Abstract
Members of the Asterinaceae and Parmulariaceae are obligate biotrophic fungi with a pantropical distribution that grow in direct association with living plant tissues and produce external ascomata and bitunicate asci. These fungi are poorly known, with limited information about their taxonomic position in the Dothideomycetes. Much of what is known is conjectural and based on observation of morphological characters. An assessment of the phylogenetic position of the Asterinaceae and Parmulariaceae is provided based on a phylogenetic analysis of the nrDNA operon (ITS) and the large subunit rDNA (LSU) sequence data obtained from fresh material of selected species collected in Brazil. Three key species were included and epitypified, namely Asterina melastomatis, which is the type species for the type genus of the Asterinaceae; Prillieuxina baccharidincola (Asterinaceae); and Parmularia styracis, which is the type species for the type genus of the Parmulariaceae. An LSU rDNA phylogenetic analysis was performed indicating the correct phylogenetic placement of the Asterinales within the Dothideomycetes. From this initial analysis it is clear that the Parmulariaceae as currently circumscribed is polyphyletic, and that the Asterinaceae and Parmulariaceae are related, which justifies the maintenance of the order Asterinales. Asterotexis cucurbitacearum is recognised as distinct from other Dothideomycetes and placed in the newly proposed family and order (Asterotexiaceae, Asterotexiales), while the higher order phylogeny of Inocyclus angularis remains unresolved. Additionally, Lembosia abaxialis is introduced as a novel species and the phylogenetic placement of the genera Batistinula and Prillieuxina is clarified.
Keywords: Asterinales, epitype, Neotropical fungi, taxonomic novelties, type species
INTRODUCTION
The Parmulariaceae (Ascomycota) was informally proposed by Müller & von Arx (1962) to accommodate plant parasitic fungi with superficial, dimidiate shield-shaped or crust-like, pulvinate stromata, strongly flattened ascomata that open by irregular disintegration, or by lateral to radial, or ring-like splits. The externally visible stromata usually originate from internal hyphae or internal hypostroma (von Arx & Müller 1975). Asci in this family are ovoid to clavate, with fissitunicate or rostrate dehiscence with a hamathecium composed of pseudoparaphyses. Ascospores of members of this family are hyaline or brown, usually septate and, with or without a mucilaginous sheath. Asexual morphs of fungi in this group are poorly known. The family was formally described by Barr (1979). A more detailed account of the Parmulariaceae was provided in the monograph published by Inácio & Cannon (2008).
The Parmulariaceae together with families of foliicolous ascomycetes such as Asterinaceae and Aulographaceae, has tra-ditionally been treated as a group with uncertain placement (incertae sedis) in the Dothideomycetes (Hyde et al. 2013). The Parmulariaceae differs from the supposedly closely related Asterinaceae, by having an apical stroma formed by several layers of pigmented cells, and a basal hypostroma formed by fungal hyphae, as well as by the absence of appressoria (Inácio et al. 2012a, Hongsanan et al. 2014). Superficial hyphae are absent in species of Parmulariaceae with the exception of Antoniomyces, Aulacostroma, Mintera and Symphaeophyma, although commonly found in the Asterinaceae (Inácio et al. 2012a). The taxonomic value of this feature was considered an artificial criterion for distinguishing the two families (von Arx & Müller 1975). Nevertheless as a matter of convenience, this morphological feature is still widely used to recognise whether a taxon belongs to one family or the other. The hypothesis of affinity between these two families has never been tested with modern molecular tools.
Léveillé (1845) described eight species in two genera, Asterina and Lembosia. In 1899, Asterina was included in Microthyriaceae and the family was divided into two subfamilies, Asterineae and Microthyrieae, based on the presence or absence of superficial mycelium (Theissen 1913a, b). Subsequently, the family Asterinaceae was described and 18 genera were included (Hansford 1946).
Currently the Asterinaceae includes species that are either epiphytic or obligate biotrophs. Fungi in this family have dimidiate ascomata that open irregularly at maturity by means of stellar, longitudinal or irregular slits. Ascomata contain bitunicate upright asci, which are globose to oval or cylindrical. Colonies are formed on the surface of leaves or green stems of plants. When present, superficial mycelium is composed of hyphae that have opposite, alternate or irregular branches with uni- or bi-cellular appressoria that are either alternate, unilateral or a mixture of these forms and with shapes that vary between oval, ampulliform, lobate or variable. Haustoria are present in many genera (von Arx & Müller 1975, Eriksson 1981, Bezerra 2004, Hofmann et al. 2010, Hofmann & Piepenbring 2011, Hosagoudar 2012).
Recent studies have shown that morphological features alone are not a reliable basis for a natural classification that reflects true phylogenetic relationships. Some examples are found at the generic level in taxa such as Cladosporium, Microcyclosporella, Phaeomoniella, Radulidium, Ramichloridium and Septoria, among others (Arzanlou et al. 2007, Schubert et al. 2007, Frank et al. 2010, Quaedvlieg et al. 2013) and at the family level in Botryosphaeriaceae and Teratosphaeriaceae (Slippers et al. 2013, Quaedvlieg et al. 2014). Delimitation and affiliation of both the Asterinaceae and Parmulariaceae and the genera they contain have relied entirely on morphological features such as ascospore septation, hamathecium reaction to iodine, presence and shape of internal stromata, plectenchyma texture and colour, ascomata and ascus dehiscence.
Morphological features are often combined with conjectured host specificity. However, the host specificity of fungi in these families has never been experimentally tested (Hofmann et al. 2010). The Asterinaceae and Parmulariaceae were regarded as probably polyphyletic both by Inácio & Cannon (2008) and Hongsanan et al. (2014), respectively. Practical difficulties related to DNA extraction from old herbarium material and difficulties with recollection of type specimens have hampered a reappraisal of these two families.
Inácio & Cannon (2008) included 35 genera as members of the Parmulariaceae, while Lumbsch & Huhndorf (2010) recognised 34 genera, with the inclusion of Hemigrapha and exclusion of Apoa and Parmulariella. The latest publication mentioning this family (Hyde et al. 2013) added Antoniomyces and excluded four genera (Coccodothis, Dothidasteroma, Englerodothis and Perischizon) from the Parmulariaceae based on the shape of the ascomata, reducing the total number to 31 genera. Now, with the addition of the recently described genus Rhagadolobiopsis (Guatimosim et al. 2014a), the Parmulariaceae include 32 genera and 114 synonyms (Inácio & Cannon 2008, Lumbsch & Huhndorf 2010, Inácio et al. 2012b, Hyde et al. 2013, Guatimosim et al. 2014a, b).
Lumbsch & Huhndorf (2010) included 38 genera in the Asterinaceae but, more recently, Hongsanan et al. (2014) revised the Asterinaceae, and recognised only 17 genera and 42 synonyms as belonging to the family. These revisions were mostly based on morphological observations, and were not substantiated by molecular data.
Molecular phylogenetic studies of the Parmulariaceae are difficult because of their biotrophic nature as well as the difficulties involved in DNA extraction from herbarium specimens. The pioneering study of the phylogenetic placement of Asterinaceae (Hofmann et al. 2010) and recent successful DNA extraction from the Meliolales (Pinho et al. 2012, 2014), shows that phylogenetic approaches can be applied to obligate biotrophs, even when only old herbarium material is available.
The aim of this study was to assess the phylogenetic placement of the Asterinaceae and Parmulariaceae based on the study of newly collected epitype materials of Parmularia styracis (the type species of Parmulariacae), Asterina melastomatis (the type species of Asterinaceae) and Prillieuxina baccharidincola (Asterinaceae). Asterotexis cucurbitacearum, formerly placed in the Asterinaceae, was re-examined and found to represent a separate family, described here as new. Additionally, a new species of Lembosia is introduced and the phylogenetic placement of B. gallesiae and P. baccharidincola is elucidated.
MATERIALS AND METHODS
Sample collection and morphology
Leaf samples bearing black fungal colonies were collected in Brazil in different biomes between 2009 and 2014. These were dried in a plant press and later examined under a stereo microscope. Freehand sections of fungal colonies were prepared and fungal structures mounted in clear lactic acid, lactophenol, lactofuchsin, and/or Melzer’s reagent. When necessary, sections were made using a Microm HM 520 freezing microtome. Observations were made with a Zeiss V20 Discovery stereo microscope and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and an MRc5 camera and ZEN imaging software. Representative specimens were deposited at the herbarium of the Universidade Federal de Viçosa (VIC) and CBS Herbarium (CBS H).
Scanning electron microscopy
Samples of dried material containing fungal structures were mounted on stubs with double-sided adhesive tape and gold-coated using a Balzer’s FDU 010 sputter coater. A Carl-Zeiss Model LEO VP 1430 scanning electron microscope (SEM) was used to analyse and generate images from the samples.
DNA isolation
Leaves harbouring fertile ascomata were examined under a stereo-microscope to check for possible contamination by other fungi, including yeasts. The leaves were then soaked in sterile water for 1 h in order to hydrate and remove the ascomata. Thirty fertile ascomata were removed from the leaves with a sterile fine pointed needle, and placed into a microcentrifuge tube (1.5 mL). Total genomic DNA was extracted by using Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following the manufacturer’s instructions and the steps described by Pinho et al. (2012).
PCR amplification
The LSU region of each fungus included in the study was sequenced with the primers LR0R + LR5 (Vilgalys & Hester 1990). For the Parmulariaceae, two additional loci, including the internal transcribed spacer regions and intervening 5.8S rDNA (ITS) and the translation elongation factor 1-alpha (EF-1 α) were amplified and sequenced with the primer pairs ITS1-F (Gardes & Bruns 1993) + ITS4 (White et al. 1990), EF2-Fd (Groenewald et al. 2013) or EF1-728F (Carbone & Kohn 1999) + EF-2 (O’Donnell et al. 1998). PCR amplifications were performed in a total volume of 12.5 μL solution containing 10–20 ng of template DNA, 1× PCR buffer, 0.63 μL DMSO (99.9 %), 1.5 mM MgCl2, 0.5 μM of each primer, 0.25 mM of each dNTP, 1.0 U BioTaq DNA polymerase (Bioline GmbH Luckenwalde, Germany). PCR conditions for ITS and LSU were set as follows: an initial denaturation temperature of 95 °C for 5 min, followed by 35 cycles of denaturation temperature of 95 °C for 30 s, primer annealing at 52 °C for 30 s, primer extension at 72 °C for 1 min and a final extension step at 72 °C for 1 min. PCR conditions for EF-1 α were set as follows: an initial denaturation temperature of 94 °C for 5 min, followed by 45 cycles of denaturation temperature of 94 °C for 45 s, primer annealing at 52 °C for 30 s, primer extension at 72 °C for 90 s and a final extension step at 72 °C for 6 min.
DNA sequencing and phylogenetic inference
PCR amplicons of the regions targeted in this study served as templates for DNA sequencing reactions with the BigDye® Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA) following the protocol of the manufacturer. DNA sequencing reactions used the same primers as those for the PCR reactions. DNA sequencing amplicons were purified through Sephadex® G-50 Superfine columns (Sigma Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were run on an ABI Prism 3730xl DNA Sequencer (Life Technologies, Carlsbad, CA, USA).
DNA sequence data were analysed in MEGA (Molecular Evolutionary Genetics Analysis) v. 6.0 (Tamura et al. 2013). Consensus sequences were generated and imported into MEGA for initial alignment and the construction of sequence datasets. Sequences obtained from Schoch et al. (2009), TreeBASE study S10245, and from GenBank (www.ncbi.nlm.nih.gov) and the novel sequences generated on this study were aligned using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html; Katoh et al. 2002) and manually improved in MEGA as indicated.
Phylogenetic analysis
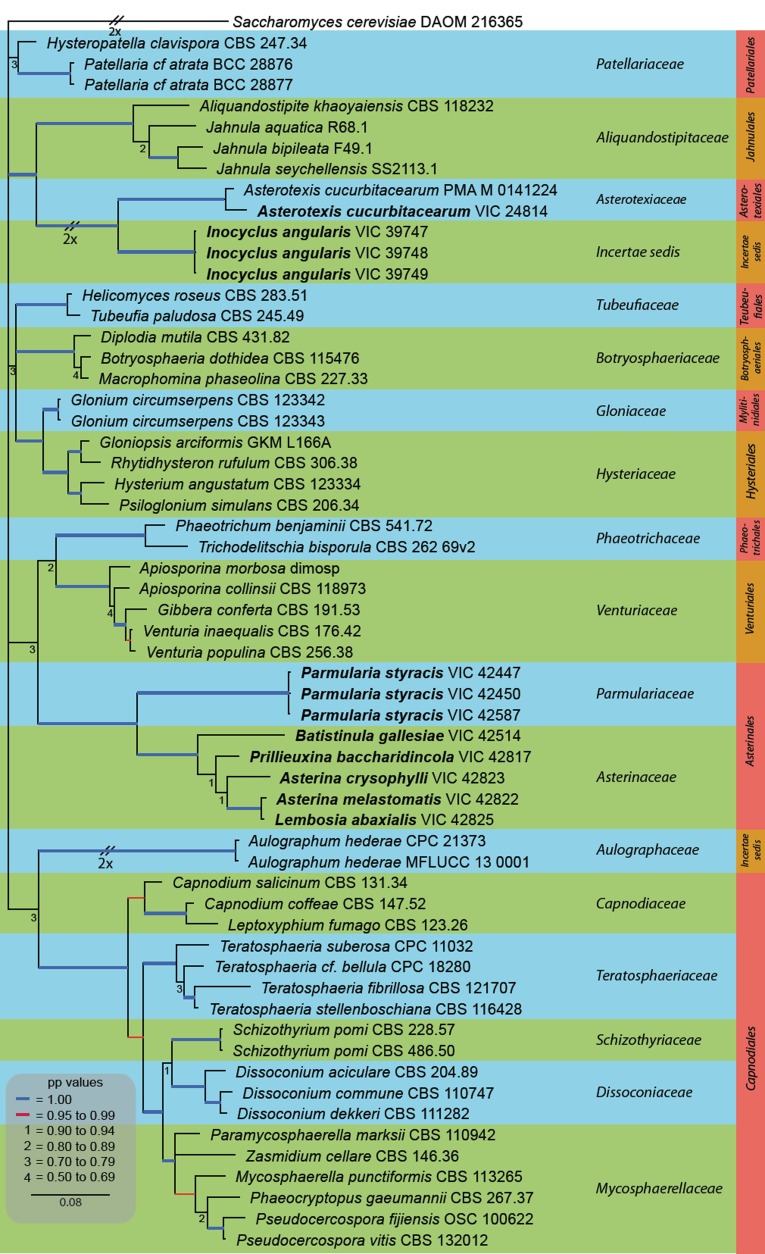
Appropriate gene models were selected using MrModeltest v. 2.3 (Nylander 2004) and applied to the gene partition. Based on the results of MrModeltest, a Bayesian phylogenetic analysis was performed with MrBayes v. 3.1.2 applying a general time-reversible (GTR+I+G) substitution model with inverse gamma rates and dirichlet base frequencies and a heating parameter set at 0.01. Saccharomyces cerevisiae DAOM 216365 (JN938921) served as outgroup for the phylogenetic analyses. Posterior probabilities were determined by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v. 3.2.1 (Ronquist et al. 2012). Six simultaneous Markov chains were run for 10 000 000 generations and trees were sampled every 100th generation and 10 000 trees were obtained. The first 2 000 trees, representing the burn-in phase were discarded, while the remaining 8 000 trees were used for calculating posterior probabilities. Bayesian posterior probabilities are presented on the left of each node (Fig. 1). Sequences derived in this study were lodged in GenBank (http://www.ncbi.nlm.nih.gov/genbank) (Table 1), the alignment and tree in TreeBASE (www.MycoBank.org) (study number 17355) and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Fig. 1.
A Bayesian 50 % majority rule tree based on a full length LSU alignment, containing all strains generated in this study. Bayesian posterior probabilities support values for the respective nodes are displayed in the tree. The tree was rooted to Saccharomyces cerevisiae. The scale bar indicates 0.08 expected changes per site. New sequence data are in bold.
Table 1.
Strains and NCBI GenBank accessions generated in this study. Type specimens are in bold.
| Species | Accession number | Host / Substrate | Locality | Collector | GenBank accessions |
||
|---|---|---|---|---|---|---|---|
| LSU | ITS | EF-1 α | |||||
| Asterina crysophylli | VIC 42823 | Henriettea succosa | Brazil | A.L. Firmino | KP143738 | – | – |
| A. melastomatis | VIC 42822 | Miconia sp. | Brazil | A.L. Firmino | KP143739 | – | – |
| Asterotexis cucurbitacearum | VIC 24814 | Cucurbita pepo | Brazil | O.L. Pereira & A.L. Firmino | KP143734 | – | – |
| Batistinula gallesiae | VIC 42514 | Caesalpinia echinata | Brazil | A.L. Firmino, D.B. Pinho & O.L. Pereira | KP143736 | – | – |
| Inocyclus angularis | VIC 39747 | Pleopeltis astrolepis | Brazil | R.W. Barreto | KP143731 | KP273233 | KP289328 |
| VIC 39748 | Pleopeltis astrolepis | Brazil | R.W. Barreto | KP143732 | KP273234 | KP289329 | |
| VIC 39749 | Pleopeltis astrolepis | Brazil | R.W. Barreto | KP143733 | KP273235 | KP289330 | |
| Lembosia abaxialis | VIC 42825 | Miconia jucunda | Brazil | R.W. Barreto | KP143737 | – | – |
| Parmularia styracis | VIC 42447 | Styrax ferrugineus | Brazil | M.S. Silva & O.L. Pereira | KP143728 | KP273230 | KP289325 |
| VIC 42450 | Styrax ferrugineus | Brazil | M.S. Silva & O.L. Pereira | KP143729 | KP273231 | KP289326 | |
| VIC 42587 | Styrax ferrugineus | Brazil | R.W. Barreto | KP143730 | KP273232 | KP289327 | |
| Prillieuxina baccharidincola | VIC 42817 | Baccharis sp. | Brazil | O.L. Pereira | KP143735 | – | – |
RESULTS
Taxonomy
Parmulariaceae M.E. Barr, Mycologia 71: 944. 1979
Type species. Parmularia styracis Lév., Ann. Sci. Nat., Bot. 5: 286. 1846.
This family includes fungi forming foliicolous or lichenicolous, superficial, dark brown to black colonies. Haustoria coralloid, hyaline, numerous in each host-cell. Ascomata solitary to gregarious, superficial (or rarely immersed), shield-like, starshaped, ellipsoidal or boat-shaped, strongly flattened, membranaceous to carbonaceous, originating from emerging hyphae or from an erumpent hypostroma, covered by a dark wall composed of often radiating rows of cells and opening by fissure or by deliquescence, containing numerous asci, dark brown to black. Asci 8-spored, thick-walled, fissitunicate, variously shaped, short stalked, with a distinct ocular chamber. Ascospores oblong, ellipsoidal or ovoid, ends rounded, 1-septate, constricted or not at the septum, hyaline to dark brown, smooth to verrucose (Inácio & Cannon 2008, Hyde et al. 2013).
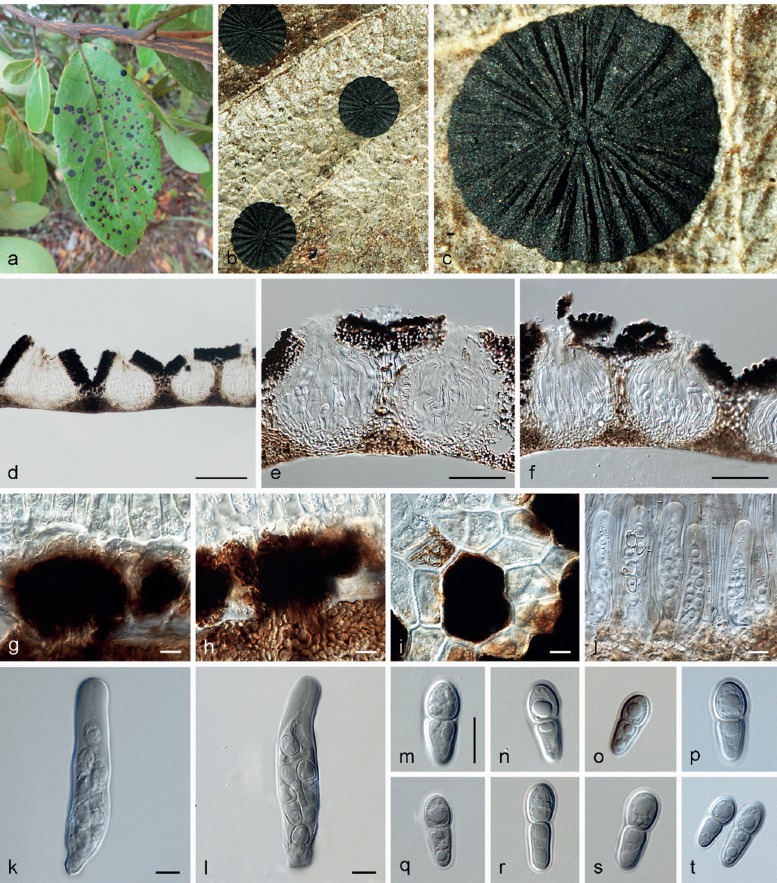
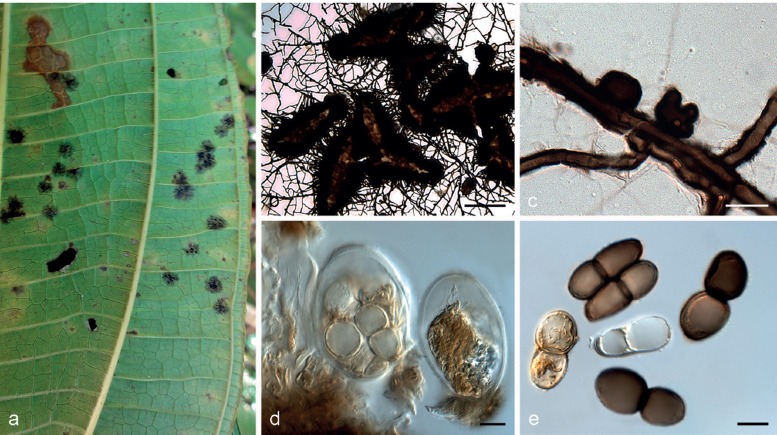
Parmularia styracis Lév., Ann. Sci. Nat., Bot. 5: 286. 1846 — Fig. 2
Fig. 2.
Parmularia styracis VIC 42447. a. Living leaves of Styrax ferrugineus with epiphyllous colonies; b, c. detail of the mature colony, opening by radiating fissures; d. vertical section showing entirely superficial ascoma with fertile locules; e, f. detail of the fertile locules; g, h. hyphal columns which connect the colony with the host tissue; i. horizontal section showing the detail of a tuft of internal mycelium that ruptures the cuticle and produce the initial stages of the ascostromata; j. detail of the fertile locule with fully developed asci and pseudoparaphyses; k, l. asci; m–t. ascospores. — Scale bars: d = 100 μm; e, f = 50 μm; g–m = 10 μm.
= Schneepia guaranitica Speg., Anales Soc. Ci. Argent. 19: 259. 1885.
≡ Parmularia guaranitica (Speg.) Henn., Hedwigia 36: 230. 1897.
= Schneepia arechavaletae Speg., Bol. Acad. Nac. Ci. 11: 581. 1889.
≡ Parmularia arechavaletae (Speg.) G. Arnaud, Ann. Ecole Natl. Agric. Montpellier 16: 116. 1918.
= Parmularia styracis var. minor Henn., Hedwigia 34: 112. 1895.
Colonies visible as superficial, epiphyllous, black discoid structures, numerous and scattered over leaves, not associated with necrosis, 2–3 mm diam. External mycelium absent. Internal mycelium intra- and intercellular, deeply penetrating the mesophyll, branched, 1.5–3.5 μm diam, sub-hyaline to dark brown, smooth. Haustoria coralloid, hyaline, several per host cell occupying both the subcuticular and the lacunar parenchymal cells. Internal stromata globular, 27–67 μm diam, located at the central portion of the colony, erupting through the cuticle, cells composed of a combination of textura angularis and textura prismatica, 3–8 × 2–5 μm. External stromata epiphyllous, superficial, discoid, laciniate at the edges, 1–3 mm diam, cells composed of textura prismatica, 3–7 × 1.5–3.5 μm. Ascomata, producing locules arranged in radiating lirellae-like slits, with undulated surface. In vertical section: ascomata entirely superficial, loosely connected to the leaf, delimited by a covering layer (above the fertile locules) and a lower layer. Covering layer black, 27–63 μm thick, consisting of dense dark brown radiating cells of textura angularis, 3–7 × 2–4 μm. Lower layer beneath the hymenium adjacent to the host cuticle, colourless to pale brown, intimately mingled with hyphal cells of the basal cushion, 13–46 μm thick, composed of pale brown to brown textura angularis (cells 2–7 × 1.5–4 μm). Locules with a thin basal cushion above the lower layer, asci and hamathecium immersed in a non-amyloid gelatinous stratum, 76–353 μm diam, 100–320 μm high. Pseudoparaphyses mostly colourless and pale brown at the rounded and slightly swollen, slightly verrucose tips, sometimes with brown to dark brown external material adhering, 49–115 × 1.5–3 μm, septate, thin-walled, filiform, sometimes dichotomously branched near the base. Asci bitunicate, maturing sequentially, with young and mature asci in the same locule; young asci variable in shape before spores can be distinguished, truncated at the base, subcylindrical; mature asci thick-walled (particularly in the upper portion), cylindric-clavate to clavate, 47–81 × 9–18 μm, non-amyloid, 6–8-spored, biseriate (with colourless hyaline ascospores) or unordered but becoming uniseriate at maturity (the stage containing pale brown ascospores), dehiscence through a large apical fracture in the outer wall, with the inner layer extending through it. Ascospores ellipsoidal to clavate, mostly hyaline to pale brown, thin-walled, verrucose, 1-septate, constricted at the septum, the upper cell broader and rounded, and the lower cell tappering towards a rounded end, 14–20 × 5–7 μm, smooth. Asexual morph unknown.
Type material. BRAZIL, Planaltina, on living leaves of Styrax, Clauseen, 1846 (PC!, holotype); on living leaves of Styrax ferrugineus, vicinities of the Estação Ecológica de Águas Emendadas, Cerrado biome, 16 Apr. 2013, M. Silva & O.L. Pereira (VIC 42447 = CBS H-22026, epitype designated here, MBT200333).
Additional materials examined. BRAZIL, Planaltina, on living leaves of Styrax ferrugineus, vicinities of the Estação Ecológica de Águas Emendadas, Cerrado biome, 18 Apr. 2013, M. Silva & O.L. Pereira, VIC 42450 = CBS H-22025; Minas Gerais, Capitólio, Furnas, on living leaves of S. ferrugineus, S20°38’54.5" W46°13’36.8", 9 Nov. 2012, R.W. Barreto, VIC 42587 = CBS H-22027.
Notes — The ontogeny of ascomata of P. styracis resembles that recently described for the genus Rhagadolobiopsis, in that mature ascostromata are produced from several ascostromatal primordia that coalesce to form a multiloculate structure (Guatimosim et al. 2014a) (Fig. 2b, c). In contrast, Parmularia produces a column of internal mycelium in the centre of the colony that ruptures through the cuticle (Fig. 2i). When the ascomatal disk is removed, the hyphal columns are limited to the central portion of the area below the colony (Fig. 2g, h).
Asterinaceae Hansf., Mycol. Pap. 15: 188. 1946
Type species. Asterina melastomatis Lév., Ann. Sci. Nat., Bot. 3: 59. 1845.
Foliicolous, epiphytic, obligately biotrophic. Sexual morph: Ex-ternal mycelium usually with or without appressoria, opposite, alternate or irregular branches, blackened. Appressoria uni- or bi-cellular, lateral and/or intercalary, and opposite, alternate or alternate and opposite, oval, ampulliform, lobate or variable, brown to dark brown, with penetration peg piercing through cuticle and invading the epidermic cells or on top of guard cells, forming stomatopodia. Haustoria present in various genera. Ascomata dimidiate, superficial, growing on the surface of plant leaves or stems, circular, elongate or linear, dehiscence non-ostiolate, opening by radiating star-like, longitudinal or irregular slits. Scutellum radiate, composed of isodiametric to cylindrical cells, with straight to dichotomously branched hyphae. Hypostroma (internal stroma or internal hyphae) present in some members. Pseudoparaphyses present or not, cylindrical, septate, branched or unbranched, hyaline to yellowish. Asci fissitunicate, upright and parallel, globose, ovoid or cylindrical, 4–8-spored, usually lacking a stalk, hyaline. Ascospores ellipsoidal, occasionally cylindrical, 2–6-celled, yellowish to brown (mostly brown when mature), walls smooth or with capitate ornamentation. Setae present or not on the ascomata and/or mycelium. Asexual morph hyphomycetous or coelomycetous states with pycnothyria. Conidiophores solitary, unbranched, brown. Conidiogenous cells monoblastic or proliferating percurrently, hyaline or brown. Conidia ovoid, cylindrical, conical or staurosporous, brown (von Arx & Müller 1975, Eriksson 1981, Bezerra 2004, Hofmann et al. 2010, Hofmann & Piepenbring 2011, Hosagoudar 2012, Hyde et al. 2013, Hongsanan et al. 2014).
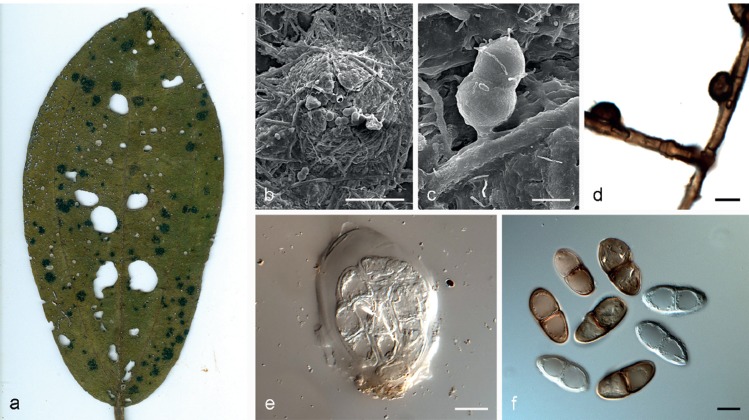
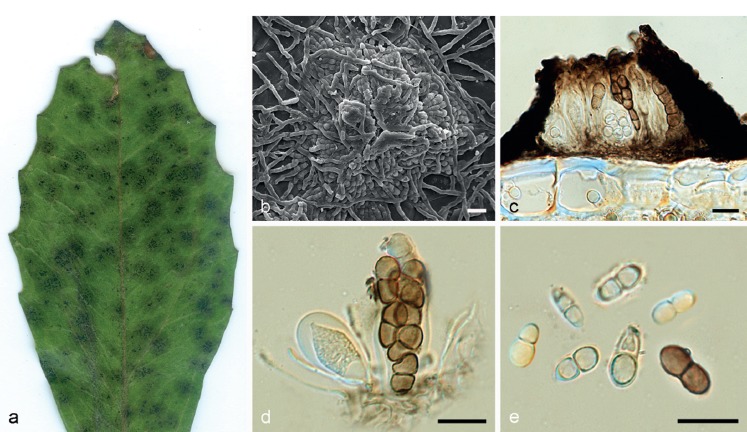
Asterina melastomatis Lév., Ann. Sci. Nat., Bot. 3: 59. 1845. — Fig. 3
Fig. 3.
Asterina melastomatis VIC 42822. a. Living leaves of Miconia sp. with epiphyllous colonies; b. colony with open thyriothecia and external mycelium; c. appressoria cylindrical to long-ovate, unicellular; d. asci ovoid to slightly clavate; e. ascospores hyaline, becoming pale brown to brown at maturity. — Scale bars = 10 μm.
≡ Parasterina melastomatis (Lév.) Theiss., Syd. & P. Syd., Ann. Mycol. 15: 246. 1917.
Colonies epiphyllous, irregular to circular, single to confluent, black, 2–6 mm diam. External mycelium straight to flexuous, branching alternate to unilateral, rarely opposed, pale brown to brown, septate, hyphal cells cylindrical, 4–5 μm diam, smooth. Appressoria numerous, entire, sessile, straight to angular, rarely crooked, rectangular to long-ovate, unicellular, alternate to unilateral, never opposed, 6–7.5 × 7–8 μm, brown, penetration peg in middle part of appressorial cell. Ascomata thyriothecia, dimidiate, superficial, developing below external mycelium, circular, single to confluent, in small clusters, fringed at margins, 165–220 μm diam, dark brown to blackish, opening by a central star-shaped fissure. Pseudoparaphyses cylindrical, septate, unbranched, hyaline to yellowish. Scutellum radiate, composed of isodiametric to cylindrical cells. Asci bitunicate, ovoid to slightly clavate, 8-spored, 47.5–57.5 × 27.5–30 μm, hyaline. Ascospores 2-celled, cylindrical, straight, constricted at the septum, hyaline initially, pale brown to brown at maturity, smooth, 19.5–21 × 9.5–11 μm. Asexual morph absent.
Type material. BRAZIL, locality unknown, on living leaves of Miconia sp., date unknown, Guillemin, (herbarium specimen not preserved); Minas Gerais, Lavras Novas, on living leaves of Miconia sp., on the track of the Cachoeira das Três Quedas, S20°28’39.63" W43°29’42.27", 26 Oct. 2013, A.L. Firmino (VIC 42822, neotype designated here MBT200348). – FRENCH GUIANA, Cayene, on leaves of Melastomataceae, Nov. 1800, Leprieur (herb. Montagne 1133, Crypt. Guyan. 582); PC0084477. Referred to by Hongsanan et al. (2014) as a neotype designated by Theissen (1912 – actually 1913), but that author only referred to species being represented by that collection.
Asterina chrysophylli Henn., Hedwigia 48: 12. 1908. — Fig. 4
Fig. 4.
Asterina chrysophylli VIC 42823. a. Living leaves of Henriettea succosa with epiphyllous colonies; b, c. SEM images: b. thyriothecium opened by a central star-shaped fissure; c. ascospore oblong, smooth, constricted at the septum; d. appressoria straight, globose to pyriform, unicellular; e. asci globose to ovoid; f. ascospores hyaline, becoming brown at maturity. — Scale bars = 10 μm.
Colonies epiphyllous, irregular to circular, solitary to confluent, black 0.5–6 mm diam. External mycelium straight to slightly flexuous, branching irregularly, pale brown to brown, septate, hyphal cells cylindrical, 4.5–5 μm diam, smooth. Appressoria numerous, entire, sessile, straight, globose to pyriform, unicellular, alternate to unilateral, never opposed, 7.5–9.5 × 11–12.5 μm, brown, penetration peg in the middle portion of the appressorial cell. Ascomata superficial, thyriothecioid dimidiate, developing below external mycelium, circular, solitary to confluent, fringed at margins, 162–253 μm diam, opening through central star-shaped fissures, dark brown to black. Scutellum radiate, composed of somewhat isodiametric to cylindrical cells, straight. Asci bitunicate, globose to ovoid, 8-spored, 52.5–57.5 × 32.5–35 μm, hyaline, smooth. Ascospores oblong to slightly fusiform, straight to slightly curved, constricted at the septum, 27–30 × 14–15 μm, 2-celled, hyaline, becoming brown at maturity, smooth. Asexual morph absent.
Material examined. BRAZIL, Espírito Santo, Sooretama, Reserva Natural Vale, on living leaves of Henriettea succosa, 19 June 2012, A.L. Firmino, VIC 42823.
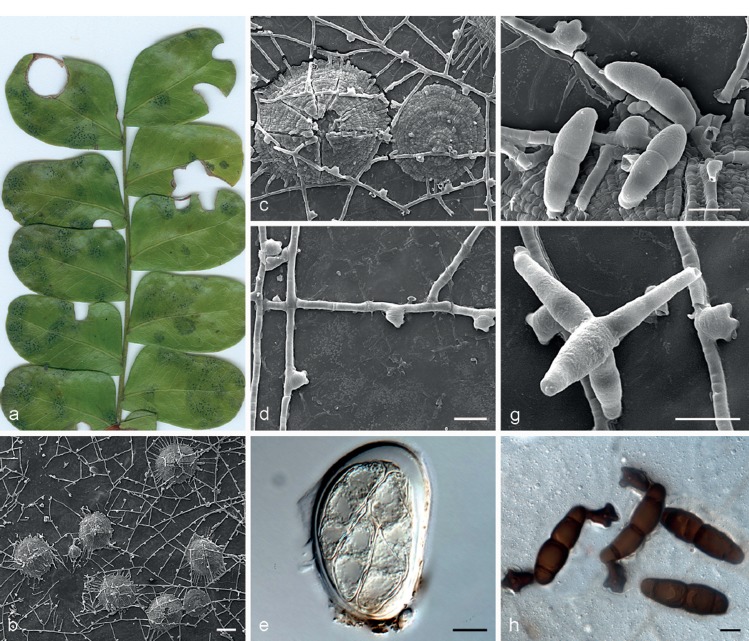
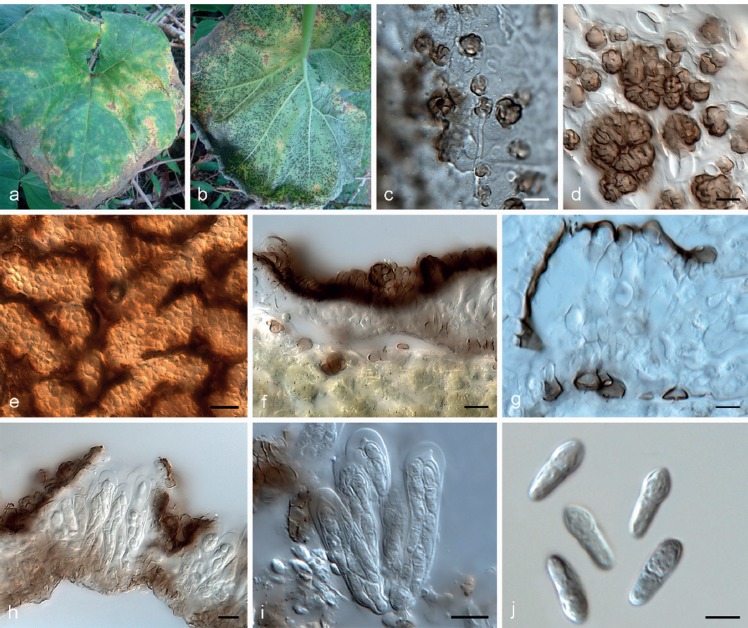
Batistinula gallesiae Arx, Publicações Inst. Micol. Univ. Recife 287: 6. 1960. — Fig. 5
Fig. 5.
Batistinula gallesiae VIC 42514. a. Living leaves of Caesalpinia echinata with epiphyllous colonies; b–d, f, g. SEM images: b. colony with open thyriothecia and external mycelium; c. thyriothecium opened by a central star-shaped fissure; d. appressoria straight, lobate, cylindrical, unicellular; e. asci ovoid, showing immature ascospores; f. ascospores oblong, with ends broadly rounded, constricted at the septum; g. conidia of Triposporium (asexual morph) and erect conidiophore; h. ascospores with lobate appressoria. — Scale bars: b = 100 μm; c, d, f, g = 20 μm; e, h = 10 μm.
Colonies amphigenous, irregular to circular, solitary becoming confluent, black, 1–7 mm diam. External mycelium straight, branching alternate, unilateral or opposite, pale brown to brown, septate, composed of cylindrical hyphal cells, 4.5–5 μm diam, smooth. Appressoria numerous, sessile, straight, cylindrical, 2–3 lobate, 9.5–15 × 9.5–14 μm, unicellular, alternate or unilateral, never opposed, brown, penetration peg centrally on the appressorial cell. Ascomata thyriothecioid dimidiate, isolated, superficial, developed below external mycelium, circular, fringed at margins, 152–213 μm diam, opening by a central star-shaped fissure, dark brown to black. Scutellum radiated, composed of isodiametric to cylindrical cells, straight. Asci bitunicate, globose, 50–67.5 × 32.5–47.5 μm, 4–8-spored, smooth, hyaline. Ascospores oblong, straight to slightly curved, 40–48 × 11–15 μm, base and apex broadly rounded, 4-celled, constricted at median septum, pale brown to brown, smooth. Asexual morph: Colonies superficial, developing above the external mycelium, brown to dark brown. Conidiophores arising from the hyphae, monoblastic, erect, cylindrical, unbranched, 33–60 × 9–13.5 μm, septate, brown. Conidia solitary, staurospores with three arms, 31–42.5 × 9.5–14 μm, brown, smooth, germinating at the ends of arms.
Type material. BRAZIL, Pernambuco, Recife, Poço das Maçãs, on living leaves of Gallesiae gorazemae, 7 Aug. 1960, O.S. Silva (URM 19988, holotype).
Additional material examined. BRAZIL, Espírito Santo, Sooretama, Reserva Natural Vale, on living leaves of Caesalpinia echinata, S19°19’03.28" W40°05’42.10", 15 July 2012, A.L. Firmino, D.B. Pinho & O.L. Pereira VIC 42514.
Notes — Batistinula gallesiae was originally described from living leaves of Gallesia gorazema (Phytolaccaceae) in the state of Pernambuco (Brazil). The present collection was from living leaves of Caesalpinia echinata (Fabaceae) collected in the state of Espírito Santo (Brazil). This specimen has the same morphological and biometric characteristics of the type. Caesalpinia echinata is a new host of B. gallesiae and the genus remains monotypic, with distribution restricted to Brazil.
Lembosia abaxialis Firmino & R.W. Barreto, sp. nov. — MycoBank MB812000; Fig. 6
Fig. 6.
Lembosia abaxialis VIC 42825. a. Living leaves of Miconia jucunda with hypophyllous colonies; b. colony with open hysterothecia and external mycelium; c. appressoria straight to angular, entire to irregularly lobate, unicellular; d. asci ovoid to slightly clavate; e. ascospores hyaline becoming pale brown to brown at maturity. — Scale bars: b = 20 μm; c–e = 10 μm.
Etymology. Name derived from the observation that colonies of this taxon are only formed abaxially.
Colonies hypophyllous, irregular to circular, solitary to confluent, black, 2–6 mm diam. External mycelium straight to flexuous, branching irregularly, septate, composed of cylindrical hyphal cells, 3–5 μm diam, brown, smooth. Appressoria numerous, entire to irregularly lobate, sessile, straight to angular, 7–10 × 10–10.5 μm, unicellular, unilateral to alternate, never opposed, brown, penetration peg centrally on the appressorial cell. Ascomata hysterothecioid, superficial, developed below external mycelium, mostly linear, rarely Y-shaped, solitary to grouped, fringed at margins, 340–550 × 160–250 μm, dark brown to black, opening by longitudinal fissures. Scutellum radiated, composed of isodiametric to cylindrical cells, straight. Asci bitunicate, slightly clavate, 52.5–57.5 × 25–37.5 μm, 8-spored, hyaline. Pseudoparaphyses cylindrical, septate, unbranched, hyaline. Ascospores oblong to cylindrical, 25–29 × 12.5–15 μm, 2-celled, constricted at the septum, hyaline, becoming pale brown to brown at maturity, smooth. Asexual morph absent.
Type material. BRAZIL, Rio de Janeiro, Bosque da Barra, Barra da Tijuca, on living leaves of Miconia jucunda, 22 Mar. 2014, R.W. Barreto (VIC 42825, holotype).
Notes — Twelve species of Lembosia have been recorded on Melastomataceae (Montagne 1855, 1856, Hennings 1904, Theissen 1913c, Arnaud 1918, Petrak & Ciferri 1930, Petrak & Sydow 1931, Song & Hosagoudar 2003, Hosagoudar & Appaiah 2005, Hosagoudar 2012, Farr & Rossman 2014). Only three of these have been reported from Brazil, namely, L. catervaria, L. melastomatum and L. miconiicola. All are distinct from L. abaxialis (Table 2).
Table 2.
Morphological characteristics of Lembosia spp. from Melastomataceae1.
| Taxon | Appressoria (μm) | Ascomata (μm) | Asci (μm) | Ascospores (μm) |
|---|---|---|---|---|
| Lembosia abaxialis2 | 7–10 × 10–10.5 | 340–550 × 160–250 | 52.5–57.5 × 25–37.5 | 25–29 × 12.5–15 |
| Lembosia catervaria | 6–8 diam | 500–700 × 70–100 | 40 × 70 | 30–38 × 15–19 |
| Lembosia domingensis | 5–6 × 7–9 | 300–800 × 150–250 | 40–52 × 28–35 | 25–33 × 11–15 |
| Lembosia gigantea | 12–17 × 9 | 784–1064 × 302–504 | 84–96 × 33–41 | 26–29 × 14 |
| Lembosia melastomacearum | 14 × 9 | 784 × 336 | 55–72 × 41–48 | 26–29 × 12 |
| Lembosia melastomatum | 6–8 diam | 700 × 250 | 70–96 × 42–52 | 35–40 × 16–20 |
| Lembosia memecyli | – | 200–450 × 120–150 | 35–55 × 26–35 | 20–23 × 8–10 |
| Lembosia memecylicola | 4–12 × 6–8 | 294–882 × 176–300 | up to 45 diam | 22–26 × 11–13 |
| Lembosia miconiae-prasinae | 7 wide | 470–860 × 313–448 | 69–84 × 33–43 | 24–29 × 12 |
| Lembosia miconiicola | – | 500–800 high | 22 × 11.5 | 23–28 × 11–13 |
| Lembosia rolliniae | 5–7 wide | 300–350 × 100 | 50–60 × 30 | 24–26 × 10–11 |
| Lembosia ryanii | 7–17 × 5 | 235–425 × 145–168 | 36–46 × 21–31 | 20–21 × 9–12 |
| Lembosia sclerolobii | – | up to 1000 × 140–180 | 35–50 × 30–40 | 17–23 × 6–9 |
Based on morphological characters, L. domingensis shows similarities with L. abaxialis, but differs by epiphyllous colonies, few, sparse, entire and conic appressoria, hysterothecia that are Y–X-shaped, with scarce, smaller asci, and slightly clavate ascospores (Petrak & Ciferri 1930). Additionally, L. catervaria differs from L. abaxialis by epiphyllous colonies, thicker hyphae, smaller appressoria, longer and narrower hysterothecia, wider asci and larger ascospores (Montagne 1855). Lembosia melastomatum differs from L. abaxialis by epiphyllous colonies, smaller appressoria, larger asci and ascospores (Montagne 1856). Finally, L. miconiicola differs from L. abaxialis, by epiphyllous colonies, larger hysterothecia and smaller asci (Arnaud 1918). Lembosia abaxialis is the first asterinaceous fungus reported on Miconia jucunda (Melastomataceae).
Prillieuxina baccharidincola (Rehm) Petr., Sydowia 4: 536. 1950. — Fig. 7
Fig. 7.
Prillieuxina baccharidincola VIC 42817. a. Living leaves of Baccharis sp. with epiphyllous colonies; b. SEM image; thyriothecium opened by a central star-shaped fissure; c. vertical section of the ascoma; d. asci ovoid to subclavate showing pseudoparaphyses; e. ascospores hyaline becoming pale brown to brown at maturity. — Scale bars = 20 μm.
Basionym. Lembosia drimydis var. baccharidincola Rehm, Ann. Mycol. 5: 532. 1907.
≡ Echidnodes baccharidincola (Rehm) Theiss. & Syd., Ann. Mycol. 15: 422. 1926.
Colonies epiphyllous, irregular to circular, solitary becoming confluent, black, 1–6.5 mm diam. External mycelium straight to flexuous, branching irregularly, septate, hyphal cells cylindrical, 3–4 μm diam, pale brown, smooth. Appressoria absent. Ascomata thyriothecioid, single to confluent, superficial, developed below external mycelium, circular to ellipsoid, 102–160 μm diam, dark brown to blackish, opening by a central star-shaped fissure. Asci bitunicate, ovoid to subclavate, 37.5–50 × 20–30 μm, 8-spored, hyaline. Ascospores cylindrical to oblong, straight, 15–22 × 9–11.5 μm, base and apex broadly rounded, 2-celled, constricted at the septum, brown, smooth. Asexual morph absent.
Type materials. BRAZIL, São Paulo, on living leaves of Baccharis sp., unknown date, A. Usteri 8 (Z+ZT, syntype, here designated lectotype MBT200871); São Paulo, on living leaves of Baccharis sp., 5 July 1907, Usteri 41 (Z+ZT, syntype); ibid., 24 July 1907, Usteri 5 (Z+ZT, syntype); Minas Gerais, Nova Lima, on living leaves of Baccharis sp., 18 July 2012, O.L. Pereira (VIC 42817, epitype designated here MBT200345).
Additional material examined. BRAZIL, Minas Gerais, Lavras Novas, on living leaves of Baccharis sp., 10 Sept. 2012, A.L. Firmino, VIC 42818.
Asterotexiales Firmino, O.L. Pereira & Crous, ord. nov. — MycoBank MB812001
Type family. Asterotexiaceae Firmino, O.L. Pereira & Crous, fam. nov.
Description as for the constituent family Asterotexiaceae (see below).
Notes — Representative sequences of the major orders in the Dothideomycetes support Asterotexiales as a separate entity (Fig. 1). Within Asterotexiales, two lineages can be defined, one that contains the Asterotexiaceae, and another that contains I. angularis, which is maintained as incertae sedis at the family level. The type species of Inocyclus needs to be recollected and its phylogenetic position resolved.
Asterotexiaceae Firmino, O.L. Pereira & Crous, fam. nov. — MycoBank MB812002
Type genus. Asterotexis Arx, Fungus 28: 6. 1958.
Type species. Asterotexis cucurbitacearum (Rehm) Arx (as ‘cucurbitarum’), Fungus 28: 6. 1958.
Foliar pathogens, asterinaceae-like, obligately biotrophic, colonies irregular to star-shaped, solitary to confluent, sometimes extending along the veins, dark brown to black. External mycelium growing through ascomatal cavity towards the host epidermis, connecting the neighbouring ascomata, septate, hyaline (unlike members of Asterinaceae), smooth. Appressoria formed underneath the ascomata, solitary or forming in small clusters, globose, cone-shaped, ovoid to elongate, brown, with a central, hyaline penetration peg. Ascomata superficial, scutellate, dimidiate, brown to blackish. Scutellum formed by radially arranged rows of cells, opening by numerous irregular fissures, smooth. Asci bitunicate, fissitunicate, clavate to cylindrical, 8-spored, hyaline, numerous, parallel, vertically oriented within ascomata. Ascospores ellipsoidal to slipper-shaped, unequally 2-celled, slightly constricted at the septum, upper cell subglobose, lower cell smaller, subcylindrical to subcuneate, hyaline to slightly yellowish (unlike members of the Asterinaceae), smooth. Asexual morph unknown.
Asterotexis cucurbitacearum (Rehm) Arx, Fungus 28: 6. 1958. — Fig. 8
Fig. 8.
Asterotexis cucurbitacearum VIC 42814. a, b. Symptoms on leaves of Cucurbita pepo: a. adaxial side; b. abaxial side, showing the hypophyllous colonies; c. external mycelium hyaline, connecting the ascomata in formation; d. immature ascomata in formation; e. fertile locules exposed on irregular fissures; f, g. vertical section of the ascomata, showing the appressoria with a central hyaline penetration peg, covered by the mature ascomata; h. vertical section of a fully developed ascoma, showing parallel and vertically orientated asci; i. asci; j. ascospores. — Scale bars: c–i = 10 μm; j = 5 μm.
Basionym. Dothidella cucurbitacearum Rehm, Hedwigia 36: 376. 1897.
≡ Rhagadolobium cucurbitacearum (Rehm) Theiss. & Syd., Ann. Mycol. 12: 275 .1914.
Colonies hypophyllous, irregular to star-shaped, solitary to confluent, sometimes extending along the veins, dark brown to black, 1–3 mm. External mycelium growing through ascomatal cavity towards the host epidermis, connecting the neighbouring ascomata, 3–4 μm diam, hyaline, septate, smooth. Appressoria formed underneath the ascomata, solitary or forming small groups, globose, cone-shaped, ovoid to elongate, 8–10 × 5–7 μm, brown, with a hyaline central penetration peg. Ascomata superficial, solitary to confluent, sometimes growing to surround the basis of individual trichomes of the host, scutellate, dimidiate, circular to irregular, 1–3 mm diam, upper cells irregularly shaped and thin-walled, brown to black. Scutellum formed by radially arranged rows of cells, opening by numerous irregular fissures, pale brown, smooth. Asci bitunicate, fissitunicate, clavate to cylindrical, 40–45 × 9.5–12.5 μm, 8-spored, numerous, parallel, vertically orientated within ascomata, hyaline, smooth. Ascospores ellipsoidal to slipper-shaped, 10–14 × 4–5 μm, unequally 2-celled, slightly constricted at the septum, upper cell subglobose, lower cell smaller, subcylindrical to subcuneate, hyaline to slightly yellowish, smooth. Asexual morph unknown.
Type materials. BRAZIL, Blumenau, on living leaves of Cucurbita pepo, May 1887, E. Ule 1415 (S F47805 syntype, here designated lectotype MBT200872); Rio de Janeiro, on living leaves of Cucurbita pepo, May 1887, E. Ule 676 (S F7565, syntype); Bahia, Igrapiúna, Reserva Ecológica Michelin, on living leaves of Cucurbita pepo, 15 July 2010, O.L. Pereira & A.L. Firmino, S13°49’17.90" W39°10’16.31" (VIC 42814, epitype designated here MBT200349).
Notes — Asterotexis cucurbitacearum has been recorded on living leaves of Cayaponia americana in the Dominican Republic and West Indies; on Cucurbita moschata in Venezuela and West Indies; on Cucurbita pepo in Brazil, Panama, Trinidad & Tobago and West Indies; on Cucurbita sp. in Brazil and Grenada; on Gurania sp. in the Dominican Republic; on Trichosanthes sp. in the Dominican Republic and on Sechium edule in Costa Rica; Asterotexis quercina has been recorded on Quercus glauca in Nepal (Guerrero et al. 2011, Farr & Rossman 2014).
INCERTAE SEDIS
Inocyclus angularis Guatimosim & R.W. Barreto, IMA Fungus 5: 52. 2014. — MB805976
Description and illustrations — Guatimosim et al. (2014b).
Materials examined. BRAZIL, Rio de Janeiro, Nova Friburgo, Mury, Sítio Colonial, on living leaves of Pleopeltis astrolepis, 30 Mar. 2013, R.W. Barreto (VIC 39747, holotype; CBS H-22028, isotype); ibid., 8 June 2013, R.W. Barreto VIC 39748, CBS H-22029; Rio de Janeiro, Nova Friburgo, Riograndina, Fazenda Barreto, on living leaves of P. astrolepis, 9 June 2013, R.W. Barreto VIC 39749, CBS H-22030.
Notes — Although I. angularis is not the type species of the genus Inocyclus, it is presently the only species from which DNA is available. A fresh collection of the type species, I. psychotriae, is required to clarify the correct placement of this genus.
DISCUSSION
The order Asterinales was included within Dothideomycetes based on the SSU and LSU analyses of five species of Asterina and a related asexual morph (Hofmann et al. 2010). In recent years, Asterinales was thought to comprise the families Asterinaceae, Parmulariaceae and Aulographaceae (Wu et al. 2011, Hyde et al. 2013). Recently, Hongsanan et al. (2014) provided a reassessment of the order. Based on LSU maximum likelihood and Bayesian analysis, and, despite the absence of molecular data for the Parmulariaceae, the authors concluded that only Asterinaceae should be included within Asterinales.
In the present study, we provide a robust molecular dataset that includes the type species of Asterina, as well as three other genera of Asterinaceae, the type species of the Parmulariaceae and a genus formerly assigned to the Parmulariaceae. The resulting LSU rDNA tree (Fig. 1) agrees in general with recent multigene analysis of the Dothideomycetes (Schoch et al. 2009) and demonstrated that the Asterinales comprises both Asterinaceae and Parmulariaceae as proposed by Barr & Huhndorf (2001), clustering with Phaeotrichiaceae and Venturiaceae.
A second analysis (available in TreeBASE), was done aiming at verifying if the former molecular studies involving species of Asterina and Lembosia (Hofmann et al. 2010, Hongsanan et al. 2014) correctly assigned the taxa included to the Asterinaceae. Based on these studies we conclude that these taxa, although considered by the authors as representative of species in the Asterinaceae, are in fact misplaced, and should be treated as incertae sedis, since they do not group with A. melastomatis – the type species of this family. The Asterinaceae, including the genera Asterina, Batistinula, Lembosia and Prillieuxina may, therefore, be polyphyletic, requiring a thorough reassessment. Nevertheless, it is important to note that all studies performed until now (Hofmann et al. 2010, Hongsanan et al. 2014), used relatively short LSU sequences (c. 490 bp) that may not provide the necessary resolution needed.
Asterotexis cucurbitacearum was initially classified in the Parmulariaceae (Theissen & Sydow 1914) and then transferred to Asterinaceae (Inácio & Cannon 2008, Kirk et al. 2008, Guerrero et al. 2011). This species is clearly not a member of the Asterinaceae (contradictory to what was shown by Hongsanan et al. 2014) and is transferred here to the newly proposed family Asterotexiaceae. This new family grouped (Fig. 1) with Inocylus angularis (originally described as a member of the Parmulariaceae).
Nuclear DNA of P. styracis, the type species of the Parmulariaceae was isolated and studied for the first time here. DNA was successfully isolated from I. angularis, allowing a preliminary assessment of the Parmulariaceae. Although involving only two taxa, the finding that I. angularis does not group with the type of Parmulariaceae, confirm that the Parmulariaceae is polyphyletic (Inácio & Cannon 2008, Hongsanan et al. 2014). The status of I. angularis within the genus Inocyclus requires confirmation, ideally with a molecular assessment of the type species of Inocyclus.
The molecular phylogenetic analysis presented here clearly indicates that both the Parmulariaceae and Asterinaceae are polyphyletic. Only the epitypification of the taxa in these and other families of thyriothecioid ascomycetes, followed by molecular phylogenetic analysis will resolve their taxonomic placement and produce a more natural classification for these neglected tropical fungi.
Acknowledgments
We would like to thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional do Desenvolvimento Científico e Tecnológico (CNPq) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support. Electron microscopy studies were performed at the Núcelo de Microscopia e Microanálise da Universidade Federal de Viçosa (NMM-UFV). O.L. Pereira wishes to thank the administration and scientific staff of Reserva Ecológica Michelin, Reserva Natural Vale and Estação Ecológica de Águas Emendadas for providing facilities and permits for the exploratory surveys of the mycodiversity on their protected areas.
REFERENCES
- Arnaud G. 1918. Les Astérinées. Annales de l’École Nationale d’Agriculture de Montpellier 16: 1–288. [Google Scholar]
- Arx JA von, Müller E. 1975. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Studies in Mycology 9: 1–159. [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, et al. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. 1979. A classification of Loculoascomycetes. Mycologia 71: 935–957. [Google Scholar]
- Barr ME, Huhndorf SM. 2001 Loculoascomycetes. In: McLaughlin DJ, McLaughlin EG, Lemke PA. (eds), The Mycota VII, Part A. Systematics and evolution: 28–305. Springer Verlag, Germany. [Google Scholar]
- Bezerra JL. 2004. Taxonomia de Ascomicetos. Ordem Asterinales. Revisão Anual de Patologia de Plantas 11: 15–28. [Google Scholar]
- Carbone I, Kohn LM. 1999 A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Eriksson O. 1981 The families of bitunicate ascomycetes. Nordic Journal of Botany 1: 1–800. [Google Scholar]
- Farr D, Rossman A. 2014 Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved 6 Nov. 2014; http://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Frank J, Crous PW, Groenewald JZ, et al. 2010. Microcyclospora and Microcyclosporella: novel genera accommodating epiphytic fungi causing sooty blotch on apple. Persoonia 24: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Groenewald J, Nakashima C, Nishikawa J, et al. 2013. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology 75: 115–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guatimosim E, Pinto HJ, Barreto RW, et al. 2014a Rhagadolobiopsis, a new genus of Parmulariaceae from Brazil with a description of the ontogeny of its ascomata. Mycologia 106: 276–281. [DOI] [PubMed] [Google Scholar]
- Guatimosim E, Schwartsburd PB, Barreto RW. 2014b A new Inocyclus species (Parmulariaceae) on the neotropical fern Pleopeltis astrolepis. IMA Fungus 4: 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero Y, Hofmann TA, Williams C, et al. 2011. Asterotexis cucurbitacearum, a poorly known pathogen of Cucurbitaceae new to Costa Rica, Grenada and Panama. Mycology 2: 87–90. [Google Scholar]
- Hansford CG. 1946 The foliicolous ascomycetes, their parasites and associated fungi: especially as illustrated by Uganda specimens. Mycological Papers 15: 1–240. [Google Scholar]
- Hennings P. 1904. Fungi Amazonici a cl. Ernesto Ule collecti II. Hedwigia 43: 242–273. [Google Scholar]
- Hofmann TA, Kirschner R, Piepenbring M. 2010 Phylogenetic relationships and new records of Asterinaceae (Dothideomycetes) from Panama. Fungal Diversity 43: 39–53. [Google Scholar]
- Hofmann TA, Piepenbring M. 2011 Biodiversity of Asterina species on Neotropical host plants: new species and records from Panama. Mycologia 103: 1284–1301. [DOI] [PubMed] [Google Scholar]
- Hongsanan S, Li Y-M, Liu J-K, et al. 2014 Revision of genera in Asterinales. Fungal Diversity 68: 1–68. [Google Scholar]
- Hosagoudar VB. 2012. Asterinales of India. Mycosphere 2: 617–852. [Google Scholar]
- Hosagoudar VB, Appaiah KAA. 2005 Foliar fungi of Western Ghats found on the plants of sacred groves in Dakshina Kannada and Udupi districts of Karnataka. Journal of Mycopathological Research 43: 167–174. [Google Scholar]
- Hyde KD, Jones EG, Liu J-K, et al. 2013 Families of Dothideomycetes. Fungal Diversity 63: 1–313. [Google Scholar]
- Inácio CA, Araúz K, Piepenbring M. 2012a A new genus of Parmulariaceae from Panama. Mycological Progress 11: 1–6. [Google Scholar]
- Inácio CA, Cannon PF. 2008. The genera of the Parmulariaceae. CBS Biodiversity Series No 8. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Inácio CA, Pereira-Carvalho RC, Souza ESC, et al. 2012b A new Hysterostomella species from the Cerrado in Brasília National Park. Mycotaxon 119: 307–313. [Google Scholar]
- Katoh K, Misawa K, Kuma K, et al. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. 2008 Ainsworth & Bisby’s Dictionary of the Fungi. 10th ed CABI, UK. [Google Scholar]
- Léveillé JH. 1845 Champignons exotiques. Annales des Sciences Naturelles Botanique 3: 38–71. [Google Scholar]
- Lumbsch HT, Huhndorf SM. 2010 Notes on Ascomycete Systematics. Fieldiana Life and Earth Sciences 1: 1–64. [Google Scholar]
- Montagne J. 1855. Cryptogamia Guyanensis seu plantarum cellularium in Guyana gallica annis 1835–1849 a cl. Leprieur collectarum enumeration universalis. Annales des Sciences Naturelles Botanique 3: 91–144. [Google Scholar]
- Montagne J. 1856. Septième centurie de plantes cellulaires nouvelles, tant indigènes qu’exotiques. Annales des Sciences Naturelles Botanique 5: 333–374. [Google Scholar]
- Müller E, Arx JA von. 1962. Die gattungen der didymosporen Pyrenomyceten. Kommissionsverlag Buchdruckerei Büchler & Co., Switzerland. [Google Scholar]
- Nylander J. 2004 MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University; 2. [Google Scholar]
- O’Donnell K, Kistler HC, Cigelnik E, et al. 1998. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America 95: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak F, Ciferri R. 1930 Fungi Dominicani. Annales Mycologici 28: 377–420. [Google Scholar]
- Petrak F, Sydow H. 1931. Micromycetes philippinenses. Series secunda. Annales Mycologici 29: 145–279. [Google Scholar]
- Pinho DB, Firmino AL, Ferreira-Junior WG, et al. 2012 An efficient protocol for DNA extraction from Meliolales and the description of Meliola centellae sp. nov. Mycotaxon 122: 333–345. [Google Scholar]
- Pinho DB, Honorato J, Junior, Firmino AL, et al. 2014. Reappraisal of the black mildews (Meliolales) on Hevea brasiliensis. Tropical Plant Pathology 39: 89–94. [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, et al. 2013. Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Mark P van der, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, et al. 2007. Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Boissin E, Phillips AJL, et al. 2013. Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Studies in Mycology 76: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Hosagoudar V. 2003. A list of Lembosia species based on the literature. Guizhou Science 21: 93–101. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA 6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen F.1912Fragmenta Brasilica. IV nebst Bemerkungen über einige andere Asterina-Arten. Annales Mycologici 10: 1–32. [Google Scholar]
- Theissen F.1913aDie Gattung Asterina. Abhandlungen der kaiserlich-koniglichen zoologisch-botanisch Gesellschaft in Wien 7: 1–130. [Google Scholar]
- Theissen F.1913bLembosia-Studien. Annales Mycologici 11: 425–467. [Google Scholar]
- Theissen F.1913cÜber Membranstructuren bei den Microthyriaceen als Grundlage für den Ausbau der Hemisphaeriales. Mycologisches Centralblatt 3: 273–286. [Google Scholar]
- Theissen F, Sydow H.1914. Dothideazeen-studien: I–II. Annales Mycologici 12: 268–281. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, San Diego, California, USA. [Google Scholar]
- Wu HX, Schoch CL, Boonmee S, et al. 2011. A reappraisal of Microthyriaceae. Fungal Diversity 51: 189–248. [DOI] [PMC free article] [PubMed] [Google Scholar]