Abstract
The learning capacities of males and females may differ with sex-specific behavioural requirements. Bumblebees provide a useful model system to explore how different lifestyles are reflected in learning abilities, because their (female but sterile) workers and males engage in fundamentally different behaviour routines. Bumblebee males, like workers, embark on active flower foraging but in contrast to workers they have to trade off their feeding with mate search, potentially affecting their abilities to learn and utilize floral cues efficiently during foraging. We used a serial colour-learning task with freely flying males and workers to compare their ability to flexibly learn visual floral cues with reward in a foraging scenario that changed over time. Male bumblebees did not differ from workers in both their learning speed and their ability to overcome previously acquired associations, when these ceased to predict reward. In all foraging tasks we found a significant improvement in choice accuracy in both sexes over the course of the training. In both sexes, the characteristics of the foraging performance depended largely on the colour difference of the two presented feeder types. Large colour distances entailed fast and reliable learning of the rewarding feeders whereas choice accuracy on highly similar colours improved significantly more slowly. Conversely, switching from a learned feeder type to a novel one was fastest for similar feeder colours and slow for highly different ones. Overall, we show that behavioural sex dimorphism in bumblebees did not affect their learning abilities beyond the mating context. We discuss the possible drivers and limitations shaping the foraging abilities of males and workers and implications for pollination ecology. We also suggest stingless male bumblebees as an advantageous alternative model system for the study of pollinator cognition.
Keywords: bumblebee, colour distance, learning speed, male, operant serial learning, sex-specific cognitive abilities
Highlights
-
•
Bumblebee males are highly efficient in associating colour cues with sucrose reward.
-
•
Males and workers (females) are similar in learning ability of novel rewarding cues.
-
•
Learning performance is determined by the feeder colours but not by sex.
-
•
Sex-specific behavioural repertoire does not affect foraging cognitive abilities.
-
•
Male bumblebees are an advantageous model system to study pollinator cognition.
Learning enables animals to store relevant information about their environment and can be assumed to vary, at least to some extent, with the animals' behavioural requirements (Healy, Bacon, Haggis, Harris, & Kelley, 2009). Males and females in many species substantially differ in their behaviour, providing a powerful tool to study how sex-specific differences in lifestyle shape the performance of the cognitive system (Beani and Zuk, 2014, Healy et al., 2009, Jacobs, 1996, Jacobs et al., 1990). Numerous studies have compared the cognitive abilities of males and females in humans (Grön et al., 2000, Halpern and LaMay, 2000, Levy et al., 2005, Saucier et al., 2002) and a wide range of other animals such as primates (Greeno and Semple, 2009, Lonsdorf et al., 2004), birds (Vicario, Naqvi, & Raksin, 2001), rodents (Dalla et al., 2009, Dalla and Shors, 2009, Gaulin and FitzGerald, 1986, Jonasson, 2005) and, more recently, some invertebrates (Aquino et al., 2015, Beani et al., 2014, Dinges et al., 2013, Leonard and Hedrick, 2009, Pankiw and Page, 1999, Sammut et al., 2015, Sanford and Tomberlin, 2011). While typically exhibiting at least some common behaviours that can be used to assess cognitive ability (Rowe and Healy, 2014, Sanford and Tomberlin, 2011), males and females often pursue fundamentally different interests and face different selective pressures that may require the utilization of different environmental information (Beani and Zuk, 2014, Galea et al., 1996, Healy et al., 2009, Leonard and Hedrick, 2009). For example, a link between differences in learning abilities between males and females and their environmental needs has been proposed in some rodent species. Male deer mice, Peromyscus maniculatus, and meadow voles, Microtus pennsylvanicus, have high dispersal rates, large home ranges and are highly promiscuous, which distinguishes them from conspecific females (Galea et al., 1996). Correspondingly, the males in both species have significantly superior spatial abilities during the breeding season. In pine voles, Microtus pinetorum, on the other hand, mating strategies of males and females are more similar and irrespective of breeding season no performance difference in spatial tasks between males and females could be found (Gaulin & FitzGerald, 1986).
Bumblebees, a well-established model species in animal cognition, also provide an ideal study system to investigate the question of how lifestyle differences might be reflected in cognitive abilities. The female workers differ profoundly from males in their behavioural needs and repertoire. Worker bumblebees are typically sterile and engage in nest construction and defence, brood provisioning, climate control and, outside the colony, forage for floral resources which they deposit in the nest for the common good (Allen et al., 1978, Cartar, 1992, Heinrich, 1976, Heinrich, 1979, Weidenmüller et al., 2002). On the other hand, workers never engage in mating behaviour, and their activities outside the colony are mostly limited to floral foraging (Goulson, 2003, Heinrich, 1979). In stark contrast, bumblebee males, just like solitary animals of other species, have to trade off multiple activities including mating behaviour (Heinrich, 1979) and active foraging. However, other than workers, males only forage for themselves, and not for the communal pantry. Living a solitary life outside the colony, males of most bumblebee species disperse (Kraus et al., 2009, Wolf et al., 2012) and pursue an energetically expensive patrolling behaviour to look for virgin queens (Alcock et al., 1978, Ayasse et al., 2001, Eickwort and Ginsberg, 1980, Frank, 1941, Haas, 1949, Svensson, 1979) which requires repeated collection of nectar from flowers (Jennersten, Morse, & O'Neil, 1991). In light of these fundamental differences in lifestyle, comparing the ability of male and worker bumblebees to utilize floral cues provides a unique opportunity to investigate how sex-specific behavioural adaptations affect cognitive performance in a nonmating context.
It is likely that bumblebee males will display some learning ability in a foraging context. Learning abilities in male pollinators were first suggested for honeybee, Apis mellifera, drones (Chittka, Beier, Hertel, Steinmann, & Menzel, 1992) in the context of colour learning at the hive entrance. Additionally, similarities between honeybee drones and workers were shown in response to sucrose concentration (Pankiw & Page, 1999) and olfactory cues (Aquino et al., 2015, Benatar et al., 1995), although, over evolutionary time, the drones have lost any active foraging behaviour (and are fed within the colony; Winston, 1991). Proboscis extension response (PER) experiments also showed the principal ability of male bumblebees for associative visual learning (Lichtenstein, Sommerlandt, & Spaethe, 2015). On the other hand, a comparison of aversive learning abilities of workers and drones suggested male deficits in the ability to avoid stress under some circumstances (Dinges et al., 2013).
Even though male and female bumblebees differ in their primary reasons for visiting flowers, the ability to forage efficiently can be assumed to provide advantages for both sexes. Minimized time investment (Burns, 2005, Chittka et al., 2003, Dyer and Chittka, 2004b), optimized energy intakes (Cartar and Dill, 1990, Waddington et al., 1981) and lower exposure to parasites (Fouks & Lattorff, 2011) and predators (Abbott, 2006, Dukas, 2001, Dukas, 2005, Ings and Chittka, 2008, Ings and Chittka, 2009) are only some factors that have shaped the foraging behaviour of workers. Likewise, in male bees, flower choice patterns reminiscent of those of females have been recorded or inferred in orchid bees (Euglossini; Ackerman, 1982) and some bumblebee species (Alcock et al., 1978, Jennersten et al., 1991, Ogilvie and Thomson, 2015a, Ostevik et al., 2010, Wolf and Moritz, 2014) indicating that male bees are at least capable of some behavioural adjustment to the foraging conditions. Yet, it remains unknown whether the males' need to accommodate both mate search and foraging in their cognitive system have compromised their foraging abilities. Conceivably, the behavioural specialization to foraging might have given rise to superior learning abilities in workers setting them apart from the males.
In this study we used a serial colour-learning task in an operant learning paradigm to compare the abilities of male and worker bumblebees to learn colour cues and flexibly adjust these associations in accordance with the characteristics of the foraging resources. This allowed us to compare the cognitive abilities of males in a dynamic nonmating task to that of the highly specialized workers in a well-controlled operant colour-learning test.
Methods
Bees and Experimental Set-up
Bumblebees, Bombus terrestris audax, originated from four commercial colonies provided by BIOBEST (Biobest Belgium N.V., Westerlo, Belgium). Each nestbox was connected to a bifurcated Perspex tunnel system leading to two identical flight arenas (100 × 70 cm and 30 cm high), in which males and workers were pretrained to freely forage for 20% sucrose solution (w/w) from 12 clear square Perspex chips (25 × 25 mm and 5 mm high) placed horizontally on top of an upright clear glass tube (height: 40 mm; Fig. 1a). Males and workers successfully feeding on these Perspex feeders were individually marked with number tags (Opalithplättchen, Warnholz & Bienenvoigt, Ellerau, Germany). Pollen was provided ad libitum directly into the colony.
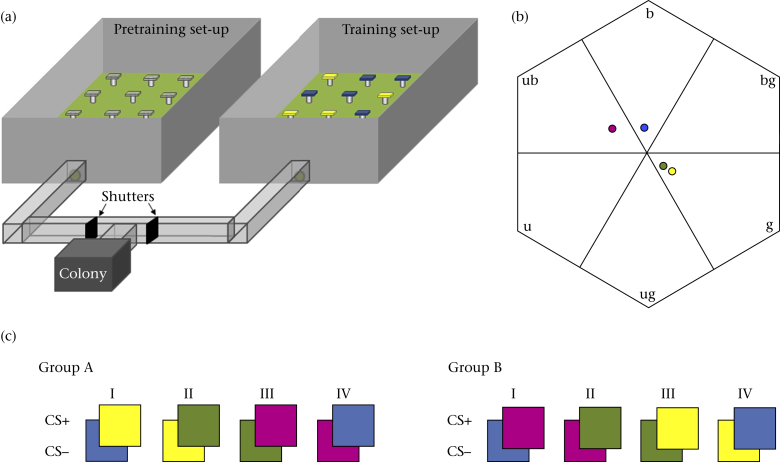
Figure 1.
(a) Experimental set-up consisting of two flight arenas (100 × 70 cm and 30 cm high) randomly used for either pretraining foraging (with Perspex feeders) or training (two differently coloured feeder types) to which a colony was connected via a bifurcated Perspex tunnel allowing for controlled access to the arenas by means of shutters. (b) The positions of the feeder colour loci in the hexagonal bee colour space determined by the responses each colour elicits on the bee's ultraviolet (u), blue (b) and green (g) photoreceptors (Chittka, 1992). The distances (hexagon units) between these colour loci were 0.061 (yellow-green), 0.189 (purple-blue), 0.333 (blue-yellow) and 0.391 (purple-green). (c) The four colour pairs comprising the two training groups (A, B). CS+ and CS− indicate the rewarding and nonrewarding conditioned colour stimulus, respectively. Each colour set was used until 50 feeder visits (after the first correct choice) were recorded, after which the next colour set in the sequence was presented. Each bee was trained on one of the two colour sequences only.
Artificial Flowers
For the training we replaced the Perspex feeder chips in one of the two flight arenas, the training arena, with coloured square plastic chips (25 × 25 mm and 5 mm high), while the other arena remained set up with Perspex feeders as a foraging arena (Fig. 1a). With shutters in the tunnel system connecting the colony to the two arenas we could divert workers for the colour training individually into the training arena, while allowing normal colony foraging on the clear feeders in the other arena.
The training arena contained 12 feeders of two types. One feeder type (six feeders) was rewarding (CS+) and provided 2 μl of sucrose solution (20% w/w), whereas the unrewarding feeder type (CS−) contained a 2 μl droplet of water. The small reward volume was chosen to encourage multiple feeder choices during each foraging bout in both workers and males, while the low sucrose concentration ensured that satiated males would become hungry again after short periods of food deprivation.
We used four different feeder colours in this study. To human observers these were yellow, green, blue and purple. The reflectance for each of those colours was measured in 1 nm increments over a wavelength range from 300 to 700 nm using a spectrophotometer (Ocean Optics S2000, Dunedin, FL, U.S.S.) with a deuterium/halogen light source with feeder reflectance values varying from 0 (no reflectance) to 1 (100% reflectance). Combining these reflectance curves with the spectral sensitivity functions of the three photoreceptors of B. terrestris (maximal sensitivity λmax: UV: 348 nm; blue: 435 nm; green: 533 nm; Chittka et al., 2001, Skorupski et al., 2007) we determined the positions of the feeder colours within the bee colour hexagon (Chittka, 1992). The distances between the feeder colour loci range from a small colour distance of 0.061 hexagon units between yellow and green, a medium colour distance between purple and blue of 0.189 to large colour distances of >0.3 hexagon units for blue-yellow (0.333) and purple-green (0.391; Fig. 1b).
Feeder Colour Sequences
Mimicking a temporal change in the floral market similar to that experienced by both workers and males, we used two sequences of successively presented feeder colour pairs. Colour sequence A started with rewarding yellow feeders (yellow+) and nonrewarding blue feeders (blue−) and subsequently progressed to green+/yellow−, purple+/green− and blue+/purple−. In sequence B we reversed the colour sets and rewarding colours starting with purple, rewarding (purple+), and blue, nonrewarding feeders (blue−) followed by green+/purple−, yellow+/green− and blue+/yellow− (Figure 1, Figure 2). In this way we balanced the use of feeder colours controlling for potential differences in colour preferences between males and workers.
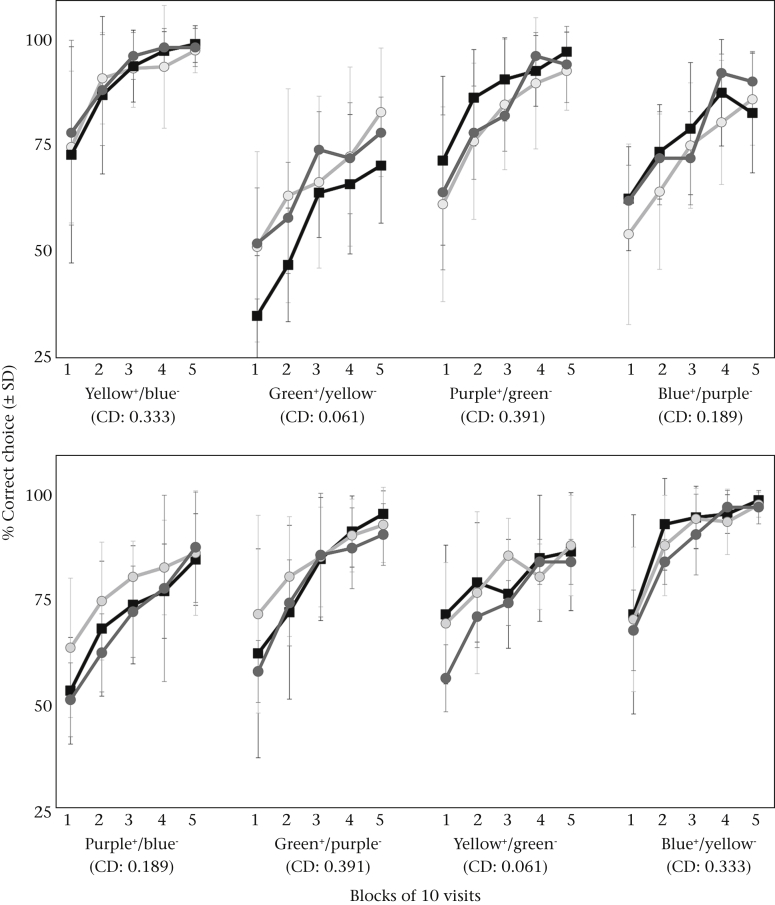
Figure 2.
The learning curves (mean choice accuracy ± SD) for the males (black squares and line, N = 30) and workers, with the latter trained either using a 2 h food deprivation period between foraging bouts (dark grey circles and line, N = 11) or allowing for continuous foraging between colony and feeders (light grey circles and line, N = 40) over 50 visits for each of four serially presented colour pairs. (a) Training sequence A started with yellow rewarding feeders (CS+) and blue nonrewarding (CS−) followed by green+/yellow−, purple+/green− and blue+/purple−. (b) Sequence B consisted of purple+ and blue− followed by green+/purple−, yellow+/green− and blue+/yellow−. CD denotes the colour distance in the hexagonal bee colour space between the feeder colours in the presented colour pair ranging from difficult to discriminate (<0.1) to highly discriminable (>0.2).
Training Procedure
Workers and males differ fundamentally in their foraging behaviour. Workers repeatedly move between the foraging grounds and the colony (Sladen, 1912), where they regurgitate their harvest before they resume foraging again. Although hunger may also be a contributing factor, their motivation to forage is largely determined by the nutritional status of the colony (Dornhaus and Chittka, 2001, Dornhaus and Chittka, 2005, Molet et al., 2008). For the colour training we only chose workers that commuted between the feeders and the colony at least three times during pretraining, indicating an active and motivated forager. Self-provisioning male bumblebees, on the other hand, only forage to satisfy their own energetic needs and commence foraging as soon as they are sufficiently hungry (Goulson, 2003). For the experiment we selected only those males that had been observed feeding on the Perspex feeders on at least three different occasions.
To provide comparable training conditions we not only tested workers in their usual foraging mode (shuttling between colony and artificial flower meadow) but we also trained isolated workers such as the males under a regime of controlled food deprivation of 2 h prior to testing and between foraging bouts to motivate them to visit the feeders. Once a bee entered the training arena it had to distinguish between six rewarding feeders indicated by a colour and six differently coloured nonrewarding ones. We recorded the number of landings on nonrewarding feeders before the first correct choice (‘latency to switch’). After a rewarding feeder had been visited for the first time, we recorded feeder choice (defined as landings on the feeder) and feeding events for the next 50 visits. Depleted rewarding feeders were refilled by hand with 2 μl of sucrose solution from an electronic dispenser pipette (HandyStep electronic, BrandTech Scientific, Inc., Essex, CT, U.S.A.) after the bee had left the feeder and had landed on another rewarding feeder. Recording continued until the bee was satiated and ceased foraging (foraging bout) or reached 50 visits and was caught.
Workers tested in their natural foraging mode (e.g. Raine and Chittka, 2008, Raine and Chittka, 2012, Raine et al., 2006, Wolf et al., 2015) were free to return to the colony after completing a foraging bout or were returned to the colony after 50 visits were completed. Their re-entering the training arena from the colony was regulated by means of shutters in the connecting tunnels. In contrast, all males and the group of workers trained using between-bout food deprivation were transferred into a separate and empty flight arena where they were allowed to move about freely but had no access to food for 2 h before being transferred back to the training arena.
Between foraging bouts all feeders were cleaned and the locations of the rewarding and unrewarding feeder types were randomized. If the bee had not completed the training on a colour pair (i.e. visited less than 50 feeders) in the previous bout the bee was allowed to complete training on the previously encountered feeder colours. Alternatively, if the training on the previous colour pair had been completed, the feeders' colours were exchanged for the next colour pair in the training sequence (Fig. 1c).
Sixty males were trained, of which 30 completed the full training sequence. In addition we trained 59 workers (48 with natural foraging and 11 with food deprivation). Forty of the natural foragers and 10 of the food-deprived individuals engaged with the full training sequence.
After completion of the last colour pair in the training sequence each bee was allowed to continue foraging to satiation (no limitation of visits) on these feeders while the recording of visits and feeding events continued. Using the largest volume of sucrose collected in a single bout by a bee we estimated the bees' crop capacity even if this exceeded 100 μl (maximum collectable volume in 50 visits) as a potential factor influencing foraging behaviour.
Data Analysis and Statistics
Learning curves were generated from the mean choice accuracies (±SD) of the bees for every consecutive block of 10 visits starting from the first correct visit in each of the four colour sets per training sequence (Fig. 2a, b). Latencies to switch were not plotted in the learning curve.
Following the approach of previous studies on bumblebee learning (Ings et al., 2009, Raine and Chittka, 2008, Raine and Chittka, 2012) we estimated individual learning speed (LS) from the rate at which the number of incorrect visits decreased over the course of the training fitting first-order exponential decay functions of the form y = y0 + Ae−x/t using Origin Pro 8 v.8.0724 (OriginLab Corporation, Northampton, MA, U.S.A.). In this function x represents the number of flower choices starting from the first correct choice, y is the number of errors, y0 is the number of incorrect choices at the end of the training and was constrained to 0 (100% correct feeder choices). Learning speed is reflected by the decay constant t where high t values indicate slow learning and vice versa. The curve amplitude A corresponds to the maximal number of incorrect choices the bee made and was constrained to 10 (100% incorrect choices). An exponential decay function could be successfully fitted to the behavioural performance in 71.9% of the workers' and 76.4% of the males' learning curves, respectively. The proportion of cases allowing for a successful curve fitting versus cases where no curve could be fitted served as an additional measure for potential performance difference.
We used a general linear model (GLM) fitted to a normal distribution with training procedure (‘TP’: with and without food deprivation), training sequence (‘seq’: A, B), colour distance of each feeder colour pair (‘CD’) and sex (‘sex’: male, worker) as fixed models to compare the choice accuracies in increments of 10 visits over the course of the training and learning speeds and latencies to switch (LAT) of the bees on each training regime (feeder colour pairs). Furthermore, using the global data set, we analysed the relationship of learning speed and latency to switch for both males and workers using a linear regression model. All statistical tests were done using Statistica 7.0 (Statsoft, Tulsa, OK, U.S.A.).
Ethical Note
Prior to the experiments all bees were in their natal colony environment and were able to freely forage in a flight arena. Colonies were kept in naturally dark conditions and the only experimenter actions at the colony were the provisioning with pollen every 2nd day. Males voluntarily left the colony and spent most of their time foraging, flying around or resting on the arena walls. Casual observations indicated that the time spent between actual bouts of flower visits was about 2 h; hence we used this interval of 2 h for our controlled food deprivation periods to ensure that individuals were not starved for periods that exceeded their voluntary breaks between bouts of foraging activity.
Workers, likewise, were not forced to leave the nest and were only trained and tested if they voluntarily engaged in foraging behaviour. In workers, a 2 h interval between foraging bouts has also been successfully used in other experimental work (Ravi, Crall, Fisher, & Combes, 2013). Food deprivation typically resulted in the bees ceasing to fly around and resting on the arena walls. They spontaneously commenced foraging when given the opportunity after 2 h. All bee handling procedures and transfers were carefully done using plastic pots rather than forceps to reduce stress of handling.
Results
Effects of Training Protocol
Both males and workers could be trained on the coloured feeders. Comparing the choice accuracies of workers in the context of the training procedure (‘TP’), we found no significant difference between the 11 workers subjected to the interbout food deprivation of 2 h and the 40 workers allowed to return to the colony between bouts (choice accuracy during the initial and final 10 visits per colour pair: GLM: blue-yellow/yellow-blue: initial: t56 = 0.39, P = 0.69; final: t55 = 0.31, P = 0.75; yellow-green/green-yellow: initial: t55 = 0.06, P = 0.93; final: t55 = −0.74, P = 0.46; green-purple/purple-green: initial: t56 = 0.25, P = 0.80; final: t54 = 0.33, P = 0.74, purple-blue/blue-purple: initial: t57 = 0.87, P = 0.38; final: t56 = 0.65, P = 0.52; overall: t1133 = 1.04, P = 0.30; Fig. 2). We thus used the more natural uninterrupted training procedure to collect the majority of the worker data.
Foraging Characteristics of Males and Workers
Overall, males visited more feeders per foraging bout than workers, requiring an average of 1.3 ± 0.46 bouts to complete training on a colour set (50 visits) whereas workers needed 1.6 ± 0.54 bouts (t test: t207 = 5.84, P < 0.001). While 84% of the males completed more than 40 visits in the first bout per colour set (70.3% finished the task in a single bout), only 41.3% of the workers accomplished more than 40 visits in the first bout (39.4% single bout tasks). Within the first bout on each colour pair, males accomplished on average more visits than workers (41.2 ± 10.9 visits) finishing the bout after 46.9 ± 7.9 visits (t test: t109 = 3.144, P < 0.01). These differences can be explained by the significantly larger crop capacities we found in males than workers: the mean maximal crop fill in males (N = 20) was 118.5 ± 45.8 μl whereas workers (N = 20) only collected 80.9 ± 20.5 μl in a single bout (t test: t18 = 3.35, P < 0.01).
Learning Performance of Males and Workers
Looking at feeder choice, we found that, independent of training protocol, both males and workers clearly improved their choice accuracy over the course of the training for each colour pair used (Fig. 2).
Throughout the training there was no significant difference in the choice accuracy of males and workers (effect of sex on choice accuracy on the initial and final 10 visits of the sequentially presented colour pairs in the sequence: first colour pair: initial: t112 = 0.51, P = 0.61; final: t110 = 0.04, P = 0.97; second: initial: t97 = 0.65, P = 0.52; final: t93 = 0.95, P = 0.35; third: initial: t89 = −1.59, P = 0.12; final: t85 = −0.84, P = 0.41; fourth: initial: t81 = −0.47, P = 0.64; final: t79 = 0.11, P = 0.91; Fig. 2). Already at the end of the first bout on each colour pair both sexes reached similarly high mean choice accuracies (% correct of the last 10 visits) with 87.7 ± 12.9% (males) and 86.5 ± 13.9% (workers) correct choices (t109 = 0.48, P < 0.63). There was no significant difference between the sexes in the learning speed (GLM: Wald test = 0.08, df = 1, P = 0.77) or in the latency to switch (GLM: Wald test = 0.05, df = 1, P = 0.82) for any of the colour pairs (Fig. 3a, b).
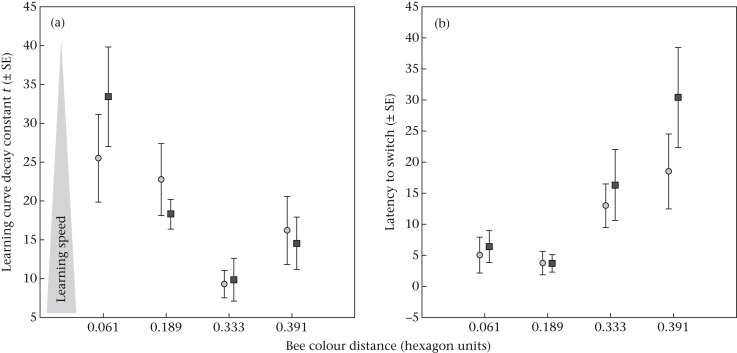
Figure 3.
(a) Mean decay constant t in the learning curve (± SE) of males (dark grey squares) and workers (light grey circles) as a function of colour distance in the hexagonal bee colour space. The t value is inversely correlated with the learning speed with high t values representing slow learning speeds and vice versa (as illustrated by the grey arrow). The colour distance of 0.061 is very small and close to the limits of discriminability (Dyer & Chittka, 2004c) whereas colour distances of >0.2 hexagon units are large and allow easy discrimination. (b) Mean number (±SE) of incorrect visits before first landing on a rewarding feeder (latency to switch) per colour distance.
In addition to our analyses based on bees for which the learning speed could be quantified using exponential decay curve fitting with Microcal Origin (OriginLab Corporation), we also found no significant difference between the sexes in the prevalence of learning curves, to which no decay function could be successfully fitted, which was the case for 42 of 178 (males) and 47 of 167 (workers) learning curves (Χ21 = 0.93, P = 0.33).
Effects of Colour Distance on Foraging Performance
In both sexes the overall foraging performance highly significantly depended on the colour distances (CD) of the feeder colours presented (GLM: LS: Wald test = 76.53, df = 3, P < 0.001; LAT: Wald test = 54.61, df = 3, P < 0.001). Similar feeder colours (smaller colour distances in the hexagonal bee colour space) entailed faster switching than highly different colours in both males and workers, with colour distance being highly significantly correlated with the latency to switch to a novel colour (LAT-CD: males: R2 = 0.25, t173 = 7.63, P < 0.001; workers: R2 = 0.22, t180 = 7.41, P < 0.001). Conversely, small colour distance caused higher numbers of incorrect visits throughout the training and was highly significantly negatively correlated with learning speed in both workers (LS–CD: R2 = 0.12, t125 = −4.10, P < 0.001) and males (LS–CD: R2 = 0.25, t127 = −6.58, P < 0.001).
We found a significant difference in overall learning speed between the two training sequences (GLM: Wald test = 5.71, df = 1, P = 0.02) associated with asymmetrical learning performances on feeder types with similar colours. For both small-distance colour pairs (yellow-green, CD: 0.061; blue-purple, CD: 0.189) initial choice accuracies were significantly different depending on which of the two colours in the pair was rewarded. The choice accuracies on green rewarding and yellow nonrewarding feeders was significantly lower for the first 30 visits than those achieved on the reverse challenge (10 visits: t92 = 3.48, P < 0.001; 20 visits: t91 = 2.45, P = 0.02; 30 visits: t91 = 4.67, P < 0.001). Similarly, yet less pronounced, there was a difference in choice accuracy for the blue and purple colour combination for the initial 20 visits (10 visits: t105 = 2.08, P = 0.04; 20 visits: t105 = 2.45, P = 0.02). In both cases these differences diminished as training progressed (green-yellow: 40 visits: t90 = 1.83, P = 0.07; 50 visits: t88 = 1.47, P = 0.14; blue-purple: 30 visits: t104 = 1.55, P = 0.12; 40 visits: t104 = 0.81, P = 0.42; 50 visits: t102 = 0.34, P = 0.74). No significant asymmetries in choice accuracy were found for the two colour pairs consisting of highly different colours (purple-green, blue-yellow). This effect, however, was not affected by sex and was similarly seen in males and workers (GLM: seq×sex: Wald test = 0.66, df = 1, P = 0.42). The differences also did not extend to the latency to switch (GLM: sex: Wald test = 0.67, df = 1, P = 0.41; seq×sex: Wald test = 0.32, df = 1, P = 0.57).
Overall learning speeds for colour-naïve bees presented with a colour set for the first time (training sequence A: yellow+/blue− (CD: 0.333); sequence B: purple+/blue− (CD: 0.189)) were significantly faster than the overall learning speeds recorded for non-naïve bees (colour pairs 2–4 in the sequence), which needed to unlearn previously made associations (CDs: group A: 0.061, 0.391, 0.181; group B: 0.333, 0.061, 0.391; GLM: Wald test = 5.76, df = 1, P = 0.016). Again, this response to colour and previous experience was not significantly different between males and workers for learning speed and latency to switch (GLM: sex×naivety: LS: Wald test = 0.11, df = 1, P = 0.74; LAT: Wald test = 0.06, df = 1, P = 0.81).
Interestingly, we found for both males and workers a weak inverse relationship between the initial latency to visit the correct feeder and the subsequent learning speed with the new feeder cue (LS-LAT: workers: R2 = 0.04, t125 = −2.30, P = 0.02; males: R2 = 0.11, t127 = −3.99, P < 0.001), i.e. individuals that were highly reluctant to abandon a learned or innately preferred feeder type were significantly faster at learning the new cue once the reward was discovered.
In addition to our analyses based on bees for which the learning speed could be quantified using exponential decay curve fitting, we also found no significant difference between the sexes in the prevalence of learning curves to which no decay function could be successfully fitted, which was the case for 42 of 178 (males) and 47 of 167 (workers) learning curves (Χ21 = 0.93, P = 0.33).
Discussion
Our results show that the ability to respond adequately to changes in foraging conditions was highly similar in males and workers, with the bees' sex not having significant effects on the recorded behavioural responses. In contrast, colour distance and experience proved to have highly significant but sex-independent effects on the foraging behaviour.
This study provides the first assessment of the learning abilities of freely foraging bumblebee males, to explore how sex-specific behavioural differences may affect the cognitive abilities in a nonmating context. Comparing the learning performance of bumblebee males on temporally changing floral colour cues with that of the much better studied workers (Clarke et al., 2013, Dyer et al., 2006, Nicholls and de Ibarra, 2014, Raine and Chittka, 2007, Raine and Chittka, 2008, Raine et al., 2006, Raine et al., 2006, Wolf et al., 2015), we found no difference between the two sexes. This is supported by recent findings on PER conditioning to colour stimuli of harnessed bumblebee males and workers (Lichtenstein et al., 2015). The similarity in learning ability between the sexes is surprising considering the workers' near exclusive focus on foraging in contrast to the males' need to integrate foraging abilities into a much broader behavioural repertoire. Trading off foraging behaviour with mate searching, self-provisioning males clearly benefit from being able to efficiently exploit available floral resources. Males face considerable energy costs of patrolling behaviour which is a prerequisite for mating involving sustained flights over large distances (Alcock et al., 1978, Frank, 1941, Haas, 1949, Svensson, 1979). Bertsch (1984) showed that males in a flight room fly up to 17 km a day, incurring substantial energetic costs. In our study, the ability to learn and memorize flower rewards facilitated the acquisition of high-quality food while also freeing up time for mate search. Bumblebee males have to frequently interrupt their patrol routes for foraging trips (Frank, 1941). During these breaks males will have no access to potentially available queens and may miss mating opportunities. This is aggravated by the fact that queens of most European bumblebee species mate only once (Schmid-Hempel & Schmid-Hempel, 2000). With several males often sharing patrol routes (Ayasse et al., 2001, Stiles, 1976) competition for mating opportunities might well be severe. Assuming that queen encounters are rare events, efficient male foraging will be tightly linked to male mating success.
Of course behavioural differences do not inevitably translate into predictable variation in cognitive abilities, which may be the result of the interplay between multiple facilitating and limiting genetic, ontogenetic and environmental factors (Rowe & Healy, 2014). One possibility is that the efficiency of colour learning relies on identical genetic pathways in both sexes. This genetic correlation may be the ‘default scenario’, and any departure from this scenario (i.e. a sexual dimorphism) requires that the respective traits are controlled by different (or differentially expressed) genes in males and females. This may explain not only the males' good learning abilities but also why workers, behaviourally highly specialized for near exclusive foraging, did not evolve superior learning abilities after losing the behavioural requirements of reproduction.
As in foraging workers, male foraging performance was largely determined by the colour characteristics of the two feeder types presented. In line with previous studies on worker bees (Dyer and Chittka, 2004a, Dyer and Chittka, 2004b), highly similar flower colours (<0.1 hexagon units) resulted in slow learning and lower performance maxima in both workers and males as a result of poor colour discriminability (colour contrasts of only 0.04 hexagon units can only be discriminated by worker bumblebees in differential but not in absolute conditioning; Dyer & Chittka, 2004c) and bees generalizing over the two choices (Chittka, Gumbert, & Kunze, 1997). Under these conditions both males and workers very readily explored the novel feeder colour and learned this new rewarding colour only slowly. Additionally, as indicated by performance differences between the two training sequences, innate colour preferences seemed to play a role in choosing from highly similar feeders as compared to feeder types with large colour differences.
In contrast, males and workers were highly reluctant to abandon a previously learned colour in favour of a highly different novel colour (>0.18 hexagon units). The latency to switch was inversely correlated with the subsequently expressed learning speed. This is similar to the findings of Ings et al. (2009), showing that in workers of two B. terrestris subspecies, longer latencies to abandon innately preferred blue feeders also entailed faster learning rates of the rewarding nonblue feeders (Ings et al., 2009). In the current study, the high flower constancy for highly different feeder types induced bees to make significantly more unrewarded visits (to the previously learnt colour). This increasingly disassociated the previously formed reward expectation and the colour cue even before the novel rewarding feeder was discovered, ultimately facilitating the fast learning of the novel rewarding feeder type. In line with this, both sexes elicited fast and precise learning of the rewarding colour. Similar behavioural responses to medium to large colour distances have been reported for workers of B. terrestris (Dyer and Chittka, 2004a, Dyer and Chittka, 2004b) and several other bee species (Chittka et al., 2001) translating into high levels of flower constancy (Chittka et al., 1999, Waser, 1986), which facilitates conspecific pollen transfer. The similarity of the foraging behaviour of bumblebee males and workers supports the recent recognition of bumblebee males as valuable pollinators (Ogilvie and Thomson, 2015b, Ostevik et al., 2010, Wolf and Moritz, 2014). Thus, our findings also have implications for the study of pollination ecology of both wild and commercial plants, which has thus far rarely considered the functional contribution male bees may make to the behavioural diversity of pollinator assemblages and, in consequence, to the quality of the pollination service (Garibaldi et al., 2013, Hoehn et al., 2008, Klein et al., 2003).
Additionally, our findings highlight the possibility of using male bumblebees as an experimental model for learning and memory. In comparison to bee workers, a classical model organism for the study of learning and cognition, males have numerous practical advantages. For one, their foraging motivation does not rely on colony level factors and can be easily and reliably manipulated in the laboratory. The only amendment to traditional procedures with freely flying bees might be the use of very small reward quantities for males, as we have done here. More importantly, however, males do not have a stinger and the usual occupational hazards of bee stings and the common development of allergies to bee venom that often occur in experimenters can thus be avoided, which provides for ease in handling the animals, while possibly not forgoing any of the cognitive capacities that are typically studied in worker bees.
In summary, we have shown that worker and male bumblebees, although fundamentally different in lifestyle and behavioural needs, are very similar in their learning performances. This is possibly a result of similar underlying mechanisms facilitating and potentially limiting these cognitive abilities and the common need to exploit available resources efficiently irrespective of the natural behavioural repertoire.
Acknowledgments
We thank J.P. Abraham and S. Malik for their help in collecting the data. We are also grateful to M. Roper and two anonymous referees for constructive comments on the manuscript. This work was supported by the ERC Advanced Grant “SpaceRadarPollinators” to L.C. and the Deutsche Forschungsgemeinschaft (DFG, grant number WO 1745/1-1) to S.W.
MS. number: 15-00435R
References
- Abbott K.R. Bumblebees avoid flowers containing evidence of past predation events. Canadian Journal of Zoology. 2006;84(9):1240–1247. [Google Scholar]
- Ackerman J.D. Food-foraging behavior of male Euglossini vagabonds or trapliners. Biotropica. 1982;14(4):241–248. [Google Scholar]
- Alcock J., Barrows E., Gordh G., Hubbard L., Kirkendall L., Pyle D. The ecology and evolution of male reproductive behaviour in the bees and wasps. Zoological Journal of the Linnean Society. 1978;64(4):293–326. [Google Scholar]
- Allen T., Cameron S., McGinley R., Heinrich B. The role of workers and new queens in the ergonomics of a bumblebee colony (Hymenoptera: Apoidea) Journal of the Kansas Entomological Society. 1978;51(3):329–342. [Google Scholar]
- Aquino I. d. S., Silva M.C., Barbosa A. d. S., Abramson C.I. Learning of proboscis extension in harnessed Africanized honeybee drones (Apis mellifera L.) Ciência Animal Brasileira. 2015;16(1):14–23. [Google Scholar]
- Ayasse M., Paxton R.J., Tengo J. Mating behavior and chemical communication in the order Hymenoptera. Annual Review of Entomology. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- Beani L., Dessì-Fulgheri F., Cappa F., Toth A. The trap of sex in social insects: from the female to the male perspective. Neuroscience & Biobehavioral Reviews. 2014;46:519–533. doi: 10.1016/j.neubiorev.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Beani L., Zuk M. Beyond sexual selection: the evolution of sex differences from brain to behaviour. Neuroscience and Biobehavioral Reviews. 2014;46:497–500. doi: 10.1016/j.neubiorev.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Benatar S.T., Cobey S., Smith B.H. Selection on a haploid genotype for discrimination learning performance: correlation between drone honey bees (Apis mellifera) and their worker progeny (Hymenoptera: Apidae) Journal of insect behavior. 1995;8(5):637–652. [Google Scholar]
- Bertsch A. Foraging in male bumblebees (Bombus lucorum L.): maximizing energy or minimizing water load? Oecologia. 1984;62(3):325–336. doi: 10.1007/BF00384264. [DOI] [PubMed] [Google Scholar]
- Burns J.G. Impulsive bees forage better: the advantage of quick, sometimes inaccurate foraging decisions. Animal Behaviour. 2005;70(6):e1–e5. [Google Scholar]
- Cartar R.V. Adjustment of foraging effort and task switching in energy-manipulated wild bumblebee colonies. Animal Behaviour. 1992;44(1):75–87. [Google Scholar]
- Cartar R.V., Dill L.M. Colony energy requirements affect the foraging currency of bumblebees. Behavioral Ecology and Sociobiology. 1990;27(5):377–383. [Google Scholar]
- Chittka L. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. Journal of Comparative Physiology A. 1992;170(5):533–543. [Google Scholar]
- Chittka L., Beier W., Hertel H., Steinmann E., Menzel R. Opponent colour coding is a universal strategy to evaluate the photoreceptor inputs in Hymenoptera. Journal of Comparative Physiology A. 1992;170(5):545–563. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- Chittka L., Dyer A.G., Bock F., Dornhaus A. Psychophysics: bees trade off foraging speed for accuracy. Nature. 2003;424(6947):388. doi: 10.1038/424388a. [DOI] [PubMed] [Google Scholar]
- Chittka L., Gumbert A., Kunze J. Foraging dynamics of bumblebees: correlates of movements within and between plant species. Behavioral Ecology. 1997;8(3):239–249. [Google Scholar]
- Chittka L., Spaethe J., Schmidt A., Hickelsberger A. Adaptation, constraint, and chance in the evolution of flower colour and pollinator colour vision. In: Chittka L., Thomson J.D., editors. Cognitive ecology of pollination. Cambridge University Press; Cambridge, U.K.: 2001. pp. 106–126. [Google Scholar]
- Chittka L., Thomson J.D., Waser N.M. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86(8):361–377. [Google Scholar]
- Clarke D., Whitney H., Sutton G., Robert D. Detection and learning of floral electric fields by bumblebees. Science. 2013;340(6128):66–69. doi: 10.1126/science.1230883. [DOI] [PubMed] [Google Scholar]
- Dalla C., Papachristos E.B., Whetstone A.S., Shors T.J. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C., Shors T.J. Sex differences in learning processes of classical and operant conditioning. Physiology & Behavior. 2009;97(2):229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges C.W., Avalos A., Abramson C.I., Craig D.P.A., Austin Z.M., Varnon C.A. Aversive conditioning in honey bees (Apis mellifera anatolica): a comparison of drones and workers. Journal of Experimental Biology. 2013;216(21):4124–4134. doi: 10.1242/jeb.090100. [DOI] [PubMed] [Google Scholar]
- Dornhaus A., Chittka L. Food alert in bumblebees (Bombus terrestris): possible mechanisms and evolutionary implications. Behavioral Ecology and Sociobiology. 2001;50(6):570–576. [Google Scholar]
- Dornhaus A., Chittka L. Bumble bees (Bombus terrestris) store both food and information in honeypots. Behavioral Ecology. 2005;16(3):661–666. [Google Scholar]
- Dukas R. Effects of perceived danger on flower choice by bees. Ecology Letters. 2001;4(4):327–333. [Google Scholar]
- Dukas R. Bumble bee predators reduce pollinator density and plant fitness. Ecology. 2005;86(6):1401–1406. [Google Scholar]
- Dyer A.G., Chittka L. Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a case study. Journal of Comparative Physiology A. 2004;190(2):105–114. doi: 10.1007/s00359-003-0475-2. [DOI] [PubMed] [Google Scholar]
- Dyer A.G., Chittka L. Bumblebees (Bombus terrestris) sacrifice foraging speed to solve difficult colour discrimination tasks. Journal of Comparative Physiology A. 2004;190(9):759–763. doi: 10.1007/s00359-004-0547-y. [DOI] [PubMed] [Google Scholar]
- Dyer A.G., Chittka L. Fine colour discrimination requires differential conditioning in bumblebees. Naturwissenschaften. 2004;91(5):224–227. doi: 10.1007/s00114-004-0508-x. [DOI] [PubMed] [Google Scholar]
- Dyer A.G., Whitney H.M., Arnold S.E., Glover B.J., Chittka L. Behavioural ecology: bees associate warmth with floral colour. Nature. 2006;442(7102):525. doi: 10.1038/442525a. [DOI] [PubMed] [Google Scholar]
- Eickwort G.C., Ginsberg H.S. Foraging and mating behavior in Apoidea. Annual Review of Entomology. 1980;25(1):421–446. [Google Scholar]
- Fouks B., Lattorff H.M.G. Recognition and avoidance of contaminated flowers by foraging bumblebees (Bombus terrestris) PLoS One. 2011;6(10):e26328. doi: 10.1371/journal.pone.0026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. Eigenartige Flugbahnen bei Hummelmännchen. Zeitschrift für vergleichende Physiologie. 1941;28(4):467–484. [Google Scholar]
- Galea L., Kavaliers M., Ossenkopp K.-P. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. Journal of Experimental Biology. 1996;199(1):195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- Garibaldi L.A., Steffan-Dewenter I., Winfree R., Aizen M.A., Bommarco R., Cunningham S.A. Wild pollinators enhance fruit set of crops regardless of honeybee abundance. Science. 2013;339(6127):1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Gaulin S.J., FitzGerald R.W. Sex differences in spatial ability: an evolutionary hypothesis and test. American Naturalist. 1986;127(1):74–88. [Google Scholar]
- Goulson D. Oxford University Press; Oxford, U.K.: 2003. Bumblebees: Their behaviour and ecology. [Google Scholar]
- Greeno N.C., Semple S. Sex differences in vocal communication among adult rhesus macaques. Evolution and Human Behavior. 2009;30(2):141–145. [Google Scholar]
- Grön G., Wunderlich A.P., Spitzer M., Tomczak R., Riepe M.W. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nature Neuroscience. 2000;3(4):404–408. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- Haas A. Arttypische Flugbahnen von Hummelmännchen. Zeitschrift für vergleichende Physiologie. 1949;31(3):281–307. [PubMed] [Google Scholar]
- Halpern D.F., LaMay M.L. The smarter sex: a critical review of sex differences in intelligence. Educational Psychology Review. 2000;12(2):229–246. [Google Scholar]
- Healy S., Bacon I., Haggis O., Harris A., Kelley L. Explanations for variation in cognitive ability: behavioural ecology meets comparative cognition. Behavioural Processes. 2009;80(3):288–294. doi: 10.1016/j.beproc.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Foraging specializations of individual bumblebees. Ecological Monographs. 1976;46(2):105–128. [Google Scholar]
- Heinrich B. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia. 1979;40(3):235–245. doi: 10.1007/BF00345321. [DOI] [PubMed] [Google Scholar]
- Hoehn P., Tscharntke T., Tylianakis J.M., Steffan-Dewenter I. Functional group diversity of bee pollinators increases crop yield. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1648):2283–2291. doi: 10.1098/rspb.2008.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings T.C., Chittka L. Speed-accuracy tradeoffs and false alarms in bee responses to cryptic predators. Current Biology. 2008;18(19):1520–1524. doi: 10.1016/j.cub.2008.07.074. [DOI] [PubMed] [Google Scholar]
- Ings T.C., Chittka L. Predator crypsis enhances behaviourally mediated indirect effects on plants by altering bumblebee foraging preferences. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1664):2031–2036. doi: 10.1098/rspb.2008.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ings T.C., Raine N.E., Chittka L. A population comparison of the strength and persistence of innate colour preference and learning speed in the bumblebee Bombus terrestris. Behavioral Ecology and Sociobiology. 2009;63(8):1207–1218. [Google Scholar]
- Jacobs L.F. Sexual selection and the brain. Trends in Ecology & Evolution. 1996;11(2):82–86. doi: 10.1016/0169-5347(96)81048-2. [DOI] [PubMed] [Google Scholar]
- Jacobs L.F., Gaulin S., Sherry D.F., Hoffman G.E. Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proceedings of the National Academy of Sciences. 1990;87(16):6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennersten O., Morse D.H., O'Neil P. Movements of male and worker bumblebees on and between flowers. Oikos. 1991;62(3):319–324. [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neuroscience & Biobehavioral Reviews. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Klein A.M., Steffan–Dewenter I., Tscharntke T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proceedings of the Royal Society B: Biological Sciences. 2003;270(1518):955–961. doi: 10.1098/rspb.2002.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus F.B., Wolf S., Moritz R.F. Male flight distance and population substructure in the bumblebee Bombus terrestris. Journal of Animal Ecology. 2009;78(1):247–252. doi: 10.1111/j.1365-2656.2008.01479.x. [DOI] [PubMed] [Google Scholar]
- Leonard A.S., Hedrick A.V. Male and female crickets use different decision rules in response to mating signals. Behavioral Ecology. 2009;20(6):1175–1184. [Google Scholar]
- Levy L.J., Astur R.S., Frick K.M. Men and women differ in object memory but not performance of a virtual radial maze. Behavioral Neuroscience. 2005;119(4):853. doi: 10.1037/0735-7044.119.4.853. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L., Sommerlandt F.M., Spaethe J. Dumb and lazy? A comparison of colour learning and memory retrieval in drones and workers of the buff-tailed bumblebee, Bombus terrestris, by means of PER conditioning. PLoS One. 2015;10(7):e0134248. doi: 10.1371/journal.pone.0134248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf E.V., Eberly L.E., Pusey A.E. Sex differences in learning in chimpanzees. Nature. 2004;428(6984):715–716. doi: 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- Molet M., Chittka L., Stelzer R.J., Streit S., Raine N.E. Colony nutritional status modulates worker responses to foraging recruitment pheromone in the bumblebee Bombus terrestris. Behavioral Ecology and Sociobiology. 2008;62(12):1919–1926. [Google Scholar]
- Nicholls E., de Ibarra N.H. Bees associate colour cues with differences in pollen rewards. Journal of Experimental Biology. 2014;217(15):2783–2788. doi: 10.1242/jeb.106120. [DOI] [PubMed] [Google Scholar]
- Ogilvie J.E., Thomson J.D. Male bumblebees are important pollinators of a late-blooming plant. Arthropod-Plant Interactions. 2015;9:205–213. [Google Scholar]
- Ogilvie J.E., Thomson J.D. Male bumblebees are important pollinators of a late-blooming plant. Arthropod-Plant Interactions. 2015;9:1–9. [Google Scholar]
- Ostevik K.L., Manson J.S., Thomson J.D. Pollination potential of male bumblebees (Bombus impatiens): movement patterns and pollen transfer-efficiency. Journal of Pollination Ecology. 2010;2:21–26. [Google Scholar]
- Pankiw T., Page R., Jr. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honeybees (Apis mellifera L.) Journal of Comparative Physiology A. 1999;185(2):207–213. doi: 10.1007/s003590050379. [DOI] [PubMed] [Google Scholar]
- Raine N.E., Chittka L. Pollen foraging: learning a complex motor skill by bumblebees (Bombus terrestris) Naturwissenschaften. 2007;94(6):459–464. doi: 10.1007/s00114-006-0184-0. [DOI] [PubMed] [Google Scholar]
- Raine N.E., Chittka L. The correlation of learning speed and natural foraging success in bumblebees. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1636):803–808. doi: 10.1098/rspb.2007.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine N.E., Chittka L. No trade-off between learning speed and associative flexibility in bumblebees: a reversal learning test with multiple colonies. PLoS One. 2012;7(9):e45096. doi: 10.1371/journal.pone.0045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine N.E., Ings T.C., Dornhaus A., Saleh N., Chittka L. Adaptation, genetic drift, pleiotropy, and history in the evolution of bee foraging behavior. Advances in the Study of Behavior. 2006;36:305–354. [Google Scholar]
- Raine N.E., Ings T.C., Ramos-Rodriguez O., Chittka L. Intercolony variation in learning performance of a wild British bumblebee population (Hymenoptera: Apidae: Bombus terrestris audax) Entomologia Generalis. 2006;28(4):241–256. [Google Scholar]
- Ravi S., Crall J.D., Fisher A., Combes S.A. Rolling with the flow: bumblebees flying in unsteady wakes. Journal of Experimental Biology. 2013;216(22):4299–4309. doi: 10.1242/jeb.090845. [DOI] [PubMed] [Google Scholar]
- Rowe C., Healy S.D. Measuring variation in cognition. Behavioral Ecology. 2014;25(6):1287–1292. [Google Scholar]
- Sammut M., Cook S.J., Nguyen K.C.Q., Felton T., Hall D.H., Emmons S.W., …, Barrios A. Glia-derived neurons are required for sex-specific learning in C. elegans. Nature. 2015;526(7573):385–390. doi: 10.1038/nature15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford M.R., Tomberlin J.K. Conditioning individual mosquitoes to an odor: sex, source, and time. PLoS One. 2011;6(8):e24218. doi: 10.1371/journal.pone.0024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier D.M., Green S.M., Leason J., MacFadden A., Bell S., Elias L.J. Are sex differences in navigation caused by sexually dimorphic strategies or by differences in the ability to use the strategies? Behavioral Neuroscience. 2002;116(3):403. doi: 10.1037//0735-7044.116.3.403. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel R., Schmid-Hempel P. Female mating frequencies in Bombus spp. from Central Europe. Insectes Sociaux. 2000;47(1):36–41. [Google Scholar]
- Skorupski P., Döring T.F., Chittka L. Photoreceptor spectral sensitivity in island and mainland populations of the bumblebee, Bombus terrestris. Journal of Comparative Physiology A. 2007;193(5):485–494. doi: 10.1007/s00359-006-0206-6. [DOI] [PubMed] [Google Scholar]
- Sladen F.W.L. MacMillan; London, U.K.: 1912. The humble-bee: Its life-history and how to domesticate it with descriptions of all the British species of Bombus and Psithyrus. [Google Scholar]
- Stiles E.W. Comparison of male bumblebee flight paths: temperate and tropical (Hymenoptera: Apoidea) Journal of the Kansas Entomological Society. 1976;49(2):266–274. [Google Scholar]
- Svensson B.G. Patrolling behaviour of bumblebee males (Hymenoptera, Apidae) in a subalpine-alpine area, Swedish Lapland. Zoon. 1979;7(2):67–94. [Google Scholar]
- Vicario D.S., Naqvi N.H., Raksin J.N. Sex differences in discrimination of vocal communication signals in a songbird. Animal Behaviour. 2001;61(4):805–817. [Google Scholar]
- Waddington K.D., Allen T., Heinrich B. Floral preferences of bumblebees (Bombus edwardsii) in relation to intermittent versus continuous rewards. Animal Behaviour. 1981;29(3):779–784. [Google Scholar]
- Waser N.M. Flower constancy: definition, cause, and measurement. American Naturalist. 1986;127(5):593–603. [Google Scholar]
- Weidenmüller A., Kleineidam C., Tautz J. Collective control of nest climate parameters in bumblebee colonies. Animal Behaviour. 2002;63(6):1065–1071. [Google Scholar]
- Winston M.L. Harvard University Press; Cambridge, MA: 1991. The biology of the honeybee. [Google Scholar]
- Wolf S., Moritz R.F. The pollination potential of free-foraging bumblebee (Bombus spp.) males (Hymenoptera: Apidae) Apidologie. 2014;45:440–450. [Google Scholar]
- Wolf S., Roper M., Chittka L. Bumblebees utilize floral cues differently on vertically and horizontally arranged flowers. Behavioral Ecology. 2015;26(3):773–781. [Google Scholar]
- Wolf S., Toev T., Moritz R.L., Moritz R.F. Spatial and temporal dynamics of the male effective population size in bumblebees (Hymenoptera: Apidae) Population Ecology. 2012;54(1):115–124. [Google Scholar]