Abstract
CD4+ T-cell help (CD4 help) plays a pivotal role in CD8+ T-cell responses against viral infections. However, the role in primary CD8+ T-cell responses remains controversial. We evaluated the effects of infection route and viral dose on primary CD8+ T-cell responses to vaccinia virus (VACV) in MHC class II−/− mice. CD4 help deficiency diminished the generation of VACV-specific CD8+ T cells after intraperitoneal (i.p.) but not after intranasal (i.n.) infection. A large viral dose could not restore normal expansion of VACV-specific CD8+ T cells in i.p. infected MHC II−/− mice. In contrast, dependence on CD4 help was observed in i.n. infected MHC II−/− mice when a small viral dose was used. These data suggested that primary CD8+ T-cell responses are less dependent on CD4 help in i.n. infection compared to i.p. infection. Activated CD8+ T cells produced more IFN-γ, TNF-α and granzyme B in i.n. infected mice than those in i.p. infected mice, regardless of CD4 help. IL-2 signaling via CD25 was not necessary to drive expansion of VACV-specific CD8+ T cells in i.n. infection, but it was crucial in i.p. infection. VACV-specific CD8+ T cells underwent increased apoptosis in the absence of CD4 help, but proliferated normally and had cytotoxic potential, regardless of infection route. Our results indicate that route of infection and viral dose are two determinants for CD4 help dependence, and intranasal infection induces more potent effector CD8+ T cells than i.p. infection.
Keywords: CD4 help, MHC II−/− mice, primary CD8+ T-cell response, vaccinia virus
Introduction
CD8+ T cells are one of the key effectors in adaptive immunity and they play a critical role in protection from various pathogens. Viruses entering the body via various routes will face different microenvironments where different cells reside and interact. T-cell responses to a typical acute viral infection can be characterized into three major phases: effector T-cell expansion and differentiation, contraction, and memory T-cell formation. These phases are precisely driven and controlled by T-cell receptor engagement, costimulation and inflammatory cytokines as well as CD4+ T-cell help (CD4 help).1
T-cell receptors of naive CD8+ T cells recognize a specific epitope presented by MHC class I (MHC I) on antigen-presenting cells (APCs), constituting ‘signal 1', which initiates a primary response and starts clonal expansion and differentiation.2 Once activated, the expansion of CD8+ T cells is preprogrammed and does not require further contact with antigen (Ag).3,4 Ag-independent expansion is supported by IL-2 and further augmented by IL-7 or IL-15.3 Costimulation provided by APCs, acting as ‘signal 2', is essential to induce full activation of T cells and prevents them from becoming refractory to Ag stimulation.5 The most important costimulatory pathways include CD28/CD80–CD86,6 CD40L/CD40,7 CD27/CD70,8 4-1BB/4-1BBL9 and OX-40/OX40L.10 Furthermore, inflammatory cytokines, such as IL-12 and type I IFNs, provide ‘signal 3' at distinct stages of the response for optimal generation of effector and memory populations.11
In addition to these three signals, CD4 help plays a pivotal role in CD8+ T-cell responses.12 A number of studies have confirmed that CD4 help is required for development of CD8+ T-cell memory and secondary expansion of CD8+ T cells.13,14,15,16 However, the role of CD4 help in the primary CD8+ T-cell response remains controversial, since differing and even contradictory results are frequently observed. CD4 help is necessary in priming CD8+ T cells with non-infectious agents (such as minor histocompatibility Ags, tumor Ags, Ag-loaded splenocytes, grafts, alloantigens and soluble protein Ags), but is variably required for CD8+ T-cell responses to infectious agents.17 CD4 help is required for the primary CD8+ T-cell response to herpes simplex virus,18 but not to vesicular stomatitis virus infection.19 It is required for sustaining cytotoxic T lymphocyte (CTL) responses during chronic infection with lymphocytic choriomeningitis virus variants, but not for resolving acute lymphocytic choriomeningitis virus infection.20 These data indicate that the identity of the pathogen is an important variable. Furthermore, divergent results regarding the role for CD4 help have been reported with the same pathogen.17 In vaccinia virus (VACV) infection, primary CD8+ T-cell responses have been shown to be dependent on CD4+ T cells in some reports,21,22,23 while other studies report CD4 help independence.14,24 Different experimental conditions were used in these studies, including the VACV strain, inoculum dose and route of infection as well as the mouse model.
VACV, a dsDNA virus, belongs to the family Poxviridae and the genus Orthopoxvirus and shares high homology with other orthopoxviruses, such as variola virus (the smallpox in humans), ectromelia virus (mousepox) and monkeypox.25 The natural reservoir of VACV is not known, but it can replicate in mice. VACV vaccination was one of the most important medical practices in human history, resulting in the eradication of smallpox. Attenuated VACV has been used as a vaccine vector against infectious agents and cancers, and as a gene delivery system to study biological functions of foreign genes.26 VACV-infected mouse models have been used extensively in the study of acute virus infection. Depending on experimental settings, VACV is administered by various routes, e.g., intranasal,24 intraperitoneal,14,21,22,23,27 intradermal,28 intracranial29 and intravenous.30
Despite numerous previous studies, there lacks a side-by-side comparison of the CD4 dependency of the CD8+ T-cell response when either the viral dose or route of VACV infection is varied. Here we used MHC class II knockout (MHC II−/−) mice to determine the CD4 help dependence of primary CD8+ T-cell response to VACV Western Reserve strain infection by comparing the response in intranasal and intraperitoneal infection. Our data demonstrate that the requirement for CD4 help in primary CD8+ T-cell response against VACV infection varies depending on the route of infection and viral dose, and intranasal infection induces more potent effector CD8+ T cells than intraperitoneal infection.
Materials and methods
Mice and generation of mixed bone marrow chimeric mice
C57BL/6 (B6) and congenic B6-Ly5.2/Cr mice were purchased from the National Cancer Institute (Frederick, MD, USA). MHC class II knockout (MHC II−/−) mice that lack CD4+ lymphocytes31 were obtained from Taconic Biosciences (Albany, NY, USA). CD25−/−mice (B6.129S4-Il2ratm1Dw/J) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). For generating mixed bone marrow (BM) chimeric mice, B6-Ly5.2 recipient mice were lethally irradiated with 1000 rads (500 rads twice given 24 h apart), and subsequently injected intravenously (i.v.) with a total of 4.5×106 BM cells containing a mixture of B6-Ly5.2 (CD45.1+) and CD25−/− (CD45.2+) cells at a 1∶2 ratio. Reconstitution of the BM was confirmed at 50 days post-BM transfer by staining blood cells for the congenic markers. All mice were bred or housed in the Dartmouth-Hitchcock Medical Center mouse facility. The Animal Care and Use Program of Dartmouth College approved all animal experiments.
Virus, viral infection and plaque assay
Vaccinia virus Western Reserve strain (VACV-WR) was obtained from Dr William R Green (Geisel School of Medicine at Dartmouth, Lebanon, NH, USA), and was propagated and titered in the 143B cell line. Mice were infected with 103 PFU of VACV in 30 µl of PBS for intranasal inoculation (i.n.) or in 300 µl of PBS for intraperitoneal inoculation (i.p.) unless otherwise noted. For i.n. infection, mice were anesthetized with isoflurane. Virus titers in the lungs and ovaries were measured by plaque-forming assay as previously described.32 Plaques were counted microscopically.
Tissue and cell preparations
To obtain single cell suspensions, spleens and livers were homogenized by passing through cell strainers, and red blood cells were lysed using Gey's solution. Lungs and livers were digested with collagenase (2.33 mg/ml) (Sigma-Aldrich, Milwaukee, WI, USA) and DNase (0.2 mg/ml) (Roche Diagnostics, Indianapolis, IN, USA) for 30 min.
Antibodies (Abs) and flow cytometry
Abs for flow cytometric analysis were purchased from eBioscience or BioLegend (San Diego, CA, USA) unless otherwise noted: CD8α PerCP-eFluor 710 (53-6.7), CD127 FITC (SB/199), KLRG1 PE (2F1 KLRG1), IFN-γ APC (XMG1.2), TNF-α FITC (MP6-XT22), granzyme B (GzmB) PE (GB12; Invitrogen, Carlsbad, CA, USA). Samples were analyzed using Accuri flow cytometers or MacsQuant flow cytometers in the Dartlab core facility at Geisel School of Medicine at Dartmouth. Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA) or Accuri software (BD Biosciences).
MHC/peptide tetramer
The VACV-specific epitope B8R20–27, TSYKFESV, was made as a synthetic peptide based on the original MVA (modified vaccinia virus Ankara) sequence.33 Peptide MHC I tetramers consisting of B8R20–27/Kb conjugated to allophycocyanin were obtained from the NIH Tetramer Core Facility (Emory University, Atlanta, GA, USA). Cells were stained with the tetramer together with Fc block (2.4G2) for 1 h at room temperature in the dark in PBS with 2% bovine growth serum, followed by surface staining at 4 °C for 20 min in 96-well U-bottom plates and were analyzed by flow cytometry.
Staining of surface markers and intracellular cytokines/effector molecules
Surface markers were stained with Abs in PBS with 2% bovine growth serum for 20 min at 4 °C. For intracellular cytokine/effector molecule detection, splenocytes from infected mice were restimulated ex vivo with 1 µg/ml B8R peptide for 5 h at 37 °C in complete medium with 10 U/ml rIL-2 and 10 µg/ml brefeldin A (Sigma-Aldrich). Subsequently, cells were stained with Abs against surface markers, followed by fixation with 1% formaldehyde for 20 min at 4 °C and then stained with Abs against IFN-γ, TNF-α or GzmB in 0.5% saponin solution for 30 min at 4 °C.
Depletion of CD4+ T cells
Mice were administered i.p. 500 µg of anti-CD4 Ab (GK1.5), at days −1 and 0 of infection, followed by 250 µg twice weekly thereafter until the mice were sacrificed. Control mice were either untreated or given rat IgG (Jackson ImmunoResearch Laboratories, West Groove, PA, USA). No differences in T-cell responses were observed in the two groups of control mice (data not shown).
Blockade of CD40L
CD40L blocking Ab MR-1 (500 µg) (BioXcell, West Lebanon, NH, USA) was administrated i.p., starting at days −1 and 0 of infection, followed by every 2 days throughout the duration of the experiments. Control mice were either untreated or given rat IgG.
Measurement of cell proliferation
To measure turnover of B8R-specific CD8+ T cells in vivo, cell proliferation was measured by 5-bromo-2′-deoxyuridine (BrdU) staining. Mice were injected 1 mg BrdU (i.p.) twice at 12 h apart, starting at day 8 post infection (pi). Splenocytes were prepared 18 h later, and cells were stained with anti-CD8α Ab and B8R tetramer, and then stained with the anti-BrdU Ab according to the protocol provided (BD Pharmingen G, San Jose, CA, USA).
Apoptosis assay by Annexin V staining
Splenocytes were prepared from infected mice at day 10 pi. Cells were stained with B8R tetramer, anti-CD8α Ab and Annexin V using an Apoptosis Detection Kit (BD Biosciences). The Annexin V staining was performed in conjunction with 7-aminoactinomycin D (7-AAD), since viable cells with intact membranes can exclude 7-AAD.
Cytotoxicity assays in vivo and ex vivo
For in vivo cytotoxicity assay, splenocytes were prepared from B6 mice and were incubated with 1 µg/ml B8R peptide in complete medium at 37 °C for 1 h. The B8R-pulsed and -unpulsed cells were labeled with 2.5 µM and 0.25 µM CFSE, respectively, in HBSS at room temperature for 10 min. The B8R-pulsed (CFSEhi) and -unpulsed (CFSElo) cells were mixed at a 1∶1 ratio and used as target cells. 2×107 target cells were injected i.v. into i.n. infected wild-type (WT) or MHC II−/− mice at day 9 pi, or naive WT mice. Six hours later, mice were killed and splenocytes were stained with 10 µM 7-AAD at room temperature for 15 min. CFSE-positive and 7AAD-negative cells were analyzed by flow cytometry. Specific lysis was evaluated with percent specific killing, calculated according to the formulas: % specific lysis=(1−[ratio of infected recipients/ratio of naive recipients])×100%, where ratio=number of CFSEhi/number of CFSElo.
For ex vivo cytotoxicity assay, effector CD8+ T cells were prepared from splenocytes of i.p. infected WT or MHC II−/− mice at day 10 pi by staining with anti-CD8α Ab and B8R tetramer. Target cells were prepared as described in in vivo cytotoxicity assay. Effector B8R-specific CD8+ T cells were cultured with target cells at a ratio of 5∶1 (105:2×104, normalized to the same total number of B8R-specific CD8+ T cells) in complete media in 96 well plates at 37 °C for 6 h. Cells were then stained with 10 µM 7-AAD at room temperature for 15 min and CFSE-positive and 7AAD-negative cells were analyzed by flow cytometry. Specific lysis was calculated based on the formulas: % specific lysis=[1−(ratio of WT or MHC II−/− B8R+CD8+ group/ratio of target alone group)]×100%, where ratio=number of CFSEhi/number of CFSElo.
Statistical analysis
Student's t-tests (two-tailed, unpaired) were performed using GraphPad Prism 5 (GraphPad, La Jolla, CA, USA). Values of P<0.05 were considered statistically significant.
Results
CD4 help deficiency impairs primary VACV-specific CD8+ T-cell responses in intraperitoneally infected mice but not in intranasally infected mice
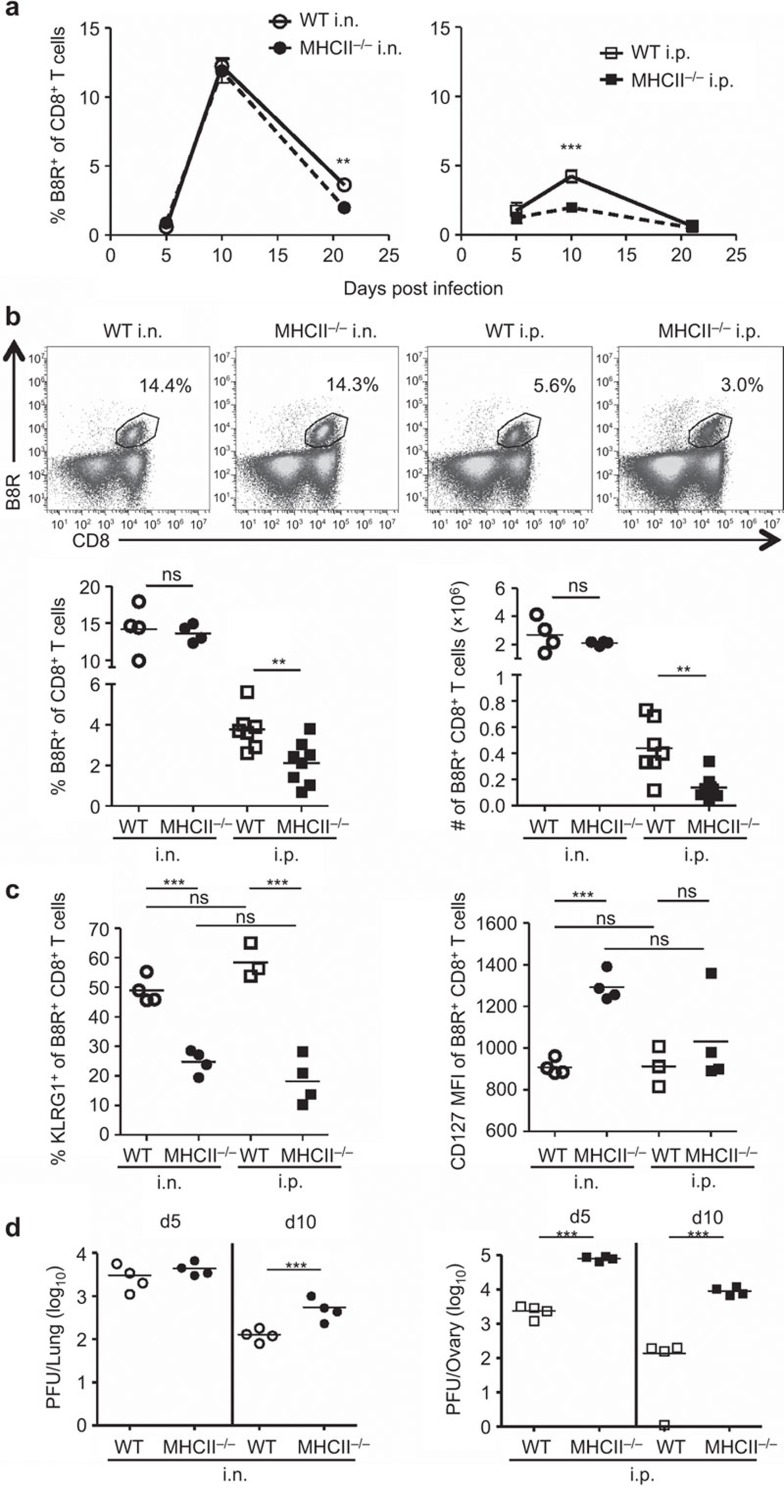
Clonal expansion of Ag-specific CD8+ T cells is essential for adaptive immunity to viral infection. Upon infection, Ag-specific CD8+ T cells can undergo 104- to 105-fold expansion in a week.34 It is estimated that there are approximately 1070 naive CD8+ T cells/spleen specific for B8R20–27, a dominant epitope of VACV in mice.35 We first studied the kinetics of the VACV-specific CD8+ T-cell response by tetramer staining of B8R-specific CD8+ T cells. WT B6 mice and MHC II−/−mice were infected i.n. or i.p. with 103 PFU of VACV, a dose less than the lethal dose of 105 PFU via i.n. infection.32 WT and MHC II−/− mice infected i.n. mounted a robust primary CD8+ T-cell response, and expansion of B8R-specific CD8+ T cells reached a peak at d10 pi in the spleen (Figure 1a and b) and the lung (Supplementary Figure 1). Over time, B8R-specific CD8+ T-cell populations contracted, and the population in MHC II−/− mice had a faster contraction than that had in the WT cohort by d21 pi. In contrast, the i.p. infected MHC II−/− mice had an attenuated primary CD8+ T-cell response, and the number of B8R-specific CD8+ T cells was significantly reduced, compared with infected WT mice in the spleen (Figure 1a and b) and the lung (Supplementary Figure 1). Similar results were also observed in mice that were depleted of CD4+ T cells using anti-CD4 Ab (GK1.5) (data not shown).
Figure 1.
Primary CD8+ T-cell responses in intranasally or intraperitoneally infected mice in the absence of CD4 help. C57BL/6 (WT) and MHC class II knockout (MHC II−/−) mice were infected with 103 PFU of VACV-WR i.n. or i.p.. B8R-specific CD8+ T cells were identified by staining with B8R tetramer and anti-CD8α Ab at the indicated times. (a) Kinetics of B8R-specific CD8+ T-cell response. Frequencies of B8R-specific cells within the CD8+ T-cell population in the spleens of WT and MHC II−/− mice infected i.n. or i.p. are shown. (b) B8R-specific CD8+ T-cell populations in the spleens at day 10 post infection (pi). Representative FACS plots (top panel); frequency and total numbers (bottom panel). (c) Expression of KLRG1 and CD127 on B8R-specific CD8+ T cells at d9 pi. (d) Viral titers in lungs and ovaries, determined by plaque-forming assays. Each point on the graph represents a single mouse, and horizontal bars indicate the means. Data are representative of two to three independent experiments with three to eight mice per group. **P<0.01; ***P<0.001. Ab, antibody; i.n., intranasally; i.p., intraperitoneally; ns, not significant; pi, post infection; VACV-WR, vaccinia virus Western Reserve; WT, wild-type.
Upon first Ag encounter, naive CD8+ T cells can expand and develop into short-lived effector cells (SLECs), which confer immediate protection, but decline following Ag clearance. Alternatively, CD8+ T cells can become memory precursor effector cells.36,37 KLRG1hiCD127lo and KLRG1loCD127hi CD8+T cells are considered SLECs and memory precursor effector cells, respectively.38 In this study, the frequency of cells expressing KLRG1 was reduced and CD127 expression was at a higher level on B8R-specific CD8+ T cells in MHC II−/− mice compared to WT mice, and there were no significant differences between the i.p. and i.n. infections (Figure 1c). These data indicate that SLECs are diminished in the absence of CD4 help.
To study the effect of the diminished primary CD8+ T-cell response on viral control, we detected viral load by plaque-forming assay. Lungs and ovaries are the major organs for VACV replication in the i.n. and i.p. infection, respectively.27,39 In this experiment, infection led to high viral titers in both WT and MHC II−/− mice at d5 pi, regardless of infection route. The viral titers declined by d10 pi, but higher viral burdens remained in the organs of MHC II−/− mice in both i.n. and i.p. infected groups (Figure 1d). This indicates that the absence of CD4 help impairs viral control, but does not strictly correlate with the size of the B8R-specific CD8+ T-cell population. The high affinity Ab response is also impaired in MHC II−/− mice,27 and this likely contributes to the reduction in virus control observed in these mice.
Viral dose determines the CD4 help dependence of VACV-specific CD8+ T-cell expansion in intranasally infected mice
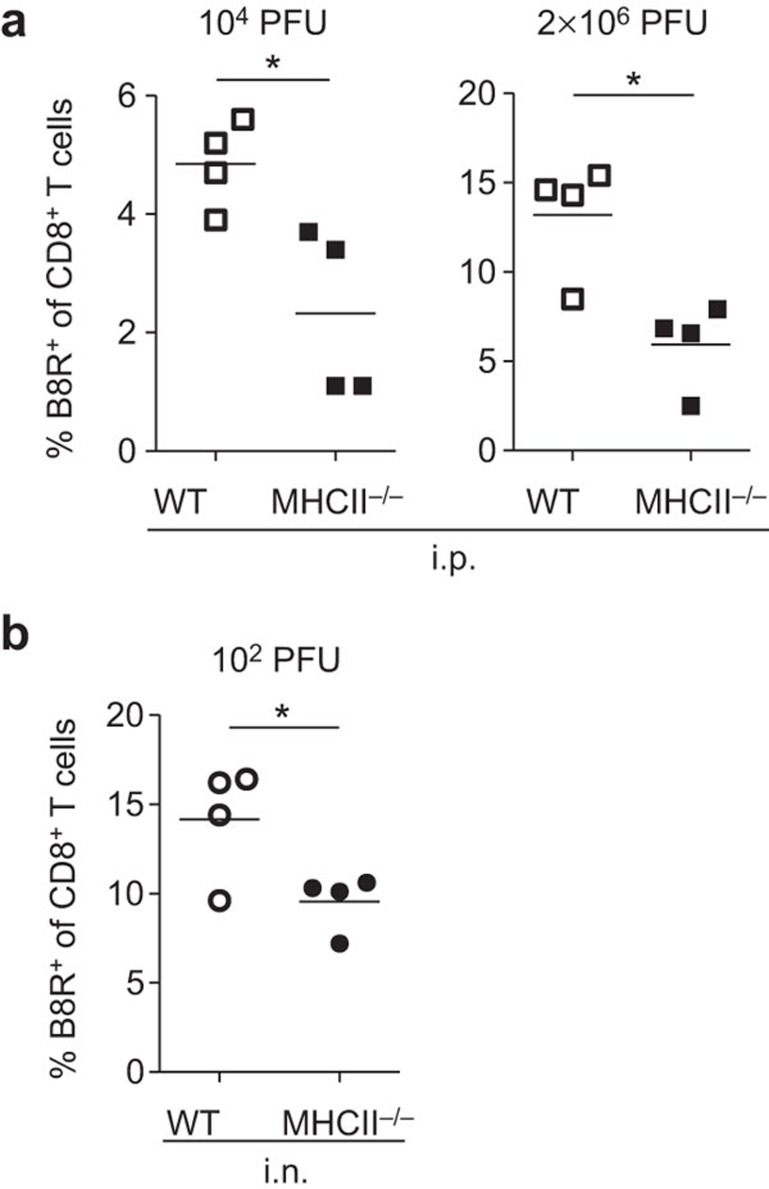
We wished to explore factors contributing to the difference in the requirement for CD4 help in clonal expansion between i.n. infection and i.p. infection. First, we analyzed the effect of viral load by administering different doses of virus. In previous experiments (Figure 1), our standard inoculating dose of virus was 103 PFU/mouse in both i.n. and i.p. infections. For i.p. infection, both 104 PFU and 2×106 PFU doses manifested the same CD4 help dependence as was seen with the standard 103PFU dose (Figure 2a). The data indicate that an increased initial viral load does not preclude the requirement of CD4 help for primary CD8+ T-cell responses in i.p. infection.
Figure 2.
Influence of amount of viral inoculum on generation of VACV-specific CD8+ T cells. B8R-specific CD8+ T cells were prepared from the spleens of infected mice at d10 pi. (a) Frequency of B8R-specific cells within the CD8+ T-cell population in mice infected i.p. with 104 or 2×106 PFU of VACV. (b) Frequency of B8R-specific cells within the CD8+ T-cell population in mice infected i.n. with 1×102 PFU of VACV. Data are representative of two independent experiments with four mice per group. *P<0.05. pi, post infection; VACV, vaccinia virus.
Unexpectedly, when reducing inoculated dose (102 PFU of VACV) in i.n. infected MHC II−/− mice, generation of B8R-specific CD8+ T cells did need CD4 help. The population of B8R-specific CD8+ T cells in MHC II−/− mice was smaller compared to WT mice (Figure 2b). These data suggest that while the requirement for CD4 help in the i.p. infection cannot be compensated by a high virus load, CD4 help in i.n. infected mice is required when a low virus dose is administered. Accordingly, inoculated viral dose is another factor determining dependence on CD4 help for primary CD8+ T-cell responses.
Enhanced effector capacity in VACV-specific CD8+ T cells following intranasal infection, compared with intraperitoneal infection
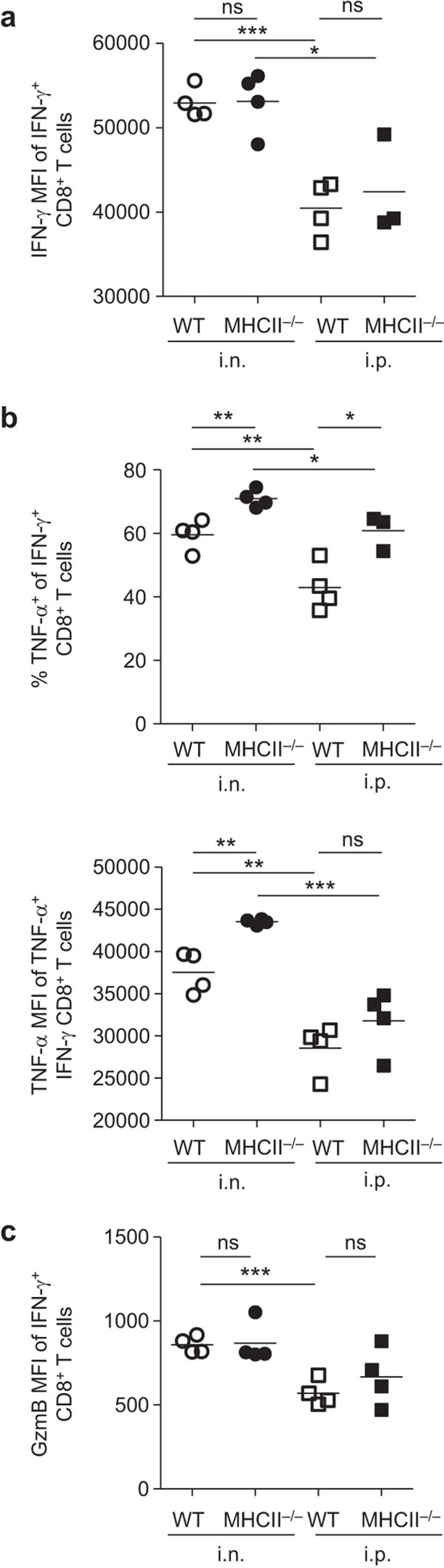
To test the influence of infection route and CD4 help on effector molecule production, we measured production of cytokines IFN-γ and TNF-α as well as effector molecule GzmB by activated CD8+ T cells. IFN-γ+CD8+ T cells from i.n. infected mice produced more IFN-γ on a per cell basis, as determined by mean fluorescence intensity (MFI), compared with i.p. infected mice, regardless of CD4 help (Figure 3a). The frequency of TNF-α+ cells (Figure 3b, top), and production of TNF-α per cell (Figure 3b, bottom) were significantly higher in the IFN-γ+CD8+ T cells from i.n. infected mice than those from i.p. infected mice. Interestingly, regardless of infection route, mice lacking CD4 help contained a higher frequency of cells producing both IFN-γ and TNF-α than WT mice (Figure 3b). Production of GzmB was higher on a per cell basis in the IFN-γ+CD8+ T cells from i.n. infected mice than those from i.p. infected mice (Figure 3c). These results indicate that i.n. infection can induce enhanced production of IFN-γ, TNF-α and GzmB from IFN-γ+CD8+ T cells, compared to i.p. infection. In addition, the absence of CD4 help did not reduce the effector cytokine/molecule production by B8R-specific CD8+ T cells.
Figure 3.
Production of IFN-γ, TNF-α and GzmB by VACV-specific CD8+ T cells in the presence or absence of CD4 help. Splenocytes were prepared at d9 pi from mice which were infected with 103 PFU of VACV. The cells were restimulated ex vivo with B8R peptide and stained with anti-CD8α, -IFN-γ, -TNF-α and -GzmB Abs. (a) IFN-γ MFI of IFN-γ+CD8+ T cells. (b) Frequency of TNF-α-producing cells within IFN-γ+CD8+ T cells (top panel) and TNF-α MFI of TNF-α+IFN-γ+CD8+ T cells (bottom panel). (c) GzmB MFI of IFN-γ+CD8+ T cells. Data are representative of two independent experiments with three to four mice per group. *P<0.05; **P<0.01; ***P<0.001. Ab, antibody; GzmB, granzyme B; MFI, mean fluorescence intensity; ns, not significant; pi, post infection; VACV, vaccinia virus.
CD4 help-deficiency does not affect cytotoxic activity of VACV-specific CD8+ T cells
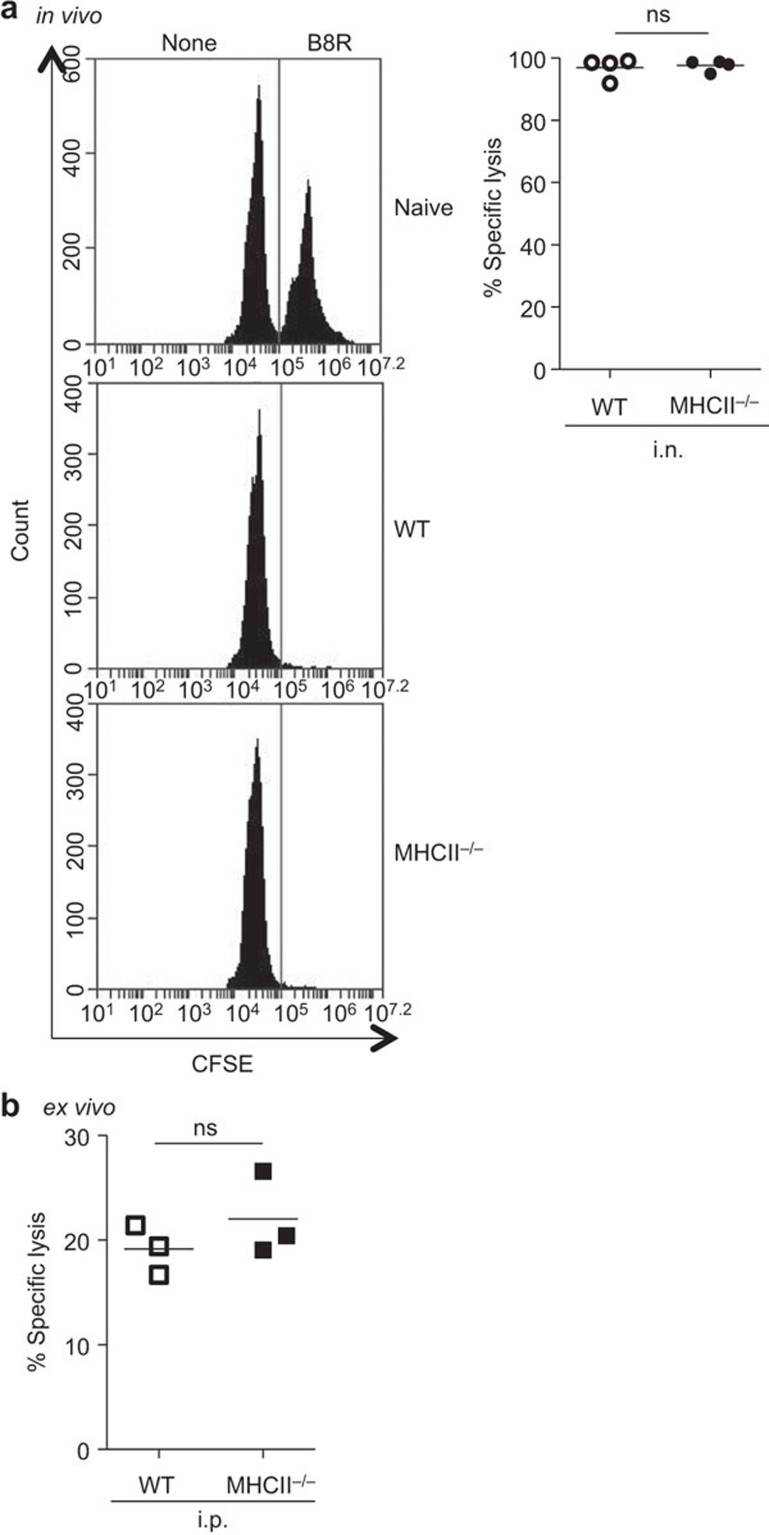
We performed an in vivo cytotoxicity assay to assess effect of CD4 help on the activity of VACV-specific CD8+ T cells in i.n infected WT and MHC II−/− mice. As these mice had the same frequency of B8R-specific CD8+ T cells (Figure 1b), a direct comparison between the two groups could be made using this assay. B8R-pulsed (CFSEhi) and -unpulsed (CFSElo) splenocytes from B6 mice were mixed as target cells, and 2×107 of the target cells were injected to WT and MHC II−/− mice at d10 pi. Six hours later, spleens were harvested and target cells were detected by flow cytometry. B8R-pulsed target cells were completely cleared from VACV-infected mice, and there was no difference between WT and MHC II−/− mice (Figure 4a). This indicated highly effective cytotoxic function in both WT and CD4 help-deficient groups.
Figure 4.
Cytotoxic activity of VACV-specific CD8+ T cells is not affected by the absence of CD4 help. (a) In vivo cytotoxic activity. Target cells, B8R-pulsed (CFSEhi) and-unpulsed (CFSElo) splenocytes (1∶1), were transferred to i.n. infected mice (103 PFU) at d10 pi. Six hours later, spleens were harvested and CFSE-labeled cells were analyzed by flow cytometry. Representative FACS histograms of live target cells and percent specific lysis are shown. (b) Ex vivo cytotoxic activity. Effector cells (B8R-specific CD8+ T cells) were prepared from splenocytes of i.p. infected mice (103 PFU) at d10 pi. Effector cells were cultured with target cells at 5∶1 ratio (normalized to same total number of B8R-specific CD8+ T cells) for 6 h, and CFSE-labeled target cells were analyzed. Percent specific lysis is shown. Data are representative of two independent experiments with three to four mice per group. i.n., intranasally; i.p., intraperitoneally; ns, not significant; pi, post infection; VACV, vaccinia virus.
Since B8R-specific CD8+ T cells in i.p. infected MHC II−/−mice were present at a much lower level than that in i.p. infected WT mice (Figure 1b), the in vivo cytotoxicity assay could not be used to compare these groups directly. Thus, we performed an ex vivo cytotoxicity assay to assess effect of CD4 help on CTL activity in i.p. infected WT and MHC II−/− mice. Target cells were prepared in the same way as those in the in vivo cytotoxicity assay. Effector B8R-specific CD8+ T cells were prepared from the splenocytes of i.p. infected WT and MHC II−/− mice at d10 pi. Effector cells were cocultured with target cells at a ratio of 5∶1 for 6 h. The specific lysis was approximately 20% and there was no difference in CTL activity between the two groups (Figure 4b). This result is in agreement with previous work with 51Cr release assays in which cytotoxicity toward VACV-infected target cells is not diminished in i.p. infected CD4+ T cell-depleted mice.27 These data suggest that cytotoxic activity of VACV-specific CD8+ T cells is not affected by the absence of CD4 help.
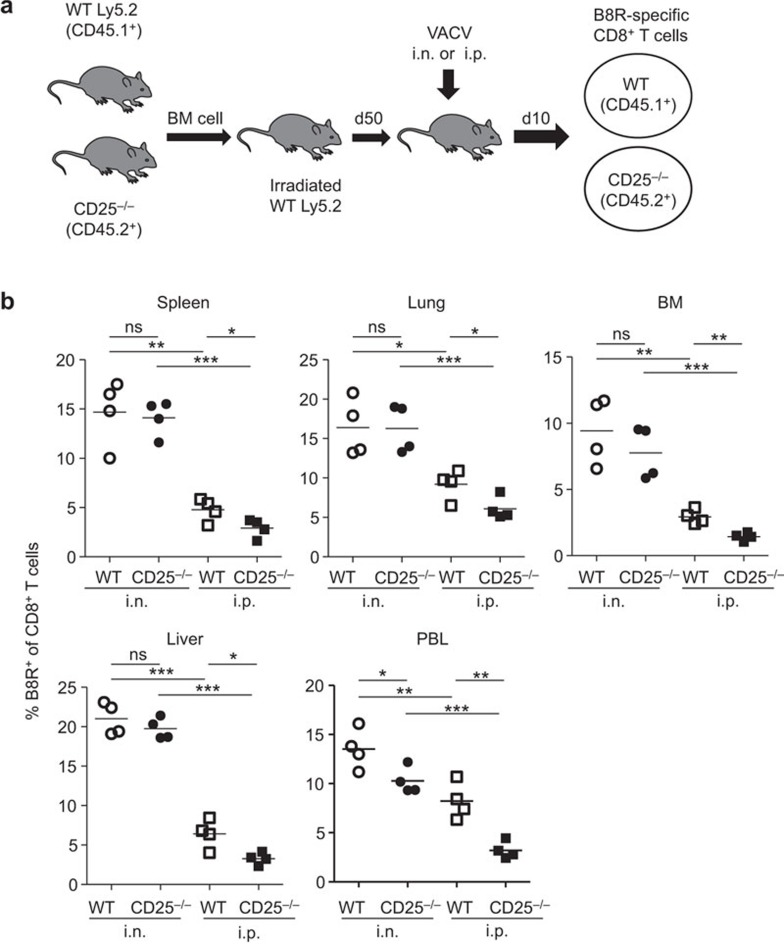
Differential requirement for IL-2 signaling through CD25 in i.n. versus i.p. infection
CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T-cell responses,40 and CD25 (IL-2 receptor α) is necessary to form the high-affinity IL-2 receptor. We investigated whether there were the same requirements for the high-affinity IL-2 receptor in CD8+ T-cell responses in mice infected by either the i.n. or i.p. route. Mixed BM chimeric mice were used, that contained both WT CD25+/+ (CD45.1+) and CD25−/− (CD45.2+) cells. CD25−/− mice cannot be used directly due to severe autoimmune disease in these mice because they lack regulatory T cells.41 Spleens, lungs, peripheral blood lymphocytes (PBLs), BM and livers were collected from the chimeric mice at d10 pi. Cells were stained with B8R tetramer and anti-CD8α Ab. The expansion of CD25−/− B8R-specific CD8+ T cells was significantly diminished compared to WT cells in i.p. infected chimeric mice, which is consistent with our previous studies.22 In contrast, i.n. infected chimeric mice mounted strong CD8+ T-cell responses from both the WT and CD25−/− compartments and no statistical differences could be detected in the spleens, lungs, BM or livers (Figure 5b). A small but statistically significant difference was detected in the PBL; however, this difference was smaller than that observed in the PBL of i.p. infected chimeric mice. The data demonstrate that CD25-deficiency significantly diminishes the expansion of the VACV-specific CD8+ T-cell pool in mice infected i.p. but not i.n., suggesting that there are compensatory mechanisms in play during lung infection.
Figure 5.
Signaling through CD25 is required to drive CD8+ T-cell responses in i.p. but not i.n infected mice. (a) Design of CD25−/− chimeric mice generation and infection with 103 PFU of VACV. (b) Frequency of B8R-specific CD8+ T cells in WT and CD25−/−chimeric mice at d10 pi. B8R-specific CD8+ T cells were detected in various organ/tissues, including the spleens, lungs, BM, livers and PBLs. *P<0.05; **P<0.01; ***P<0.001. BM, bone marrow; i.n., intranasally; i.p., intraperitoneally; ns, not significant; PBL, peripheral blood lymphocyte; pi, post infection; VACV, vaccinia virus; WT, wild-type.
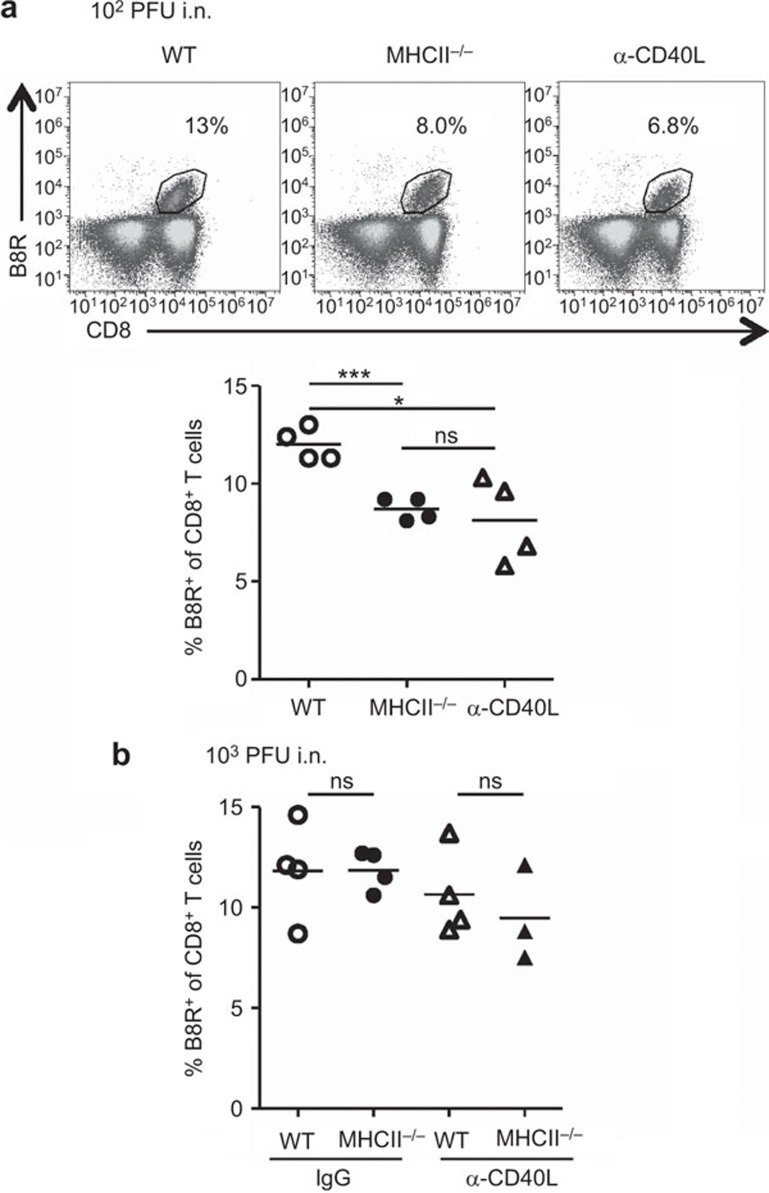
Role of CD40–CD40L pathway in the generation of VACV-specific CD8+ T-cell population in i.n. infection
It has been reported that signaling via the CD40–CD40L pathway can replace CD4 help in priming the CTL response.42,43 In this experiment, we measured generation of B8R-specfic CD8+ T cells in mice that were administered CD40L blocking Ab (MR-1) and infected i.n. with 102 PFU of VACV. This viral dose was used as it induces a CD4 help-dependent response after i.n. infection (Figure 2b). Blockade of CD40L resulted in populations of B8R-specific CD8+ T cells that were significantly smaller than in WT mice, and similar to MHC II−/− mice (Figure 6a). The data indicate that CD40–CD40L signaling is required for expansion of B8R-specific CD8+ T cells in i.n. infection with as mall viral dose. In contrast, for i.n. infection with 103 PFU of VACV, blockade of CD40–CD40L signaling did not significantly affect the expansion of the B8R-specific CD8+ T-cell population in both WT and MHC II−/− mice (Figure 6b). This is consistent with the CD4 help-independent status of the response after i.n. infection with 103 PFU of VACV (Figure 1a and b). Thus, whether CD40–CD40L signaling is required for expansion of B8R-specific CD8+ T cells depends upon viral dose after i.n. infection.
Figure 6.
Effect of CD40L signaling deficiency on the generation of VACV-specific CD8+ T-cell population in i.n. infection. Splenocytes were prepared from WT, MHC II−/−, CD40L-blocked (α-CD40L) WT or α-CD40L MHC II−/−mice at d9 pi. Blockade of CD40L signaling was performed with a CD40L-blocking Ab, MR-1. (a) Representative FACS plots and frequencies of B8R-specific cells within CD8+ T cells in mice infected with 102 PFU of VACV. (b) Frequency of B8R-specific cells within CD8+ T cells in mice infected with 103 PFU of VACV. Data are representative of two independent experiments with three to four mice per group. *P<0.05; ***P<0.001. i.n., intranasally; ns, not significant; pi, post infection; VACV, vaccinia virus; WT, wild-type.
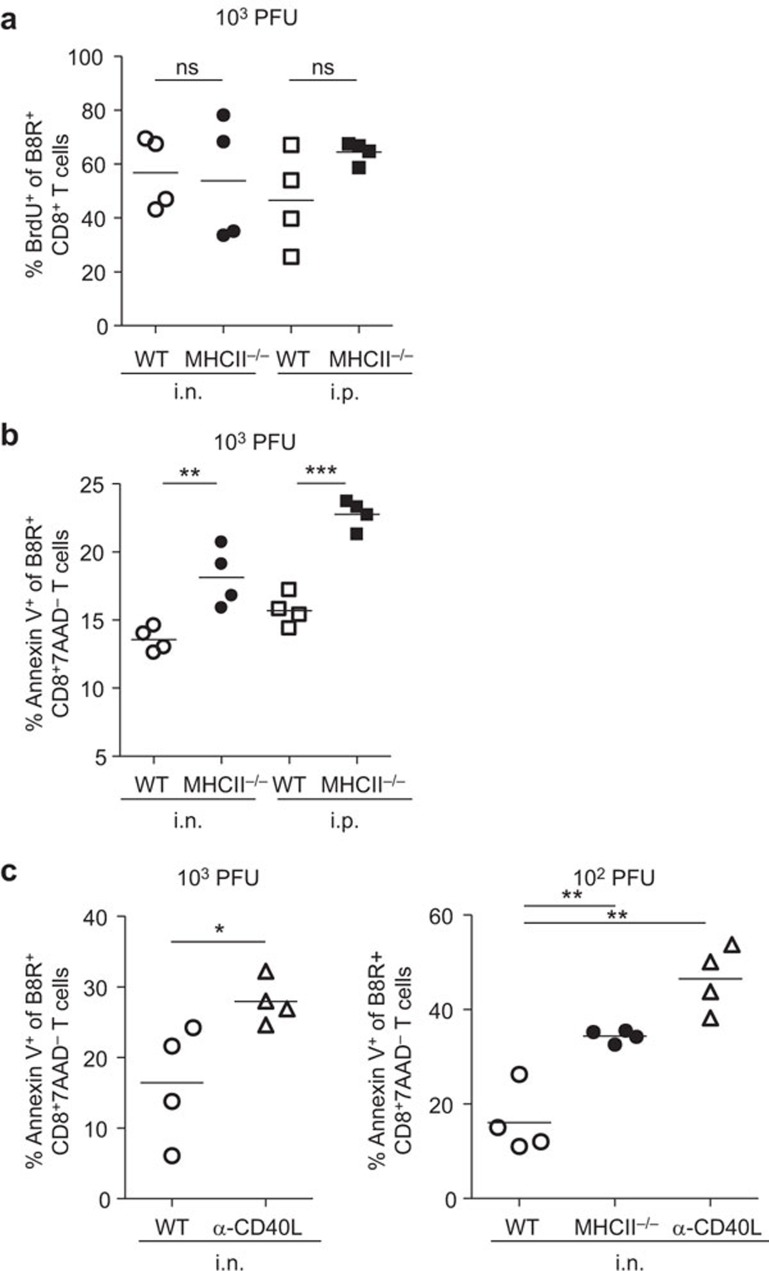
CD4 help deficiency results in increased apoptosis of VACV-specific CD8+ T cells
We measured the proliferation of B8R-specific CD8+ T cells in vivo by BrdU incorporation. Mice were administered BrdU at d8 pi, and 18 h later, splenocytes were prepared and stained with anti-CD8α Ab and B8R tetramer, and then with anti-BrdU Ab. B8R-specific CD8+ T cells from WT and MHC II−/− mice had similar rates of BrdU incorporation, regardless of infection route, indicating that Ag-specific CD8+ T cells were able to proliferate equivalently in CD4 help-deficient mice and WT mice (Figure 7a).
Figure 7.
Proliferation and apoptosis of VACV-specific CD8+ T cells. In vivo proliferation was measured by detecting incorporation of BrdU. Apoptosis was measured by Annexin V staining in conjunction with 7-AAD. B8R-specific CD8+ T cells were prepared from the spleens of infected mice. (a) Frequency of BrdU+ cells within B8R-specific CD8+ T-cell population in WT or MHC II−/− mice at d9 pi. (b) Frequency of Annexin V+ cells within B8R-specific CD8+7AAD− T-cell population in WT or MHC II−/− mice at d10 pi. (c) Frequency of Annexin V+ cells within B8R-specific CD8+7AAD− T-cell population in WT, MHC II−/− or α-CD40L WT mice at d9 pi. Data are representative of two independent experiments with four mice per group. *P<0.05; **P<0.01; ***P<0.001. 7-ADD, 7-aminoactinomycin D; BrdU, 5-bromo-2′-deoxyuridine; ns, not significant; pi, post infection; VACV, vaccinia virus; WT, wild-type.
Next, we determined the proportion of B8R-specific CD8+ T cells undergoing apoptosis in the absence of CD4 help, using Annexin V staining in conjunction with 7-AAD, since viable cells with intact membranes can exclude 7-AAD. Splenocytes were prepared from WT and MHC II−/− mice at d9 pi. The proportions of Annexin V+ cells in B8R-specific CD8+7-AAD− T cells were higher in cells from MHC II−/− mice than those from WT mice in both i.n. and i.p. infection (Figure 7b). This indicates that VACV-specific CD8+ T cells undergo more apoptosis in the absence of CD4 help.
We also determined the proportion of cells undergoing apoptosis in the absence of CD40L signaling in i.n. infected mice with different viral doses. Blockade of CD40L resulted in more apoptosis in mice infected with 103 PFU (Figure 7c, left) and 102 PFU (Figure 7c, right) compared to the WT cohort. These data suggest that CD40–CD40L pathway is responsible for preventing apoptosis of VACV-specific CD8+ T cells.
Discussion
Several studies have indicated that the entry route of pathogens influences immune responses. Intravenous and intranasal infections with Listeria monocytogenes induce Th1 and Th17 CD4+ effector T cells, respectively.44 Intravenous and intrarectal infections with simian immunodeficiency virus result in difference in time of virus appearance in the blood and size of the virus-specific immune response, and the natural mucosal barrier may delay viral spreading.45 By examining intradermal, subcutaneous, i.p. or i.v. infection, studies have shown that the immunodominance hierarchy is also affected by the route of infection.46 Additionally, the role of CD4 help in the CD8+ T-cell responses does not follow a simple on–off rule. In herpes simplex virus 1 infection, the primary CD8+ T-cell response is strictly CD4 help-dependent with subcutaneous (footpad) infection,47,48 but is largely independent with ocular infection.49 In this study with 1×103 PFU VACV-WR infection, B8R-specific CD8+ T cells displayed a poor expansion (Figure 1a and b) in i.p. infected CD4 help deficient mice. In contrast, after i.n. infection, B8R-specific CD8+ T cells expanded equivalently in either the presence or absence of CD4 help. These data indicate that the route of infection can markedly affect Ag-specific CD8+ T-cell responses in the absence of CD4 help.
We studied the effect of Ag dose by inoculating various doses of VACV. An increased viral load could not restore expansion of B8R-specific CD8+ T cells in i.p. infection, even when the dose was increased to 2000-fold more than the standard dose used in this study (Figure 2a). This suggested that for VACV-WR infection, dependency on CD4 help could not be overcome by providing more Ag stimulation. Consistent with our data, another group also demonstrated that primary CD8+ T-cell expansion is dependent on CD4 help when mice were infected i.p with 5×106 PFU of VACV (VV-G2).23 While another report found CD8+ T-cell expansion was not dependent on CD4 help after i.p. infection with 107 PFU of VACV (rVV33),14 this study used CD4−/− mice, which we now know contain an aberrant population of ‘helper' CD8+ T cells.50 Our studies show that reduction in the viral load, from 103 to 102 PFU, did result in dependence on CD4 help following i.n. infection (Figure 2b). These data indicate that in addition to route of infection, viral dose is also an important factor determining the degree to which primary CD8+ T-cell responses require CD4 help.
We analyzed the profiles of antiviral effector molecules or cytokines. B8R-specific CD8+ T cells produced more IFN-γ, TNF-α and GzmB in i.n. infected mice than in i.p. infected mice, regardless of CD4 help (Figure 3). Although the underlying mechanism is not clear, higher expression of effector molecules by B8R-specific CD8+ T cells in i.n. infection may be a result of the mucosal microenvironment in the lung. It would therefore be interesting to determine if the same is true for other mucosal routes of infection. Additionally, we analyzed cytotoxicity of CD8+ T cells in the absence of CD4 help. The VACV-specific CD8+ T cells showed similar cytotoxic activity in WT and MHC II−/− mice (Figure 4). Therefore cytotoxic function is not impaired in the absence of CD4 help, regardless of the route of infection.
T helper cell-derived IL-2 is a central component of help for CD8+ T-cell responses.40 Our lab has previously reported that CD4+ T cells upregulate CD25 expression on Ag-specific CD8+ T cells, thereby promoting the expansion of the virus-specific CD8+ T-cell population, specifically SLECs.22 Other studies have shown that IL-2 signaling promotes expression of the transcription factor Blimp-1, which is necessary for differentiation of SLECs.51 In this study, we confirmed that signaling through CD25 was essential for driving expansion of the population after i.p. infection, but not after i.n. infection (Figure 5). The data suggest that deficiency of IL-2/CD25 signaling may be partially responsible for the reduced primary B8R-specific CD8+ T-cell response in the absence of CD4 help following i.p. infection. However, i.n. infected mice did not show dependence on the IL-2/CD25 pathway, suggesting that some pathways or signaling in lung infection can replace or compensate for CD4 help.
Dendritic cells (DCs) are potent professional APCs, bridging the innate and adaptive immune systems to induce primary immune responses.52,53 A CD4 help signal is transmitted from DCs to CD8+ T cells partly via CD40–CD40L interactions between DCs and CD4+ T cells.42 Immunization with non-infectious agents requires the CD4 help,17 and CD40–CD40L signaling can replace CD4 help in priming of CTL responses to Ag-loaded splenocytes and alloantigen.42,43 In addition, upregulating CD40L on DCs can promote optimal priming of CD8+ T cells against influenza in the absence of CD4 help.54 Work from our lab has shown that expansion of the VACV-specific CD8+ T-cell population in CD40−/− mice is equivalent to WT mice after i.p. infection.22 However, Wiesel et al.23 found that expansion of VACV-specific CD8+ T cells is impaired in the absence of CD40–CD40L in i.p. infection. Differences in the infectious dose, the viral strain and mouse model might partly explain the discrepancy. In this study, expansion of virus-specific CD8+ T-cell population was dependent on CD4 help (Figure 2b) and the CD40–CD40L pathway (Figure 6a) in i.n. infected mice with 102 PFU of VACV. This suggests that CD4 help through CD40–CD40L signaling is required for optimal primary CD8+ T-cell responses when mice are infected i.n. at a small viral dose. In contrast, after i.n. infection with 103 PFU of VACV, the expansion was independent of CD4 help (Figure 1a and b) and the CD40–CD40L pathway (Figure 6b). Recognition of microbial products by Toll-like receptors directly activate APCs and thus, bypass the need for CD4 help.55 We are currently investigating whether higher viral doses result in better DC maturation, which may reduce the requirement for CD4 help.
To explore the mechanism by which the absence of CD4 help resulted in a smaller population of B8R-specific CD8+ T cells, we measured proliferation and apoptosis of VACV-specific CD8+ T cells. The B8R-specific CD8+ T cells proliferated normally in the absence of CD4 help regardless of the route of infection (Figure 7a). However, CD4 help deficiency resulted in increased apoptosis of the B8R-specific CD8+ T cells in mice infected either i.n. or i.p. (Figure 7b). This agrees with previous reports in which an i.p. infected mouse model was used.21,23 Moreover, CD40L signaling-deficiency also resulted in increased apoptosis of the B8R-specific CD8+ T cells in i.n. infected mice regardless of viral dose (Figure 7c). Increased apoptosis may contribute to the reduced clonal expansion observed in i.p. infected mice lacking CD4 help. However, a similar increase in apoptosis was observed following i.n. infection, but this was insufficient to reduce the clonal expansion. Currently, we do not know the mechanism underlying this discrepancy. Possibly B8R-specific cell death rates are not increased in organs other than the spleen, and there may be differential recruitment of these cells to the spleen in the presence or absence of CD4+ T cells. Alternatively, there may be another, as yet undefined, mechanism that may compensate for reduced CD8+ T-cell viability during respiratory infection in the absence of CD4 help.
As the front line exposed to the air, the lung is equipped with an elaborate network of DCs to sense entering pathogens.56 Studies have demonstrated that mucosal vaccination is more effective than systemic vaccination in establishing protective mucosal immune responses.57 This is because an optimum mucosal vaccine strategy can activate local innate immune responses for generating neutralizing sIgA Abs, polyfunctional CD8+ CTLs and CD4+ T-cell effector memory responses.57 Our findings demonstrate that there is remarkable variability in the dependence of the CD8+ T-cell response on CD4 help. The identical pathogen can have differing requirements for CD4 help owing to a different route of infection or viral dose. In addition, infection in the lung microenvironment may favor superior effector activity in virus-specific CD8+ T cells. Natural infections with orthopox viruses occur through the respiratory tract; thus, the i.n. infection model may provide some important insights into the host response to VACV infection. This information is also very relevant for vaccine design, as it highlights the route of immunization and dose of immunogen must be selected with great care to achieve an optimal effector CD8+ T-cell response.
Acknowledgments
This work was supported by National Institutes of Health Grants R01AI069943 and R01CA103642.
The authors declare no conflict of interest.
Footnotes
Supplementary information accompanies the paper on Cellular & Molecular Immunology's website(http://www.nature.com/cmi/).
Supplementary Information
References
- 1Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol 2007; 25: 171–192. [DOI] [PubMed] [Google Scholar]
- 2van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol 2001; 2: 423–429. [DOI] [PubMed] [Google Scholar]
- 3Wong P, Pamer EG. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol 2001; 166: 5864–5868. [DOI] [PubMed] [Google Scholar]
- 4Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol 2001; 2: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol 2004; 16: 185–196. [DOI] [PubMed] [Google Scholar]
- 6Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol 2002; 20: 29–53. [DOI] [PubMed] [Google Scholar]
- 7Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 1998; 16: 111–135. [DOI] [PubMed] [Google Scholar]
- 8Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol 2005; 17: 275–281. [DOI] [PubMed] [Google Scholar]
- 9Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229: 192–215. [DOI] [PubMed] [Google Scholar]
- 10Croft M So T Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009; 229: 173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006; 211: 81–92. [DOI] [PubMed] [Google Scholar]
- 12Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol 2004; 4: 595–602. [DOI] [PubMed] [Google Scholar]
- 13Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003; 421: 852–856. [DOI] [PubMed] [Google Scholar]
- 14Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003; 300: 337–339. [DOI] [PubMed] [Google Scholar]
- 15Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 2003; 300: 339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol 2004; 5: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Wiesel M, Oxenius A. From crucial to negligible: functional CD8+ T-cell responses and their dependence on CD4+ T-cell help. Eur J Immunol 2012; 42: 1080–1088. [DOI] [PubMed] [Google Scholar]
- 18Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol 1991; 133: 234–252. [DOI] [PubMed] [Google Scholar]
- 19Marzo AL, Vezys V, Klonowski KD, Lee SJ, Muralimohan G, Moore M et al. Fully functional memory CD8 T cells in the absence of CD4 T cells. J Immunol 2004; 173: 969–975. [DOI] [PubMed] [Google Scholar]
- 20Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol 1994; 68: 8056–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Novy P, Quigley M, Huang X, Yang Y. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J Immunol 2007; 179: 8243–8251. [DOI] [PubMed] [Google Scholar]
- 22Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci USA 2010; 107: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Wiesel M, Joller N, Ehlert AK, Crouse J, Sporri R, Bachmann MF et al. Th cells act via two synergistic pathways to promote antiviral CD8+ T cell responses. J Immunol 2010; 185: 5188–5197. [DOI] [PubMed] [Google Scholar]
- 24Fuse S, Tsai CY, Molloy MJ, Allie SR, Zhang W, Yagita H et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol 2009; 182: 4244–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25McFadden G. Poxvirus tropism. Nat Rev Microbiol 2005; 3: 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Gomez CE, Najera JL, Krupa M, Esteban M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr Gene Ther 2008; 8: 97–120. [DOI] [PubMed] [Google Scholar]
- 27Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol 2004; 172: 6265–6271. [DOI] [PubMed] [Google Scholar]
- 28Tscharke DC, Smith GL. A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J Gen Virol 1999; 80: 2751–2755. [DOI] [PubMed] [Google Scholar]
- 29Lee MS, Roos JM, McGuigan LC, Smith KA, Cormier N, Cohen LK et al. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol 1992; 66: 2617–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Benning N, Hassett DE. Vaccinia virus infection during murine pregnancy: a new pathogenesis model for vaccinia fetalis. J Virol 2004; 78: 3133–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C et al. Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA 1999; 96: 10338–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86–CD28 costimulation and restoration by IL-2 during the recall response. J Immunol 2008; 180: 1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med 2005; 201: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 2007; 27: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med 2009; 206: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Stemberger C, Neuenhahn M, Buchholz VR, Busch DH. Origin of CD8+ effector and memory T cell subsets. Cell Mol Immunol 2007; 4: 399–405. [PubMed] [Google Scholar]
- 37Yuzefpolskiy Y, Baumann FM, Kalia V, Sarkar S. Early CD8 T-cell memory precursors and terminal effectors exhibit equipotent in vivo degranulation. Cell Mol Immunol 2014; in press. [DOI] [PMC free article] [PubMed]
- 38Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 2007; 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Reading PC, Smith GL. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J Gen Virol 2003; 84: 1973–1983. [DOI] [PubMed] [Google Scholar]
- 40Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol 2008; 181: 7445–7448. [DOI] [PubMed] [Google Scholar]
- 41Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 1995; 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 42Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature 1998; 393: 480–483. [DOI] [PubMed] [Google Scholar]
- 43Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 1998; 393: 478–480. [DOI] [PubMed] [Google Scholar]
- 44Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol 2010; 11: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Stevceva L, Tryniszewska E, Hel Z, Nacsa J, Kelsall B, Washington Parks R et al. Differences in time of virus appearance in the blood and virus-specific immune responses in intravenous and intrarectal primary SIVmac251 infection of rhesus macaques; a pilot study. BMC Infect Dis 2001; 1: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Lin LC, Flesch IE, Tscharke DC. Immunodomination during peripheral vaccinia virus infection. PLoS Pathog 2013; 9: e1003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Rajasagi NK, Kassim SH, Kollias CM, Zhao X, Chervenak R, Jennings SR. CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1. J Virol 2009; 83: 5256–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat Immunol 2004; 5: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 49Frank GM, Lepisto AJ, Freeman ML, Sheridan BS, Cherpes TL, Hendricks RL. Early CD4+ T cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of Herpes Simplex Virus 1 latency. J Immunol 2010; 184: 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med 2004; 199: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51Boulet S, Daudelin JF, Labrecque N. IL-2 induction of Blimp-1 is a key in vivo signal for CD8+ short-lived effector T cell differentiation. J Immunol 2014; 193: 1847–1854. [DOI] [PubMed] [Google Scholar]
- 52Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811. [DOI] [PubMed] [Google Scholar]
- 53Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998; 393: 474–478. [DOI] [PubMed] [Google Scholar]
- 54Johnson S, Zhan Y, Sutherland RM, Mount AM, Bedoui S, Brady JL et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 2009; 30: 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 56Neyt K, Lambrecht BN. The role of lung dendritic cell subsets in immunity to respiratory viruses. Immunol Rev 2013; 255: 57–67. [DOI] [PubMed] [Google Scholar]
- 57Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol 2009; 183: 6883–6892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.