Significance
This study uses advanced functional neuroimaging analyses to test both existing and novel psychological theories about how we understand others’ minds. Analyses show that three dimensions—rationality, social impact, and valence—account for almost half of the variation in the neural representation of mental states, the most comprehensive theory to date regarding our ability to think about others’ minds. These findings both inform long-standing debates within social psychology about theory of mind and generate testable predictions about how our neural hardware supports our ability to mentalize.
Keywords: social cognition, theory of mind, mentalizing, functional magnetic resonance imaging, multivoxel pattern analysis
Abstract
How do people understand the minds of others? Existing psychological theories have suggested a number of dimensions that perceivers could use to make sense of others’ internal mental states. However, it remains unclear which of these dimensions, if any, the brain spontaneously uses when we think about others. The present study used multivoxel pattern analysis (MVPA) of neuroimaging data to identify the primary organizing principles of social cognition. We derived four unique dimensions of mental state representation from existing psychological theories and used functional magnetic resonance imaging to test whether these dimensions organize the neural encoding of others’ mental states. MVPA revealed that three such dimensions could predict neural patterns within the medial prefrontal and parietal cortices, temporoparietal junction, and anterior temporal lobes during social thought: rationality, social impact, and valence. These results suggest that these dimensions serve as organizing principles for our understanding of other people.
The human mind plays host to a panoply of thoughts, feelings, intentions, and impressions. External observers can never directly perceive these mental states—one can never see “nostalgia” nor touch “awe.” Nevertheless, humans are quite adept at representing other people’s internal states. Our ability to perceive and distinguish among the rich set of others’ mental states serves as the bedrock of human social life. We understand the fine differences between pure joy and schadenfreude and judge a friend’s glee accordingly. Our ability to distinguish a partner’s sympathy from sarcasm can make a world of difference to a relationship. Legal decisions frequently hinge on nuanced mental distinctions such as that between inattention and intentional neglect. How do people navigate such complexities in others’ internal mental worlds?
One crucial tool for any navigator is a compass: a set of dimensions that help organize the contents of the world. By attending to the position of others’ mental states on key dimensions, humans might reduce the complexity of others’ minds to just a few essential elements—coordinates on a map. Might navigators of the world of mental states make use of such an intuitive compass? Research in other domains of cognition suggests such organization might be possible: The brain has a demonstrated capacity for extracting and capitalizing on useful regularities in the world. For example, our object representation system makes use of dimensions such as size and animacy to organize its processing tracts (1). Here, we explore the possibility that similar principles may organize our representations of other people’s minds.
Decades of research in social cognitive neuroscience, primarily using functional magnetic resonance imaging (fMRI), have already implicated a well-defined set of brain regions in the process of thinking about mental states: Thinking about the lives and minds of others reliably engages a network including the medial prefrontal cortex (MPFC), medial parietal cortex (MPC), temporoparietal junction (TPJ), superior temporal sulcus (STS), and the anterior temporal lobe (ATL) (for a review, see refs. 2 and 3). However, this relatively young field has yet to explain how the social brain’s hardware processes the richness and complexity of others’ mental states. Fortunately, research in psychology supplies a set of theories regarding how people might organize their knowledge of mental states. The dimensions of these theories include valence and arousal (4, 5), warmth and competence (6, 7), agency and experience (8), emotion and reason, mind and body (9), social and nonsocial (2, 10, 11), and uniquely human and shared with animals (12). Any of these dimensions might plausibly play a role in organizing our understanding of mental states. But which, if any, do we spontaneously use during mentalizing? If a dimension actually matters to the way people typically think about others’ mental states, we should see evidence that the brain organizes its activity around that dimension. However, merely locating where in the brain mental state processing occurs—as social neuroscience has done so well already—cannot tell us how these regions represent mental states.
Fortunately, new analytic techniques in functional neuroimaging, under the umbrella of multivariate or multivoxel pattern analysis (MVPA), enable us to bridge these levels of analysis. MVPA examines activity in distributed sets of voxels, allowing for discrimination between stimuli by their associated patterns of activity even when absolute magnitudes of activity remain constant. In this study, we use the form of MVPA known as representational similarity analysis (13) to test which psychological dimensions organize people’s understanding of mental states. These analyses work by measuring the extent to which neural patterns of activity can be predicted from theories of representational organization. To illustrate, the dimension “arousal” would predict that “ecstasy” and “rage” are represented very similarly in the brain because both are similarly intense mental states. In contrast, the dimension “valence” would predict that “ecstasy” and “rage” are represented very differently in the brain because one state is very positive, whereas the other is very negative. Both predictions can be tested by measuring the extent to which patterns of neural activity elicited by thinking about a person in ecstasy are similar to those elicited by thinking about a person in a fit of rage. Each dimension makes thousands of predictions about the similarity of each mental state compared with each other mental state; representational similarity analysis allows us to assess the accuracy of all of these predictions simultaneously. Thus, we can test which psychological dimensions capture the way the brain encodes others’ mental states.
Results
Refining Psychological Theories.
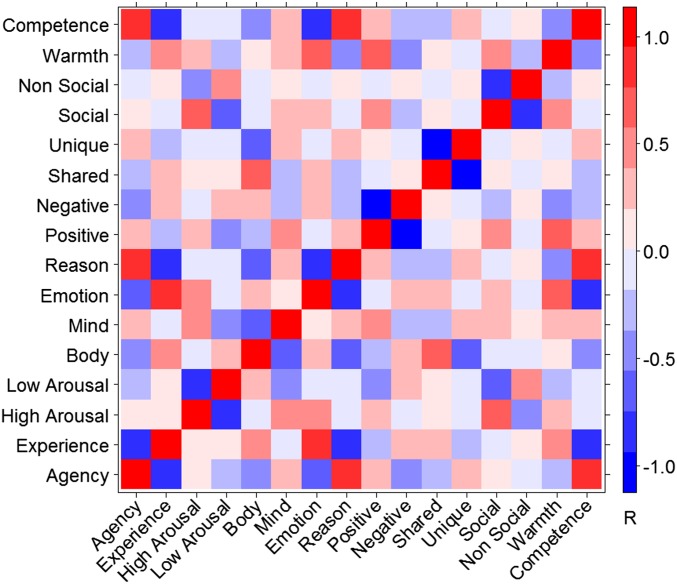
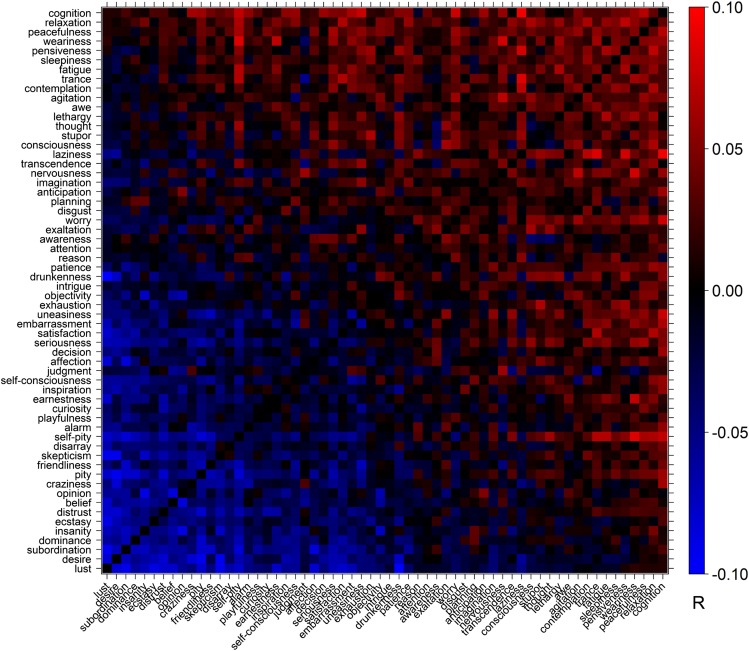
We used 16 dimensions extracted from the psychological literature as a starting point for developing a theory of mental state representation: positive, negative, high arousal, low arousal, warmth, competence, agency, experience, emotion, reason, mind, body, social, nonsocial, shared, and unique. Note that these initial dimensions are nominal—in many cases they merely represent different poles of the same underlying variable—but we initially analyze them separately to remain maximally agnostic to the possible covariance between them. To determine what predictions each dimension would make about mental state representation—that is, which mental states were predicted to be similar or different with regard to each dimension—we used a large online sample (n = 1,205) to measure the position of 166 mental states on each dimension. Ratings across many of the dimensions were highly correlated (Fig. S1). We distilled the overlapping intuitions embodied in the original dimensions down to a smaller set of nonredundant dimensions using principal component analysis (PCA).
Fig. S1.
Correlations between theoretical dimensions. Pearson product-moment correlations between participant ratings of 60 mental states on 16 potential dimensions of mental state representation derived from the existing psychological literature (n = 1,205).
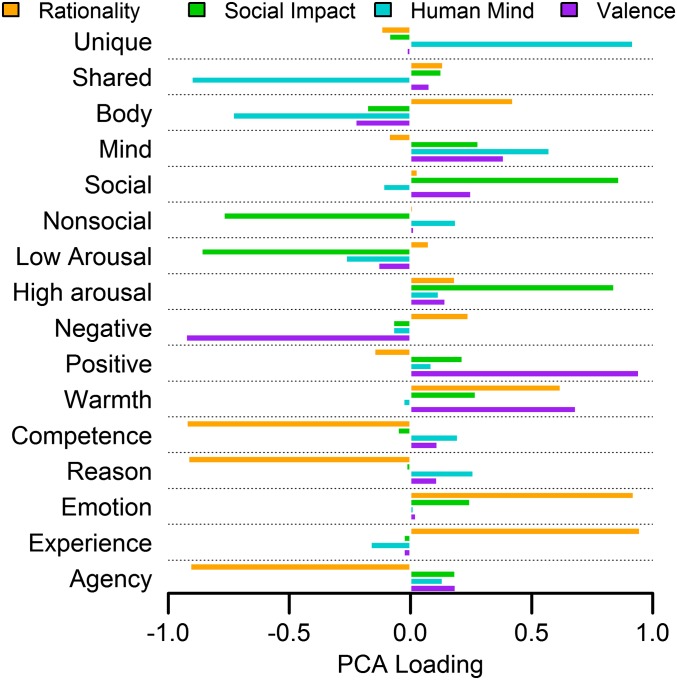
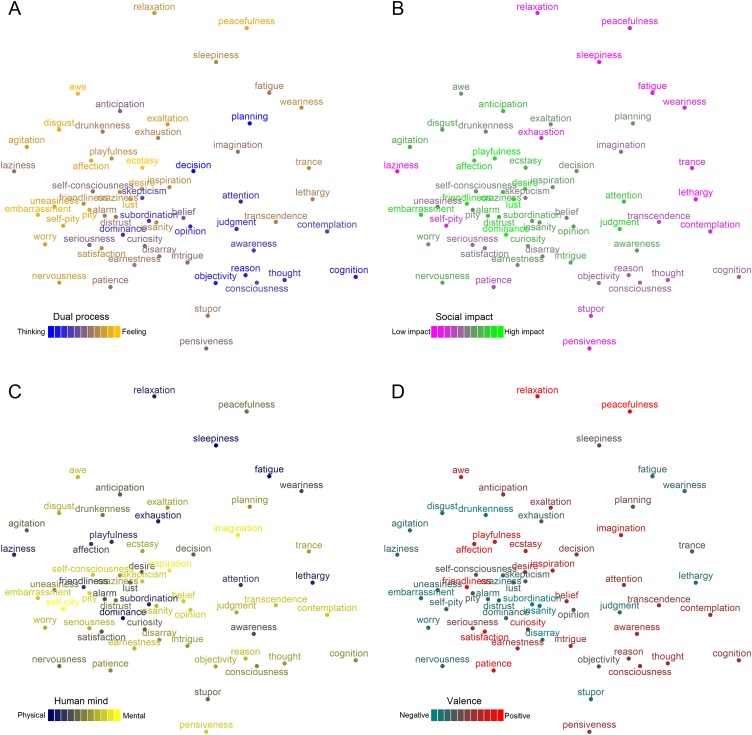
The PCA revealed a much simpler set of four orthogonal dimensions, each with easily interpretable loadings (Fig. 1). The first component, which we term “rationality,” loaded highly in one direction on the original dimensions experience, emotion, and warmth, and loaded highly in the opposite direction on competence, reason, and agency. States such as embarrassment and ecstasy occupy one pole of this dimension whereas the other pole is occupied by states such as planning and decision. The second component, which we term “social impact,” loaded positively on the dimensions high arousal and social, and negatively on low arousal and nonsocial. States such as dominance, friendliness, and lust rate highly on social impact whereas sleepiness and pensiveness rate as minimally impactful. The third component, which we term “human mind,” loaded positively on unique to humans and mind, and negatively on shared with other animals and body. States high in human mind include those like imagination or self-pity whereas states such as fatigue and stupor are considered more physical in nature. The fourth component, which we term “valence,” loaded positively on positive and warmth, and negatively on negative. Positive states include affection and satisfaction whereas negative states include disgust and disarray. From each PCA dimension, we derived predictions about the similarity of each mental state to the others by calculating their psychological similarity as the absolute difference between the positions of mental states on each dimension. These predictions were tested against the neural data using representational similarity analysis, allowing us to see whether patterns of neural activity elicited by thinking about mental states reflected each dimension.
Fig. 1.
Principal component loadings. Principal component loadings of the 16 existing theoretical dimensions onto the optimal four-dimensional solution.
Neural Patterns Representing PCA Dimensions.
Participants were scanned while performing a task designed to elicit their thoughts about 60 mental states (Table S1). On each trial, participants saw the name of a mental state (e.g., “awe”) and decided which of two scenarios would better evoke that mental state in another person (e.g., “seeing the pyramids” or “watching a meteor shower”). This task allowed us to estimate neural representations for each of 60 mental states by averaging the patterns elicited across the varied scenarios. We estimated the pairwise similarity of the neural representations of the 60 states by correlating their activity patterns. These measures of neural similarity were then regressed onto the predictions of psychological similarity made by the four PCA-derived dimensions. For example, if mental states that rated similarly on the valence dimension (such as “affection” and “inspiration”) also elicited similar neural patterns of activity, the regression would reveal that valence was a strong predictor of neural pattern similarity. We would take this result as evidence that mental state representations—embodied by these neural patterns—were indeed organized by valence. This process was conducted repeatedly using local patterns extracted from throughout the brain of each participant. Regression maps for each dimension were combined across participants using t tests, thus revealing which dimensions reliably organized mental state representations in each region of the brain.
Table S1.
The 60 mental states used in the imaging experiment
| Affection | Disgust | Intrigue | Relaxation |
| Agitation | Distrust | Judgment | Satisfaction |
| Alarm | Dominance | Laziness | Self-consciousness |
| Anticipation | Drunkenness | Lethargy | Self-pity |
| Attention | Contemplation | Lust | Seriousness |
| Awareness | Earnestness | Nervousness | Skepticism |
| Awe | Ecstasy | Objectivity | Sleepiness |
| Belief | Embarrassment | Opinion | Stupor |
| Cognition | Exaltation | Patience | Subordination |
| Consciousness | Exhaustion | Peacefulness | Thought |
| Craziness | Fatigue | Pensiveness | Trance |
| Curiosity | Friendliness | Pity | Transcendence |
| Decision | Imagination | Planning | Uneasiness |
| Desire | Insanity | Playfulness | Weariness |
| Disarray | Inspiration | Reason | Worry |
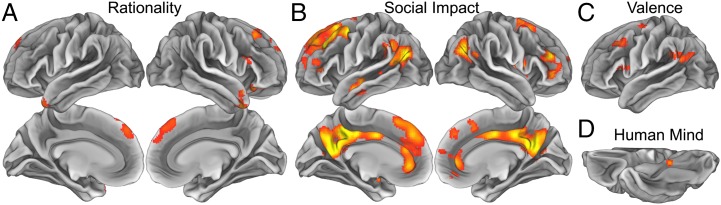
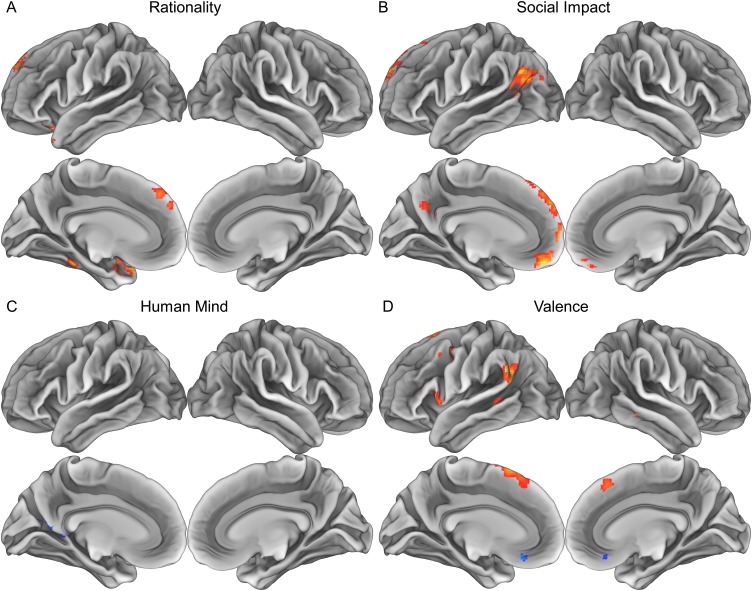
This analysis revealed that three PCA-derived psychological dimensions organize the way the brain represents mental states. Most regions implicated in mental state representation fell within a network of regions previously implicated in social cognition (Fig. 2 and Table S2). The “rationality” dimension predicted the similarity of patterns of neural activity in portions of the dorsolateral prefrontal cortex (DLPFC), ventral lateral prefrontal cortex (VLPFC), dorsal medial prefrontal cortex (DMPFC), lateral orbitofrontal cortex (OFC), and anterior temporal lobe (ATL) bilaterally (Fig. 2A). The “social impact” dimension robustly predicted neural pattern similarity in a widespread set of regions, including significant clusters in the DLPFC, VLPFC, DMPFC, VMPFC, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), precuneus, temporoparietal junction (TPJ) extending into the posterior superior temporal sulcus (pSTS) and ATL (Fig. 2B). The valence dimension predicted neural pattern similarity in a completely left-lateralized set of regions including the DLPFC, VLPFC, and TPJ (Fig. 2C). Finally, the “human mind” dimension captured a spatially restricted set of neural patterns, predicting representations in only a single region in the posterior parahippocampal cortex (Fig. 2D).
Fig. 2.
Searchlight results indicating regions sensitive to the (A) rationality, (B) social impact, (C) valence, and (D) human mind of others' mental states. Within the yellow/orange regions, the similarity of patterns elicited by thinking about mental states can be explained in terms of the corresponding social cognitive dimension extracted from existing theories via PCA (P < 0.05, corrected). Representational similarity searchlight analyses were conducted on each participant and combined through one-sample random-effects t tests.
Table S2.
Regions representing PCA-derived dimensions (cluster-corrected, P < 0.05)
| Dimension/anatomical label | x | y | z | Max T | Volume |
| Rationality | |||||
| Anterior temporal lobe | −40 | 21 | −40 | 4.83 | 123 |
| Anterior temporal lobe/orbitofrontal cortex | 46 | 13 | −36 | 5.23 | 584 |
| Ventrolateral prefrontal cortex | 48 | 21 | 22 | 4.82 | 250 |
| Dorsomedial prefrontal cortex/dorsolateral prefrontal cortex | 32 | 33 | 48 | 5.13 | 1,449 |
| Social Impact | |||||
| Anterior temporal lobe/insula | −38 | −3 | −12 | 6.08 | 890 |
| Posterior cingulate/dorsolateral prefrontal cortex/dorsomedial prefrontal cortex | −8 | −57 | 20 | 8.29 | 14,306 |
| Anterior temporal lobe | 40 | 41 | 22 | 5.88 | 1,106 |
| Insula | 48 | 3 | 10 | 8.52 | 433 |
| Temporoparietal junction | −40 | −69 | 22 | 6.62 | 2,038 |
| Posterior superior temporal sulcus | −54 | −37 | 0 | 4.28 | 78 |
| Temporoparietal junction | 46 | −63 | 26 | 6.40 | 939 |
| Dorsolateral prefrontal cortex | 30 | 11 | 60 | 5.73 | 955 |
| Human mind | |||||
| Parahippocampal gyrus | 18 | −41 | −14 | 5.29 | 108 |
| Valence | |||||
| Ventrolateral prefrontal cortex | −52 | 17 | 10 | 3.98 | 99 |
| Temporoparietal junction | −48 | −47 | 28 | 4.66 | 646 |
| Dorsolateral prefrontal cortex | −34 | 23 | 38 | 5.01 | 591 |
| Precentral gyrus | −32 | −7 | 60 | 4.58 | 387 |
Coordinates refer to the Montreal Neurological Institute stereotaxic space.
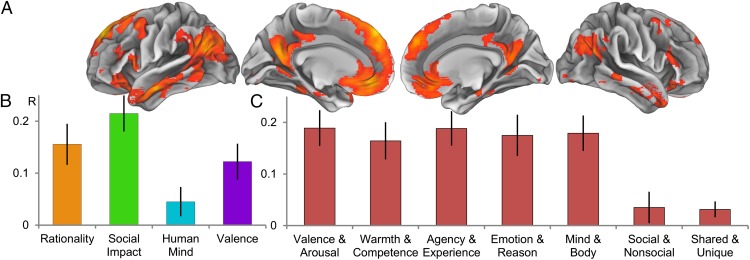
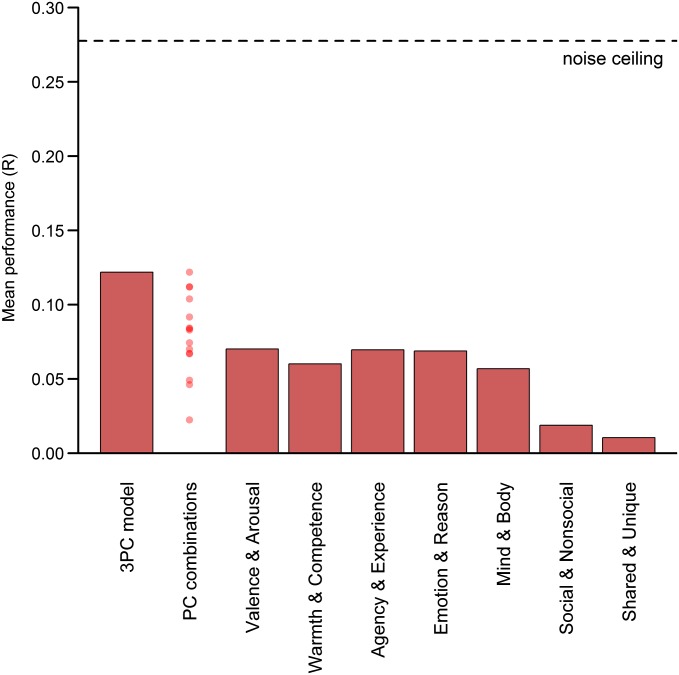
This analysis identified regions of the brain within which local patterns of activity were predicted by the PCA-based models. To test whether relevant patterns of activity were represented in a more distributed manner, we conducted a network-wide analysis. In this analysis, we extracted a single set of activity patterns from across the entirety of a neural network sensitive to mental state content. As with the whole brain analysis, the neural similarity of each pair of mental states was estimated, and the results were correlated with the predictions of the PCA-derived dimensions. Results showed that three dimensions significantly predicted network-level patterns: rationality [r = 0.16; 95% bootstrap confidence interval (CI) (0.06, 0.20)], social impact [r = 0.21; 95% bootstrap CI (0.12, 0.26)], and valence [r = 0.12; 95% bootstrap CI (0.04, 0.17)]. The human mind dimension [r = 0.05; 95% bootstrap CI (−0.01, 0.10)] did not (Fig. 3B). Results of a multidimensional scaling analysis (Fig. S2) allowed us to estimate that the dimensions of rationality, social impact, and valence collectively account for approximately one-third of the variance in neural patterns underlying mental state representation (weighted total R2 = 0.33) (SI Text). Disattenuating this value by dividing it by the reliability of the neural similarity (α = 0.69) yielded a final R2 = 0.47. The results of the network analyses were highly robust to different analytic approaches (SI Text). Statistically controlling for the influence of scenario concreteness, complexity, and familiarity did not produce any qualitative changes in the outcomes. Using independent component analysis (ICA) instead of PCA to generate dimensions, conducting the analysis with Spearman rank correlations, and using a metaanalysis-based feature selection method all produced very similar results. Further, results were not contingent on the use of statistical significance: The same three dimensions emerged from a model selection technique based on cross-validation performance (14) (Fig. S3). Finally, allowing two-way interactions between dimensions did not alter the significance of the main effects although three significant interactions were observed: human mind with rationality, human mind with social impact, and social impact with valence.
Fig. 3.
Network-wide representational similarity analysis. (A) Whole brain ANOVA used for feature selection (voxelwise P < 0.0001). Different mental states reliably elicited different levels of univariate activity within these regions. (B) Bar graphs of model fits for dimensions derived via principal component analysis from existing psychological theories. (C) Bar graphs of model fits for existing psychological models. All model fits are given in terms of Pearson product-moment correlations between neural pattern similarity and model predictions, with error bars indicating bootstrapped SEs. Note that bars in B refer to individual dimensions derived via PCA whereas bars in C indicate the performance of full multidimensional theories. The theoretical advantage of the synthetic model presented here can thus be seen by comparing any one bar in C with the combination of the three significant bars in B.
Fig. S2.
Multidimensional scaling of network-level neural similarity. Proximity between points indicates greater neural pattern similarity within the social brain network. The same 2D scaling is presented in A–D, overlaid with each of the four hypothetical dimensions of mental state representation. The 2D scaling is insufficient to fully capture the differences between patterns elicited for each mental state, but associations between neural space and psychological dimension are still visible.
Fig. S3.
Cross-validated model performance. Bars indicate performance of a representation similarity analysis based on nonnegative least-squares regression. Weights for dimensions within each theory were trained on data from 19 participants. This regression model was then tested by predicting the neural pattern similarity of the left-out participant. Each participant was left out iteratively, and results were averaged across all 20 training-testing combinations. Points in the “PC combinations” column indicate the performance of every possible combination of 1–4 of the 4 PCs. The farthest left bar indicates the performance of the best model, consisting of the PCs rationality, social impact, and valence. The noise ceiling indicates the expected performance of an ideal model for mental state representation.
Neural Patterns Representing Theoretical Models.
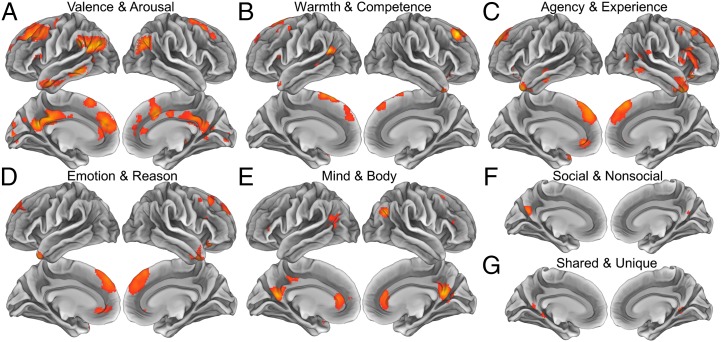
Although the primary purpose of this study was to discover the organization of mental state representation, we also tested whether the seven psychological theories from which we drew our PCA dimensions could predict neural representations of mental states. To do so, we repeated the whole brain and network-level representational similarity analysis with the original psychological dimensions. Whole brain analyses on each of the seven extant theoretical models revealed regions of the brain within which patterns of neural activity were predicted by each model (Fig. 4 and Table S3). The valence and arousal model (Fig. 4A) predicted patterns of activity in a number of regions, including the PCC, ACC, bilateral lateral temporoparietal cortex, left lateral and anterior temporal cortex, bilateral DLPFC, and both rostral and caudal portions of the DMPFC. The warmth and competence model (Fig. 4B) predicted patterns of activity in the left TPJ, rostral and caudal DMPFC, bilateral ATL, bilateral VLPFC, and bilateral DLPFC. Agency and experience (Fig. 4C) and emotion reason (Fig. 4D) produced very similar results, an unsurprising outcome given the degree of correlation between these models. These models both predicted patterns of activity in the VMPFC, rostral DMPFC, bilateral ATL, bilateral VLPFC and DLPFC, and portions of the lateral temporal cortex. The mind and body dimensions (Fig. 4E) predicted patterns in a proximal but distinct set of regions to those discussed above, including the ACC, PCC, TPJ, and portions of the lateral prefrontal cortex. Sociality (Fig. 4F) and human uniqueness (Fig. 4G) models both predicted much less extensive clusters of activity, with both appearing in the precuneus and uniqueness also appearing in a posterior portion of the parahippocampal gyrus.
Fig. 4.
Searchlight results indicating the spatial distribution of mental state representations consistent with (A) the circumplex model of affect, (B) the stereotype content model, (C) the agency and experience model of mind perception, (D) emotion and reason, (E) mind and body, (F) social and nonsocial, and (G) shared with other animals and uniquely human. The similarity of patterns within the yellow/orange regions can be explained by their proximity to each other on the dimensions of the corresponding social cognitive models (P < 0.05 corrected). Searchlight analyses were conducted on each participant and combined through one-sample random-effects t tests.
Table S3.
Regions containing patterns consistent with existing psychological theories (cluster-corrected, P < 0.05)
| Dimensions/anatomical label | x | y | z | Max T | Volume |
| Agency & experience | |||||
| Anterior temporal lobe | −42 | 19 | −40 | 6.17 | 462 |
| Anterior temporal lobe/dorsomedial prefrontal cortex/dorsolateral prefrontal cortex | 46 | 13 | −36 | 5.81 | 5,826 |
| Superior temporal sulcus | −54 | −19 | −18 | 4.98 | 340 |
| Lateral orbitofrontal cortex | −34 | 37 | −10 | 5.21 | 281 |
| Ventromedial prefrontal cortex | −6 | 41 | −6 | 5.03 | 303 |
| Temporoparietal junction/posterior superior temporal sulcus | 58 | −47 | 20 | 4.74 | 662 |
| Valence & arousal | |||||
| Anterior temporal lobe | −56 | 1 | −30 | 5.33 | 531 |
| Middle temporal gyrus | −58 | −45 | −10 | 5.37 | 608 |
| Hippocampus | −34 | −13 | −12 | 4.42 | 78 |
| Thalamus | 12 | −23 | −4 | 5.29 | 120 |
| Cuneus | −4 | −89 | 0 | 5.49 | 282 |
| Medial parietal lobe | −10 | −55 | 20 | 6.5 | 2,746 |
| Dorsolateral/dorsomedial prefrontal cortex | 8 | 21 | 34 | 6.82 | 7,608 |
| Temporoparietal junction | −46 | −51 | 26 | 7.02 | 2,311 |
| Ventrolateral prefrontal cortex | −56 | 17 | 10 | 4.18 | 113 |
| Temporoparietal junction | 52 | −69 | 30 | 6.01 | 960 |
| Midcingulate gyrus | 6 | −1 | 38 | 4.41 | 137 |
| Emotion & reason | |||||
| Anterior temporal lobe | −42 | 19 | −38 | 5.51 | 170 |
| Anterior temporal lobe/orbitofrontal cortex | 16 | 35 | −8 | 5.95 | 951 |
| Ventromedial prefrontal cortex | −8 | 41 | −4 | 4.18 | 310 |
| Lateral prefrontal cortex | 48 | 21 | 22 | 4.03 | 77 |
| Dorsomedial prefrontal cortex | 0 | 51 | 30 | 4.9 | 3,011 |
| Mind & body | |||||
| Anterior insula | −40 | 13 | −22 | 4.25 | 78 |
| Medial prefrontal/anterior cingulate cortex | 0 | 35 | 12 | 4.95 | 916 |
| Ventrolateral prefrontal cortex | −46 | 33 | 4 | 4.38 | 106 |
| Temporoparietal junction | −42 | −61 | 8 | 4.48 | 336 |
| Medial parietal lobe | 4 | −53 | 20 | 6.23 | 1,753 |
| Dorsolateral prefrontal cortex | 38 | 33 | 10 | 4.2 | 185 |
| Temporoparietal junction | 46 | −67 | 32 | 5.68 | 316 |
| Dorsolateral prefrontal cortex | 22 | 15 | 52 | 4.39 | 194 |
| Shared & unique | |||||
| Parahippocampal gyrus | −16 | −39 | −2 | 4.56 | 86 |
| Posterior cingulate cortex | 12 | −49 | 6 | 5.63 | 260 |
| Social & nonsocial | |||||
| Precuneus | −4 | −65 | 26 | 4.92 | 186 |
| Warmth & competence | |||||
| Anterior temporal lobe | 42 | 19 | −42 | 5.61 | 101 |
| Anterior temporal lobe/ventrolateral prefrontal cortex | −50 | 19 | 12 | 4.3 | 374 |
| Superior temporal gyrus | 46 | 21 | −12 | 4.05 | 94 |
| Middle temporal gyrus | −48 | −41 | −4 | 4.31 | 135 |
| Temporoparietal junction | −46 | −57 | 20 | 6.02 | 371 |
| Dorsomedial prefrontal cortex/dorsolateral prefrontal cortex | 18 | 37 | 46 | 6.6 | 2,638 |
Coordinates refer to the Montreal Neurological Institute stereotaxic space.
Finally, we tested the degree to which of each of the seven theoretical models predicted patterns of neural activity in a distributed manner. At the network level, the predictions of five of seven theoretical models were significantly correlated with neural similarity (Fig. 3C)—valence and arousal [r = 0.19, 95% bootstrap CI (0.10, 0.23)], warmth and competence [r = 0.16, 95% bootstrap CI (0.07, 0.21)], agency and experience [r = 0.19, 95% bootstrap CI (0.09, 0.22)], emotion and reason [r = 0.17, 95% bootstrap CI (0.06, 0.22)], and mind and body [r = 0.18, 95% bootstrap CI (0.09, 0.22)]—all with statistically indistinguishable effect sizes. Two theoretical models did not predict network level patterns: social vs. nonsocial [r = 0.04, 95% bootstrap CI (−0.03, 0.09)] and shared vs. unique [r = 0.03, 95% bootstrap CI (−0.003, 0.06)].
Discussion
The current study used fMRI and representational similarity analysis to explore the dimensions that organize our representations of other people’s internal mental states. We used dimensions from the existing psychological literature on mental states as a springboard for generating four nonredundant, easily interpretable dimensions and tested which dimensions organize patterns of neural activity elicited by considering others’ mental states. Results indicated that neural activity patterns within the network of regions sensitive to others’ mental states are attuned to three dimensions: rationality, social impact, and valence. These dimensions account for nearly half of the variation in the neural representation of mental states, constituting the most comprehensive theory to date regarding how we understand others’ minds.
What significance do these three dimensions hold? One of these dimensions, termed “rationality,” has arisen across disparate philosophical and psychological traditions. Here, it derives from theories in the domain of social cognition, including primarily experience and agency (8), warmth vs. competence (6, 7), and emotion vs. reason, an idea extending back at least as far as Plato. This dimension may also closely mirror theories outside the social domain, such as active vs. passive (15), system I vs. system II (16), and reflective vs. reflexive (17). The ubiquity of this distinction hints that it may reflect a deep principle of cognition. The results of the present study align with previous MVPA work (18) in suggesting that the brain spontaneously attunes to others’ rationality. Knowing whether a person is experiencing a rational state or not may be particularly useful for certain social calculations. For example, it seems plausible that rationality assessments may help guide our decisions about whether people are responsible for their actions. These decisions in turn would shape the degree to which we take those actions into account during impression formation. These functions have been repeatedly associated with the DMPFC, one of the regions implicated in representing rationality (19–22).
A second dimension, termed “social impact,” combines two well-known concepts: arousal and sociality. Social impact is the most widely represented of the three dimensions identified here, suggesting that it may serve as a crucial ingredient in many different social computations. We did not anticipate the degree of covariation that these constructs displayed although this shared variation across seemingly disparate dimensions is clearly important, because sociality alone explains little neural pattern similarity. Validating and exploring the nature of this construct should be a topic for future research. Here, we suggest one possible explanation: A key property of another’s mental state is how much that state is likely to affect one’s self. For example, intense (i.e., high arousal) states are often more impactful than more moderate states. However, another person’s rage, although highly arousing for them, may hold import for us only to the extent that it is directed outward at other people (i.e., social) rather than inward. Similarly, another’s envy, although highly social, may hold import for us only in proportion to its intensity. Thus, whereas others’ mental states might affect the self for many reasons, highly intense and social states may be most likely to do so.
The third dimension to emerge from this study, “valence,” captures the difference between positive and negative mental states. This concept has long been implicated in social and affective processing (5). As such, it may come as no surprise that valence plays an important role in the organization of mental state representations. Of note, however, is that we find a unique spatial distribution associated with this dimension. Previous work has associated the processing of positive vs. negative stimuli with specific neural networks, including the mesolimbic dopamine system (23), as well as other limbic structures, such as the amygdala (24). Supplementary univariate analyses do show that the VMPFC, a region involved in reward and value more generally, tracks the positivity of mental states (Fig. S4). However, our MVPA results did not identify these regions but instead implicated left-lateralized cortical regions in the lateral prefrontal cortex and the angular gyrus. One possible explanation is that language supports the processing of mental state valence, but not other types of valence, a hypothesis here only preliminarily supported by the lateralization and the proximity of the valence regions to known language areas.
Fig. S4.
Univariate effects of PCA-derived dimensions. Significant associations between each of the four PCA-derived dimensions and voxelwise univariate brain activity. Orange voxels indicate activity associated with greater emotionality (and less rationality) of mental states (A), greater social impact (B), or greater negativity (D). Blue voxels indicate activity associated with more shared/bodily states (C), or more positive states (D). Statistical maps resulted from random effects one-sample t tests across participants and were corrected for multiple comparisons (P < 0.05) via Monte Carlo simulation (voxelwise P < .001, k > 75).
Together, the three significant dimensions described above explain nearly half of the reliable variance in the neural representation of mental states. While much remains unexplained (Fig. S5), this result appears quite promising. The social impact dimension alone predicts more variance than any of the original theoretical models; the combination of the three significant PCA-derived dimensions explains approximately twice the variance of the circumplex model, the most successful of the original theories. At the same time, given their significance to psychological theory, it is both reassuring and unsurprising that five of the seven original theories significantly predict neural pattern similarity. Notably, even theories that were originally geared toward explaining traits or groups, such as the stereotype content model, demonstrate their efficacy in the mental state domain. This finding raises the interesting possibility that the same dimensions organize neural activity about different types of social constructs.
Fig. S5.
Residual representational dissimilarity matrix. High positive residuals (red) indicate that mental states were more dissimilar than three significant PCA-derived dimensions would predict. High negative residuals (blue) indicate pairs of mental states that were less different than the PCA-derived dimensions would predict.
In addition to informing us about the psychological question of interest—the organization of mental states—the current results also hint at the neural encoding scheme within the social brain network. By assessing the representation of mental states at two different levels of spatial organization—local activity patterns within spherical searchlights and broader activity patterns across the social brain network—the current study is well placed to bear on this issue. The results of the present study support the hypothesis that information is encoded by patterns of activity within localized brain regions, rather than across different regions. If local patterns did not encode social information but coarse patterns across the network did, the searchlight analysis would fail to produce results. Instead, we observe reliable encoding of mental state information in local patterns across the social brain, and explanatory power at the network level appears roughly in proportion to the cortical extent of their local encoding. As such, the current results provide no evidence that others’ mental states are represented by interregional activity differences above and beyond the information already contained in local patterns. Interestingly, we find that two regions, the dMPFC and TPJ, each underlie multiple dimensions. Previous work has already heavily implicated these regions in mentalizing. The convergence of multiple dimensions on these nodes may help to explain their prominence in this domain.
Here, we have identified three dimensions that organize our representations of others’ mental states. However, participants in this study thought about only the mental states of a nonspecific other. Do these same dimensions apply across different categories of “other”? For example, our understanding of a friend’s happiness likely differs considerably from our concept of a stranger’s happiness; our understanding of our own happiness likely differs considerably from others’ happiness. Future work should endeavor to understand whether the dimensions we discovered here expand or contract in their importance on the basis of the person under consideration. We might expect such changes to be asymmetric across dimensions depending on one’s relationship with the person experiencing the mental state. For instance, when considering a close friend’s mental state, we might become more sensitive to valence differences but less sensitive to social impact (because all of the friend’s states are more impactful).
We can also ask how these dimensions might apply across social cognition more generally. The current study used only lexical stimuli and tested these dimensions on only English-speaking adults. Do these dimensions apply to social cognition in other cultures? Do infants or other primates demonstrate any of the building blocks of these dimensions? Do these same dimensions apply when mentalizing about nonlinguistic content? Previous work on cross-modal emotion representation indeed suggests that visual and verbal emotional stimuli may be processed similarly (25, 26) although the full model has yet to be tested. We hope that the current data will provide a solid foundation for future research in these domains. It is also worth considering precisely which processes the imaging task taps. The task relies heavily on conceptual representations of mental states, and it is not entirely clear how strongly these concepts might guide other forms of mentalizing.
Finally, we should endeavor to ask why the social brain would organize its activity in accordance with the three dimensions discussed above and not others. The dimensions that shape mental state representations likely contribute to helping us solve problems in the social world. For example, we speculate that the three dimensions identified here might inform calculations regarding the threat posed by others: Valence could indicate the probability of help or harm; social impact would help estimate the likely magnitude of that that help or harm; and rationality would indicate the likely method of its expression (e.g., harm through a devious plot vs. an explosion of rage).
The present study derived four potential dimensions of mental state representation—rationality, social impact, human mind, and valence—from the existing psychological literature. We discovered that three of these dimensions—rationality, social impact, and valence—predicted patterns of neural activity elicited across the social brain network by consideration of others’ mental states. By discovering which dimensions the brain spontaneously uses to organize the domain of mental states, we have forged a deeper understanding of both human social cognition and its relationship to our own internal mental experience. These findings both inform long-standing debates within social psychology about theory of mind and can be used to generate novel predictions about how the brain supports our ability to mentalize.
Materials and Methods
Participants.
Participants (N = 20) were recruited via the Harvard University Study Pool (16 female; mean age, 22.7 y; range, 18–27 y). A Monte Carlo simulation was used to determine participant and trial numbers consistent with adequate statistical power (SI Text). All participants were right-handed native speakers of English, reported no history of neurological problems, and had normal or corrected-to-normal vision. Participants provided informed consent in a manner approved by the Committee on the Use of Human Subjects at Harvard University.
Experimental Design.
Participants underwent functional neuroimaging while considering another person experiencing a variety of mental states. The task elicited patterns of neural activity that reflect the representation of each state. On each trial, participants considered 1 of 60 mental states (Table S1). At the onset of the trial, one mental state term was presented for 1 s. This word remained on screen while two very brief scenarios associated with that mental state appeared for 3.75 s, one on the lower left side of the screen and one on the lower right side. Participants were instructed to report which of the two scenarios they thought would better evoke the mental state in another person. Participants indicated their response using a button box in their left hand by pressing either the middle finger for the left scenario or their index finger for the right scenario. There were no correct answers because both scenarios were pretested to elicit the scenario in question. Each trial was followed by a minimum 250-ms fixation and a randomized jittered fixation period (mean 1.67 s, range 0–10 s, in 2.5-s increments). During scanning, participants saw each of the 60 mental states on 16 occasions. Each state was presented once per run over the course of 16 consecutive runs of 405 s each. Participants judged a unique pair of scenarios on each trial; each of 16 scenarios was used only twice over the course of the experiment. Stimuli were presented with PsychoPy (27).
The 60 mental states in this study were selected to maximize observable differences based on survey ratings from a separate set of participants (n = 1,205) (SI Text). Many of the theories under consideration made similar predictions about mental state representations. We pared down the information contained in the extant models using PCA. The PCA was conducted with respect to the 16 rating dimensions described above and the 60 mental states selected for the experiment. Varimax rotation was used to maximize the interpretability of the factors while maintaining their orthogonality (oblique rotation indicated that the orthogonal solution was satisfactory) (SI Text). Parallel analysis (28) and very simple structure (29) criteria were used to determine component number, with both indicating four factor solutions. The scenarios presented to subjects in this study were all written to be concise (fewer than five words), believable, devoid of personal pronouns, in the present tense, and maximally associated with their respective mental state. We selected an optimal set of scenarios using a genetic algorithm on survey ratings from a separate set of participants (n = 795) (SI Text).
Functional Imaging Procedure.
Functional data were acquired using a gradient-echo echo-planar pulse sequence with parallel imaging and prospective motion correction [repetition time, 2,500 ms; echo time (TE), 30 ms; flip angle, 90°) on a 3T Siemens Trio with standard 32-channel headcoil. Images were acquired using 43 axial, interleaved slices with a thickness of 2.5 mm and 2.51 × 2.51-mm in-plane resolution (field of view, 216 mm2; matrix size, 86 × 86 voxels; 162 measurements per run). Functional images were preprocessed and analyzed with SPM8 (Wellcome Department of Cognitive Neurology), using SPM8w. Data were first spatially realigned to correct for head movement and then normalized to a standard anatomical space (2-mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute).
A general linear model (GLM) was used to generate participant-specific patterns of activity for each mental state. The model included one regressor for each of the mental states, for a total of 60 regressors of interest. Events were modeled using a canonical hemodynamic response function and covariates of no interest (temporal and dispersion derivatives, session mean, run mean, linear trends, outlier time points, and six motion realignment parameters). Boxcar regressors for events began at the onset of the presentation of the mental state. GLM analyses resulted in 60 t-value maps, one for each mental state, for each participant. In essence, these maps embody the average neural representation of each state.
We compared neural representations at each voxel in the brain using a searchlight procedure (30). Patterns of activity for each of the 60 mental states were extracted from participant’s GLM-derived t-value maps using a spherical searchlight with 4-voxel radius (∼9 mm). To compare the similarity of activity patterns for different mental states, we computed the Pearson correlation between each pair of patterns. Thus, two mental states that elicited highly correlated patterns of activity across the searchlight were considered to be more similar to each other. This searchlight procedure resulted in neural similarity matrices at each point in the brain: 60 × 60 matrices whose elements correspond to the correlations between the patterns of neural activity within that searchlight.
We used these estimates of neural similarity to test whether mental states were represented in a manner predicted by the four PCA-derived dimensions. To do so, we made similarity predictions for each dimension with respect to each pair of mental states by taking the absolute difference in their scores on the dimension in question. Multiple regression was used to determine how well the predictions of the PCA-derived dimensions accounted for neural similarity. These regressions generated four maps of unstandardized regression coefficients for each participant, one for each component. The participant-specific maps were smoothed (Gaussian 6-mm FWHM kernel) and entered into random effects analysis using one-sample t tests. The four resulting t-value maps indicate regions of the brain in which differences in the neural patterns elicited by mental states correspond to the differences between mental states along each component. Results were corrected for multiple comparisons via a Monte Carlo simulation using the AFNI (31) 3dClustSim script (estimates of actual smoothness obtained from the four PCA maps and averaged; whole brain mask from the contrasts constrained voxel number). This simulation indicated that, with an uncorrected threshold P < 0.001, a 76-voxel extent was sufficient to yield a corrected threshold of P < 0.05. For visualization, statistical maps were rendered on the cortical surface using Connectome Workbench (32).
To test whether relevant patterns of activity were represented in a more distributed manner, we conducted an additional network-wide similarity analysis. In this analysis, we generated a single neural similarity matrix per participant based on the pattern of activity across an independently defined network of neural regions. This network was defined using a whole brain omnibus repeated-measures ANOVA across the 60 mental states and 20 participants, which selected any voxels that showed different levels of activity across mental states (Fig. 3A). Due to the sensitivity of this analysis, voxels were selected at a conservative voxelwise threshold of P < 0.0001. The univariate nature of this approach appeared adequate as similar regions emerge from split-half searchlight reliability (Fig. S6). Note that, whereas this feature selection relied on the same data subjected to MPVA, it was independent of any of the dimensions being tested and thus did not yield biased results. Indeed, the network analysis based on these voxels produced results nearly indistinguishable from the same analysis conducted using voxels selected via a metaanalysis of mentalizing studies (SI Text).
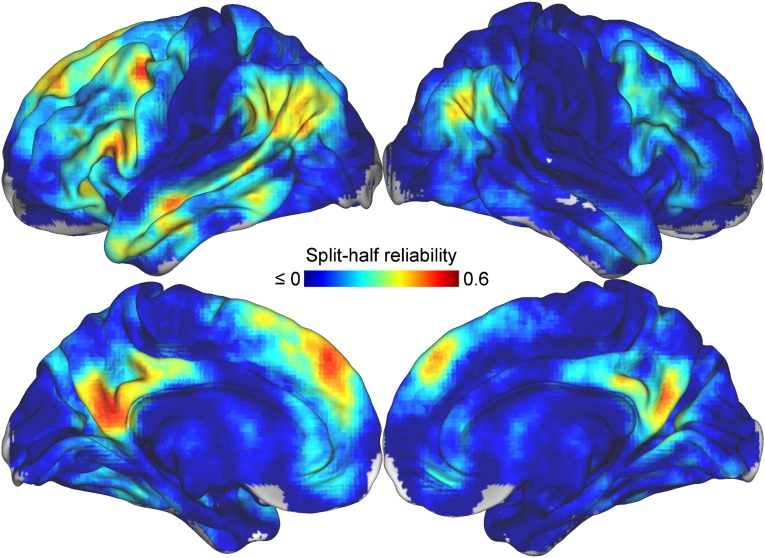
Fig. S6.
Reliability of similarity searchlights. The reliability of the neural representations of other’s mental states throughout the brain, calculated as the split-half correlation between pattern similarity estimates. Many regions typically implicated in theory of mind demonstrate relatively high reliability.
As with the searchlight analysis, in the network analysis, patterns of neural activity were extracted from the entirety of the feature selected area for each of the 60 mental states. These patterns were correlated to produce a single neural similarity matrix for each participant. These matrices were then averaged to produce a single group-level matrix. The group neural similarity matrix was Pearson-correlated with the similarity matrices generated from each of the four latent dimensions. To generate confidence intervals for these correlations, this procedure was repeated 10,000 times with group similarity matrices based on bootstrapped samples of the 20 participants.
We conducted analogous searchlight and network similarity analyses to test the seven theoretical models. The similarity between pairs of mental states was calculated as the (opposite of the) distance between the two mental states in the Euclidean space determined by the dimensions of each theory. This analysis diverged from that used for the PCA-based models only in that each theoretical model’s predictions were independently correlated with neural similarity. This divergence was due to the substantial collinearity between the models, which was absent from the PCA-based models.
SI Text
Power Analysis
A Monte-Carlo simulation in MATLAB 7 was conducted to establish a design with adequate statistical power. We simulated a behavioral similarity matrix and a neural similarity matrix that were correlated at the estimated population effect size of r = 0.15. This effect size was thought to be reasonable based on previous work (e.g., ref. 13). To generate the behavioral similarity matrix, we simulated activity in a single searchlight. That searchlight consisted of 200 voxels and 60 separate patterns of activity, to represent each of the mental states. The simulated “activity” within the voxels should be normally distributed (M = 0, SD = 1) as an approximation for the t-values used in the actual analysis. The 60 × 60 correlation matrix produced by this searchlight was considered the behavioral model. We created patterns of neural activity within the simulated searchlight by taking the 200 voxel-by-60 state matrix used to generate the behavioral model and adding additional random noise: ∼N(0, 2.4). When these neural patterns were correlated with one another, the resulting neural similarity matrix consistently correlated with the corresponding behavioral matrix at approximately r = 0.15. Because this neural pattern matrix reflects experiment-level data, we added additional noise ∼N(0, 10) to represent data from individual trials.
On each iteration of the simulation, a particular participant number and trial (per mental state) were set. Trial-wise neural patterns of searchlight activity were generated for each participant and averaged to produce a single pattern for each participant. These patterns were then converted to similarity matrices and correlated with the overall behavioral similarity matrix. The resulting r values were R-to-z transformed and entered into a t test across simulated participants. The result of this t test was tabulated to estimate power. Participant numbers between 2 and 30 and item numbers between 2 and 20 were simulated, with 100 simulation iterations at each combination of these parameters. These simulations indicated that 20 participants with 16 trials per mental state should be adequate to ensure 95% voxelwise statistical power at an uncorrected threshold of P < 0.001.
Mental State Selection
The 60 mental states in this study were selected to maximize observable differences and thus statistical power. To accomplish this outcome, a set of participants assessed how representative each of 166 mental states was of each of 16 univariate dimensions. Participants (n = 1,205) were recruited through Amazon Mechanical Turk and the Harvard University Study Pool to complete one or more of eight online surveys: emotion/reason (n = 145), mind/body (n = 140), agency/experience (n = 145), warmth/competence (n = 157), high/low arousal (n = 151), social/nonsocial (n = 137), positive/negative (n = 168), and shared/uniquely human (n = 153). In each survey, participants were provided with definitions of the two dimensions of interest and then were asked on each trial whether a particular mental state could be categorized along one, both, or neither of the two dimensions of interest in that survey. Across all participants, we could thus assess the proportion of trials in which each mental state was (or was not) associated with each dimension. This aggregation resulted in continuous ratings between 0 and 1 for each of the 166 mental states on each of 16 psychological dimensions. We used data from the ratings of the 166 mental states along the 16 nominal dimensions to select the optimal set of states. To do so, we ran the resulting 166 × 16 matrix of data through an optimization selection process, which iteratively selected a random subset of mental states (separately for subsets between 50 and 98), calculated how well they sampled each dimension using a Kolmogorov–Smirnov test (compared with a uniform distribution), calculated the redundancy of each dimension using Tolerance, and then, over 10,000,000 iterations, selected the solution that maximized the former and minimized the latter. The optimal solution of 60 mental states was used in the current study.
Analysis on Excluded Mental States
To ensure that the mental state selection process did not bias the factors derived from principal component analysis, an identical analysis was carried out with respect to the 106 mental states not included in the imaging experiment. Very simple structure indicated a four-factor solution to this analysis for the 106 mental states not included in this study as well. Factor order was not identical across the two solutions, but, when rearranged, including reflection where necessary, the factor loadings were reproduced with the following respective reliabilities (rs): 0.97, 0.96, 0.84, 0.95. The reliability of the solutions not only suggests that the 60-state model did not produce a biased factor structure, but also provides additional evidence for the importance of the identified factors.
The full set of mental states was also used to determine whether an orthogonal PCA rotation was appropriate. The 16 psychological dimensions were subjected to PCA across all 166 rated mental states, with four components retained. These components were then allowed to correlate with each other via an oblique direct oblimin rotation. The resulting factor correlation matrix indicated little tendency for the components to correlate. The highest correlation was between social impact and valence (r = 0.27), and the mean (absolute value) of the intercomponent correlations was r = 0.12. This result suggests that the orthogonal varimax rotation and its concomitant interpretational simplicity may be retained without substantially distorting the relationship between components.
Scenario Selection Algorithm
The scenarios presented to participants in this study were all written to be concise (fewer than five words), believable (e.g., “finding $5 on the sidewalk” rather than “winning the lottery”), devoid of personal pronouns, in the present tense, and maximally associated with their respective mental state. To select an optimal set of scenarios, a separate set of participants (n = 795) were recruited through Amazon Mechanical Turk and the Harvard University Study Pool to complete an online survey that assessed how well each mental state was associated with each scenario. On each trial, participants saw one of the 60 mental states selected using the procedure described above and one of 36 scenarios specific to that mental state. Their task was to rate the degree to which the mental state was associated with the scenario on a scale from 1 (mildly) to 5 (highly). Each participant was presented with 180 such items.
The sets of 16 scenarios for each mental state used in this study were selected (out of a larger set of 36 for each state) by a custom genetic algorithm using participant ratings. Genetic algorithms are optimization programs intended to achieve a desired result by mimicking the mechanisms of organic evolution by natural selection. The algorithm was initiated by randomly generating 100 “strains,” with strain defined as any set of 16 scenarios for each mental state. On each of 10,000 iterations, the “fitness” of each strain was evaluated (as described below), and strains were selected for reproduction proportional to their fitness raised to the power of 100 (to increase selection pressure) through stochastic universal sampling. Imitating sexual reproduction, two-parent two-child crossover of scenarios within mental state was used to generate a new generation of strains. In this process, each scenario in a “child” strain had an equal probability of being drawn from either of the two “parent” strains. During reproduction, each scenario also had 0.001 probability of “mutating” to a different scenario within the same mental state (even if that scenario appeared in neither of the parent strains). Additionally, the best strain from each generation persisted unchanged to avoid discarding a potential optimum solution.
The “fitness” in this algorithm was determined by four equally weight parts: (i) The scenarios should maximally evoke the mental state of interest—to ensure the appropriateness of each scenario to the mental state in question; (ii) they should minimize variability in how well different mental states are evoked—to make sure we were not left with many good examples of one state and bad examples of another; (iii) they should minimize variability in how variably scenarios evoke mental states across scenarios—to ensure that choices were not easier for one state (with high variability between scenarios) than another (with lower variability); and (iv) they should minimize average character length variability across mental states—to ensure that low level features such as size on retina did not differ across states. The strain with the best fitness at the end of the simulation dictated the scenarios ultimately used in the experiment.
Text Analysis of Scenarios
To control the scenarios more closely, an automated text analysis was performed to assess several midlevel linguistic properties. In particular, we aimed to control for the concreteness, complexity, and familiarity of the scenarios associated with each mental state. Concreteness and complexity norms were taken from large rating sets (33, 34), with the latter measured using age of acquisition as a proxy. Familiarity was based on the SUBTLEXus word frequency measure. For a given scenario, each word’s concreteness, complexity, and familiarity values were determined and then averaged to produce a single measure. These measures were then averaged across scenarios to provide a single score along each linguistic dimension for each of the 60 mental states.
The network-level representational similarity analysis was repeated after partialing out the influence of the linguistic variables on neural pattern similarity. In other words, each linguistic variable was converted to a set of similarity predictions by taking absolute differences, and neural pattern similarity was then regressed onto these predictions. The residuals of this regression were then correlated with the four dimensions derived from psychological theories. These correlations remained very similar to the values obtained before removing the influence of the linguistic variables: rationality (r = 0.15), social impact (r = 0.21), human mind (r = 0.05), and valence (r = 0.12). Moreover, the statistical significance (or lack thereof) at P < 0.05 of the PCA-derived dimensions remained unchanged. This result suggests that the influence of these linguistic features cannot account for the predictive power of the psychological dimensions.
ICA Network Representational Similarity Analysis
To further assess the robustness of the results to varying analytic techniques, we repeated the feature-selected network representational similarity analysis using dimensions extracted from the behavioral ratings via independent component analysis (ICA) instead of PCA. The four components that emerged from the ICA analysis of the 60 mental states closely resembled those extracted via PCA in terms of simple structure. Adjusting for changes in order, and ignoring arbitrary sign reflections, ICA and PCA components expressed the following correlations for rationality, social impact, human mind, and valence, respectively: rs = 0.86, 0.95, 0.94, and 0.89. The ICA components were converted to similarity predictions and correlated with neural pattern similarity from the ANOVA-selected social brain network. Unsurprisingly, given the high correlations with the PCA components, similar correlations with the neural data obtained: rationality (r = 0.10), social impact (r = 0.23), human mind (r = 0.03), and valence (r = 0.18). The statistical significance of these values—as determined through bootstrapping participants—did not differ qualitatively from that reported for the PCA dimensions.
Spearman Correlation Network Analysis
Again, to assess the robustness of the results reported in the main text, we repeated the representational similarity analyses on the feature-selected network, this time using Spearman rank correlation instead of Pearson correlation to associate the behavioral and neural data. Spearman correlations have the advantages of being more robust to outliers than Pearson correlations, and also of being able to detect any (monotonic) nonlinear relationships that might exist. The analysis, including bootstrapping, otherwise proceeded exactly as in the original Pearson case. Results were nearly indistinguishable from those in the Pearson correlation analysis: rationality (r = 0.13), social impact (r = 0.22), human mind (r = 0.04), and valence (r = 0.12). For the original theories, correlations also remained quite stable: valence and arousal (r = 0.19), warmth and competence (r = 0.16), agency and experience (r = 0.19), emotion and reason (r = 0.17), mind and body (r = 0.18), social and nonsocial (r = 0.04), and shared and unique (r = 0.03). The statistical significance of the results remained unchanged. This outcome suggests that neither outliers nor nonlinearity is likely to have contributed substantially to the results we present.
Interdimensional Interaction Network Analysis
The primary network analysis we present features only the main effects of the four PCA-derived dimensions. It is quite possible that these dimensions interact with each other in terms of their influence on neural pattern similarity. Such an interaction would effectively indicate that the importance of one dimension for distinguishing mental states changes depending on how different those states were along another dimension. To test for this possibility here, we repeated the ANOVA-selected network representational similarity analysis, including interaction effects. Although the possibility exists for highly complex interactions between the four dimensions, we considered only two-way interactions for the sake of interpretability and limiting multiple comparisons.
As before, the four dimensions were converted to similarity predictions by taking the absolute distance between points. These distances were then z-scored and multiplied to produce the six possible two-way interaction terms. The main effects and interaction terms were simultaneously entered into a multiple regression predicting neural similarity. The resulting coefficients were bootstrapped across participants—as in the original correlation analyses—to determine their statistical significance. The main effects of rationality, social impact, and valence remained statistically significant whereas the main effect of human mind remained nonsignificant. In addition, three interaction terms emerged as significant at the P < 0.05 level: rationality × human mind [b = −0.003, 95% bootstrap CI (−0.004, −0.002)], social impact × human mind [b = −0.002, 95% bootstrap CI (−0.004, −0.001)], and social impact × valence [b = 0.001, 95% bootstrap CI (0.0005, 0.002)].
Interestingly, two of these results involve the human mind despite the fact that this variable has no main effect on neural pattern similarity. This result may help clarify the role of this dimension with respect to mental state representation. Rather than distinguishing between mental states, the human mind may primarily play a modulatory role, moderating the importance of other dimensions on mental state representation. In both cases, these interactions were negative; thus, they can be interpreted as follows: The more mental states differ in terms of their human mind, the fewer differences in rationality and social impact predict neural pattern differentiation. The third interaction term runs in the opposite direction: The greater the difference in social impact between two mental states, the more differences in valence can predict pattern differences.
Network Analysis with Alternative Feature Selection
The feature selection approach we used to isolate the social brain network was based on a univariate ANOVA over the same data analyzed in the subsequent representational similarity analysis. This ANOVA is agnostic to the theories we are testing and not statistically biased to produce a spurious correlation between neural similarity and the behavioral ratings. It is in essence similar to choosing voxels based on their univariate reliability. A biased, circular analysis selects voxels based on their correlation with the external measure with which they will ultimately be correlated; here, our approach selected voxels based on their correlation with themselves (i.e., their reliability) producing an orthogonal contrast.
Nonetheless, we consider it worthwhile to repeat the network analysis using a completely independent feature selection method. In this case, we turned to Neurosynth (neurosynth.org/), an online tool for performing automated metaanalyses over large numbers of imaging studies. Using the “theory mind” reverse inference map, Neurosynth produced a metaanalysis of 140 studies involving theory of mind. We used this map [at the default false discovery rate (FDR) q = 0.01] to produce a feature selection mask. This approach has the virtues of being both completely statistically independent of our current data, and also coming about as close as possible to producing a set of canonical voxels involved in thinking about other minds. From this point on, the representational similarity analysis proceeded as previously described in Functional Imaging Procedure in the main text. The correlations between the PCA-derived dimensions and neural similarity within the network selected by metaanalysis remained similar to the values previously observed, with no changes in statistical significance at the P < 0.05 level: rationality [r = 0.19; 95% bootstrap CI (0.08, 0.22)], social impact [r = 0.20; 95% bootstrap CI (0.10, 0.24)], human mind [r = 0.02; 95% bootstrap CI (−0.03, 0.08)], and valence [r = 0.10; 95% bootstrap CI (0.02, 0.15)]. The fact that these values have not uniformly decreased serves as further evidence for the unbiased nature of the ANOVA feature selection technique. Results from the seven original theories also remained qualitatively unchanged: valence and arousal [r = 0.16; 95% bootstrap CI (0.8, 0.21)], warmth and competence [r = 0.17; 95% bootstrap CI (0.07, 0.21)], agency and experience [r = 0.23; 95% bootstrap CI (0.12, 0.25)], emotion and reason [r = 0.21; 95% bootstrap CI (0.10, 0.24)], mind and body [r = 0.16; 95% bootstrap CI (0.08, 0.20)], social and nonsocial [r = 0.04; 95% bootstrap CI (−0.03, 0.10)], and shared and unique [r = 0.02; 95% bootstrap CI (−0.02, 0.05)].
Multidimensional Scaling
Nonmetric multidimensional scaling (MDS) was used to estimate the proportion of variance in representational space of mental states that could be explained by the three significant PCA-derived dimensions from the representational similarity analysis. We took this approach to better assess the proportion of variance our model explains in the true dimensions underlying mental state representation. The raw R2 between the similarity predictions of the PCA-derived dimensions and neural similarity estimates would systematically underestimate this quantity by a quadratic factor, leading us to use this MDS approach. A 5D scaling yielded an acceptable stress (0.13) below the conventional threshold of 0.15. Unfortunately, due to the arbitrary orientation of MDS solutions and the high dimensionality of this particular solution, it is beyond the scope of this article to explore the nature of the unidentified dimensions. However, we do present the results of a 2D scaling solution to allow the reader to explore the neural similarity more directly (Fig. S2). The relative importance of the five dimensions was assessed by regressing the original dissimilarity matrix onto similarity predictions made by the five MDS dimensions and partitioning the resulting R2. These estimates were normalized by the total R2 of the regression and later used as weights. The 5D scaling dimensions were then individually regressed onto the three significant PCA-based dimensions from the network analysis. The resulting R2 values were summed into a final estimate of total R2, with weights based upon the relative importance of each dimension to overall neural similarity as calculated via regression of the MDS dimensions onto the original neural dissimlarity matrix.
Model Selection by Cross-Validated Performance
The principal aim of this study has been to establish which psychological dimensions contribute to shaping the neural representation of mental states. To that aim, we have used the standard null hypothesis significance testing framework to assessing the contribution of each of our four PCA-derived dimensions in turn. However, it is also worth considering a model selection technique that holistically assesses the representational spaces dictated by different possible combinations of our hypothetical dimensions. Moreover, such a technique need not necessarily rely on the concept of statistical significance to determine which dimensions ought to be included in the optimal model. Instead, it might rely on cross-validated prediction performance as a measure of support for different potential models.
To this end, we conducted an alternative to the network representational similarity analysis described in the main text. This technique proceeded as follows. First, a set of dimensions consisting of between one and four of the PCA-derived dimensions was selected. This step was repeated exhaustively to ultimately include all possible unique combinations of principal components (PCs) (15 in total). Next, the regression coefficients were simultaneously estimated for all of the selected dimensions with respect to the neural data using nonnegative linear least squares. Squared similarity values were used for both behavioral and neural data to allow for later comparison with models containing nonorthogonal dimensions (distances in Euclidean spaces do not sum unless dimensions are orthogonal, but their squares do, regardless). The regression coefficients estimated using this approach with the 19 “training” participants were used to weight the squared behavioral similarity values from each dimension. These values were then summed to form a single predictor for the neural similarity space. This predictor was then correlated with the squared neural similarity values of the left-out participant to produce a measure of predictive performance. This process was repeated leaving out each participant in turn, and the 20 correlations for each combination of PCs were averaged to produce an overall measure of model performance for that combination. The cross-validated performance of all possible PC combinations could then be directly compared. Consistent with the findings from the earlier representational similarity analyses, the highest performing model consisted of the three dimensions of rationality, social impact, and valence. This model achieved a cross-validated r = 0.12 (note that this value is considerably lower than those previously reported because it corresponds to the prediction of an individual participant rather than the group average).
This cross-validation approach also provided an alternative approach to comparing the PCA-based model with the original theoretical models. This approach might favor the original models more than the representational similarity analysis reported earlier because it allows the weights of their dimensions to differ rather than effectively fixing them to equality. The original theoretical models achieved the following performance: valence and arousal (r = 0.07), warmth and competence (r = 0.06), agency and experience (r = 0.07), emotion and reason (r = 0.07), mind and body (r = 0.06), social and nonsocial (r = 0.02), and shared and unique (r = 0.01). Although again the values are smaller due to the fact that they apply to single participants, the overall pattern of results remains qualitatively quite similar, with most of the original models performing in the r = 0.06–0.07 range and while sociality and human uniqueness perform close to r = 0. Unsurprisingly, the best PCA-derived model also retained its substantial lead over the original theories in explaining neural pattern similarity (Fig. S3).
A noise ceiling for cross-validated model performance was obtained by iteratively correlating the neural similarity matrix of each participant with the average neural similarity matrix of the other 19. The result was a ceiling of r = 0.28. This ceiling is an indication of the expected single-participant predictive performance of a “perfect” or “complete” model of mental state representation, as approximated by the group average similarity matrix. Consistent with the R2 results reported in the main text, comparison of the noise ceiling with the performance of the best “3 PC” model indicates that the dimensions of rationality, social impact, and valence explain slightly less than half of the neural representation of others mental states. Although we consider this result surprisingly high given the early state of this research, this result also highlights the need to uncover the dimensions that can explain the remaining variance in mental state representation.
Split-Half Searchlight Reliability Mapping
We performed a whole brain mapping of the split-half reliability of searchlight similarity matrices. The value of this analysis is twofold. First, it illustrates which regions contain patterns with reliable structure with respect to mental states, agnostic to any particular theory regarding their representation. Second, it allows us to determine whether differing effects across the cortical surface might be attributed to differences in correlation attenuation. For instance, the proportion of variance explained by a particular psychological dimension, such as valence, might be the same across two regions with differing correlation between valence and neural similarity if the reliability of the neural similarity differs between the regions. A less reliable region would produce greater correlation attenuation, reducing the apparent effect size of valence whereas a more reliable region would be relatively disattenuated, and therefore manifest a larger effect, all else equal. Thus, the ultimate correlation between neural and behavioral similarity in a particular region might be viewed as the result of two different qualities: pattern similarity reliability, which reflects the degree to which a region represents mental state at all, and the disattenuated brain–behavior correlation, which indicates how well any mental state representation is modeled by the behavioral ratings in question.
To perform the whole brain reliability mapping, representational similarity matrices were calculated for each searchlight in the brain of each participant. The participants were then randomly divided into two groups of 10, and the similarity matrices from corresponding voxels were averaged within each half. The averaged similarity matrices were then correlated across the halves to produce split-half reliability values at each point in the brain. The resulting values were visualized on the cortical surface using Connectome Workbench (Fig. S6). The results from this mapping were highly consistent with those from the univariate feature selection ANOVA, as might be expected. In general, regions associated with social cognition, such as medial prefrontal and parietal cortices, the TPJ, and the ATL, demonstrated high representational reliability whereas other regions, such as visual and somatosensory cortices, did not.
Parallel Univariate Whole Brain Mapping
To supplement the MVPA, we also carried out whole brain univariate analyses with respect to the four PCA-derived dimensions. To maximize the parallelism between these analyses and the MVPA, we used the same contrast maps produced by the MVPA GLM (i.e., one pattern of betas for each of the 60 mental states for each participant). These maps were smoothed with a 6-mm FWHM Gaussian kernel and then entered into a multiple regression analysis. At each voxel, activity levels from the 60 mental states were regressed onto the scores of the four PCA-derived dimensions. Whole brain regression maps were combined across participants via voxelwise t tests. The same voxelwise and cluster correction thresholds were retained for consistency with the multivariate results.
The results were visualized on the cortical surface using Connectome Workbench (Fig. S4). The spatial distribution of univariate activity was largely similar to that of the searchlight information mapping, albeit generally less extensive. One deviation is worthy of particular consideration, however, because it may reconcile an apparent discrepancy between our findings and other results. Although the MVPA results indicate no relationship between VMPFC patterns and valence, we do observe a univariate correlation, such as more positive mental states elicit greater activity in this region. The latter finding is consistent with other work on mental state representation and VMPFC’s role in reward and value more generally.
Analysis of Residuals
To determine where the PCA-based representational similarity analysis was failing to account for differences between mental states, we constructed a representational dissimilarity matrix of the residuals from a multiple regression featuring the three significant dimensions (Fig. S4). Additionally, we calculated the average residual for each mental state and correlated these averaged residuals with three significant PCs. The rationality of a mental state did not predict whether its pattern was chronically predicted to be more or less different from that of other states (r = −0.03). The pattern dissimilarity between negative states tended to be slightly overestimated (r = 0.18). Finally, pattern dissimilarity between highly socially impactful states tended to be substantially underestimated (r = −0.66).
Acknowledgments
We thank Talia Konkle, Brenda Li, Radhika Rastogi, Eve Wesson, and Ava Zhang. D.I.T. was supported by NIH Blueprint for Neuroscience Research Training Grant T90DA022759. M.A.T. and J.M.C. were supported by Graduate Research Fellowships from the National Science Foundation (DGE 1144152). M.A.T. was also supported by The Sackler Scholar Programme in Psychobiology. The views expressed in this article do not necessarily reflect the views of the General Services Administration or the United States Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The neuroimaging data have been deposited in the Harvard University Dataverse, https://dataverse.harvard.edu (accession ELLLZM).
See Commentary on page 19.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511905112/-/DCSupplemental.
References
- 1.Konkle T, Caramazza A. Tripartite organization of the ventral stream by animacy and object size. J Neurosci. 2013;33(25):10235–10242. doi: 10.1523/JNEUROSCI.0983-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JP. Contributions of functional neuroimaging to the study of social cognition. Curr Dir Psychol Sci. 2008;17(2):142–146. [Google Scholar]
- 3.Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage. 2009;48(3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 2005;17(3):715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell JA. A circumplex model of affect. J Pers Soc Psychol. 1980;39(6):1161–1178. [Google Scholar]
- 6.Cuddy AJ, Fiske ST, Glick P. Warmth and competence as universal dimensions of social perception: The stereotype content model and the BIAS map. Adv Exp Soc Psychol. 2008;40:61–149. [Google Scholar]
- 7.Fiske ST, Cuddy AJ, Glick P, Xu J. A model of (often mixed) stereotype content: Competence and warmth respectively follow from perceived status and competition. J Pers Soc Psychol. 2002;82(6):878–902. [PubMed] [Google Scholar]
- 8.Gray HM, Gray K, Wegner DM. Dimensions of mind perception. Science. 2007;315(5812):619. doi: 10.1126/science.1134475. [DOI] [PubMed] [Google Scholar]
- 9.Forstmann M, Burgmer P. Adults are intuitive mind-body dualists. J Exp Psychol Gen. 2015;144(1):222–235. doi: 10.1037/xge0000045. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JP. Social psychology as a natural kind. Trends Cogn Sci. 2009;13(6):246–251. doi: 10.1016/j.tics.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britton JC, et al. Neural correlates of social and nonsocial emotions: An fMRI study. Neuroimage. 2006;31(1):397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Haslam N. Dehumanization: An integrative review. Pers Soc Psychol Rev. 2006;10(3):252–264. doi: 10.1207/s15327957pspr1003_4. [DOI] [PubMed] [Google Scholar]
- 13.Kriegeskorte N, Mur M, Bandettini P. Representational similarity analysis: Connecting the branches of systems neuroscience. Front Syst Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khaligh-Razavi SM, Kriegeskorte N. Deep supervised, but not unsupervised, models may explain IT cortical representation. PLOS Comput Biol. 2014;10(11):e1003915. doi: 10.1371/journal.pcbi.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osgood CE, Suci GJ, Tannenbaum PH. The Measurement of Meaning. Univ of Illinois Press; Oxford, England: 1957. [Google Scholar]
- 16.Kahneman D. Maps of bounded rationality: Psychology for behavioral economics. Am Econ Rev. 2003;93(5):1449–1475. [Google Scholar]
- 17.Heckhausen H, Gollwitzer PM. Thought contents and cognitive functioning in motivational versus volitional states of mind. Motiv Emot. 1987;11(2):101–120. [Google Scholar]
- 18.Corradi-Dell’Acqua C, Hofstetter C, Vuilleumier P. Cognitive and affective theory of mind share the same local patterns of activity in posterior temporal but not medial prefrontal cortex. Soc Cogn Affect Neurosci. 2014;9(8):1175–1184. doi: 10.1093/scan/nst097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mende-Siedlecki P, Cai Y, Todorov A. The neural dynamics of updating person impressions. Soc Cogn Affect Neurosci. 2013;8(6):623–631. doi: 10.1093/scan/nss040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell JP, Cloutier J, Banaji MR, Macrae CN. Medial prefrontal dissociations during processing of trait diagnostic and nondiagnostic person information. Soc Cogn Affect Neurosci. 2006;1(1):49–55. doi: 10.1093/scan/nsl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: Social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12(4):508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- 23.Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98(3):1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- 24.Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12(12):2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- 25.Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. J Neurosci. 2010;30(30):10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skerry AE, Saxe R. A common neural code for perceived and inferred emotion. J Neurosci. 2014;34(48):15997–16008. doi: 10.1523/JNEUROSCI.1676-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peirce JW. PsychoPy: Psychophysics software in Python. J Neurosci Methods. 2007;162(1-2):8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- 29.Revelle W, Rocklin T. Very simple structure: An alternative procedure for estimating the optimal number of interpretable factors. Multivariate Behav Res. 1979;14(4):403–414. doi: 10.1207/s15327906mbr1404_2. [DOI] [PubMed] [Google Scholar]
- 30.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA. 2006;103(10):3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 32.Marcus DS, et al. Informatics and data mining tools and strategies for the human connectome project. Front Neuroinform. 2011;5:4. doi: 10.3389/fninf.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brysbaert M, Warriner AB, Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behav Res Methods. 2014;46(3):904–911. doi: 10.3758/s13428-013-0403-5. [DOI] [PubMed] [Google Scholar]
- 34.Kuperman V, Stadthagen-Gonzalez H, Brysbaert M. Age-of-acquisition ratings for 30,000 English words. Behav Res Methods. 2012;44(4):978–990. doi: 10.3758/s13428-012-0210-4. [DOI] [PubMed] [Google Scholar]