Abstract
Study Objectives:
The aim of this study was to describe and analyze the association between bilateral leg movements (LMs) during sleep in subjects with restless legs syndrome (RLS), in order to eventually support or challenge the current scoring rules defining bilateral LMs.
Methods:
Polysomnographic recordings of 100 untreated patients with RLS (57 women and 43 males, mean age 57 y) were included. In each recording, we selected as reference all LMs that occurred during sleep and that were separated from another ipsilateral LM by at least 10 sec of EMG inactivity. For each reference LM and an evaluation interval from 5 sec before the onset to 5 sec after the offset of the reference LM, we evaluated (1) the presence or absence of contralateral leg movement activity and (2) the distribution of the onset-to-onset and (3) the offset-to-onset differences between bilateral LMs.
Results:
We selected a mean of 368 (± 222 standard deviation [SD]) reference LMs per subject. For 42% (± 22%) of the reference LMs no contralateral leg movement activity was observed within the evaluation interval. In 55% (± 22%) exactly one and in 3% (± 2%) more than one contralateral LM was observed. A further evaluation of events where exactly one contralateral LM was observed showed that in most (1) the two LMs were overlapping (93% ± 9% SD) and (2) were classified as bilateral according to the World Association of Sleep Medicine and the International Restless Legs Syndrome Study Group (WASM/ IRLSSG) (96% ± 6% SD) and (3) the American Academy of Sleep Medicine scoring rules (99% ± 2% SD). Although there was a systematic and statistically significant difference in standard LM indices during sleep based on the two different definitions of bilateral LMs, the size of the difference was not clinically meaningful (maximum individual, absolute difference in LM indices ± 2.5). In addition, we found that the duration of LMs within bilateral LM pairs was longer compared to monolateral LMs and that the duration of the single LMs in bilateral LM pairs tended to correlate.
Conclusions:
The results of this study indicate that the two current standard scoring rules for the definition of bilateral LMs during sleep provide largely corresponding classifications in subjects with RLS and, in a clinical context, can be considered to be equivalent.
Citation:
Ferri R, Manconi M, Rundo F, Zucconi M, Aricò D, Bruni O, Ferini-Strambi L, Fulda S. A data-driven analysis of the rules defining bilateral leg movements during sleep. SLEEP 2016;39(2):413–421.
Keywords: bilateral leg movements, periodic leg movements during sleep, PLMS, restless legs syndrome, sleep scoring
Significance.
The two standard scoring rules for periodic leg movements during sleep, i.e. the WASM/IRLSSG and the AASM rules, differ in the definition of bilateral leg movements. We show that the choice of defining bilateral leg movements by either the WASM/IRLSSG or the AASM rules does not affect leg movement and periodic leg movement counts during sleep in a clinically meaningful way in patients with RLS.
INTRODUCTION
Currently, two sets of somewhat similar rules for the scoring of periodic leg movements during sleep (PLMS) are considered to be the current standards.1–3 The first were proposed by the World Association of Sleep Medicine and the International Restless Legs Syndrome (RLS) Study Group (WASM/ IRLSSG),1 whereas the second set of rules was issued by the American Academy of Sleep Medicine (AASM).2,3 Both sets have been based partially on algorithms proposed for the automatic detection of leg movements (LMs) in polysomnographic recordings (PSG)4 and incorporated mathematical parameters such as thresholds, intervals, and amplitude.4,5
Notwithstanding the efforts to base the rules on data-driven measures, the current criteria still contain various unchallenged rules that had been introduced without a formal assessment of their validity. As an example, only recently has the rule to define the association between a periodic LM and arousals been systematically evaluated by an evidence-based analysis and substantially confirmed6; on the contrary, the rules that define the association between PLMS and respiratory events have similarly been challenged and shown to be little supported by the statistical analysis, suggesting a possible need for change and reconceptualization.7
The current paper focuses on the definition of bilateral LMs. The WASM/IRLSSG criteria1 consider LMs as bilateral if the LMs overlap or if the offset of the first LM is < 0.5 sec before the onset of the subsequent LM in the contralateral leg. This differs from the AASM rules,2,3 which consider LMs as bilateral if the onset of the first LM is < 5 sec before the onset of the subsequent LM in the contralateral leg. In addition to the evident difference between the two sets of rules, neither of them has been formally validated, both having been established by expert consensus.
Therefore, the aim of this study is to analyze statistically the association between contralateral LMs during sleep, in order to eventually support or challenge the current scoring rules defining bilateral LMs.
METHODS
Subjects
For this study we retrospectively identified and included recordings that followed a standardized protocol of patients with RLS who participated in previous studies published by our groups.8–17 To exclude recordings with predominantly unilateral LMs, we selected only recordings where at least 30 right and 30 left LMs during the total sleep time had been observed, each separated by at least 10 sec from a preceding or following leg movement of the same leg. These right and left LMs did not have to be bilateral or periodic. Selecting patients who had a minimum of left and right LMs ensured that there was at least a theoretical possibility that these LMs were bilateral.
In agreement with the International RLS Study Group,18,19 the minimal criteria accepted for the diagnosis of RLS were: (1) an urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs; (2) the urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying or sitting; (3) the urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching; (4) the urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night. Routine blood tests and neurophysiological investigation (electromyography [EMG] and electroneurography of the lower limbs) were also normal. The sleep respiratory pattern of each patient was assessed by means of oral and/or nasal airflow (thermistor and/or nasal pressure cannula), thoracic and abdominal respiratory effort (strain gauge), and oxygen saturation (pulse oximetry), in a previous recording (within 1 w) or during the respective study; subjects with an apnea-hypopnea index > 5 were not included. Neurological examination was un-remarkable in all patients. For each patient included, the International RLS Severity Scale (IRLS) score20 was also obtained.
The original studies were approved by the local ethics committees and all subjects had provided informed consent before entering the study.
Polygraphic Sleep Recording
Each subject underwent a full night PSG after an adaptation night, carried out in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory. Subjects were not allowed to have beverages containing caffeine during the afternoon preceding the recording and were allowed to sleep until their spontaneous awakening in the morning.
The following parameters were included in the PSG study: EEG (at least three channels, one frontal, one central, and one occipital, referred to the contralateral earlobe); electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to A1), EMG of the submentalis muscle, EMG of the right and left tibialis anterior muscles (bipolar derivations with two electrodes placed 3 cm apart on the belly of the anterior tibialis muscle of each leg, impedance was kept less than 10 KΩ), and electrocardiography (ECG, one derivation). EMG signals, in particular, were digitally band-pass filtered at 10–100 Hz, with a notch filter at 50 Hz.
At the beginning of each session and before the start of recording, the sleep technician checked that the amplitude of the EMG signal from the two tibialis anterior muscles was below 2 μV at rest.
Sleep Scoring and Detection of LMs
Sleep stages were visually scored following standard criteria on 30-sec epochs.21 LMs were scored according to AASM and WASM/IRLSSG criteria as any leg EMG increase ≥ 8 μV above the resting baseline and lasting between 0.5 and 10 sec.1–3 The onset of the LM was defined as the beginning of the EMG increase ≥ 8 μV above the resting baseline, and the end of the LM was defined as the beginning of the period where the EMG decreases for at least 0.5 sec to < 2 μV above resting baseline. LMs during sleep were detected by the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy). With this software, the detection is performed by means of a human-supervised automatic approach controlled by the scorer.4 For this study, one scorer (RF) visually edited all detections proposed by the automatic analysis.
Analysis of the Bilateral LMs
For the current analysis, we considered all LMs, irrespective of whether they were classified as periodic or not. In each recording, we selected as reference LMs (refLMs) all LMs that met the following inclusion criteria: (1) the LM occurred during sleep and (2) the LM was separated from another ipsilateral LM (preceding or following) by at least 10 sec of EMG inactivity. For each refLM and an evaluation interval from 5 sec before the onset to 5 sec after the offset of the refLM, we investigated the presence or absence of contralateral LM activity, i.e., whether any part of a contralateral LM was detected within the evaluation interval.
For all refLM with at least one contralateral LM within the evaluation interval we investigated whether the refLM and the contralateral LM:
were overlapping (i.e., any part of the contralateral LM coincided with any part of the refLM) and/or
were considered as bilateral according to the WASM/ IRLSSG scoring rules, i.e., when the two LMs were overlapping or the onset of the second movement was < 0.5 sec after the offset of the first LM and/or
were considered as bilateral according to the AASM rules, i.e., when the onset of the second LM was < 5 sec after the onset of the first LM.
In addition, for each refLM with at least one contralateral LM within the evaluation interval we computed the latency from the onset of the refLM to the onset of the contralateral LM and from the offset of the refLM to the onset of the contralateral LM. Because the number of analyzed LMs differed widely between individual subjects, all results reported are based on weighted summary statistics given equal weight to each subject. For example, for the computation of the average percentage of refLMs classified as monolateral, we first computed the percentage for each subject and then averaged across subjects.
RESULTS
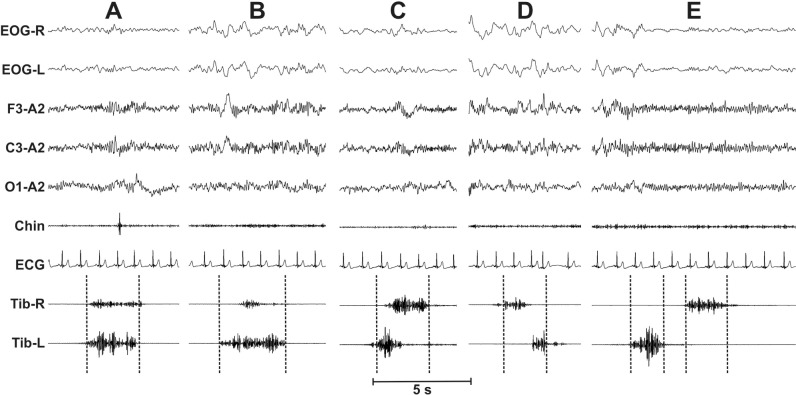
We included 100 subjects with RLS (57 female, 43 male) with a mean age of 57.0 y (± 13.2 y, SD) and an average IRLS score of 23.9 (± 7.05 SD). Figure 1 shows some examples of the different types of combinations between two LMs occurring in time proximity, over the two legs. Cases A-D show different types of overlapping between the two legs, whereas in case E two nonoverlapping but close LMs are shown, separated by a short EMG inactivity lag.
Figure 1.
Some examples of the different types of combinations between two leg movements (LMs) occurring in time proximity, over the two legs. Cases A–D show different types of overlapping between the two legs whereas in case E two nonoverlapping but close LMs are shown, separated by a short electromyography (EMG) inactivity lag.
Individual participants contributed on average 470.5 LMs (± 275.1 SD, range 84–1,496) during sleep to the analyses. Of these, on average 368.2 (± 221.9, 69–1,207) LMs were selected as refLMs, which represented 78.9% (± 11.2 SD, 26.6% to 96.8%) of all LMs during sleep. On average, refLMs were equally distributed between left and right LMs (percentage of right LMs 50.4% ± 12.9 SD) with wide variation between participants (range: 20.9% to 84.1%).
Classification of LMs
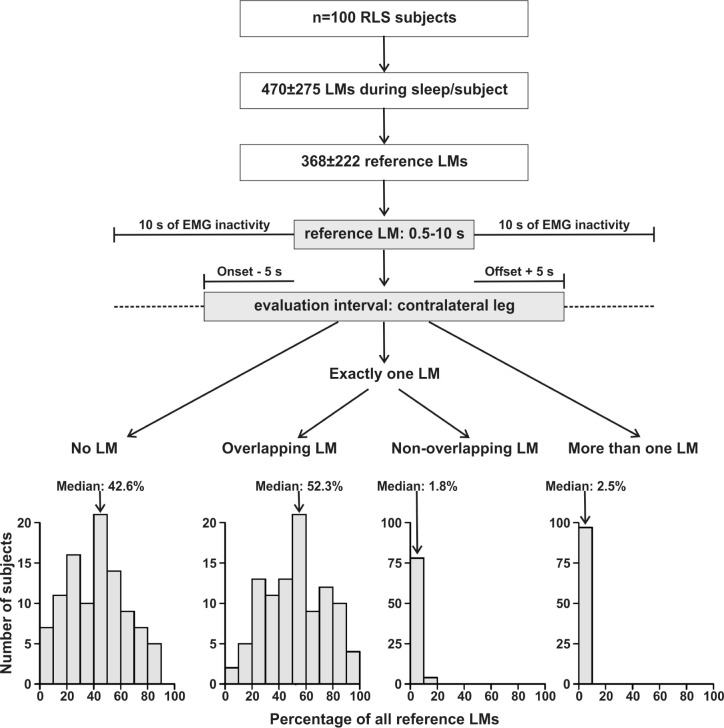
On average, 41.9% (± 21.6 SD, 0.9–89.4%) of refLMs were classified as monolateral LMs, i.e., there was no contralateral LM within 5 sec before the onset to 5 sec after the offset of the LM. For 55.2% of the refLMs (± 22.4 SD, 9.7–98.5%) there was exactly one contralateral LM in this interval, which was overlapping with the refLM in 52.6% (of all refLMs, ± 22.5 SD, 7.1–98.4%) and in only 2.6% (± 3.1 SD, 0–16.3%) started or ended in the 5 sec after the refLM offset or before the refLM onset, respectively. In 2.8% (± 2.1% SD, 0–9.5%) more than one contralateral LM was observed in this interval (Figure 2).
Figure 2.
Flow chart of analysis strategy and classification of leg movements (LMs). For each reference LM, we evaluated the presence or absence of contralateral leg movement activity in an interval from 5 sec before the onset to 5 sec after the offset of the reference LM. For each subject separately, the percentage of all reference LMs with no, one, or more contralateral LMs were computed. The distribution of these individual classification is shown in the lowest panels. EMG, electromyography; RLS, restless legs syndrome.
Onset-to-Onset and Onset-to-Offset Latencies of Bilateral LMs
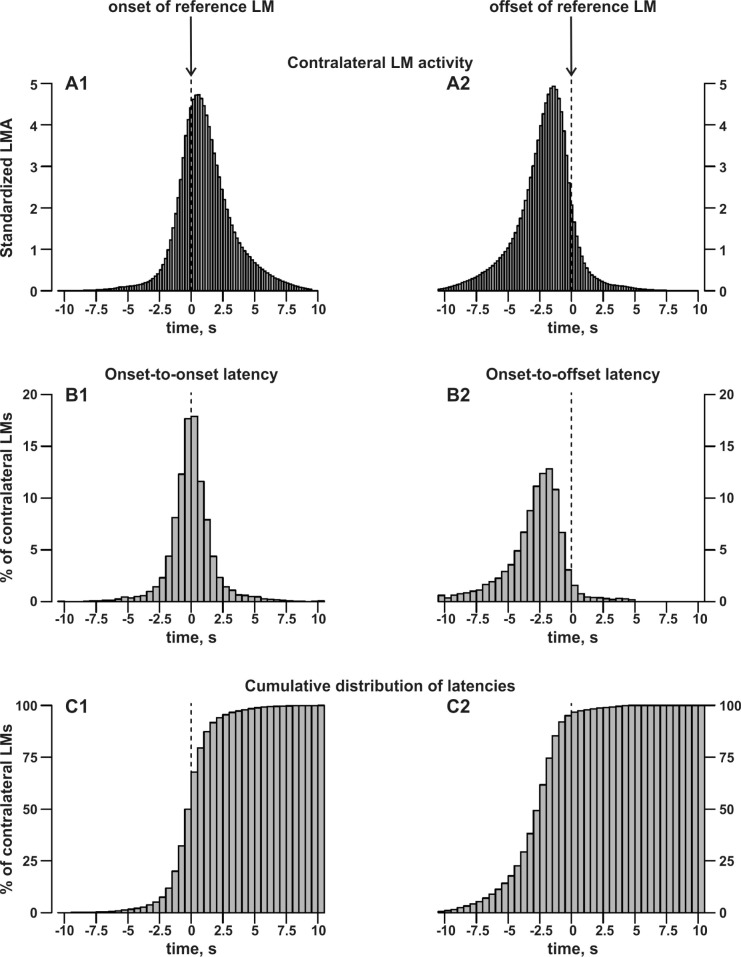
Figure 3 shows a detailed analysis of the contralateral LM activity (A1, A2) and the onset-to-onset (B1) and onset-to-offset (B2) differences between bilateral LMs. As before, all LMs that occurred within the interval from 5 sec before the onset to 5 sec after the offset of refLMs were considered. Figure 3 B1 shows the distribution of onset-to-onset differences which, although symmetric around zero, did not pass the Shapiro-Wilk test of normality (W = 0.5696, P < 0.001). The majority of onset-to-onset differences were within ± 5 sec, fulfilling the criteria for bilateral LMs set by the AASM,2,3 with only 2.9% (± 2.9 SD, 0 –16.3%) of differences outside this interval. Figure 3 B2 details the onset-to-offset differences showing that the onset of contralateral LMs was mostly before the offset of the refLM (95.1%, ± 4.9 SD, 72.1% to 100%). The onset of a further 1.6% (± 2.2 SD, 0–13.1%) of contralateral LMs was within 0.5 sec after the offset of the refLM. In 3.3% (± 3.2 SD, 0–16.4%), the onset of the contralateral LM was more than 0.5 sec after the offset of the refLM.
Figure 3.
Top: Distribution of averaged, individually standardized contralateral leg movement (LM) activity at the onset (A1) and offset (A2) of reference LMs. For each subject and each reference LM, we evaluated the presence of contralateral LM activity in each 0.2 sec-interval from −15 sec to +15 sec around the onset or offset of the reference LM. The presence of LM activity in a specific interval was counted as one and summed up over all reference LMs. The total sum over all intervals and all reference LMs was standardized to 100 for each subject before averaging across subjects. Middle: Distribution of averaged, individually standardized differences between the onset of the contralateral LM and the onset (B1) or offset (B2) of the reference LM. The distribution of all onset-to-onset and onset-to-offset latencies was first derived for each subject individually. The total number of available latencies represents 100% for each subject, which were then averaged across subjects. Bottom: Cumulative distribution of averaged, individually standardized differences (as shown in B1 and B2) between the onset of the contralateral LM and the onset (C1) or offset (C2) of the reference LM.
Classification of Bilateral LMs According to WASM/IRLSSG and AASM Rules
In the 55.2% (± 21.7 SD) of events where exactly one contralateral LM was observed in the evaluation interval from 5 sec before the onset to 5 sec after the offset of the refLM, we further evaluated the differences between the WASM/ IRLSSG1 and the AASM rules.2,3 In most events (93.5%, ± 8.8 SD, 55.8–100%) the two LMs were overlapping. Consequently, a comparative percentage (96.2%, ± 5.6 SD) of refLMs was classified as bilateral according to the WASM/IRLSSG rules. According to the AASM rules, an even higher percentage of refLMs was considered as bilateral (98.6%, ± 2.4 SD; AASM versus WASM/IRLSSG: t = 4.84, P < 0.001). Notably, 0.5% (± 1.2 SD, 0–6.8%) of events where the contralateral LM overlapped with the refLM would not be considered as bilateral according to the AASM rules.
The overlap between the two sets of rules was substantial (see Figure 4). On average, 95.7% (± 5.8 SD, 72.1–100%) of refLMs were considered as bilateral with both set of rules. Only a minority was labeled as bilateral according to WASM/ IRLSSG but not AASM rules (0.5%, ± 1.2 SD, 0–6.8%) whereas the opposite case was more frequent (2.9%, ± 4.8 SD, 0–25.8%, V = 303, P < 0.001 for between-rules comparison). Finally, in only 0.9% (± 1.9 SD) of events where a LM was observed within the evaluation interval, was the refLM not classified as bilateral according to one or the other set of rules (Figure 4).
Figure 4.
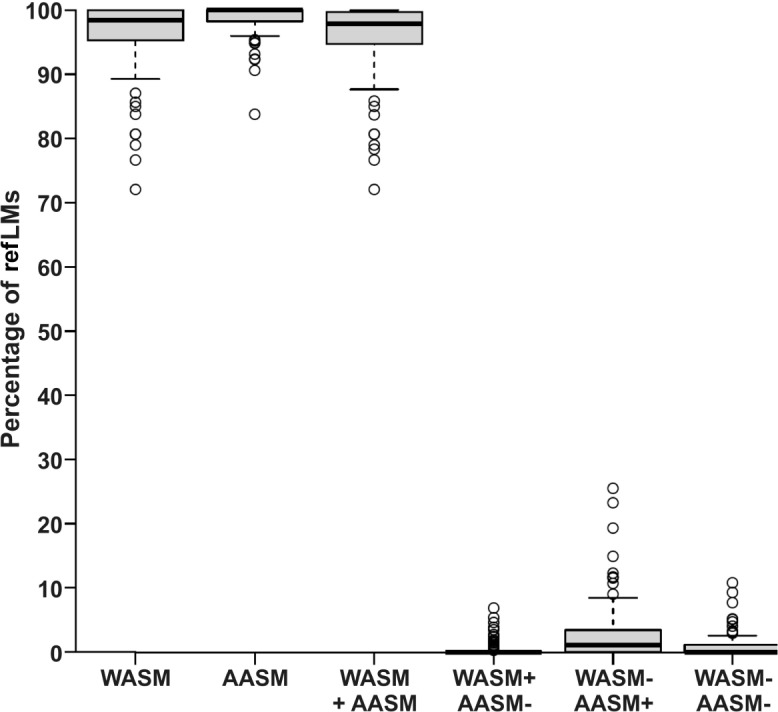
Individual percentages of reference leg movements (refLMs) with a contralateral LM classified as bilateral according to the definition of bilateral movements of the World Association of Sleep Medicine and the International Restless Legs Syndrome Study Group. (WASM/IRLSSG) and the American Academy of Sleep Medicine (AASM). For each subject, 100% represents the total number of reference LMs where a contralateral LM was observed within the interval from 5 sec before the onset to 5 sec after the offset of the reference LM. From left to right: Percentage of reference LMs classified as bilateral according to (1) the WASM/ IRLSSG rules, (2) the AASM rules, (3) both rules, (4) according to the WASM/IRLSSG but not the AASM rules, (5) according to the AASM but not the WASM/IRLSSG, or (6) not classified as bilateral by either set of rules. The bottom and the top of the boxes represent the first and third quartile (25%/75%), the thick black band inside the box denotes the median. Whiskers extent to ± 1.58 times the interquartile range divided by the square root of the number of observations. Any data point outside this range is depicted as an individual open circle.
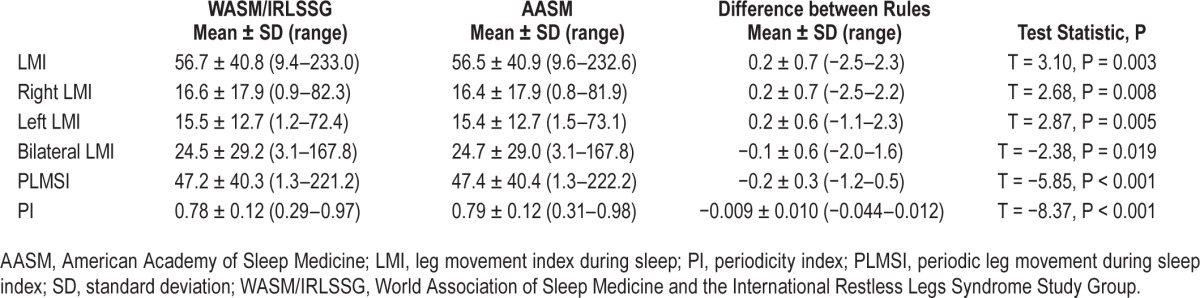
In a last step, we explored whether the difference between the WASM/IRLSSG and AASM definitions of bilateral LMs had any effect on standard indices of leg movement activity during sleep. The results are given in Table 1 and show that although the difference between the resulting indices was highly systematic and statistically significant, the absolute difference between any two indices was numerically small. In fact, the maximum individual differences between indices were equal or less than 2.5. Interestingly, switching from one definition to another had also only a minor effect on the resulting periodicity index.
Table 1.
Influence of different definitions of bilateral leg movements on standard leg movement indices during sleep in 100 subjects with restless legs syndrome.
Duration of Bilateral LM
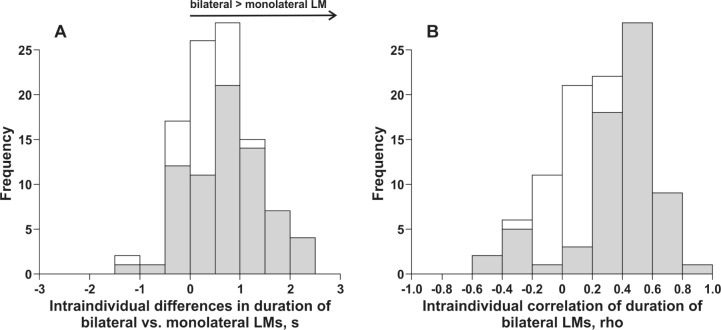
To further describe bilateral LM we explored first whether bilateral LMs differed in duration from monolateral LMs. As bilateral LMs we selected those refLMs that overlapped with another contralateral LM, monolateral refLMs had no contra-lateral leg movement activity in an interval ranging from 5 sec before the onset to 5 sec after the offset of the refLM. Average durations of monolateral and bilateral LMs were computed and statistical significance of the difference in duration (Mann-Whitney U test) was explored individually for each subject. Figure 5A summarizes these results across all subjects. The average within-subject difference in duration varied between −1.1 sec and 2.3 sec, across all subjects the bilateral LMs were 0.61 sec (± 0.68 SD) longer than monolateral LMs (P < 0.001 for differences at the group level). In 71 of the 100 subjects, the difference in duration was statistically significant at the intra-individual level.
Figure 5.
(A) Distribution of the average intraindividual difference in the duration of reference leg movements (LMs) that were classified as bilateral versus monolateral. A positive difference signifies that on average the duration of the bilateral LMs was larger than that of monolateral LMs. Statistical significance of the difference in durations was explored individually within each subject (Mann-Whitney U test) and the gray shaded distribution refers to group of subjects with P < 0.05. (B) Distribution of intraindividual correlations (Spearman rho) of the durations of the bilateral LMs with the gray shaded distribution referring to individual correlations with P < 0.05.
Finally, we also explored whether there was a correlation of the duration of bilateral LMs. Again we selected as bilateral LMs those refLMs that overlapped with a contralateral LM. Spearman rho correlations were computed for each subject and Figure 5B summarizes the results. Intraindividual correlations were significant (P < 0.05) in 67 of 100 subjects and varied widely between −0.56 and 0.84 (Fisher weighted mean correlation: 0.27) with a mode around 0.5 (Figure 5B).
Interindividual Difference in the Time Lag Between Bilateral LMs
Finally, we explored interindividual differences in the time lag between bilateral LMs. For each subject we selected all refLMs where exactly one contralateral LM was observed within the interval from 5 sec before the onset to 5 sec after the offset of the refLM. The onset-to-onset (log-transformed to achieve approximate normality) and offset-to-onset time lag was computed for each event. Within- and between-subject variance was derived from a linear mixed-model analysis and the intraclass correlation coefficient (ICC)22 was computed as the ratio of the between-subject variance to the total variance (i.e., sum of between- and within-subject variance).
The ICC was 0.13 for onset-to-onset and 0.09 for offset-to-onset time lags corresponding to a low stability of interindividual differences according to standard benchmarks.22
DISCUSSION
Our findings indicate that in the overwhelming majority of events, bilateral LMs are overlapping with each other and at the same time are correctly identified by both the current WASM/IRLSSG1 and AASM2,3 rules. Surprisingly, although both rules differ considerably in their definition of bilateral LMs, we found that they provided largely corresponding classifications in subjects with RLS. Indeed, between rule differences in standard LM indices during sleep including the PLMS index were numerically minor (≤ 2.5 in all subjects) and could even be considered negligible in a clinical context.
A detailed analysis of the onset-to-onset differences between bilateral LMs showed that in 97% of events these were < 5 sec, thereby satisfying the AASM definition of bilateral LM. At the same time, in 96% of events the two LMs overlapped or were separated by < 0.5 sec from offset to onset, fulfilling the WASM/ IRLSSG definition. Therefore, although these definitions were not based on physiological considerations, our findings lend statistical support to both rules. In deciding between the two set of rules we can therefore point out only the difference at the conceptual level: since the maximum duration of LMs is 10 sec and the AASM rules define bilateral LMs by an onset-to-onset difference of less than 5 sec,2,3 there is the possibility that LMs that are overlapping would be classified as monolateral. This case, although not frequent, was nevertheless observed in 0.5% (± 1.2 SD) of events and in individual subjects accounted for up to 6.8%.
As already seen for the association between PLMS and arousals,6 the close but not perfect synchronization between LMs within the bilateral pairs and their correlated durations seem to indicate that both events are regulated by a complex mechanism including them and other sleep events,23 such as heart rate and blood pressure,9,24–26 involving several and potentially different generators interacting with each other in a more complex way than in a simple unidirectional causal-effect mechanism.27–30 Both, the observation of PLMS in patients with complete transverse cervical spinal lesions28 and the recently reported pharmacological dissociation of cortical arousals from PLMS,17 suggest the existence of different generators for each phenomenon, possibly located in different areas of the central nervous system.
The rules that govern a synchronization between different generators during sleep are still unclear but this question also applies to the current study that focused on the monolateral/ bilateral occurrence of LMs. Indeed, it is an unanswered question whether we are dealing with two different spinal generators, one for each leg, or with one single pacemaker. The existence of monolateral LMs (around 50% of all LMs) suggests the presence of two functionally distinct generators that work simultaneously in generating bilateral LMs or independently in generating monolateral ones. The view that two different spinal generators needing the integrity of descending central pathways are at play to generate bilateral LMs has already been suggested for consideration31 and received some support.27 We have also found that bilateral LMs tend to be longer than monolateral LMs and that there is a correlation of the durations of the bilateral LMs in many subjects. This finding could support the notion of a reciprocal influence between two hypothetical generators or of a common modulation mechanism.
The duration of each of these events might also be interpreted as a feature describing their “intensity”, a parameter that has no current formal definition. Notwithstanding this, it appears reasonable to believe that LMs have different intensities, possibly correlated with different degrees of sleep disruption or supraspinal disinhibition, and reflected in differences in associated EEG, motor, and autonomic changes.9,32 A possible candidate for a measure of LM intensity might be the area under the curve that integrates both duration and amplitude into a single value8; however, because the EMG is an essentially noncalibrated signal, this needs to be implemented with great care and solid statistical support in the future. In this respect, it should be assessed if the intensity of a bilateral LM corresponds to the sum of the intensities of the two single-leg movements or not. Two other possibilities exist: that the total intensity is intermediate between the two single-leg intensities or that a potentiation leads to an intensity that is greater than the sum of the two single-leg intensities. Of note, it has already been reported that bilateral PLMS are accompanied by larger heart rate and spectral EEG changes than monolateral movements.9 Nevertheless, it must be stressed that our definition of monolaterality and bilaterality is exclusively based on a temporal association and is not a functional concept. Indeed, two separate phenomena might be functionally or even causally related while occurring at very different time points. In this regard, the current results can be considered relevant for scoring purposes, but need further neurophysiological studies to address the possible governing mechanisms of monolateral and bilateral LMs during sleep.
Regarding the scoring of LMs, our results showed that the two different definitions of bilateral LMs gave largely equivalent results concerning the standard LM indices of sleep. Concerning the PLMS index, this was to be expected based on the current rules that specify that bilateral LMs are counted as one LM and if not bilateral but in close proximity, i.e., with an intermovement interval below 5 sec, the second LM is ignored and therefore the classification has no practical consequence. In contrast, the periodicity index considers all LMs and quantifies the proportion, over the total, of intermovement intervals with length 10 < i ≤ 90 sec, that are preceded and followed by another interval with the same length (this is equivalent to a series of 4 LMs all separated by intervals with length 10 < i ≤ 90 sec).8 Therefore, the classification as one bilateral versus two monolateral LMs has a direct effect on the resulting periodicity index. However, in this study also the periodicity index remained largely invariant regarding the different definitions of bilateral LMs, further reinforcing our primary finding of a close temporal relationship between bilateral LMs.
The definition of PLMs is an ongoing process. The first scoring system was proposed by Coleman in 198233 which later served as the basis for the first international scoring criteria in 1993.34 The established criteria were to a large extent based on expert consensus, foremost because they were introduced during a time when paper recordings were the standard, and signal analysis such as the one in the current study was not feasible. With the current level of technology these restrictions no longer apply. Indeed, the current rules1–3 have begun to incorporate criteria such as those for the amplitude of PLMS that had been empirically derived4 and are better suited to computer-based polysomnography. Nevertheless, unchallenged criteria remain as well as differences between the two current standard set of rules, with the definition of bilateral LMs being one of them. Because any study on PLMS will be based on those leg movements that are defined as PLMS according to one or the other rule, then should these rules significantly differ in which LMs are identified, the results are likely to differ and results across studies cannot be compared. The exploration, such as in the current paper, to what extent PLMS are sensitive to the differences in rules is therefore a necessary step in the study of this fascinating phenomenon.35 There is a need to critically and empirically investigate all criteria used for the scoring of PLMS. Such an evidence base could then be incorporated into the decisional process regarding an eventual improvement of these criteria. Hopefully, in the long term it would also obliterate the need to have more than one set of standard rules.
As univocal as our results are, there are several important caveats to consider. First, we only investigated LMs during sleep and it can in no way be assumed that these findings also apply to LMs during wakefulness. Second, we only included patients with RLS. Future studies are needed to explore whether our results also hold true for other groups of sleep disordered patients.
Nevertheless, the results of this study suggest that in subjects with RLS the two current standard scoring rules for the definition of bilateral LMs during sleep give largely concurrent information and could be considered equivalent in a clinical context.
DISCLOSURE STATEMENT
This was not an industry-supported study. This work was performed at the Sleep Research Centre, Department of Neurology IC, Oasi Institute (IRCCS), Troina, Italy. This study (Drs. Ferri and Rundo) was partially supported by a grant of the Italian Ministry of Health (“Ricerca Corrente”). Drs. Manconi and Fulda are supported by the Swiss National Science Foundations (Grant No.:320030_144007). The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan S American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 3.Berry R, Brooks R, Gamaldo C, et al. Darien, IL: American Academy of Sleep Medicine; 2013. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.0.1. www.aasmnet.org. [Google Scholar]
- 4.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 5.Wetter TC, Dirlich G, Streit J, Trenkwalder C, Schuld A, Pollmächer T. An automatic method for scoring leg movements in polygraphic sleep recordings and its validity in comparison to visual scoring. Sleep. 2004;27:324–328. doi: 10.1093/sleep/27.2.324. [DOI] [PubMed] [Google Scholar]
- 6.Ferri R, Rundo F, Zucconi M, et al. An evidence-based analysis of the association between periodic leg movements during sleep and arousals in restless legs syndrome. Sleep. 2015;38:919–24. doi: 10.5665/sleep.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manconi M, Zavalko I, Fanfulla F, Winkelman JW, Fulda S. An evidence-based recommendation for a new definition of respiratory-related leg movements. Sleep. 2015;38:295–304. doi: 10.5665/sleep.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 9.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Manconi M, Ferri R, Zucconi M, Fantini ML, Plazzi G, Ferini-Strambi L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. Sleep. 2007;30:1779–85. doi: 10.1093/sleep/30.12.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Ferri R, Manconi M, Lanuzza B, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9:790–8. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Manconi M, Ferri R, Feroah TR, Zucconi M, Ferini-Strambi L. Defining the boundaries of the response of sleep leg movements to a single dose of dopamine agonist. Sleep. 2008;31:1229–37. [PMC free article] [PubMed] [Google Scholar]
- 14.Ferri R, Manconi M, Aricò D, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33:793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–7. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 16.Manconi M, Ferri R, Zucconi M, et al. Pramipexole versus ropinirole: polysomnographic acute effects in restless legs syndrome. Mov Disord. 2011;26:892–5. doi: 10.1002/mds.23543. [DOI] [PubMed] [Google Scholar]
- 17.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 18.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–9. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 19.Allen RP, Picchietti D L, Garcia-Borreguero D, et al. Restless legs syndrome/Willis–Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria – history, rationale, description, and significance. Sleep Med. 2014;15:860–73. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Walters A S, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 21.Washington, DC: Public Health Service, US Government Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 23.Ferri R. Two legs, one heart, one sleeping brain. Sleep Med. 2006;7:299–300. doi: 10.1016/j.sleep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Ali NJ, Davies RJO, Fleetham JA, Stradling JR. Periodic movements of the legs during sleep associated with rises in systemic blood pressure. Sleep. 1991;14:163–5. [PubMed] [Google Scholar]
- 25.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–30. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Gosselin N, Lanfranchi P, Michaud M, et al. Age and gender effects on heart rate activation associated with periodic leg movements in patients with restless legs syndrome. Clin Neurophysiol. 2003;114:2188–95. doi: 10.1016/s1388-2457(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Proserpio P, Rundo F, et al. Neurophysiological correlates of sleep leg movements in acute spinal cord injury. Clin Neurophysiol. 2015;126:333–8. doi: 10.1016/j.clinph.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Salminen AV, Manconi M, Rimpilä V, et al. Disconnection between periodic leg movements and cortical arousals in spinal cord injury. J Clin Sleep Med. 2013;9:1207–9. doi: 10.5664/jcsm.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clemens S, Rye D, Hochman S. Restless legs syndrome: revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–30. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 30.Ferri R. The time structure of leg movement activity during sleep: the theory behind the practice. Sleep Med. 2012;13:433–41. doi: 10.1016/j.sleep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Raggi A, Ferri R. Sleep disorders in neurodegenerative diseases. Eur J Neurol. 2010;17:1326–38. doi: 10.1111/j.1468-1331.2010.03034.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferrillo F, Beelke M, Canovaro P, et al. Changes in cerebral and autonomic activity heralding periodic limb movements in sleep. Sleep Med. 2004;5:407–12. doi: 10.1016/j.sleep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Coleman R. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. In: Guilleminault C, editor. Sleeping and waking disorders: indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 265–95. [Google Scholar]
- 34.American Sleep Disorders Association. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 35.Fulda S, Wetter TC. The influence of different definition criteria on the PLM index. Sleep Med. 2007;8:484–90. doi: 10.1016/j.sleep.2006.06.013. [DOI] [PubMed] [Google Scholar]