Abstract
Bacteremia is currently one of the infections with the highest mortality in hospitals [1]. Acinetobacter lwoffii and Acinetobacter baumannii are gram-negative bacteria and both represent opportunistic pathogens. In certain cases, the management can be challenging since these organisms can be highly resistant to antimicrobial agents. Clinical illnesses associated with Acinetobacter include pneumonia, meningitis, peritonitis, endocarditis and infections of the urinary tract and skin [1]. Acinetobacter bacteremia represents a serious and ever increasing problem because of the high associated morbidity and mortality.
Keywords: Acinetobacter bacteremia, Disseminated intravascular coagulation, Acinetobacter lwoffii, Acinetobacter baumannii
Introduction
The source of bacteremia with Acinetobacter species is frequently unknown. In 21–70% of cases, the source remains undetermined [2]. The crude death rate associated with Acinetobacter spp. bacteremia is high, usually ranging from 22% to 52% [2]. Seifert et al. reported a 19% mortality directly associated with Acinetobacter baumannii bacteremia, and found that the independent factors associated with a higher mortality were the presence of a rapidly fatal underlying disease, mechanical ventilation, initial septic shock, and pneumonia as the primary source of infection [2]. Other factors associated with mortality were the presence of DIC and the use of inappropriate antimicrobial therapy [2]. We present a case of disseminated intravascular coagulation (DIC) triggered by Acinetobacter infection in an asplenic patient.
Case
A 46-year old Caucasian female with a history of intravenous drug abuse, hypertension and traumatic splenectomy (after a motor vehicle accident 12 years prior), presented to our institution with altered mental status for the past one day. She was also a chronic smoker and had a history of noncompliance to vaccinations. On arrival to the emergency department, she had multiple episodes of coffee ground emesis and tarry colored stools.
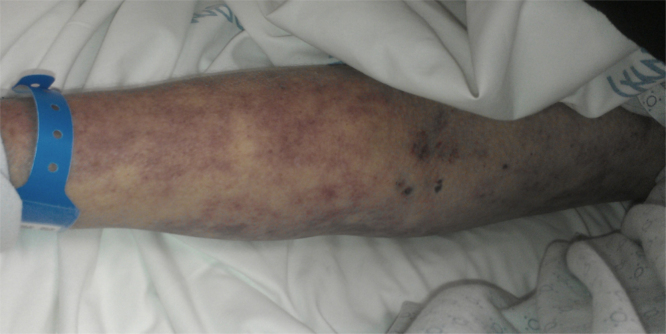
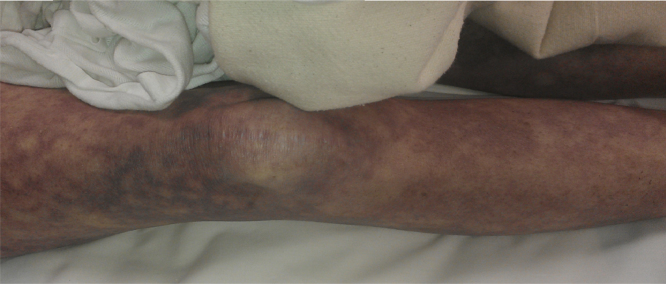
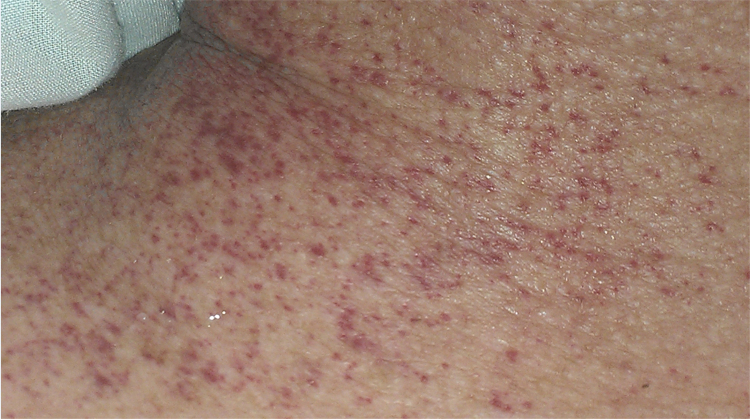
On examination, she was febrile up to a temperature 101.7 F and persistently hypotensive with blood pressure (BP) 74/22, and heart rate 70 beats per min (bpm). A petechial rash was noted initially on her face, but then rapidly became generalized spreading to her arms, legs and trunk (see Fig. 1, Fig. 2, Fig. 3). She also had multiple track marks on both upper extremities. Digital rectal examination was guaiac positive. Gastric lavage was also significant for 800cc coffee ground fluid.
Fig. 1.
Showing maculopapular rash located on patient's arm with track marks.
Fig. 2.
Showing maculopapular rash located on patient's leg.
Fig. 3.
Showing petechial rash on patient's neck.
Since she did not respond to aggressive intravenous fluid resuscitation, pressors had to be started. She also required intubation for airway protection. Vancomycin, cefepime and metronidazole were empirically started. Chest X-ray (CXR) did not show any evidence of cardiopulmonary disease. Head computer tomography (CT) without contrast did not reveal any abnormalities. Laboratory data showed a lactic acid of 8.5 and also evidence of coagulopathy consistent with DIC. Blood cultures revealed gram negative rods on gram stain. Despite prompt antibiotic treatment and administration of fresh frozen plasma and platelets, she passed away on the day of admission. Soon after her demise, the blood cultures resulted A. baumannii and Acinetobacter lwoffii. The microbiological result was confirmed by molecular methods. Both species of Acinetobacter were pansusceptible. Family declined autopsy.
Discussion
The recent taxonomy of the genus Acinetobacter was proposed by Bouvet and Grimont [5], [7] in 1986 and expanded later [6], [7] to include the seven named genomospecies (Acinetobacter calcoaceticus, A. baumannii, Acinetobacter haemolyticus, Acinetobacter junii, Acinetobacter johnsonii, A. lwoffii and Acinetobacter radioresistens) and nine unnamed genomospecies [7].
It has been noted that the mortality rate from bacteraemia is one of the highest among infections in hospitals, especially in the intensive care [1] unit (ICU). Acinetobacter species are widely distributed in nature and are recoverable readily from moist and dry surfaces [5]. This organism can colonize almost any human body site either transiently or as normal flora [5]. Both A. baumannii and A. wolffii can be found as part of normal skin flora in approximately 25% of the healthy individuals [4].
Our patient was an intravenous (IV) drug user. She had multiple track marks on her upper extremities, suggestive of active drug use. This was likely the source of her bacteremia since other sources were considered to be much less likely. She did not have any gastrointestinal complaints prior to arrival to the hospital and her last hospitalization was more than five months prior to this presentation precluding that this could have been related to an IV line from a previous admission.
Inherent bacterial virulence factors of Acinetobacter spp. are not well elucidated, although it is known that the organism is encapsulated, which may enable it to “escape” phagocytosis. Our patient was asplenic, therefore this likely contributed to the severity of her infection.
The production of an exopolysaccharide protects Acinetobacter spp. from other innate immune mechanisms [5]. The ability of this organism to participate in production of biofilms at epithelial cell interfaces and its innate iron acquisition systems for survival in a host's iron-poor environment also contribute to its pathogenicity [5].
Acinetobacter bacteremia represents a grave problem because of its high associated morbidity and mortality and, especially, because of the development of antimicrobial resistance. This resistance is developing so rapidly that it can almost be seen as a harbinger of the so-called post-antibiotic era [2]. Measures are needed to prevent and control infections caused by Acinetobacter species and to improve the use of antimicrobial agents to avoid, or at least delay, the appearance of newly resistant strains. The resistance pattern of the A. lwoffii isolate could be similar to other Acinetobacter species, which produce an AmpC beta-lactamase that inactivates aminopenicillins and narrow-spectrum cephalosporins [8].
In the case presented above, both species of Acinetobacter were pansusceptible, therefore antibiotic coverage was appropriate. Likely the only factor that might have changed the outcome is if the patient would have presented sooner to the hospital.
While A. baumannii, which is frequently associated with outbreaks, is still by far the most common of the Acinetobacter species among clinical isolates [3], it is clear that the lesser-known species, such as A. lwoffii have been associated with serious infections, therefore may represent emerging pathogens.
In conclusion, to our knowledge, we present the first case of intravenous drug use related A. lwoffii and baumannii bacteremia. The poor outcome of this case was likely due to an increased severity of infection due to asplenia and late presentation of the patient to the hospital.
Conflicts of interest/disclosures
None.
Acknowledgement
None.
References
- 1.Valero C., Garcıa Palomo J.D., Matorras P., Fernandez-Mazarrasa C., Gonzalez Fernandez C., Farinas M.C. Acinetobacter bacteraemia in a teaching hospital, 1989–1998. Eur J Intern Med. 2001;12:425–429. doi: 10.1016/s0953-6205(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 2.Cisneros J.M., Reyes M.J., Pachon J., Becerril B., Caballero F.J., Garcia-Garmendia J.L. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin Infect Dis. 1996;22:1026–1032. doi: 10.1093/clinids/22.6.1026. [DOI] [PubMed] [Google Scholar]
- 3.Turton J.F., Shah J., Ozongwu C., Pike R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol. 2010;(April):1445–1449. doi: 10.1128/JCM.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guide to the elimination of multidrug-resistant Acinetobacter baumannii transmission in healthcare settings. An APIC guide; 2010.
- 5.Bouvet P.J.M., Grimont P.A.D. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonii sp. nov., and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int J Syst Bacteriol. 1986;36:228–240. [Google Scholar]
- 6.Bouvet P.J.M., Jeanjean S. Delineation of new proteolytic genomic species in the genus Acinetobacter. Res Microbiol. 1989;140:291–299. doi: 10.1016/0923-2508(89)90021-1. [DOI] [PubMed] [Google Scholar]
- 7.Ku S.C., Hsueh P.R., Yang P.C., Luh K.T. Article clinical and microbiological characteristics of bacteremia caused by Acinetobacter lwoffii. Eur J Clin Microbiol Infect Dis. 2000;19:501–505. doi: 10.1007/s100960000315. [DOI] [PubMed] [Google Scholar]
- 8.Roy R., Das D., Kumar S., Mukherjee A. Postcataract surgery endophthalmitis caused by Acinetobacter lwoffii. Middle East Afr J Ophthalmol. 2015;22(2):253–254. doi: 10.4103/0974-9233.151974. [DOI] [PMC free article] [PubMed] [Google Scholar]