Abstract
Introduction
The HIV-1 gp120 envelope (Env) glycoprotein mediates attachment of virus to human target cells that display requisite receptors, CD4 and co-receptor, generally CCR5. Despite high affinity interactions with host receptors and proof-of-principle by the drug maraviroc that interference with CCR5 provides therapeutic benefit, no licensed drug currently targets gp120.
Areas covered
An overview of the role of gp120 in HIV-1 entry and of sites of potential gp120 vulnerability to therapeutic inhibition is presented. Viral defenses that protect these sites and turn gp120 into a moving labyrinth are discussed together with strategies for circumventing these defenses to allow therapeutic targeting of gp120 sites of vulnerability.
Expert opinion
The gp120 envelope glycoprotein interacts with host proteins through multiple interfaces and has conserved structural features at these interaction sites. In spite of this, targeting gp120 for therapeutic purposes is challenging. Env mechanisms evolved to evade the humoral immune response also shield it from potential therapeutics. Nevertheless, substantial progress has been made in understanding HIV-1 gp120 structure and its interactions with host receptors, and in developing therapeutic leads that potently neutralize diverse HIV-1 strains. Synergies between advances in understanding, needs for therapeutics against novel viral targets, and characteristics of breadth and potency for a number of gp120-targetting lead molecules bodes well for gp120 as a HIV-1 therapeutic target.
Keywords: HIV antibodies, miniprotein mimetics, natural products, receptor mimetics, small molecule inhibitors, structure-based design
1. Introduction
Entry into host cells is an essential step in HIV-1 life cycle that can be targeted by anti-HIV-1 drugs. HIV-1 entry is mediated by the viral spike (Env), which comprises three gp120 and three gp41 subunits (Figure 1A) [1]. The gp120 envelope glycoprotein, which HIV uses for attachment, undergoes receptor-driven conformational changes, first upon engaging the CD4 receptor, then on CCR5 co-receptor binding, to trigger fusion of viral and host cell membranes. Apart from being critical in entry of cell-free virus particles into host cells, interaction of gp120 with CD4 and CCR5 is also implicated in spread of virus through cell-to-cell transfer via the virological synapse [2].
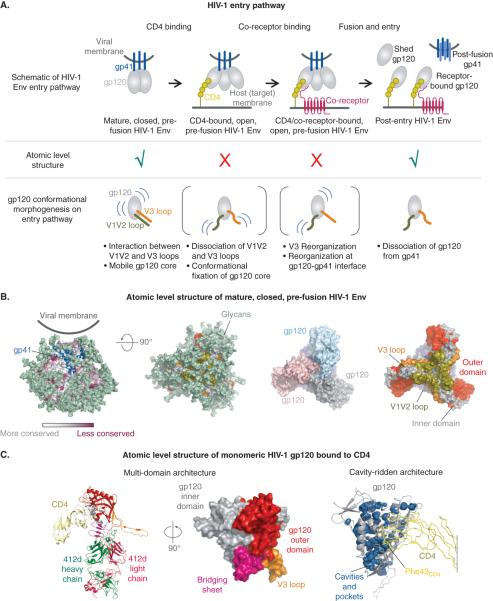
Figure 1. Conformational dynamics of HIV-1 entry.
(A) Schematic representation of HIV-1 entry pathway. Mature HIV-1 Env prior to receptor activation adopts a closed conformation resistant to neutralization by antibodies. On engaging CD4 receptor the trimer adopts an open conformation with the gp120 subunits undergoing rigid body motion, along with reorganization at the trimer association domain, made up by the variable loops V1V2 and V3, and at the gp120-gp41 interface. The trimer then interacts with co-receptor and undergoes additional conformational changes to effect viral and host cell membrane fusion.(B) Atomic level structure of pre-fusion closed HIV-1 BG505 SOSIP.664 trimer (PDB ID 4TVP). Panels from left to right show: gp120 in surface representation, colored by residue conservation with gp41 in blue cartoon, and glycans in pale green surface representation; a 90° rotated view looking down the viral membrane distal trimer apex with gp120 outer domain colored red, inner domain grey, V3 loop orange and V1V2 olive; HIV-1 Env with glycans removed to allow visualization of the underlying protein with gp120 protomers colored grey, pink and cyan, respectively; and gp120 subunits colored by domain. (C) Atomic level structure of monomeric HIV-1 YU2 gp120 in complex with CD4 and CD4-induced co-receptor mimetic antibody 412d. Left panel shows gp120 colored by domains, with outer domain colored red, inner domain grey, bridging sheet magenta and V3 loop orange. CD4 is shown in yellow and the antibody heavy and light chains in green and dark pink, respectively. Middle panel shows a 90° rotated view of gp120 colored by domains as described above. Right panel depicts gp120 cavities and pockets, which are shown in blue surface representation, with the gp120 polypeptide shown as white cartoon. CD4 is shown in yellow ribbon representation with its Phe 43 residue shown in stick representation.
Key findings highlighting entry inhibition as an effective therapeutic strategy include (1) a 32 base-pair deletion (Δ32) in the entry co-receptor CCR5 gene that confers resistance to HIV infection in populations that carry this mutant allele [3], (2) an HIV-1-positive, ART-experienced patient (Berlin, Germany) who received a hematopoietic stem cell transplant from a donor homozygous for the Δ32 CCR5 mutation, and subsequently went off antiretrovirals, has survived HIV-free without the need for antiretroviral therapy for more than 5 years [4,5], (3) favorable efficacy of drugs maraviroc [6] and enfurvirtude [7], each targeting different steps in HIV-1 entry, and (4) emerging data predicting efficacy of the gp120-targeting drug BMS-663068 in ART experienced HIV-1 positive patients [8]. Thus multiple lines of evidence indicate that blocking entry can provide therapeutic benefit to combat HIV-1 infection.
Of the two licensed entry inhibitors currently in market, maraviroc targets the HIV-1 co-receptor CCR5, and enfurvitide targets the gp41 glycoprotein. In spite of its critical role in HIV-1 entry and exposed location on viral surface, currently no FDA-approved drug targets the HIV-1 gp120 glycoprotein. Recent advances in Env understanding, multi-target efficacy, and lead characteristics, however, have heightened interest in gp120 as a therapeutic target. First, with Env understanding, atomic-level structures of soluble trimeric HIV-1 Env, in the pre-fusion mature closed conformation, were recently published (Figure 1B) [9-11], and single-molecule analyses of functional HIV-1 Env trimers on virion surface [12] showed that this pre-fusion ground state is targeted by many broadly neutralizing gp120-directed antibodies and drugs. Second, with multi-target efficacy, treatment with combinations of antibodies that target different gp120 sites were shown to reduce viremia to undetectable levels in a subpopulation of rhesus macaques infected with a simian-HIV chimera [13]. Moreover, HIV-1 antibodies have recently been shown to work synergistically with HAART to suppress viremia [14]. Together, these results indicate that use of multiple entry inhibitors or entry inhibitors in combination with other therapeutics might be an effective therapeutic strategy [13,15]. Third, with lead characteristics, a number of recently described leads against different sites of vulnerability on gp120 have demonstrated favorable neutralization breadth and potency (Table 1). These include (i) at the CD4-binding site, BMS-626529 that targets the pre-fusion closed conformation of HIV-1 Env [8,12], and a CD4-mimetic miniprotein named M48U1 with picomolar affinity for gp120 that has demonstrated in vitro efficacy including near-pan reactive neutralization [16], and in vivo efficacy as a microbicide [17], (ii) antibodies such as VRC01 that neutralize over 90% of circulating HIV-1, providing proof-of-principle that near pan-neutralization can be achieved despite the vaunted diversity of gp120 [18], (iii) lectins such as cyanovirin that can neutralize most strains of HIV-1 indicating that targeting gp120 evasion, in this case, its N-linked glycosylation, can effectively inhibit HIV-1 entry in vitro [19] and prevent rectal transmission of SHIV in rhesus macaques [20]. The goal of this review is to explore the potential of HIV-1 gp120 as a therapeutic target, to discuss challenges and opportunities for therapeutics development against various target sites on gp120, and to review new developments and promising leads.
Table 1.
HIV-1 gp120 directed entry inhibitors: Classification, structural determination, antiviral and therapeutic efficacy.
| Target | Inhibitor type | Molecules | Binding-site determination | Antiviral activity |
Clinical trials | |
|---|---|---|---|---|---|---|
| In vitro efficacy | In vivo efficacy | |||||
| CD4-binding site | Antibodies | VRC01-like antibodies (VRC01, 12A21, 3BNC117, etc.), HJ16, b12 | Crystal structures, EM structures, mutagenesis and resistance mutation [35,43,130,131] | VRC01-like antibodies neutralize > 90% of circulating HIV-1 isolates [43,44] | VRC01 protects macaques against SHIV mucosal challenge [132]. A cocktail of bnAbs including 3BNC117 reduces plasma viral loads to undetectable levels in monkeys infected with a pathogenic SHIV [133]. | |
| Peptides and miniproteins | M48U1, M48, M47, M48U7, M48U12, etc. | Crystal structures, mutagenesis, resistance mutation [17,50,52,53,134] | M48U1 neutralizes 90.4 % of a 180-isolate representative panel of circulating HIV-1 isolates with geometric IC50 mean of 0.13 μg/ml [50], and inhibits HIV-1(Ba-L) in human mucosal explants of cervical and colorectal tissues[18]. | Used as microbicide, M48U1 blocked vaginal transmission of SHIV(162P3) in 5 out of 6 cynomolgus macaques [17] . | ||
| Small molecules | NBD-class, DMJ-class | Crystal structures , mutagenesis, [56,135-137] | DMJ-II-121 neutralizes 83% of a 42-isolate panel of Clade B and Clade C viruses with geometric IC50 mean of 2.3 ± 0.05 μM. [56] | N.D. | ||
| BMS-class (BMS-806, BMS-626529) | mutagenesis, resistance mutations [8,48,138] | BMS-626529 neutralizes R5-tropic viruses with EC50 of 0.4-1.7 nM, X4-tropic viruses with EC50 between 0.7 and 16.2 nM, and dual tropic virus 89.6 with EC50 57.6 nM. [8] | Oral administration of BMS-663068 for 8 days resulted in substantial declines in plasma HIV-1 RNA levels. [8] | PhaseIIb trial to investigate safety, efficacy and dose-response in ART experienced HIV-1 subjects [8] | ||
| Co-receptor binding site | Antibody fragments and nanobodies | m36, 17b scFv | Crystal structures, mutagenesis [28,31,33,67,139-144] | Yes [28] | N.D. | |
| Peptides Small molecules | CCR5 Nt and ECL-derived peptides | NMR data, mutagenesis [61,62,65] | Yes [59,61,62,65] | N.D. | ||
| Tyrosine sulfate mimetic molecules | Competition assay, CD4-induction, mutagenesis [69] | Yes [69] | N.D. | |||
| Variable loops and glycan shield | Antibodies | PG9, PG16, 2G12, PGT122, 10-1074, CAP 256, VRC26.01 | Crystal structures, mutagenesis, resistance mutation [79,80,145] | 10-1074 and PGT121 inhibit cell-free and cell-to-cell transmission[146] | PG9 protects macaques against SHIV mucosal challenge [132]. | |
| Yes | Used as a microbicide blocks SHIV transmission in rectal challenge [20] | |||||
| CBAs: Lectins | CVN, GRFT Benzoboroxazoles | Crystal structures, mutagenesis, resistance mutation [19,92,102,107,113,115] | Yes | Preclinical toxicity [20] | ||
| CBAs: Synthetic | Yes | Preclinical toxicity | ||||
2. HIV-1 gp120: a moving labyrinth
Functional constraints necessitate both a high degree of conservation of host-receptor binding sites on HIV-1 gp120, as well as exposure of these conserved sites during viral entry to enable receptor engagement. HIV-1 employs a number of evasion techniques to protect these vulnerable sites from surveillance and attack by the host immune system [21-28]. Evasion mechanisms include 1) high degree of overall sequence variability with sequence conservation limited to functional regions such as receptor binding sites, 2) rapid emergence of escape variants under selection pressure of antibodies and drugs, facilitated by low-fidelity HIV-1 replication, 3) steric occlusion of receptor sites, 4) extensive glycosylation that limits access to conserved receptor binding sites and allows the virus to masquerade as self , and 5) conformational masking that necessitates receptor-triggered conformational changes for exposure of some of the conserved receptor-binding elements. These elaborate defense mechanisms turn gp120 into a fortified, moving labyrinth [29], with recessed receptor-binding pockets and a multi-domain, cavity-ridden structure further protected by a mobile architecture involving large flexible loops and glycans, and conformational masking of gp120 itself (Figure 1). This ability of gp120 to morph shape and structure has recently been experimentally observed by single molecule fluorescence resonance energy transfer (smFRET) measurements that show HIV-1 Env trimers on virus surface as intrinsically dynamic with conformation changing in response to host receptors [12]. While the entire HIV-1 Env, including the gp120 and gp41 subunits, undergoes conformational changes during entry, much of the receptor-driven conformational transitions occur within and are driven by gp120 (Figure 1A). These conformational dynamics confound not only the human immune system, but also challenge the development of effective gp120-targeting drugs. This review focuses on the gp120 subunit of the HIV-1 Env. The next two sections summarize current knowledge of gp120 in the pre-fusion mature ground state and receptor-activated states of HIV-1.
2.1 gp120 architecture in pre-fusion, mature HIV-1 Env
Advances in structural determination of native gp120, both on intact virion [30] and within soluble trimeric mimics of the HIV-1 spike [9-11] have revealed its structure in the conformation presented to the immune system prior to its interactions with host cellular receptors (Figure 1B). Accompanying smFRET measurements showed that the conformation captured in the 3.5 Å crystal structure of the HIV-1 Env, obtained in complex with broadly neutralizing antibodies PGT122 and 35O22, closely resembled its pre-fusion, closed conformation. Within the HIV-1 trimer, gp120 subunits form the blades of a 3-blade propeller, and are held by interactions with gp41 at one end and by interactions between its variable loops, V1V2 and V3 at the other. V1V2 and V3 loops within each monomer tightly wrap against each other, while also interacting with the V1V2 loop of an adjacent monomer, creating a “wound-up” pre-fusion ground state [11].
2.2 gp120 in receptor-activated HIV-1 Env
While atomic level structures of receptor-activated trimeric HIV-1 Env are yet to be determined, details of the interface between gp120 and the CD4 receptor were revealed by structures of monomeric gp120 bound to interactive domains of the CD4 receptor and co-receptor-mimetic antibodies [31-33] (Figure 1C and 2B). Additionally, insights into overall subunit motions in receptor-activated Env have emerged from cryo-EM studies [30,34]. The CD4-binding site is accessible on the native trimer, although stable CD4 engagement and formation of the co-receptor binding site require gp120 structural rearrangements [11,30,34,35] (Figures 1 and 2). In the absence of an atomic resolution structure of CD4/co-receptor-bound HIV-1 spike, comparison of structural features of monomeric gp120 bound to CD4 with that of gp120 within the pre-fusion closed trimer reveals regions likely to undergo conformational changes to transition the HIV-1 spike from its pre-fusion closed state to its receptor-activated conformation. Although relative positions of gp120 outer and inner domains appeared similar in both closed and CD4-activated conformations, transition to CD4-activated conformation would likely require substantial Env structural rearrangements including a) dissociation of interactions between the gp120 V1V2 and V3 loops, forcing the Env trimer to lose its tight packing and adopt an open conformation [30,34], thereby exposing immunodominant epitopes in the V3 loop [36], b) reorganization of the base of the V1V2 loop to form the bridging sheet [11,31], c) formation of the Phe43 cavity and a solvent channel adjacent to it at the CD4-binding site [31], and d) disengagement of gp120 from gp41 that accompanies structural rearrangements in gp120 resulting in the formation of the immunodominant epitope for the A32-like antibodies [16] (Figure 1).
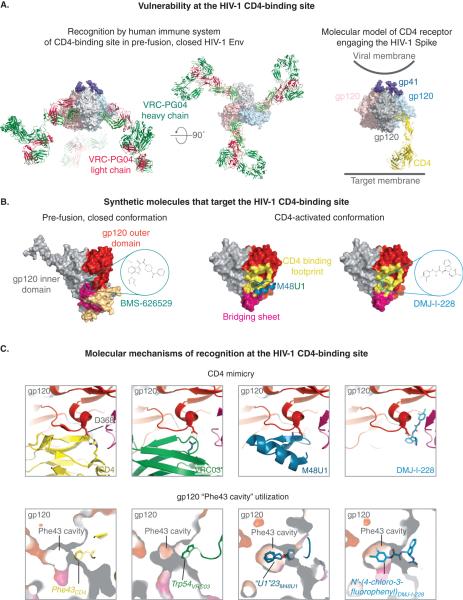
Figure 2. Targeting the CD4-binding site of HIV-1 gp120.
The initial site of CD4 engagement on HIV-1 Env is, by functional necessity, highly conserved and accessible on the viral surface. Naturally occurring antibodies target this site to neutralize virus. Synthetic molecules have been developed that target the CD4 binding site on gp120. Atomic resolution structures of CD4-binding site directed ligands reveal unifying mechanisms of recognition. (A) (Left) Model of IgG VRC-PG04 binding to the CD4-binding site. The model was constructed by superposing coordinates of b12 IgG (1HZH) on VRC-PG04 Fab in the BG505 SOSIP.664-VRC-PG04 complex (PDB ID: 3J5M). (Right) Model of HIV-1 trimeric spike bound to CD4 receptor. The model was constructed by superposing the gp120 outer domain from PDB ID 1RGK to the outer domain in PDB 4TVP (EM structure of BG505 SOSIP.664 bound to VRC-PG04), followed by superposition of 4-domain CD4 (PDB ID 1WIP). (B) Synthetic molecules that target the CD4 binding site to neutralize HIV-1. HIV-1 gp120 is shown in surface representation with its domains colored as in Figure 1C middle panel, and with the CD4-binding footprint overlaid in yellow. The left panel shows residues implicated in BMS-806 binding by mutagenesis studies in lime green. The predicted binding site is circled and the chemical structure of class member BMS-626529 is shown. In the gp120-M48U1 complex (PDB ID: 4JZZ) (middle) M48U1 is shown in teal cartoon representation with the U1 moiety that inserts into the gp120 Phe43 cavity shown in stick representation. In the gp120-DMJ-I-228 complex (PDB ID: 4DKQ) (right) DMJI-228 is shown in cyan stick representation. (C) Mechanism of CD4-binding site recognition. Molecular mechanisms of recognition conserved among diverse inhibitors include recognition of Asp 368 of gp120 via a salt bridge interaction, and utilization of the gp120 Phe43 cavity.
3.0 Targeting gp120 sites of vulnerability
In the following sections we describe vulnerable sites on gp120 that emerge during HIV-1 entry, and strategies to target these sites for therapy and prevention.
3.1 CD4-binding site
The CD4-binding site on HIV-1 gp120 is a well-established site of Env vulnerability (Figure 2). Present on the surface of the native viral spike as the initial site of attachment for the CD4 receptor (Figure 2A) [35], the CD4-binding site, together with other regions on gp120, morphs upon CD4 engagement, into an altered structure that can stably engage CD4. The CD4-binding site, therefore, is an example of a region on the gp120 “moving labyrinth” that alters itself in response to an incoming ligand. Each of these presentations of the CD4-binding site has distinct structural features and vulnerabilities that can be exploited for therapeutics development.
3.1.1 CD4-binding site on the native trimer
In the pre-fusion, closed HIV-1 Env, the initial site of CD4 attachment allows access to CD4, which binds gp120 via the membrane distal subunit of a 4-domain tandem immunoglobulin repeat, but severely restricts access of antibodies through glycan shielding and quaternary constraints [26]. Despite these barriers, the human immune system has found ways to breach viral defenses to target this site on gp120 (Figure 2A). That this site is a natural target is highlighted by the discovery that antibodies from multiple patients effectively neutralize HIV-1 by targeting the CD4-binding site (Table 1) [37]. Over the last few years, the invention of new antibody discovery and isolation techniques have led to a remarkable increase in the number of broadly neutralizing antibodies identified from HIV-1 infected patients [18,38], and have generated an arsenal that can potentially be used for therapy and prevention.
In some patients broadly neutralizing antibodies arise within a few years of HIV-1 infection in an ongoing battle between the virus and the immune system, with the immune system working to clear infection and the virus fighting to escape antibody recognition. In most cases, this battle culminates in viral escape [21,22], showing that emergence of broadly neutralizing antibodies in patients following infection is ultimately ineffective at controlling infection. The question then is - with such evidence of ineffective protection during natural infection- can antibodies be effectively used in therapeutic regimens?
3.1.1.2 Antibodies targeting the CD4-binding site on pre-fusion, closed HIV-1 spike
Earlier studies on passive administration of antibodies had shown limited success in viremic control due to emergence of resistant viruses [39,40]. More recent studies (reviewed in [41]) in humanized mice [15], and in macaques [13,42] using cocktails of newer broadly neutralizing antibodies (bNabs) with extremely high potency and breadth, have demonstrated effective control of HIV-1 infection, suppression of viral load below detection, and delayed viral rebound upon cessation of therapy (compared to ARV drugs). The antibody-cocktail used for therapy included members of the VH1-2 germline derived VRC01-class of antibodies that potently neutralize >90% of HIV-1 isolates by targeting the CD4-binding site in the mature, closed, pre-fusion conformation of the HIV-1 spike (Table 1) .
CD4 mimicry is a common mechanism adopted by antibodies to target the CD4-binding site (Figure 2C). Antibody recognition by this mechanism is achieved by copying two key CD4 interactions with gp120- i) the interaction of the CDR2 loop of CD4 via a β sheet interaction with the β15 strand of gp120, and ii) salt bridge interaction with the highly conserved Asp368gp120. Discovery of a number such CD4-mimetic antibodies [18,43,44] from different patients emphasize the generality of this mechanism of recognition. Apart from this characteristic mode of recognition by precise CD4 mimicry, other antibodies that originate from diverse germline genes and use long loops to reach into the recessed CD4-binding site can also effectively target the CD4-binding site on the native HIV-1 spike [45].
CD4-binding site antibodies such as VRC01 are among the most potent and broad, with VRC01 neutralizing greater than 90% of circulating HIV-1 isolates (Table 1) [18]. While precise mimicry of the CD4 receptor has resulted in high virus neutralization efficacy, antibodies still need to contend with their size, which is considerably larger than the single headed immunoglobulin-like CD4-receptor. Consequently, resistance mutations resulting from antibody selection pressure typically emerge at antibody contact sites that lie outside the CD4-recognition region. In the case of the VRC01-like antibodies, for example, resistance mutations have been mapped to the gp120 variably loop 5 (V5 loop) [43]. Passive immunization strategies that use antibody combinations targeting different epitopes on gp120 are, therefore, necessary to prevent rapid viral escape under selection pressure from a therapeutic antibody regimen [13,15,42,46].
3.1.1.2 Small molecules targeting the CD4-binding site on pre-fusion, closed HIV-1 spike
Apart from naturally elicited antibodies, a class of indoleoxoacetyl piperazine compounds developed by Bristol Myers Squibbs targets the pre-fusion, closed HIV-1 envelope and potently inhibits viral entry by preventing receptor-induced structural changes of the envelope (Figure 2B) [12,47]. Prodrug BMS-663068 that metabolizes into active drug BMS-626529 has shown in vivo efficacy in ART-experienced patients (Table 1) [48]. Resistance mutations against BMS-626529 typically develop within and near the Phe43 cavity at the CD4 binding site consistent with its predicted binding site. Recent structural studies on class member BMS-806 [49] should allow structure-guided design of variants with improved potency, breadth and/or tolerance for commonly occurring resistance mutations. The success of BMS-626529 demonstrates that it is possible for a small molecule to achieve high efficacy by preventing receptor binding to HIV-1 gp120, and effectively places gp120 on the roadmap for small molecule drug development.
3.1.2 CD4-binding site on receptor-activated gp120
HIV-1 gp120 alters its architecture in response to CD4-enagagement, with its CD4-activated conformation characterized by formation of the bridging sheet and the Phe43 cavity at the CD4-binding site (Figure 1C and 2B) [31]. Due to energetic requirement for conformational change, molecules that target the CD4-activated conformation of gp120 were considered unlikely candidates for development of potent antivirals. This was exemplified by CD4 itself; although CD4-engagement is an essential step in viral entry, soluble CD4 fragments do not neutralize HIV-1 potently [50]. The sections below explore the utility of the CD4-activated conformation as a therapeutic target.
3.1.2.1 CD4-mimetic miniproteins and small molecules
Structural solution of gp120 in complex with a soluble CD4 fragment, and a CD4-induced (CD4i) antibody 17b, yielded the first atomic level image of the gp120 core in its CD4-trigerred conformation [31] and elucidated details of the interactions between gp120 and CD4. CD4 binds a large (~800 Å2) depression on the gp120 surface by using 22 residues in the span of amino acids 25-64 of the CD4 D1 domain and centered on the CDR-2-like 36-47 loop. The critical elements in the CD4 interface include i) Phe43CD4, the side chain of which protrudes and plugs the entrance of a deep cavity in gp120, ii) Arg59CD4, which makes multiple contacts with gp120, and iii) strand C”, which interacts with strand ß15 of gp120 in a ß -sheet alignment. These critical functional elements of the CD4-binding site were transplanted first onto a structurally compatible scorpion charybdotoxin scaffold, and next onto a scorpion scyllatoxin scaffold to produce a molecule that prevented HIV-1 attachment to cells and subsequently, HIV-1 entry [51]. Using iterative feedback from structural methods- first NMR and subsequently X-ray crystallography- this inhibitor was progressively improved by optimization of the scaffold backbone, and gp120-interactive residues, including the critical residue 23 of the scaffold- a position analogous to Phe43CD4 [52] [53] to yield M48U1 (Figure 2B and 2C), which binds gp120 with pM affinity, neutralizes with extraordinary potency and breadth [50], and has demonstrated efficacy as a microbicide in a macaque challenge study (Table 1) [17]. X-ray crystallographic analyses on M48U1 bound to gp120 at 1.5 Å resolution revealed details of its interaction with the gp120 Phe43 cavity, and its mechanism of action. Because M48U1 reaches deep into the Phe43 cavity and interacts with residues that do not contact CD4 contact region, emergence of resistance mutations within the Phe43 cavity is a possibility and has been shown to occur in vitro [54]. Overall, the success of M48U1 as an entry inhibitor shows that the CD4-activated state can be a drug target.
Another class of CD4-mimetics that utilize the Phe43 cavity includes drugs that belong to the NBD/DMJ series [55,56] (Table 1). A recent review [57] covers progress in the development of this class of drugs, including the structure-guided conversion of an agonist that facilitated HIV-1 entry into CD4- cells to an antagonist that does not cause such entry.
3.2 Co-receptor binding site
The co-receptor binding site is one of the most conserved regions of gp120, and is protected from the human immune system by conformational and steric restrictions (Figures 1 and 3) [28]. Unlike the CD4-binding site, where the initial site of CD4 attachment is exposed on the viral surface in its pre-fusion closed conformation, the co-receptor binding site is not accessible in the pre-fusion, closed conformation of the HIV-1 spike, and becomes exposed only after gp120 binding to cell surface CD4 triggers conformational changes in Env (Figures 1 and 3) [27]. After CD4 binds, proximity of viral and cellular membranes restricts access of antibodies to the co-receptor binding site [28]. While full-length antibodies cannot effectively target this site, smaller antibody fragments have been shown to have better access (Figure 3) [28,58], suggesting that targeting this highly conserved region with small molecules may be a potential intervention strategy. While the structure of gp120 in complex with co-receptor has not yet been determined, structural, biochemical and virological analyses with co-receptor fragments [32,59-65] and co-receptor-mimetic antibodies [32,66-68] have provided information on gp120 interactions with its co-receptors, and these insights can be exploited for developing inhibitors that target the gp120 co-receptor binding site (Figure 3).
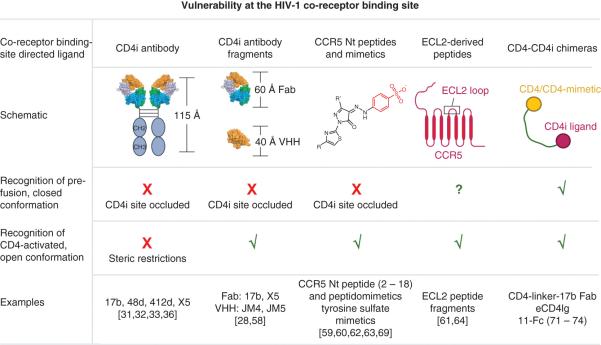
Figure 3. Targeting the co-receptor binding site of HIV-1 gp120.
The highly conserved co-receptor binding site is occluded in the mature, pre-fusion, closed HIV-1 Env trimer. Hence this state cannot be targeted by co-receptor binding-site directed ligands that need CD4-activation to bind the HIV-1 spike. Once the spike engages cell surface CD4, the space between the viral and cellular membranes do not allow access to full-length human antibodies. Smaller antibody fragments such as Fab and VHH can, however, access the CD4-induced epitope. Peptides and small molecules that mimic the CCR5 N terminus (Nt) bind to a CD4i binding surface, although they have been shown to be active without CD4 induction with enhancement of activity in presence of CD4, suggesting that these molecules may be able to induce the CD4i state. Peptides derived from the second extracellular loop (ECL2) of CCR5, however, can work on both states and are not CD4-induced, suggesting that some regions on the co-receptor binding site may be accessible on the mature, closed, pre-fusion spike. Synthetic chimeras that include CD4 (or a CD4-mimetic) and a CD4-induced ligand within the same molecule can target the closed spike, with the CD4 component of the ligand first binding to induce the CD4i conformation, thus allowing the CD4i component to bind. This figure shows schematics of various molecules that bind to the gp120 co-receptor binding-site, and indicate whether each can target the pre-fusion, closed and the CD4-activated states of the viral spike. A (√) indicates that the target conformation can be accessed by the inhibitor molecule indicated, and (X) indicates that the molecule cannot target the conformational state.
Similar to the CD4-binding site, receptor-mimicry is a common mechanism of inhibition at the co-receptor-binding site, both by antibodies [32] and synthetic molecules [69]. GPCR coreceptors utilize tyrosine sulfated N-terminus and extracellular loops to interact with gp120. HIV-1 gp120 harbors highly conserved tyrosine sulfate binding sites at its co-receptor interactive region, which are critical for engaging co-receptor N-terminus [32,59,60]. Naturally elicited antibodies use post-translationally modified sulfated tyrosine residues to target this region on gp120, which includes two sulfated tyrosine binding sites, one at the base of the V3 loop centered around Arg 298, and the other around Arg 327, both highly conserved gp120 residues [32,67]. This mimicry has been further exploited to develop lead inhibitors against this site [69]. An in silico structure-guided, ligand-based search of chemical libraries, coupled to a rapid ELISA-based screen has yielded small molecule inhibitors that extend the natural mimicry of CCR5 N terminus shown by monoclonal antibody 412d to synthetically derived molecules (Table 1) (reviewed in [70]). Apart from small molecules, synthetically derived receptor fragments have the potential to inhibit HIV-1 entry, and in the absence of an atomic level structure, have allowed fine-mapping of co-receptor interaction with the HIV-1 envelope [60,61,65] and provided a framework for development of receptor-mimicking peptide or peptidomimetic inhibitors.
3.3 Simultaneous targeting of CD4 and co-receptor binding sites
Inhibitors that bind the highly conserved co-receptor binding site target a transient, receptor-activated conformation on the HIV-1 entry pathway, and depend on prior CD4-triggering for activity. One strategy to circumvent the need for Env triggering by cell surface CD4 is to use chimeric molecules that link soluble CD4 (or a CD4-mimetic) with a co-receptor binding site ligand (Figure 3). Such a molecule would first engage the pre-fusion, closed Env via its CD4 component, thus triggering the spike and allowing the co-receptor site directed moiety to bind. A number of inhibitors that exploit this conformational synergy between the CD4 and co-receptor binding sites have been developed [71-74]. Through tandem targeting of both receptor-binding sites on gp120 these inhibitors effect broad and potent HIV-1 neutralization.
3.4 Targeting loops and glycan: breaching viral defenses
HIV gp120 is a highly unusual glycoprotein with its solvent exposed surface almost entirely covered with N-linked glycans (Figures 1B and 4) [75]. These glycans have important functions for the virus: they are essential for correct folding and assembly, thereby contributing to viral fitness; they shield the most immunogenic epitopes on gp120; and because the glycans are of host origin, they can as decoy to the immune system. Though precise structures of each glycan can vary as a function of viral strain or gp120 sequence, as well as the cell type in which the gp120 was produced, biochemical and mass spectrometry studies have shown that the majority of glycans present on gp120 comprise high mannose types (namely Man-5 to Man-9) [76,77]. The gp120 glycan coat was long pictured as a shield covering conserved regions on HIV-1 Env, but is also potentially vulnerable, and is recognized by broadly neutralizing antibodies [75].
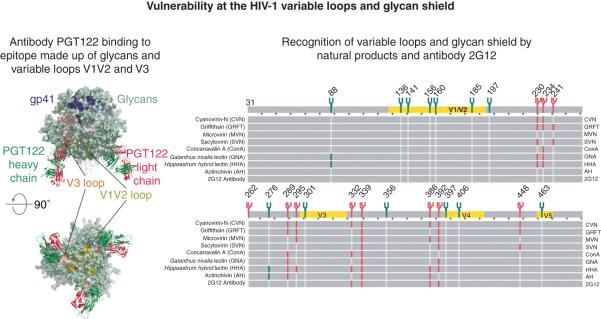
Figure 4. Targeting the variable loops and glycans of HIV-1 gp120.
(Left) Antigen binding fragment of antibody PGT122 bound to an epitope on gp120 that combines glycans and elements of the variable loops. (PDB ID 4TVP). (Right) Chart showing glycan type and observed glycan deletions as a function of gp120 amino acid sequence. Lectins for which resistance profiles have been determined are shown at the beginning and end of rows. Complex and high-mannose type glycans are shown at their respective sites and are depicted as green and red branches. Red or green vertical bars indicate loss of that glycosylation site when treated with the corresponding lectin.
3.4.1 Carbohydrate-binding agents that target gp120's glycan shield
3.4.1.1 Glycan-dependent Antibodies
A substantial proportion of recently identified, bnAbs present in certain HIV-infected donors recognize glycan-dependent epitopes on HIV-1 gp120 (Figure 4) [38,78-80]. Generally speaking, these glycan-reactive antibodies can be classified as those that bind with high affinity to discrete epitopes featuring a Man8-9 at position N332, exemplified by 2G12, PGT121, PGT122 and PGT128; and those whose epitope comprises glycan at position N160 and protein surface, exemplified by PG9 and PG16. Atomic level structures of glycan-dependent antibodies with various glycosylated Env constructs, including a soluble trimer mimic [9,11,79], have provided structural definition for antibody recognition of these conserved structural motifs on the HIV-1 Env.
3.4.1.2 Lectins
Lectins are carbohydrate-binding proteins ubiquitous in Nature and abundant in plants, invertebrates and bacteria. By the late 1980's, it had been shown that select plant lectins such as concanavalin A (ConA) and wheat germ agglutinin (WGA) could inhibit HIV [81,82]. Those early studies were the first to establish that inhibition correlated with mannose-binding. In contrast, lectins with specificities toward galactose (Gal), N-acetyl galactosamine (GalNAc) and sialic acid (Sial), the components of complex-type glycans, were less effective or inactive. More recently targeted searches for mannose binding lectins have uncovered several that exhibit very potent (picomolar) and broad inhibition profiles toward HIV [83-85]. Several previously published reviews comprehensively described antiviral lectins [86-88]; here we focus only on those that show the highest potency and whose mechanisms of inhibition have been established through high resolution structural and biophysical studies or by generating resistant viruses.
Among the most potent anti-HIV lectins reported to date are the cyanobacterial and algal lectins cyanovirin-N (CV-N), griffithsin (GRFT), Microcystis viridis lectin (MVL), microvirin (MVN), Oscillatoria agghardi agglutinin (OAA), and scytovirin (SVN); the streptomycete-derived lectin actinohivin (AH) ; and the plant lectins banana lectin (BanLec), ConA, Galanthus nivalis lectin (GNA), and the amaryllis-derived lectin HHA [83,89-98] (Table 1). Although none of these proteins share amino acid sequence, or three-dimensional structural, homology, they can be grouped according to carbohydrate specificities (Table 1). ConA, BanLec and GRFT are mannose-binding lectins [84,91,98]. GNA and OAA are reported to bind the inner branching mannose structures Manα1-3Man and Manα1-6Man with or without the GlcNAc2 core [99,100], while MVL requires a mixed epitope comprising GlcNAc2 and at least two additional Man units [93,101]. CVN, MVN and SVN, on the other hand, are unique in that they only recognize and bind to terminal Manα1-2Man units present on the branches of Man-7 to Man-9 [102-104]. As each of these small saccharide structures is present within high mannose-type glycans, these lectins can bind to gp120 and inhibit viral entry.
Affinity for the individual epitopes is a variable feature among lectins; whereas some lectins, like CVN, can bind their carbohydrate ligands with submicromolar affinity, others bind their ligands with only millimolar Kds, as is the case for ConA. To augment affinity and confer specificity, lectins are usually oligomeric and/or contain more than one carbohydrate-binding site per protein unit allowing for multivalent interactions. Depending on the spatial arrangement of carbohydrate binding sites within the lectin as well as glycans on the target molecule or surface, multivalent binding can occur through different mechanisms. For example, when all binding sites are positioned in the same orientation allowing them to be engaged simultaneously, face-to-face or chelating type interactions occur. In contrast, when carbohydrate-binding sites are opposed and surface glycan densities are high, intermolecular cross-linking can occur and contributes to the potency of some carbohydrate-binding agents (CBAs) [105]. High resolution structures have been obtained for all of the lectins listed in Table 2, and their stoichiometries of binding to individual saccharides has been determined by ITC or SPR. Interestingly, while multi-site lectins tend to bind gp120 with low nanomolar affinities, their effects in neutralization assays is much more variable with IC50 values ranging from nanomolar to picomolar. At least two lectins, CV-N and GRFT [20,106], have shown outstanding efficacy as microbicides in SIV models. For reasons that are not yet understood, some lectins are cytotoxic to cells while others show no signs of toxicity [103,106,107].
Table 2.
Biochemical and virological properties of lectins.
| Lectin | Oligomeric state | # Sites per monomer | Total valency | Carbohydrate specificity | EC50, IC50 (nM) | Kd, gp120 (nM) | References | |
|---|---|---|---|---|---|---|---|---|
| ConA | Concanavalin A | tetramer | 1 | 4 | Man | 98 | [114] | |
| OAA | O. agardhii agglutinin | monomer | 2 | 2 | Man | 45 | 2.5a | [95,147] |
| MVL | M. viridis lectin | homodimer | 2 | 4 | Man3GlcNAc2 | 30 | [93] | |
| BanLec | Banana lectin | homodimer | 2 | 4 | Man | 20 | [148,149] | |
| GNA | G. nivalis lectin | tetramer | 3 | 12 | α-1,3 Man; α-1,6 Man | 7 | 0.3a | [99,112] |
| HHA | Hippeastrum hybrid | tetramer | 3 | 12 | α-1,3 Man; α-1,6 Man | 6 | 0.4a | [150,151] |
| AH | Actinohivin | monomer | 3 | 3 | α-1,2 Man | 12 | 13a | [152,153] |
| MVN | Microvirin | monomer | 1 | 1 | α-1,2 Man | 12 | [103,107] | |
| SVN | Scytovirin | monomer | 2 | 2 | α-1,2 Man | 0.3 | [119] | |
| CV-N | Cyanovirin-N | monomer | 2 | 2 | α-1,2 Man | 0.1 | 37 (5:1)b | [114,119,154] |
| GRFT | Griffithsin | homodimer | 3 | 6 | Man, Man-9 | 0.04 | 0.12a | [106,119,155] |
| 8.2 (10:1)b |
Kds measured by SPR, data fit to a 1:1 binding model
Kds determined by ITC, with stoichiometries of binding in parantheses.
3.4.1.3 Glycan-binding synthetic molecules and natural products
Design or discovery of small molecules that can bind carbohydrates with high affinity/avidity presents an ongoing challenge in glycobiology. To date two such molecules have been reported; they include the pradimicin family of natural products, and synthetic benzoboroxazoles. The pradimicins are actinomycete derived napthacenquinones funtionalized with a D-amino acid and hexose sugars. In the presence of Ca2+ the pradimicins form dimers and higher order oligomers that lead to high avidity binding to mannosides and potent inhibition of HIV-1 infection [108]. The pradimicins were originally discovered due to their potent antifungal activity and have since been shown to have in vivo efficacy as antifungal agents [109], Thus development of the pradimicins as HIV microbicides may be facilitated.
Kiser and co-workers took a different approach by synthesizing a series of benzoboroxazoles with the aim of designing artificial carbohydrate receptors. Boronic acids are known to form reversible covalent adducts with 1,2- and 1,3-diols, present in most sugars. By generating a stable, masked boronic acid in the form of benzoboroxazole, and incorportating these units into water soluble polymers, the Kiser team achieved low nanomolar inhibition in single round HIV infectivity assays. These formulations are currently being investigated as microbicides [110].
3.3.3 Resistance toward carbohydrate-binding therapeutics
Given their breadth and potency, and the absence of cross resistance toward other HIV drug-resistant strains, carbohydrate-binding agents (CBAs) represent promising microbicide candidates. As a prerequisite to development, several groups have been studying the effects of CBAs on HIV by selecting CBA-resistant viruses. Cumulatively those studies reveal that multiple rounds of selection must generally occur before resistant viruses develop. Further, in all cases the resistant viruses undergo mutations that delete multiple sites of N-linked glycosylation rather than single site mutants (Figure 4). When these mutations are viewed in the context of the gp120 trimer structure, several patterns emerge. First, loss of sites bearing complex type glycans are less common, and have only been observed for some CBAs such as the lectins AH, GNA and HHA [111,112]. Second, while culture in the presence of lectins such as GRFT, CVN or SVN that preferentially bind to Man-8 or Man-9 leads to the loss of multiple glycosylation sites, some of which e.g. the glycan at gp120 residue 339 are uniformly deleted (Figure 4) [113-116]. Interestingly, these glycan deletions overlap with residues that have been shown to bear high mannose structures, and to be the targets of glycan-dependent neutralizing antibodies such as 2G12, PGT121, PGT122, and PGT128 [114,117,118]. In addition to consistency between glycan specificity by the lectin and glycan type on gp120, the location of these sites suggests that the Man-9-binding lectins inhibit HIV through multivalent interactions with gp120 that involves this residue 332-clustered group of glycans in particular [116,119]. With regard to therapeutic potential, it is noteworthy that viruses that are resistant to glycan-targeting antibodies remain sensitive to lectins. For example, HIV-1 strain YU2 as well as HIV-1 subtype C viruses are resistant to the antibody 2G12 due to lack of glycans at Asn positions 295 or 332 on gp120, yet these viruses are still potently inhibited by lectins such as CV-N and GRFT [119]. Additionally, the loss of multiple glycosylation sites that is needed to confer lectin resistance has the potential to expose other immunogenic epitopes, which are otherwise protected by the glycan shield [120,121]. The high genetic barrier to developing resistance to CBA, the limited cross resistance to glycan-targeting mAbs, and the observation that viruses with reduced numbers of glycans can show increased sensitivity to some gp120-directed antibodies [122] makes a number of these entities promising microbicide candidates, emphasizing the importance of gp120 as a therapeutic target.
3.5 Expert Opinion
HIV-1 gp120 is a complex target for inhibitor development with elaborate and intricate defenses built to combat constant surveillance and attack by the human immune system. Therefore, despite its critical role in HIV-1 entry, it has not been a preferred therapeutic target. Unlike enzyme targets, disrupting gp120 function involves interfering with protein-protein interactions that usually have larger and more complex interfaces than enzyme-substrate pairs. Despite these challenges, the quest for an HIV vaccine, with the HIV-1 Env at its center, has yielded a large volume of structural knowledge of gp120, as well as its interactions with host receptors and antibodies. This wealth of knowledge has spurred development of inhibitors, which currently are at different stages of development ranging from lead molecules with μM potency to receptor mimetics capable of broad and potent neutralization. ART is continuously evolving with new drugs being sought to address various issues with existing regimens including toxicity and treatment failure. New therapeutics against gp120 would most immediately benefit patients who have exhausted current antiretroviral regimens due to emergence of resistant viruses.
One area that is expected to see rapid development is passive therapy with broadly neutralizing antibodies. The large number of antibodies targeting various sites of vulnerability on gp120 provides a valuable arsenal for multi-“drug” therapy, which has proven critical in ARV to prevent emergence of resistant viruses. Antibodies have a number of unique advantages including low toxicity, which may benefit treatment of specific populations such as infants. Apart from the advantage of longer serum half-life, full-length antibodies also have the unique advantage of Fc-mediated functions, which have been shown to have an effect on viral control through non-neutralizing protective mechanisms. Significant drawbacks toward development of preventive and therapeutic regimens using antibodies involve their route of administration through injections, which can limit adherence, and the high cost of manufacturing compared to synthetic small molecules. Enhancing potency of antibodies can reduce the amount of antibody that needs to be administered thereby reducing cost, and strategic efforts to improve the breadth and potency of existing broadly neutralizing antibodies through structure based design have been pursued [123-125]. Despite some difficulties, passive therapy with antibodies holds immediate promise, especially for treatment of select populations, such as infants where doses are expected to be lower. Finally, emerging gene therapy approaches using adeno-associated vectors can, in the future, make treatments involving antibody delivery more effective and less expensive [126,127].
Precise atomic-level definition of gp120 interactions with host molecules, such as receptors and antibodies, has opened doors to rational, structure-guided development of inhibitors that target the conserved, functional regions of gp120. The symbiosis between structural analyses, rational design and chemical synthesis that combine the precision of structure-guided design and the manipulative power of synthetic chemistry have great potential to deliver a diverse arsenal of HIV-1 entry inhibitors. Notwithstanding the challenges inherent in disrupting large protein-protein interactions with smaller chemical moieties, this strategy has a unique advantage of being unencumbered by the biological repertoire and can use synthetic compounds to reach where natural molecules cannot [128]. Any antiretroviral therapy has to take into account the latent viral reservoir since the ultimate goal of therapy is to purge the reservoirs and effect a cure, thus enabling patients to end life-long therapy. Emerging ideas in this area include targeted cytotoxicity and coupling inhibitors and targeting molecules with immunotoxins and cytolytic agents [129].
In conclusion, HIV-1 gp120, notwithstanding its complexities and the challenges, is an emerging therapeutic target, with a rich arsenal of therapeutic leads that can enrich the current ART repertoire. Two recent technological advances that will likely impact the development of inhibitors targeting HIV-1 gp120 are i) the atomic level definition of the pre-fusion closed state of HIV-1 Env and availability of a robust crystallization lattice for structural determination [11], and ii) an ability to visualize the gp120 moving labyrinth by smFRET [12]. While not all the activated states revealed by smFRET analyses of the HIV-1 Env have been resolved structurally, the definition of the pre-fusion, closed, ground state, which is targeted by a number of broadly neutralizing antibodies and inhibitors [12] is clear. Apart from this closed conformation of HIV-1 Env, receptor-activated Env conformations can also be accessed and targeted by broad and potent inhibitors [50,72]. Taken together, the convergence of structural and mechanistic knowledge, emerging synergy between vaccine and cure research fields, and the availability of several potent lead inhibitors targeting different binding sites and conformations of HIV-1 gp120 bode well for the development of gp120-targeting therapeutics in the next few years.
Acknowledgments
The authors were supported by the AIDS Targeted Antiviral Program, Office of the Director, NIH, Intramural Research program, NIDDK, Intramural Research Program, Vaccine Research Center, NIAID, NIH.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Bibliography
Papers of special note have been highlighted as either of interest (.) or of considerable interest (..) to readers.
- 1.Wilen CB, Tilton JC, Doms RW. HIV: cell binding and entry. Cold Spring Harbor perspectives in medicine. 2012;2(8) doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolly C, Kashefi K, Hollinshead M, et al. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199(2):283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nature medicine. 1996;2(11):1240–1243. doi: 10.1038/nm1196-1240. [Reports strong resistance of homozygous CCR5delta32 mutant allele, and weaker effect of heterozygosity at this allele, in HIV-1 transmission and disease progression.] [DOI] [PubMed] [Google Scholar]
- 4••.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. The New England journal of medicine. 2009;360(7):692–698. doi: 10.1056/NEJMoa0802905. [Reports functional HIV-1 cure by CCR5delta32/delta32 stem cell transplantation.] [DOI] [PubMed] [Google Scholar]
- 5.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 6.Maraviroc reduces viral load in naive patients at 48 weeks. AIDS Patient Care STDS. 2007;21(9):703–704. [PubMed] [Google Scholar]
- 7.Lalezari JP, Eron JJ, Carlson M, et al. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS. 2003;17(5):691–698. doi: 10.1097/00002030-200303280-00007. [DOI] [PubMed] [Google Scholar]
- 8••.Lalezari J, Latiff GH, Brinson C, et al. Attachment Inhibitor Prodrug BMS-663068 in ARV-Experienced Subjects: Week 24 Analysis.. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. Abs 131. [Reports efficacy of gp120 CD4-binding site targeting drug BMS-663068.] [Google Scholar]
- 9••.Julien JP, Cupo A, Sok D, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1477–1483. doi: 10.1126/science.1245625. [Crystal structure at 4.7 Å of gp120 in the context of the pre-fusion HIV-1 Env trimer bound by antibody PGT122.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Lyumkis D, Julien JP, de Val N, et al. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342(6165):1484–1490. doi: 10.1126/science.1245627. [Cryo-EM structure at 5.8 Å of soluble, closed HIV-1 Env trimer bound to broadly neutralizing CD4-binding site antibody VRC-PG04.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Pancera M, Zhou T, Druz A, et al. Structure and immune recognition of trimeric pre-fusion HIV-1 Env. Nature. 2014;514(7523):455–461. doi: 10.1038/nature13808. [Crystal structure at 3.5 Å of pre-fusion HIV-1 Env bound by antibodies PGT122 and 35O22.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Munro JB, Gorman J, Ma X, et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science. 2014 doi: 10.1126/science.1254426. [smFRET studies demonstrate HIV-1 Env trimers on virus surface as intrinsically dynamic with host receptors, broadly neutralizing antibodies and drugs recognizing and stabilizing pre-existing conformations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–228. doi: 10.1038/nature12744. [First report demonstrating therapeutic efficacy of broadly neutralizing antibodies in rhesus monkeys.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balazs A. Durable Suppression of Established Transmitted Founder Replication in Infected BLT Humanized Mice by Vectored ImmunoTherapy.
- 15•.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi: 10.1038/nature11604. [First report of HIV therapy using broadly neutralizing antibodies in humanized mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Acharya P, Tolbert WD, Gohain N, et al. Structural Definition of an Antibody-Dependent Cellular Cytotoxicity Response Implicated in Reduced Risk for HIV-1 Infection. J Virol. 2014;88(21):12895–12906. doi: 10.1128/JVI.02194-14. [First structural definition of ADCC activity at conserved epitopes in HIV-1 gp120 C1-region.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Dereuddre-Bosquet N, Morellato-Castillo L, Brouwers J, et al. MiniCD4 microbicide prevents HIV infection of human mucosal explants and vaginal transmission of SHIV(162P3) in cynomolgus macaques. PLoS pathogens. 2012;8(12):e1003071. doi: 10.1371/journal.ppat.1003071. [Demonstrated in vivo efficacy of synthetic CD4-mimetic miniproteins as microbicides.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [Used atomic level structural information to design gp120 probes to isolate broadly neutralizing CD4-binding site directed antibodies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esser MT, Mori T, Mondor I, et al. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73(5):4360–4371. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Tsai CC, Emau P, Jiang Y, et al. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19(7):535–541. doi: 10.1089/088922203322230897. [First report showing that CV-N prevents rectal transmission of SHIV89.6 in macaques, demonstrating in vivo efficacy of CBAs as microbicides.] [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Wang C, O'Dell S, et al. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol. 2012;86(10):5844–5856. doi: 10.1128/JVI.07139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wibmer CK, Bhiman JN, Gray ES, et al. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS pathogens. 2013;9(10):e1003738. doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roche M, Jakobsen MR, Sterjovski J, et al. HIV-1 escape from the CCR5 antagonist maraviroc associated with an altered and less-efficient mechanism of gp120-CCR5 engagement that attenuates macrophage tropism. J Virol. 2011;85(9):4330–4342. doi: 10.1128/JVI.00106-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascola JR, Montefiori DC. HIV-1: nature's master of disguise. Nature Med. 2003;9(4):393–394. doi: 10.1038/nm0403-393. [DOI] [PubMed] [Google Scholar]
- 25.Roche M, Salimi H, Duncan R, et al. A common mechanism of clinical HIV-1 resistance to the CCR5 antagonist maraviroc despite divergent resistance levels and lack of common gp120 resistance mutations. Retrovirology. 2013;10:43. doi: 10.1186/1742-4690-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Kwon YD, Zhou T, et al. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science. 2009;326(5956):1123–1127. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Kwong PD, Doyle ML, Casper DJ, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420(6916):678–682. doi: 10.1038/nature01188. [Describes gp120 immune evasion by conformational masking.] [DOI] [PubMed] [Google Scholar]
- 28•.Labrijn AF, Poignard P, Raja A, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77(19):10557–10565. doi: 10.1128/JVI.77.19.10557-10565.2003. [Describes steric restriction at the co-receptor binding site in CD4-activated gp120.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Rowling JK. Harry Potter and the Goblet of Fire. 2000:4. [Describes a moving maze from which we draw an analogy to the mobile gp120 architecture in this review.] [Google Scholar]
- 30•.Liu J, Bartesaghi A, Borgnia MJ, et al. Molecular architecture of native HIV-1 gp120 trimers. Nature. 2008;455(7209):109–113. doi: 10.1038/nature07159. [First structural definition by Cryo-EM of subunit motions upon activation of HIV-1 Env.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Kwong PD, Wyatt R, Robinson J, et al. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–659. doi: 10.1038/31405. [First atomic level structure of gp120, in complex with CD4 receptor and a CD4-induced antibody.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Huang CC, Lam SN, Acharya P, et al. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317(5846):1930–1934. doi: 10.1126/science.1145373. [Structural definition of CCR5 N terminus binding to gp120.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pancera M, Majeed S, Ban YE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proc Natl Acad Sci USA. 2010;107(3):1166–1171. doi: 10.1073/pnas.0911004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran EE, Borgnia MJ, Kuybeda O, et al. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS pathogens. 2012;8(7):e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou T, Xu L, Dey B, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CC, Tang M, Zhang MY, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310(5750):1025–1028. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Migueles SA, Welcher B, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nature Med. 2007;13(9):1032–1034. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LM, Phogat SK, Chan-Hui PY, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poignard P, Sabbe R, Picchio GR, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10(4):431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 40.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 41.West AP, Jr., Scharf L, Scheid JF, et al. Structural Insights on the Role of Antibodies in HIV-1 Vaccine and Therapy. Cell. 2014;156(4):633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shingai M, Nishimura Y, Klein F, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou T, Zhu J, Wu X, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39(2):245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao F, Bonsignori M, Liao HX, et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell. 2014;158(3):481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shingai M, Donau OK, Plishka RJ, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211(10):2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin PF, Blair W, Wang T, et al. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci USA. 2003;100(19):11013–11018. doi: 10.1073/pnas.1832214100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowicka-Sans B, Gong YF, McAuliffe B, et al. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother. 2012;56(7):3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pancera M, Druz A, Zhou T, et al. Structure of BMS-806, a Small-molecule HIV-1 Entry Inhibitor, Bound to BG505 SOSIP.664 HIV-1 Env Trimer. AIDS Res Hum Retroviruses. 2014;30(Suppl 1):A151. [Google Scholar]
- 50•.Acharya P, Luongo TS, Louder MK, et al. Structural basis for highly effective HIV-1 neutralization by CD4-mimetic miniproteins revealed by 1.5 Å cocrystal structure of gp120 and M48U1. Structure. 2013;21(6):1018–1029. doi: 10.1016/j.str.2013.04.015. [Reports broad and potent HIV-1 neutralization and 1.5 Å crystal structure of CD4-mimetic miniprotein M48U1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Vita C, Drakopoulou E, Vizzavona J, et al. Rational engineering of a miniprotein that reproduces the core of the CD4 site interacting with HIV-1 envelope glycoprotein. Proc Natl Acad Sci USA. 1999;96(23):13091–13096. doi: 10.1073/pnas.96.23.13091. [Used atomic level structural information to design a CD4-mimetic miniprotein.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stricher F, Huang CC, Descours A, et al. Combinatorial optimization of a CD4-mimetic miniprotein and cocrystal structures with HIV-1 gp120 envelope glycoprotein. J Mol Biol. 2008;382(2):510–524. doi: 10.1016/j.jmb.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang CC, Stricher F, Martin L, et al. Scorpion-toxin mimics of CD4 in complex with human immunodeficiency virus gp120 crystal structures, molecular mimicry, and neutralization breadth. Structure. 2005;13(5):755–768. doi: 10.1016/j.str.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Selhorst P, Grupping K, Tong T, et al. M48U1 CD4 mimetic has a sustained inhibitory effect on cell-associated HIV-1 by attenuating virion infectivity through gp120 shedding. Retrovirology. 2013;10(12) doi: 10.1186/1742-4690-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao Q, Ma L, Jiang S, et al. Identification of N-phenyl-N'-(2,2,6,6-tetramethylpiperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology. 2005;339(2):213–225. doi: 10.1016/j.virol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 56.LaLonde JM, Kwon YD, Jones DM, et al. Structure-based design, synthesis, and characterization of dual hotspot small-molecule HIV-1 entry inhibitors. J Med Chem. 2012;55(9):4382–4396. doi: 10.1021/jm300265j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Courter JR, Madani N, Sodroski J, et al. Structure-based design, synthesis and validation of CD4-mimetic small molecule inhibitors of HIV-1 entry: conversion of a viral entry agonist to an antagonist. Acc Chem Res. 2014;47(4):1228–1237. doi: 10.1021/ar4002735. [Reports on structure-guided synthesis to convert an HIV-1 entry agonist CD4-mimetic small molecule to an antagonist.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matz J, Kessler P, Bouchet J, et al. Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved CD4 and coreceptor binding sites of HIV-1 gp120. J Virol. 2013;87(2):1137–1149. doi: 10.1128/JVI.00461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cormier EG, Persuh M, Thompson DA, et al. Specific interaction of CCR5 amino-terminal domain peptides containing sulfotyrosines with HIV-1 envelope glycoprotein gp120. Proc Natl Acad Sci USA. 2000;97(11):5762–5767. doi: 10.1073/pnas.97.11.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cormier EG, Tran DN, Yukhayeva L, et al. Mapping the determinants of the CCR5 amino-terminal sulfopeptide interaction with soluble human immunodeficiency virus type 1 gp120-CD4 complexes. J Virol. 2001;75(12):5541–5549. doi: 10.1128/JVI.75.12.5541-5549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dogo-Isonagie C, Lam S, Gustchina E, et al. Peptides from second extracellular loop of CC chemokine receptor type 5 (CCR5) inhibit diverse strains of HIV-1. J Biol Chem. 2012;287(18):15076–15086. doi: 10.1074/jbc.M111.332361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lam SN, Acharya P, Wyatt R, et al. Tyrosine-sulfate isosteres of CCR5 N-terminus as tools for studying HIV-1 entry. Bioorg Med Chem. 2008;16(23):10113–10120. doi: 10.1016/j.bmc.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farzan M, Chung S, Li W, et al. Tyrosine-sulfated peptides functionally reconstitute a CCR5 variant lacking a critical amino-terminal region. J Biol Chem. 2002;277(43):40397–40402. doi: 10.1074/jbc.M206784200. [DOI] [PubMed] [Google Scholar]
- 64.Berger EA, Alkhatib G. HIV gp120 interactions with coreceptors: insights from studies with CCR5-based peptides. European J Med Res. 2007;12(9):403–407. [PubMed] [Google Scholar]
- 65.Agrawal L, VanHorn-Ali Z, Berger EA, et al. Specific inhibition of HIV-1 coreceptor activity by synthetic peptides corresponding to the predicted extracellular loops of CCR5. Blood. 2004;103(4):1211–1217. doi: 10.1182/blood-2003-08-2669. [DOI] [PubMed] [Google Scholar]
- 66.Xiang SH, Farzan M, Si Z, et al. Functional mimicry of a human immunodeficiency virus type 1 coreceptor by a neutralizing monoclonal antibody. J Virol. 2005;79(10):6068–6077. doi: 10.1128/JVI.79.10.6068-6077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang CC, Venturi M, Majeed S, et al. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci USA. 2004;101(9):2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choe H, Li W, Wright PL, et al. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell. 2003;114(2):161–170. doi: 10.1016/s0092-8674(03)00508-7. [DOI] [PubMed] [Google Scholar]
- 69•.Acharya P, Dogo-Isonagie C, LaLonde JM, et al. Structure-based identification and neutralization mechanism of tyrosine sulfate mimetics that inhibit HIV-1 entry. ACS chemical biology. 2011;6(10):1069–1077. doi: 10.1021/cb200068b. [Reports structure-guided identification of small molecule leads that inhibit gp120-CCR5 binding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Debnath AK. Rational design of HIV-1 entry inhibitors. Methods in molecular biology. 2013;993:185–204. doi: 10.1007/978-1-62703-342-8_13. [DOI] [PubMed] [Google Scholar]
- 71.Quinlan BD, Joshi VR, Gardner MR, et al. A double-mimetic peptide efficiently neutralizes HIV-1 by bridging the CD4- and coreceptor-binding sites of gp120. J Virol. 2014;88(6):3353–3358. doi: 10.1128/JVI.03800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dey B, Del Castillo CS, Berger EA. Neutralization of human immunodeficiency virus type 1 by sCD4-17b, a single-chain chimeric protein, based on sequential interaction of gp120 with CD4 and coreceptor. J Virol. 2003;77(5):2859–2865. doi: 10.1128/JVI.77.5.2859-2865.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gardner MR, Kattenhorn LM, Kondur HR, et al. Durable protection of rhesus macaques from multiple SHIV challenges through AAV expression of an exceptionally broad and potent HIV-1 entry inhibitor. Nature. 2015 [Google Scholar]
- 74.Chen W, Feng Y, et al. Exceptionally potent and broadly cross-reactive, bispecific multivalent HIV-1 inhibitors based on single human CD4 and antibody domains. J Virol. 2014;88(2):1125–1139. doi: 10.1128/JVI.02566-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250(1):180–198. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doores KJ, Bonomelli C, Harvey DJ, et al. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc Natl Acad Sci USA. 2010;107(31):13800–13805. doi: 10.1073/pnas.1006498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Go EP, Hewawasam G, Liao HX, et al. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J Virol. 2011;85(16):8270–8284. doi: 10.1128/JVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79•.McLellan JS, Pancera M, Carrico C, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. [Describes structural basis for immune recognition of a gp120 site of vulnerability at the V1V2 domain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pancera M, Shahzad-Ul-Hussan S, Doria-Rose NA, et al. Structural basis for diverse N-glycan recognition by HIV-1-neutralizing V1-V2-directed antibody PG16. Nat Struct Mol Biol. 2013;20(7):804–813. doi: 10.1038/nsmb.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen JE, Nielsen CM, Nielsen C, et al. Correlation between carbohydrate structures on the envelope glycoprotein gp120 of HIV-1 and HIV-2 and syncytium inhibition with lectins. AIDS. 1989;3(10):635–641. doi: 10.1097/00002030-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Robinson WE, Jr., Montefiori DC, Mitchell WM. Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res Hum Retroviruses. 1987;3(3):265–282. doi: 10.1089/aid.1987.3.265. [DOI] [PubMed] [Google Scholar]
- 83.Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mori T, O'Keefe BR, Sowder RC, 2nd, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. The Journal of Biological Chemistry. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 85.Bokesch HR, O'Keefe BR, McKee TC, et al. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42(9):2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 86.Balzarini J. Carbohydrate-binding agents: a potential future cornerstone for the chemotherapy of enveloped viruses? Antiviral Chem Chemother. 2007;18(1):1–11. doi: 10.1177/095632020701800101. [DOI] [PubMed] [Google Scholar]
- 87.Huskens D, Schols D. Algal Lectins as Potential HIV Microbicide Candidates. Mar Drugs. 2012;10(7):1476–1497. doi: 10.3390/md10071476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.François KO, Balzarini J. Potential of carbohydrate-binding agents as therapeutics against enveloped viruses. Medicinal Research Reviews. 2012;32(2):349–387. doi: 10.1002/med.20216. [Comprehensive review of the scope of lectins among other CBA against different enveloped viruses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoorelbeke B, Van Damme EJ, Rouge P. Antiviral chemistry & chemotherapy Differences in the mannose oligomer specificities of the closely related lectins from Galanthus nivalis and Zea mays strongly determine their eventual anti-HIV activity. Retrovirology. 2011;8(1):10. doi: 10.1186/1742-4690-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saidi H, Nasreddine N, Jenabian MA. Antiviral chemistry & chemotherapy Differential in vitro inhibitory activity against HIV-1 of alpha-(1-3)- and alpha-(1-6)-D-mannose specific plant lectins: implication for microbicide development. J Transl Med. 2007;5:28. doi: 10.1186/1479-5876-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Swanson MD, Winter HC, Goldstein IJ, et al. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem. 2010;285(12):8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mori T, O'Keefe BR, Sowder RC, 2nd, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 93.Bewley CA, Cai M, Ray S, et al. New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL: NMR, ITC and sedimentation equilibrium studies. J Mol Biol. 2004;339(4):901–914. doi: 10.1016/j.jmb.2004.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huskens D, Ferir G, Vermeire K, et al. Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem. 2010;285(32):24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sato Y, Okuyama S, Hori K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J Biol Chem. 2007;282(15):11021–11029. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- 96.Xiong C, O'Keefe BR, Byrd RA, et al. Potent anti-HIV activity of scytovirin domain 1 peptide. Peptides. 2006;27(7):1668–1675. doi: 10.1016/j.peptides.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 97.Chiba H, Inokoshi J, Okamoto M, et al. Actinohivin, a novel anti-HIV protein from an actinomycete that inhibits syncytium formation: isolation, characterization, and biological activities. Biochem Biophys Res Comm. 2001;282(2):595–601. doi: 10.1006/bbrc.2001.4495. [DOI] [PubMed] [Google Scholar]
- 98.Gattegno L, Ramdani A, Jouault T, et al. Lectin-carbohydrate interactions and infectivity of human immunodeficiency virus type 1 (HIV-1). AIDS Res Hum Retroviruses. 1992;8(1):27–37. doi: 10.1089/aid.1992.8.27. [DOI] [PubMed] [Google Scholar]
- 99.Wright CS, Hester G. The 2.0 A structure of a cross-linked complex between snowdrop lectin and a branched mannopentaose: evidence for two unique binding modes. Structure. 1996;4(11):1339–1352. doi: 10.1016/s0969-2126(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 100.Koharudin LM, Kollipara S, Aiken C, et al. Structural insights into the anti-HIV activity of the Oscillatoria agardhii agglutinin homolog lectin family. J Biol Chem. 2012;287(40):33796–33811. doi: 10.1074/jbc.M112.388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams DC, Jr., Lee JY, Cai M, et al. Crystal structures of the HIV-1 inhibitory cyanobacterial protein MVL free and bound to Man3GlcNAc2: structural basis for specificity and high-affinity binding to the core pentasaccharide from n-linked oligomannoside. J Biol Chem. 2005;280(32):29269–29276. doi: 10.1074/jbc.M504642200. [DOI] [PubMed] [Google Scholar]
- 102.Bewley CA. Solution structure of a cyanovirin-N:Man alpha 1-2Man alpha complex: structural basis for high-affinity carbohydrate-mediated binding to gp120. Structure. 2001;9(10):931–940. doi: 10.1016/s0969-2126(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 103.Shahzad-ul-Hussan S, Gustchina E, Ghirlando R, et al. Solution structure of the monovalent lectin microvirin in complex with Man(alpha)(1-2)Man provides a basis for anti-HIV activity with low toxicity. J Biol Chem. 2011;286(23):20788–20796. doi: 10.1074/jbc.M111.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McFeeters RL, Xiong C, O'Keefe BR, et al. The Novel Fold of Scytovirin Reveals a New Twist For Antiviral Entry Inhibitors. J Mol Biol. 2007;369(2):451–461. doi: 10.1016/j.jmb.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dam TK, Brewer CF. Multivalent Lectin—Carbohydrate Interactions. Adv Carbohydrate Chem Biochem. 2010;63:139–164. doi: 10.1016/S0065-2318(10)63005-3. [DOI] [PubMed] [Google Scholar]
- 106.Emau P, Tian B, O'Keefe BR, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. Journal of Med Primatol. 2007;36(4-5):244–253. doi: 10.1111/j.1600-0684.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 107.Huskens D, Ferir G, Vermeire K, et al. Microvirin, a novel alpha(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem. 2010;285(32):24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shahzad-ul-Hussan S, Ghirlando R, Dogo-Isonagie CI, et al. Characterization and carbohydrate specificity of pradimicin S. J Am Chem Soc. 2012;134(30):12346–12349. doi: 10.1021/ja303860m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kakushima M, Masuyoshi S, Hirano M, et al. In vitro and in vivo antifungal activities of BMY-28864, a water-soluble pradimicin derivative. Antimicrob Agents Chemother. 1991;35(11):2185–2190. doi: 10.1128/aac.35.11.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antiviral Res. 2013;99(3):391–400. doi: 10.1016/j.antiviral.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 111.Hoorelbeke B, Huskens D, Ferir G, et al. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob Agents Chemother. 2010;54(8):3287–3301. doi: 10.1128/AAC.00254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Balzarini J, Van Laethem K, Hatse S, et al. Profile of resistance of human immunodeficiency virus to mannose-specific plant lectins. J Virol. 2004;78(19):10617–10627. doi: 10.1128/JVI.78.19.10617-10627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alexandre KB, Gray ES, Pantophlet R, et al. Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J Virol. 2011;85(17):9039–9050. doi: 10.1128/JVI.02675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Witvrouw M, Fikkert V, Hantson A, et al. Resistance of human immunodeficiency virus type 1 to the high-mannose binding agents cyanovirin N and concanavalin A. J Virol. 2005;79(12):7777–7784. doi: 10.1128/JVI.79.12.7777-7784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Balzarini J, Van Laethem K, Peumans WJ, et al. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–8421. doi: 10.1128/JVI.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu Q, Mahmood N, Shattock RJ. High-mannose-specific deglycosylation of HIV-1 gp120 induced by resistance to cyanovirin-N and the impact on antibody neutralization. Virology. 2007;368(1):145–154. doi: 10.1016/j.virol.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sadjadpour R, Donau OK, Shingai M, et al. Emergence of gp120 V3 variants confers neutralization resistance in an R5 simian-human immunodeficiency virus-infected macaque elite neutralizer that targets the N332 glycan of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 2013;87(15):8798–8804. doi: 10.1128/JVI.00878-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore PL, Gray ES, Wibmer CK, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012;18(11):1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alexandre KB, Moore PL, Nonyane M, et al. Mechanisms of HIV-1 subtype C resistance to GRFT, CV-N and SVN. Virology. 2013;446(1-2):66–76. doi: 10.1016/j.virol.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat Rev Microbiol. 2007;5(8):583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nature Med. 1998;4(6):679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 122.Hu J, Cladel NM, Balogh K, et al. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358(2):384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Diskin R, Scheid JF, Marcovecchio PM, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334(6060):1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diskin R, Klein F, Horwitz JA, et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J Exp Med. 2013;210(6):1235–1249. doi: 10.1084/jem.20130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudicell RS, Kwon YD, Ko SY, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88(21):12669–12682. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Balazs AB, Chen J, Hong CM, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Balazs AB, Ouyang Y, Hong CM, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nature Med. 2014;20(3):296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dey B, Berger EA. Blocking HIV-1 gp120 at the Phe43 cavity: if the extension fits. Structure. 2013;21(6):871–872. doi: 10.1016/j.str.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berger EA. Targeted cytotoxic therapy: adapting a rapidly progressing anticancer paradigm for depletion of persistent HIV-infected cell reservoirs. Curr Opin HIV AIDS. 2011;6(1):80–85. doi: 10.1097/COH.0b013e3283412515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klein F, Diskin R, Scheid JF, et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 2013;153(1):126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Castro S, Andrei G, Snoeck R, Balzarini J, Camarasa MJ, Velazquez S. Novel nonnucleoside human cytomegalovirus inhibitors based upon TSAO nucleoside derivatives: structure-activity relationships. Nucleosides Nucleotides Nucleic Acids. 2007;26(6-7):625–628. doi: 10.1080/15257770701490431. [DOI] [PubMed] [Google Scholar]
- 133.Maruyama T, Demizu Y, Kozai S, et al. Antiviral activity of 3-(3,5-dimethylbenzyl)uracil derivatives against HIV-1 and HCMV. Nucleosides Nucleotides Nucleic Acids. 2007;26(10-12):1553–1558. doi: 10.1080/15257770701545424. [DOI] [PubMed] [Google Scholar]
- 134.Morellato-Castillo L, Acharya P, Combes O, et al. Interfacial cavity filling to optimize CD4-mimetic miniprotein interactions with HIV-1 surface glycoprotein. J Med Chem. 2013;56(12):5033–5047. doi: 10.1021/jm4002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kwon YD, LaLonde JM, Yang Y, et al. Crystal structures of HIV-1 gp120 envelope glycoprotein in complex with NBD analogues that target the CD4-binding site. PLoS One. 2014;9(1):e85940. doi: 10.1371/journal.pone.0085940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lalonde JM, Elban MA, Courter JR, et al. Design, synthesis and biological evaluation of small molecule inhibitors of CD4-gp120 binding based on virtual screening. Bioorg Med Chem. 2011;19(1):91–101. doi: 10.1016/j.bmc.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madani N, Schon A, Princiotto AM, et al. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure. 2008;16(11):1689–1701. doi: 10.1016/j.str.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Madani N, Perdigoto AL, Srinivasan K, et al. Localized changes in the gp120 envelope glycoprotein confer resistance to human immunodeficiency virus entry inhibitors BMS-806 and #155. J Virol. 2004;78(7):3742–3752. doi: 10.1128/JVI.78.7.3742-3752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kwong PD, Wyatt R, Majeed S, et al. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure. 2000;8(12):1329–1339. doi: 10.1016/s0969-2126(00)00547-5. [DOI] [PubMed] [Google Scholar]
- 140.Rizzuto CD, Wyatt R, Hernandez-Ramos N, et al. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280(5371):1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 141.Xiang SH, Kwong PD, Gupta R, et al. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 2002;76(19):9888–9899. doi: 10.1128/JVI.76.19.9888-9899.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiang SH, Wang L, Abreu M, et al. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology. 2003;315(1):124–134. doi: 10.1016/s0042-6822(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 143.Zhang W, Canziani G, Plugariu C, et al. Conformational changes of gp120 in epitopes near the CCR5 binding site are induced by CD4 and a CD4 miniprotein mimetic. Biochemistry. 1999;38(29):9405–9416. doi: 10.1021/bi990654o. [DOI] [PubMed] [Google Scholar]
- 144.Wan C, Sun J, Chen W, et al. Epitope mapping of M36, a human antibody domain with potent and broad HIV-1 inhibitory activity. PLoS One. 2013;8(6):e66638. doi: 10.1371/journal.pone.0066638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Julien JP, Lee JH, Cupo A, et al. Asymmetric recognition of the HIV-1 trimer by broadly neutralizing antibody PG9. Proc Natl Acad Sci USA. 2013;110(11):4351–4356. doi: 10.1073/pnas.1217537110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Broggi J, Joubert N, Aucagne V, et al. Alkyne-azide click chemistry mediated carbanucleosides synthesis. Nucleosides Nucleotides Nucleic Acids. 2007;26(10-12):1391–1394. doi: 10.1080/15257770701534139. [DOI] [PubMed] [Google Scholar]
- 147.Koharudin LM, Gronenborn AM. Structural basis of the anti-HIV activity of the cyanobacterial Oscillatoria Agardhii agglutinin. Structure. 2011;19(8):1170–1181. doi: 10.1016/j.str.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meagher JL. Crystal structure of banana lectin reveals a novel second sugar binding site. Glycobiology. 2005;15(10):1033–1042. doi: 10.1093/glycob/cwi088. [DOI] [PubMed] [Google Scholar]
- 149.Swanson MD, Winter HC, Goldstein IJ, et al. A Lectin Isolated from Bananas Is a Potent Inhibitor of HIV Replication. J Biol Chem. 2010;285(12):8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balzarini J, Van Laethem K, Hatse S, et al. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol Pharmacol. 2005;67(5):1556–1565. doi: 10.1124/mol.104.005082. [DOI] [PubMed] [Google Scholar]
- 151.Hoorelbeke B, van Montfort T, Xue J, et al. HIV-1 envelope trimer has similar binding characteristics for carbohydrate-binding agents as monomeric gp120. FEBS Lett. 2013;587(7):860–866. doi: 10.1016/j.febslet.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 152.Takahashi A, Inokoshi J, Hachiya A, et al. The high mannose-type glycan binding lectin actinohivin: dimerization greatly improves anti-HIV activity. J Antibiot (Tokyo) 2011;64(8):551–557. doi: 10.1038/ja.2011.51. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi A, Inokoshi J, Tsunoda M, et al. Actinohivin: specific amino acid residues essential for anti-HIV activity. J Antibiot (Tokyo) 2010;63(11):661–665. doi: 10.1038/ja.2010.106. [DOI] [PubMed] [Google Scholar]
- 154.Shenoy SR, O'Keefe BR, Bolmstedt AJ, et al. Selective interactions of the human immunodeficiency virus-inactivating protein cyanovirin-N with high-mannose oligosaccharides on gp120 and other glycoproteins. J Pharmacol Exp Ther. 2001;297(2):704–710. [PubMed] [Google Scholar]
- 155.O'Keefe BR, Giomarelli B, Barnard DL, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. Journal of Virology. 2010;84(5):2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]