Abstract
Objective:
Mycobacterium tuberculosis and Cryptococcus neoformans are major causes of meningitis in HIV-1-infected patients. Identifying differences in the inflammatory profiles of HIV-1-associated tuberculous meningitis (TBM) and cryptococcal meningitis may inform differences in immunopathogenic mechanisms in these diseases. In this study we compared the clinical and inflammatory features of HIV-1-associated TBM, and cryptococcal meningitis.
Methods:
A prospective study of HIV-1-infected adults who presented with either TBM [antiretroviral therapy (ART)-naive] or cryptococcal meningitis (regardless of ART prescription). Clinical and laboratory findings and concentrations of 40 inflammatory mediators measured in cerebrospinal fluid (CSF, 33 paired with blood) were compared between TBM and cryptococcal meningitis patients regardless of ART prescription and between TBM and cryptococcal meningitis patients not receiving ART.
Results:
Clinical and laboratory findings were similar in TBM (n=34) and cryptococcal meningitis (n = 19; ART prescribed: n = 10, no ART prescribed: n = 9). Exceptions included a higher median CD4+ cell count [interquartile: 113 (69–199) vs. 25 (8–49) cells/μl, P = 0.0001] and higher HIV-1 median viral load [plasma: 5.46 (4.82–5.89) vs. 4.87 (4.36–5.17) log10copies/ml, P = 0.037; CSF: 6.05 (5.43–6.56) vs. 5.56 (4.52–5.80) log10copies/ml, P = 0.03] in TBM vs. cryptococcal meningitis patients not receiving ART. CSF interleukin (IL)-17A was lower in TBM compared with cryptococcal meningitis [1.00 (0.25–2.35) vs. 9.31 (1.24–23.36) pg/ml, P-adjusted = 0.03].
Conclusion:
Despite presenting with higher peripheral CD4+ cell counts, TBM patients also presented with higher HIV-1 viral loads compared with cryptococcal meningitis patients, suggesting a greater propensity of M. tuberculosis compared with C. neoformans to increase HIV-1 replication in vivo. CSF IL-17A was lower in TBM; its role in the immunopathogenesis of TBM and cryptococcal meningitis deserves further research.
Keywords: central nervous system, Cryptococcus neoformans, meningitis, pathogenesis, tuberculosis
Introduction
Mycobacterium tuberculosis and Cryptococcus neoformans are two major causes of meningitis in HIV-1-infected patients and contribute substantially to neurological disease burdens in high HIV-1 prevalence settings [1–5]. Recent treatment trials report high mortality rates for HIV-1-associated cryptococcal meningitis (30–50%) [6,7] and tuberculous meningitis (TBM, 58%) [8]. Several studies have compared clinical and cerebrospinal fluid (CSF) findings in patients with cryptococcal meningitis and TBM: both present subacutely (days to weeks after neurological symptom onset) and CSF findings of high protein, low glucose and lymphocytosis, are frequently indistinguishable in these groups [5,9–13].
The immunopathogenesis of cryptococcal meningitis and TBM remains unclear. Studies investigating correlates of human immunity to cryptococcal infection have reported associations between high pretreatment CSF interleukin (IL)-6, interferon (IFN)-γ, tumor necrosis factor (TNF) and IL-8 concentrations and 2-week survival in patients with HIV-1-associated cryptococcal meningitis [14,15]. In patients with HIV-1-associated TBM, one study found an independent association between lower CSF IFN-γ (but not other cytokines such as TNF, IL-6 or IL-8) at presentation and death [16]. Others report correlations between higher IFN-γ and TNF and disease severity in HIV-1-infected and -uninfected TBM patients combined [17].
Studies that compare inflammatory mediators in patients with cryptococcal meningitis and TBM, which may inform differences in immunopathogenic mechanisms in these diseases, are limited. Patel et al. compared CSF IFN-γ and C-X-C chemokine ligand (CXCL)10 between patients with TBM and controls with other causes of meningitis, 58% (28/48) of whom had cryptococcal meningitis [18]. Other studies investigated inflammatory markers simultaneously in cryptococcal meningitis and TBM, such as TNF [19,20], IFN-γ [20], TGF-β1 [20], matrix metalloproteinases (MMP)-2 and -9 [20–22] and tissue inhibitors of MMP (TIMP)-1 and -2 [22]. However, these studies did not present statistical comparisons between findings in TBM and cryptococcal meningitis [18–22] or included a limited number (n < 4) in either of these patient groups [19,22]. In this study we report the clinical and laboratory findings, including an analysis of a wide range of inflammatory mediators in patients with HIV-1-associated TBM compared with HIV-1-associated cryptococcal meningitis.
Methods
Setting and participants
A prospective, observational study at a public sector hospital in Cape Town, South Africa from March 2009 to January 2011. We enrolled antiretroviral therapy (ART)-naive HIV-1-infected adults (≥ 18 years) who presented with TBM diagnosed according to a published case definition [23]. As control participants we enrolled HIV-1-infected adults with cryptococcal meningitis, regardless of ART prescription, diagnosed by a positive CSF India ink stain and/or cryptococcal antigen latex agglutination test (CLAT); central nervous system (CNS) infection with C. neoformans was subsequently confirmed by a positive CSF culture. We further compared findings between cryptococcal meningitis patients and a control group of ART-naive HIV-1-infected patients who did not have meningitis (referred to as the ‘no-meningitis’ group). The details of comparisons of findings between the TBM group and the no-meningitis group [24], as well as comparisons of findings in TBM patients who did and did not developed TBM-immune reconstitution inflammatory syndrome (IRIS), have previously been described [24,25]. The University of Cape Town Human Research Ethics Committee approved the study (HREC 232/2008) and written informed consent was obtained from all patients or their relatives.
Procedures
Demographic data, history of TB and cryptococcal disease, and HIV-1 infection were recorded and a neurological examination performed. Paired CSF and blood samples were then collected. Blood investigations included full blood count, electrolytes and renal function, C-reactive protein, CD4+ cell count and HIV-1 viral load. CSF analysis included biochemistry, microbiology (microscopy and culture for fungi, pyogenic bacteria and M. tuberculosis), syphilis serology and CLAT. CSF HIV-1 viral load was performed in TBM patients and cryptococcal meningitis patients not receiving ART. Aliquots of CSF and blood were stored at −80 °C and analyzed for inflammatory mediators as detailed below. At diagnosis, TBM patients commenced TB treatment (prescribed for a total duration of 9 months) and prednisone (starting dose: 1.5 mg/kg per day, weaned over 6 weeks). Patients with cryptococcal meningitis commenced amphotericin B (0.7 mg/kg per day for 2 weeks), followed by daily oral fluconazole (400 mg for 8 weeks then 200 mg until CD4+ cell count >200 cells/μl for more than 6 months on ART). TBM patients were followed up for the duration of TB treatment, whilst cryptococcal meningitis patients and no-meningitis controls were seen once only (at presentation). We subsequently used the South African National Health Laboratories Service database and the electronic hospital register to trace cryptococcal meningitis patients to determine their in-hospital and 9-month outcome.
Luminex multiplex and enzyme-linked immunosorbent assay performed on blood and cerebrospinal fluid samples
As previously described for TBM patients and no-meningitis controls [24], mediators analyzed in CSF and serum by Luminex multiplex included TNF, IFN-γ, IFN-α2, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-13, IL-17, C-C chemokine 2 ligand (CCL2), CCL3, CCL4, CXCL1–3, CXCL8, granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage (GM)-CSF. MMP-1, -2, -3, -7, -9, -10, -12 and -13, and TIMP-1 and -2 were analyzed by Luminex multiplex in CSF and plasma. Mediators measured by enzyme-linked immunosorbent assay (ELISA) in CSF and serum samples included IL-12p70, IL-17A, IL-21, IL-22, IL-23 and CXCL10. CSF was also analyzed by ELISA for IL-18 and neutrophil-associated mediators: cathepsin G, lipocalin-2, LL-37, human neutrophil peptides (HNP) 1–3, complement (C) 5a and S100A8/A9.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software, Inc., San Diego, California, USA) and R version 3.0 [26]. Findings were compared between patients with TBM and all cryptococcal meningitis (CM) patients (i.e. including patients who were receiving ART at the time of presentation and those who were not, referred to as the ‘combined-CM’ group). As none of the TBM patients were receiving ART at presentation, subgroup analyses were also performed for TBM patients and cryptococcal meningitis patients who were not receiving ART at time of cryptococcal meningitis presentation (including ART-naive patients and patients who defaulted ART, referred to as the ‘CM-off-ART’ group). Findings were further compared between CM-off-ART patients and cryptococcal meningitis patients receiving ART at presentation (referred to as the ‘CM-on-ART’ group), and between cryptococcal meningitis patients (combined-CM and CM-off-ART groups) and no-meningitis controls. Between groups, categorical variables were compared using Fischer's exact test and continuous variables were compared using Wilcoxon rank-sum tests. Continuous variables between blood and CSF compartments, within groups, were compared using Wilcoxon matched-pairs tests. P-values were adjusted for a false discovery rate (FDR, Benjamini-Hochberg) for analyses of inflammatory mediators; an adjusted P-value (P-adj) < 0.05 was considered to be statistically significant [27]. Correlations were estimated using Spearman's rho.
Results
Demographic and clinical results
Thirty-four TBM patients and 19 cryptococcal meningitis patients, including 10 of 19 (53%) who had received ART for a median of 31 days (IQR, 18–87) at the time of presentation, were included in this study. The demographic and baseline findings for TBM, combined-CM and CM-off-ART groups are presented in Table 1, and comparisons of findings between CM-on-ART and CM-off-ART patients are presented in Table S1, Supplemental Digital Content (SDC) 1. Six CM-off-ART patients were ART-naive whereas three had defaulted ART 2, 6 and 19 months prior to cryptococcal meningitis presentation, respectively. Three cryptococcal meningitis patients presented with a recurrence of cryptococcal meningitis; one developed CM-IRIS 4, and 2, months after initial cryptococcal meningitis diagnosis and ART initiation, respectively, whilst two were not receiving ART at the time of cryptococcal meningitis presentation. Prior TB was common in both groups; 11 (32%) TBM and 9 (47%) cryptococcal meningitis patients had a history of previous successfully treated pulmonary TB (P = 0.38) and five cryptococcal meningitis patients were receiving treatment for pulmonary TB (duration, range: 3–10 months) at cryptococcal meningitis presentation; all five cryptococcal meningitis patients on TB treatment had shown an appropriate symptomatic response to TB treatment. Neurological symptoms and examination findings were similar between the TBM and cryptococcal meningitis groups. Baseline characteristics and routine investigation findings of 14 ART-naive HIV-1-infected controls without meningitis, compared with findings in cryptococcal meningitis and TBM patients, are presented in Table S2, SDC1.
Table 1.
Baseline demographic, clinical and investigative findings in patients with tuberculous meningitis (TBM) and cryptococcal meningitis (CM).
| TBM (n = 34) | Combined-CM (n = 19)a | TBM vs. combined-CM | CM-off-ART (n = 9) | TBM vs. CM-off-ART | ||||
| Median or n | (IQR or %) | Median or n | (IQR or %) | P-value | Median or n | (IQR or %) | P-value | |
| Demographic characteristics | ||||||||
| Age (years) | 33 | (28–44) | 34 | (27–39) | 0.61 | 34 | (25–38) | 0.61 |
| Sex (female) | 15 | (44) | 8 | (42) | 1.00 | 2 | (22) | 0.28 |
| Medical history | ||||||||
| History of previous TB | 11 | (32) | 9 | (47) | 0.38 | 5 | (56) | 0.26 |
| On TB treatment at presentation | 0 | (0) | 5 | (26) | 0.0041 | 1 | (11) | 0.21 |
| History of previous CM | 0 | (0) | 3 | (16) | 0.041 | 2 | (22) | 0.040 |
| Neurological symptoms | ||||||||
| Duration neurological symptoms (days) | 15 | (6–30) | 11 | (7–31) | 0.80 | 14 | (9–44) | 0.52 |
| Headache | 30 | (88) | 19 | (100) | 0.28 | 9 | (100) | 0.56 |
| Confusion | 13 | (38) | 2 | (11) | 0.05 | 1 | (11) | 0.23 |
| Vomiting | 21 | (61) | 10 | (53) | 0.57 | 5 | (56) | 1.00 |
| Visual disturbance | 11 | (32) | 10 | (53) | 0.24 | 6 | (67) | 0.12 |
| Clinical findings | ||||||||
| BMI | 19.95 | (18.33–22.7) | 19.73 | (18.03–23.07) | 0.97 | 18.72 | (17.55–19.53) | 0.14 |
| Neck stiffness | 27 | (79) | 16 | (84) | 1.00 | 8 | (89) | 1.00 |
| BMRC grade IIb | 16 | (47) | 5 | (26) | 0.16 | 3 | (33) | 0.71 |
| Focal neurological signs | 7 | (21) | 3 | (16) | 1.00 | 2 | (22) | 1.00 |
| Blood investigations | ||||||||
| Hemoglobin (g/dl) | 11.4 | (8.8–13.1) | 11.9 | (9.8–13.5) | 0.28 | 12.9 | (10.6–15) | 0.086 |
| Sodium (mmol/l) | 129 | (123–131) | 128 | (126–132) | 0.61 | 129 | (128–136) | 0.37 |
| C-reactive protein (mg/l) | 40 | (6–78) | 23 | (6–66) | 0.58 | 23 | (1–51) | 0.22 |
| CD4+ (cells/μl) | 113 | (69–199) | 55 | (23–77) | 0.0012 | 25 | (8–49) | 0.0001 |
| HIV viral load (log10 copies/ml) | 5.46 | (4.82–5.89) | 3.93 | (2.18–4.93) | < 0.0001 | 4.87 | (4.36–5.17) | 0.037 |
| CSF investigations | ||||||||
| Neutrophils, cells (×106/l) | 21 | (2–43) | 5 | (0–44) | 0.32 | 13 | (0–75) | 0.74 |
| Lymphocytes, cells (×106/l) | 177 | (87–339) | 90 | (7–132) | 0.031 | 90 | (4–182) | 0.14 |
| Protein (g/l) | 1.94 | (1.28–3.06) | 1.82 | (0.74–2.48) | 0.32 | 1.9 | (1.28–2.71) | 0.94 |
| Glucose (mmol/l) | 1.8 | (1.1–2.7) | 1.4 | (1.3–2.5) | 0.57 | 1.6 | (1.2–2.9) | 0.78 |
| CSF : blood glucose ratio | 0.3 | (0.2–0.53) | 0.24 | (0.21–0.45) | 0.42 | 0.24 | (0.22–0.48) | 0.78 |
| HIV viral load (log10 copies/ml)c | 6.05 | (5.43–6.56) | − | − | − | 5.56 | (4.52–5.80) | 0.03 |
ART, antiretroviral therapy, n; number; BMRC, British Medical Research Council; CSF, cerebrospinal fluid; IQR, interquartile range; TB, tuberculosis.
aCentral nervous system infection with Cryptococcus neoformans was confirmed by CSF culture in all but one CM patient in whom fungal culture was omitted in error; this patient had a positive CSF India ink stain and a CSF cryptococcal latex antigen test titer of 1 : 4096.
bNo patients had BMRC grade III disease. BMRC grade I: Glasgow Coma Scale (GCS) score of 15 with no focal neurologic signs; grade II: GCS score of 11–14 or GCS score of 15 with focal neurologic signs; grade III: GCS score of 10 or less.
cIn CM patients, HIV viral load was only performed in CSF of patients not receiving ART.
Blood and cerebrospinal fluid findings
Blood and CSF findings (Table 1) were similar between TBM and cryptococcal meningitis patients with the following exceptions: CSF lymphocyte counts were higher in TBM patients compared with combined-CM patients [177 (87–339) vs. 90 (7–132) cells/μl, P = 0.031; Fig. 1h]. TBM patients had higher blood CD4+ cell counts compared with combined-CM patients [median (IQR), 113 (69–199) vs. 55 (23–77) cells/μl, P = 0.0012]. This difference between TBM and cryptococcal meningitis was more pronounced when analyzing only CM-off-ART patients [CD4+: 25 (8–49) cells/μl, P = 0.0001]. TBM patients also presented with higher HIV-1 viral loads, both in plasma [5.46 (4.82–5.89) vs. 4.87 (4.36–5.17) log10 copies/ml, P = 0.037] and in CSF [6.05 (5.43–6.56) vs. 5.56 (4.52–5.80) log10 copies/ml, P = 0.031] compared with CM-off-ART patients. As migratory leukocytes may contribute to CSF HIV viral load, we assessed correlation between CSF HIV viral loads and total CSF leukocyte counts (lymphocytes and neutrophils) and between CSF HIV viral loads and CSF lymphocyte counts, in TBM and CM-off-ART patients (Table S3, SDC1). No significant correlations were observed in either group in any of these analyses.
Fig. 1.
Cerebrospinal fluid Luminex multiplex/ELISA showing results for inflammatory mediators and cells different by univariate analysis in tuberculous meningitis (TBM) and cryptococcal meningitis (CM).
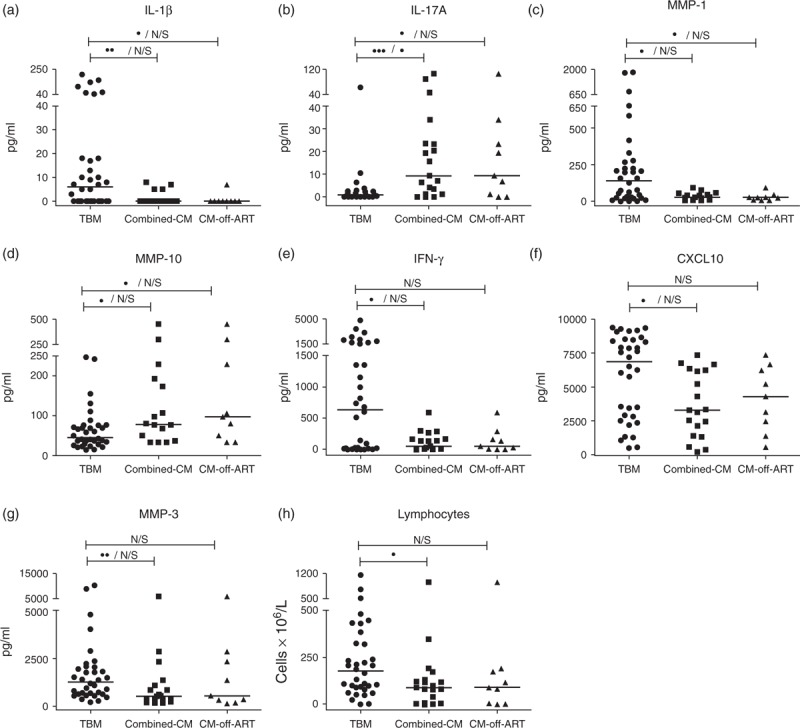
Results for TBM, combined-CM and CM-off-antiretroviral therapy (ART) groups are shown. Horizontal lines within graphs represent median concentrations. Statistical analysis between TBM and CM groups displayed as unadjusted/adjusted P-values on horizontal bars: N/S P ≥ 0.05, •0.05 < P ≤ 0.01. ••0.01 < P ≤ 0.001. •••0.001 < P ≤ 0.0001. P-values were adjusted for a false discovery rate (Benjamini-Hochberg) for analysis of inflammatory mediators.
As previously described for the TBM group [24] and shown in Table S2, SDC1, combined-CM patients had lower sodium [128 (126–132) vs. 135 (133–137) mmol/l, P = 0.0009] and higher C-reactive protein [23 (6–66) vs. 5 (1–8) mg/l, P = 0.02] concentrations compared with no-meningitis patients. Both CD4+ cell counts and HIV viral loads were lower in combined-CM patients compared with controls [CD4+: 55 (23–77) vs. 129 (75–180) cells/μl, P = 0.0042; HIV viral load: 3.93 (2.18–4.93) vs. 4.87 (4.44–5.44) log10 copies/ml, P = 0.029]. CM-off-ART patients had lower CD4+ cell counts (P = 0.0013), but comparable HIV viral loads (P = 0.76) compared with no-meningitis patients. Similar to patients with TBM [24], cryptococcal meningitis patients had higher CSF lymphocyte counts, neutrophil counts and protein concentrations, and lower CSF : blood glucose ratios, compared with the no-meningitis group (P < 0.01 for all).
Management and outcome
In addition to amphotericin B, TB treatment was started in three cryptococcal meningitis patients for suspected pulmonary TB. In none of these patients was a diagnosis of TB confirmed microbiologically; TB treatment was started empirically based on chest radiograph findings that could have been consistent with either TB or cryptococcosis in two patients, and in the third, chest radiograph findings showed fibrotic changes (the patient had previous TB) and active disease could not be excluded. None of the no-meningitis patients commenced TB treatment. None of the TBM patients but three cryptococcal meningitis patients died during hospitalization and 9-months mortality was 12% (n = 4) and 37% (n = 7) (P = 0.41) for TBM and combined-CM patients, respectively with no loss-to-follow-up.
Inflammatory mediators measured by enzyme-linked immunosorbent assay or Luminex multiplex
IL-12p70 and IL-23 were undetectable in any blood or CSF sample. These mediators were therefore excluded from further analysis.
Table 2 shows univariate analyses of CSF and blood inflammatory mediator findings comparing TBM and combined-CM patients; comparisons of results between TBM and CM-off-ART are presented in Table S4, SDC1. As we described previously for TBM [24], cryptococcal meningitis patients presented with a compartmentalized inflammatory response with higher CSF concentrations of G-CSF, IFN-α2, TNF, IL-6, IL-17A, CCL2, CCL3, CXCL8, CXCL10, MMP-9 and TIMP-1 (P-adj≤0.0001); IFN-γ, IL-10, CCL4 (0.001<P-adj<0.01); and IL-13 (0.01<P-adj<0.05), compared with blood in the combined-CM group (Table 2). Conversely, CXCL1–3 (P-adj = 0.0075) and MMP-1, -2, -3, -7 and -10 (P-adj≤0.0045) were higher in blood compared with CSF in these patients.
Table 2.
Cerebrospinal fluid (CSF) and blood mediator concentrations in patients with tuberculous meningitis (TBM) and combined cryptococcal meningitis (CM) patients.
| Mediator pg/mla | TBM (n = 34) | Combined cryptococcal meningitis (n = 19) | TBM vs. CM | TBM vs. CM | ||||||||||
| CSF | Blood | CSF vs. Blood | CSF | Blood | CSF vs. Blood | CSF vs. CSF | Blood vs. Blood | |||||||
| Median | (IQR) | Median | (IQR) | P-adj | Median | (IQR) | Median | (IQR) | P-adj | P-value | P-adj | P-value | P-adj | |
| G-CSF | 1748 | (362–6536) | 80 | (45–169) | <0.0001 | 1067 | (288–3494) | 70 | (21–95) | <0.0001 | 0.34 | 0.20 | ||
| GM-CSF | 21 | (0–69) | 0 | (0–5) | 0.0001 | 29 | (0–59) | 0 | (0–14) | 0.035 | 0.76 | − | − | |
| IFN-α2 | 97 | (62–114) | 11 | (0–37) | <0.0001 | 116 | (83–135) | 13 | (0–34) | <0.0001 | 0.18 | 0.99 | ||
| IL-12p40 | 23 | (0–91) | 0 | (0–34) | 0.086 | 21 | (0–61) | 0 | (0–28) | 0.26 | 0.86 | − | − | |
| TNF | 130 | (31–264) | 18 | (12–42) | <0.0001 | 111 | (46–206) | 18 | (13–36) | 0.0001 | 0.72 | 0.99 | ||
| IFN-γ | 640 | (16–1701) | 8 | (0–21) | <0.0001 | 55 | (6–169) | 0 | (0–15) | 0.0029 | 0.018 | 0.10 | 0.042 | 0.33 |
| IL-1β | 6 | (0–18) | 0 | (0–1) | 0.0015 | 0 | (0–0) | 0 | (0–0) | − | 0.0027 | 0.055 | − | − |
| IL-2 | 0 | (0–6) | 0 | (0–0) | − | 0 | (0–6) | 0 | (0–0) | − | − | − | − | − |
| IL-4 | 0 | (0–5) | 0 | (0–0) | − | 0 | (0–8) | 0 | (0–0) | − | − | − | − | − |
| IL-6 | 2504 | (58–8883) | 7 | (4–14) | <0.0001 | 976 | (39–7463) | 6 | (0–9) | <0.0001 | 0.47 | 0.37 | ||
| IL-10 | 96 | (51–196) | 9 | (4–19) | <0.0001 | 104 | (36–157) | 13 | (0–62) | 0.0025 | 0.80 | 0.41 | ||
| IL-13 | 0 | (0–18) | 0 | (0–0) | − | 3 | (0–7) | 0 | (0–0) | 0.017 | 0.95 | − | − | |
| IL-17A | 1 | (0.25–2.35) | 0 | (0–0) | <0.0001 | 9.31 | (1.24–23.36) | 0 | (0–0) | <0.0001 | 0.0007 | 0.030 | − | − |
| IL-21 | 0 | (0–0) | 0 | (0–0) | − | 0 | (0–0) | 0 | (0–0) | − | − | − | − | − |
| IL-22 | 42 | (0–160) | 0 | (0–12) | 0.0057 | 0 | (0–114) | 0 | (0–11) | − | − | − | − | − |
| CXCL1–3 | 442 | (63.5–1558) | 1246 | (772–2150) | 0.011 | 300 | (88–1010) | 1023 | (532–2438) | 0.0075 | 0.72 | 0.79 | ||
| CCL2 | 2935 | (870–7936) | 302 | (180–412) | <0.0001 | 4913 | (1821–8686) | 457 | (270–660) | <0.0001 | 0.22 | 0.040 | 0.33 | |
| CCL3 | 82 | (49–132) | 0 | (0–0) | <0.0001 | 85 | (55–126) | 0 | (0–0) | <0.0001 | 0.61 | − | − | |
| CCL4 | 90 | (38–151) | 16 | (0–36) | <0.0001 | 80 | (52–124) | 28 | (16–76) | 0.0086 | 0.69 | 0.029 | 0.33 | |
| CXCL8 | 1959 | (204–5353) | 24 | (12–44) | <0.0001 | 1744 | (280–5343) | 19 | (13–30) | <0.0001 | 0.83 | 0.37 | ||
| CXCL10 | 6844 | (2731–8499) | 1113 | (739–1620) | <0.0001 | 3284 | (1382–6239) | 669 | (316–1178) | 0.0001 | 0.010 | 0.08 | 0.013 | 0.33 |
| MMP-1 | 142 | (23–269) | 710 | (287–1681) | <0.0001 | 28 | (9–53) | 472 | (242–1053) | <0.0001 | 0.011 | 0.08 | 0.27 | |
| MMP-2 | 28790 | (19410–40760) | 45495 | (33600–53920) | 0.0002 | 24917 | (21050–38559) | 45684 | (39468–54531) | 0.0045 | 0.69 | 0.81 | ||
| MMP-3 | 1280 | (632–2040) | 11290 | (7258–20430) | <0.0001 | 529 | (310–1160) | 8772 | (6350–24784) | <0.0001 | 0.0099 | 0.08 | 1.00 | |
| MMP-7 | 314 | (205–642) | 21260 | (14040–34860) | <0.0001 | 440 | (267–564) | 26861 | (10260–41875) | <0.0001 | 0.53 | 0.67 | ||
| MMP-9 | 25390 | (7110–69610) | 7914 | (5822–12550) | 0.0052 | 34617 | (20451–62142) | 7106 | (4826–11525) | <0.0001 | 0.41 | 0.56 | ||
| MMP-10 | 46 | (27–75) | 584 | (461–1064) | <0.0001 | 79 | (40–188) | 650 | (421–1112) | <0.0001 | 0.020 | 0.10 | 0.81 | |
| MMP-12 | 0 | (0–0) | 0 | (0–0) | − | 0 | (0–0) | 0 | (0–273) | − | − | − | − | − |
| MMP-13 | 0 | (0–19) | 0 | (0–0) | − | 0 | (0–13) | 0 | (0–0) | − | − | − | − | − |
| TIMP-1 | 420100 | (188500–589900) | 123600 | (77620–170900) | <0.0001 | 287021 | (209050–649605) | 136314 | (91010–162949) | <0.0001 | 0.72 | 0.89 | ||
| TIMP-2 | 48260 | (36330–68640) | 49800 | (40300–58040) | 0.65 | 63039 | (42295–78860) | 52502 | (34492–64463) | 0.29 | 0.38 | 0.54 | ||
| IL-18 | 0 | (0–250) | − | − | − | 0 | (0–0) | − | − | − | − | − | − | − |
| C5a | 0 | (0–1321) | − | − | − | 0 | (0–0) | − | − | − | − | − | − | − |
| HNP 1–3 | 3578 | (803–4892) | − | − | − | 851 | (607–5247) | − | − | − | 0.17 | − | − | |
| LL-37 | 568 | (0–949) | − | − | − | 228 | (0–739) | − | − | − | 0.19 | − | − | |
| Cathepsin G | 11 | (0–15) | − | − | − | 9 | (0–11) | − | − | − | 0.36 | − | − | |
| Lipocalin-2 | 1066 | (748–1169) | − | − | − | 1094 | (804–1274) | − | − | − | 0.47 | − | − | |
| S100A8/A9 | 29000 | (6000–48000) | − | − | − | 16884 | (0–70859) | − | − | − | 0.63 | − | − | |
IQR, interquartile range; MMP, metalloproteinases; TIMP, tissue inhibitors of MMP. P-values for analysis within and between disease groups: P-values are not reported if the medians of both comparator groups are zero. P-values were adjusted for a false discovery rate (P-adj, Benjamini-Hochberg). Only adjusted P-values are shown for comparisons between CSF and blood within TBM and cryptococcal meningitis groups. Significant differences (P-adj < 0.05) for comparisons between patients groups are shown in bold. Results shown are mediator concentrations assayed with Luminex multiplex or ELISA. IL-18, C5a, HNP 1–3, LL-37, Cathepsin G, Lipocalin-2 and S100A8/A9 were only measured in CSF. aUnits are picograms per milliliter except for Cathepsin G that is presented as units per milliliter.
When comparing CSF findings in TBM to those in combined-CM patients, TBM patients had higher IFN-γ (P = 0.018), IL-1β (0.0027), CXCL10 (P = 0.01), MMP-1 (P = 0.011) and MMP-3 (P = 0.0099), and lower IL-17A (P = 0.0007) and MMP-10 (P = 0.02) by univariate analysis (Table 2, Fig. 1). However, only IL-17A remained significantly different between these groups (P-adj = 0.03) after adjustment for FDR. Similar trends in differences were seen when comparing IL-1β, IL-17A, MMP-1 and MMP-10 between TBM and CM-off-ART groups (Table S4, SDC1 and Fig. 1); none of these remained significantly different after adjustment for FDR. Table S5, SDC1 shows Spearman's rho and P-values for correlations between CSF IL-17A concentrations and the following: blood HIV viral loads, CSF HIV viral loads and blood CD4+ cell counts, in TBM and cryptococcal meningitis groups. Notably, no significant correlation was observed in either group in any of the analyses performed.
Concentrations of mediators measured in blood were similar in TBM and cryptococcal meningitis patients, with the exception of CXCL10 and IFN-γ that were higher, and CCL2 and CCL4 that were lower (0.01<P < 0.05 for all) in TBM patients compared with combined-CM patients (Table 2). CCL2 and CXCL10 were similarly different when TBM was compared with CM-off-ART (0.01<P < 0.05 for both; Table S4, SDC1). None of these differences remained significant after adjustment for FDR.
CSF and blood concentrations of mediators were similar between cryptococcal meningitis patients irrespective of ART (Table S6, SDC1) with only two significant differences between these groups in univariate analysis: higher plasma MMP-10 [median (IQR): 1018 (608–1372) vs. 527 (159–578) pg/ml, P = 0.031] and lower CSF TIMP-1 [242297 (154658–417305) vs. 477480 (287021–794524) pg/ml, P = 0.040] in CM-off-ART patients; neither of these differences were significant after adjustment for FDR.
As was previously described for TBM patients [24] (Table S7, SDC1), combined-CM patients had significantly higher concentrations of most mediators measured in CSF compared with no-meningitis patients, both when comparing combined-CM and CM-off-ART groups to these controls (Tables S8 and Table S9, SDC1). The only mediators that were not significantly higher in combined-CM patients compared with no-meningitis controls after adjustment for FDR were MMP-3 that were similar, and IL-1β, IL-2, IL-4, IL-21, IL-22, MMP-12, MMP-13, IL-18 and C5a that had medians equal to zero, in both groups (Table S8, SDC1). Similar differences were seen when comparing CM-off-ART patients to no-meningitis controls (Table S9, SDC1). There were no significant differences between mediators measured in blood after adjustment for FDR between both combined-CM and CM-off-ART groups and no-meningitis controls (Tables S8 and S9, SDC1).
Discussion
As previously reported in studies comparing HIV-1-associated cryptococcal meningitis and TBM [5,10], the clinical and basic laboratory features in these groups were similar; notable exceptions that were also found in a study of ART-naive meningitis patients from a high TB/HIV-1 prevalence setting [12] were higher CD4+ cell counts and higher CSF lymphocyte counts in TBM patients. Despite presenting with higher peripheral CD4+ cell counts, TBM patients also presented with higher HIV-1 viral loads, both in blood and CSF, compared with cryptococcal meningitis patients not receiving ART. Leucocytes that have migrated from peripheral blood into the CNS could potentially increase the CSF HIV viral load and in this study, TBM patients had higher CSF lymphocyte counts compared with cryptococcal meningitis patients. However, we did not find any correlation between leukocyte counts and HIV viral loads in either TBM or CM-off-ART patients. Both M. tuberculosis[28] and C. neoformans[29] accelerate HIV-1 replication in vitro, and findings from bronchoalveolar lavage fluid suggest a similar effect of M. tuberculosis on HIV-1 replication in the lungs of pulmonary TB patients [30]. Based on our findings, we hypothesize that of the two pathogens, M. tuberculosis more significantly stimulates HIV-1 replication in vivo in the periphery as well as at the site-of-disease, potentially resulting in increased HIV-1-associated CNS pathology in TBM compared with cryptococcal meningitis patients.
Our results reveal a pathologically important difference in IL-17A concentrations at the site-of-disease in patients with TBM and cryptococcal meningitis. CSF IL-17A was significantly higher in combined-CM compared with TBM patients in univariate analysis after adjustment for FDR. It is unlikely that this difference was related to the higher peripheral blood CD4+ cell counts and HIV viral loads (in both blood and CSF) observed in the TBM group compared with patients with cryptococcal meningitis, as no significant correlation was observed between the concentrations of IL-17A and any of these factors in either TBM or cryptococcal meningitis patients. As previously reported [24], we have also not found any significant correlation between the concentrations of the mediators measured in CSF and CSF leucocyte counts in TBM patients. Through the induction of neutrophil recruitment, IL-17 promotes inflammatory pathology in autoimmune disease but protects the host against many pathogens [31,32]. In a murine model of cryptococcal lung disease, IL-17A promoted host defense against C. neoformans by increasing leukocyte recruitment, IFN-γ production and activation of lung myeloid cells [33]. In patients with HIV-1-associated cryptococcal meningitis not on ART, increased concentrations of CSF IL-17A in combination with increased concentrations of other cytokines (IL-10 and classical Th1 cytokines) was associated with a IFN-γ/TNF-α producing cryptococcal antigen-specific peripheral blood T cells response that, in turn, associated with lower 2-week mortality [34]. A protective role for IL-17 against TB infection has also been inferred from studies in mice [32]. However, IL-17 may potentially be detrimental in TB as over-expression of IL-17 through induction of S100A8/A9 mediated neutrophil recruitment and exacerbated lung inflammation in TB-infected mice [35]. A harmful role for IL-17 in TBM was also suggested by CSF findings in TBM-IRIS, a potentially life-threatening complication of ART in TB patients characterized by severe CNS inflammation [24]; IL-17A concentrations increased significantly after starting TB treatment in TBM-IRIS patients. Given these findings, it is intriguing to consider that the inflammatory response associated with IL-17 may play different and possibly opposite roles in the immunopathogenesis of TBM and cryptococcal meningitis, being harmful in the former and protective in the latter.
In both TBM and cryptococcal meningitis patients the inflammatory response was highly compartmentalized in the CSF, with concentrations of the majority of cytokines and chemokines measured being significantly higher in CSF compared with blood. This is consistent with previous reports of other forms of extrapulmonary TB [36,37] and cryptococcal meningitis [38]. Furthermore, we found no significant differences in mediators measured in blood of TBM compared with cryptococcal meningitis patients. These findings emphasize the importance of investigating mediators at the site-of-disease (i.e. CSF), rather than solely relying on blood samples, when investigating immunopathogenic mechanisms in TBM and cryptococcal meningitis.
Although this is the most extensive comparison of inflammatory mediators in HIV-1-associated TBM vs. cryptococcal meningitis, we acknowledge several limitations. We were unable to include a control group of HIV-uninfected patients without TB and cryptococcal meningitis as such patients are less common in our high HIV/TB setting. Due to the relatively small sample size, some biologically relevant differences between groups may have passed undetected by univariate analysis. We also made conservative choices (using less powerful nonparametric tests) as even log transformed mediators had nonnormal distributions, typical for this type of data. Given further the large number of mediators analyzed and the large interindividual differences in mediator concentrations, straightforward multivariate analysis was not suitable for this dataset. In particular, logistic regression relies on relatively large sample sizes for good approximation of parameter estimates and is not suitable in this case. Our findings should therefore be considered preliminary, requiring validation in larger studies.
In conclusion, we demonstrated potentially important immunological differences between TBM and cryptococcal meningitis in spite of similar clinical characteristics. A propensity for M. tuberculosis to increase HIV replication compared with C. neoformans may have important implications for the control of HIV infection in co-infected patients. Furthermore, IL17 may play different roles in the pathogenesis of TBM and cryptococcal meningitis. We anticipate that our findings will guide future studies to investigate different pathogenic mechanisms that may ultimately inform host-directed treatment strategies in HIV-1-associated TBM and cryptococcal meningitis.
Acknowledgements
We thank the patients who participated in the study and Monica Magwayi for providing counseling and support to patients during the study. We are grateful to the clinical and administrative staff of the Provincial Government of the Western Cape Department of Health for their support of this study.
Author contributions: S.M., R.J.W. and G.M. conceived and designed the clinical study; S.M. and G.M. performed clinical data collection; S.M., R.J.W., K.A.W. and G.M. designed laboratory experiments; S.M. and K.A.W. performed laboratory experiments; S.M., G.M., R.J.W. and M.L. analyzed and interpreted the data; S.M. wrote the manuscript; R.J.W., K.A.W., G.M. and M.L. critically revised the manuscript.
Sources of funding: This work was supported by the Carnegie Corporation Training Award and Discovery Foundation Academic Fellowship Award (S.M.); the Perinatal HIV-1 Research Unit, the US Agency for International Development, and the President's Emergency Plan for AIDS Relief (S.M.); the Wellcome Trust (grants WT 097254, 084323, 098316 and 104803 to S.M., G.M. and R.J.W.); National Institutes of Health and Fogarty International Center South Africa TB/AIDS training awards (grant NIH/FIC U2RTW007373-01A to G. M.); the National Research Foundation of South Africa (grant UID 85858 to G.M. and 96841 to R.J.W.); European Union (grant FP-7-HEALTH-F3-2012-305578 and FP7-PEOPLE-2011-IRSES to R.J.W.); and the Medical Research Council (UK) (grant U.1175.02.002.00014.01 to R.J.W.).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Berhe T, Melkamu Y, Amare A. The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther 2012; 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asselman V, Thienemann F, Pepper DJ, Boulle A, Wilkinson RJ, Meintjes G, et al. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS 2010; 24:2871–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One 2011; 6:e20077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai L, Mahajan SD, Guo C, Zhang T, Wang W, Li T, et al. Spectrum of central nervous system disorders in hospitalized HIV/AIDS patients (2009-2011) at a major HIV/AIDS referral center in Beijing, China. J Neurol Sci 2014; 342:88–92. [DOI] [PubMed] [Google Scholar]
- 5.Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS 2000; 14:1401–1407. [DOI] [PubMed] [Google Scholar]
- 6.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 2013; 368:1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torok ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis 2011; 52:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checkley AM, Njalale Y, Scarborough M, Zjilstra EE. Sensitivity and specificity of an index for the diagnosis of TB meningitis in patients in an urban teaching hospital in Malawi. Trop Med Int Health 2008; 13:1042–1046. [DOI] [PubMed] [Google Scholar]
- 10.Cohen DB, Zijlstra EE, Mukaka M, Reiss M, Kamphambale S, Scholing M, et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health 2010; 15:910–917. [DOI] [PubMed] [Google Scholar]
- 11.Helbok R, Pongpakdee S, Yenjun S, Dent W, Beer R, Lackner P, et al. Chronic meningitis in Thailand. Clinical characteristics, laboratory data and outcome in patients with specific reference to tuberculosis and cryptococcosis. Neuroepidemiology 2006; 26:37–44. [DOI] [PubMed] [Google Scholar]
- 12.Silber E, Sonnenberg P, Ho KC, Koornhof HJ, Eintracht S, Morris L, et al. Meningitis in a community with a high prevalence of tuberculosis and HIV infection. J Neurol Sci 1999; 162:20–26. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Lv K, Bao J, Lu C, Lu Z. Clinical and laboratory factors in the differential diagnosis of tuberculous and cryptococcal meningitis in adult HIV-negative patients. Intern Med 2013; 52:1573–1578. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol 2005; 174:1746–1750. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog 2015; 11:e1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons CP, Thwaites GE, Quyen NT, Torok E, Hoang DM, Chau TT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol 2006; 176:2007–2014. [DOI] [PubMed] [Google Scholar]
- 17.Patel VB, Bhigjee AI, Bill PL, Connolly CA. Cytokine profiles in HIV seropositive patients with tuberculous meningitis. J Neurol Neurosurg Psychiatry 2002; 73:598–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel VB, Singh R, Connolly C, Kasprowicz V, Ndung’u T, Dheda K. Comparative utility of cytokine levels and quantitative RD-1-specific T cell responses for rapid immunodiagnosis of tuberculous meningitis. J Clin Microbiol 2011; 49:3971–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastroianni CM, Paoletti F, Valenti C, Vullo V, Jirillo E, Delia S. Tumour necrosis factor (TNF-alpha) and neurological disorders in HIV infection. J Neurol Neurosurg Psychiatry 1992; 55:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown HC, Chau TT, Mai NT, Day NP, Sinh DX, White NJ, et al. Blood-brain barrier function in cerebral malaria and CNS infections in Vietnam. Neurology 2000; 55:104–111. [DOI] [PubMed] [Google Scholar]
- 21.Liuzzi GM, Mastroianni CM, Santacroce MP, Fanelli M, D’Agostino C, Vullo V, et al. Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological diseases. J Neurovirol 2000; 6:156–163. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura E, Umehara F, Hashiguchi T, Fujimoto N, Okada Y, Osame M. Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J Neurol Sci 2000; 173:45–52. [DOI] [PubMed] [Google Scholar]
- 23.Bhigjee AI, Padayachee R, Paruk H, Hallwirth-Pillay KD, Marais S, Connoly C. Diagnosis of tuberculous meningitis: clinical and laboratory parameters. Int J Infect Dis 2007; 11:348–354. [DOI] [PubMed] [Google Scholar]
- 24.Marais S, Wilkinson KA, Lesosky M, Coussens AK, Deffur A, Pepper DJ, et al. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2014; 59:1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marais S, Meintjes G, Pepper DJ, Dodd LE, Schutz C, Ismail Z, et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 2013; 56:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org [Accessed 10 March 2015]. [Google Scholar]
- 27.Hochberg YBY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57:289–300. [Google Scholar]
- 28.Pathak S, Wentzel-Larsen T, Asjo B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect Immun 2010; 78:4022–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison TS, Nong S, Levitz SM. Induction of human immunodeficiency virus type 1 expression in monocytic cells by Cryptococcus neoformans and Candida albicans. J Infect Dis 1997; 176:485–491. [DOI] [PubMed] [Google Scholar]
- 30.Nakata K, Rom WN, Honda Y, Condos R, Kanegasaki S, Cao Y, et al. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med 1997; 155:996–1003. [DOI] [PubMed] [Google Scholar]
- 31.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 2009; 9:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology 2009; 126:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and gamma interferon production. Infect Immun 2014; 82:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, Levitz SM, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis 2013; 207:1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna K, et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med 2013; 188:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, et al. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis 2005; 40:184–187. [DOI] [PubMed] [Google Scholar]
- 37.Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis 2011; 91:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CC, Omarjee S, Lim A, Spelman T, Gosnell BI, Carr WH, et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis 2013; 208:1604–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


