Abstract
The aim of this study was to evaluate the quality of B cell responses in patients with Inflammatory Bowel Disease (IBD) and healthy individuals of different ages, vaccinated with the pandemic (p)2009 influenza vaccine. The in vivo response was measured by the hemagglutination inhibition (HAI) assay, which represents the most established correlate with vaccine protectiveness. The in vitro response was measured by activation-induced cytidine deaminase (AID) in cultures of vaccine-stimulated PBMC. Both responses are somewhat impaired in IBD patients undergoing anti-TNF-α treatment but these are much more decreased in IBD patients undergoing treatment with anti-TNF-α and immunosuppressive (IS) drugs. These latter patients had in vivo and in vitro B cell responses similar to those of elderly individuals. Moreover, as we have previously demonstrated in healthy subjects, the in vitro response to the polyclonal stimulus CpG may be used as a biomarker for subsequent vaccine response and AID activation is correlated with the serum response in IBD patients, as it is in healthy individuals. These results altogether indicate that IBD patients on anti-TNF-α and IS have significantly impaired in vivo and in vitro B cell responses, as compared to those on monotherapy. This is the first report to demonstrate that B cell defects, as measured by the autonomous AID reporter, in IBD patients contribute to reduced humoral responses to the influenza vaccine, as we have previously shown for elderly individuals.
Keywords: AID (activation-induced cytidine deaminase), B lymphocytes, IBD (Inflammatory Bowel Disease), p2009 (pandemic 2009) influenza vaccine, aging
Introduction
Activation-induced cytidine deaminase (AID) is the essential enzyme inducing DNA cleavage required for both somatic hypermutation (SHM) and class switch recombination (CSR) of Ig genes [1,2]. These processes are crucial for the generation of high affinity antibodies and robust humoral immunity. AID is not required for normal B cell development [3,4], but is expressed by activated B cells, mainly in germinal centers (GCs) of peripheral lymphoid organs [5]. SHM and CSR occur in GCs in response to both T-dependent and T-independent stimuli [5,6]. AID can also be induced in vitro in human purified B lymphocytes or peripheral blood mononuclear cells (PBMCs) and is a B cell biomarker which recapitulates the quality of the in vivo antibody response [7–10].
AID triggers SHM and CSR by deaminating cytosines in the variable and switch regions of the Ig locus [11,12]. Individuals unable to class switch have been described and include those with hyper-IgM (HIGM) syndromes: HIGM1, due to a genetic defect in the CD40L expressed on T cells [13,14]; HIGM2, due to mutations in Aicda, the gene coding for AID [15]; HIGM3, due to mutations in the cd40 gene [16]; HIGM4, due to a defect in CSR downstream of AID which does not affect SHM [17], and HIGM5, due to mutations in the ung gene, coding for the DNA repair enzyme uracil-DNA glycosylase which is involved in early steps of CSR and SHM [18]. From studies in humans, it has been suggested that AID plays a role in inducing B cell tolerance, as indicated by the fact that HIGM patients which have high levels of serum anti-nuclear IgM antibodies are prone to develop autoimmune diseases [19].
However, an alternative interpretation of these findings can be supported by the following results. AID-deficient MRL/lpr mice have high levels of auto-reactive IgM antibodies and these are protective for survival [20–23]. These results suggest that autoreactive IgM antibodies are in general protective natural antibodies but in HIGM patients these may suffer from the same kind of autoimmunity that is seen across the board with immunodeficient patients regardless of the defect: they expand inflammatory cell populations to deal with infection since they are immunodeficient.
We [10,24] and others [25] have shown that specific B cell defects occur in aging mice and humans and these include decreases in CSR, AID and the transcription factor E47 which activates Aicda [26,27]. Defects in AID levels in patients with Inflammatory Bowel Disease (IBD) have not been reported yet. IBD patients are often placed on long term anti-inflammatory therapies (anti-TNF-α), alone or with immunosuppressive (IS) drugs (azathioprine, methotrexate). Azathioprine is known to control all cellular proliferation including the overwhelming activation of lamina propria T lymphocytes in the colon of IBD patients through pro-apoptotic and anti-proliferative effects [28,29], therefore affecting mainly adaptive immunity. Methotrexate also inhibits T cell activation and suppresses adhesion molecule expression [30]. Anti-TNF-α antibodies act through different mechanisms: by blocking TNF-α, they block TNFR signalling and they also bind to transmembrane TNF-α inducing apoptosis of activated lymphocytes and monocytes.
Additionally, they induce antibody-dependent cell mediated cytotoxicity in TNF-α expressing cells. Therefore, they affect both adaptive and innate immunity [31]. Although a majority of IBD patients do not develop serious infections, reports of life-threatening infections have been published and these occur primarily in patients receiving IS [32,33]. Based on our previous work with the elderly, where there is also an increase in inflammation [34–38], we hypothesized that increased inflammation would also lead to decreased B cell function in IBD patients.
In the present study we performed an evaluation of the humoral B cell/serum antibody response to the pandemic (p)2009 influenza vaccine in IBD patients undergoing anti-TNF-α therapy alone, or in combination with IS, and correlated this with specific in vitro B cell measures to assess the contribution of B cells to the serum response in these individuals at risk for infections. Responses in IBD patients were compared to those in healthy elderly individuals. Results of this study show that IBD patients on monotherapy have a reduced B cell response to the p2009 vaccine, both in vivo and in vitro. IBD patients under combined therapy, have an even more reduced response, as compared to healthy adult controls and this response is similar to that in elderly individuals.
Methods
Subjects
Experiments were conducted using blood isolated from volunteers after appropriate written informed consent. The IBD patients and controls were recruited at the Universita’ Cattolica del Sacro Cuore, Rome (Italy), whereas other healthy young and elderly individuals were recruited at the University of Miami Miller School of Medicine. Both protocols were approved by internal committees. We have analyzed here a subgroup of those who have participated in our previous studies, one on IBD patients [39] and another on healthy individuals of different ages [9], with the aim to correlate in vivo and in vitro B cell responses to the p2009 vaccine, which was done only on those patients and controls which were able to also give blood to perform the in vitro tests.
All individuals in the study were screened for diseases known to alter the immune response or for consumption of medications that could alter the immune response, as in our previously published work on seasonal influenza vaccination [8]. In order to have a random sampling of subjects, we selected only those recruited in both locations in December 2009. All subjects were influenza-free at the time of enrollment and at the time points of blood draws and were also without symptoms associated with respiratory infections. Moreover, presence of relevant co-morbidities was recorded and no IBD patients had cancer or infectious diseases in the 6 months prior to enrollment.
Influenza vaccination
The study was conducted during the pandemic 2009 influenza season. Two vaccines were used: Novartis Focetria (MF59-adjuvanted) and Novartis NDC 66521-200-10 (non-adjuvanted). The first was given to the patients and controls of the Universita’ Cattolica del Sacro Cuore, Rome, whereas the second one was given to the young and elderly individuals recruited at the University of Miami. The adjuvanted vaccine was the only p2009 influenza vaccine available in Italy. Vaccine was well tolerated in IBD patients and no one reported a flare of IBD 1, 3 or 6 months after vaccination. All IBD patients and elderly individuals received the seasonal influenza vaccine in the previous 3 years and in the current season. Only 20% of the young subjects, conversely, were vaccinated with seasonal vaccine in the previous and/or in the current season.
Hemagglutination inhibition (HAI) assay
We evaluated the immunogenicity of the influenza vaccine by comparing sera before (t0) and after vaccination (t28) by HAI assay, as we have previously described [8,9]. The HAI assay is based on the ability of certain viruses or viral components to hemagglutinate the red blood cells of specific animal species. Antibodies specific to influenza can inhibit this agglutination. The HAI test is useful for the measurement of antibody titers of sera and is the most established correlate with vaccine protectiveness [40,41]. Paired pre- and post-immunization serum samples from the same individual were tested simultaneously. Briefly, sera were pretreated with receptor destroying enzyme (RDE, Denke Seiken Co Ltd., Tokyo, Japan) for 20 hours at 37°C; in order to inactivate this enzyme, sera were then heated at 56°C for 60 minutes.
Two-fold serial dilutions were done; 25 μl of diluted sera were incubated with an equal volume of 4 HA units of whole vaccine, for 1 hour at room temperature and then 50 μl of a 1.25% suspension of chicken red blood cells were added. After 2 hours of incubation at room temperature titers were determined. Serum inhibiting titers of 1/40 or greater are the defined positive measure of seroprotection against infection, whereas a four-fold rise in the reciprocal of the titer from t0 to t28 indicates a positive response to the vaccine and indicates seroconversion [8,9,40,42].
PBMC cultures
PBMC were collected by density gradient centrifugation on Ficoll-Paque premium solution (GE Healthcare, Uppsala, Sweden). Cells were then washed three times with PBS and cyropreserved (frozen) with 90% FBS and 10% DMSO. Frozen PBMC (10 × 106) were thawed in a 37°C waterbath and washed twice with medium (RPMI 1640), resuspended, rested for 1 hour and then counted in trypan blue to evaluate cell viability which was usually over 80%. PBMC (1 × 106/ml) at t0 and t28 were cultured at the same time in complete medium (RPMI 1640, supplemented with 10% FCS, 10 μg/ml Pen-Strep, 1 mM Sodium Pyruvate, and 2 × 10−5 M 2-ME and 2 mM L-glutamine), stimulated for 3 days in 24-well culture plates with CpG (ODN 2006 InvivoGen) at the concentration of 1 μg/106 cells or with influenza vaccine (2 μl/106 cells). We have previously shown that the percentages of B cells do not change between t0 and t28.
Moreover, B cell percentages in PBMC cultures were comparable at least for young and elderly individuals (8 ± 0.54 and 6 ± 0.65, respectively). For each subject, the vaccine used to stimulate PBMC in vitro was the same as that given in vivo. Vaccines contained comparable amounts of H1N1 protein (~0.75 μg/μl). At the end of stimulation, cells were harvested, and RNA extracted for quantitative (q)PCR to evaluate AID and GAPDH mRNA expression. Although B cells in the PBMC cultures have been stimulated in the presence of other cell types, primarily T cells and monocytes - macrophages, our endpoint is to measure a B cell response, as AID is exclusively expressed in B cells. In previous studies we have compared the response of purified B cells with PBMC and the fold-increase (t28/t0) in vitro to the vaccine is comparable [9]. Therefore, the contribution of T cells to this is not significant.
RNA extraction and quantitative (q)PCR
mRNA was extracted from stimulated PBMC (106/ml) using the μMACS mRNA isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer’s protocol, eluted into 75 μl of preheated elution buffer, stored at −80°C until use, and qPCR performed as described. Calculations were made as follows. We determined the cycle number at which transcripts reached a significant threshold (Ct) for AID and GAPDH as control. A value for the target gene, relative to GAPDH, was calculated and expressed as ΔCt. We have chosen a two-fold rise in AID mRNA from t0 to t28 to indicate a positive response to the vaccine.
Statistical analyses
Non-parametric analyses of the variables were performed by the Mann-Whitney test (two-tailed), whereas correlations were performed by the Spearman test, using GraphPad Prism 5 software.
Results
Decreased serum response to the p2009 vaccine in IBD patients and in the elderly
Demographic and serological information on the participants are in Table 1. IBD patients were divided into 2 groups: those undergoing anti-TNF-α therapy (n = 10) and those on anti TNF-α combined with IS (n = 6). Combined therapy was with azathioprine/6MP (2–2.5 mg/kg) or methotrexate (10–15 mg/week), same doses for at least 4 months.
Table 1.
Demographic and serological characteristics of the individuals participating in the study.
| IBD anti-TNF-α | IBD anti-TNF-α + IS | Controls | Elderly | Young | |
|---|---|---|---|---|---|
| Participants (n) | 10 | 6 | 5 | 12 | 21 |
| Age (mean yrs ± SE) | 45 ± 4 | 50 ± 8 | 33 ± 6 | 68 ± 1 | 37 ± 3* |
| Gender (M/F) | 3/7 | 1/5 | 1/5 | 7/5 | 10/11 |
| Vaccine | adjuvanted | adjuvanted | adjuvanted | non-adjuvanted | non-adjuvanted |
| Type of disease (Crohn’s/UC) | 5/5 | 3/3 | na | na | na |
| Disease duration (yrs) | 9 ± 2 | 11 ± 2 | na | na | na |
| anti-TNF-α (INFX/Adalimumab) | 7/3 | 3/3 | na | na | na |
| IS (AZA/MTX) | na | 4/2 | na | na | na |
| ESR | 28 ± 5 | 25 ± 8 | na | na | na |
| TNF-α (pg/ml) | 4.5 ± 0.4 | 3.9 ± 0.3 | 3 ± 0.2 | 7 ± 0.9 | 2 ± 0.3** |
| CRP (pg/ml) | 658 ± 37 | 657 ± 26 | 563 ± 35 | 1709 ± 33 | 693 ± 29** |
| IL-6 (pg/ml) | 66 ± 3 | 65 ± 3 | 56 ± 3 | 98 ± 7 | 48 ± 2* |
p-values refer to differences between elderly and young individuals:
p < 0.05,
p < 0.01 (Mann-Whitney, two-tailed);
na: not applicable; INFX: Infliximab; IS: immunosuppressants; AZA: azathioprine; MTH: methotrexate; ESR: erythrocyte sedimentation rate. Please, see also Methods for further description of the subjects.
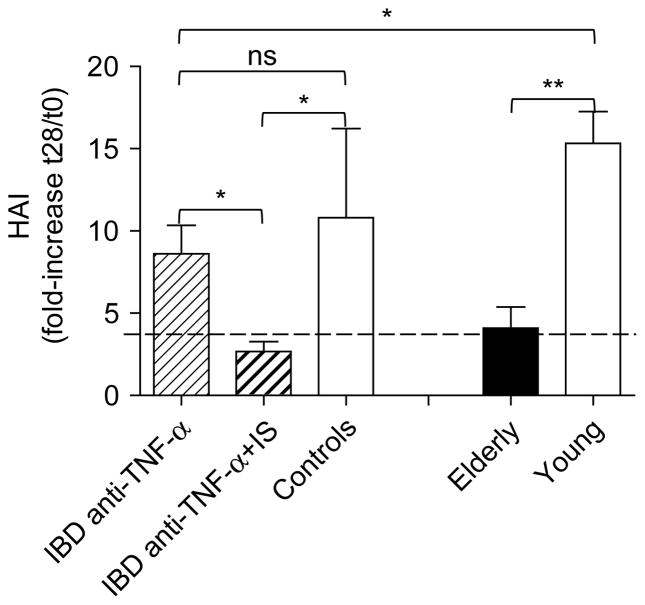
We analyzed the serum response to the p2009 influenza vaccine in IBD patients and controls, vaccinated with the adjuvanted vaccine, as well as in young and elderly individuals, vaccinated with the non-adjuvanted vaccine. Among IBD patients, 5/10 under monotherapy and 4/6 under combined therapy had a seroprotective titer (1/40) at t0. Among elderly individuals, 10/12 had a seroprotective titer at t0. Controls and young individuals were all seroprotected at t0. The specific response to the p2009 vaccine, expressed as fold-increase in reciprocal of the titers after vaccination, is shown in Figure 1.
Figure 1.
Decreased serum response to the p2009 vaccine in IBD patients and in the elderly. Sera isolated before (t0) and after vaccination (t28) were evaluated in HAI. Results are expressed as fold-increase in the reciprocal of the titer after vaccination, calculated as follows: reciprocal of titer values after vaccination/reciprocal of titer values before vaccination. The line indicates a positive response (4-fold increase in reciprocal of the titers). Non-parametric analyses were calculated by Mann-Whitney test (two-tailed), using GraphPad Prism 5 software. *p < 0.05, **p < 0.01. The numbers of individuals are given in Table I.
Due to the small number of individuals in the control group vaccinated with the adjuvanted vaccine and to the high variability in their response, the difference with IBD patients on monotherapy is not significant. However, IBD patients on monotherapy showed a significantly reduced response as compared to healthy young individuals. IBD patients on combined therapy had a significantly reduced response as compared to either the group on mono-therapy or controls, and this response is comparable to that of healthy elderly individuals. We previously published in a larger number of these IBD patients that those on monotherapy had a suboptimal serum response but those on the combination therapy had an even worse response to the p2009 vaccine [39].
Decreased in vitro B cell response to the p2009 vaccine in IBD patients and in the elderly
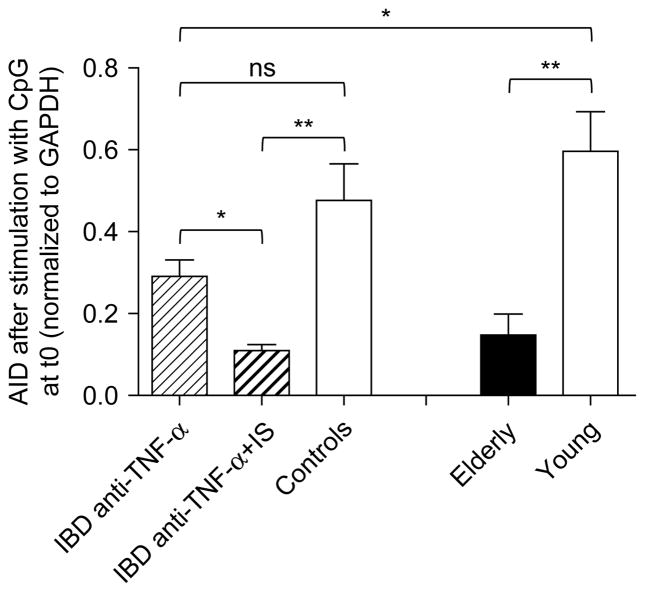
We next analyzed the in vitro B cell response to the vaccine in the same individuals as above. We used AID as a biomarker for optimal B cell function because we have shown that it correlates with the ability of B cells to undergo CSR [10]. Moreover, we have previously shown that B cells can be efficiently stimulated in vitro by the influenza vaccine to induce AID and this response is up-regulated in young as compared to elderly subjects and more importantly it correlates with the in vivo HAI response [8,9]. In order to evaluate the B cell response to the vaccine in vitro, we stimulated frozen PBMC with the p2009 influenza vaccine, an approach that we have previously validated [9].
Results in Figure 2, expressed as fold-increase in AID mRNA expression after vaccination, show that IBD patients on combined therapy had a significantly reduced AID response as compared to either the group on monotherapy or controls, and this response is comparable to that of healthy elderly individuals. Again, the difference between IBD patients on monotherapy and controls is not significant. However, IBD patients on monotherapy showed a significantly reduced in vitro B cell response to the p2009 vaccine as compared to healthy young individuals. We should note that the IBD patients were 40–58 years of age (Table 1) and we have previously shown that healthy individuals of this age maintain a “young phenotype”, similar to the controls here [10]. Therefore, controls in Table 1 should not be different from the IBD patients based on age.
Figure 2.
Decreased in vitro B cell response to the p2009 vaccine in IBD patients and in the elderly. Frozen PBMC (106 cells/ml), isolated from the peripheral blood at t0 and t28, were thawed and cultured with the vaccine for 3 days. At the end of this time, cells were processed as described in Methods. Results are expressed as fold-increase in AID mRNA expression after vaccination, calculated as follows: qPCR values (ΔCt) after vaccination/qPCR values before vaccination. The line indicates a positive response (2-fold increase in AID mRNA). Non-parametric analyses were performed by Mann-Whitney test (two-tailed), using GraphPad Prism 5 software. *p < 0.05, **p < 0.01. The numbers of individuals are given in Table I.
Decreased CpG-induced B cell stimulation in IBD patients and in the elderly at t0
We then measured AID mRNA expression induced by CpG in the same individuals as above. The CpG response was measured at t0, as we have shown that this can predict the robustness of the in vivo vaccine response. Results in Figure 3, expressed as raw qPCR values of AID mRNA expression in response to CpG at t0, show that IBD patients on combined therapy respond significantly less than those on monotherapy or controls, and also this response is comparable to that of healthy elderly individuals.
Figure 3.
Decreased CpG-induced B cell stimulation in IBD patients and in the elderly at t0. Frozen PBMC (106 cells/ml), isolated from the peripheral blood at t0, were thawed and cultured with CpG, for 3 days. At the end of this time, cells were processed as described in Methods. Results are expressed as raw qPCR values (ΔCt) of AID mRNA normalized to GAPDH. Non-parametric analyses were performed by Mann-Whitney test (two-tailed), using GraphPad Prism 5 software. *p < 0.05, **p < 0.01. The numbers of individuals are given in Table I.
Again, the difference between IBD patients on monotherapy and controls is not significant. However, IBD patients on monotherapy showed a significantly reduced in vitro B cell response to the p2009 vaccine as compared to healthy young individuals. As opposed to our previous studies [7–10,43], the elderly individuals here did not have significantly fewer B cells (5–14% in the young and 3–12% in the elderly). Even so, we have also normalized the AID values here to the pecentage of B cells in each culture (data not shown) and the results are still significantly different between young and elderly (p = 0.0012). B cell data were not available for IBD patients and controls.
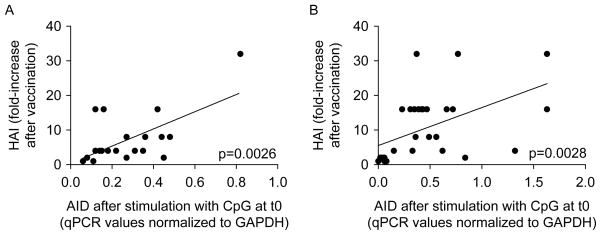
CpG-induced AID at t0 and the serum response are correlated in IBD patients and healthy individuals
We then correlated the CpG-induced AID response at t0 with the fold-increase in the HAI serum response. Results in Figure 4 show that these were significantly correlated in both IBD patients and healthy controls. These results confirm our previous evidence that CpG-induced AID measures the competence of B cells and can effectively predict the ability to generate optimal specific humoral responses.
Figure 4.
CpG-induced AID at t0 and the serum response are correlated in IBD patients and healthy individuals. Frozen PBMC were cultured with CpG, for 3 days. IBD patients and controls (A) as well as healthy young and elderly individuals (B) were evaluated. IBD patients and controls in A were recruited at the Universita’ Cattolica del Sacro Cuore, Rome (Italy) and received the MF-59 adjuvanted vaccine, whereas healthy young and elderly individuals in B were recruited at the University of Miami Miller School of Medicine and received the non-adjuvanted vaccine. Correlations between CpG-induced AID at t0 and HAI were calculated by Spearman test, using GraphPad Prism 5 software. p-values (two-tailed) are indicated in each graph.
Discussion and Conclusions
Only a few studies have evaluated the influenza vaccine response in adult IBD patients. These studies have found that the serum response to vaccination is lower [39,44] or similar [45,46] in patients on combined therapy as compared to those on mono-therapy. No studies have been performed to correlate B cell function with the serum influenza vaccine response. Here we show that the in vivo and the in vitro B cell responses to the p2009 influenza vaccine, as well as the non specific response of B cells to CpG, are decreased in IBD patients on monotherapy and more in those on combined therapy, as compared to young controls, and IBD patients on combined therapy respond in a similar way as elderly individuals do. We have also measured the response of B cells to CpG before vaccination, because we have previously shown in healthy young and elderly individuals that this response predicts the robustness of the vaccine. We have extended our findings showing that also in IBD patients AID can be used as a predictive marker for B cell responsiveness.
IS therapies have been widely used for several chronic immune-mediated diseases, including IBD, which results from an abnormal immune response against intestinal bacteria [47]. Although beneficial for the control of the disease, these therapies represent an increased risk of infections for the patients. Guidelines have been established to vaccinate immunosuppressed patients with vaccines against infectious diseases which can be prevented using available vaccines [48]. Similar guidelines have been established for IBD patients [49,50]. However, despite these recommendations, vaccination rates in patients at risk for infectious diseases are still low [51].
The ability to make optimal immune responses to vaccines declines with age as we [8,9] and others [40,41,52,53] have previously shown. Age-related changes in immune cells have been shown to be responsible for a suboptimal response. The importance of intrinsic B cell defects to this decline and some molecular biomarkers for B cell deficiencies with age have been elucidated in our laboratory and include decreases in CSR, AID and E47 [8–10]. Conversely, the contribution of B cells to vaccine responses in IBD patients has not been addressed so far. Mucosal inflammation in IBD patients has been shown to generate activated B cells which can recirculate in blood.
These activated B cells express high levels of TLR2, produce large amounts of pro-inflammatory cytokines and show increased ex vivo levels of phosphorylated signaling proteins such as Btk, p38 and Syk [54]. The presence of circulating anti-microbial antibodies in IBD patients (e.g., anti-flagellin antibodies and anti-Saccharomyces cerevisiae) has also been described and indicates B cell activation [55]. However, we have shown in mice [56] and we also have data in humans (Frasca et al., manuscript in preparation) that the basal hyperactivation status of B cells and the constitutive levels of B cell-derived pro-inflammatory cytokines are negative regulators of CSR and humoral responses.
In conclusion, our results show an impairment of in vivo and in vitro B cell responses in IBD patients and more in those patients undergoing combined therapy. These patients on combined therapy had in vivo and in vitro B cell responses similar to those of elderly individuals. As we have previously demonstrated, the intrinsic defect in AID in aged B cells is a general phenomenon, and therefore not limited to the influenza vaccine response. We have used the AID response as a biomarker for specific B cell response to the vaccine as we have before demonstrated that it correlates with the in vivo serum response. Moreover, AID activation in response to the polyclonal stimulus CpG can predict the serum response in IBD patients, as it is in healthy individuals. These results suggest possible screening protocols to identify those individuals who could benefit from more robust vaccination protocols, e.g., double dose or adjuvant or other immune-boosting interventions.
Acknowledgments
We would like to express our gratitude to the people who participated in this study. We thank nurses and doctors at the Universita’ Cattolica del Sacro Cuore in Rome and at the Family Medicine Department of the University of Miami Miller School of Medicine, in particular Dr. Robert Schwartz, chairman and Susie Batista, for the recruitment of healthy volunteers; and Dr. Sandra Chen-Walta, Employee Health Manager. This study was supported by NIH AG-32576 (BBB).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Okazaki IM, Kinoshita K, Muramatsu M, Yoshikawa K, Honjo T. The AID enzyme induces class switch recombination in fibroblasts. Nature. 2002;416:340–345. doi: 10.1038/nature727. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa K, I, Okazaki M, Eto T, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, Kinoshita K, Fagarasan S, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pone EJ, Zan H, Zhang J, Al-Qahtani A, Xu Z, Casali P. Toll-like receptors and B-cell receptors synergize to induce immunoglobulin class-switch DNA recombination: relevance to microbial antibody responses. Crit Rev Immunol. 2010;30:1–29. doi: 10.1615/critrevimmunol.v30.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;31:430–435. doi: 10.1007/s10875-010-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasca D, Diaz A, Romero M, et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine. 2010;28:8077–8084. doi: 10.1016/j.vaccine.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frasca D, Diaz A, Romero M, et al. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012;24:175–182. doi: 10.1093/intimm/dxr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasca D, Landin AM, Lechner SC, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu M, Nagaoka H, Shinkura R, Begum NA, Honjo T. Discovery of activation-induced cytidine deaminase, the engraver of antibody memory. Adv Immunol. 2007;94:1–36. doi: 10.1016/S0065-2776(06)94001-2. [DOI] [PubMed] [Google Scholar]
- 12.Rada C, Williams GT, Nilsen H, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 13.Levy J, Espanol-Boren T, Thomas C, et al. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 14.Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol Rev. 2005;203:48–66. doi: 10.1111/j.0105-2896.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Durandy A. Hyper-IgM syndromes: a model for studying the regulation of class switch recombination and somatic hypermutation generation. Biochem Soc Trans. 2002;30:815–818. doi: 10.1042/bst0300815. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari S, Giliani S, Insalaco A, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci USA. 2001;98:12614–12619. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai K, Zhu Y, Revy P, et al. Analysis of class switch recombination and somatic hypermutation in patients affected with autosomal dominant hyper-IgM syndrome type 2. Clin Immunol. 2005;115:277–285. doi: 10.1016/j.clim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Imai K, Slupphaug G, Lee WI, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 19.Meyers G, Ng YS, Bannock JM, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci USA. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Foley J, Clayton N, et al. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C, Zhao ML, Scearce RM, Diaz M. Activation-induced deaminase-deficient MRL/lpr mice secrete high levels of protective antibodies against lupus nephritis. Arthritis Rheum. 2011;63:1086–1096. doi: 10.1002/art.30230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannoor K, Matejuk A, Xu Y, Beardall M, Chen C. Expression of natural autoantibodies in MRL-lpr mice protects from lupus nephritis and improves survival. J Immunol. 2012;188:3628–3638. doi: 10.4049/jimmunol.1102859. [DOI] [PubMed] [Google Scholar]
- 24.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 25.Gibson KL, Wu YC, Barnett Y, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quong MW, Romanow WJ, Murre C. E protein function in lymphocyte development. Annu Rev Immunol. 2002;20:301–322. doi: 10.1146/annurev.immunol.20.092501.162048. [DOI] [PubMed] [Google Scholar]
- 27.Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Horin S, Goldstein I, Fudim E, et al. Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut. 2009;58:396–403. doi: 10.1136/gut.2008.157339. [DOI] [PubMed] [Google Scholar]
- 29.Poppe D, Tiede I, Fritz G, et al. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol. 2006;176:640–651. doi: 10.4049/jimmunol.176.1.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114:154–163. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch DE, Olson AD, Kraker S, Dickinson CJ. Overwhelming varicella pneumonia in a patient with Crohn’s disease treated with 6-mercaptopurine. J Pediatr Gastroenterol Nutr. 1995;20:351–353. doi: 10.1097/00005176-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Korelitz BI, Fuller SR, Warman JI, Goldberg MD. Shingles during the course of treatment with 6-mercaptopurine for inflammatory bowel disease. Am J Gastroenterol. 1999;94:424–426. doi: 10.1111/j.1572-0241.1999.871_w.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Franceschi C, Valensin S, Bonafe M, et al. The network and the remodeling theories of aging: historical background and new perspectives. Exp Gerontol. 2000;35:879–896. doi: 10.1016/s0531-5565(00)00172-8. [DOI] [PubMed] [Google Scholar]
- 37.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 38.Wikby A, Nilsson BO, Forsey R, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Andrisani G, Frasca D, Romero M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: Effects of combined therapy with immunosuppressants. J Crohns Colitis. 2012 doi: 10.1016/j.crohns.2012.05.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murasko DM, Bernstein ED, Gardner EM, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 41.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 42.Ito T, Suzuki Y, Mitnaul L, et al. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology. 1997;227:493–499. doi: 10.1006/viro.1996.8323. [DOI] [PubMed] [Google Scholar]
- 43.Frasca D, Diaz A, Romero M, Landin AM, Blomberg BB. Age effects on B cells and humoral immunity in humans. Ageing Res Rev. 2011;10:330–335. doi: 10.1016/j.arr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2012;61:385–391. doi: 10.1136/gutjnl-2011-300256. [DOI] [PubMed] [Google Scholar]
- 45.Gelinck LB, van der Bijl AE, Beyer WE, et al. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis. 2008;67:713–716. doi: 10.1136/ard.2007.077552. [DOI] [PubMed] [Google Scholar]
- 46.Molnar T, Farkas K, Jankovics I, et al. Appropriate response to influenza A (H1N1) virus vaccination in patients with inflammatory bowel disease on maintenance immunomodulator and/or biological therapy. Am J Gastroenterol. 2011;106:370–372. doi: 10.1038/ajg.2010.395. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 48.ACIP. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins for persons with altered immunocompetence. MMWR Recomm Rep. 1993;42:1–18. [PubMed] [Google Scholar]
- 49.Rahier JF, Yazdanpanah Y, Viget N, Travis S, Colombel JF. Review article: influenza A (H1N1) virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31:5–10. doi: 10.1111/j.1365-2036.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- 50.Sands BE, Cuffari C, Katz J, et al. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677–692. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 51.Melmed GY, Ippoliti AF, Papadakis KA, et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840. doi: 10.1111/j.1572-0241.2006.00646.x. [DOI] [PubMed] [Google Scholar]
- 52.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–429. doi: 10.1007/978-3-540-92165-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29:2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noronha AM, Liang Y, Hetzel JT, et al. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007–1016. doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frasca D, Romero M, Diaz A, et al. A molecular mechanism for TNF-alpha-mediated downregulation of B cell responses. J Immunol. 2012;188:279–286. doi: 10.4049/jimmunol.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]