Summary
The Aggregatibactor actinomycetemcomitans cytolethal distending toxin (Cdt) induces G2 arrest and apoptosis in lymphocytes and other cell types. We have shown that the active subunit, CdtB, exhibits phosphatidylinositol-3,4,5-triphosphate (PIP3) phosphatase activity and depletes lymphoid cells of PIP3. Thus we propose that Cdt toxicity results from depletion of this signaling lipid and perturbation of PI-3K/PIP3/Akt signaling. We have now focused on the relationship between cell susceptibility to CdtB and differences in the status of baseline PIP3 levels. Our studies demonstrate that the baseline level of PIP3, and likely the dependence of cells on steady-state activity of the PI-3K signaling pathway for growth and survival, influence cell susceptibility to the toxic effects of Cdt. Jurkat cells with known defects in both PIP3 degradative enzymes, PTEN and SHIP1, not only contain high baseline levels of PIP3, pAkt and pGSK3β, but also exhibit high sensitivity to Cdt. In contrast, HUT78 cells, with no known defects in this pathway contain low levels of PIP3, pAkt and pGSK3β and likely minimal dependence on the PI-3K signaling pathway for growth and survival exhibit reduced susceptibility to Cdt. These differences in susceptibility to Cdt can not be explained by differential toxin binding or internalization of the active subunit. Indeed, we now demonstrate that Jurkat and HUT78 cells bind toxin at comparable levels and internalize relatively equal amounts of CdtB. The relevance of these observations to the mode of action of Cdt and its potential role as a virulence factor is discussed.
Keywords: Host-parasite interactions, bacterial toxins, lymphocytes, PI-3K signaling
Introduction
Over the course of our investigations, we have demonstrated that Aggregatibactor actinomycetemcomitans, a putative pathogen implicated in the etiology and pathogenesis of localized juvenile periodontitis, produces an immunotoxin that affects human T-cell responses (Shenker et al., 1982;Shenker et al., 1990;Shenker et al., 1999). This toxin was shown to interfere with normal cell cycle progression resulting in G2 arrest as well as induction of apoptosis (Shenker et al., 1995). Moreover, we have shown that the toxin is the product of the cytolethal distending toxin (Cdt) operon (Shenker et al., 1999). Cdts are a family of heat-labile protein cytotoxins produced by several different bacterial species including diarrheal disease-causing enteropathogens such as some Escherichia coli isolates as well as Campylobacter jejuni, Shigella species, Haemophilus ducreyi and A. actinomycetemcomitans (Comayras et al., 1997;Mayer et al., 1999;Okuda et al., 1995;Okuda et al., 1997;Pickett et al., 1994;Pickett et al., 1996;Pickett and Whitehouse, 1999;Shenker et al., 1999). The Cdt operon contains three genes: cdtA, cdtB and cdtC which specify three polypeptides with molecular masses of 18, 32 and 20kDa, respectively. The three subunits form a heterotrimeric holotoxin that functions as an AB2 toxin.
Our studies have focused on Cdt subunit function, events critical to toxin-cell interactions, fate of Cdt-treated-cells and mode of action by which Cdt induces G2 arrest and apoptosis. In particular, we have demonstrated that Cdt subunits, CdtA and CdtC, comprise the binding unit which involves recognition and binding of CdtC to cholesterol in the context of lipid rafts (Boesze-Battaglia et al., 2006;Boesze-Battaglia et al., 2009). Other studies have also implicated glycans and glycoproteins on target cells as a site for toxin binding (Eshraghi et al., 2010;Nesic et al., 2004). The active subunit, CdtB, functions as a PIP3 phosphatase mimicking the tumor suppressors, PTEN (ptase and tensin homolog deleted on chromosome ten) or SHIP (src homology 2-containing inositol phosphatase), and is able to block the PI3K/PIP3/Akt signaling cascade (Shenker et al., 2007;Shenker et al., 2010a). It should be noted, however, that several investigators have demonstrated that in nonlymphoid cells CdtB functions as a DNase inducing DNA damage and thereby activating the DNA damage response pathway leading to G2 arrest and cell death (Cortes-Bratti et al., 2001a;Cortes-Bratti et al., 2001b;Frisan et al., 2003;Guerra et al., 2008;Lara-Tejero and Galan, 2000). We have not been able to confirm similar events in lymphoid cells (Shenker et al., 2006; Shenker et al., 2015).
Several cell types and cell lines have been shown to be susceptible to the toxic actions of Cdt, however, tropism for specific cells and/or tissue remains to be identified. Consistent with the ability of Cdt to intoxicate a large number of potential cell targets is its ability to bind to cholesterol, a ubiquitous membrane lipid. This is in opposition to many bacterial toxins that target specific cells via their ability to utilize cell-specific receptors. Nonetheless, it is notable that we have demonstrated that lymphocytes in vitro are most susceptible to Cdt, requiring very low concentrations of Cdt (pg/ml) to induce cell cycle arrest and apoptosis versus other cell types that typically require as much as microgram quantities (Shenker et al., 2007). Therefore, in order to account for differential cell susceptibility to Cdt, we chose to focus on the molecular mode of action by which the active subunit, CdtB, acts to induce cell cycle arrest and apoptosis. In this study we have specifically addressed the relationship between sensitivity to Cdt and the baseline status of the PI-3K signaling pathway with particular attention to the levels of CdtB’s substrate, PIP3 and the phosphorylation status of downstream signaling components Akt and GSK3β. Moreover, we have taken advantage of our previous observations demonstrating that susceptibility to Cdt varies among lymphocytes according to the presence or absence of the PIP3 degradative enzymes PTEN and SHIP (Shenker et al., 2007). Indeed, we now demonstrate that lymphoid cells exhibiting an active baseline level of the PI-3K signaling pathway characterized by heightened levels of PIP3, pAkt and pGSK3β, are more susceptible to Cdt toxicity than cells exhibiting a relatively quiescent pathway.
Methods
Cell culture and cell cycle analysis
The human leukemic T cell lines, Jurkat (E6-1) and the T-lymphoblastoid cell line CCRF-CEM, were maintained in RPMI-1640 supplemented with 10% FCS, 2 mM glutamine, 10 mM HEPES, 100 U/ml penicillin and 100 μg/ml streptomycin. HUT78 cells were maintained in Iscoves modified Dulbecco medium containing 4 mM L-glutamine and 20% FCS. The cells were harvested in mid-log growth phase and plated at 5 × 105 cells/ml, or as indicated, in 24-well tissue culture plates. Cells were exposed to medium or Cdt holotoxin and incubated for times indicated. The PTEN-inducible (PIJ-12) and control clone (Con18) Tet-on Jurkat cell lines were kindly provided by Simonetta Camandola, Ph.D. (National Institute of Aging, NIH) and have previously been described and characterized (Seminario et al., 2003). These cells were cultured in complete RPMI-1640 (described above) containing the antibiotics G418 and Hygromycin B; the expression of PTEN was induced by the addition of doxycycline (1 μg/ml).
To measure Cdt-induced cell cycle arrest, cells were incubated for the times indicated and then washed and fixed for 60 min with cold 80% ethanol (Shenker et al., 2005). The cells were stained with 10 μg/ml propidium iodide containing 1 mg/ml RNase (Sigma Chemical) for 30 min. Samples were analyzed on a Becton-Dickinson LSRII flow cytometer (BD Biosciences) as previously described (Shenker et al., 2005). A minimum of 15,000 events were collected for each sample; cell cycle analysis was performed using Modfit (Verity Software House).
DNA fragmentation in Cdt-treated cells was employed to determine the percentage of apoptotic cells using the TUNEL assay [In Situ Cell Death Detection Kit; (Boehringer Mannheim; Indianapolis, IN)]. Cell cultures were prepared as described above; at the end of the incubation period cells were centrifuged, resuspended in 1 ml of freshly prepared 4% formaldehyde and vortexed gently. After 30 min at RT, the cells were washed with PBS and permeabilized in 0.1% Triton X-100 for 2 min at 4_C. The cells were then washed with PBS and incubated in a solution containing FITC labeled nucleotide and terminal deoxynucleotidyl transferase (TdT) according to the manufacturers specifications. Following the final wash, the cells were resuspended in PBS and analyzed by flow cytometry.
Measurement of cellular PIP3
Cells (1 × 106/ml) were incubated in the presence of medium or Cdt for 4 hrs. Replicate cultures (0.5–1 × 107 cells) were pooled and harvested. The cell pellet was treated with cold 0.5 TCA for 5 min, centrifuged and the pellet washed twice with 5% TCA containing 1 mM EDTA. Neutral lipids were extracted twice with methanol:chloroform (2:1) at RT. Acidic lipids were extracted with methanol:chloroform:12M HCl (80:40:1) for 15 min at RT; the samples were centrifuged for 5 min and the supernatant recovered. The supernatant was then treated with 0.75 ml chloroform andb0.1 M HCl and centrifuged to separate organic and aqueous phases; the organic phase was collected and dried. The dried lipids were resuspended in 120 μl 50 mM HEPES buffer (pH 7.4) containing 150 mM NaCl and 1.5% sodium cholate, and left overnight at 4°C. PIP3 levels were then determined using commercially available competitive ELISA according to the manufacturers directions (Echelon).
Western blot analysis
Cells were treated as described and solubilized in 20mM Tris-HCl buffer (pH7.5) containing 150 mM NaCl, 1mM EDTA, 1% NP-40, 1% sodium deoxycholate and protease inhibitor cocktail (Pierce). Samples (30 μg) were separated on 12% SDS-PAGE and then transferred to nitrocellulose. The membrane was blocked with BLOTTO and then incubated with one of the following primary antibodies for 18 hrs at 4°C (Shenker et al., 1999): anti-Akt, anti-pAkt (S473), anti-GSK3β, anti-pGSK3β(S9) (Cell Signaling) or anti-GAPDH (Santa Cruz Biotechnology). Membranes were incubated with goat anti-mouse immunoglobulin conjugated to horseradish peroxidase (Southern Biotech Technology). The Western blots were developed using chemiluminescence and analyzed by digital densitometry (LICOR) as previously described (Shenker et al., 2010b).
Immunofluorescence of toxin binding and internalization
Jurkat cells or HUT78 cells (1 × 106) were incubated for 30 min (surface staining) or 1 hr (intracellular staining) in the presence of medium or 2 μg/ml of Cdt at 37°C (internalization) or 5°C (surface binding). Surface CdtC was detected by washing cells, exposure to normal mouse IgG (Zymed Labs; San Franscisco, CA) and then stained (30 min) for CdtC peptides with anti-CdtC subunit mAb (11B12) conjugated to AlexaFuor 488 (Molecular Probes; Eugene, OR) according to the manufacturers directions. After washing, the cells were fixed in 2% paraformaldehyde and analyzed by flow cytometry as previously described (Boesze-Battaglia et al., 2006). Intracellular CdtB was detected after exposure of cells to toxin (above) and fixation with 2% formaldehyde for 30 min followed by permeabilization with 0.1% Triton X-100 in 0.1% sodium citrate and stained with anti-CdtB mAb (17A1) conjugated to Alexafluor 488 (Molecular Probes).
Statistical analysis
Mean ± SEM were calculated for replicate experiments. Significance was determined using a Student’s t-test; differences between multiple treatments were compared by ANOVA paired with Tukeys HSD posttest; a P-value of less than 0.05 was considered to be statistically significant.
Results
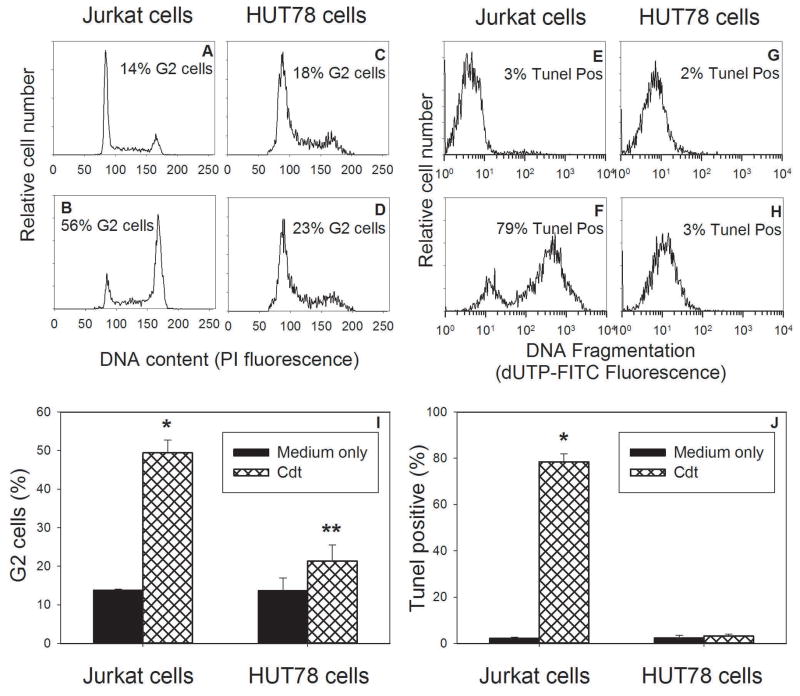
PTEN and SHIP1, both PIP3 phosphatases, regulate cell proliferation and survival by opposing the action of PI-3K, thereby, maintaining low levels of PIP3 and blocking phosphorylation and activation of Akt (Krystal, 2000;March and Ravichandran, 2002;Seminario and Wange, 2003). As a consequence of this mode of action, PTEN and SHIP function as tumor suppressors (Horn et al., 2004;Seminario et al., 2003). Indeed, somatic deletions or mutations have been identified in a variety of cancers making them, along with p53, the most commonly mutated genes in human cancers (Cantley et al., 1999). Of particular relevance, cell lines derived from leukemia and lymphoma patients often exhibit defects in either one or both of these lipid phosphatases (Horn et al., 2004;Seminario et al., 2003). We previously demonstrated that Jurkat cells, known to be deficient in both PTEN and SHIP (Horn et al., 2004;Seminario et al., 2003), exhibit the highest sensitivity to Cdt relative to other lymphoid cell lines which contain normal levels of PTEN and/or SHIP (Shenker et al., 2007). In Figure 1, the relative susceptibility of Jurkat cells to Cdt versus that of the cutaneous T-cell lymphoma cell line, HUT78, which contain normal levels of both PTEN and SHIP is shown. A representative experiment of the ability of Cdt (2 pg/ml) to induce G2 arrest in Jurkat cells and HUT78 cells is shown (Figs 1A–D); this dose of Cdt was determined to be optimal to induce cell cycle arrest in Jurkat cells. In the presence of Cdt, Jurkat cells exhibited an increase in the percentage of G2 cells to 56% versus 14% observed in control cells. In contrast, HUT78 cells exhibited 23% G2 cells in the presence of Cdt versus 18% in control cells. Results from multiple experiments are shown in Fig 1I and demonstrate that the increase in the percentage of G2 cells in toxin-treated Jurkat cells to 49.4±3.3% is statistically significant over the mean value of 13.8±0.2% observed with control cells. In contrast, HUT78 cells demonstrate consistent resistance to Cdt as this concentration induced 21.3±4.1% G2 cells versus 13.6±3.3% in control cells. Moreover, the difference between the percentage of G2 cells observed in the presence of Cdt for HUT78 and Jurkat cells is also statistically significant.
Figure 1.
Comparison of the effects of Cdt on toxicity in Jurkat and HUT78 cells. Jurkat cells were treated with medium alone (panel A) or Cdt (2.0 pg/ml) for 16–20 hr and cell cycle distribution determined following staining with propidium iodide (PI) and analysis by flow cytometry. Panels C and D show HUT78 cells in the presence of medium alone or Cdt, respectively. In addition to cell cycle, the effects of Cdt on apoptosis was assessed using the TUNEL assay; Panels E and F show the results from Jurkat cells in the presence of medium and Cdt (25 pg/ml), respectively, 48 hr after exposure to toxin. Results with HUT78 cells are shown in panels G (medium only) and H (Cdt). Numbers in each panel represent the percentage of G2 cells or apoptotic cells. Panels I and J show the aggregate results from three experiments each performed in duplicate; *indicate P<0.05 when compared to control Jurkat cells and ** indicates P<0.05 when compared to Cdt-treated Jurkat cells.
As demonstrated above, Cdt induces cell cycle arrest within 16–20 hrs following exposure of lymphocytes to the toxin. In addition to cell cycle arrest, exposure of lymphocytes to Cdt (25 pg/ml) for 48 hrs leads to activation of the apoptotic cascade. In order to detect apoptosis, we employed the TUNEL assay to assess the presence of chromosomal fragmentation. As shown in Fig. 1E–H, Cdt induced apoptosis in 79% of Jurkat cells versus 3% in control cells; these results are in contrast to HUT78 cells which did not become apoptotic as they exhibited 3% TUNEL positive cells in the presence of toxin versus 2% in control cells. Aggregate results for multiple experiments are shown in Fig. 1J and demonstrate a statistically significant increase in the percentage of apoptotic Jurkat cells in the presence of Cdt (79.4±3.5 %). HUT78 cells consistently failed to become apoptotic in the presence of Cdt (3.1±0.9 %).
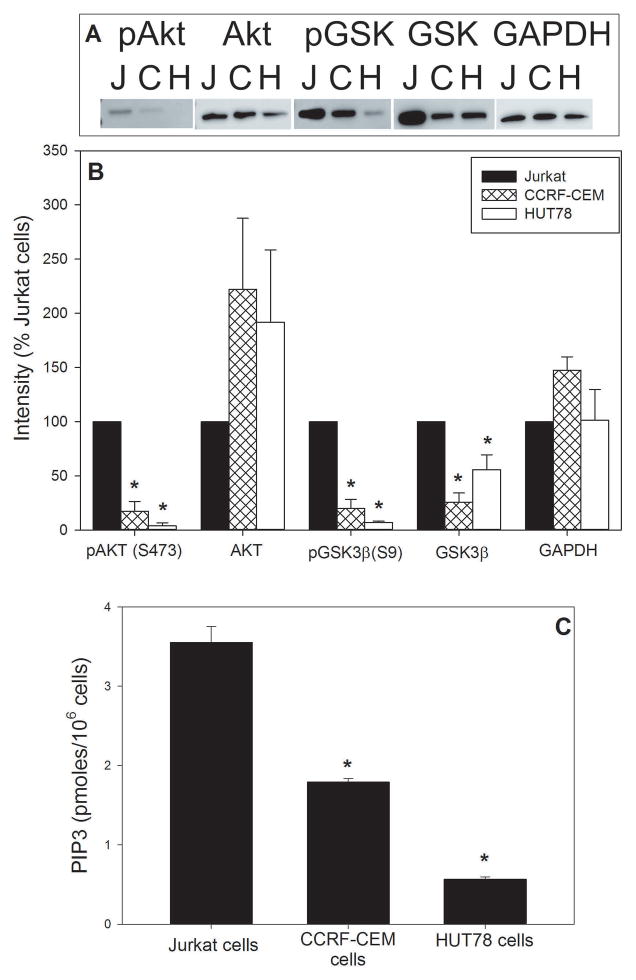
Analysis of cell cycle arrest and apoptosis demonstrates a clear difference in susceptibility between Jurkat cells and HUT78 cells. In order to relate these observations to the baseline status of the PI-3K signaling pathway, we assessed three components of this signaling pathway: PIP3 levels and the phosphorylation status of both Akt and GSK3β. As shown in Figure 2A and 2B, Jurkat cells exhibit Akt and GSK3β phosphorylation status that is clearly different from that observed with HUT78 cells. Akt is expressed in both cells, although total Akt levels in HUT78 cells is almost twice that observed in Jurkat cells (191.8±66.9). In contrast Jurkat cells contain very high levels of pAkt relative to HUT78; mean density for HUT78 cells is 3.8±2.9% of that observed for Jurkat cells. Likewise, both cells expressed GSK3β, although HUT78 cells contained 55.9±13.7% of this kinase relative to Jurkat cells. In contrast, HUT78 cells contained 7.0±1.2% of pGSK3β observed in Jurkat cells. Results are also shown for a third lymphoid cell line, CCRF-CEM which contain only SHIP1; we previously found these cells to exhibit a level of sensitivity to Cdt less than that observed with Jurkat cells but greater than HUT78 cells (Shenker et al., 2007). Likewise, the CCRF-CEM cells exhibited pAkt and pGSK3β levels that were less than that observed with Jurkat cells, but slightly higher than HUT78 cells. In addition to phosphorylation status of the downstream kinases, we assessed the levels of the upstream signaling lipid, PIP3 (see Fig. 2C). PIP3 levels were relatively high in Jurkat cells, 3.6±0.2 pmole/106 cells, versus 0.57±0.03 pmoles in HUT78 cells; CCRF-CEM cells exhibited 1.8±0.04 pmole/106 cells.
Figure 2.
Baseline analysis of components of the PI-3K signaling pathway. Panel A shows a representative Western blot for pAkt, Akt, pGSK3β (pGSK), GSK3β (GSK) and GAPDH for Jurkat cells, CCRM-CEM cells and HUT78 cells. Panel B shows the aggregate results for three experiments; results are expressed as a percentage of Jurkat cell density. Panel C shows the results of PIP3 levels and represent the mean SEM. *indicate P<0.05 when compared to control Jurkat cells
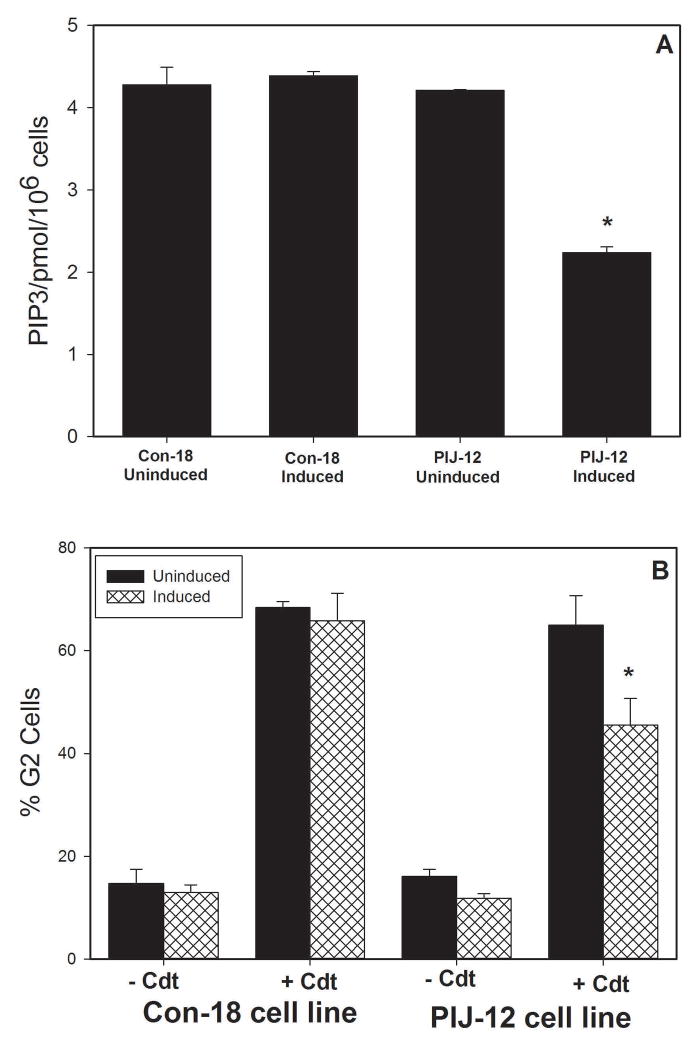
In the next series of experiments, we wanted to determine if we could change the status of Jurkat cells with respect to both PIP3 levels and susceptibility to Cdt by reconstituting them with PTEN. For these experiments, we utilized Jurkat cells, which are PTEN-null, containing a stable transfection of a tet-inducible PTEN plasmid [PIJ, PTEN-Inducible Jurkat; expression of PTEN in these cells have been previously demonstrated (Seminario et al., 2003)]. The effect of PTEN expression on PIP3 levels and susceptibility to Cdt was analyzed in PIJ clone 12 and a non PTEN-expressing control clone, Con18. As shown in Fig. 3A, PIP3 levels in un-induced PIJ-12 and Con18 were 4.2±0.01 and 4.3±0.02pmol/106 cells, respectively. Following tet-induced expression of PTEN, the levels were reduced to 2.2 ± 0.07 pmol/106 cells; no change was observed in similarly treated Con18 cells. In the absence of PTEN expression, both clones exhibited similar susceptibility to Cdt (Fig 3B); exposure to 200 pg/ml Cdt resulted in 68.4±1.2% (Con18) and 64.9±5.8% (PIJ-12) G2 cells. Induction of PTEN expression in PIJ-12 cells resulted in reduced susceptibility to Cdt; the percentage of G2 cells was 45.5±5.2 a statistically significant reduction in cell cycle arrest. In contrast, similarly treated Con18 cells exhibited no change in susceptibility to Cdt-induced cell cycle arrest (65.8±5.4% G2).
Figure 3.
Analysis of Jurkat cells reconstituted with PTEN. Panel A shows the baseline PIP3 levels were measured in Jurkat cells reconstituted with PTEN: controls (Con18) and pten transfection (PIJ-12); these cells were assessed under non-induced and induced conditions. Values are pmol PIP3/106 cells and represent the mean ± SEM of three replicate experiments; *indicate P<0.05 when compared to uninduced cells treated with Cdt. Panel B shows the effect of Cdt holotoxin on PIJ-12 and Con 18 cell lines which were treated with doxycycline as well as cells maintained under non-induced conditions. The percentage of G2 cells is plotted versus Cdt concentration. The mean ± SEM is shown for three experiments; *indicate P<0.05 when compared touninduced cells treated with Cdt.
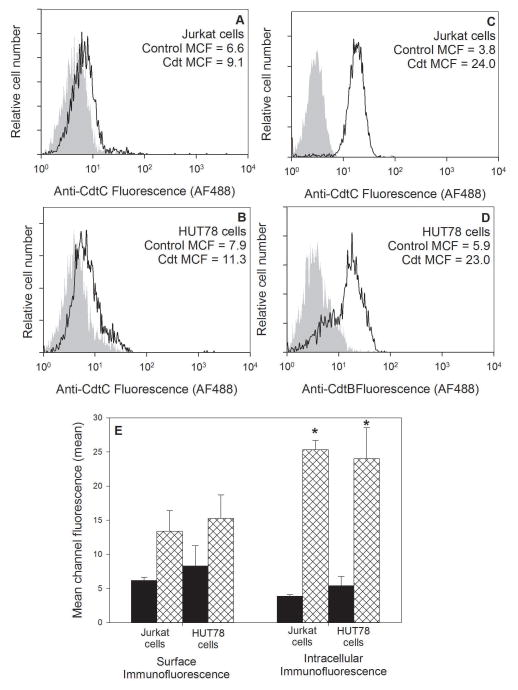
Cdt binding to the cell surface and subsequent internalization of CdtB are requirements for inducing toxicity; we demonstrate that the differential susceptibility to Cdt between Jurkat cells and HUT78 cells was not simply due to differences in these events. To measure toxin binding, cells were treated with Cdt holotoxin as described in Materials and Methods; the cells were then washed and stained with anti-CdtC monoclonal antibody (mAb) conjugated to AF488. As shown in Fig. 4A,B, both cells bound equivalent mount of Cdt as reflected in the mean channel fluorescence (MCF): 9.1 (Jurkat cells) and 11.3 (HUT78 cells) versus MCF of 6.6 and 7.9 in cells not treated with Cdt. Results from multiple experiments are shown in Fig. 4E; both cells bound significant and comparable amounts of toxin, MCF was 13.4±3.0 (Jurkat cells) and 15.3±3.5 (HUT78 cells). Intracellular CdtB was detected following treatment of cells with toxin, followed by fixation, permeabilization and staining with anti-CdtB mAb. As shown in Figs. 4C–4E, both lymphoid cells internalized comparable levels of CdtB: MCF of 24 (Jurkat cells) and 23 (HUT78 cells) versus MCF of 3.8 and 5.9 in untreated cells. Aggregate results from multiple experiments confirm that both Jurkat cells and HUT78 cells internalize comparable levels of CdtB; the MCF was 25.3±1.3 and 24.0±4.5 for Jurkat cells and HUT78 cells, respectively.
Figure 4.
Flow cytometric analysis of the binding of Cdt holotoxin and internalization of CdtB in Jurkat cells and HUT78 cells. Jurkat cells (panels A and B) and HUT78 cells (panels C and D) were exposed to medium (panels A and C) or Cdt (panels B and D). Cells were then stained for surface association of Cdt holotoxin (anti-CdtC mAb) or internalization of CdtB (anti-CdtB mAb). The mean channel fluorescence (MCF) is indicated in each panel for both control cells not exposed to Cdt (grey curve) and toxin treated cells (black line). The mean MCF ± SEM for three experiments is shown in panel E; *indicate P<0.05 when compared to control cells.
Discussion
Phosphoinositides (PIs) are derivatives of phosphatidylinositol and while they represent minor components of membrane lipids, PIs regulate a wide range of cellular functions by serving as site-specific membrane signals that mediate membrane recruitment and regulation of effector/signaling proteins (Kraub and Haucke, 2007;Sasaki et al., 2007). The best characterized of the PIs, PIP3, is synthesized from PI(4,5)P2 following the activation of phosphatidylinositol-3-kinase (PI-3K) and has received much attention for its critical role as a second messenger in the PI-3K/PIP3/PTEN/Akt signaling pathway. PIP3 recruits pleckstrin homology domain containing proteins such as BTK, Akt and PDK1 to the plasma membrane where they become activated and coupled to upstream signals and in turn transduce those signals to downstream signaling components (reviewed in (Martini et al., 2014). For example, activation of Akt (phosphorylation) leads to inhibition of GSK3β (phosphorylation) which in turn regulates glycogen metabolism and cell cycle progression. Activated Akt (pAkt) also inactivates pro-apoptotic targets such as Bad, Bim and Fox03a to promote cell survival. Additionally, pAkt regulates the mTOR signaling pathway which in turn promotes cell growth and protein synthesis. PIP3 is negatively regulated by the tumor suppressor phosphatase, PTEN or SHIP. It is noteworthy that mutations leading to activation of the PI-3K/PIP3/Akt pathway are highly oncogenic contributing to increased cell growth, proliferation and survival (Yuan and Cantley, 2008).
The critical role of PIP3 in the regulation of cell function including, proliferation and survival, is particularly relevant to the toxic effects of Cdt as we have previously demonstrated that the active toxin subunit, CdtB, functions as a PIP3 phosphatase, similar in function to PTEN and SHIP (Shenker et al., 2007). Furthermore, in addition to PIP3 depletion, we have recently demonstrated that Cdt treatment results in PI-3K signaling blockade and that a key element to toxicity is the downstream de-phosphorylation of Akt and GSK3β; this results in a loss of Akt kinase activity and a concomitant increase in GSK3β kinase activity (Shenker et al., 2015). In order to determine if cell susceptibility to Cdt is linked to differences in PIP3 content and activity of the PI-3K signaling pathway, we took advantage of lymphoid cell lines that differ in respect to their ability to express the lipid phosphatases. Specifically, we employed two cell lines with clear differences in PI-3K pathway activity. Jurkat cells, which lack both PIP3 degradative enzymes, PTEN and SHIP1, and HUT78 cells which do not have any known mutations associated with the PI-3K pathway. Indeed, we demonstrate a clear distinction in the baseline PI-3K pathway activity. Jurkat cells exhibit evidence of hyperactivity of the PI-3K pathway exemplified by relatively high levels of the signaling lipid, PIP3; these levels were almost four times higher than that observed in HUT78 cells. Consistent with these findings we also demonstrated that Jurkat cells exhibit elevated levels of pAkt and pGSK3β. This phosphorylation status reflects increased Akt kinase activity and decreased GSK3β kinase activity; collectively, these findings are consistent with and indicative of an active PI-3K signaling pathway in Jurkat cells relative to a quiescent signaling pathway associated with HUT78 cells.
In addition to differences in relative PI-3K signaling activity, we also observed that Jurkat cells exhibited high sensitivity to Cdt toxicity as the cells underwent both cell cycle arrest (16–20 hrs) and apoptosis (48 hrs) in the presence of Cdt. In contrast, HUT78 cells exhibited resistance to intoxication by Cdt under similar conditions and require significantly higher concentrations of toxin to exhibit signs cell cycle arrest (Shenker et al., 2007). It is also interesting to note that we have previously shown that cells harboring a single defect in PTEN, such as CCRF-CEM, exhibit a moderate sensitivity to Cdt that is between that observed for Jurkat and HUT78 cells (Shenker et al., 2007). This previous observation is of particular interest as we now show that the CCRF-CEM cells exhibit PIP3levels and exhibit an Akt and GSK3β phosphorylation status that is also less than that observed for Jurkat cells but greater than that for HUT78 cells suggesting a moderate level of PI-3K signaling activity relative to the other two cell lines.
To further demonstrate that baseline PIP3 levels, and likely, the status of PI-3K signaling contributes to toxin sensitivity, we employed Jurkat cells reconstituted with PTEN. PTEN expression in the PTEN-null Jurkat cell resulted in a reduction of baseline PIP3 levels along with a reduced susceptibility to Cdt-induced G2 arrest. It should be noted that Seminario et al (Seminario et al., 2003) demonstrated that these PTEN reconstituted Jurkat cells do not undergo cell cycle arrest, but instead exhibit a reduced rate of progression throughout each phase of the cell cycle. The differences in cell growth retardation versus Cdt-induced G2 arrest likely reside in distinct differences between PTEN and CdtB. First, PTEN represents an endogenous enzyme for which cells contain mechanisms to modify and limit its activity. Thus, PTEN expression did reduce PIP3, however, the levels were not as low as that which we previously observed with Cdt (Shenker et al., 2007). Second, it is possible that CdtB has distinct effects on the PI-3K pathway. In this context it should be noted that whereas PTEN degrades PIP3 resulting in the formation of PI-4,4-P2, CdtB metabolizes PIP3 to PI-3,4-P2 (Shenker et al., 2007). Thus the formation of polyinositol di-phosphates may be critical to the toxic effects of Cdt.
Collectively, our results demonstrate that lymphocyte susceptibility and sensitivity to Cdt is at least, in part due to the baseline status of PI-3K signaling, and in particular, the cellular content of PIP3. This conclusion is based upon results from lymphoid cells with oncogenic molecular lesions that contribute to disparate levels of PIP3 and in turn, PI-3K signaling activity. Further support for this conclusion is derived from our ability to modulate lymphocyte susceptibility to Cdt by reconstitution of Jurkat cells with PTEN and the accompanying reduction in PIP3 and PI-3K signaling activity and a decrease in sensitivity to Cdt. It should also be noted that differences in susceptibility to Cdt could not be explained by differential toxin binding or CdtB internalization. These findings are consistent with our previous studies that a critical component of Cdt toxicity is the ability of the active subunit, CdtB, to function as a PIP3 phosphatase (Shenker et al., 2007;Shenker et al., 2010a;Shenker et al., 2014). The requirement for this enzymatic activity was demonstrated through the use of mutational analysis, specific inhibitors of components of the PI-3K signaling pathway and by direct assessment of Cdt-treated cells. For instance, toxin-treated Jurkat cells exhibit a decline in substrate (PIP3) levels and a concomitant increase in enzymatic product, PI-3,4-P2. Likewise, the toxin was able to block mitogen-induced proliferation of primary lymphoid cultures (Shenker et al., 1999); these effects were accompanied by failure to activate the PI-3K signaling pathway, a requirement for the activation of these cells. (Buckler et al., 2008;Huang and Sauer, 2010;Luo, 2009;Rayasam et al., 2009;Shenker et al., 2007;Yuan and Cantley, 2008). In conclusion, this study advances our understanding of the underlying molecular mechanism by which Cdt acts to intoxicate cells and further defines the likely host target(s) of this potential virulence factor.
Acknowledgments
We thank the SDM Flow Cytometry Facility for its support of these studies. This work was supported by the National Institutes of Health grant DE06014.
References
- Boesze-Battaglia K, Besack D, McKay TL, et al. Cholesterol-rich membrane microdomains mediate cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cell Microbiol. 2006;8:823–836. doi: 10.1111/j.1462-5822.2005.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Brown A, Walker L, et al. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J Biol Chem. 2009;284:10650–10658. doi: 10.1074/jbc.M809094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler J, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunol Rev. 2008;224:239–248. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L, Neel B. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3 kinase/Akt pathway. Proc Natl Acad SCi. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comayras C, Tasca C, Peres SY, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Bratti X, Frisan T, Thelestam M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon. 2001a;39:1729–1736. doi: 10.1016/s0041-0101(01)00159-3. [DOI] [PubMed] [Google Scholar]
- Cortes-Bratti X, Karlsson C, Lagergard T, Thelastam M, Frisan T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J Biol Chem. 2001b;276:5296–5302. doi: 10.1074/jbc.M008527200. [DOI] [PubMed] [Google Scholar]
- Eshraghi A, Maldonado-Arocho F, Gargi A, et al. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J Bio Chem. 2010;285:18199–18207. doi: 10.1074/jbc.M110.112912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisan T, Cortes-Bratti X, Chaves-Olarte E, Stenerlow B, Thelastam M. The Haemophilus ducreyi cytolethal distending toxin induces DNA double-strand breaks and promotes ATM-dependent activation of RhoA. Cellular Microbiology. 2003;5:695–707. doi: 10.1046/j.1462-5822.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- Guerra L, Carr H, Richter-Dahlfors A, et al. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS ONE. 2008;3:e2254. doi: 10.1371/journal.pone.0002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S, Endl E, Fehse B, Weck M, Mayr G, Jucker M. Restoration of SHIP activity in a huma leukemia cell line downregulates constitutively activated phosphatidylinositol 3-kinase/Akt/BSK-3β signaling and leads to an increased transit time through the G1 phase of the cell cycle. Leukemia. 2004;18:1839–1849. doi: 10.1038/sj.leu.2403529. [DOI] [PubMed] [Google Scholar]
- Huang Y, Sauer K. Lipid signaling in T cell development and function. Cold Spring Harb Perspect Biol. 2010;2:1–25. doi: 10.1101/cshperspect.a002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraub M, Haucke V. Phosphoinositides: regulators of membrane traffic and protein function. FEBS Lett. 2007;581:2105–2111. doi: 10.1016/j.febslet.2007.01.089. [DOI] [PubMed] [Google Scholar]
- Krystal G. Lipid phosphatases in the immune system. Sem in Immunol. 2000;12:397–403. doi: 10.1006/smim.2000.0222. [DOI] [PubMed] [Google Scholar]
- Lara-Tejero M, Galan JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354–357. doi: 10.1126/science.290.5490.354. [DOI] [PubMed] [Google Scholar]
- Luo J. Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Letters. 2009;273:194–200. doi: 10.1016/j.canlet.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March M, Ravichandran K. Regulation of the immune response by SHIP. Sem in Immunol. 2002;14:37–47. doi: 10.1006/smim.2001.0340. [DOI] [PubMed] [Google Scholar]
- Martini M, DeSantis M, Braccini L, Gulluni F, Hirsch E. PI3K/Akt signaling pathway and cancer: an updated review. Annals of Med. 2014;46:372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bueno L, Hansen E, DiRienzo JM. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect Immun. 1999;67:1227–1237. doi: 10.1128/iai.67.3.1227-1237.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429:429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to the products of the cdtABC genes of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Kurazono H, Takeda Y. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb Pathog. 1995;18:167–172. doi: 10.1016/s0882-4010(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Cottle DL, Pesci EC, Bikah G. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect Immun. 1994;62:1046–1051. doi: 10.1128/iai.62.3.1046-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Pesci EC, Cottle DL, Russell G, Erdem AN, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CL, Whitehouse CA. The cytolethal distending toxin family. Trends in Microbiol. 1999;7:292–297. doi: 10.1016/s0966-842x(99)01537-1. [DOI] [PubMed] [Google Scholar]
- Rayasam G, Tulasi V, Sokhi R, Davis J, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Sasaki J, Sakai T, Takasuga S, Suzuki A. The physiology of phosphoinositides. Biol Pharm Bull. 2007;30:1599–1604. doi: 10.1248/bpb.30.1599. [DOI] [PubMed] [Google Scholar]
- Seminario M, Wange R. Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial. Immunol Cell Biol. 2003;192:80–97. doi: 10.1034/j.1600-065x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Seminario MC, Precht P, Wersto R, Gorospe M, Wange RL. PTEN expression in PTEN-null leukeamic T cell lines leads to reduced proliferation via slowed cell cycle progression. Oncogene. 2003;22:8195–8204. doi: 10.1038/sj.onc.1206872. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Besack D, McKay TL, Pankoski L, Zekavat A, Demuth DR. Induction of cell cycle arrest in lymphocytes by Actinobacillus actinomycetemcomitans cytolethal distending toxin requires three subunits for maximum activity. J Immunol. 2005;174:2228–2234. doi: 10.4049/jimmunol.174.4.2228. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Demuth DR, Zekavat A. Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect Immun. 2006;74:2080–2092. doi: 10.1128/IAI.74.4.2080-2092.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, Dlakic M, Walker L, et al. A novel mode of action for a microbial-derived immunotoxin: the cytolethal distending toxin subunit B exhibits phosphatidylinositol (3,4,5) tri-phosphate phosphatase activity. J Immunol. 2007;178:5099–5108. doi: 10.4049/jimmunol.178.8.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker BJ, McArthur WP, Tsai CC. Immune Suppression Induced by Actinobacillus actinomycetemcomitans. I. Effects on human peripheral blood lymphocyte responses to mitogens and antigens. J Immunol. 1982;128:148–154. [PubMed] [Google Scholar]
- Shenker BJ, McKay TL, Datar S, Miller M, Chowhan R, Demuth DR. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- Shenker BJ, Vitale L, King C. Induction of human T cells that coexpress CD4 and CD8 by an immunomodulatory protein produced by Actinobacillus actinomycetemcomitans. Cell Immunol. 1995;164:36–46. doi: 10.1006/cimm.1995.1140. [DOI] [PubMed] [Google Scholar]
- Shenker BJ, Vitale LA, Welham DA. Immune suppression induced by Actinobacillus actinomycetemcomitans: Effects on immunoglobulin production by human B cells. Infect Immun. 1990;58:3856–3862. doi: 10.1128/iai.58.12.3856-3862.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B, Boesze-Battaglia K, Scuron D, Walker L, Zekavat A, Dlakic M. The toxicity of the Aggregatibacter actinomycetemcomitans cytolethal distending toxin correlates with its phosphatidylinositol (3,4,5) triphosphate phosphatase activity. Cell Microbiol. 2015 doi: 10.1111/cmi.12497. [DOI] [PubMed] [Google Scholar]
- Shenker B, Boesze-Battaglia K, Zekavat A, Walker L, Besack D, Ali H. Inhibition of mast cell degranulation by a chimeric toxin containing a novel phosphatidylinositol-3,4,5-triphosphate phosphatase. Molec Immunol. 2010a;48:203–210. doi: 10.1016/j.molimm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B, Boesze-Battaglia K, Zekavat A, Walker L, Besack D, Ali H. Inhibition of mast cell degranulation by a chimeric toxin containing a novel phosphatidylinositol-3,4,5-triphosphate phosphatase. Molecular Immunology. 2010b;48:203–210. doi: 10.1016/j.molimm.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B, Walker L, Zekavat A, Dlakic M, Boesze-Battaglia K. Blockade of the PI-3K signaling pathway by the Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces macrophages to synthesize and secrete pro-inflammatory cytokines. Cell Microbiol. 2014;16:1391–1404. doi: 10.1111/cmi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T, Cantley L. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]