported by grants from the Kaohsiung Medical University Research Foundation (KMU-Q104001) and the Ministry of Science and Technology, Republic of China (MOST 104-2321-B-037-002).
Authors' addresses: Sheng-Fan Wang and Sung-Pin Tseng, Department of Laboratory Science and Biotechnology, Kaohsiung Medical University, Kaohsiung, Taiwan, E-mails: wasf1234@kmu.edu.tw and tsengsp@kmu.edu.tw. Wen-Hung Wang and Yi-Ming Arthur Chen, Center for Infectious Disease and Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan, E-mails: bole0918@gmail.com and arthur@kmu.edu.tw. Ko Chang and Deng-Chyang Wu, Division of Internal Medicine, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, E-mails: johnsonckk@yahoo.com.tw and dechwu@yahoo.com. Yen-Hsu Chen, Department of Medicine, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan, E-mail: d810070@kmu.edu.tw. Chia-Hung Yen, Graduate Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan, E-mail: chyen@kmu.edu.tw.
References
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang JH, Su CL, Yang CF, Liao TL, Hsu TC, Chang SF, Lin CC, Shu PY. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2008–2010. Am J Trop Med Hyg. 2012;87:349–358. doi: 10.4269/ajtmh.2012.11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu PY, Su CL, Liao TL, Yang CF, Chang SF, Lin CC, Chang MC, Hu HC, Huang JH. Molecular characterization of dengue viruses imported into Taiwan during 2003–2007: geographic distribution and genotype shift. Am J Trop Med Hyg. 2009;80:1039–1046. [PubMed] [Google Scholar]
- 4.Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CH, Schioler KL, Jepsen MR, Ho CK, Li SH, Konradsen F. Dengue outbreaks in high-income area, Kaohsiung City, Taiwan, 2003–2009. Emerg Infect Dis. 2012;18:1603–1611. doi: 10.3201/eid1810.111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao DY, Lin TH, Hwang KP, Huang JH, Liu CC, King CC. 1998 dengue hemorrhagic fever epidemic in Taiwan. Emerg Infect Dis. 2004;10:552–554. doi: 10.3201/eid1003.020518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC . Taiwan National Infectious Disease Statistics System for Dengue Virus. Center for Disease Control; Taiwan: 2015. http://nidss.cdc.gov.tw/en/SingleDisease.aspx?dc=1&dt=2& disease=061 Available at. [Google Scholar]
- 8.Sui C. Taiwan Gas Blasts in Kaohsiung Kill at Least 25. BBC News. 2014. http://www.bbc.com Available at.
- 9.Banu S, Hu W, Hurst C, Tong S. Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop Med Int Health. 2011;16:598–607. doi: 10.1111/j.1365-3156.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 10.Sang S, Gu S, Bi P, Yang W, Yang Z, Xu L, Yang J, Liu X, Jiang T, Wu H, Chu C, Liu Q. Predicting unprecedented dengue outbreak using imported cases and climatic factors in Guangzhou, 2014. PLoS Negl Trop Dis. 2015;9:e0003808. doi: 10.1371/journal.pntd.0003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez-Lazaro P, Muller-Karger FE, Otis D, McCarthy MJ, Pena-Orellana M. Assessing climate variability effects on dengue incidence in San Juan, Puerto Rico. Int J Environ Res Public Health. 2014;11:9409–9428. doi: 10.3390/ijerph110909409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin X, Lee M, Shu J. Dengue fever in China: an emerging problem demands attention. Emerg Microbes Infect. 2015;4:e3. doi: 10.1038/emi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong YY, Thay CH, Tin TC, Devi S. Rapid detection, serotyping and quantitation of dengue viruses by TaqMan real-time one-step RT-PCR. J Virol Methods. 2006;138:123–130. doi: 10.1016/j.jviromet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Egger JR, Coleman PG. Age and clinical dengue illness. Emerg Infect Dis. 2007;13:924–925. doi: 10.3201/eid1306.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thai KT, Nishiura H, Hoang PL, Tran NT, Phan GT, Le HQ, Tran BQ, Nguyen NV, de Vries PJ. Age-specificity of clinical dengue during primary and secondary infections. PLoS Negl Trop Dis. 2011;5:e1180. doi: 10.1371/journal.pntd.0001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang SF, Chang K, Lu RW, Wang WH, Chen YH, Chen M, Wu DC, Chen YM. Large dengue virus type 1 outbreak in Taiwan. Emerg Microbes Infect. 2015;4:e46. doi: 10.1038/emi.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuan MM, Chang FY. Airport sentinel surveillance and entry quarantine for dengue infections following a fever screening program in Taiwan. BMC Infect Dis. 2012;12:182. doi: 10.1186/1471-2334-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuda Y, Maekawa Y, Ogawa K, Itokawa K, Komagata O, Sasaki T, Isawa H, Tomita T, Sawabe K. Jpn J Infect Dis. 2015. Biting density and distribution of Aedes albopictus during the September 2014 outbreak of dengue fever in Yoyogi Park and the vicinity in Tokyo Metropolis, Japan. [Epub ahead of print, 2015 Mar 13] [DOI] [PubMed] [Google Scholar]
- 19.Ng LC, Chem YK, Koo C, Mudin RN, Amin FM, Lee KS, Kheong CC. 2013 dengue outbreaks in Singapore and Malaysia caused by different viral strains. Am J Trop Med Hyg. 2015;92:1150–1155. doi: 10.4269/ajtmh.14-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.