Abstract
Introduction
There is a growing body of evidence demonstrating frailty as an important predictor of surgical outcomes in older adults undergoing major surgeries. The age-related onset of many symptoms of female pelvic floor dysfunction (PFD) in women suggests that many women seeking treatment for PFD may also have a high prevalence of frailty, which could potentially impact the risks and benefits of surgical treatment options. Our primary objective was to determine the prevalence of frailty, cognitive impairment, and functional disability in older women seeking treatment for PFD.
Methods
We conducted a cross-sectional study with prospective recruitment between 9/2011 and 9/2012. Women, age 65 years and older, were recruited at the conclusion of their new patient consultation for PFD at a tertiary center. A comprehensive geriatric screening including frailty measurements (Fried Frailty Index), cognitive screening (Saint Louis University Mental Status Score), and functional status evaluation for activities of daily living (Katz ADL score) was conducted.
Results
Sixteen percent (n/N = 25/150) of women were categorized as frail according to the Fried Frailty Index Score. After adjusting for education level, 21.3% of women (n/N =32/150) screened positive for dementia and 46 (30.7%) reported functional difficulty or dependence in performing at least one Katz ADL. Sixty-nine women (46.0%) chose surgical options for treatment of their PFD at the conclusion of their new patient visit with their physician.
Conclusion
Frailty, cognitive impairment, and functional disability are common in older women seeking treatment for PFD.
Keywords: female pelvic floor dysfunction, frailty, functional disability, pelvic organ prolapse, urinary incontinence
INTRODUCTION
Frailty is a common biologic syndrome of decreased reserve and resistance to stressors that increases with age. Frailty has been shown to be an important predictor of adverse outcomes of aging (long-term nursing home stay, injurious falls, and death) in community-dwelling older adults independent of medical comorbidities and age.(1) There is a growing body of evidence demonstrating frailty is an important predictor of surgical outcomes in older adults undergoing major surgeries requiring admission to intensive care units and general surgical procedures.(2–6) A joint best-practice guideline statement from the American College of Surgeons and the American Geriatrics Society recommends that, in addition to routine assessment and optimization of medical conditions, older adults undergoing surgical procedures should be assessed for frailty, cognitive ability, and functional status in the preoperative period.(7) Combining measurements of frailty, cognitive impairment, functional disability with medical comorbidities have been demonstrated to have increased prognostic ability to predict postoperative complications after major non-cardiac surgery than medical comorbidities or American Society of Anesthesiologists (ASA) status alone.(8, 9)
Urinary incontinence (UI), pelvic organ prolapse (POP) and fecal incontinence., and are common conditions that increase dramatically with age.(10, 11) Female pelvic floor dysfunction can cause life-altering symptoms that impact women’s quality of life with significant associations with increased depression, embarrassment and social isolation.(12, 13) Treatment of female PFD can be with either surgical or non-surgical interventions. These various treatments are elective and performed with the primary intention of increasing a women’s overall quality of life. The age-related onset of PFD symptoms in women suggests that many women seeking treatment for these disorders may also have a high prevalence of frailty, which could potentially impact the risks and benefits of different treatment options.
Our primary aim was to determine the occurrence of frailty, cognitive impairment, and functional disability in older women seeking treatment for PFD. Our secondary aim was to determine if differences existed in frailty, cognitive impairment and functional disability between older women choosing surgical compared to non-surgical treatments for PFD.
METHODS
After obtaining institutional review board approval from the Yale University Human Investigation Committee, we recruited community-dwelling older women (≥65 years) seeking treatment for PFD. Non-consecutive recruitment occurred between September 2011 and September 2012 at a tertiary center for Female Pelvic Medicine and Reconstructive Surgery. The referral base of this center included patients referred from general gynecologists and primary care physicians, however referrals from physicians were not required for a new patient appointments. Women were recruited at the conclusion of a standard new patient visit with one of four physicians after a treatment plan was established. The standard new patient evaluation included history, physical examination, including the pelvic organ prolapse-quantification (POP-Q) measurements, education about pelvic floor disorders and a decision about treatment options. All treatment options, both non-surgical and surgical treatments were routinely offered to patients as a part of routine clinical care. A comprehensive panel of non-surgical treatment options were offered including lifestyle and behavioral changes, medications, pessaries, percutaneous tibial nerve stimulation (PTNS), and pelvic floor physical therapy (including myofascial release, electrical stimulation, and biofeedback as needed). Onabotulinumtoxin A injection and transurethral bulking agents were also offered and categorized as “non-surgical treatment” as both procedures were performed in the office setting and neither required anesthesia. After obtaining written informed consent, women were asked if they and their physician had decided to pursue surgical or non-surgical treatment options. We asked women about treatment decision only after their consultation with a physician, but prior to our study assessments to mirror routine clinical care for women without the addition of geriatric assessment. A comprehensive geriatric assessment was performed. In addition, women completed quality of life questionnaires related to symptom distress and life impact of PFDs. Finally, a medical record review was performed by study personnel to obtain past medical history.
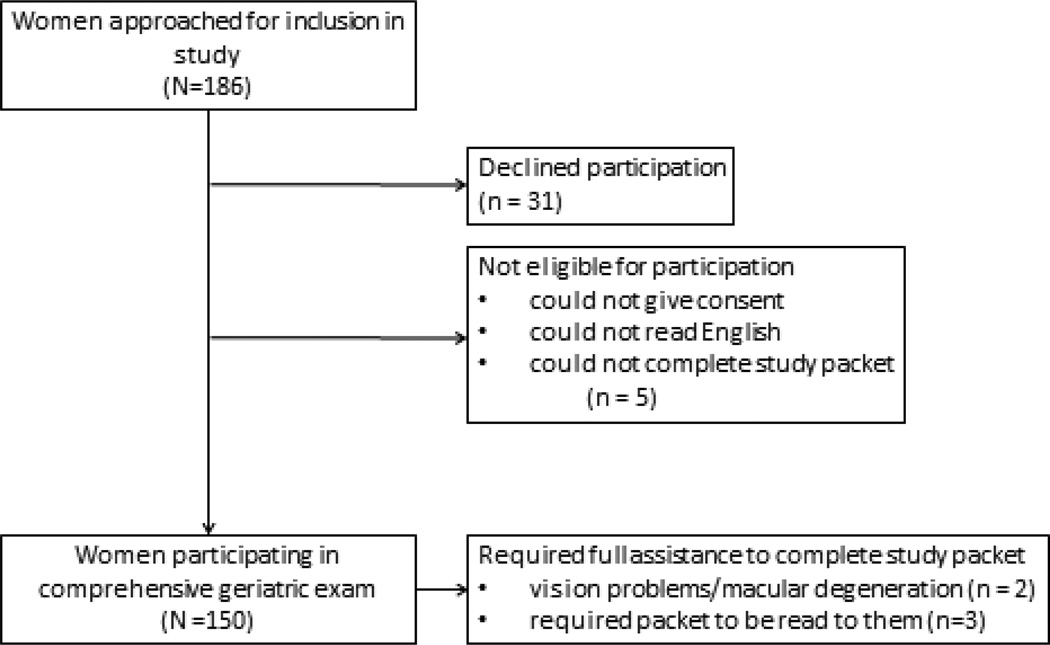
Women were excluded if they could not read English at a 5th grade reading level and if they were not able to legally give consent. During the enrollment period for this study, 713 new women were evaluated as new patients. Of these, 357 were ≥ 65 years old. 186 eligible women were approached to participate in this study due to study personnel availability, 31 declined to participate, 5 women were unable to given consent, complete study questionnaires, or could not read at a 5th grade level and were excluded. (Figure 1) Five women required assistance to complete questionnaires, 2 due to vision problems/macular degeneration and 3 required the written study questions to be read out loud. The average time to complete the screening was approximately 20 minutes. The longest time to complete the full screening was approximately 50 minutes (full assistance required). Any woman participating in this study who had any questions about the results of the study or any worries about memory or cognition were referred for evaluation to a comprehensive Geriatric Assessment Center.
Figure 1.
Flow diagram for women approached for recruitment to undergo comprehensive geriatric assessment at the conclusion of their new patient visit
Comprehensive geriatric screening included frailty measurements, cognitive testing, medical comorbidities, functional status, and depressive symptoms. Five components of Fried frailty index were measured including unintended weight loss in the previous year, self-reported exhaustion (measured by two endurance related questions from the Center for Epidemiological Studies–Depression scale [CES-D]), low physical activity level (measured by the Physical Activity Scale for the Elderly [PASE]) (14), slow walking speed, and decreased grip strength. Women were categorized as normal (0 frailty components present), prefrail (1–2 components present), or frail women (≥3 components present). Cognitive testing was performed with the Saint Louis University Mental Status (SLUMS) examination.(15) The SLUMS was been validated against the Mini-Mental Status Exam and does not require copyright permission for its use in clinical research. SLUMS scores ranged from 0 to 30 with lower scores indicating greater cognitive impairment. SLUMS scores ≤19 in women who have not completed high school and ≤20 in women who completed high school are consider a positive screen test, although not diagnostic, for dementia. Medical comorbidities were measured with the Charlson Comorbidity Index.(16) Functional status was measured by asking about seven basic activities of daily living (ADLs) according to Katz’s classification (Katz ADL score) including walking across a room, walking one block, dressing, bathing, eating, toileting, and getting in and out of bed.(17, 18) Women’s functional status for each basic activity of daily living was categorized into one of three categories: independent, independent with difficulty if they reported difficulty with performing any of the ADL but did not require assistance and dependent if they reported inability to perform ≥ 1 ADL without assistance.(18, 19) Depressive symptoms were measured with the Geriatric Depression Scale-Short (GDS-S) form.(20, 21) The Geriatric Depression Scale-Short has demonstrated ease of use and been validated in geriatric populations. The Geriatric Depression Scale-Short form is a 15 question Yes or No format with scores of 0 to 15. Three women screened scored above 10 on the GDS-S and these results were discussed with these participants who were also referred for further treatment.
Principal urogynecologic diagnosis was determined by the primary International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) billing code assigned to a woman’s visit. Women commonly have more than one diagnosis; however, we recorded the principal billing code as a representation of their primary diagnosis. Subjective measurements of symptom distress and life-impact of PFD was assessed utilizing the Pelvic Floor Distress Inventory Short Form-20 (PFDI-20) and the Pelvic Floor Impact Questionaire-7 (PFIQ-7). Scores for both the PFDI-20 and the PFIQ-7 range from 0 to 300 with higher scores indicating more symptom distress or greater life-impact from pelvic floor disorders.(22, 23)
Clinical characteristics were recorded. Data on race and ethnicity was obtained from women’s self-identified written answers to questions about race and ethnicity as a part of the full written study packet. Full data was available on all participants for frailty measures, cognitive function, medical comorbidities, and functional status. Missing data on PFDI-21 and PFIQ-7 were handled according to the survey scoring system recommendations.(22) Descriptive statistics were performed. Student’s t-test, Fisher’s exact test, χ2, and analysis of variance testing with post-hoc analysis utilizing the Scheffe method were used as appropriate. Point estimates, standard deviations, interquartile ranges (IQR) and 95% confidence intervals (CI) were reported. Given our sample of 150 women with confidence level of 95%, the precision of our point estimate for the prevalence of frailty is 11.9%.
RESULTS
We prospectively recruited 150 women ≥65 years seeking treatment for female pelvic floor dysfunction. Mean age was 76.2 (±7.2) years (range 65 to 97 years). (Table 1) Seven percent of women were Black and 4.0% Hispanic. Median vaginal parity was 3. Median Charlson Comorbidity Index score was 1 (IQR 0, 5). Sixteen percent (n/N = 25/150) of women were categorized as frail according to the Fried Frailty Index Score (Frailty prevalence = 16.7% (95% CI 10.7%, 22.6%). Forty-two percent (n/N= 63/150) of women were identified as prefrail.
Table 1.
Demographics of older women seeking treatment for pelvic floor dysfunction
| Variable | Frail (≥3 criteria) N= 25 |
PreFrail (1–2 criteria) N = 63 |
Normal N = 62 |
P |
|---|---|---|---|---|
| Age (Year, Mean, ±SD) | 82.5 (±7.9) | 76.2 (± 6.6) | 73.3 (±5.6) | |
| Age Category: | <.001 | |||
| 65–74 years | 5 (20.0) | 26 (41.3) | 35 (56.4) | |
| 75–84 years | 10 (40.0) | 29 (46.0) | 26 (42.0) | |
| ≥85 years | 10 (40.0) | 8 (12.7) | 1 (1.6) | |
| Race/Ethnicity | .77 | |||
| White | 23 (92.0) | 55 (87.3) | 57 (91.9) | |
| Black | 1 (4.0) | 6 (9.5) | 2 (3.2) | |
| Hispanic, non-black | 1 (4.0) | 1 (1.6) | 2 (3.2) | |
| Other | 0 | 1 (1.6) | 1 (1.6) | |
| Education | .26 | |||
| Less than high school | 3 (12.0) | 2 (3.2) | 3 (4.8) | |
| High school degree or higher | 22 (88.0) | 61 (96.8) | 59 (95.2) | |
| BMI (kg/m2, Mean, ±SD) | 31.8 (±9.0) | 28.4 (±6.6) | 26.5 (±4.2) | <.001 |
| Prior hysterectomy | 14 (56.0) | 27 (42.9) | 30 (48.4) | .52 |
| Vaginal parity (median, IQR) | 3 (2,3) | 3 (2,4) | 3 (2,3) | .26 |
All values listed as n (%) unless otherwise specified.
SD = standard deviation
BMI = body mass index
IQR = Interquartile range
Principal urogynecologic diagnosis was POP (65.3%), UI (20.7%), overactive bladder (9.3%), and anal incontinence (0.7%). Prior to presentation to our clinic, 46 women (30.6%) had undergone prior surgical procedures: 30 (20%) for POP and 16 (10.7%) for UI. Twenty-eight women had prior anti-cholinergic use recorded in their medical record. There was no difference in women choosing surgical vs. non-surgical treatment options based on prior anti-cholinergic use: 18.8% (n/N = 13/69) vs. 17.2% (n/N =14/81) p=.80. Symptom distress from PFD, as measured by the PFDI-20 score, was not significantly different between normal (mean 89.4 (±82.4)), prefrail (mean 54.8 (±47.8), and frail women (mean 60.2 (±45.1)); p=.17. (Table 2) Life-impact of female PFD, measured by PFIQ-21 score, was significantly higher in frail women (mean 59.1(±82.4)) compared with prefrail (mean 18.4 (±34.0)) and normal women (mean 11.3 (±15.6); p<.001.
Table 2.
Urogynecologic diagnoses in older women seeking treatment
| Variable | Frail (≥3 criteria) N= 25 |
PreFrail (1–2 criteria) N = 63 |
Normal N = 62 |
P |
|---|---|---|---|---|
| Primary Diagnosis | .77 | |||
| Pelvic Organ Prolapse | 16 (64.0) | 40 (63.5) | 42 (67.7) | |
| Urinary Incontinence | 5 (20.0) | 13 (20.6) | 13 (21.0) | |
| Overactive bladder | 2 (8.0) | 8 (12.7) | 4 (6.5) | |
| Fecal Incontinence | 0 | 1 (1.6) | 0 | |
| Other | 2 (8.0) | 1 (1.6) | 3 (4.8) | |
| POP-Q Stage | .58 | |||
| Stage 0/I—No prolapse | 10 (43.5) | 24 (38.7) | 21 (33.9) | |
| Stage II—mild prolapse | 11 (47.8) | 25 (10.3) | 26 (41.9) | |
| Stage III—moderate prolapse | 2 (8.7) | 8 (12.9) | 13 (21.0) | |
| Stage IV—severe prolapse | 0 | 5 (8.1) | 2 (3.2) | |
| PFDI-20 Score (Mean, ±SD) | 89.4 (±82.4) | 54.8 (±47.8) | 60.2 (±45.1) | .17 |
| POPDI-6 Score (Mean, ±SD) | 22.3 (±25.6) | 14.7 (± 20.2) | 16.4 (±15.8) | .02 |
| CRADI-8 Score (Mean, ± SD) | 24.3 (±20.3) | 16.7 (±18.6) | 14.2 (±12.8) | .007 |
| UDI-6 Score (Mean, ±SD) | 41.1 (±27.2) | 22.9 (±20.7) | 26.5 (±26.1) | .14 |
| PFIQ-7 Score (Mean, ±SD) | 59.1 (±82.4) | 18.4 (±34.0) | 11.3 (±15.6) | <.001 |
| UIQ-7 Score (Mean, ±SD) | 30.1 (±31.1) | 11.3 (±17.4) | 8.0 (±15.1) | <.001 |
| CRAIQ-7 Score (Mean, ±SD) | 16.2 (±27.6) | 4.3 (±11.1) | 4.2 (±14.7) | <.001 |
| POPIQ-7 Score (Mean, ±SD) | 12.6 (±28.8) | 2.8 (±10.6) | 1.8 (±4.5) | <.001 |
Primary diagnosis and POP-Q Stage values listed as n(%)
POP-Q = Pelvic Organ Prolapse Quantification
SD = standard deviation
PFDI-20= Pelvic Floor Distress Inventory Short Form 20
POPDI-6 = Pelvic Organ Prolapse Distress Inventory
CRADI-6 = Colorectal Anal Distress Inventory
UDI-6 = Urinary Distress Inventory
PFIQ-7 = Pelvic Floor Impact Questionaire-7
UIQ-7 = Urinary Impact Questionaire-7
CRAIQ-7 = Colorectal Anal Impact Questionaire-7
POPIQ-7 = Pelvic Organ Prolapse Impact Questionaire-7
PFDI-20 and PFIQ-7—scores between 0 and 300 with higher scores indicating increased symptom bother or life impact
POPDI-6, CRADI-6, UDI-6, IUQ-7, CRAIQ-7, & POPIQ-7—subscale scores between 0 and 100 with higher scores indicating increased symptom bother or life impact
After adjusting for education level, 21.3% of women (n/N =32/150) screened positive for dementia based on Saint Louis University Mental Status Score. Among community-dwelling older women seeking treatment for pelvic floor disorders, 46 (30.7%) reported functional difficulty or dependence in performing at least one activity of daily living. Fifteen percent of women (n/N = 10/150) reported functional dependence for at least one activity of daily living.
Sixty-nine women (46.0%) chose surgical options for treatment of their PFD at the conclusion of their new patient visit with their physician. Fried Frailty Index Score, frailty components, cognitive function, functional status and Charlson comorbidity index in older women choosing surgery and non-surgical treatments for pelvic floor disorders are shown in Table 3. There was no significant difference in the women with and without frailty electing for surgical management (40.0% (n/N =10/25) vs. 47.2% (n/N=59/125), p=.66), although this study was not powered to detect a difference for this secondary objective. Among the 5 frailty components, only decreased strength, measured by grip strength adjusted for BMI, was significantly different between women electing for surgical compared with non-surgical treatments (21 (30.4%) vs. 39 (48.2%), p=.03). There were no significant differences in unintentional weight loss, exhaustion, low activity level and slow walking speed between women choosing different treatments. There were no significant differences in cognitive function, measured by Saint Louis University Mental Status scores, between women electing surgical compared with non-surgical treatments (mean 24.4(±3.8) vs. mean 23.5(±4.8); p= .19).(Table 3)
Table 3.
Frailty, cognitive function, and functional status in older women choosing surgery and non-surgical treatments for pelvic floor dysfunction
| Surgery N= 69 |
Non-surgical treatments N = 81 |
P | |
|---|---|---|---|
| Frailty Components | |||
| Unintentional weight loss | 10 (14.5) | 11 (13.6) | .87 |
| Exhaustion (self-report) | 12 (17.4) | 14 (17.3) | .98 |
| Low activity (PASE score) | 10 (14.5) | 21 (25.9) | .08 |
| Slow walking speed | 11 (15.9) | 15 (18.5) | .68 |
| Decreased grip strength | 21 (30.4) | 39 (48.2) | .03 |
| Hopkins Frailty Index Score | .35 | ||
| Frail (≥ 3 components) | 10 (14.5) | 15 (18.5) | |
| Prefrail (1–2 components) | 26 (37.7) | 37 (45.7) | |
| Normal (0 components) | 33 (47.8) | 29 (35.8) | |
| Cognitive Function | |||
| SLUMS Score (mean, ±SD) | 24.4 (±3.8) | 23.5 (±4.8) | .19 |
| Screen positive for dementia | 11 (15.9) | 21 (25.9) | .30 |
| Medical Comorbidities | |||
| Charlson Comorbidity index (≥3) | 9 (13.0) | 16 (19.8) | .38 |
| Depressive Symptoms | |||
| GDS-Short (mean, ±SD) | 5.3(±1.1) | 5.7 (±1.5) | .11 |
| Composite Functional Disability (for 7 ADLs) | .03 | ||
| Independent for all 7 ADLs | 55 (79.7) | 49 (60.5) | |
| Functional difficulty with ≥ 1 ADL | 12 (17.4) | 24 (29.6) | |
| Functional dependence for ≥ 1 ADL | 2 (2.9) | 8 (9.9) | |
All values listed as n(%) unless otherwise noted
Unintentional weight loss ≥10lbs in last year
Exhaustion was defined as self-report of exhaustion using two validated questions
Low activity defined as the lowest quintile of weekly physical activity (kcal/week) utilizing the Physical Activity Scale for the Elderly (PASE)
Slowness defined as a slow walking speed over 10 feet adjusted for height
Decreased grip strength defined as grip strength measured by hand dyanometer (Kg) adjusted by BMI
SLUMS = Saint Louis Mental Status Examination. Scores ranged from 0 to 30. Scores ≤19 in women who have not completed high school and ≤20 in women who completed high school are consider a positive screen for dementia.
Women who were dependent for ≥1 ADLs were more likely to elect for non-surgical treatment than surgery ((9.9%, n/N=8/81) vs. (2.9%, n/N=2/69), p =.02). (Table 3) We then examined specific activities of daily living between women electing for surgical treatments for their pelvic floor disorders vs. non-surgical treatments. (Table 4) Women who reported dependence or difficulty with walking, dressing, bathing, and toileting were more likely to elect for non-surgical treatment options than surgery p =.005 −.05. (Table 4)
Table 4.
Activities of daily living in older women choosing surgery and non-surgical treatments for pelvic floor dysfunction
| Surgery N= 69 |
Non-surgical treatments N = 81 |
P | |
|---|---|---|---|
| Walking across the room | .07 | ||
| Independent | 65 (94.2) | 67 (82.7) | |
| Independent with difficulty | 4 (5.8) | 12 (14.8) | |
| Dependent | 0 | 2 (2.5) | |
| Walking one block | .005 | ||
| Independent | 58 (84.1) | 49 (60.5) | |
| Independent with difficulty | 9 (13.0) | 25 (30.9) | |
| Dependent | 2 (2.9) | 7 (8.6) | |
| Dressing | .05 | ||
| Independent | 66 (95.7) | 68 (84.0) | |
| Independent with difficulty | 3 (4.3) | 12 (14.8) | |
| Dependent | 0 | 1 (1.2) | |
| Bathing or showering | .005 | ||
| Independent | 69 (100) | 71 (87.6) | |
| Independent with difficulty | 0 | 7 (7.4) | |
| Dependent | 0 | 4 (5.0) | |
| Eating | .13 | ||
| Independent | 69 (100) | 77 (95.1) | |
| Independent with difficulty | 0 | 4 (4.9) | |
| Dependent | 0 | 0 | |
| Getting in and out of bed | .18 | ||
| Independent | 67 (97.1) | 74 (91.4) | |
| Independent with difficulty | 2 (2.9) | 7 (8.6) | |
| Dependent | 0 | 0 | |
| Using the toilet | .02 | ||
| Independent | 69 (100) | 74 (91.4) | |
| Independent with difficulty | 0 | 7 (8.6) | |
| Dependent | 0 | 0 | |
All values listed as n(%)
DISCUSSION
Frailty, cognitive impairment, and functional disability are common in older women seeking treatment for female pelvic floor dysfunction. Symptom distress from PFD was not different among frail, pre-frail and normal women; however women with frailty reported a significantly greater life-impact from PFD than normal and pre-frail women.
We measured frailty in 17% of community-dwelling women ≥65 years old seeking treatment which is similar to the prevalence of frailty measured in community-dwelling older adults of between 7% to 25%.(1, 24) No significant difference was observed between women electing for surgical management in the prevalence of frailty or cognitive impairment, although this study was not powered to detect a difference for this secondary objective. We demonstrated that women with overt functional disability with activities of daily living were significantly more likely to choose a non-surgical treatment option. The implication of these results suggests that overt functional impairment may be associated with different treatment choices for women with PFD; however, frailty, which is more subtle for a woman and her surgeon to recognize, may not always be considered when deciding on a treatment plan.
Geriatric assessments may be beneficial for older women considering surgical treatments for PFD in four very important ways. The first is to identify patients who are at risk of increased postoperative complications and present these increased risks to the patient prior to surgery as an opportunity for enhanced decision making. The second is to use the preoperative period for elective surgery as a powerful motivator for behavioral change in older patients. Certain modifiable risk factors can then be identified and addressed preoperatively to reduce a patient’s unique postoperative risks. This includes reducing polypharmacy, substance abuse interventions, smoking cessation, nutritional improvement, and increasing preoperative activity or “prehabilitation”. Increased preoperative physical activity, either respiratory muscle training or aerobic activity over 3 weeks to 6 months, has some data to support decrease postoperative complications.(25, 26) Currently, the best exercises, timing and duration of exercises to optimize postoperative outcomes are not known. The third is that women identified preoperatively with frailty could be managed differently in the postoperative period by implementing evidence-based pathways to reduce complications such as delirium and falls.(27) Finally, identifying women with frailty preoperatively can allow for better assessment of disposition and needs at the time of discharge to prevent morbidity in the transition to home.(27)
Measurements of frailty prior to surgery are proving important in cardiac and colorectal surgery for preoperative risk adjustment to predict outcomes.(4, 28, 29) The frailty phenotype defined by Fried et al. as having ≥ 3 frailty traits includes unintentional weight loss, exhaustion, low activity, slowness and decreased strength. Frailty was demonstrated to be a predictor of common adverse outcomes of aging (long-term nursing home stay, injurious falls, and death).(1) Additionally, Fried et al. demonstrated that pre-frail individuals, having 1–2 frailty traits were at intermediate risk for these adverse outcomes of aging.(1) Robinson et al. developed a predictive tool combining clinical measures of frailty with cognitive status and functional disability (Mini-Cog score, Charlson comorbidity index, functional disability, history of falls, preoperative serum albumin, and preoperative serum hematocrit) to predict mortality after surgery.(29) This prediction tool has been demonstrated to predict 6-month mortality with 81% sensitivity and 86% specificity after major surgery with postoperative intensive care unit admission.(29) We did not collect laboratory values or fall data on women seeking treatment for pelvic floor disorders. However we did find that frailty, cognitive impairment, medical comorbidities, functional disability, and depressive symptoms were not rare in women seeking treatment for pelvic floor disorders. There are two major differences between Robinson et al.’s population and the population of women we examined. First, Robinson et al.’s study was conducted at a Veterans Administration hospital and 96% of the subjects studied were male. (29) Second, Robinson et al.’s population was undergoing major surgery requiring postoperative intensive care unit admissions. Due to the minimally-invasive nature of pelvic reconstructive surgery, postoperative intensive care unit admissions are uncommon. Prior investigations have described favorable outcomes in older women undergoing surgery for PFD.(30–33) These studies suggest that well-selected procedures for PFD can have very favorable impact on quality-of-life of older women. However, these studies are limited as single-institution case series,(31–33) by dichotomizing age categories as ≥ 65 years,(32) and by not measuring preoperative frailty or functional status. More research needs to determine the impact of preoperative geriatric screening in older women undergoing surgery for PFD on outcomes, both immediate postoperative complications and overall quality-of-life.
Our study has several limitations. This was essentially a cross-sectional study. We were able to elicit patient and surgeon’s treatment choices at the conclusion of an initial new patient visit; however, we were not able to follow women with and without frailty to see if they did, in fact, have increased postoperative complications or longer-term morbidity after surgery. Additionally, this study was not powered to show a difference in treatment selection for women choosing surgical vs. non-surgical treatment options. Women were asked about treatment selection only after comprehensive consultation with a physician. Utilizing routine clinical judgment, surgeons will not always offer surgery to every older patient perhaps due to cardiac history, an inability to walk without an assistive device, difficulty recalling details of a medical history, or due to advanced age. We purposely conducted the comprehensive geriatric assessment only after this routine clinical care to add objective measurements to augment routine care. We anticipate this study will lay the groundwork for a future prospective cohort study of older women deciding on surgical and non-surgical treatment option for their PFD. This preliminary work does demonstrate feasibility of data collection and recruitment as well as a common occurrence of frailty and cognitive impairment in this population. We were able to recruit 150 women; however these women were not recruited consecutively. Due to funding constraints, we were only able to recruit on days that study personnel was available so not all 357 women ≥ 65 years old were approached for participation (e.g. non-consecutive recruitment). We do not have information on women who were not approached for participation. However, since women were recruited on days with study personnel available, we do not believe this introduced systematic bias to our study. Finally, we were also limited in sampling women counseled by one practice that included four physicians. Preoperative counseling may vary significantly from across both surgeons and practices. We did not detect a difference in the number of patients choosing surgical or non-surgical treatments or in the number of patients with frailty, cognitive impairment, or functional disability across the 4 surgeons in the practice in this study. Additionally, this tertiary practice offered a comprehensive panel of both surgical and non-surgical treatment options to patients; therefore, we do not believe treatment choices were made by patients due to lack of availability.
We demonstrated frailty, cognitive impairment, and functional disability are present in older women who seek treatment for pelvic floor dysfunction. Although women with functional disability were less likely to choose surgical treatment options, women with frailty and cognitive impairment did not differ in their treatment choices from women without frailty and women with normal cognition. Identification of frailty, cognitive impairment, and functional disabilities in the preoperative period may help better predict a woman’s unique risks for complications. This knowledge could empower women with a more informed decision about treatment, create opportunities for preoperative behavioral change, and identify women who may need specialized care in the immediate postoperative period and at the transition to discharge.
Acknowledgments
Funding
This research was supported in part by a grant from the American Urogynecologic Society (AUGS) Foundation. Dr. Erekson was supported through a grant from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (#P30AG021342 NIH/NIA). The funding agreement ensured the authors independence in designing the study, interpreting the data, and writing and publishing the article.
Footnotes
The abstract for this paper was accepted for poster presentation at the 2014 Annual Scientific Meeting of the American Geriatrics Society in Orlando, FL May 15th to 17th, 2014.
Conflict of Interest: No authors have any conflict of interest to disclose.
Author Contributions:
Elisabeth A. Erekson, MD MPH: study design, analysis and interpretation of data, critical revision of manuscript for important intellectual content
Terri R Fried, MD: critical revision of manuscript for important intellectual content
Deanna K Martin, MPH: subject recruitment, analysis and interpretation of data
Thomas J Rutherford, MD, PhD: critical revision of manuscript for important intellectual content
Kris Strohbehn, MD, interpretation of data and critical revision of manuscript for important intellectual content
Julie P W Bynum, MD, MPH: interpretation of data and critical revision of manuscript for important intellectual content
References
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009 Jan-Feb;48(1):78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011 Nov;202(5):511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011 Jul;213(1):37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. discussion 42-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: Morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005 Mar;53(3):424–429. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010 Jun;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Chow WB, Merkow RP, Cohen ME, Bilimoria KY, Ko CY. Association between postoperative complications and reoperation for patients undergoing geriatric surgery and the effect of reoperation on mortality. Am Surg. 2012 Oct;78(10):1137–1142. [PubMed] [Google Scholar]
- 8.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: Risk factors and outcomes. Ann Surg. 2009 Jan;249(1):173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 9.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008 Dec;56(12):2211–3116. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: The norwegian EPINCONT study. Obstet Gynecol. 2001 Dec;98(6):1004–1010. doi: 10.1016/s0029-7844(01)01566-6. [DOI] [PubMed] [Google Scholar]
- 12.Yip SO, Dick MA, McPencow AM, Martin DK, Ciarleglio MM, Erekson EA. The association between urinary and fecal incontinence and social isolation in older women. Am J Obstet Gynecol. 2013 Feb;208(2):146.e1–146.e7. doi: 10.1016/j.ajog.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: A population-based study. Am J Obstet Gynecol. 2005 Dec;193(6):2071–2076. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 15.Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the saint louis university mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006 Nov;14(11):900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Spitzer WO. State of science 1986: Quality of life and functional status as target variables for research. J Chronic Dis. 1987;40(6):465–471. doi: 10.1016/0021-9681(87)90002-6. [DOI] [PubMed] [Google Scholar]
- 18.Gill TM. Assessment of function and disability in longitudinal studies. J Am Geriatr Soc. 2010 Oct;58(Suppl 2):S308–S312. doi: 10.1111/j.1532-5415.2010.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: Two components of the disability continuum among community-living older persons. Ann Intern Med. 1998 Jan 15;128(2):96–101. doi: 10.7326/0003-4819-128-2-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: Criterion validity of the 10-item center for epidemiological studies depression scale (CES-D) Arch Intern Med. 1999 Aug 9–23;159(15):1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 21.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the center for epidemiologic studies-depression scale and the geriatric depression scale. Arch Intern Med. 1997 Feb 24;157(4):449–454. [PubMed] [Google Scholar]
- 22.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005 Jul;193(1):103–113. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 23.Barber MD, Chen Z, Lukacz E, Markland A, Wai C, Brubaker L, et al. Further validation of the short form versions of the pelvic floor distress inventory (PFDI) and pelvic floor impact questionnaire (PFIQ) Neurourol Urodyn. 2011 Apr;30(4):541–546. doi: 10.1002/nau.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006 Feb 27;166(4):418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 25.O'Doherty AF, West M, Jack S, Grocott MP. Preoperative aerobic exercise training in elective intra-cavity surgery: A systematic review. Br J Anaesth. 2013 May;110(5):679–689. doi: 10.1093/bja/aes514. [DOI] [PubMed] [Google Scholar]
- 26.Jack S, West M, Grocott MP. Perioperative exercise training in elderly subjects. Best Pract Res Clin Anaesthesiol. 2011 Sep;25(3):461–472. doi: 10.1016/j.bpa.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Robinson TN, Finlayson E. How to best forecast adverse outcomes following geriatric trauma: An ageless question? JAMA Surg. 2014 Jun 11; doi: 10.1001/jamasurg.2014.304. [DOI] [PubMed] [Google Scholar]
- 28.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011 Nov;202(5):511–514. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009 Sep;250(3):449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald MP, Richter HE, Bradley CS, Ye W, Visco AC, Cundiff GW, et al. Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008 Dec;19(12):1603–1609. doi: 10.1007/s00192-008-0696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker DY, Burke JJ, 2nd, Gallup DG. Gynecological surgery in octogenarians and nonagenarians. Am J Obstet Gynecol. 2004 May;190(5):1401–1403. doi: 10.1016/j.ajog.2004.01.065. [DOI] [PubMed] [Google Scholar]
- 32.Stepp KJ, Barber MD, Yoo EH, Whiteside JL, Paraiso MF, Walters MD. Incidence of perioperative complications of urogynecologic surgery in elderly women. Am J Obstet Gynecol. 2005 May;192(5):1630–1636. doi: 10.1016/j.ajog.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Toglia MR, Nolan TE. Morbidity and mortality rates of elective gynecologic surgery in the elderly woman. Am J Obstet Gynecol. 2003 Dec;189(6):1584–1587. doi: 10.1016/s0002-9378(03)00940-2. discussion 1587-9. [DOI] [PubMed] [Google Scholar]