Abstract
Fetal exposure to endocrine disruptors (EDs) is believed to predispose males to reproductive abnormalities. Although males are exposed to combinations of chemicals, few studies have evaluated the effects of ED mixtures at environmentally relevant doses. Our previous work showed that fetal exposure to a mixture of the phytoestrogen genistein (GEN) and the plasticizer di-(2-ethylhexyl) phthalate (DEHP) induced unique alterations in adult testis. In this follow-up study, we examined Postnatal Day 3 (PND3) and PND6 male offspring exposed from Gestational Day 14 to parturition to corn oil, 10mg/kg GEN, DEHP, or their combination, to gain insight into the early molecular events driving long-term alterations. DEHP stimulated the mRNA and protein expression of the steroidogenic enzyme HSD3B, uniquely at PND3. DEHP also increased the mRNA expression of Nestin, a Leydig progenitor/Sertoli cell marker, and markers of Sertoli cell (Wt1), gonocyte (Plzf, Foxo1), and proliferation (Pcna) at PND3, while these genes were unchanged by the mixture. Redox (Nqo1, Sod2, Sod3, Trx, Gst, Cat) and xenobiotic transporter (Abcb1b, Abcg2) gene expression was also increased by DEHP at PND3, while attenuated when combined with GEN, suggesting the involvement of cellular stress in short-term DEHP effects and a protective effect of GEN. The direct effects of GEN and mono-(2-ethylhexyl) phthalate, the principal bioactive metabolite of DEHP, on testis were investigated in PND3 organ cultures, showing a stimulatory effect of 10 μM mono-(2-ethylhexyl) phthalate on basal testosterone production that was normalized by GEN. These effects contrasted with previous reports of androgen suppression and decreased gene expression in perinatal rat testis by high DEHP doses, implying that neonatal effects are not predictive of adult effects. We propose that GEN, through an antioxidant action, normalizes reactive oxygen species-induced neonatal effects of DEHP. The notion that these EDs do not follow classical dose-response effects and involve different mechanisms of toxicity from perinatal ages to adulthood highlights the importance of assessing impacts across a range of doses and ages.
Keywords: endocrine disruptor; gene expression; genistein; germ cells; gonadal function; Leydig; mixture; phthalate; rodents (mice, guinea pigs, rats, voles); ROS; Sertoli; testis; toxicology
INTRODUCTION
The developmental origins of disease hypothesis states that adverse events or influences early in life can lead to permanent changes in physiology and predispose adults to disease [1, 2]. In that regard, fetal and neonatal exposure to endocrine disruptors (EDs) is thought to contribute to reported declines in male reproductive potential and increased incidence of developmental abnormalities such as testicular cancer and genital birth defects. A concerning 15% of couples have primary infertility, up to half of which can be attributed solely to male cause, and an estimated 40% of men are currently presenting sperm counts in the subfertile range [3, 4]. Evidence suggests that semen quantity and quality have been decreasing steadily since early 19th century, alongside increasing incidence of cryptorchidism and hypospadias, now affecting 2%–9% and 0.2%–1% of male newborns, respectively [5]. These aberrations, collectively termed testicular dysgenesis syndrome, have shared risk factors and are thought to arise from alterations in fetal androgen levels, androgen/estrogen balance or hormone action, and impaired development and reprogramming of progenitors during a developmentally sensitive fetal and neonatal window [3, 5–12].
Following SRY-induced sex determination of the fetus, somatic cells proliferate and differentiate to form supporting Sertoli and steroidogenic Leydig cells and an environment suitable for fetal primordial germ cells. Fetal Sertoli and Leydig cells play a fundamental role in generating hormones required for differentiation of the urogenital tract and testis decent, including androgens, anti-Mullerian hormone (AMH), and insulinlike growth factor 3 (INSL3) [13]. Once in the interstitium of the gonad primordia, germ cells, now termed gonocytes, undergo active DNA remethylation and establishment of new paternal imprints prior to forming a critical pool of renewable spermatogonial stem cells that will support spermatogenesis throughout adulthood [14–17]. Spermatogenesis is regulated by factors produced by Sertoli cells, themselves dependent on the gonadotropin FSH and on Leydig cell-produced testosterone (T). Interestingly, adult Leydig cells are not derived from fetal Leydig cells, but rather from neonatal mesenchymal precursors [18, 19]. It is therefore thought that affecting the pool of stem Leydig precursors, either directly or indirectly by altering the fetal testis environment, can ultimately impair adult Leydig development and function.
The default developmental program of the fetus is female and largely hormone independent, whereas hormone production is absolutely necessary for masculinization [20]. The masculinization programming window is driven principally by fetal Leydig T production, and in humans, it begins at 8 wk gestation, peaks around 12–14 wk, and steadily declines after 20 wk [21]. Correspondingly, well-established rat developmental models have an equivalent window with T production starting at Gestational Day (GD) 14.5–15.5, peaking around GD18.5–19.5, and declining shortly after parturition [21]. Thus, the hormonal dependence and developmental intricacy of the fetal period, in combination with a comparatively permeable fetal skin surface, minimal detoxifying capacity, and lack of blood-testis barrier, make the developing male gonads particularly sensitive to EDs capable of crossing the placenta [5, 9, 10, 20]. Interestingly, several common environmental pollutants, including derivatives of paints, plastics, and resins as well as natural soy derived phytoestrogens, have been detected in amniotic fluid, cord blood, breast milk, and semen, and have also been shown to possess antiandrogenic or estrogenic properties [22–26].
Although EDs have been extensively studied in the context of single high-dose chemical exposures, few studies have examined the additive or synergistic effects of ED coexposure during critical periods of development. In reality, humans and animals are exposed not to one, but a myriad of potentially harmful substances throughout their lifetimes. Previous work from our laboratory demonstrated that environmentally relevant in utero coexposure to two common EDs, the plant derived phytoestrogen, genistein (GEN), found in soy-based infant formula and antiandrogenic plasticizer, di-(2-ethylhexyl) phthalate (DEHP), primarily used in the production of polyvinyl chloride plastics, can induce alterations in testis development different or potentially more harmful than exposure to a single chemical at the same dose [27]. GEN is reported to have broad biochemical interactions, including estrogenicity, peroxisome proliferator-activated receptor (PPAR) activation, direct or indirect antioxidant action, and modulation of important signaling molecules and DNA methylation [28–30]. DEHP or its principal bioactive metabolite, mono-(2-ethylhexyl) phthalate (MEHP), has been demonstrated to act as an antiandrogen via PPARs and alters DNA methylation status and estradiol-regulated proteins [31–34].
The current follow-up study was conducted to evaluate GEN and DEHP acute toxicity in early postnatal animals and gain insight into the early cellular and molecular events driving long-term changes. We hypothesized that early postnatal hormonal alterations and reproductive toxicity of this ED mixture would correlate with or at least clarify the roots of the long-term effects and that the mixture would pose a greater risk than individual compounds. A combination of in vivo, ex vivo, and in vitro approaches in rat, however, demonstrated that these EDs do not follow classical dose-response effects and involve different mechanisms of toxicity from perinatal ages to adulthood.
MATERIALS AND METHODS
Treatments and Tissue Collection
Pregnant Sprague Dawley rats were purchased from Charles River Laboratories and kept on a 12L:12D photoperiod with ad libitum access to food and water. To address early life alterations in testis development and function, pregnant Sprague Dawley rats were treated from GD14 to parturition by gavage with either control corn oil or corn oil + GEN, DEHP, or GEN + DEHP, both used at a dose of 10 mg/kg, a dose within the range of human exposure. This dose was selected from previous dose-response studies in which we had examined the short- and long-term effects of in utero exposure to GEN or DEHP used separately [35–39]. These studies had shown that a fetal exposure to 10 mg/kg/day GEN did not induce changes in circulating T nor germ cell numbers in adult rats [37], while long-term effects of DEHP were seen at much higher doses [35, 36].
Gestationally treated male offspring were raised on a normal diet to defined ages, euthanized, and analyzed. Treatment doses were adjusted according to changes in dam weights. Male offspring were euthanized at specific postnatal ages to evaluate testis function and development, including fetal Leydig activity and the initiation of gonocyte proliferation/migration on Postnatal Day 3 (PND3) and differentiation to form a critical pool of spermatogonial stem cells (PND6) following in utero exposure. Testes were collected, weighed, and either snap frozen and fixed in paraformaldehyde or dissected for organotypic cultures (each end-point was determined using randomly selected offspring of four dams [N] per treatment, where N is defined as the number of offspring from independent litters). Animals were handled according to protocols approved by the McGill University Animal Care and Use Committee.
RNA Extraction and Quantitative Real-Time PCR
Testis RNA was extracted using QIAGEN RNeasy Plus Mini kit (Qiagen). Complementary DNA was synthesized from isolated RNA using the single-strand cDNA transcriptor synthesis kit (Roche Diagnostics). Quantitative real-time PCR (qPCR) was performed as previously described using the LightCycler 480 Real-Time PCR System (LC480; Roche Diagnostics) [40]. Alpha tubulin was used as an endogenous control and for normalization of gene targets. A minimum of three male offspring from different litters were evaluated in triplicate. The comparative Ct method was used to calculate relative expression. Data are represented as mean relative mRNA expression in arbitrary units. Complete primer sequences for all gene targets can be found in Table 1.
TABLE 1.
Primer sets used for quantitative real-time PCR.
BTB, BR-C, ttk and bab domain (found in Zinc finger proteins).
Histology, Hematoxylin and Eosin Staining, and Immunohistochemistry
Testis from male offspring were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin or used for immunohistochemical analysis as previously described [37]. Briefly, tissue sections were dewaxed and rehydrated, followed by antigen retrieval using a DAKO solution (code #S1699). Primary antibodies, HSD3B (ab65156; Abcam) and NQO1 (ab28947; Abcam), were incubated at 1:300 and 1:100 dilutions, respectively, overnight at 4°C and revealed using species-appropriate secondary antibodies (mouse or rabbit), horseradish peroxidase colorimetry using the horseradish peroxidase chromogen 3-amino-9-ethylcarbazole, and hematoxylin counterstaining. Representative images are shown. For quantification, digital images of stained sections were loaded into ImageJ analysis software and RGB stacks/montages were generated (n ≥ 3). Uniquely for HSD3B quantification, seminiferous tubules were manually removed using the cut feature, leaving on the interstitium. The threshold feature was adjusted to specifically identify positive red/brown immunostaining. Once set, identical settings were used for all the images quantified. Percent of positive staining relative to total area was calculated using the measure feature and subsequently expressed in terms of fold change over control.
Ex Vivo and In Vitro Testis Organ Culture
Ex vivo organ culture of PND3 testis was performed to evaluate basal and hormone-stimulated androgen production following in utero exposure as reported previously [35]. Briefly, PND3 testes were excised and cut into 8–10 small fragments, placed on filter papers and cultured on trans-well inserts in normal Dulbecco-modified Eagle medium (GIBCO by Life Technologies) or Dulbecco-modified Eagle medium + hCG for 3 days. Supernatant containing steroids was collected daily and replenished with new medium. Testosterone levels in supernatant were measured by radioimmunoassay (RIA), expressed in ng/testis/3 days or fold change relative to control. In vitro organ culture of testes from PND3-untreated rats was performed in the presence of either 0.2% dimethyl sulfoxide (DMSO) serving as the control, GEN, MEHP, or GEN + MEHP at 10 μM. A paradigm similar to that described above was followed, with T measured by RIA. After organ cultures, testis fragments were collected, fixed, and sent for embedding and cutting. Histological and morphological alterations were evaluated following hematoxylin and eosin counterstaining.
Statistical Analysis
All the statistical analysis was performed using unpaired two-tailed t-test with statistical analysis functions in GraphPad Prism version 5.0 software (GraphPad Inc.). Asterisks indicate a significant change relative to control (P ≤ 0.05).
RESULTS
In Utero GEN Exposure Antagonizes Proandrogenic Effects of DEHP in Young Male Offspring
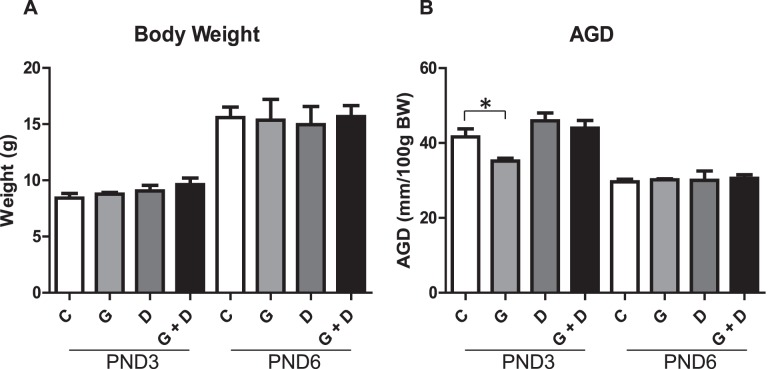
Litters of treated dams did not display any abnormalities in pup number or sex ratio (data not shown). Examination of PND3 and PND6 body weights (Fig. 1A) and testes weights (data not shown) revealed no significant differences between control and treated animals. Anogenital distances (AGD) normalized to body weight (Fig. 1B) were significantly reduced uniquely in PND3 GEN but not DEHP or combined GEN and DEHP offspring, suggesting early feminizing effects. Initial alterations in AGD were resolved with age, however, because no significant alterations were observed at PND6 in any treatment group. The overall tissue morphology appeared normal (data not shown).
FIG. 1.
Effects of in utero exposure to genistein (GEN) and DEHP on general and reproductive health parameters. PND3 and PND6 average body weight (A) and anogenital distance (AGD) normalized to body weight (B). Data are represented as mean measurements (± SEM) of parameters measured in the offspring of four dams per treatment. Asterisk indicates a significant difference relative to control in respective age groups (P ≤ 0.05); C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
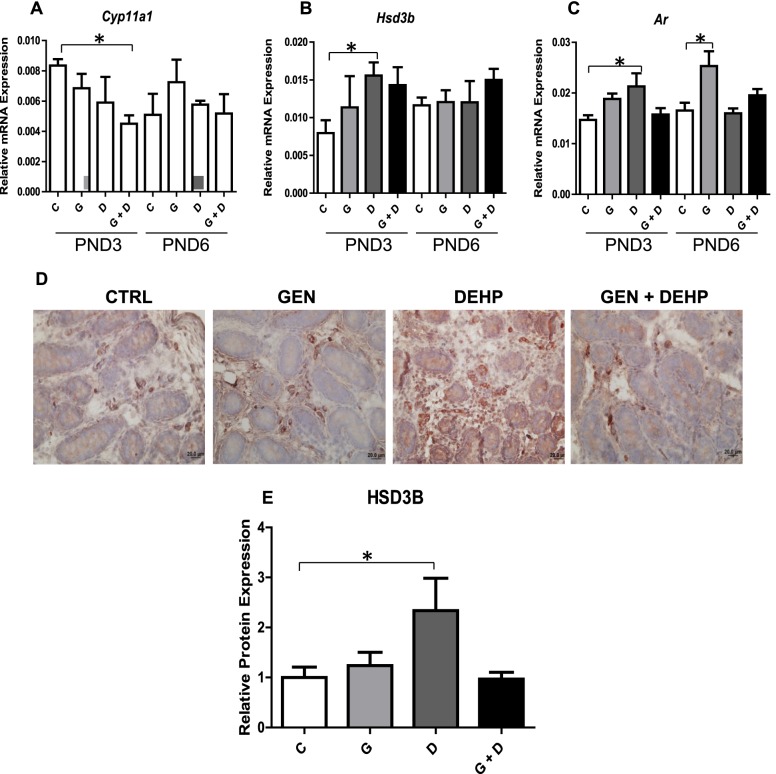
The qPCR analysis of PND3 and PND6 testes revealed a significant decrease in Cyp11a1 (Fig. 2A) only in combined GEN and DEHP-treated PND3 testes. Two other steroidogenenic mediators, Hsd3b (Fig. 2B) and Ar (Fig. 2C), were increased uniquely in PND3 DEHP-treated animals, suggesting an early proandrogenic effect of DEHP that is resolved by cotreatment with GEN. Similar trends were not observed in PND6 testes, with Ar (Fig. 2C) being the only significantly increased gene, unique to GEN-treated offspring. Alterations in PND3 Hsd3b mRNA were further validated at the protein level by immunohistochemistry and subsequent quantitative image analysis (Fig. 2, D and E). Consistent with gene expression data, HSD3B stained strongest in the interstitial Leydig cells of DEHP-treated PND3 testis (Fig. 2D). Quantitative image analysis of interstitial HSD3B also revealed a significant increase only in PND3 DEHP-treated offspring relative to control (Fig. 2E).
FIG. 2.
Effects of in utero exposure to genistein (GEN) and DEHP on steroidogenic mediators. Relative mRNA expression of steroidogenic enzymes Cyp11a1 (A) and Hsd3b (B), and androgen receptor (Ar) (C) in PND3 and PND6 testes. Data are expressed as mean relative mRNA levels ± SEM normalized to alpha-tubulin (n = 4 per treatment). To further validate mRNA alterations, immunohistochemical analysis of HSD3B (D) was performed on PND3 testes and positive interstitial staining was quantified by image analysis (E); photos taken at 40× magnification. Immunohistochemical quantification is represented by mean fold change ± SEM of HSD3B staining relative to control (n ≥ 3). Omission of the primary antibody was used as a negative control (data not shown). Representative pictures are presented. Asterisks in both mRNA and protein analyses indicate a significant difference relative to control (P ≤ 0.05); C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
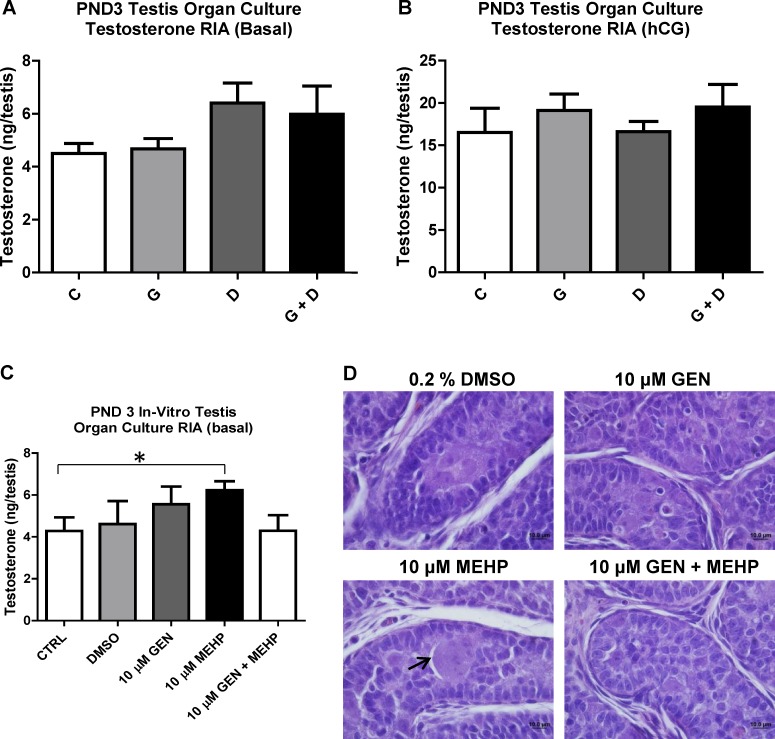
PND3 testes basal and hCG-stimulated androgen production were evaluated using ex vivo organ culture (Fig. 3). Testosterone measurement demonstrated no significant alterations in any treatment group compared to control (Fig. 3A). Testes from all treatment groups responded well to hCG stimulation, with no significant alterations or obvious trends (Fig. 3B).
FIG. 3.
Effects of in utero and in vitro exposure to genistein (GEN) and DEHP on PND3 testes T production. Ex vivo testis organ culture was performed over 3 days in basal (A) or hCG-containing (B) medium using PND3 testes from in utero treated offspring. C) Control (untreated) PND3 testes were also treated in vitro over 3 days with either plain medium (control), vehicle (0.2% DMSO), 10 μM GEN, 10 μM MEHP, or combined 10 μM GEN + MEHP. Testosterone levels in supernatant medium for both ex vivo and in vitro organ cultures were determined by radioimmunoassay (RIA) and expressed in ng/testes. Graphs represent the sum of T produced over 3 days (supernatant collected once daily). Asterisk indicates a significant difference relative to control (P ≤ 0.05, n = 4). To assess histological alterations, testes from ex vivo (data not shown) and in vitro organ cultures (D) were collected for processing and hematoxylin and eosin staining (photos taken at 100× magnification). Arrow indicates multinucleated germ cell. Representative pictures are presented; C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
To decipher between direct effects on testis and effects involving the hypothalamus-pituitary-testis axis, untreated PND3 testes were exposed in vitro over 3 days to medium, DMSO (0.2%), GEN, MEHP (the principal bioactive metabolite of DEHP), or combined GEN and MEHP, both at 10 μM, a concentration within the range of reported human blood levels [42–45] (Fig. 3C) under basal conditions. Consistent with the observed in vivo proandrogenic gene expression data, MEHP significantly stimulated PND3 testes T production in vitro, an effect that was attenuated by combination treatment with GEN. Interestingly, histological analysis of paraffin sections from the cultured testes fragments also revealed the presence of large multinucleated germ cells (MGCs) uniquely in the seminiferous tubules treated with 10 μM MEHP (Fig. 3D).
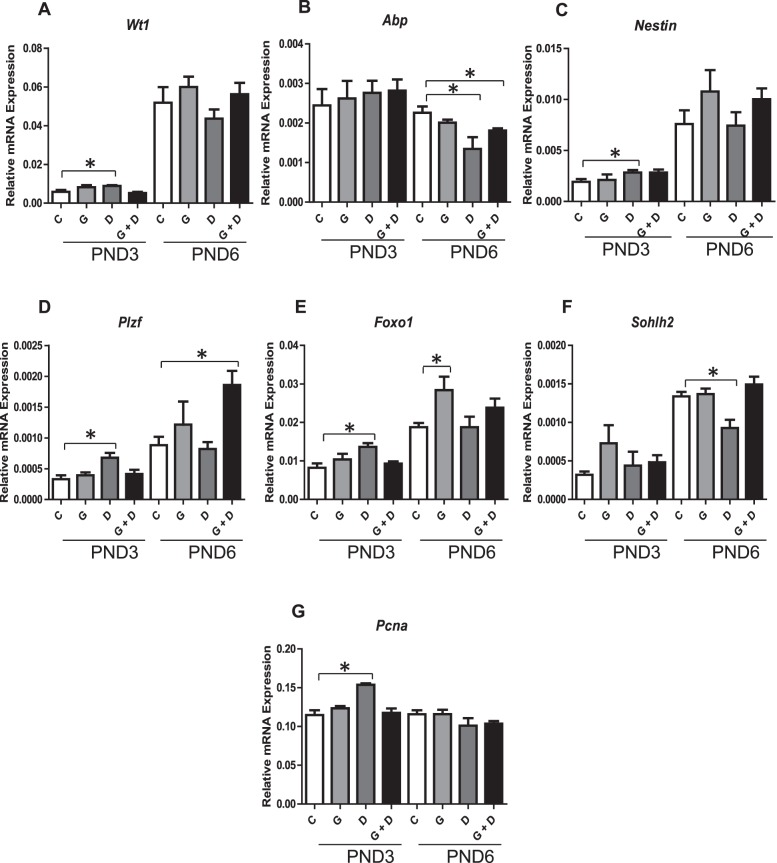
Further qPCR analysis of PND3 and PND6 testes was conducted to evaluate the impact on Sertoli cell, proliferation, and early germ cell markers (Fig. 4). The Sertoli cell marker Wt1 and the Sertoli/Leydig progenitor marker Nestin (Fig. 4, A–C) were significantly increased uniquely in PND3 DEHP-treated offspring. By contrast, the marker of Sertoli cell differentiation Abp was reduced in DEHP-exposed PND6 pups, an effect partially corrected in pups exposed to GEN + DEHP mixture. Early germ cell markers Plzf and Foxo1 (Fig. 4, D and E) displayed a similar trend, being significantly increased in PND3 DEHP but not GEN or combined GEN and DEHP. Interestingly, the marker of differentiating spermatogonia, Sohlh2, was significantly decreased in DEHP-exposed pups at PND6, an effect alleviated by combination with GEN (Fig. 4F). The cell proliferation marker Pcna, was significantly increased uniquely in PND3 DEHP-treated offspring (Fig. 4G).
FIG. 4.
Effects of in utero exposure to genistein (GEN) and DEHP on Sertoli, proliferation, and early germ cell markers. Relative mRNA expression of Sertoli cell markers Wt1 (A) and Abp (B), Sertoli cell and Leydig marker Nestin (C), early germ cell markers Plzf (D), Foxo1 (E), and Sohlh2 (F), and proliferation marker Pcna (G) in PND3 and PND6 testes. Data are expressed as mean relative mRNA levels ± SEM normalized to alpha-tubulin (n = 4 per treatment). Asterisks indicate a significant difference relative to control (p ≤ 0.05); C, Control; G, GEN; D, DEHP; G + D, GEN + DEHP.
In Utero Exposure to GEN and DEHP Up-Regulates Cellular Junction Markers in Early Postnatal Testes
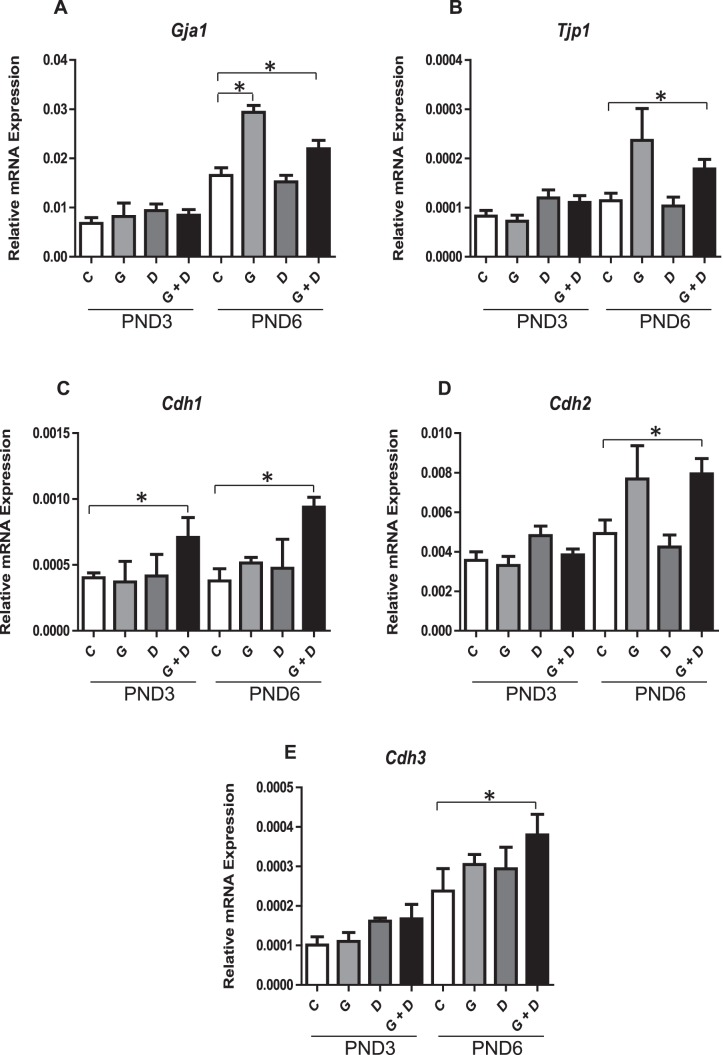
The qPCR analysis of PND3 and PND6 testes revealed a stimulatory effect of the ED mixture on several gap, tight, and adherens junction markers (Fig. 5). Gap junction marker Gja1 (Connexin 43) was significantly up-regulated in GEN and combined (GEN + DEHP)-treated offspring at PND6, but not in animals treated with DEHP alone (Fig. 5A). Similarly, tight junction maker Tjp1 (Fig. 5B) and several adherens junctions Cdh1, Cdh2, and Cdh3 (Fig. 5, C–E) were increased uniquely by combined GEN and DEHP at PND6. Excluding a consistent up-regulation of Cdh3 in PND3 animals exposed to combined GEN and DEHP, no significant alterations or trends were observed for cellular junction markers immediately following exposure at PND3.
FIG. 5.
Effects of in utero exposure to genistein (GEN) and DEHP on cellular junctions. Relative mRNA expression of gap junction Gja1 (A), tight junction protein Tjp1 (B), and adherens junctions Cdh1 (C), Cdh2 (D), and Cdh3 (E) in PND3 and PND6 testes. Data are expressed as mean relative mRNA levels ± SEM normalized to alpha-tubulin (n = 4 per treatment). Asterisks indicate a significant difference relative to control (P ≤ 0.05); C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
In Utero GEN Coexposure Antagonizes Prooxidant Cellular Stress of DEHP in Early Postnatal Testes
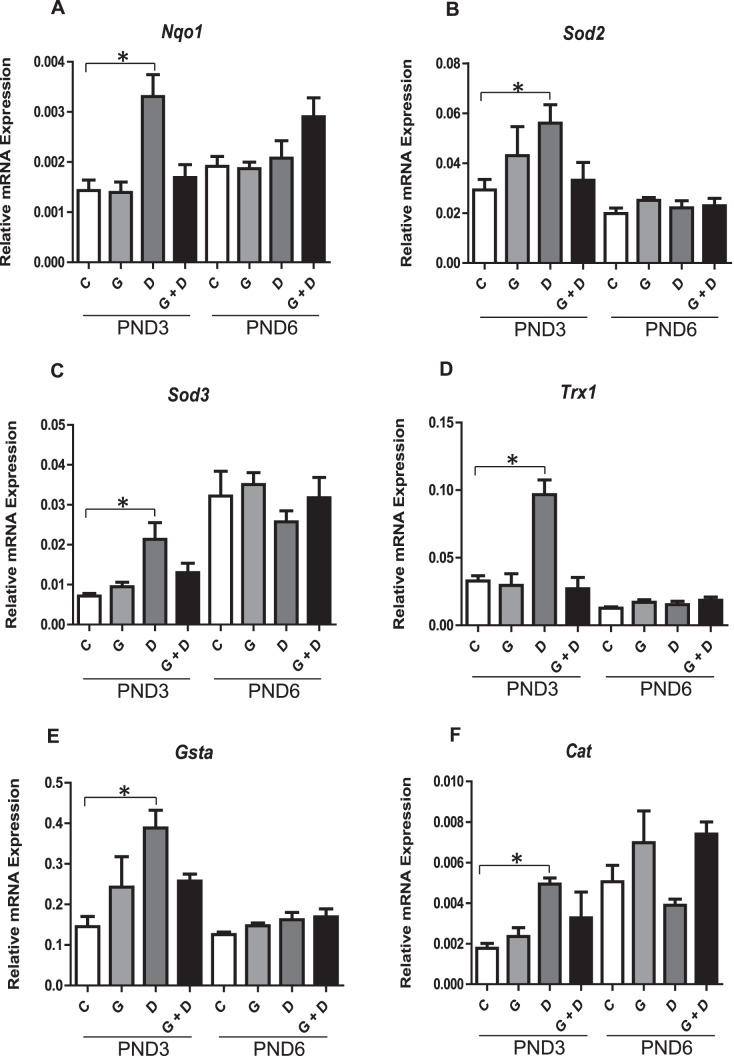
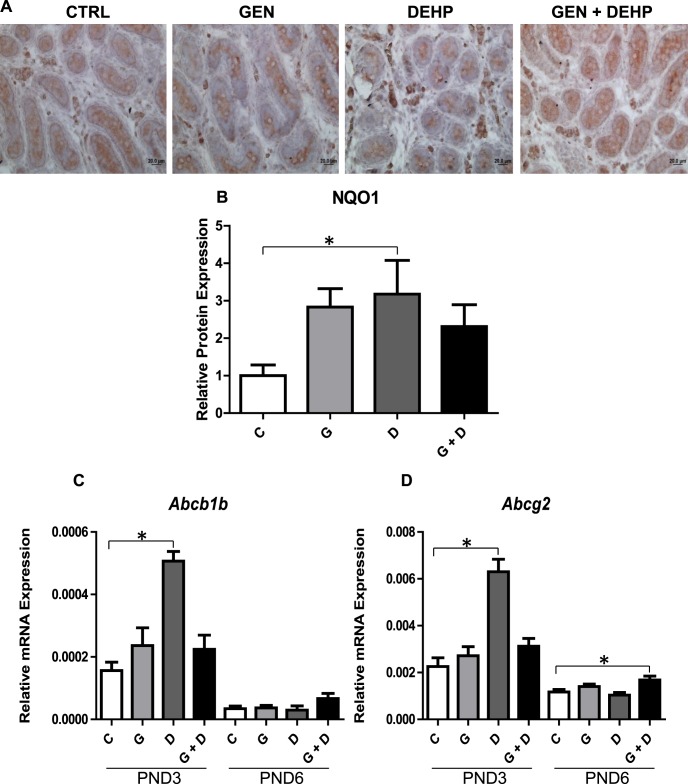
The qPCR analysis of PND3 and PND6 testes revealed a significant (2- to 3-fold) up-regulation of numerous downstream NRF2 antioxidant gene targets, including Nqo1, Sod2, Sod3, Trx1, Gsta1, and Cat (Fig. 6, A–F) specifically in PND3 testes of DEHP-treated animals. This up-regulation was attenuated in PND3 testes of animals treated with combined GEN and DEHP and was not observed in GEN-treated animals. No obvious trends for any antioxidant makers evaluated were observed in PND6 testes. Nqo1 gene alterations were further validated at the protein level by immunohistochemistry and subsequent quantitative image analysis (Fig. 7, A and B): NQO1 protein stained strongest in the PND3 interstitial Leydig cells of DEHP-treated animals, an effect that was not present in animals receiving combined GEN and DEHP or GEN alone (Fig. 7A). Quantitative image analysis of NQO1 also revealed a consistent, significant increase only in PND3 DEHP-treated offspring relative to control (Fig. 7B). Lastly, consistent with antioxidant expression data, qPCR analysis of PND3 and PND6 xenobiotic transporters Abcb1b and Abcg2 (Fig. 7, C and D) revealed a significant up-regulation uniquely in PND3 DEHP-treated testes, suggesting a concerted effort to detoxify cells by physically eliminating chemical stressors. The effects of DEHP on the transporters were normalized in pups exposed to the GEN and DEHP combination.
FIG. 6.
Effects of in utero exposure to genistein (GEN) and DEHP on cellular defense mediators. Relative mRNA of downstream NRF2 antioxidant and detoxifying enzymes Nqo1 (A), Sod2 (B), Sod3 (C), Trx1 (D), Gsta (E), and Cat (F) in PND3 and PND6 testes. Data are expressed as mean relative mRNA levels ± SEM normalized to alpha-tubulin (n = 4 per treatment). Asterisks indicate a significant difference relative to control (P ≤ 0.05); C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
FIG. 7.
Effects of in utero exposure to genistein (GEN) and DEHP on antioxidant protein and xenobiotic transporter gene expression. Immunohistochemical analysis of NQO1 was performed on PND3 testes (A) and positive staining was quantified by image analysis (B) (photos taken at 40× magnification). Immunohistochemical quantification is represented by mean fold change ± SEM of NQO1 staining relative to control (n ≥ 3). Omission of the primary antibody was used as a negative control (data not shown). Representative pictures are presented. Relative mRNA expression of xenobiotic transporters Abcb1b (C) and Abcg2 (D) in PND3 and PND6 testes. Data are expressed as mean relative mRNA levels ± SEM normalized to alpha-tubulin (n = 4 per treatment). Asterisks in both mRNA and protein analyses indicate a significant difference relative to control (P ≤ 0.05); C, control; G, GEN; D, DEHP; G + D, GEN + DEHP.
DISCUSSION
Previous work from our laboratory demonstrated that gestational exposure (GD14 to parturition) to combined 10mg/kg/day GEN and DEHP induces long-term alterations in testis function and gene and protein expression of critical germ and somatic cell markers [27]. This dose corresponds to 1.6–2.0 mg/kg/day in human equivalents, after conversion using the body surface area normalization method [41]. Under normal conditions, human exposure to GEN and DEHP ranges from 0.01 to 0.2 mg/kg/day and 0.003 to 0.03 mg/kg/day, respectively. Neonates fed soy-based infant formula or undergoing medical intervention however, can experience levels that are 10- to 100-fold higher than the general population [23, 25, 42–46]. In search of mechanistic clues for the previously observed long-term effects in testis, we hypothesized that GEN and DEHP would target the early postnatal precursors of adult somatic and germ cells by altering similar genes/pathways in these cells and that the mixture of the two EDs would have stronger effects than single compounds. The present results showed that this is not the case.
Unexpected Gene Profile Alterations in Response to DEHP, but Not GEN, or Their Combination
PND3 and PND6 gross measurements revealed a significant decrease in PND3 AGD of GEN-exposed animals, suggesting possible feminizing effects in response to this soy-derived phytoestrogen. An intriguing effect of GEN was its consistent induction of Ar expression between PND6 and adulthood. Interestingly, GEN has been reported to exert a biphasic effect on Ar expression in prostate cancer cells, inducing its expression at 0.5–5 μM and inhibiting it at 25–50 μM [47]. Thus, it is possible that a similar process takes place in testis. No other significant gene expression or protein alterations were found in GEN-treated offspring for the endpoints evaluated. Paradoxically, the effects of GEN on AGD were normalized in rats treated in utero with combined GEN and DEHP, a previously reported antiandrogen [48]. The expression of genes altered in adult testes by fetal exposure to the mixture revealed different gene profiles in neonatal testes. Noticeably, exposure to DEHP, but not GEN and DEHP mixture, induced a significant up-regulation of Hsd3b and Ar, whose expression was previously shown to be induced by T [49]. Concomitantly, DEHP up-regulated the expression of the Sertoli cell marker Wt1, early germ cell markers (Plzf, Foxo1), and proliferation marker Pcna, which at these early ages reflects the behavior of Sertoli, germ, and myoid cells [50], suggesting a general stimulatory effect of DEHP on neonatal testis development. Our finding that the transcript of Nestin, an intermediate filament protein found in Sertoli and progenitor Leydig cells [51, 52], was increased in DEHP-exposed rats is reminiscent of an earlier study where we found that in utero exposure to higher DEHP doses (≥ 250 mg/kg/day) increased nestin expression in fetal Leydig cells right before birth [53]. Interestingly, these DEHP-induced effects were normalized by adding GEN, implying that GEN-activated pathways opposed those triggered by DEHP in neonatal testes.
At PND6, the decreased expression of the Sertoli cell differentiation marker Abp [54] and the differentiating spermatogonia marker Sohlh2 [55] in DEHP-exposed rats only suggested that DEHP had altered the differentiation program of juvenile Sertoli and germ cells. Moreover, Cyp11a1, the enzyme responsible for cholesterol conversion to pregnenolone, was decreased uniquely by combined GEN and DEHP, indicating a selective targeting of specific steroidogenic mediators by the ED mixture.
Despite the significant up-regulation of androgen-related genes by DEHP at PND3, ex vivo T production was not altered, potentially reflecting the functional recovery of testis fragments after 3 days in culture without DEHP, similarly to the normalization of gene expression observed in PND6 rats. By contrast, direct treatment of unexposed PND3 testis fragments over 3 days with 10 μM MEHP, a concentration in the range of those measured in human blood under specific conditions [46, 56], revealed a proandrogenic effect of MEHP, which was attenuated by combining GEN with MEHP, matching in vivo PND3 expression data. These results contrast with previous studies where the same window of DEHP exposure using higher doses showed reduced basal and hormone-induced T production for ≥100 and ≥900 mg/kg/day DEHP, respectively, suggesting differential DEHP sensitivity of basal and hormone-induced steroidogenesis mechanisms [35]. Moreover, studies using testis organ cultures from GD14–GD16 fetuses reported the suppression of basal T production by high doses of dibutyl phthalate and an inhibitory effect of MEHP, but stimulatory effect of DEHP, on basal and LH-stimulated T production, implying that MEHP and DEHP have direct modes of action on fetal Leydig cells [57, 58].
Occurrence of MGCs In Vitro
A recent study using organ cultures of human fetal testes incubated with 100 μM 14C-MEHP determined that similar concentrations were present in the medium and testis fragments after 24 h [59], suggesting that intratesticular MEHP levels were close to the 10 μM added in PND3 testis cultures, where abnormal MNGs were observed in vitro in the present study. Considering these data, together with the facts that in vivo MNGs were observed in rats treated in utero with ≥500 mg/kg/day DEHP, and that 10 mg/kg/day DEHP would elicit blood levels ∼1 μM [60], the absence of MNGs in neonatal testes from rats treated in utero with 10 mg/kg/day DEHP is not surprising. Nonetheless, MNG formation is a phthalate-induced in vitro phenotype consistent across rat, mouse, and human fetal testis and independent of hormone alterations [57, 61, 62]. Although this phenotype still remains poorly understood, selective targeting of critical cellular junctions has been proposed as a mechanism contributing to endocrine-dependent or -independent phthalate reproductive toxicity [63, 64].
In the adult, dynamically regulated tight junctions between Sertoli cells form a functional blood-testis barrier that physically divides basal and apical compartments of the seminiferous epithelium, creating a microenvironment that allows the maturation and movement of dividing germ cells [65–70]. Although no MNG was observed at the dose used in the current in vivo study, combined GEN and DEHP significantly up-regulated PND6 testis mRNA levels of several cellular junction-related genes, including cadherins (Cdh1 and Cdh2), tight junction protein 1 (Tjp1), and gap junction gene Gja1. Interestingly, an up-regulation of N-cadherin and catenins was also reported in rats displaying testicular atrophy and degeneration of the seminiferous epithelium following high-dose DEHP treatment [71]. Inhibition or up-regulation of adhesion proteins by combined GEN and DEHP during early testis development may be related to long-term reproductive toxicity and be a contributing factor to the observed MNG phenotype in vitro. Further research is necessary to determine the cell specificity of junctional changes and their implications, and potential relationship with phthalate-induced MNGs, in which organ culture may serve as a valuable toxicological tool.
Are Reactive Oxygen Species Involved in DEHP Biphasic Effect?
The in vitro data suggest that MEHP stimulates T production, a process abrogated by GEN cotreatment. Other studies have reported nonmonotonic or biphasic effects of EDs, including phthalates. Rats treated gestationally (GD2–GD20) with DEHP had increased T at 10mg/kg/day and decreased T at higher doses [72]. Nonmonotonic effects of DEHP on fetal T were also observed at GD20 and in vitro following MEHP treatment [73–75]. Several studies point to the involvement of reactive oxygen species (ROS) in modifying Leydig mitochondrial function: low concentrations of H2O2 and MEHP increased ROS, T production, and STAR/CYP11A1 activities in isolated rat Leydig cells, an effect that was attenuated by cotreatment with the antioxidant vitamin C [76]. Interestingly, high concentrations of H2O2 and MEHP had a suppressive effect on T production and steroidogenic enzyme activity, suggesting a biphasic effect of ROS on androgen production. Although not fully understood, research using MA-10 Leydig cells demonstrated a potential role for ROS in cAMP-mediated modulation of cellular signaling pathways, an effect that was attenuated by an uncoupler of oxidative phosphorylation [77]. Alternatively, or perhaps in parallel, evidence suggests that oxysterols generated in part by an oxidative cellular environment can regulate STAR expression and also serve as ligands for liver x nuclear receptors (LXR), critical mediators of steroid and lipid metabolism and male reproductive function [78, 79].
Does GEN Protect Against DEHP Mediated Cellular Stress?
Although we have been investigating GEN in the context of a phytoestrogen with potential endocrine-disrupting properties, GEN is also a relatively promiscuous compound with broad biological interactions, including PPAR activation, modulation of signaling molecules, as well as direct or indirect antioxidant action [28, 30]. It is therefore plausible that GEN may attenuate DEHP-mediated oxidative stress during the fetal period independently of the long-term effects of GEN and DEHP on male reproductive development.
To test this ROS hypothesis in PND3 and PND6 animals, gene and protein expression profiles of several downstream targets of the KEAP1/NRF2-mediated antioxidant response element were examined [80]. In the event of oxidative environment changes, stress-sensing cysteine in cytoplasmic KEAP1 results in a conformational change and subsequent dissociation and migration of NRF2 to the nucleus, activating a battery of antioxidant and detoxifying gene targets intended to restore cell homeostasis.
Quantitative real-time PCR analysis revealed a significant up-regulation of downstream Nrf2 antioxidant and detoxifying gene targets, including Nqo1, Sod2, Sod3, Gst, Trx, and Cat, specifically in PND3 testes of DEHP-treated rats. Cotreatment with GEN attenuated these effects, suggesting a protective effect of GEN in neonatal testes. The DEHP-induced oxidative stress responses were no longer visible at PND6, implying that the prooxidant effects of DEHP occurred only in the presence of the chemical or its metabolite MEHP in the tissues, in agreement with pharmacokinetic studies reporting that the total elimination of DEHP/MEHP and GEN can take 5–7 days [81]. Our data concur with previous studies reporting changes in oxidative stress genes in fetal testes exposed to various phthalates [82]. Immunohistochemical and quantitative image analysis indicated that DEHP-induced NQO1 up-regulation occurred primarily in interstitial Leydig cells, fitting with the observed phenotype and ROS hypothesis, given the close proximity of these cells to interstitial macrophages and endothelial cells of blood vessels trafficking toxicants.
In addition to detoxifying enzymes and the blood-testis and blood-epididymal barriers created by tight junction proteins at later ages, specialized xenobiotic efflux transporters actively regulate the type and quantity of compounds entering the male reproductive tract. Several members of the ATP-binding cassette (ABC) family of energy-dependent efflux transporters, including ABCB1A/B (P-GP), ABCBG2 (BCRP), and ABCC1 (MRP1), are expressed in testicular germ, Sertoli, Leydig and endothelial cells, epididymal principal cells, and maturing spermatozoa [83–87]. In our study, the xenobiotic transporters Abcb1b and Abcg2 were significantly up-regulated in PND3, but not PND6, testes of DEHP-exposed rats, suggesting a concerted effort to detoxify the cells by physically eliminating chemical stressors. Interestingly, DEHP and MEHP are not known substrates for these xenobiotic transporters. However, oxidative stress can up-regulate ABCB1, ABCG2, and ABCC1 expression via NRF2, P53, and NFKB pathways [88–90]. DEHP has also been proposed as a potential chemosensitizer, capable of modifying drug entry in multidrug resistant tumors [91]. BRCP−/− mice displayed increased bioavailability and plasma levels of GEN metabolites and accumulation of several phytoestrogens in testis and epididymis, suggesting an important role in GEN toxicokinetics [85, 92]. Our results therefore reaffirm an important role for xenobiotic transporters in mediating testicular bioavailability and detoxification, endpoints that are often overlooked and warrant more investigation.
The results of the present study were somewhat unexpected because they identified DEHP as the main inducer of changes in neonatal testis and GEN as a protective agent. This contrasts with the effects observed in adult testis, where fetal exposure to the mixture of DEHP and GEN induced deleterious long-term effects in various testicular cell types, and GEN acted with DEHP to induce effects unique to their mixture. Although much of the literature has focused on EDs in relation to androgen suppression, research suggests that any deviation from normal hormonal programming, including androgen up-regulation or altering the balance of androgens and estrogens, can have profound consequences on male reproductive development [20, 93, 94]. To our knowledge, this is the first in vivo study to provide mechanistic insight into low-dose DEHP-induced early responses and the differential effects of GEN and DEHP mixture at doses below their known thresholds of toxicity.
We propose a mechanism by which GEN, through its antioxidant action, normalizes the ROS-mediated and proandrogenic effects of DEHP in early postnatal testes. Thus, cotreatment of antioxidants devoid of endocrine-disrupting side effects may help attenuate DEHP-mediated alterations during the fetal and neonatal periods. Paralleling our findings, low-dose GEN antagonized the neonatal germ cell toxicity of vinclozolin, an antiandrogenic food contaminant, while conversely exacerbating adult toxicity in a synergistic manner [95, 96]. It is likely that GEN long-term effects are not related to its antioxidant properties, but rather to its estrogenic properties, because protection against PND3 oxidative stress did not prevent long-term effects.
Discrepancies between acute and delayed long-term effects may be related to the nonmonotonic nature of the effects. The latent effects of our mixture may be independent of hormonal or testicular environment aberrations triggered at much higher doses. Longitudinal effects likely reflect permanent alterations in early versions or progenitors of the testicular cells affected in adulthood. For example, adult Leydig cells are derived not from fetal Leydig cells but rather from neonatal mesenchymal precursors [18, 19]. Similarly, damages of primordial germ cells and gonocytes in utero can lead to disrupted spermatogenesis in adulthood [14, 15, 97]. GEN and DEHP may have epigenotoxic effects in early fetal development that affect the developmental progression of key testicular cell types. Thus, future work will focus at identifying correlative epigenetic alterations, such as DNA methylation and histone modifications, between fetal precursors and adult cell types that may be driving long-term toxicity.
Finally, this study highlights how short-term studies of GEN and DEHP testicular effects cannot predict their long-term effects. The notion that these EDs do not follow classical dose-response effects with consistent mechanisms of toxicity from perinatal ages to adulthood further stresses the importance of assessing impacts across a range of doses during appropriate windows of exposure and at different ages. We feel that these findings raise pertinent questions for regulatory agencies performing chemical risk assessments and determining acceptable exposure levels.
Footnotes
Supported by a grant from the Canadian Institutes of Health Research (CIHR) (no. MOP-312268) to M.C., a scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC) to S.J., and a visiting program scholarship from the Graduate school of Xi'an Jiaotong University to L.Z. The Research Institute of McGill University Health Centre is supported in part by a center grant from Fonds de la Recherche en santé Quebec.
REFERENCES
- de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman AJ, Keiding N, Skakkebaek NE. Declining semen quality and increasing incidence of testicular cancer: is there a common cause? Environ Health Perspect. 1995;103 Suppl 7:137–139. doi: 10.1289/ehp.95103s7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Virtanen HE, Main KM, Skakkebaek NE. Cryptorchidism and hypospadias as a sign of testicular dysgenesis syndrome (TDS): environmental connection. Birth Defects Res a Clin Mol Teratol. 2010;88:910–919. doi: 10.1002/bdra.20707. [DOI] [PubMed] [Google Scholar]
- DiVall SA. The influence of endocrine disruptors on growth and development of children. Curr Opin Endocrinol Diabetes Obes. 2013;20:50–55. doi: 10.1097/MED.0b013e32835b7ee6. [DOI] [PubMed] [Google Scholar]
- Phillips KP, Tanphaichitr N. Human exposure to endocrine disrupters and semen quality. J Toxicol Environ Health B Crit Rev. 2008;11:188–220. doi: 10.1080/10937400701873472. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Jorgensen N, Asklund C, Carlsen E, Kristensen TS, Holm M, Skakkebaek NE. Self-rated health and semen quality among 3,457 young Danish men. Fertil Steril. 2007;88:1366–1373. doi: 10.1016/j.fertnstert.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Joensen UN, Jorgensen N. Rajpert-De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin Pharmacol Toxicol. 2008;102:155–161. doi: 10.1111/j.1742-7843.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Joensen UN, Jorgensen N, Skakkebaek NE. Testicular dysgenesis syndrome and carcinoma in situ of the testes. Nat Clin Pract Urol. 2007;4:402–403. doi: 10.1038/ncpuro0859. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci. 2010;365:1571–1579. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73:241–278. doi: 10.1002/jemt.20783. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Perey B. The stages of the cycle of the seminiferous epithelium of the rat: practical definitions in PA-Schiff-hematoxylin and hematoxylin-eosin stained sections. Rev Can Biol. 1957;16:451–462. [PubMed] [Google Scholar]
- Clermont Y, Perey B. Quantitative study of the cell population of the seminiferous tubules in immature rats. Am J Anat. 1957;100:241–267. doi: 10.1002/aja.1001000205. [DOI] [PubMed] [Google Scholar]
- Culty M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res C Embryo Today. 2009;87:1–26. doi: 10.1002/bdrc.20142. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Matsui Y. Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet. 2008;9:129–140. doi: 10.1038/nrg2295. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Lording DW, De Kretser DM. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil. 1972;29:261–269. doi: 10.1530/jrf.0.0290261. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Pathways of endocrine disruption during male sexual differentiation and masculinization. Best Pract Res Clin Endocrinol Metab. 2006;20:91–110. doi: 10.1016/j.beem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, Herrick R, Swan SH. Maternal urinary metabolites of di-(2-ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169:1015–1024. doi: 10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113:e429–e434. doi: 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Slakman AR, Silva MJ, Herbert AR, Needham LL. Automated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:49–56. doi: 10.1016/j.jchromb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Williams CJ. Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes. Reprod Toxicol. 2011;31:272–279. doi: 10.1016/j.reprotox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Ku HY, Su PH, Chen JW, Huang PC, Angerer J, Wang SL. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere. 2011;82:947–955. doi: 10.1016/j.chemosphere.2010.10.073. [DOI] [PubMed] [Google Scholar]
- Jones S, Boisvert A, Duong TB, Francois S, Thrane P, Culty M. Disruption of rat testis development following combined in utero exposure to the phytoestrogen genistein and antiandrogenic plasticizer di-(2-ethylhexyl) phthalate. Biol Reprod. 2014;91:64. doi: 10.1095/biolreprod.114.120907. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S. Soy, phyto-oestrogens and male reproductive function: a review. Int J Androl. 2010;33:304–316. doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Zimmermann C, Beny JL, Schaad O, Combepine C, Descombes P, Doerge DR, Pralong FP, Vassalli JD, Nef S. Potential detrimental effects of a phytoestrogen-rich diet on male fertility in mice. Mol Cell Endocrinol. 2010;321:152–160. doi: 10.1016/j.mce.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Qian Y, Guan T, Huang M, Cao L, Li Y, Cheng H, Jin H, Yu D. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-kappaB activation in a cerebral ischemia mouse model. Neurochem Int. 2012;60:759–767. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Klinefelter GR, Laskey JW, Winnik WM, Suarez JD, Roberts NL, Strader LF, Riffle BW, Veeramachaneni DN. Novel molecular targets associated with testicular dysgenesis induced by gestational exposure to diethylhexyl phthalate in the rat: a role for estradiol. Reproduction. 2012;144:747–761. doi: 10.1530/REP-12-0266. [DOI] [PubMed] [Google Scholar]
- Maloney EK. Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, Xiong J, Liu X, Xu M, Zhao D, Ma C, Li X, et al. Dynamic epigenetic changes involved in testicular toxicity induced by di-2-(ethylhexyl) phthalate in mice. Basic Clin Pharmacol Toxicol. 2010;106:118–123. doi: 10.1111/j.1742-7843.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhu J, Li Y, Lin T, Gan L, Yuan X, Xu M, Wei G. Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: epigenetic changes and their impact on gene expression. Int J Toxicol. 2010;29:193–200. doi: 10.1177/1091581809355488. [DOI] [PubMed] [Google Scholar]
- Culty M, Thuillier R, Li W, Wang Y, Martinez-Arguelles DB, Benjamin CG, Triantafilou KM, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol Reprod. 2008;78:1018–1028. doi: 10.1095/biolreprod.107.065649. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Guichard T, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to the antiandrogen di-(2-ethylhexyl) phthalate decreases adrenal aldosterone production in the adult rat. Biol Reprod. 2011;85:51–61. doi: 10.1095/biolreprod.110.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuillier R, Manku G, Wang Y, Culty M. Changes in MAPK pathway in neonatal and adult testis following fetal estrogen exposure and effects on rat testicular cells. Microsc Res Tech. 2009;72:773–786. doi: 10.1002/jemt.20756. [DOI] [PubMed] [Google Scholar]
- Thuillier R, Wang Y, Culty M. Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptors alpha and beta in neonatal rat testis: identification of gonocytes as targets of estrogen exposure. Biol Reprod. 2003;68:867–880. doi: 10.1095/biolreprod.102.009605. [DOI] [PubMed] [Google Scholar]
- Wang Y, Thuillier R, Culty M. Prenatal estrogen exposure differentially affects estrogen receptor-associated proteins in rat testis gonocytes. Biol Reprod. 2004;71:1652–1664. doi: 10.1095/biolreprod.104.030205. [DOI] [PubMed] [Google Scholar]
- Manku G, Wing SS, Culty M. Expression of the ubiquitin proteasome system in neonatal rat gonocytes and spermatogonia: role in gonocyte differentiation. Biol Reprod. 2012;87:44. doi: 10.1095/biolreprod.112.099143. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Foster WG, Chan S, Platt L, Hughes CL., Jr Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol Lett. 2002;129:199–205. doi: 10.1016/s0378-4274(02)00018-8. [DOI] [PubMed] [Google Scholar]
- Shelby MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of di (2-ethylhexyl) phthalate (DEHP) NTP CERHR MON 2006. (18) v: vii 7 [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL, Jr, Needham LL, Calafat AM. Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol. 2004;72:1226–1231. doi: 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- McCarver G, Bhatia J, Chambers C, Clarke R, Etzel R, Foster W, Hoyer P, Leeder JS, Peters JM, Rissman E, Rybak M, Sherman C, et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res B Dev Reprod Toxicol. 2011;92:421–468. doi: 10.1002/bdrb.20314. [DOI] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AM, Zhu T, Parray A, Siddique HR, Yang W, Saleem M, Bosland MC. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS One. 2013;8:e78479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V. Fetal origin of endocrine dysfunction in the adult: the phthalate model. J Steroid Biochem Mol Biol. 2013;137:5–17. doi: 10.1016/j.jsbmb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kostic TS, Stojkov NJ, Bjelic MM, Mihajlovic AI, Janjic MM, Andric SA. Pharmacological doses of testosterone upregulated androgen receptor and 3-beta-hydroxysteroid dehydrogenase/delta-5-delta-4 isomerase and impaired Leydig cells steroidogenesis in adult rats. Toxicol Sci. 2011;121:397–407. doi: 10.1093/toxsci/kfr063. [DOI] [PubMed] [Google Scholar]
- Hadjiolova KV, Martinova YS, Yankulov KY, Davidov V, Kancheva LS, Hadjiolov AA. An immunocytochemical study of the proliferating cell nuclear matrix antigen p125/6.5 during rat spermatogenesis J Cell Sci 1989. 93 (Pt 1): 173 177 [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Muller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–249. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Culty M, Zirkin BR, Papadopoulos V. In utero exposure to di-(2-ethylhexyl) phthalate decreases mineralocorticoid receptor expression in the adult testis. Endocrinology. 2009;150:5575–5585. doi: 10.1210/en.2009-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus GL, Larrea F, Musto NA, Becker RR, Mather JP, Bardin CW. Androgen binding protein as a marker for Sertoli cell function. J Steroid Biochem. 1981;15:99–106. doi: 10.1016/0022-4731(81)90263-6. [DOI] [PubMed] [Google Scholar]
- Hao J, Yamamoto M, Richardson TE, Chapman KM, Denard BS, Hammer RE, Zhao GQ, Hamra FK. Sohlh2 knockout mice are male-sterile because of degeneration of differentiating type A spermatogonia. Stem Cells. 2008;26:1587–1597. doi: 10.1634/stemcells.2007-0502. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, Gaido K, Hodgson E, Marcus M, Shea K, Williams P. NTP-CERHR. Expert Panel Update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22:291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Heger NE, Hall SJ, Sandrof MA, McDonnell EV, Hensley JB, McDowell EN, Martin KA, Gaido KW, Johnson KJ, Boekelheide K. Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ Health Perspect. 2012;120:1137–1143. doi: 10.1289/ehp.1104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvigne F, Menuet A, Lesne L, Chagnon MC, Chevrier C, Regnier JF, Angerer J, Jegou B. Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro. Environ Health Perspect. 2009;117:515–521. doi: 10.1289/ehp.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muczynski V, Cravedi JP, Lehraiki A, Levacher C, Moison D, Lecureuil C, Messiaen S, Perdu E, Frydman R, Habert R, Rouiller-Fabre V. Effect of mono-(2-ethylhexyl) phthalate on human and mouse fetal testis: In vitro and in vivo approaches. Toxicol Appl Pharmacol. 2012;261:97–104. doi: 10.1016/j.taap.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Makinodan F, Shimamura N, Katoh M. Metabolism of di (2-ethylhexyl) phthalate (DEHP): comparative study in juvenile and fetal marmosets and rats. J Toxicol Sci. 2012;37:33–49. doi: 10.2131/jts.37.33. [DOI] [PubMed] [Google Scholar]
- Lambrot R, Muczynski V, Lecureuil C, Angenard G, Coffigny H, Pairault C, Moison D, Frydman R, Habert R, Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ Health Perspect. 2009;117:32–37. doi: 10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kim KH. Effects of mono-(2-ethylhexyl) phthalate on fetal and neonatal rat testis organ cultures. Biol Reprod. 2003;69:1964–1972. doi: 10.1095/biolreprod.103.018895. [DOI] [PubMed] [Google Scholar]
- Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol. 2004;18:413–421. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang R, Xiang Z, Qian W, Han X, Li D. Mixture effects of nonylphenol and di-n-butyl phthalate (monobutyl phthalate) on the tight junctions between Sertoli cells in male rats in vitro and in vivo. Exp Toxicol Pathol. 2014;66:445–454. doi: 10.1016/j.etp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Pointis G, Fiorini C, Defamie N, Segretain D. Gap junctional communication in the male reproductive system. Biochim Biophys Acta. 2005;1719:102–116. doi: 10.1016/j.bbamem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci U S A. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincon-Raymond M. Cadherins: structure and signalisation protein of cell-cell junctions. Introduction and conclusions [in French] J Soc Biol. 2004;198:353–356. [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J Androl. 2007;9:463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Sobarzo CM, Lustig L, Ponzio R, Denduchis B. Effect of di-(2-ethylhexyl) phthalate on N-cadherin and catenin protein expression in rat testis. Reprod Toxicol. 2006;22:77–86. doi: 10.1016/j.reprotox.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, Sealfon SC, Bernard DJ, Hardy MP. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J Androl. 2007;28:513–520. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D, Leffler P, Ekwurtzel E, Martinsson G, Liu K, Selstam G. Mono-(2-ethylhexyl) phthalate stimulates basal steroidogenesis by a cAMP-independent mechanism in mouse gonadal cells of both sexes. Reproduction. 2008;135:693–703. doi: 10.1530/REP-07-0460. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Svechnikova I, Soder O. Inhibitory effects of mono-ethylhexyl phthalate on steroidogenesis in immature and adult rat Leydig cells in vitro. Reprod Toxicol. 2008;25:485–490. doi: 10.1016/j.reprotox.2008.05.057. [DOI] [PubMed] [Google Scholar]
- Do RP, Stahlhut RW, Ponzi D. Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34:614–621. doi: 10.1016/j.reprotox.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, Zhao LF, Liang Y, Zhang YH. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PLoS One. 2013;8:e62526. doi: 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P, Ascoli M. Reactive oxygen species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol. 2011;25:885–893. doi: 10.1210/me.2010-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SR, Matassa AA, White EK, Walsh LP, Jo Y, Rao RM, Stocco DM, Reyland ME. Oxysterols regulate expression of the steroidogenic acute regulatory protein. J Mol Endocrinol. 2004;32:507–517. doi: 10.1677/jme.0.0320507. [DOI] [PubMed] [Google Scholar]
- El-Hajjaji FZ, Oumeddour A, Pommier AJ, Ouvrier A, Viennois E, Dufour J, Caira F, Drevet JR, Volle DH, Baron S, Saez F, Lobaccaro JM. Liver X receptors, lipids and their reproductive secrets in the male. Biochim Biophys Acta. 2011;1812:974–981. doi: 10.1016/j.bbadis.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc) 2011;76:407–422. doi: 10.1134/s0006297911040031. [DOI] [PubMed] [Google Scholar]
- Coldham NG, Sauer MJ. Pharmacokinetics of [(14)C]genistein in the rat: gender-related differences, potential mechanisms of biological action, and implications for human health. Toxicol Appl Pharmacol. 2000;164:206–215. doi: 10.1006/taap.2000.8902. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Melaine N, Lienard MO, Dorval I, Le Goascogne C, Lejeune H, Jegou B. Multidrug resistance genes and p-glycoprotein in the testis of the rat, mouse, guinea pig, and human. Biol Reprod. 2002;67:1699–1707. doi: 10.1095/biolreprod.102.003558. [DOI] [PubMed] [Google Scholar]
- Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- Jones SR, Cyr DG. Regulation and characterization of the ATP-binding cassette transporter-B1 in the epididymis and epididymal spermatozoa of the rat. Toxicol Sci. 2011;119:369–379. doi: 10.1093/toxsci/kfq318. [DOI] [PubMed] [Google Scholar]
- Robillard KR, Hoque T, Bendayan R. Expression of ATP-binding cassette membrane transporters in rodent and human Sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier. J Pharmacol Exp Ther. 2012;340:96–108. doi: 10.1124/jpet.111.186916. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fang J, Huang S, Chen L, Fan G, Wang C. The chronic effects of low lead level on the expressions of Nrf2 and Mrp1 of the testes in the rats. Environ Toxicol Pharmacol. 2013;35:109–116. doi: 10.1016/j.etap.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers. J Neurosci. 2014;34:8585–8593. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini A, Centurione L, Sancilio S, Castellani ML, Conti P, Di Ilio C, Porreca E, Cuccurullo F, Di Pietro R. The effect of the plasticizer diethylhexyl phthalate on transport activity and expression of P-glycoprotein in parental and doxo-resistant human sarcoma cell lines. J Biol Regul Homeost Agents. 2011;25:203–211. [PubMed] [Google Scholar]
- Yang Z, Zhu W, Gao S, Yin T, Jiang W, Hu M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:1883–1893. doi: 10.1124/dmd.111.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. The roles of oestrogen in the male. Trends Endocrinol Metab. 1998;9:371–377. doi: 10.1016/s1043-2760(98)00089-7. [DOI] [PubMed] [Google Scholar]
- Williams K, McKinnell C, Saunders PT, Walker M, Fisher JS, Turner KJ, Atanassova N, Sharpe M. Neonatal exposure to potent and environmental oestrogens and abnormalities of the male reproductive system in the rat: evidence for importance of the androgen-oestrogen balance and assessment of the relevance to man. Hum Reprod Update. 2001;7:236–247. doi: 10.1093/humupd/7.3.236. [DOI] [PubMed] [Google Scholar]
- Eustache F, Mondon F, Canivenc-Lavier MC, Lesaffre C, Fulla Y, Berges R, Cravedi JP, Vaiman D, Auger J. Chronic dietary exposure to a low-dose mixture of genistein and vinclozolin modifies the reproductive axis, testis transcriptome, and fertility. Environ Health Perspect. 2009;117:1272–1279. doi: 10.1289/ehp.0800158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehraiki A, Messiaen S, Berges R, Canivenc-Lavier MC, Auger J, Habert R, Levacher C. Antagonistic effects of gestational dietary exposure to low-dose vinclozolin and genistein on rat fetal germ cell development. Reprod Toxicol. 2011;31:424–430. doi: 10.1016/j.reprotox.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Culty M. Gonocytes, from the fifties to the present: is there a reason to change the name? Biol Reprod. 2013;89:46. doi: 10.1095/biolreprod.113.110544. [DOI] [PubMed] [Google Scholar]