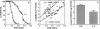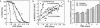Abstract
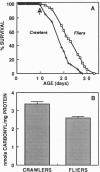
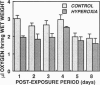
The objective of this study was to test some of the predictions of the oxidative-stress hypothesis of aging, which postulates that aging is causally associated with the molecular damage inflicted by reactive oxygen species. Protein carbonyl content was used as an index of molecular oxidative modifications. The carbonyl content was found to be associated with the physiological age or life expectancy of flies rather than with their chronological age. Exposure of flies to sublethal hyperoxia (100% oxygen) irreversibly enhanced the carbonyl content of the flies and decreased their rate of oxygen consumption. Results of this study indicate that protein carbonyl content may be a biomarker of aging and support the general concept that oxidative stress may be a causal factor in the aging process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Andrews A. D., Barrett S. F., Yoder F. W., Robbins J. H. Cockayne's syndrome fibroblasts have increased sensitivity to ultraviolet light but normal rates of unscheduled DNA synthesis. J Invest Dermatol. 1978 May;70(5):237–239. doi: 10.1111/1523-1747.ep12541383. [DOI] [PubMed] [Google Scholar]
- Barrett S. F., Robbins J. H., Tarone R. E., Kraemer K. H. Evidence for defective repair of cyclobutane pyrimidine dimers with normal repair of other DNA photoproducts in a transcriptionally active gene transfected into Cockayne syndrome cells. Mutat Res. 1991 Nov;255(3):281–291. doi: 10.1016/0921-8777(91)90032-k. [DOI] [PubMed] [Google Scholar]
- Brash D. E., Franklin W. A., Sancar G. B., Sancar A., Haseltine W. A. Escherichia coli DNA photolyase reverses cyclobutane pyrimidine dimers but not pyrimidine-pyrimidone (6-4) photoproducts. J Biol Chem. 1985 Sep 25;260(21):11438–11441. [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback R. A., Yoder F. W., Andrews A. D., Peck G. L., Robbins J. H. Normal pressure hydrocephalus. Recognition and relationship to neurological abnormalities in Cockayne's syndrome. Arch Neurol. 1978 Jun;35(6):337–345. doi: 10.1001/archneur.1978.00500300011002. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair in man. Birth Defects Orig Artic Ser. 1989;25(2):61–82. [PubMed] [Google Scholar]
- Deschavanne P. J., Chavaudra N., Fertil B., Malaise E. P. Abnormal sensitivity of some Cockayne's syndrome cell strains to UV- and gamma-rays. Association with a reduced ability to repair potentially lethal damage. Mutat Res. 1984 Feb;131(2):61–70. doi: 10.1016/0167-8817(84)90012-9. [DOI] [PubMed] [Google Scholar]
- Donato H., Jr, Hoselton M. A., Sohal R. S. An analysis of the effects of individual variation and selective mortality on population averages in aging populations. Exp Gerontol. 1979;14(3):133–140. doi: 10.1016/0531-5565(79)90028-7. [DOI] [PubMed] [Google Scholar]
- Farmer K. J., Sohal R. S. Relationship between superoxide anion radical generation and aging in the housefly, Musca domestica. Free Radic Biol Med. 1989;7(1):23–29. doi: 10.1016/0891-5849(89)90096-8. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Philpott M., Meehan C., Henning M. Recovery from oxygen poisoning in Drosophila. Am J Physiol. 1967 Sep;213(3):663–670. doi: 10.1152/ajplegacy.1967.213.3.663. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman B. W., Schaaper R. M., Haseltine W. A., Dunn R. L., Brash D. E. The C-C (6-4) UV photoproduct is mutagenic in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6945–6949. doi: 10.1073/pnas.83.18.6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar D. I., Waghorne C. DNA repair in Cockayne syndrome. Am J Hum Genet. 1978 Nov;30(6):590–601. [PMC free article] [PubMed] [Google Scholar]
- Honda S., Matsuo M. Lifespan shortening of the nematode Caenorhabditis elegans under higher concentrations of oxygen. Mech Ageing Dev. 1992 May;63(3):235–246. doi: 10.1016/0047-6374(92)90002-u. [DOI] [PubMed] [Google Scholar]
- Jiang N., Taylor J. S. In vivo evidence that UV-induced C-->T mutations at dipyrimidine sites could result from the replicative bypass of cis-syn cyclobutane dimers or their deamination products. Biochemistry. 1993 Jan 19;32(2):472–481. doi: 10.1021/bi00053a011. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987 Feb;123(2):241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Seidman M. M. Use of supF, an Escherichia coli tyrosine suppressor tRNA gene, as a mutagenic target in shuttle-vector plasmids. Mutat Res. 1989 Mar-May;220(2-3):61–72. doi: 10.1016/0165-1110(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Harcourt S. A., de Weerd-Kastelein E. A., Keijzer W., Hall-Smith P. Repair of ultraviolet light damage in a variety of human fibroblast cell strains. Cancer Res. 1977 Mar;37(3):904–910. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Mayne L. Abnormal kinetics of DNA synthesis in ultraviolet light-irradiated cells from patients with Cockayne's syndrome. Cancer Res. 1979 Oct;39(10):4237–4241. [PubMed] [Google Scholar]
- Lehmann A. R. Three complementation groups in Cockayne syndrome. Mutat Res. 1982 Dec;106(2):347–356. doi: 10.1016/0027-5107(82)90115-4. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A. G., Ahn B. W., Shaltiel S., Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Marshall R. R., Arlett C. F., Harcourt S. A., Broughton B. A. Increased sensitivity of cell strains from Cockayne's syndrome to sister-chromatid-exchange induction and cell killing by UV light. Mutat Res. 1980 Jan;69(1):107–112. doi: 10.1016/0027-5107(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. Superoxide dismutase: "positive" spectrophotometric assays. Anal Biochem. 1977 May 1;79(1-2):553–560. doi: 10.1016/0003-2697(77)90429-8. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Muriel W. J., Lamb J. R., Lehmann A. R. UV mutation spectra in cell lines from patients with Cockayne's syndrome and ataxia telangiectasia, using the shuttle vector pZ189. Mutat Res. 1991 Mar;254(2):119–123. doi: 10.1016/0921-8777(91)90002-7. [DOI] [PubMed] [Google Scholar]
- Nance M. A., Berry S. A. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992 Jan 1;42(1):68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Norris P. G., Arlett C. F., Cole J., Lehmann A. R., Hawk J. L. Abnormal erythemal response and elevated T lymphocyte HRPT mutant frequency in Cockayne's syndrome. Br J Dermatol. 1991 May;124(5):453–460. doi: 10.1111/j.1365-2133.1991.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Oliver C. N., Ahn B. W., Moerman E. J., Goldstein S., Stadtman E. R. Age-related changes in oxidized proteins. J Biol Chem. 1987 Apr 25;262(12):5488–5491. [PubMed] [Google Scholar]
- Otsuka F., Tarone R. E., Cayeux S., Robbins J. H. Use of lymphoblastoid cell lines to evaluate the hypersensitivity to ultraviolet radiation in Cockayne syndrome. J Invest Dermatol. 1984 May;82(5):480–484. doi: 10.1111/1523-1747.ep12260999. [DOI] [PubMed] [Google Scholar]
- Parris C. N., Kraemer K. H. Ultraviolet mutagenesis in human lymphocytes: the effect of cellular transformation. Exp Cell Res. 1992 Aug;201(2):462–469. doi: 10.1016/0014-4827(92)90295-j. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G. Solar-ultraviolet-induced damage to DNA. Photodermatol. 1989 Feb;6(1):1–15. [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Perera M. I., Um K. I., Greene M. H., Waters H. L., Bredberg A., Kraemer K. H. Hereditary dysplastic nevus syndrome: lymphoid cell ultraviolet hypermutability in association with increased melanoma susceptibility. Cancer Res. 1986 Feb;46(2):1005–1009. [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. Reduced repair of non-dimer photoproducts in a gene transfected into xeroderma pigmentosum cells. Photochem Photobiol. 1986 May;43(5):509–513. doi: 10.1111/j.1751-1097.1986.tb09528.x. [DOI] [PubMed] [Google Scholar]
- Ragland S. S., Sohal R. S. Ambient temperature, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1975;10(5):279–289. doi: 10.1016/0531-5565(75)90005-4. [DOI] [PubMed] [Google Scholar]
- Ragland S. S., Sohal R. S. Mating behavior, physical activity and aging in the housefly, Musca domestica. Exp Gerontol. 1973 Jun;8(3):135–145. doi: 10.1016/0531-5565(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Riely C. A., Cohen G., Lieberman M. Ethane evolution: a new index of lipid peroxidation. Science. 1974 Jan 18;183(4121):208–210. doi: 10.1126/science.183.4121.208. [DOI] [PubMed] [Google Scholar]
- Seetharam S., Kraemer K. H., Waters H. L., Seidman M. M. Mutational hotspot variability in an ultraviolet-treated shuttle vector plasmid propagated in xeroderma pigmentosum and normal human lymphoblasts and fibroblasts. J Mol Biol. 1990 Apr 5;212(3):433–436. doi: 10.1016/0022-2836(90)90319-H. [DOI] [PubMed] [Google Scholar]
- Seetharam S., Kraemer K. H., Waters H. L., Seidman M. M. Ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum lymphoblastoid cells and fibroblasts. Mutat Res. 1991 Jan;254(1):97–105. doi: 10.1016/0921-8777(91)90045-q. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Seidman M. The development of transient SV40 based shuttle vectors for mutagenesis studies: problems and solutions. Mutat Res. 1989 Mar-May;220(2-3):55–60. doi: 10.1016/0165-1110(89)90010-9. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Arnold L., Orr W. C. Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mech Ageing Dev. 1990 Dec;56(3):223–235. doi: 10.1016/0047-6374(90)90084-s. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Buchan P. B. Relationship between physical activity and life span in the adult housefly, Musca domestica. Exp Gerontol. 1981;16(2):157–162. doi: 10.1016/0531-5565(81)90040-1. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Donato H. Effects of experimentally altered life spans on the accumulation of flourescent age pigment in the housefly, Musca domestica. Exp Gerontol. 1978;13(5):335–341. doi: 10.1016/0531-5565(78)90042-6. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Farmer K. J., Allen R. G., Ragland S. S. Effects of diethyldithiocarbamate on life span, metabolic rate, superoxide dismutase, catalase, inorganic peroxides and glutathione in the adult male housefly, Musca domestica. Mech Ageing Dev. 1984 Feb;24(2):175–183. doi: 10.1016/0047-6374(84)90069-1. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Müller A., Koletzko B., Sies H. Effect of age and ambient temperature on n-pentane production in adult housefly, Musca domestica. Mech Ageing Dev. 1985 Mar;29(3):317–326. doi: 10.1016/0047-6374(85)90071-5. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Sohal B. H. Hydrogen peroxide release by mitochondria increases during aging. Mech Ageing Dev. 1991 Feb;57(2):187–202. doi: 10.1016/0047-6374(91)90034-w. [DOI] [PubMed] [Google Scholar]
- Sohal R. S., Toy P. L., Allen R. G. Relationship between life expectancy, endogenous antioxidants and products of oxygen free radical reactions in the housefly, Musca domestica. Mech Ageing Dev. 1986 Sep;36(1):71–77. doi: 10.1016/0047-6374(86)90140-5. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Stadtman E. R., Starke-Reed P. E., Oliver C. N., Carney J. M., Floyd R. A. Protein modification in aging. EXS. 1992;62:64–72. doi: 10.1007/978-3-0348-7460-1_7. [DOI] [PubMed] [Google Scholar]
- Starke-Reed P. E., Oliver C. N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989 Dec;275(2):559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Oliver C. N., Stadtman E. R. Modification of hepatic proteins in rats exposed to high oxygen concentration. FASEB J. 1987 Jul;1(1):36–39. doi: 10.1096/fasebj.1.1.2886388. [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Toyoda M., Hashimoto T., Furuyama J., Kurihara T., Inoue M., Takebe H. Differential hypersensitivity of xeroderma pigmentosum lymphoblastoid cell lines to ultraviolet light mutagenesis. Carcinogenesis. 1987 Jan;8(1):53–57. doi: 10.1093/carcin/8.1.53. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Levitt J. G., Crapo J. D. The effect of hyperoxia on superoxide production by lung submitochondrial particles. Arch Biochem Biophys. 1982 Sep;217(2):401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- Venema J., Mullenders L. H., Natarajan A. T., van Zeeland A. A., Mayne L. V. The genetic defect in Cockayne syndrome is associated with a defect in repair of UV-induced DNA damage in transcriptionally active DNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4707–4711. doi: 10.1073/pnas.87.12.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]