Abstract
The flavor profile of Chinese liquor is the result of the metabolic activity of its microbial community. Given the importance of the microbial interaction, a novel way to control the liquor's flavor is by regulating the composition of the community. In this study, we efficiently improved the liquor's flavor by perturbing the intrinsic microbial metabolism with extrinsic microbes. We first constructed a basic microbial group (intrinsic) containing Saccharomyces cerevisiae, Wickerhamomyces anomalus, and Issatchenkia orientalis and added special flavor producers (extrinsic), Saccharomyces uvarum and Saccharomyces servazzii, to this intrinsic group. Upon the addition of the extrinsic microbes, the maximum specific growth rates of S. cerevisiae and I. orientalis increased from 6.19 to 43.28/day and from 1.15 to 14.32/day, respectively, but that of W. anomalus changed from 1.00 to 0.96/day. In addition, most volatile compounds known to be produced by the extrinsic strains were not produced. However, more esters, alcohols, and acids were produced by S. cerevisiae and I. orientalis. Six compounds were significantly different by random forest analysis after perturbation. Among them, increases in ethyl hexanoate, isobutanol, and 3-methylbutyric acid were correlated with S. cerevisiae and I. orientalis, and a decrease in geranyl acetone was correlated with W. anomalus. Variations in ethyl acetate and 2-phenylethanol might be due to the varied activity of W. anomalus and S. cerevisiae. This work showed the effect of the interaction between the intrinsic and extrinsic microbes on liquor flavor, which would be beneficial for improving the quality of Chinese liquor.
INTRODUCTION
Traditional fermented foods are mainly prepared by a spontaneous fermentation process carried out by complex communities of microorganisms (1, 2). These microbial systems are resistant to certain environmental stresses to some extent, and they have stable structures and metabolic activities (3, 4). However, the metabolic activity of the natural microbial community can be variable. Therefore, inoculation of specially formulated starter cultures into the natural fermentation community has been used to improve its activity (5–7). This method has become attractive in the fermented food industry because it has several excellent advantages, including shortening of fermentation times, reducing the risk of spoilage, improving sensory qualities, and increasing safety attributes (6). For example, to improve the aroma and color of cheese, some yeast and bacterial strains have been inoculated to inhibit or eliminate undesired microorganisms that cause quality defects (8). The use of Saccharomyces cerevisiae resulted in the suppression of potential spoilage yeasts in fermentation, ensuring constant product characteristics and quality (9). The inoculation of Bacillus licheniformis also improved the flavor profile of Chinese Maotai-flavored liquor by increasing the amount of acids, aromatic compounds, phenols, and pyrazines (10).
However, the use of selected microbes did not always achieve positive qualities, because the dominance of the inoculated strains was not always assured. The most important reason is that the microbial interaction between the inoculated microbes (extrinsic) and the naturally occurring microbes (intrinsic) is poorly understood. There are various types of microbial interactions, including neutralism, commensalism, mutualism or synergism, amensalism or antagonism, predation or parasitism, and competition (11). These interactions can be classified into two groups in food fermentation: promotion and inhibition (11). These interactions influence the metabolic activity of each microbe and, hence, influence the total function of the community. Therefore, the extrinsic microbes might be inhibited by the natural microbes, or they might change the original interactions, resulting in unsuccessful fermentation due to suboptimal structure and function of the community. As a result, microbial interactions play an important role in the structure and function of microbial communities (5, 12), and it is important to examine the microbial interactions and their variations with perturbation from extrinsic microbes and to investigate the effects of perturbation on the structure and function of microbial communities.
Chinese light-style liquor is one of the oldest traditional alcoholic beverages in China (13). A typical example is Fen liquor, with its renowned “light-fragrance” flavor. In our previous work, we reported the microbial community structure in Fen liquor fermentation. We found that S. cerevisiae, Issatchenkia orientalis, and Wickerhamomyces anomalus were the most important species in the production of the characteristic Fen liquor (14). As a result, we were able to produce Fen liquor using a starter culture containing only these three strains. Recently, we isolated two additional flavor producers, Saccharomyces uvarum and Saccharomyces servazzii, from another type of Chinese light-style liquor with a flavor different from that of Fen liquor. We hypothesized that these two strains might contribute to the flavor difference between these two liquors. In addition, there might also be two different reasons for this contribution: direct production of flavor compounds by the additional strains and their effects on the metabolism of the intrinsic microbes. In this study, we investigated whether these two extrinsic strains might influence the flavor profile of the liquor and how this effect influences the microbial metabolism and the liquor flavor profile.
MATERIALS AND METHODS
Strains.
The following strains were isolated from the Chinese light-style liquor-making process (15) and deposited in the China General Microbiological Culture Collection Center (CGMCC): S. cerevisiae CGMCC 8130, I. orientalis CGMCC 4741, W. anomalus CGMCC 4740, S. servazzii CGMCC 8132, and S. uvarum CGMCC 8134.
Mixed-culture fermentations.
The fermentation medium was prepared with sorghum as previously reported (16). The original total reducing sugar in the extract was about 45.5 g/kg. This medium was sealed in 2-liter beakers and autoclaved at 121°C for 15 min. Yeast cultures were inoculated by sterile loops in 250-ml Erlenmeyer flasks with 50 ml sorghum extract, which was prepared as previously reported (16). Fermentations were conducted at 150 rpm and 30°C for 48 h. Yeast cell numbers were determined using a hemocytometer (16). These strains were then inoculated into solid-state sorghum in beakers for mixed fermentation, with the initial cell density of each strain adjusted to 1 × 105 CFU/g. The total added volume was made the same by addition of sorghum extract. All fermentations were conducted without agitation at 30°C for 20 days.
Fermentations were conducted with single components, double combinations of S. cerevisiae, I. orientalis, and W. anomalus, and higher-order combinations (basal cocultures of S. cerevisiae, I. orientalis, and W. anomalus; basal cultures with S. servazzii and S. uvarum alone and in combination). A noninoculated sample of fermentation medium was prepared as the control. All experiments were performed in triplicate.
Enumeration of different yeast strains.
Each sample (10 g) was mixed with 90 ml sterile saline (0.85% NaCl) and soaked at 4°C for 30 min. Yeast enumeration was carried out on Wallerstein Laboratory nutrient (WLN) medium (17), in which the five strains showed different macroscopic features, which were detailed in a previous report (15). According to the macroscopic features (texture, surface, margin, elevation, and color), colonies of different types on the WLN medium were counted separately (15, 16). Standard deviations were calculated from triplicate repetitions of the fermentation.
Analysis of reducing sugar and ethanol.
Fermented sorghum (10 g) was mixed with 90 ml distilled water, ultrasonically treated at 0°C for 30 min, and centrifuged at 8,000 × g at 4°C for 5 min. The supernatant obtained was used to determine the contents of reducing sugar and ethanol. The reducing sugar was analyzed using the 3,5-dinitrosalicylic acid method (18). The ethanol content was determined by high-performance liquid chromatography (Agilent 1200 system) using a column (Aminex HPX-87H, 300 mm by 7.8 mm; Bio-Rad) and a refractive index (RI) detector (Schambeck SFD GmbH) (19). Standard deviations were calculated from triplicate repetitions of the fermentation.
Volatile compound analysis.
The fermented grain (5 g) was mixed with 20 ml sterile saline (0.85% NaCl, 1% CaCl2) and soaked overnight. After ultrasonic treatment at 0°C for 30 min in a capped bottle, the mixture was centrifuged at 8,000 × g at 4°C for 10 min. The supernatant was filtered through a 0.22-μm filter and stored at −20°C until analysis (15). Volatile compounds in fermented sorghums were assayed by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (6890N gas chromatograph and 5975 mass selective detector; Agilent) and on a DB-Wax column (30 m by 0.25-mm inside diameter [i.d.], 0.25-μm film thickness; J&W Scientific). The internal standard was 4-methyl-2-pentanol, and the final concentration was 54.50 μg/liter. Standard deviations were calculated from triplicate repetitions of the fermentation.
Statistical analysis.
Heat maps were generated and cluster analyses of the flavor compounds were performed in the statistical environment R, version 2.15.3. All flavor data were normalized by SPSS 19.0 (IBM, Armonk, NY, USA). The specific growth rate was calculated by Origin 8.0 (OriginLab) with the derivative equation reported previously (20), with cell number/(cell number · day) as a complete unit (abbreviated here as per day).
Random forest analysis was used to measure the importance of flavor compounds and to obtain the significant differentiated flavor compounds among different mixed cultures. It was used to compute two qualitative measures. One value (increased mean square error [%IncMSE]) measured the importance of the variables (flavor compounds) in the ability to reduce the mean square error. If the random permutation of the compounds drastically changed the mean square error, then the compound was considered critical. The other value (increased impurity index [IncNodePurity]) measures the total increase in the homogeneity of the data samples from splitting them in a given compound. A higher IncNodePurity value represents higher importance of the compound (21). Volatile compounds, which had %IncMSE values of >6% and IncNodePurity values of >10, were considered to be critical to differentiate different mixed cultures. These analyses were performed in the statistical environment R, version 2.15.3 (R Development Core Team, 2010) using the “random forest” library.
RESULTS
Influence of extrinsic yeasts on cell growth in intrinsic mixed-culture fermentation.
The main intrinsic yeast species for making Chinese Fen liquor are S. cerevisiae, W. anomalus, and I. orientalis. We constructed mixed cultures using combinations of two or all three strains under simulated liquor fermentation conditions. The growth curves of these three yeast strains in mixed-culture fermentation are shown in Fig. 1A to D. Except for the culture of W. anomalus-I. orientalis, the growth of all yeast strains slightly declined in the initial time of this mixed-culture fermentation. This might be due to the adaptation of the cells to this environment.
FIG 1.
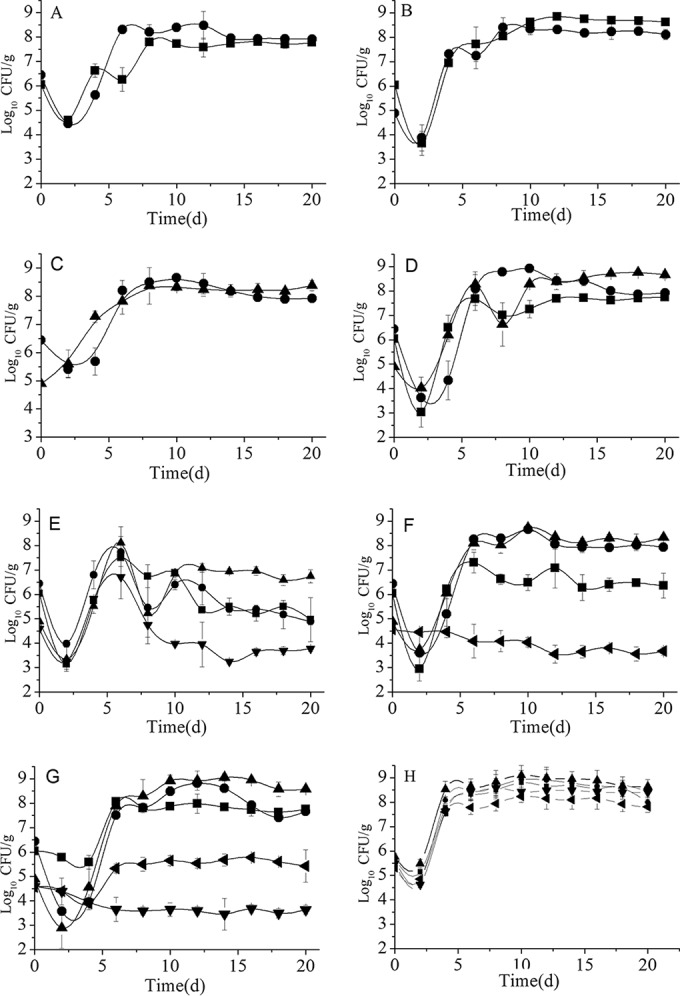
Yeast growth profiles in different mixed-culture fermentations. (A) S. cerevisiae-W. anomalus; (B) S. cerevisiae-I. orientalis; (C) W. anomalus-I. orientalis; (D) S. cerevisiae-W. anomalus-I. orientalis; (E) S. cerevisiae-W. anomalus-I. orientalis-S. uvarum; (F) S. cerevisiae-W. anomalus-I. orientalis-S. servazzii; (G) S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii; (H) single cultures. ■, S. cerevisiae; ▲, I. orientalis; ●, W. anomalus; ◀, S. servazzii; ▼, S. uvarum.
W. anomalus reached maximum populations of 3.0 × 108 and 4.5 × 108 CFU/g at 12 and 10 days, respectively, and then decreased to the final population of 8.3 × 107 CFU/g when grown in the presence of S. cerevisiae or I. orientalis (Fig. 1A and C). However, it grew better and the maximum population reached 8.3 × 108 CFU/g when all three strains were present (Fig. 1D). The growth curve of I. orientalis was unaffected by the presence of S. cerevisiae or W. anomalus. It reached the maximum value of 2.5 × 108 to 2.3 × 108 CFU/g at 8 days, respectively, and then was nearly stable until the end of fermentation (Fig. 1B and C). However, in the presence of S. cerevisiae and W. anomalus (Fig. 1D), its growth quickly increased to 1.9 × 108 CFU/g at 6 days and further increased to 5.6 × 108 CFU/g at 18 days, indicating that the growth of I. orientalis is more vigorous in the triple combination than in the double combinations. The growth of S. cerevisiae was lower than that of the other two strains when grown in the presence of W. anomalus or W. anomalus and I. orientalis, and its maximum populations reached 6.1 × 107 and 5.1 × 107 CFU/g, respectively (Fig. 1A and D). However, it grew better in the coculture with I. orientalis and maintained a population of around 1.1 × 108 to 6.9 × 108 CFU/g after 8 days (Fig. 1B). Therefore, W. anomalus and I. orientalis grew better than S. cerevisiae in the basal coculture of the three intrinsic strains.
S. uvarum and S. servazzii were unique strains in another type of Chinese light-style liquor, and they produced special flavors in liquor fermentation (15). To improve flavor, they were added to this intrinsic Fen liquor coculture. The effect of these two strains on the cell growth of intrinsic yeast strains was investigated. As shown in Fig. 1E, when S. uvarum was added, the growth of all of the intrinsic yeast strains decreased after 6 days. However, the growth of W. anomalus and I. orientalis slightly increased after 8 days, and I. orientalis became the dominant strain with a population of 5.5 × 106 to 1.2 × 107 CFU/g at the end of fermentation, while W. anomalus slightly decreased to 7.9 ×104 CFU/g and S. cerevisiae decreased to 9.2 × 104 CFU/g. This indicated that the addition of S. uvarum inhibited the growth of all three intrinsic strains. When S. servazzii was added (Fig. 1F), it was unable to propagate. However, the growth of S. cerevisiae was still affected, and its population decreased from 5.6 × 107 to 2.3 × 106 CFU/g at the end of fermentation. The final population number of I. orientalis also decreased about 2-fold, from 3.6 × 108 to 1.8 × 108 CFU/g, while W. anomalus was unaffected. When S. uvarum and S. servazzii were both added to the basal coculture of the three intrinsic strains (Fig. 1G), the population of S. uvarum gradually decreased about 10-fold until the end of fermentation, while that of S. servazzii gradually increased 10-fold at 6 days and then stayed stable. Compared with the population in the basal intrinsic culture, the maximum populations of S. cerevisiae and I. orientalis in the five-strain mixed culture increased from 5.6 × 107 to 9.5 × 107 CFU/g and 5.6 × 108 to 8.9 × 108 CFU/g, respectively. However, the growth of W. anomalus was slightly inhibited and the final population decreased from 8.31 × 107 to 4.64 × 107 CFU/g at the end of fermentation. The more vigorous growth of S. cerevisiae and I. orientalis in the five-strain combination indicated that the inhibition from S. uvarum and S. servazzii might be weakened to some extent.
In order to determine the effects of S. uvarum and S. servazzii on the microbial interactions among the three intrinsic yeast strains, we determined the maximum specific growth rate of each strain in different cultures (Table 1). The specific growth rate of S. cerevisiae is nearly the same in single culture and double cocultures. However, it increased upon the addition of both extrinsic microbes and was much higher than those of W. anomalus and I. orientalis, although the final biomass of S. cerevisiae was less than those of the other two strains. The higher maximum specific growth rate of S. cerevisiae might be due to the increasing metabolic activity when it was challenged with the disturbance from the combination of W. anomalus and I. orientalis. However, the initial fast growth consumes much more energy and leads to less biomass at the end of fermentation. While the maximum specific growth rates of W. anomalus and I. orientalis were higher in double cocultures than in single cultures, they decreased upon the addition of both extrinsic microbes, which might be due to the stronger competition from S. cerevisiae. This suggests that different combinations lead to different growth rates and metabolic activities.
TABLE 1.
Maximum specific cell growth rates in different mixed-culture fermentations
| Culture | Maximum specific growth rate (day−1) for: |
||||
|---|---|---|---|---|---|
| S. cerevisiae | W. anomalus | I. orientalis | S. uvarum | S. servazzii | |
| Single culture | 1.03 | 0.88 | 0.89 | 1.47 | 1.47 |
| S. cerevisiae-W. anomalus | 0.90 | 2.11 | |||
| S. cerevisiae-I. orientalis | 1.38 | 2.20 | |||
| W. anomalus-I. orientalis | 1.88 | 1.88 | |||
| S. cerevisiae-W. anomalus-I. orientalis | 6.19 | 1.00 | 1.15 | ||
| S. cerevisiae-W. anomalus-I. orientalis-S. uvarum | 2.62 | 7.52 | 3.07 | 8.45 | |
| S. cerevisiae-W. anomalus-I. orientalis-S. servazzii | 7.77 | 1.11 | 0.94 | 0 | |
| S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii | 43.28 | 0.96 | 14.32 | 0 | 0.84 |
When S. uvarum was added in the basal intrinsic coculture, the maximum specific growth rate of S. cerevisiae decreased, while those of W. anomalus and I. orientalis increased, which might be due to decreased competition from S. cerevisiae. However, when S. servazzii was added to the intrinsic coculture, the maximum specific growth rate of S. cerevisiae increased slightly, while those of W. anomalus and I. orientalis remained nearly the same. This suggests a contradictory relationship between the growth activities of S. cerevisiae and W. anomalus/I. orientalis. However, when both extrinsic strains were added, the maximum specific growth rate of W. anomalus changed from 1.00 to 0.96/day, while those of S. cerevisiae and I. orientalis increased remarkably from 6.19 to 43.28/day and 1.15 to 14.32/day, respectively. This suggests that S. uvarum and S. servazzii influence the competition between S. cerevisiae and I. orientalis.
Influence of extrinsic yeasts on ethanol production in the mixed-culture fermentation.
The influence of S. uvarum and S. servazzii on ethanol production in the mixed-culture fermentation was investigated, and the results are shown in Table 2. The fermentation efficiencies of W. anomalus-I. orientalis and S. cerevisiae-I. orientalis were lower than those of other cultures, and the reducing sugar contents were about 15% and 20% of the total reducing sugar at the end of fermentation, respectively. Besides these two cultures, all other cultures grew and fermented well, and the reducing sugar was <10% of the total reducing sugar after 14 days.
TABLE 2.
Fermentation parameters in different mixed-culture fermentationsa
| Culture | Fermentation time (day) | Maximum biomass (108 CFU/kg) | Maximum ethanol concn (g/kg) | Consumed reducing sugar (g/kg) | Consumed sugar (10−8 g/cell) | Ethanol per reducing sugar (g/g) | Ethanol (10−8 g/cell) |
|---|---|---|---|---|---|---|---|
| S. cerevisiae | 16 | 3.05 ± 0.42 B | 18.58 ± 1.21 H | 41.2 ± 1.8 C | 13.29 | 0.45 | 5.99 |
| W. anomalus | 18 | 4.27 ± 0.64 B | 6.13 ± 0.70 D | 41.0 ± 2.1 C | 9.53 | 0.15 | 1.43 |
| I. orientalis | 20 | 6.77 ± 0.79 C | 1.78 ± 0.21 A | 36.2 ± 1.4 A | 5.32 | 0.05 | 0.26 |
| S. uvarum | 20 | 3.77 ± 0.64 B | 16.29 ± 1.02 G | 35.9 ± 1.6 A | 9.45 | 0.45 | 4.29 |
| S. servazzii | 20 | 0.88 ± 0.22 A | 5.90 ± 0.42 C | 39.1 ± 1.2 B | 43.44 | 0.15 | 6.56 |
| W. anomalus-I. orientalis | 20 | 6.60 ± 0.72 C | 4.12 ± 0.36 B | 38.6 ± 2.0 B | 5.85 | 0.11 | 0.62 |
| S. cerevisiae-W. anomalus | 14 | 3.39 ± 0.53 B | 11.03 ± 0.96 F | 42.1 ± 1.7 C | 12.42 | 0.26 | 3.25 |
| S. cerevisiae-I. orientalis | 20 | 8.96 ± 0.64 D | 11.86 ± 0.72 F | 36.0 ± 1.0 A | 4.02 | 0.33 | 1.32 |
| S. cerevisiae-W. anomalus-I. orientalis | 14 | 10.30 ± 0.42 E | 7.89 ± 0.61 E | 41.8 ± 2.2 C | 4.06 | 0.19 | 0.77 |
| S. cerevisiae-W. anomalus-I. orientalis-S. uvarum | 14 | 2.21 ± 0.39 B | 6.80 ± 0.73 D | 41.0 ± 1.2 C | 18.55 | 0.17 | 3.08 |
| S. cerevisiae-W. anomalus-I. orientalis-S. servazzii | 14 | 9.78 ± 0.60 E | 9.63 ± 0.88 E | 41.3 ± 1.6 C | 4.22 | 0.23 | 0.98 |
| S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii | 14 | 16.08 ± 0.69 F | 15.36 ± 1.21 G | 41.2 ± 1.7 C | 2.56 | 0.37 | 0.96 |
n = 3. Values followed by different letters are significantly different for different cultures at a P of <0.05.
In single-culture fermentation, the ethanol content of all cultures reached a maximal value at 15 days and then decreased. S. cerevisiae and S. uvarum produced 18.58 and 16.29 g/kg ethanol in single cultures, respectively, indicating that they were excellent ethanol producers, while W. anomalus, I. orientalis, and S. servazzii produced only 6.13, 1.78, and 5.90 g/kg ethanol, respectively, indicating that they were not active ethanol producers.
The total biomass in the S. cerevisiae-W. anomalus-I. orientalis-S. uvarum culture was the lowest among those for the mixed cultures. The ethanol production and conversion rate of sugar to ethanol were also the lowest in this culture, except for the culture with nonactive ethanol producers W. anomalus and I. orientalis. In the S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii culture, the total microbial population and ethanol production were the highest among those for the mixed cultures. Compared with the basal intrinsic coculture of three yeast strains, the sugar consumed per cell was the lowest and the conversion rate of sugar to ethanol was the highest for the S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii culture, even though the total consumed sugar was nearly the same for the two cultures. Since S. uvarum and S. servazzii strongly improved the growth of S. cerevisiae, S. cerevisiae grew much better and might produce more ethanol in the five-strain culture than in the basal coculture of the three intrinsic strains. Additionally, the five-strain culture had a high conversion rate of sugar to ethanol, because W. anomalus and I. orientalis have weak ethanol productivity and S. uvarum and S. servazzii had their maximal biomasses of 5.76 × 103 CFU/g and 6.00 × 105 CFU/g, which were far lower than that of S. cerevisiae. This indicates that a more harmonious interaction was beneficial for the function of the microbial community as less substrate is consumed in competition between the microbes; consequently, more substrate is used for ethanol production.
Influence of extrinsic yeasts on flavor metabolism in mixed-culture fermentation.
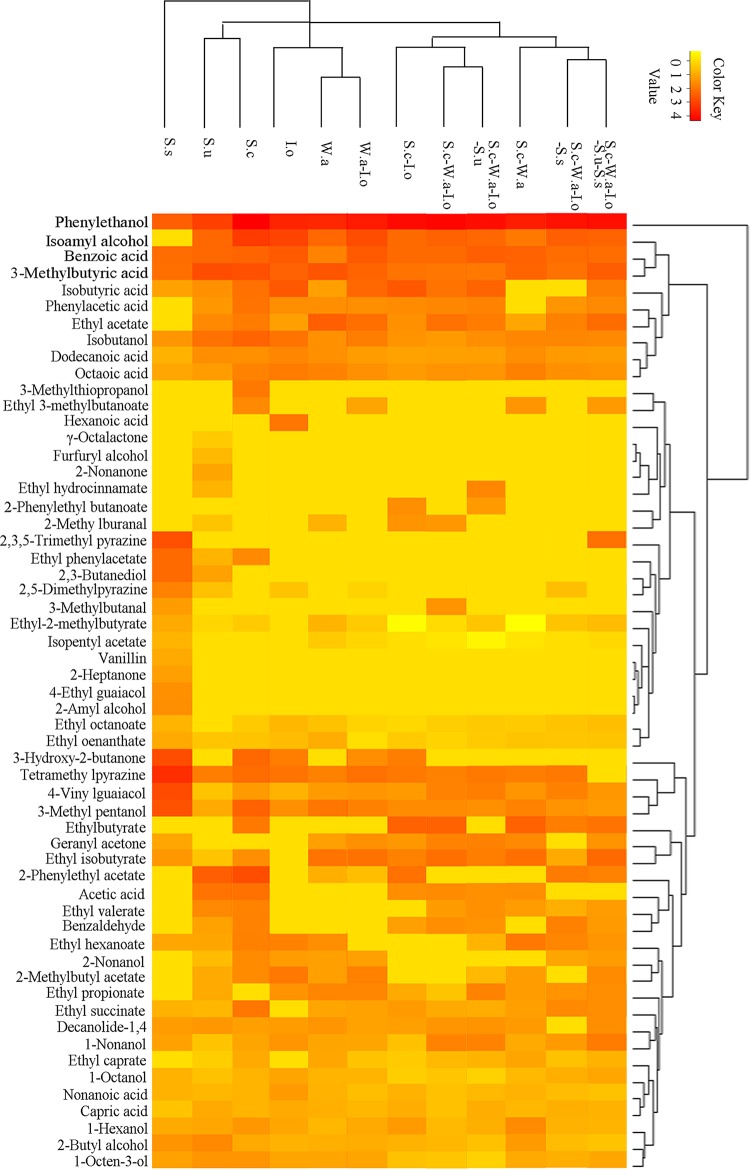
The volatile compound profile is a main characteristic of a microbial community in the making of fermented foods. To investigate the effect of extrinsic yeast strains on the metabolic activity of the intrinsic mixed culture, the volatile compounds in different mixed cultures were determined. A total of 57 volatile compounds were detected. Hierarchical clustering analysis was performed based on the volatile compounds in different cultures. The tree represents the cluster of 12 different mixed-culture fermentations. The distance indicates the close association of different cultures, and cultures appearing close in the tree are those that had a close proximity in the cluster. As shown in Fig. 2, based on the volatile compounds, cultures were efficiently differentiated.
FIG 2.
Hierarchical clustering of volatile compounds in mixed fermentations. Yellow indicates no correlation between the cultures and the flavor compounds, and red indicates high correlation between the cultures and the flavor compounds. S.c, S. cerevisiae; I.o, I. orientalis; W.a, W. anomalus; S.s, S. servazzii; S.u, S. uvarum.
S. servazzii is a special flavor-producing strain. Its profile of flavor metabolites was significantly different from those in the other cultures. It uniquely produced 2-amyl alcohol, 2-heptanone, and 4-vinylguaiacol, and it was characterized by high production of various flavor compounds, including ethyl 2-methylbutyrate, ethyl 3-methylbutanoate, ethyl oenanthate, 3-methyl pentanol, 2,3-butanediol, ethyl phenylacetate, 3-hydroxy-2-butanone, 2,5-dimethyl pyrazine, 2,3,5-trimethyl pyrazine, and tetramethyl pyrazine. S. uvarum uniquely produced 2-nonanone, furfuryl alcohol, 3-methylthiopropanol, and ethyl hydrocinnamate. Compared with other single-strain cultures, it was characterized by high production of 2-butyl alcohol and 3-methylbutyric acid. However, most of the special volatile compounds produced by S. servazzii and S. uvarum were not produced in the mixed culture. This is most likely a result of their weak growth in the mixed-culture fermentation. Thus, the effects of S. uvarum and S. servazzii on flavor production are probably not due to their specific metabolic activities when added to the intrinsic cultures.
S. uvarum and S. cerevisiae were classified in one cluster and showed similar metabolic activities. They were both characterized by high production of ethyl valerate, isobutanol, 1-octen-3-ol, acetic acid, 3-methylbutyric acid, and 2-phenylethyl acetate.
W. anomalus-I. orientalis and W. anomalus were grouped in one cluster. They were characterized by high contents of ethyl acetate and ethyl propionate. They were further classified in one higher cluster with I. orientalis. The three cultures were special, with high contents of 2-methylbutyl acetate, octanoic acid, and 2-phenylethanol.
The other cultures were classified into two different clusters. S. cerevisiae-W. anomalus-I. orientalis-S. uvarum, S. cerevisiae-W. anomalus-I. orientalis, and S. cerevisiae-I. orientalis were in the same cluster, with uniquely high contents of benzaldehyde and geranyl acetone. When the growth of S. uvarum was inhibited, the metabolic activities of W. anomalus and S. uvarum were not exhibited, while I. orientalis and S. cerevisiae were not inhibited by other strains, and they still exhibited excellent metabolic activities. This indicated that S. cerevisiae and I. orientalis are the main flavor producers in these three cultures.
The S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii, S. cerevisiae-W. anomalus-I. orientalis-S. servazzii, and S. cerevisiae-W. anomalus cultures were in the same cluster and had uniquely high contents of ethyl succinate. There were not many differentially produced compounds in this cluster because the combinations of different strains were also the combinations of these special compounds from different single strains.
In addition, S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii and S. cerevisiae-W. anomalus-I. orientalis samples were in a different cluster. The five-strain coculture contained larger amounts of esters, alcohols, and acids than the three-strain cultures but smaller amounts of aromatic compounds, aldehydes and ketones, and other compounds. The maximal biomasses of S. cerevisiae and I. orientalis were about 2-fold higher and that of W. anomalus was about 2-fold lower in the five-strain coculture than in the basal culture.
Although S. servazzii and S. uvarum might be metabolically active, the maximal biomass of each strain was only 5.76 × 103 CFU/g and 6.00 × 105 CFU/g, respectively, which was far lower than that of the intrinsic strains. Therefore, the metabolic activities in the two cultures might be mainly attributed to the three intrinsic yeast strains. This indicates that S. cerevisiae and I. orientalis might be responsible for the higher productivities of esters, alcohols, and acids.
Significant different volatile compounds between the intrinsic and the disturbed culture fermentations.
Random forest analysis was used to measure the importance of flavor compounds among different mixed cultures. Among the 57 volatile compounds, 6 compounds had a %IncMSE value of >6%, and 10 compounds had an IncNodePurity value of >10 (see Table S1 in the supplemental material). Six compounds (ethyl acetate, ethyl hexanoate, isobutanol, 3-methylbutyric acid, 2-phenylethanol, and geranyl acetone) were significant by the combination of the two qualitative measures. These compounds are also the main flavor compounds in Chinese light-style liquor (15). The intrinsic species culture produced more geranyl acetone than any of the single cultures. However, it produced less of the other 5 compounds, compared with the highest values of the 3 single cultures. Specifically, it did not produce ethyl hexanoate. The contents of these compounds in the S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii and S. cerevisiae-W. anomalus-I. orientalis cultures were also compared. As shown in Fig. 3, when S. servazzii and S. uvarum were both added to the basal culture, the concentrations of ethyl acetate, ethyl hexanoate, isobutanol, and 3-methylbutyric acid increased from 127.5 to 164.0 μg/liter, from 0 to 25.6 μg/liter, from 20.3 to 47.7 μg/liter, and from 110.0 to 326.8 μg/liter, respectively. Meanwhile, the concentrations of 2-phenylethanol and geranyl acetone decreased from 16,315.9 to 10,875.1 μg/liter and from 60.7 to 27.8 μg/liter, respectively. This suggests that S. servazzii and S. uvarum may stimulate production of volatile compounds by the intrinsic microbes.
FIG 3.
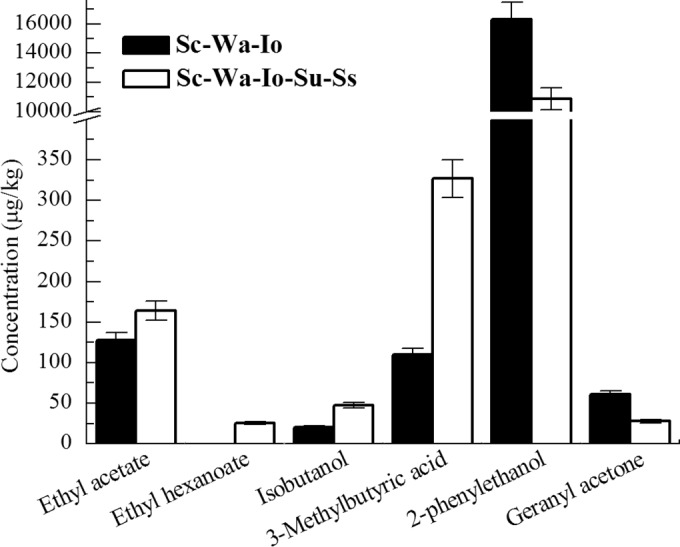
Comparison of the contents of important volatile compounds between cultures of S. cerevisiae-W. anomalus-I. orientalis-S. uvarum-S. servazzii and S. cerevisiae-W. anomalus-I. orientalis. Sc, S. cerevisiae; Io, I. orientalis; Wa, W. anomalus; Ss, S. servazzii; Su, S. uvarum.
DISCUSSION
This work revealed that the flavor profile of Chinese liquor could be altered via the addition of extrinsic microbes. This effect was not due to the flavor production ability of the extrinsic microbes but to the interaction between the extrinsic and intrinsic microbes. In natural and inoculated microbial communities, different microbial strains coexist and interact with each other, and the interactions in the microbial community are essential for the formation of specific microbial structures and the variations in functions. Variations in microbial interactions lead to variations in the microbial structure and metabolism. Therefore, it is important to gain deep insight into the interactions among the component strains.
In this work, when the three intrinsic species (S. cerevisiae, I. orientalis, and W. anomalus) were grown in the presence of two specific flavor producers (S. uvarum and S. servazzii), the maximum specific growth rate of W. anomalus slightly decreased, while those of S. cerevisiae and I. orientalis remarkably increased. This indicates the alleviation of the competition between S. cerevisiae and I. orientalis: the intrinsic microbes might turn from competition to cooperation to compete together against the extrinsic microbes to suppress the extrinsic perturbation. This type of interaction has also been observed in the coculture of Chinese sesame-flavored liquor: S. cerevisiae strongly inhibited B. licheniformis in the coculture of B. licheniformis, S. cerevisiae, and I. orientalis, but the inhibition was slightly alleviated when Bacillus amyloliquefaciens was added (22). As a result, the extrinsic strains grew weakly or might not survive and exhibited little metabolic activity because of inhibition from the intrinsic microbes. This might be the reason for the resilience and robustness of the structure of the microbial community in the presence of different extrinsic microbes.
The inhibition by the intrinsic microbes on the extrinsic microbes is important for the stability of the microbial community. It might be caused by competition for nutrients, cell-cell contact-mediated mechanisms, and inhibition by metabolites such as acids and ethanol (23, 24). In a previous study, we investigated the mechanism of inhibition between S. cerevisiae and W. anomalus isolated from Chinese liquor. The inhibition was not alleviated when glucose and new fermentation medium were added to the fermentation broth, which indicated that the inhibition was not due to the competition for nutrients (25). We also found that the inhibition still existed when we separated them by a dialysis bag for coculture or when the culture supernatant of S. cerevisiae was added to the W. anomalus fermentation medium. These results indicated that the inhibition might be mainly due to the metabolites produced by the inhibitor.
Furthermore, although the extrinsic microbes grew poorly, they might still influence the overall metabolic activity of the intrinsic microbes. There has been increasing interest in understanding the interaction based on the aromatic profile analysis, such as the interaction between non-Saccharomyces species and S. cerevisiae (26–28). This effect was classified into three types: neutral, positive, and negative. An example of a neutral interaction is that between S. cerevisiae and Torulaspora delbrueckii, which had nearly the same aromatic profile in monocultures and in cocultures (26). A positive effect leads to increases in the types and amounts of metabolites, such as fatty acids, ethyl esters and acetates, and terpenol, found in the cocultures of Metschnikowia pulcherrima and S. cerevisiae (26), or production of sulfur compounds, higher alcohols, and esters found in the cocultures of Hanseniaspora uvarum, Hanseniaspora guilliermondii, and S. cerevisiae (28). In contrast, a negative interaction results in a decrease in metabolism. For example, terpenols, norisoprenoids, and lactones were significantly lower in the coculture than in the monoculture of S. cerevisiae and Candida zemplinina (26). However, the effects of microbial interactions on metabolism are investigated mainly via the double combination culture, and the key function of the individual microbe on the final flavor profile in the mixed culture is still unclear. Since cultures found in liquor making are more complex, it is important to carry out further experiments in these more complex cultures to understand how to improve the metabolism of the community.
This work investigated the microbial interactions in cocultures with up to 5 strains, and more complicated interactions were observed, including the contribution of each single strain to the final flavor profile. After inoculation of both S. servazzii and S. uvarum, there were 6 significant volatile compounds: ethyl acetate, ethyl hexanoate, isobutanol, 3-methylbutyric acid, 2-phenylethanol, and geranyl acetone. S. cerevisiae and I. orientalis were both characterized by high concentrations of ethyl hexanoate. S. cerevisiae was characterized by high concentrations of isobutanol, 3-methylbutyric acid, and 2-phenylethanol, while W. anomalus was characterized by high concentrations of ethyl acetate and geranyl acetone, both in the single fermentation (Fig. 2). Because the maximum population numbers of S. cerevisiae and I. orientalis in the five-species combined culture were about 2-fold greater than those in the basal intrinsic culture (Fig. 1), the increases in ethyl hexanoate, isobutanol, and 3-methylbutyric acid might be correlated with the increased growth of S. cerevisiae and I. orientalis, while the decrease of geranyl acetone might be correlated with the slightly decreased growth of W. anomalus. However, the increase in ethyl acetate was not consistent with that of W. anomalus. S. cerevisiae can also efficiently produce ethyl acetate and its precursor acetic acid, which might transform to ethyl acetate by the esterification reaction, explaining the increased ethyl acetate. The decreased 2-phenylethanol was not consistent with the increase in the growth of S. cerevisiae. Although S. cerevisiae efficiently produced 2-phenylethanol in the single-culture fermentation, the production of 2-phenylethanol decreased in all of the cocultures. S. cerevisiae might retain 2-phenylethanol in the five-strain coculture fermentation, which might be beneficial for its growth. Moreover, this decrease is beneficial for the overall liquor flavor, as a high concentration of 2-phenylethanol leads to defective liquor.
Therefore, we concluded that the function of the additional inoculation of extrinsic microbes in this work did not follow the 1 + 1 ≥ 2 principle because most of the special volatile compounds produced by S. servazzii and S. uvarum were not produced in the mixed cultures. The flavor profile produced by the intrinsic strains varied due to the perturbation by the extrinsic strains. The additional inoculation might follow the 1 + x = 1′ principle, which might be another type of emergent property of the community because the intrinsic microbes were stimulated by the extrinsic microbes.
Although there are many methods to improve microbial metabolism, such as imposing stress by changing the environmental conditions (pH and temperature), they might lead to weak growth of the culture. Application of additional cultures to the original culture increases compound production in a more moderate manner. With the use of a suitable extrinsic strain, microbial growth would be unaffected, but the intrinsic microbial metabolic activity might increase. Thus, selection of a suitable extrinsic starter is also important. The general principle for selection is the microbial behavior under defined conditions for making fermented foods (6, 9), such as the stresses from high temperature (about 40 to 50°C), low pH (pH 2.0 to 3.0), and ethanol (about 4.5 to 5.5% [vol/vol]) in the Chinese liquor fermentation process (29). However, interactions between the extrinsic and intrinsic microbes should also be considered. Once we better understand the nature of the interactions, we can select suitable extrinsic microbes and design strategies to alleviate the inhibition among the intrinsic microbes and improve the intrinsic microbial metabolism. This might become an attractive method for improving the fermentation efficiency and the qualities of fermented products and play an important role in the development of the fermentation industry.
Furthermore, it is important to reveal the details of the mechanism of the microbial interactions in the community to allow more scientific designs and control of their interactions to improve the metabolism in mixed-culture fermentations. However, the mechanism of the metabolic activity shift in the perturbed microbial community in this work remains elusive. Although there are many reports about the individual transcription shift in a mixed culture containing two microbes (30, 31), the individual metabolism shift in a two-microbe coculture is not enough to represent the changes of the whole community. The recent development of metatranscriptome sequencing provides an attractive method to reveal the systems-level transcriptional response of the microbial community (32). Therefore, our future work will be focused on the investigation of the transcription and metabolic shift of each microbial community member with the extrinsic microbial perturbation and the interactions between the microbes by use of a metatranscriptomic approach.
In conclusion, traditional fermented foods that are prepared by spontaneous fermentation or by artificial mixed-culture fermentation do not always meet the expectations of consumers. Applying additional inoculated cultures is an attractive way to improve these products. However, since the microbial interactions are poorly understood, the use of selected microbes does not always have a positive result. This work investigated the microbial interactions in a perturbed microbial community. We found that the microbial interactions varied when the intrinsic-species mixed culture was perturbed by extrinsic microbes, and the intrinsic strains turned competition to cooperation to suppress the extrinsic perturbation. In addition, the effects of the extrinsic yeasts on the metabolic activity of the mixed culture were also investigated. The special metabolic activities of the extrinsic yeasts were not retained, and the flavor profiles varied because of the variations in the population and metabolism of the intrinsic strains. This indicates that the microbial interactions play important roles in the metabolic activities of microbial communities. Our work furthers the understanding of the interactions within microbial communities and can be used to increase the effectiveness of added cultures in fermented food production.
Supplementary Material
ACKNOWLEDGMENTS
We thank Pete Chandrangsu for revising the language of the manuscript.
Funding Statement
Funding was provided by the Jiangsu Province "Collaborative Innovation Center for Advanced Industrial Fermentation" industry development program.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02518-15.
REFERENCES
- 1.Fleet GH. 1999. Microorganisms in food ecosystems. Int J Food Microbiol 50:101–117. doi: 10.1016/S0168-1605(99)00080-X. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Lerma GK, Gutiérrez-Moreno K, Cárdenas-Manríquez M, Botello-Álvarez E, Jiménez-Islas H, Rico-Martínez R, Navarrete-Bolaños JL. 2011. Microbial ecology studies of spontaneous fermentation: starter culture selection for prickly pear wine production. J Food Sci 76:M346–M352. doi: 10.1111/j.1750-3841.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 3.Little AEF, Robinson CJ, Peterson SB, Raffa KF, Handelsman J. 2008. Rules of engagement: interspecies interactions that regulate microbial communities. Annu Rev Microbiol 62:375–401. doi: 10.1146/annurev.micro.030608.101423. [DOI] [PubMed] [Google Scholar]
- 4.Kato S, Haruta S, Cui Z, Ishii M, Igarashi Y. 2008. Network relationships of bacteria in a stable mixed culture. Microb Ecol 56:403–411. doi: 10.1007/s00248-007-9357-4. [DOI] [PubMed] [Google Scholar]
- 5.Sieuwerts S, de Bok FAM, Hugenholtz J, van Hylckama Vlieg JET. 2008. Unraveling microbial interactions in food fermentations: from classical to genomics approaches. Appl Environ Microbiol 74:4997–5007. doi: 10.1128/AEM.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holzapfel WH. 2002. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int J Food Microbiol 75:197–212. doi: 10.1016/S0168-1605(01)00707-3. [DOI] [PubMed] [Google Scholar]
- 7.Lefeber T, Papalexandratou Z, Gobert W, Camu N, De Vuyst L. 2012. On-farm implementation of a starter culture for improved cocoa bean fermentation and its influence on the flavour of chocolates produced thereof. Food Microbiol 30:379–392. doi: 10.1016/j.fm.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira AD, Viljoen BC. 2003. Yeasts as adjunct starters in matured Cheddar cheese. Int J Food Microbiol 86:131–140. doi: 10.1016/S0168-1605(03)00252-6. [DOI] [PubMed] [Google Scholar]
- 9.Ciani M, Comitini F, Mannazzu I, Domizio P. 2010. Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in wine making. FEMS Yeast Res 10:123–133. doi: 10.1111/j.1567-1364.2009.00579.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Wu Q, Xu Y. 2013. Aroma characteristics of Moutai-flavour liquor produced with Bacillus licheniformis by solid-state fermentation. Lett Appl Microbiol 57:11–18. doi: 10.1111/lam.12087. [DOI] [PubMed] [Google Scholar]
- 11.Fleet GH. 2003. Yeast interactions and wine flavor. Int J Food Microbiol 86:11–22. doi: 10.1016/S0168-1605(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 12.Larsen PE, Gibbons SM, Gilbert JA. 2012. Modeling microbial community structure and functional diversity across time and space. FEMS Microbiol Lett 332:91–98. doi: 10.1111/j.1574-6968.2012.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Ma E, Yan L, Meng H, Du X, Zhang S, Quan Z. 2011. Bacterial and fungal diversity in the traditional Chinese liquor fermentation process. Int J Food Microbiol 146:31–37. doi: 10.1016/j.ijfoodmicro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Zhu W, Wang W, Xu Y. 2015. Effect of yeast species on the terpenoids profile of Chinese light-style liquor. Food Chem 168:390–395. doi: 10.1016/j.foodchem.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 15.Kong Y, Wu Q, Zhang Y, Xu Y. 2014. In situ analysis of metabolic characteristics reveals the key yeast in the spontaneous and solid-state fermentation process of Chinese light-style liquor. Appl Environ Microbiol 80:3667–3676. doi: 10.1128/AEM.04219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Ling J, Xu Y. 2014. Starter culture selection for Chinese sesame-flavor liquor making: based on microbial metabolic activity in mixed-culture fermentation. Appl Environ Microbiol 80:4450–4459. doi: 10.1128/AEM.00905-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallmann CL, Brown JA, Olineka TL, Cocolin L, Mills DA, Bisson LF. 2001. Use of WL medium to profile native flora fermentations. Am J Enol Vitic 52:198–203. [Google Scholar]
- 18.Wang Y, Yuan B, Ji Y, Li H. 2013. Hydrolysis of hemicellulose to produce fermentable monosaccharides by plasma acid. Carbohyd Polym 97:518–522. doi: 10.1016/j.carbpol.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Chen L, Xu Y. 2013. Yeast community associated with the solid state fermentation of traditional Chinese Maotai-flavor liquor. Int J Food Microbiol 166:323–330. doi: 10.1016/j.ijfoodmicro.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Juang R, Tsai S. 2006. Growth kinetics of Pseudomonas putida in the biodegradation of single and mixed phenol and sodium salicylate. Biochem Eng J 3:133–140. doi: 10.1016/j.bej.2006.05.025. [DOI] [Google Scholar]
- 21.Kuhn S, Egert B, Neumann S, Steinbeck C. 2008. Building blocks for automated elucidation of metabolites: machine learning methods for NMR prediction. BMC Bioinformatics 9:400. doi: 10.1186/1471-2105-9-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bok FA, Janssen PW, Bayjanov JR, Sieuwerts S, Lommen A, van Hylckama Vlieg JET, Molenaar D. 2011. Volatile compound fingerprinting of mixed-culture fermentations. Appl Environ Microbiol 77:6233–6239. doi: 10.1128/AEM.00352-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Nevado F, Albergaria H, Hogg T, Girio F. 2006. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int J Food Microbiol 108:336–345. doi: 10.1016/j.ijfoodmicro.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Nissen P, Nielsen D, Arneborg N. 2003. Viable Saccharomyces cerevisiae cells at high concentrations cause early growth arrest of non-Saccharomyces yeasts in mixed cultures by a cell-cell contact-mediated mechanism. Yeast 20:331–341. doi: 10.1002/yea.965. [DOI] [PubMed] [Google Scholar]
- 25.Tang J, Wang H, Xu Y. 2012. Effect of mixed culture of Saccharomyces cerevisiae and Pichia anomala on fermentation efficiency and flavor compounds in Chinese liquor. Microbiol China 39:921–930. (In Chinese.) doi: 10.1111/1567-1364.12175. [DOI] [Google Scholar]
- 26.Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón JJ, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H. 2012. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32:243–253. doi: 10.1016/j.fm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Medina K, Boido E, Fariña L, Gioia O, Gomez ME, Barquet M, Gaggero C, Dellacassa E, Carrau F. 2013. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem 141:2513–2521. doi: 10.1016/j.foodchem.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Moreira N, Mendes F, Guedes de Pinho P, Hogg T, Vasconcelos I. 2008. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int J Food Microbiol 124:231–238. doi: 10.1016/j.ijfoodmicro.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Wu Q, Xu Y, Chen L. 2012. Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavour liquor making. Lett Appl Microbiol 55:301–307. doi: 10.1111/j.1472-765X.2012.03294.x. [DOI] [PubMed] [Google Scholar]
- 30.Sieuwerts S, Molenaar D, van Hijum SAFT, Beerthuyzen M, Stevens MJA, Janssen PWM, Ingham CJ, de Bok FAM, de Vos WM, van Hylckama Vlieg JET. 2010. Mixed-culture transcriptome analysis reveals the molecular basis of mixed-culture growth in Streptococcus thermophilus and Lactobacillus bulgaricus. Appl Environ Microbiol 76:7775–7784. doi: 10.1128/AEM.01122-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouaille S, Even S, Charlier C, Loir YL, Cocaign-Bousquet M, Loubière P. 2009. Transcriptomic response of Lactococcus lactis in mixed culture with Staphylococcus aureus. Appl Environ Microbiol 75:4473–4482. doi: 10.1128/AEM.02653-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Embree M, Nagarajan H, Movahedi N, Chitsaz H, Zengler K. 2014. Single-cell genome and metatranscriptome sequencing reveal metabolic interactions of an alkane-degrading methanogenic community. ISME J 8:757–767. doi: 10.1038/ismej.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.