Abstract
In women, obesity is associated with decrements in reproductive health that are improved with weight loss. Due to the difficulty of maintaining weight loss through lifestyle interventions, surgical interventions have become popular treatments for obesity. We examined how weight loss induced by Roux-en Y gastric bypass surgery (RYGB) or calorie restriction impacted expression of hypothalamic genes related to energy intake and reproduction. RYGB and calorie restriction induced equivalent weight loss; however, expression of the anorexigenic melanocortin pathway decreased only in calorie restricted mice. Serum estradiol concentrations were lower in calorie restricted mice relative to RYGB during proestrous, suggesting that RYGB maintained normal estrous cycling. Thus, effects of RYGB for female mice, and possibly humans, extend beyond weight loss to include enhanced reproductive health.
Keywords: Roux-en-Y gastric bypass (RYGB), 17beta-estradiol, estrogen receptor alpha (Erα), Proopiomelanocortin (Pomc), Agouti-related peptide (Agrp), neuropeptide Y (Npy), Kisspeptin (Kiss1), reproduction
Introduction
In the United States, 61.9% of adult women are overweight, while the prevalence of obesity has increased to 33.9% of women older than 20 years of age [1]. In addition to increasing the risks of developing cardiovascular disease, diabetes, and cancer, obesity significantly impairs reproductive health in women. The relative risk of infertility due to ovulatory disorders increases with BMI, and excess body weight is associated with abnormal levels of reproductive hormones [2-4]. For example, hyperinsulinemia secondary to insulin resistance (a common feature of obesity) depresses hepatic secretion of sex hormone-binding globulin (SHBG), leading to reflexive upregulation of androgen synthesis. The resultant hyperandrogenaemia perturbs menstrual cycling and ovulation [5]. Weight loss remediates insulin resistance and improves menstrual cycling in women; thus, weight loss represents a primary goal for the return of reproductive function in obese women. However, significant lifestyle modifications or pharmacological therapies have proven to be inefficacious for long-term weight loss and weight loss maintenance [6].
By contrast, Roux-en-Y gastric bypass (RYGB), one of the most commonly performed bariatric procedures in the world [7], has demonstrated superiority versus conventional dietary intervention for weight loss and diabetes resolution in randomized, controlled studies [6, 8-10]. Moreover, Sarwer et al. recently characterized significant improvements in overall sexual function, reproductive hormone levels, and psychosocial status in women following RYGB surgery [3]. Interestingly, many of these beneficial effects have been observed independently of significant weight loss, implying that the surgery itself has a beneficial effect on reproductive health; however, despite widespread clinical use of the surgery, the mechanisms underlying this effect remain incompletely understood.
Hypothalamic neurons sense perturbations in energy status and alter secretion of reproductive hormones accordingly; thus, the hypothalamus integrates nutrient and reproductive signaling. To better understand how the mode of weight loss (i.e. reductions of total body energy stores) influences reproductive health, we compared hypothalamic gene expression patterns in female mice undergoing either RYGB surgery or calorie restriction-induced weight loss. A more complete characterization of how differing modes of weight loss impact hormonal and hypothalamic signaling will facilitate the development of alternative therapies to bariatric surgery that can be more broadly applied to the wider problem of obesity and obesity-related comorbidities.
Materials and methods
Animal Care
Studies were conducted in accordance with UT Southwestern Institutional Animal Care and Use Committee and the Association of Assessment and Accreditation of Laboratory Animal Care policies. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Mice were individually housed in a temperature-controlled environment at 22°C-24°C using 12-hour light/12-hour dark cycles (Light cycle: 0600-1800 hours). Female C57/Bl6 mice were placed on high fat diet (HFD) (D12492, Research Diets) at six weeks of age in order to induce obesity.
Study design
Mice were maintained on HFD diet from six weeks of age. Upon reaching approximately 40-45g (12-14 weeks on HFD), mice were randomized to receive RYGB or sham operations (SO). To control for the effects of weight loss per se, a subset of female sham-operated diet induced obese (DIO) mice were weight-matched to the RYGB group by calorie restriction (WM-SO). After recovery from surgery, mice were provided HFD ad libitum. Body weight was monitored daily and body composition evaluated using a Minispec mq10 NMR (Bruker Optics). Food intake was measured over four consecutive days during week four.
Surgical intervention
RYGB surgery was performed as described [11]. Briefly, RYGB involved gastrointestinal reconstruction such that ingested nutrients pass from a proximal gastric pouch into a jejunal afferent limb. Distal stomach and proximal intestine were excluded from alimentary flow using a vascular clip (Ethicon) placed just distal to the gastro-jejunostomy. The sham procedure involved gastrotomy, enterotomy, and repair. Mice were maintained using a scavenged circuit of isoflurane and anesthesia time was standardized between groups. Subsequent to the surgery, mice were maintained on a standardized feeding protocol in which liquid diet was provided from post-operative days 2-7. On post-operative day 6, 0.25g of HFD was provided on a daily basis until consumed in its entirety. Subsequently, solid diet was re-introduced ad libitum.
Metabolic Chamber Analyses
Respiratory Exchange Ratio (RER) was determined by assessment of VO2 and CO2 consumption while mice were housed in a combined indirect calorimetry system (TSE Systems). Mice were singly housed and adapted to the metabolic cages for six days prior to measurements. During the measurement periods, heat production, VO2, and CO2 consumption were simultaneously determined. The mice were permitted to eat their usual diet while in the metabolic chamber and food intake was monitored.
Determination of Estrus Cycle, Euthanasia, Serum and Tissue Collection
Prior to sacrifice the mice underwent vaginal lavage with sterile phosphate buffered saline, and vaginal cytology was used to determine phase of the estrus cycle [12]. At sacrifice, mice were fasted for 3-4 hours, deeply sedated with isoflourane (Aerrane, Baxter), and euthanized by decapitation. Whole trunk blood was collected. The brain was removed and the basal medial hypothalamus was dissected and snap-frozen in liquid nitrogen for gene expression analysis.
Hormone and metabolite measurements
Serum was obtained by centrifugation and assayed using enzyme–linked immunosorbent assays (Invitrogen) for 17beta-estradiol and leptin.
Tissue mRNA Analyses
Tissue samples were homogenized in Trizol (Invitrogen) using a TissueLyser (Qiagen). Total mRNA was extracted using the RNeasy RNA extraction kit and protocol (Qiagen). Quality and quantity of RNA were determined by UV Spectroscopy (absorbance at 260/280 nm). cDNA was prepared from 2.0 μg mRNA using Superscript III reverse transcriptase (Invitrogen) and oligo(dT) (Invitrogen). Real time quantitative polymerase chain reactions (qPCR) assays were performed according to published protocols [13] using an ABI 7900HT system. TaqMan gene expression assays (Life Technologies) were used to determine expression levels of the following genes: Estrogen Receptor Alpha (ERalpha), Estrogen Receptor Beta (ERbeta), Proopiomelanocortin (Pomc), Neuropeptide Y (Npy), Agouti-related Peptide (Agrp), Kisspeptin 1(Kiss1), and Kisspeptin 1 Receptor (Kiss1r). The identification and catalog numbers of these assays are available upon request.
Statistics
The data are presented as mean ± SEM. Only p-values less than .05 were considered statistically significant. For all data sets, experiments comparing two means were analyzed using Student's t-test, with Welch's correction as appropriate; experiments comparing three or more means were analyzed using one-way ANOVA followed by Tukey-Kramer Post-Hoc. The post-operative body weight curve of the three treatments groups was analyzed using repeated measures two-way ANOVA followed by Tukey Post-Hoc (Fig. 1a). Statistical significance between SO and RYGB or WM-SO groups is denoted by *. Statistical significance between RYGB and WM-SO groups is denoted by #.
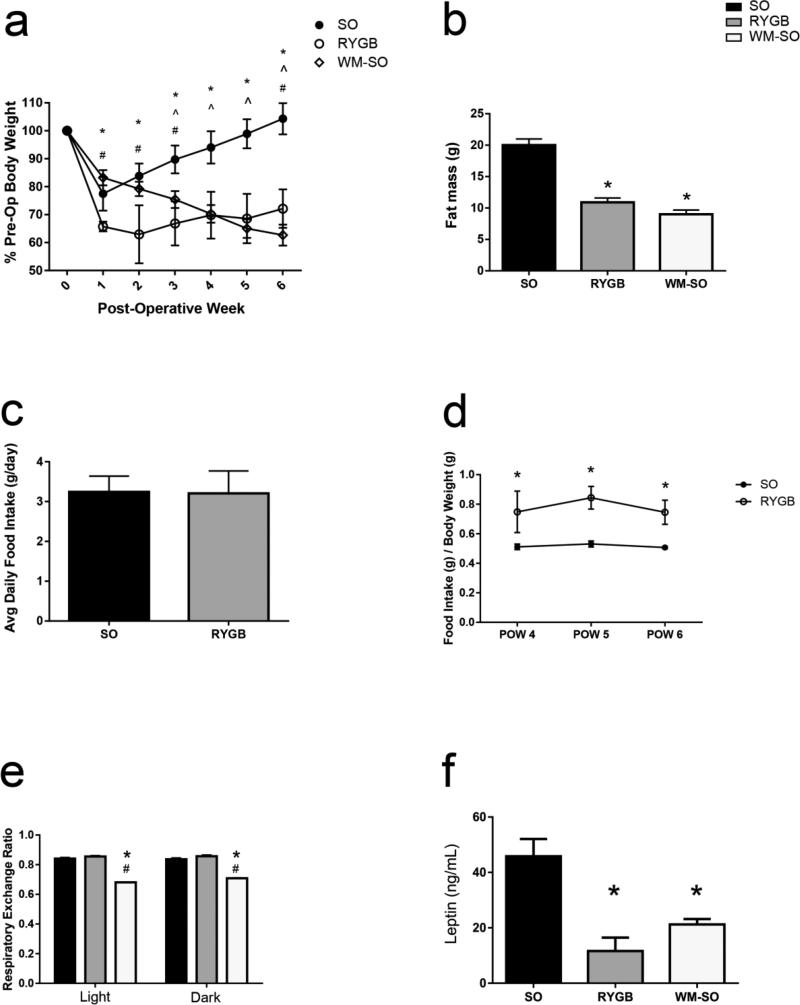
Figure 1.
Physical effect of RYGB and calorie restriction. Three groups of mice received either sham operation (SO), Roux-en-Y gastric bypass surgery (RYGB), or sham operation with subsequent caloric restriction (WM-SO). Effect on (a) body weight and (b) adipose mass following the surgeries. Comparison of (c) total food intake and (d) food intake per gram of body weight in SO and RYGB groups in the post-surgical period. (g) Respiratory Exchange Ratio (RER) in SO, RYGB, and WM-SO mice measured in metabolic cages. (f) Fasting serum leptin at the time of sacrifice. Data are presented as mean ± SEM with statistical significance set at P-value < 0.05. * denotes statistical significance between SO and RYGB or WM-SO groups. # denotes statistical significance between RYGB and WM-SO groups.
Results
Postsurgical Weight Loss
Over the six week postoperative course, RYGB surgery and calorie restriction induced significant body weight and fat mass loss in female DIO mice. By post-operative week six, RYGB mice lost 27.86% of their pre-operative weight, while calorie-restricted WM-SO mice lost 37.33% (Fig. 1a). Additionally, fat mass was significantly reduced in both RYGB and WM-SO groups (Fig. 1b).
During post-operative week four we assessed food intake in SO, RYGB, and WM-SO groups. Food intake did not differ between RYGB and SO groups (Fig. 1c). Indeed, when viewed on a per gram body weight basis, RYGB mice consumed more food than the SO group (Fig. 1d). During this time period, SO mice gained an average of 0.31g of body weight per day, while RYGB gained only an average of 0.03g body weight. Thus, feeding efficiency (calculated as the average daily change in body weight divided by average daily food intake) declined by 91.7% in the RYGB group versus SO (Supplementary Fig. 1a), further indicating that weight loss following RYGB surgery was not secondary to alterations in food intake.
Respiratory Exchange Ratio
The Respiratory Exchange Ratio indicates the relative contributions of lipid and carbohydrate to energy metabolism. In order to assess how differing modes of weight loss influence nutrient substrate use, we measured RER in SO, RYGB, and WM-SO groups. RER did not differ between RYGB and SO groups; however, it significantly decreased in WM-SO, indicating a shift toward greater fatty acid oxidation (Fig. 1e).
Serum Leptin
Plasma leptin concentrations are directly proportional to adipose tissue mass. Consistent with this, leptin concentrations were lower in RYGB and WM-SO groups compared to SO (Fig. 1f). Interestingly, leptin concentrations were lowest in the RYGB group; however, the difference between RYGB and WM-SO leptin concentrations was not statistically significant (Fig. 1f).
Estradiol and Hypothalamic Gene Expression
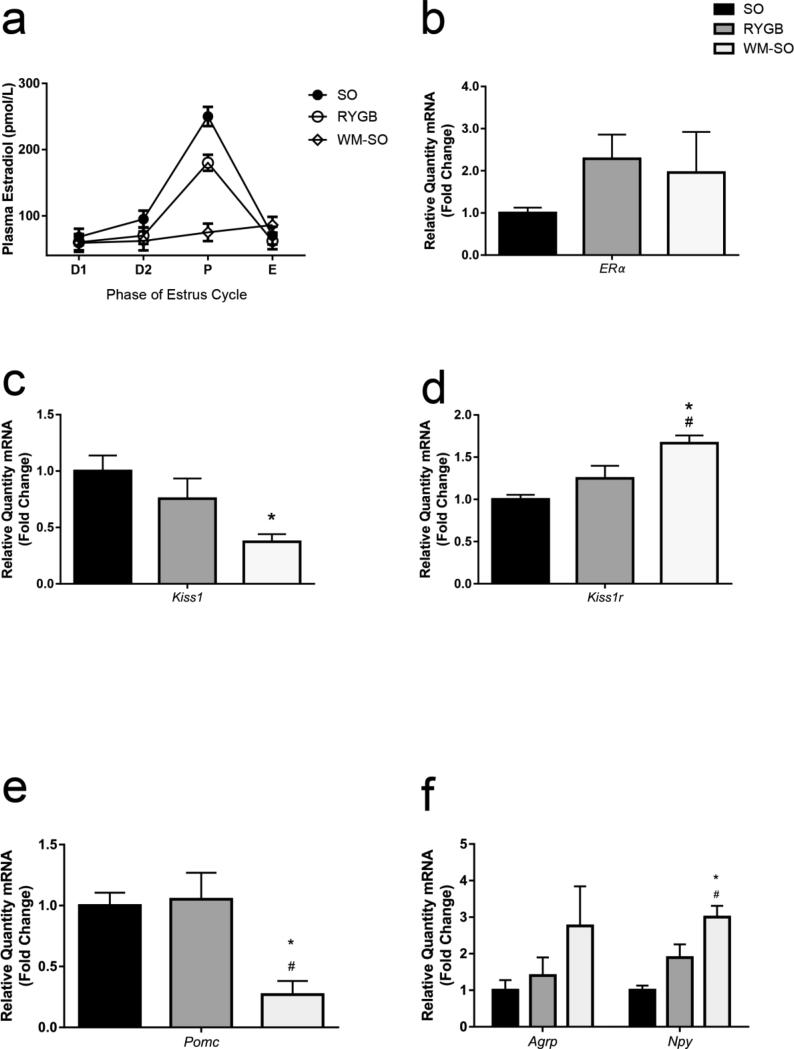
Obese women have significantly higher plasma estradiol levels than lean women [14]. Consistent with this, prior to estrous, plasma 17-beta estradiol concentrations were higher in SO versus RYGB or WM-SO (Fig. 2a).
Figure 2.
RYGB effect on estradiol and hypothalamic gene expression. (a) Concentration of 17beta–estradiol versus phase of estrous cycle in SO, RYGB, and WM-SO female mice. Relative expression levels of (b) Eralpha (c) Kisspeptin (d) Kisspeptin receptor, (c) Pomc, (f) Agrp and Npy mRNA in SO, RYGB, and WM-SO groups. Data are presented as mean ± SEM with statistical significance set at P-value < 0.05. * denotes statistical significance between SO and RYGB or WM-SO groups. # denotes statistical significance between RYGB and WM-SO groups.
Reductions in expression of estrogen receptor alpha (ERalpha) in the hypothalamus lead to increases in body weight [15]. Hypothalamic ERalpha expression was lower in SO mice compared to both RYGB and WM-SO females (Fig. 2b). Expression of ERbeta, which has not been consistently associated with the regulation of energy homeostasis or reproduction, did not differ between the groups (Supplementary Fig. 1b).
Kisspeptin (coded by the Kiss1 gene) binds the kisspeptin receptor to induce release of GnRH from hypothalamic neurons. As such, kisspeptin represents a critical mediator of reproductive function. Importantly, certain populations of hypothalamic neurons express not only Kiss1 and Kiss1r, but also the leptin receptor, indicating that these neurons also respond to leptin. In order to assess how the mode of weight loss influences Kiss1 expression, we assayed Kiss1 and Kiss1r in the hypothalamus. Kiss1 expression significantly decreased in the WM-SO group (Fig. 2c), while Kiss1r expression significantly increased (Fig. 2d).
Orexigenic and Anorexigenic Gene Expression in the Hypothalamus
To determine if differences in the hypothalamic-pituitary-gonadal axis (HPG) were associated with the mode of weight loss, we dissected the basal medial hypothalamus and determined the relative expression level of multiple genes related to energy homeostasis and reproduction. Pomc expression did not differ between SO and RYGB groups; however, it did significantly decrease in WM-SO mice (Fig. 2e). Agrp and Npy expression increased in WM-SO relative to both SO and RYGB groups (Fig. 2f); however, only Npy levels reached statistical significance. These data suggest a compensatory reduction in the anorexigenic leptin-melanocortin pathway in response to weight loss in WM-SO females, which did not occur in the RYGB group.
Discussion
Here, we demonstrate that RYGB produces significant and persistent weight loss without reductions in food intake. Furthermore, RYGB maintains a nutrient utilization profile more similar to SO than WM-SO. Finally, we show that calorie restriction but not RYGB alters Kiss1 and Pomc gene expression, as well as decreases circulating 17-beta estradiol levels prior to estrous despite similar magnitudes of weight loss between the groups. These differences suggest that the method of weight loss impacts hypothalamic circuits with consequences for both energy homeostasis and reproduction.
Consistent with human and rodent studies, both RYGB and caloric restriction reduced overall body weight and plasma leptin levels [8, 16, 17], with RYGB mice having the lowest mean concentrations of the three groups. Since both RYGB and WM-SO mice lost similar amounts of body weight by different means, this data suggests that RYGB could impact leptin secretion or sensitivity in a unique manner; indeed, in humans, leptin levels fall after bariatric surgery without concomitant increases in food intake [18], implying that gastric bypass surgeries could “re-sensitize” hypothalamic circuits to the effects of leptin. However, the lack of a statistically significant difference between leptin concentrations in RYGB and WM-SO groups weakens this notion and indicates the need for additional, higher-powered studies. Finally, while we did not observe increases in leptin receptor expression between RYGB and WM-SO mice (data not shown), an influence of RYGB on hypothalamic leptin sensitivity cannot be ruled out and should be given due consideration in future investigations.
Hypothalamic regulation of body weight involves counterbalancing anorexigenic and orexigenic neuronal signaling in response to changes in energy status, the end result being alterations in food intake and energy expenditure. For example, anorexigenic Pomc neurons produce alpha-melanocortin stimulating hormone (alpha-MSH) and beta-endorphin; alpha-MSH, in turn, stimulates the melanocortin 4 receptor (MC4R) to reduce food intake. Conversely, the orexigenic neuropeptides AGRP and NPY inhibit MC4R activation and promote food intake. In this study, Pomc expression was significantly lower in WM-SO versus SO and RYGB mice, suggesting a pronounced orexigenic response to calorie restriction and weight loss. This response was unsurprising; indeed, the natural response to caloric deficit should be hunger. However, the fact that RYGB mice had neither a pronounced reduction of Pomc gene expression, nor a precipitous increase of Agrp or Npy despite significant reductions in plasma leptin concentration, indicates that the RYGB surgical intervention alters the hypothalamic signaling response to weight loss. Human bariatric patients do not report increased hunger ratings following surgery despite significant weight loss, which is in stark contrast to patients on severe caloric restriction who report enhanced hunger [19]. This lack of overt hunger in human RYGB patients is consistent with the lack of increased food intake we observed in the RYGB group and implies that hypothalamic signaling could play a critical part in the efficacy of RYGB surgery in humans.
While it is well established that Pomc neurons regulate energy homeostasis [20, 21], evidence also indicates that Pomc neurons coordinate aspects of reproduction, as well. Pomc neurons make direct synaptic contact with Gnrh neurons [22-25] and release the neurotransmitters GABA and glutamate [26], both of which have been shown to regulate Gnrh neurons [27-29], and Pomc itself can be cleaved into either alpha-MSH or beta-endorphins [30-32]. While alpha-MSH affects excitatory inputs on Gnrh neurons by acting on central melanocortin receptors [33, 34], beta-endorphin inhibits GnRH and LH secretion [24, 35]. Furthermore, beta-endorphin levels fluctuate across the ovarian cycle, indicative of an important role in regulating negative feedback and maintenance of normal reproduction. Interestingly, it is thought Pomc neurons make a ‘choice’ to express alpha-MSH or beta-endorphin based on nutrient status, adiposity, and the presence of signaling peptides, including leptin.
In this respect, leptin acts as a metabolic signal to hypothalamic Pomc neurons in the hypothalamus, as well as a modulator of reproductive function. In anorectic females and in athletes with extreme reductions in body weight, exogenous leptin administration increases luteinizing hormone [36] and restores the menstrual cycle [37]. Our data demonstrate that weight reduction by caloric restriction but not RYGB reduces leptin levels and suppresses plasma estradiol concentrations prior to estrous. RYGB reduced leptin levels to a greater degree than in the WM-SO group, yet estradiol concentrations remained significantly higher prior to estrous for RYGB mice. Based on this evidence, it is reasonable to hypothesize that RYGB maintains leptin sensitivity, thereby triggering Pomc neurons to release beta-endorphins and maintain reproductive capacity despite significant weight loss. In contrast, weight loss induced by calorie restriction increases Pomc cleavage into alpha-MSH, thereby reducing gonadotropin secretion and, possibly, overall reproductive capacity.
We also observed significant decreases in kisspeptin expression and significant increases in kisspeptin receptor mRNA in WM-SO but not RYGB groups. In rodents, kisspeptin is expressed in the neurons of the hypothalamic arcuate nucleus and anteroventral periventricular nucleus, and binds kisspeptin receptors on Gnrh neurons to release GNRH into circulation [38]. This kisspeptin signal pathway is required for sexual maturity and reproductive function [38]. Combined with reduced plasma estradiol prior to estrous, reductions of kisspeptin in the hypothalamus of mice imply the presence of reproductive impairment in WM-SO but not RYGB mice.
Finally, estrogens and Erα regulate Pomc excitability [39], negative feedback, and reproduction. Estrogens influence reproduction and ovarian cycles; however in obese women, there are significant increases in circulating estrogen levels which are associated with changes in sexual function, alterations in testosterone, and changes in reproductive hormonal profiles. Recently, following RYGB in women, Sarwer et al. reported improvements in sexual function, circulating estradiol, total testosterone, follicle-stimulating hormone, lutenizing hormone, and sex hormone-binding globulin levels [3]. Consistent with this, we observed reduced circulating concentrations of 17 beta-estradiol levels prior to estrous in both RYGB and WM-SO groups; however, the concentrations in WM-SO were substantially lower than RYGB, indicating a reduced ability in WM-SO to ovulate. Interestingly, reductions in 17beta-estradiol corresponded to changes in hypothalamic expression of Erα: Erα expression was greater in RYGB and WM-SO than SO mice. This increase in Erα expression may be associated with the body weight reductions seen in both cohorts when compared with the SO group.
Conclusion
RYGB reduces body weight independent of changes in food intake. Despite comparable weight loss, the expression of key hypothalamic genes (notably, Pomc, Kiss1, and Erα), as well as circulating leptin and 17 beta-estradiol concentrations differ between RYGB and WM-SO mice, possibly driving a normalization of estrous cycling following RYGB surgery. Our findings begin to address the unique hormonal and hypothalamic responses to differing methods of weight loss; however, additional research is required to further understand the weight-independent mechanisms by which alterations in reproductive potential are achieved following RYGB.
Supplementary Material
Acknowledgments
We would like to acknowledge and thank Vincent Aguirre, M.D., PhD. for performing the gastric bypass and sham procedures.
Funding: This research was supported by the NIH grant, DK 073689, and the Women's Health Institute.
Footnotes
Email addresses of contributing authors:
Aaron.frank@cshs.org
Juliet.Fong@UTSouthwestern.edu
Disclosure of Previously Published Data
The present manuscript expands upon a project from which data has been previously published. Specifically, the results presented in Figure 1a-d, f and Supplementary Figure 1a can be found here [40]. We have chosen to include this previously published data in order to establish a context for the interpretation of this manuscript's novel findings.
Conflict of Interest Statement
No conflict of interest exists for any of the contributing authors, Aaron Frank, Juliet Zechner, and Deborah Clegg.
Statement of Human and Animal Rights
Studies were conducted in accordance with UT Southwestern Institutional Animal Care and Use Committee and the Association of Assessment and Accreditation of Laboratory Animal Care policies. All applicable institutional and/or national guidelines for the care and use of animals were followed.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81. doi: 10.1016/s0140-6736(14)60460-8. Epub 2014/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):482–7. doi: 10.1097/MED.0b013e3282f1d6cb. Epub 2007/11/06. [DOI] [PubMed] [Google Scholar]
- 3.Sarwer DB, Spitzer JC, Wadden TA, Mitchell JE, Lancaster K, Courcoulas A, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg. 2014;149(1):26–33. doi: 10.1001/jamasurg.2013.5022. Epub 2013/11/06. [DOI] [PubMed] [Google Scholar]
- 4.Rich-Edwards JW, Goldman MB, Willett WC, Hunter DJ, Stampfer MJ, Colditz GA, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–7. doi: 10.1016/0002-9378(94)90465-0. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali R, Gambineri A. Metabolic effects of obesity on reproduction. Reprod Biomed Online. 2006;12(5):542–51. doi: 10.1016/s1472-6483(10)61179-0. Epub 2006/06/23. [DOI] [PubMed] [Google Scholar]
- 6.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. Epub 2013/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obesity surgery. 2015;25(10):1822–32. doi: 10.1007/s11695-015-1657-z. Epub 2015/04/04. [DOI] [PubMed] [Google Scholar]
- 8.Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. Jama. 2013;309(21):2240–9. doi: 10.1001/jama.2013.5835. Epub 2013/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. The New England journal of medicine. 2012;366(17):1567–76. doi: 10.1056/NEJMoa1200225. Epub 2012/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. Epub 2014/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM, et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology. 2013;144(3):580–90. e7. doi: 10.1053/j.gastro.2012.11.022. Epub 2012/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PloS one. 2012;7(4):e35538. doi: 10.1371/journal.pone.0035538. Epub 2012/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal. 2003;1:e012. doi: 10.1621/nrs.01012. Epub 2006/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin H, Austin JM, Jr., Partridge EE, Hatch KD, Shingleton HM. Endometrial cancer, obesity, and body fat distribution. Cancer Res. 1991;51(2):568–72. Epub 1991/01/25. [PubMed] [Google Scholar]
- 15.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2501–6. doi: 10.1073/pnas.0610787104. Epub 2007/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Annals of surgery. 2004;240(2):236–42. doi: 10.1097/01.sla.0000133117.12646.48. Epub 2004/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33(7):786–95. doi: 10.1038/ijo.2009.79. Epub 2009/05/07. ijo200979 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavalda JM, Hernandez M, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obesity surgery. 2013;23(11):1790–8. doi: 10.1007/s11695-013-1033-9. Epub 2013/07/09. [DOI] [PubMed] [Google Scholar]
- 19.Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. The American psychologist. 2007;62(3):220–33. doi: 10.1037/0003-066x.62.3.220. Epub 2007/05/02. [DOI] [PubMed] [Google Scholar]
- 20.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–41. doi: 10.1016/s0092-8674(00)81865-6. Epub 1997/01/10. [DOI] [PubMed] [Google Scholar]
- 21.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10(6):211–6. doi: 10.1016/s1043-2760(99)00153-8. Epub 1999/07/17. [DOI] [PubMed] [Google Scholar]
- 22.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Catecholaminergic innervation of luteinizing hormone-releasing hormone and glutamic acid decarboxylase immunopositive neurons in the rat medial preoptic area. An electron-microscopic double immunostaining and degeneration study. Neuroendocrinology. 1988;48(6):591–602. doi: 10.1159/000125068. Epub 1988/12/01. [DOI] [PubMed] [Google Scholar]
- 23.Thind KK, Goldsmith PC. Infundibular gonadotropin-releasing hormone neurons are inhibited by direct opioid and autoregulatory synapses in juvenile monkeys. Neuroendocrinology. 1988;47(3):203–16. doi: 10.1159/000124914. Epub 1988/03/01. [DOI] [PubMed] [Google Scholar]
- 24.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between pro-opiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449(1-2):167–76. doi: 10.1016/0006-8993(88)91035-9. Epub 1988/05/24. [DOI] [PubMed] [Google Scholar]
- 25.Ward DR, Dear FM, Ward IA, Anderson SI, Spergel DJ, Smith PA, et al. Innervation of gonadotropin-releasing hormone neurons by peptidergic neurons conveying circadian or energy balance information in the mouse. PloS one. 2009;4(4):e5322. doi: 10.1371/journal.pone.0005322. Epub 2009/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29(43):13684–90. doi: 10.1523/jneurosci.3770-09.2009. Epub 2009/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd GM. The dendritic spine: a multifunctional integrative unit. J Neurophysiol. 1996;75(6):2197–210. doi: 10.1152/jn.1996.75.6.2197. Epub 1996/06/01. [DOI] [PubMed] [Google Scholar]
- 28.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037–50. doi: 10.1523/JNEUROSCI.19-06-02037.1999. Epub 1999/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABA(A) receptor signalling in native GnRH neurons. Eur J Neurosci. 2000;12(10):3497–504. doi: 10.1046/j.1460-9568.2000.00261.x. Epub 2000/10/13. [DOI] [PubMed] [Google Scholar]
- 30.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–92. doi: 10.1210/endo.138.10.5570. Epub 1997/10/10. [DOI] [PubMed] [Google Scholar]
- 31.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15043–8. doi: 10.1073/pnas.95.25.15043. Epub 1998/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrang N, Larsen PJ, Clausen JT, Kristensen P. Neurochemical characterization of hypothalamic cocaine- amphetamine-regulated transcript neurons. J Neurosci. 1999;19(10):RC5. doi: 10.1523/JNEUROSCI.19-10-j0006.1999. Epub 1999/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Celis ME. Release of LH in response to alpha-MSH administration. Acta Physiol Pharmacol Latinoam. 1985;35(3):281–90. Epub 1985/01/01. [PubMed] [Google Scholar]
- 34.Backholer K, Smith J, Clarke IJ. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology. 2009;150(12):5488–97. doi: 10.1210/en.2009-0604. Epub 2009/10/13. [DOI] [PubMed] [Google Scholar]
- 35.Chen WP, Witkin JW, Silverman AJ. beta-Endorphin and gonadotropin-releasing hormone synaptic input to gonadotropin-releasing hormone neurosecretory cells in the male rat. J Comp Neurol. 1989;286(1):85–95. doi: 10.1002/cne.902860106. Epub 1989/08/01. [DOI] [PubMed] [Google Scholar]
- 36.Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(5):2541–6. doi: 10.1073/pnas.95.5.2541. Epub 1998/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. The New England journal of medicine. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. Epub 2004/09/03. [DOI] [PubMed] [Google Scholar]
- 38.Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30(30):10205–19. doi: 10.1523/jneurosci.2098-10.2010. Epub 2010/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, et al. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature medicine. 2007;13(1):89–94. doi: 10.1038/nm1525. Epub 2007/01/02. [DOI] [PubMed] [Google Scholar]
- 40.Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF, et al. Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Molecular metabolism. 2015;4(5):427–36. doi: 10.1016/j.molmet.2015.02.006. Epub 2015/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.