Abstract
IMPORTANCE
Although breast-conserving therapy (BCT) is an accepted modality for treatment of early-stage breast cancer, many women continue to undergo mastectomy. Detailing the factors associated with choice of BCT may assist with overcoming barriers in the use of this treatment modality.
OBJECTIVE
To conduct a population-based examination of the factors that influence the use of BCT.
DESIGN, SETTING, AND PARTICIPANTS
Using the National Cancer Data Base, we examined the surgical choices of women with stage T1 or T2 breast cancer treated between 1998 and 2011. Logistic regression analysis conducted between September 19, 2013, and August 26, 2014, was used to assess the multivariate association between patient and facility variables and the probability of undergoing BCT.
MAIN OUTCOMES AND MEASURES
Factors associated with the use of BCT.
RESULTS
A cohort of 727 927 women was identified in the National Cancer Data Base. Use of BCT, determined using odds ratio (OR) and 95% CI, was greater in patients aged 52 to 61 years compared with younger patients (1.14; 1.12–1.15) and in those with the highest educational level (1.16; 1.14–1.19). Rates of BCT were lower in patients without insurance compared with those with private insurance (0.75; 0.72–0.78) and in those with the lowest median income (0.92; 0.90–0.94). Academic cancer programs, US Northeast location, and residence within 27.8 km of a treatment facility were associated with greater BCT rates than were community cancer programs (1.13; 1.11–1.15), Southern location (1.50; 1.48–1.52), and residence farther from a treatment facility (1.25; 1.23–1.27). When comparing BCT use in 1998 with use in 2011, increases were seen across age groups (from 48.2% to 59.7%), in community cancer programs (48.4% in 1998 vs 58.8% in 2011), and in facilities located in the South (45.1% in 1998 vs 55.3% in 2011).
CONCLUSIONS AND RELEVANCE
Although the use of BCT has increased during the past 14 years, nonclinical factors, including socioeconomic demographics, insurance, and travel distance to the treatment facility, persist as key barriers to receipt of BCT. Interventions that address these barriers may facilitate further uptake of BCT.
With several randomized prospective trials1,2 confirming the efficacy of breast-conserving therapy (BCT), the National Institutes of Health (NIH)3 issued a consensus statement in 1990 in support of this treatment modality. These trials and the NIH consensus statement led to a substantial decline in the rates of mastectomy and the widespread acceptance of BCT as an appropriate treatment modality for early-stage breast cancer.4 However, during the past decade, technical advances and changes in societal norms may have created new incentives other than BCT, even among patients who remain good candidates for this treatment. These incentives include genetic testing for BRCA1 and BRCA2 mutation, advances in reconstruction techniques, breast magnetic resonance imaging, and increased patient interest in contralateral prophylactic mastectomy.
Several studies have sought to address the contemporary rates of BCT in the United States. Single-institution studies from the Mayo Clinic4 and Moffitt Cancer Center5 have reported an increase in mastectomy rates in the early 2000s after the sharp decrease in the 1990s. Patient age and higher tumor stage were predictors of mastectomy in both retrospective reviews. The use of preoperative breast magnetic resonance imaging was also found to be a predictor of mastectomy in the Mayo Clinic review.4 In contrast, evaluation of national mastectomy trends using the Surveillance, Epidemiology, and End Results (SEER) database6 showed an overall decrease in mastectomy rates for ductal carcinoma in situ and stage I to III breast cancers. The factors that were associated with decreased mastectomy rates included age older than 40 years, non-Hispanic white race, small tumor size, low tumor grade, nonlobular histologic characteristics, positive estrogen receptor status, and negative lymph node findings. The difference in mastectomy rates between the single-institution Moffitt Cancer5 Center and Mayo Clinic4 studies and the SEER database was thought to be the result of differences in referral patterns and patient selection bias.5,6
The SEER report by Habermann and colleagues6 suggested practice-based disparities in the use of BCT. However, because practice-based variables are unavailable in the SEER database, this hypothesis could not be directly tested. We sought to investigate this question using the National Cancer Data Base (NCDB) (https://www.facs.org/quality%20programs/cancer/ncdb), which codes for facility-level data, such as type of practice, in addition to clinical variables and patient demographics. Furthermore, the NCDB provides socioeconomic factors, such as educational level, income, insurance, and travel distance, which we hypothesized could also influence the type of surgical treatment received.
Methods
Patient Cohort
We identified a cohort of 727 927 women from the 2 720 347 patients with breast cancer in the NCDB who had clinical T1 or T2 tumors treated between 1998 and 2011. The NCDB, a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society, is a nationwide oncology outcomes database for more than 1500 commission-accredited cancer programs in the United States and Puerto Rico. Approximately 70% of all newly diagnosed cases of cancer in the United States are captured at the institutional level and reported to the NCDB.5 The patients included in the analysis cohort met the following criteria: (1) had clinical T1 or T2, N0 to N3, or Nx breast cancer; (2) had surgery codes for mastectomy or breast-conserving surgery plus radiotherapy (ie, BCT); and (3) did not receive their therapy at an “other specified type of cancer program.”7 Because patient-level identifiers are not available to NCDB users, this study was exempted from institutional review board evaluation and approval by The University of Texas MD Anderson Cancer Center institutional review board.
Statistical Analysis
Data analysis was performed between September 19, 2013, and August 26, 2014. Univariate analyses were performed to evaluate the association between each variable and the delivery of BCT, using χ2 tests for categorical variables and t test, analysis of variance or the counterparts of the nonparametric approaches (Wilcoxon rank sum test or Kruskal-Wallis test) for continuous variables.8 Logistic regression analysis was used to assess the multivariate relationship between patient demographics, clinical characteristics, and the probability of a woman receiving BCT.9 A logistic regression model was obtained by first including aninitial set of candidate predictor variables with a significance level of P < .05 in the univariate analysis. A backward stepwise elimination was then performed, using .05 for the significance level for an effect to stay in the model. Once the list of variables to be used in the final model was selected, the functional form of each variable and multicolinearity between the variables were examined. The race/ethnicity indicator was not included in the multivariate logistic regression model owing to its strong colinear correlations with other important factors, such as insurance, educational level, and facility location. In addition, breast cancer patient volume of a facility and facility type were not included in the multivariate model simultaneously because they were strongly correlated. Breast cancer patient volume of a facility is defined by the quartiles of the range of breast cancer cases reported across all the facilities in the study cohort for this analysis. To evaluate the differential influence that various factors had on the receipt of BCT during the study period, we used a multivariate logistic regression model on data from patients with breast cancer diagnosed only during 1998 and 2011. We also included interaction terms between each factor with year of diagnosis (1998 vs 2011) to assess statistically significant differences in the ability of these predictive factors between the 2 years. In addition, annual rates of BCT by insurance status, facility type, facility location, travel distance to the treatment facility, clinical T stage, and clinical N stage were calculated, with adjustment of all other factors in the multivariate logistic model. Computations for all analyses were performed using SAS, version 9.2 (SAS Institute Inc), and S-Plus, version 8.04 (TIBCO Software Inc).
Results
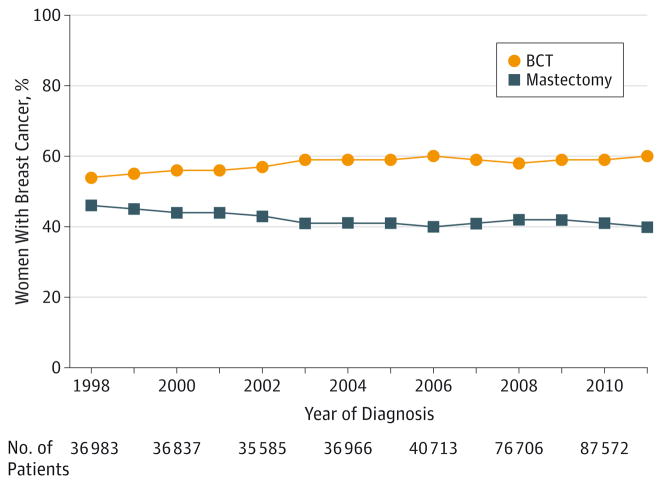
A cohort of 727 927 women met the inclusion criteria for this study. The trend of BCT during the study period is shown in Figure 1. The percentage of patients with early-stage breast cancer undergoing BCT increased from 54.3% in 1998 to 59.7% in 2006. The rate of BCT then remained relatively constant at approximately 59% thereafter.
Figure 1. Trends in Surgical Treatment Between 1998 and 2011.
BCT indicates breast-conserving therapy.
By univariate analysis, patients of white race and those aged 52 to 70 years were more likely to receive BCT compared with patients of other races and those younger (18–51 years) or older (>70 years) (all P < .01). The rates of BCT decreased from 61% to 43% in patients with more comorbidities than other patients (P < .01). Comorbidities were measured by the Charlson-Deyo score, with 0 indicating no comorbidities and 2 representing multiple comorbidities. The rate of BCT also decreased with decreasing levels of education measured (P < .01). Insurance status was significantly associated with the receipt of BCT, with patients who had private insurance most likely to undergo BCT (62.3%) compared with Medicare patients 65 years or older (53.9%), Medicare patients younger than 65 years (54.8%), women receiving Medicaid (51.5%), and those with no insurance (49.3%) (P < .01). Likewise, BCT rates were associated with income. Patients with an annual median income of $46 000 per year or more had the highest rates of BCT (61.6%), and those who made less than $30 000 per year had the lowest BCT rates (51.1%) (P < .01) (Table 1).
Table 1.
Association Between Treatment Type and Patient Characteristics
| Characteristic | No. (%)a | |
|---|---|---|
| BCT | Mastectomy | |
| Year of breast cancer diagnosis | ||
| 1998 | 20 097 (54.3) | 16 886 (45.7) |
| 1999 | 20 494 (55.3) | 16 558 (44.7) |
| 2000 | 20 613 (56.0) | 16 224 (44.0) |
| 2001 | 20 425 (56.1) | 16 015 (43.9) |
| 2002 | 20 260 (56.9) | 15 325 (43.1) |
| 2003 | 24 581 (59.2) | 16 955 (40.8) |
| 2004 | 21 745 (58.8) | 15 221 (41.2) |
| 2005 | 22 509 (59.3) | 15 425 (40.7) |
| 2006 | 24 325 (59.7) | 16 388 (40.3) |
| 2007 | 28 422 (58.6) | 20 050 (41.4) |
| 2008 | 44 745 (58.3) | 31 961 (41.7) |
| 2009 | 51 207 (58.5) | 36 341 (41.5) |
| 2010 | 51 806 (59.2) | 35 766 (40.8) |
| 2011 | 52 654 (60.1) | 34 929 (39.9) |
| Age, y | ||
| 18–51 | 110 736 (57.8) | 80 812 (42.2) |
| 52–61 | 115 078 (62.8) | 68 175 (37.2) |
| 62–70 | 99 165 (61.6) | 61 713 (38.4) |
| >70 | 98 904 (51.4) | 93 344 (48.6) |
| Race/ethnicity | ||
| Black | 39 251 (54.9) | 32 285 (45.1) |
| White | 366 767 (58.8) | 256 687 (41.2) |
| Other | 13 662 (52.9) | 12 188 (47.1) |
| Charlson/Deyo score | ||
| 0 | 282 342 (60.7) | 182 444 (39.3) |
| 1 | 33 710 (50.8) | 32 642 (49.2) |
| 2 | 5942 (42.8) | 7950 (57.2) |
| Insurance status | ||
| Medicaid | 18 099 (51.5) | 17 033 (48.5) |
| Medicare, age <65 y | 12 976 (54.8) | 10 723 (45.2) |
| Medicare, age ≥65 y | 131 112 (53.9) | 112 024 (46.1) |
| Not insured | 6956 (49.3) | 7141 (50.7) |
| Other government | 3152 (57.8) | 2301 (42.2) |
| Private | 243 843 (62.3) | 147 553 (37.7) |
| Median income, $ | ||
| <30 000 | 41 830 (51.1) | 40 068 (48.9) |
| 30 000–34 999 | 62 061 (54.5) | 51 783 (45.5) |
| 35 000–45 999 | 110 402 (57.9) | 80 407 (42.1) |
| ≥46 000 | 188 255 (61.6) | 117 211 (38.4) |
| Population without high school diploma, % | ||
| <14.0 | 172 200 (61.7) | 106 734 (38.3) |
| 14.0–19.9 | 97 137 (59.0) | 67 623 (41.0) |
| 20.0–28.9 | 81 910 (55.6) | 65 356 (44.4) |
| ≥29.0 | 51 266 (50.8) | 49 732 (49.2) |
| Facility type | ||
| Academic/research program | 122 347 (59.8) | 82 366 (40.2) |
| Community cancer program | 47 920 (55.4) | 38 624 (44.6) |
| Comprehensive community cancer program | 253 616 (58.1) | 183 054 (41.9) |
| Facility location | ||
| Midwest | 112 945 (58.9) | 78 761 (41.1) |
| Northeast | 106 350 (64.5) | 58 515 (35.5) |
| South | 124 394 (52.0) | 114 761 (48.0) |
| West | 80 194 (60.7) | 52 007 (39.3) |
| Distance from treatment facility, km | ||
| 6.4–13.2 | 105 952 (60.1) | 70 407 (39.9) |
| 13.3–27.7 | 100 019 (59.0) | 69 485 (41.0) |
| >27.8 | 92 420 (54.0) | 78 830 (46.0) |
| ≤6.3 | 108 893 (59.5) | 74 128 (40.5) |
| Clinical T stage | ||
| T1 | 330 209 (65.3) | 175 143 (34.7) |
| T2 | 93 674 (42.1) | 128 901 (57.9) |
| Clinical N stage | ||
| N0 | 357 640 (61.8) | 220 871 (38.2) |
| N1 | 32 523 (42.3) | 44 385 (57.7) |
| N2 | 4241 (32.5) | 8803 (67.5) |
| N3 | 1398 (30.9) | 3121 (69.1) |
| NX | 28 081 (51.1) | 26 864 (48.9) |
| Hormone receptor status | ||
| Negative | 49 297 (55.3) | 39 770 (44.7) |
| Positive | 242 086 (60.5) | 158 229 (39.5) |
Abbreviation: BCT, breast-conserving therapy.
All differences were significant at P < .01.
In addition to patient demographics, the rates of BCT varied based on treating facility variables, such as facility type, facility location, and travel distance to the treatment facility (Table 1). The highest percentage of BCT was performed at facilities with academic/research programs (59.8%) followed by comprehensive community cancer programs (58.1%), and the lowest percent-age of BCT was seen in community cancer programs (55.4%) (P < .01). Facilities located in the South had the lowest rate of BCT (52.0%) and those in the Northeast had the highest rate (64.5%) (P < .01). Patients who had a travel distance of more than 27.8 km to the treatment facility had the lowest BCT rate (54.0%) compared with women who lived closer to a treatment facility (59.0%–60.1%) (P < .01). The South had the largest percentage of patients with a travel distance to a treatment facility greater than 27.2 km (eTable 1 in the Supplement).
In multivariate analysis, year of diagnosis, clinical T and N stage, age, insurance status, median annual income, educational level, facility type, facility location, and distance from the treatment facility remained significantly associated with receipt of BCT (Table 2). Race/ethnicity was not included in the multivariate model owing to its strong colinear correlations with insurance status, educational level, and facility location. Similarly, breast cancer volume was not included in the multivariate model owing to its association with facility type. Nodal status was also associated with socioeconomic variables; specifically, higher nodal stage was more likely among women with lower median income, lower educational level, and those uninsured or insured by Medicaid (eTable 2 in the Supplement). However, after adjusting for these socioeconomic variables, higher nodal status remained associated with a lower likelihood of undergoing BCT.
Table 2.
Multivariate Logistic Regression Model for Receipt of BCT as a Function of Patients’ Demographic and Clinical Characteristics
| Characteristic | OR (95% CI) |
|---|---|
| Year of diagnosis | |
| 1998 | 1 [Reference] |
| 1999 | 1.04 (1.01–1.07) |
| 2000 | 1.07 (1.03–1.10) |
| 2001 | 1.07 (1.04–1.11) |
| 2002 | 1.13 (1.09–1.16) |
| 2003 | 1.24 (1.20–1.27) |
| 2004 | 1.24 (1.20–1.28) |
| 2005 | 1.29 (1.25–1.33) |
| 2006 | 1.30 (1.26–1.34) |
| 2007 | 1.24 (1.20–1.27) |
| 2008 | 1.18 (1.15–1.21) |
| 2009 | 1.18 (1.15–1.21) |
| 2010 | 1.22 (1.18–1.25) |
| 2011 | 1.25 (1.21–1.28) |
| Age, y | |
| 18–51 | 1 [Reference] |
| 52–61 | 1.14 (1.12–1.15) |
| 62–70 | 1.09 (1.07–1.11) |
| >70 | 0.73 (0.72–0.75) |
| Insurance status | |
| Private | 1 [Reference] |
| Medicaid | 0.77 (0.75–0.79) |
| Medicare, age <65 y | 0.75 (0.73–0.77) |
| Medicare, age ≥65 y | 0.84 (0.83–0.86) |
| Not insured | 0.75 (0.72–0.78) |
| Other government | 0.96 (0.91–1.02) |
| Median income, $ | |
| ≥46 000 | 1 [Reference] |
| <30 000 | 0.92 (0.90–0.94) |
| 30 000–34 999 | 0.96 (0.94–0.97) |
| 35 000–45 999 | 0.99 (0.97–1.00) |
| Population without a high school diploma, % | |
| ≥29.0 | 1 [Reference] |
| <14.0 | 1.16 (1.14–1.19) |
| 14.0–19.9 | 1.13 (1.11–1.15) |
| 20.0–28.9 | 1.09 (1.07–1.11) |
| Facility type | |
| Community cancer program | 1 [Reference] |
| Academic/research program | 1.13 (1.11–1.15) |
| Comprehensive community cancer program | 1.08 (1.06–1.10) |
| Facility location | |
| South | 1 [Reference] |
| Midwest | 1.24 (1.22–1.25) |
| Northeast | 1.50 (1.48–1.52) |
| West | 1.33 (1.31–1.35) |
| Distance from treatment facility, km | |
| >27.8 | 1 [Reference] |
| 13.3–27.7 | 1.15 (1.14–1.17) |
| 6.4–13.2 | 1.25 (1.23–1.27) |
| ≤6.3 | 1.25 (1.23–1.27) |
| Clinical T stage | |
| T2 | 1 [Reference] |
| T1 | 2.30 (2.28–2.3) |
| Clinical N stage | |
| N0 | 1 [Reference] |
| N1 | 0.58 (0.57–0.59) |
| N2 | 0.42 (0.41–0.44) |
| N3 | 0.40 (0.37–0.42) |
| NX | 0.71 (0.70–0.72) |
Abbreviations: BCT, breast-conserving therapy; OR, odds ratio.
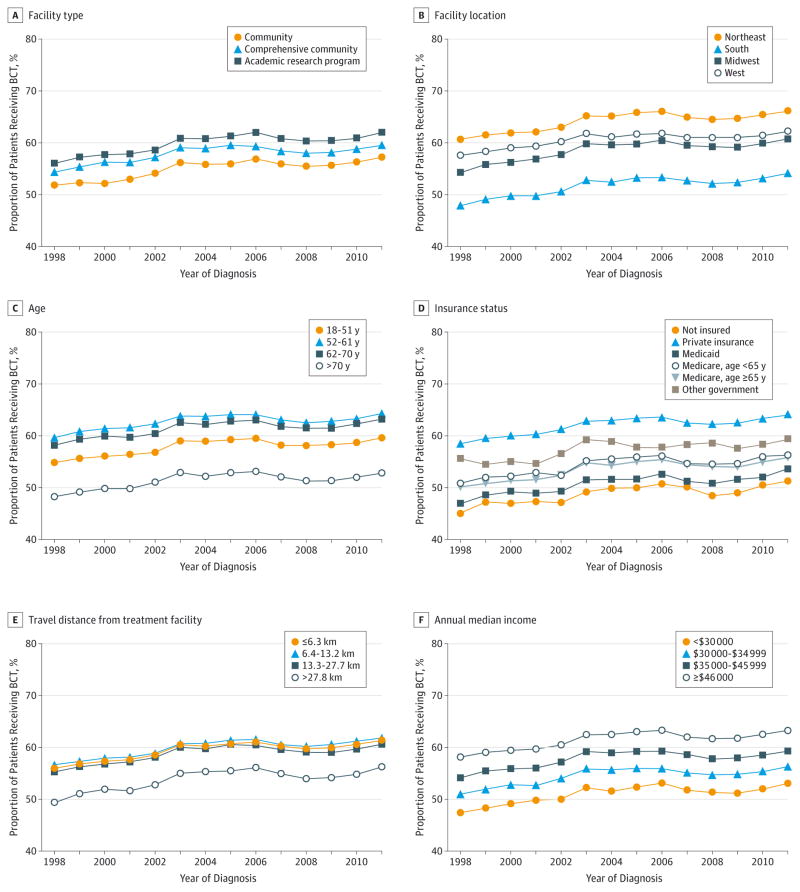
We next evaluated the change over time for the variables associated with BCT use (Table 3). There were several notable interactions between year of diagnosis and risk factors of BCT use. At the beginning of the study period in 1998, a large gap existed with most BCT being performed at academic/research programs (Figure 2A; unadjusted data available in eFigure in the Supplement). Rates of BCT use increased significantly at nonacademic programs during the study period, such that by 2011, the association between facility type and BCT was no longer statistically significant (P = .17 for association between BCT and facility type in 2011) (Table 3). This change over time was statistically significant (P < .01).
Table 3.
Comparison of Factors Related to Receipt of BCT in 1998 and 2011
| Variable | 1998
|
2011
|
P Value for Interaction | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value vs Reference Group | P Value for Overall Effect | OR (95% CI) | P Value vs Reference Group | P Value for Overall Effect | ||
| Age, y | |||||||
|
| |||||||
| 18–51 | 1 [Reference] | <.01 | 1 [Reference] | <.01 | <.01 | ||
|
|
|
||||||
| 52–61 | 1.00 (0.94–1.07) | >.99 | 1.26 (1.21–1.32) | <.01 | |||
|
|
|
||||||
| 62–70 | 0.99 (0.92–1.07) | .82 | 1.22 (1.17–1.29) | <.01 | |||
|
|
|
||||||
| >70 | 0.78 (0.72–0.86) | <.01 | 0.79 (0.75–0.84) | <.01 | |||
|
| |||||||
| Insurance status | |||||||
|
| |||||||
| Private | 1 [Reference] | <.01 | 1 [Reference] | <.01 | .03 | ||
|
|
|
||||||
| Medicaid | 0.65 (0.56–0.76) | <.01 | 0.80 (0.76–0.86) | <.01 | |||
|
|
|
||||||
| Medicare, age <65 y | 0.82 (0.71–0.94) | <.01 | 0.78 (0.72–0.85) | <.01 | |||
|
|
|
||||||
| Medicare ≥65 y | 0.86 (0.80–0.93) | <.01 | 0.90 (0.85–0.94) | <.01 | |||
|
|
|
||||||
| Not insured | 0.70 (0.60–0.81) | <.01 | 0.83 (0.75–0.92) | <.01 | |||
|
|
|
||||||
| Other government | 1.19 (0.87–1.63) | .28 | 0.92 (0.79–1.07) | .28 | |||
|
| |||||||
| Median income, $ | |||||||
|
| |||||||
| ≥46 000 | 1 [Reference] | .35 | 1 [Reference] | <.01 | .28 | ||
|
|
|
||||||
| <30 000 | 0.94 (0.85–1.03) | .19 | 0.86 (0.81–0.92) | <.01 | |||
|
|
|
||||||
| 30 000–34 999 | 1.00 (0.93–1.09) | .91 | 0.93 (0.88–0.98) | .01 | |||
|
|
|
||||||
| 35 000–45 999 | 1.01 (0.95–1.08) | .69 | 1.00 (0.96–1.04) | .93 | |||
|
| |||||||
| Population without high school diploma, % | |||||||
|
| |||||||
| ≥29.0 | 1 [Reference] | <.01 | 1 [Reference] | <.01 | .03 | ||
|
|
|
||||||
| <14.0 | 1.37 (1.26–1.50) | <.01 | 1.16 (1.09–1.23) | <.01 | |||
|
|
|
||||||
| 14.0–19.9 | 1.24 (1.13–1.35) | <.01 | 1.11 (1.04–1.17) | <.01 | |||
|
|
|
||||||
| 20.0–28.9 | 1.20 (1.11–1.30) | <.01 | 1.08 (1.03–1.14) | <.01 | |||
|
| |||||||
| Facility type | |||||||
|
| |||||||
| Community cancer program | 1 [Reference] | <.01 | 1 [Reference] | .17 | <.01 | ||
|
|
|
||||||
| Academic/research program | 1.51 (1.39–1.64) | <.01 | 1.01 (0.96–1.06) | .76 | |||
|
|
|
||||||
| Comprehensive community cancer program | 1.18 (1.01–1.27) | <.01 | 1.04 (0.99–1.09) | .15 | |||
|
| |||||||
| Facility location | |||||||
|
| |||||||
| South | 1 [Reference] | <.01 | 1 [Reference] | <.01 | <.01 | ||
|
|
|
||||||
| Midwest | 1.31 (1.23–1.39) | <.01 | 1.19 (1.14–1.23) | <.01 | |||
|
|
|
||||||
| Northeast | 1.82 (1.71–1.94) | <.01 | 1.42 (1.36–1.48) | <.01 | |||
|
|
|
||||||
| West | 1.56 (1.45–1.67) | <.01 | 1.17 (1.12–1.22) | <.01 | |||
|
| |||||||
| Distance from treatment facility, km | |||||||
|
| |||||||
| >27.8 | 1 [Reference] | <.01 | 1 [Reference] | <.01 | .10 | ||
|
|
|
||||||
| ≤6.3 | 1.11 (1.04–1.18) | <.01 | 1.21 (1.16–1.26) | <.01 | |||
|
|
|
||||||
| 6.4–13.2 | 1.15 (1.07–1.23) | <.01 | 1.16 (1.11–1.21) | <.01 | |||
|
|
|
||||||
| 13.3–27.7 | 1.08 (1.01–1.16) | .03 | 1.09 (1.05–1.14) | <.01 | |||
|
| |||||||
| Clinical T stage | |||||||
|
| |||||||
| T2 | 1 [Reference] | 1 [Reference] | <.01 | <.01 | |||
|
|
|
||||||
| T1 | 2.62 (2.49–2.75) | <.01 | 2.22 (2.15–2.29) | <.01 | |||
|
| |||||||
| Clinical N stage | |||||||
|
| |||||||
| N0 | 1 [Reference] | <.01 | 1 [Reference] | <.01 | <.01 | ||
|
|
|
||||||
| N1 | 0.55 (0.50–0.59) | <.01 | 0.56 (0.54–0.59) | <.01 | |||
|
|
|
||||||
| N2 | 0.44 (0.33–0.57) | <.01 | 0.40 (0.35–0.45) | <.01 | |||
|
|
|
||||||
| N3 | 1.73 (0.65–4.61) | .27 | 0.34 (0.28–0.41) | <.01 | |||
|
|
|
||||||
| NX | 0.72 (0.67–0.77) | <.01 | 0.64 (0.58–0.70) | <.01 | |||
Abbreviations: BCT, breast-conserving therapy; OR, odds ratio.
Figure 2. Breast-Conserving Therapy Use Over Time.
Data were adjusted for all variables: age, insurance status, median income, facility type, travel distance, facility location, and tumor (T) and nodal (N) stage.
Significant gains were also seen in the use of BCT in the South, thereby narrowing the disparity in BCT rates across geographic regions of the United States (Figure 2B). When adjusting for other variables, in 1998 patients in the Midwest, Northeast, and West had 30% to 80% higher odds of BCT use compared with patients in the South. However, by 2011 these odds decreased to 19% to 40%, which is a significant change over time in the association between geographic location of the facility and use of BCT (P < .01) (Table 3).
Compared with 1998, age remained significantly associated with BCT in 2011. However, the BCT rates increased in the older cohorts, especially among the 62- to 70-year age group (odds ratio [OR], 0.99; 95% CI, 0.92–1.07 in 1998 vs 1.22; 1.17–1.29 in 2011), resulting in significantly smaller age-based disparities over time (P < .01) (Figure 2C and Table 3).
In contrast, associations between insurance type, income, and travel distance to the treatment facility in 1998 remained unchanged or showed greater disparity compared with the 2011 associations. Throughout the entire study period, private insurance was consistently associated with higher BCT use (Figure 2D). Although trends were seen toward greater use of BCT in the Medicaid, Medicare, and uninsured groups resulting in significant decrease in insurance-based disparities over time (P = .03), overall, patients with private insurance remained the most likely to undergo BCT and those with other government insurance saw a relative decrease in 2011 compared with 1998 (Table 3).
Travel distance to a treatment facility retained a constant negative association with use of BCT across the study period (Figure 2E and Table 3). In both 1998 and 2011, travel distance greater than 27.7 km resulted in significantly less BCT use, with little improvement during the years studied (P = .10). As reported in Table 3 (with data given as OR; 95% CI), patients with a travel distance of less than 6.4 km to a treatment facility were much more likely to undergo BCT compared with those for whom the travel distance was more than 27.7 km in 1998 (1.11; 1.04–1.18); this finding persisted in 2011 (1.21; 1.16–1.26).
A median annual income of at least $46 000 was associated with more BCT use across the study period (Figure 2F). However, in the adjusted analysis presented in Table 3, no association was seen in 1998 between BCT and annual median income. In 2011, however, a substantial disparity had developed, with patients who earned less than $30 000 per year much less likely to undergo BCT than were those who earned at least $46 000 annually (OR, 0.86; 95% CI, 0.81–0.92; P < .01). However, the overall change between 1998 and 2011 did not reach significance (P = .28) (Table 3).
Discussion
To our knowledge, this comprehensive population-based review is one of the largest studies of BCT use for early-stage breast cancer in the past 15 years. Comparison between the beginnings of the study period in 1998 with the end of the study period in 2011 highlights the important dynamic nature of the demographic factors that affect the use of BCT. We found significant declines in disparities in age-, facility type–, and facility location–based factors and receipt of BCT. However, we identified several socioeconomic factors that appear to represent new and/or persistent barriers to use of BCT, and we showed that the proximity to a treatment facility remains a significant consideration for receipt of BCT.
Our findings align with several prior observations10,11 showing significantly less BCT performed in the southern regions of the United States. Using the national Medicare data set of patients who underwent surgical treatment of early-stage breast cancer in 2009, Smith et al13 found a significant variation in treatment type based on geographic location. Similar to our results, they found that the highest percentage of 29 828 patients undergoing breast-conserving surgery lived in the Northeast (78%) and Pacific West (71%) compared with the South (57%).13 Likewise, in a multi-institutional review by Chagpar et al11 of patients with early-stage breast cancer treated from 1998 to 2004, the Northeast had the highest rate of BCT independent of patient age, tumor factors, and surgeon practice. Although our study continues to show a lower rate of BCT in the South compared with other US regions, it appears that the gap has been closing between 1998 and 2011. Furthermore, our data suggest that one of the reasons for the lower rates of BCT in the South is that women in this region have disproportionately greater travel distance to treatment facilities.
Our findings suggest that travel distance may represent a surrogate for ability or willingness to access radiotherapy, a hypothesis that is supported by reports from a number of other authors.13,14 Jacobs et al15 analyzed the likelihood of an urban vs a rural patient to undergo mastectomy, which included access to radiotherapy as a variable. Using the SEER database, Jacobs and colleagues reviewed the records on women with stage I to III breast cancer and saw a significant difference in the percentage of mastectomies between 124 143 women in the urban population (44% underwent mastectomies) and 13 160 in the rural population (59% underwent mastectomies). The density of radiotherapy oncologists was found to be much higher in the urban population, with a mean of 10 radiation oncologists per county. This density was contrasted with 2 radiotherapy oncologists per county for the rural population. Similarly, in a review of the national Medicare data set performed by Smith et al,13 the median density of radiotherapy oncologists (1 per 10 000 persons) was a significant predictor of use of BCT. Therefore, our data agree with those in the literature indicating that the availability of radiotherapy is a key consideration for access to BCT and underscore the need to comprehensively consider the availability of all elements of multidisciplinary care when reviewing geographic variation in the rates of BCT vs mastectomy.
Access to health care has been a concern in this country for some time, and many reports have highlighted the disparities in care that exist between insured patients and those without health insurance.12,16 Using single-institutional data, Voti et al,17 found that privately insured women were 49% more likely to undergo BCT compared with women without health insurance, a finding similar to ours. Data from the Kentucky Cancer Registry18 showed that lack of insurance was significantly associated with the omission of adjuvant radiotherapy after breast-conserving surgery, and Ayanian et al12 demonstrated that women with breast cancer who did not have health insurance had worse overall outcomes, including decreased survival, compared with women with private health insurance. Although we demonstrated improvement in access to BCT across most groups who do not have private insurance, overall the absolute rate of BCT use remains disparate between individuals with private insurance and those with other forms of coverage.
It remains uncertain what effect the Affordable Care Act and increased patient insurance coverage will have on BCT trends. A lack of health insurance has been correlated15,19 with unemployment and, therefore, lower income status, and socioeconomic factors may contribute to patient choice of surgical treatment. A woman in a low-income family may be unwilling and unable to take the length of time off work needed for the weeks of radiotherapy required to appropriately complete BCT. In the present study, we found that low median household income was associated with less BCT use and that this disparity increased during the study period. A review20 of Medicare Part A claims in 1990 showed similar results, with a greater proportion of people living below the poverty line having a reduced chance of undergoing BCT. In addition, women from low-income families often have less education, and we showed that level of education also correlates with use of BCT.21 These data demonstrate the breadth of the socioeconomic factors that need to be considered to adequately address the disparate use of BCT across demographic groups.
Among the most encouraging findings from our analysis is the considerable improvement of disparities based on facility type and the options afforded to older populations. This improvement suggests that national guidelines on breast cancer care are effectively meeting the goal of standardizing care across the United States. However, within age groups, there remains room for improvement. Our data show that women older than 70 years continue to undergo mastectomy at higher rates than their younger counterparts. Although this higher rate may reflect a desire to simplify care by omitting the need for radiotherapy following lumpectomy, clinical trial data22 support the use of lumpectomy alone in women older than 70 years with T1N0, estrogen receptor–positive cancers—a cohort that likely constitutes a significant proportion of elderly women with breast cancer. A limitation of our study is that we did not examine rates of breast-conserving surgery alone (ie, without radiotherapy) in this cohort.
Conclusions
This comprehensive national review demonstrates that BCT rates have increased during the past 2 decades. Disparities in the use of BCT based on age, geographic location, and type of cancer program have improved since 1998. However, insurance, income, and travel distance to treatment facilities persist as key barriers to BCT use. These socioeconomic barriers are unlikely to be erased without health policy changes.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by a support grant P30 CA016672 to The University of Texas MD Anderson Cancer Center.
Footnotes
Supplemental content at jamasurgery.com
Role of the Funder/Sponsor: The University of Texas MD Anderson Cancer Center had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest Disclosures: None reported.
Author Contributions: Drs Shen and Bedrosian had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lautner, Shen, Babiera, Bedrosian.
Acquisition, analysis, or interpretation of data: Lautner, Lin, Shen, Parker, Kuerer, Shaitelman.
Drafting of the manuscript: Lautner, Lin, Kuerer, Babiera, Bedrosian.
Critical revision of the manuscript for important intellectual content: Lautner, Shen, Parker, Kuerer, Shaitelman.
Statistical analysis: Lin, Shen.
Obtained funding: Kuerer.
Administrative, technical, or material support: Shen, Shaitelman, Babiera, Bedrosian.
Contributor Information
Meeghan Lautner, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Heather Lin, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston.
Yu Shen, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston.
Catherine Parker, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Henry Kuerer, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Simona Shaitelman, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Gildy Babiera, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
Isabelle Bedrosian, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston.
References
- 1.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305(1):6–11. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Conference. NIH Consensus Conference. Treatment of early-stage breast cancer. JAMA. 1991;265(3):391–395. [PubMed] [Google Scholar]
- 4.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27(25):4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? a 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16(10):2682–2690. doi: 10.1245/s10434-009-0635-x. [DOI] [PubMed] [Google Scholar]
- 6.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J Clin Oncol. 2010;28(21):3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 7.American College of Surgeons. [Accessed May 12, 2015];CoC Accreditation Categories. https://www.facs.org/quality-programs/cancer/accredited/about/categories.
- 8.Woolson RF, Clarke WR. Statistical Methods for the Analysis of Biomedical Data. 2. New York, NY: Wiley-Interscience; 2002. [Google Scholar]
- 9.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. New York, NY: Wiley; 2000. [Google Scholar]
- 10.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326(17):1102–1107. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 11.Chagpar AB, Studts JL, Scoggins CR, et al. Factors associated with surgical options for breast carcinoma. Cancer. 2006;106(7):1462–1466. doi: 10.1002/cncr.21728. [DOI] [PubMed] [Google Scholar]
- 12.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 13.Smith GL, Xu Y, Shih YC, et al. Breast-conserving surgery in older patients with invasive breast cancer: current patterns of treatment across the United States. J Am Coll Surg. 2009;209(4):425–433. e2. doi: 10.1016/j.jamcollsurg.2009.06.363. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin LM, Taplin SH, Friedman H, Moe R. Access to multidisciplinary cancer care: is it linked to the use of breast-conserving surgery with radiation for early-stage breast carcinoma? Cancer. 2004;100(4):701–709. doi: 10.1002/cncr.20030. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs LK, Kelley KA, Rosson GD, Detrani ME, Chang DC. Disparities in urban and rural mastectomy populations: the effects of patient- and county-level factors on likelihood of receipt of mastectomy. Ann Surg Oncol. 2008;15(10):2644–2652. doi: 10.1245/s10434-008-0053-5. [DOI] [PubMed] [Google Scholar]
- 16.Roetzheim RG, Gonzalez EC, Ferrante JM, Pal N, Van Durme DJ, Krischer JP. Effects of health insurance and race on breast carcinoma treatments and outcomes. Cancer. 2000;89(11):2202–2213. doi: 10.1002/1097-0142(20001201)89:11<2202::aid-cncr8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Voti L, Richardson LC, Reis IM, Fleming LE, Mackinnon J, Coebergh JW. Treatment of local breast carcinoma in Florida: the role of the distance to radiation therapy facilities. Cancer. 2006;106(1):201–207. doi: 10.1002/cncr.21557. [DOI] [PubMed] [Google Scholar]
- 18.Dragun AE, Huang B, Tucker TC, Spanos WJ. Disparities in the application of adjuvant radiotherapy after breast-conserving surgery for early stage breast cancer: impact on overall survival. Cancer. 2011;117(12):2590–2598. doi: 10.1002/cncr.25821. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll AKBA, Bernstein AB. Health and access to care among employed and unemployed adults: United States, 2009–2010. NCHS Data Brief. 2012;(83):1–8. [PubMed] [Google Scholar]
- 20.Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79(2):314–319. [PubMed] [Google Scholar]
- 21.Day JCNE. The Big Payoff: Educational Attainment and Synthetic Estimates of Work-Life Earnings. Suitland, MD: US Census Bureau, US Dept of Commerce; 2002. [Google Scholar]
- 22.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.