Abstract
This review will provide a comprehensive overview of the interactions between dietary isoflavones and the ATP-binding cassette (ABC) G2 efflux transporter, which is also named the breast cancer resistance protein (BCRP). Expressed in a variety of organs including the liver, kidneys, intestine, and placenta, BCRP mediates the disposition and excretion of numerous endogenous chemicals and xenobiotics. Isoflavones are a class of naturally-occurring compounds that are found at high concentrations in commonly consumed foods and dietary supplements. A number of isoflavones, including genistein and daidzein and their metabolites, interact with BCRP as substrates, inhibitors, and/or modulators of gene expression. To date, a variety of model systems have been employed to study the ability of isoflavones to serve as substrates and inhibitors of BCRP; these include whole cells, inverted plasma membrane vesicles, in situ organ perfusion, as well as in vivo rodent and sheep models. Evidence suggests that BCRP plays a role in mediating the disposition of isoflavones and in particular, their conjugated forms. Furthermore, as inhibitors, these compounds may aid in reversing multidrug resistance and sensitizing cancer cells to chemotherapeutic drugs. This review will also highlight the consequences of altered BCRP expression and/or function on the pharmacokinetics and toxicity of chemicals following isoflavone exposure.
Keywords: BCRP, isoflavones, genistein, daidzein, biochanin A, phase II metabolites
Introduction
Isoflavones
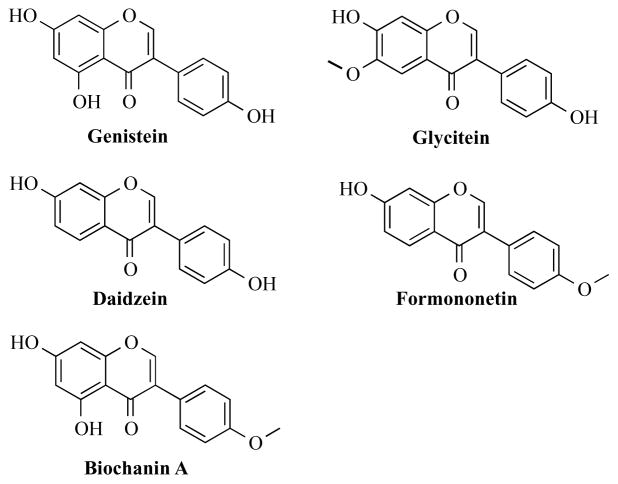
Isoflavones are secondary metabolites that occur naturally in plants of the Leguminosae family and include soybeans, red clover, peanuts, chickpeas, and alfalfa [1]. Isoflavones attract bacteria to the roots to aid in nodulation and nitrogen fixation [2]. Collectively, isoflavones represent a subset of the larger class of chemicals, flavonoids, which are defined by a polyphenolic three ring structure (A, B, and C) (Figure 1A). The location of the B ring at position C3 rather than C2 separates the isoflavones from the remaining flavonoids (Figure 1B). This review article will focus on the most commonly studied isoflavones: genistein, daidzein, biochanin A, glycitein, and formononetin (Figure 2).
Figure 1.
The parent ring structures of A) flavonoids and B) isoflavones.
Figure 2.
Chemical structures of the most commonly studied isoflavones: genistein, daidzein, biochanin A, glycitein, and formononetin.
The most abundant source of isoflavones is soybeans, which contain primarily genistein (approximately 2.3 mg/g), daidzein, and glycitein [1], in addition to trace amounts of formononetin and biochanin A [3]. It is important to note that soybeans are used in the production of numerous manufactured dietary products including tofu, tempeh, soy infant formula, miso, soybean oil, cereal, and bacon bits [1]. Red clover is another naturally abundant source of isoflavones containing primarily formononetin, in addition to biochanin A, daidzein, and genistein [3]. Within plants, isoflavones are present as two different forms: 1) glycoside (Figure 3) (i.e., genistin or genistein-7-O-β-D-glucoside) and 2) aglycone (i.e., genistein) (reviewed in [4]). Glycosides occur at greater concentrations than aglycones in soybeans and other plants [5]; however fermented foods (i.e., miso, tempeh) contain a greater proportion of aglycones compared to unfermented soy due to the hydrolysis of the glycosides to aglycones by microbial β-glucosidases during the fermentation process (reviewed in [4]).
Figure 3.
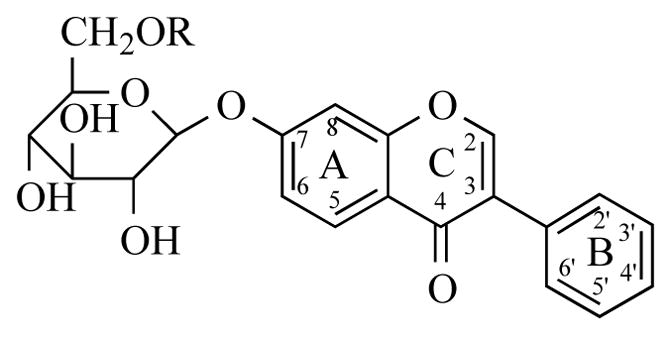
Basic chemical structure of an isoflavone-glycoside.
Following ingestion, isoflavone glycosides are converted to their aglycone form by epithelial and microbial β-glucosidases in the oral cavity [6] and small intestines [7]. This is important because it is primarily the aglycone form of isoflavones that pass across the intestinal epithelium [8–10] and possess biological activity [11, 12]. Approximately 70–90% of isoflavones are metabolized to glucuronide and sulfate conjugates by UDP-glucuronosyltransferases (UGTs; UGT1A1, 1A8, 1A9, 1A10) and sulfotransferases (SULTs; SULT1A1*2, 1E, 2E1), respectively, in the intestines and liver [13, 14]. Conjugation occurs primarily at the C7 and C4′ positions on the parent ring system (Figure 1B), to form monoconjugates (i.e., genistein-7-glucuronide, G-7-G; genistein-4′-glucuronide, G-4′-G; genistein-7-sulfate, G-7-S; genistein-4′-sulfate, G-4′-S) or diconjugates (i.e., genistein-7-glucuronide-4′-glucuronide; genistein-7-sulfate-4′-sulfate; genistein-7-glucuronide-4′-sulfate; genistein-7-sulfate-4′-glucuronide) [15]. The phase II metabolites are excreted into the intestinal lumen and bile duct and subsequently eliminated in the feces or deconjugated by gut microflora which results in reabsorption and enterohepatic recirculation of the isoflavones [16]. Though it is not the primary pathway of isoflavone metabolism, phase I oxidative metabolites of genistein, daidzein [17], and formononetin [18] have been identified in the urine of healthy volunteers. Confirmed using human liver microsomes, the cytochrome P450 enzymes are responsible for the oxidation of isoflavones in the liver [17, 18]. Additional phase I metabolism of these chemicals include oxidative demethylation, particularly for biochanin A and formononetin. Incubation of biochanin A and formononetin with human liver microsomes resulted in the formation of genistein and daidzein, respectively [18]. Another example of phase I metabolism of isoflavones is the formation of equol from daidzein by intestinal bacteria [19]. Equol is more effective in reducing prostate cancer growth in vitro than daidzein [20] and its presence in urine has been associated with a decreased risk of breast cancer [21]. Evidence suggests that approximately 30–50% of the population can produce equol from daidzein [22, 23]. This interindividual heterogeneity in the conversion of daidzein to equol may be a potential contributor to the inconclusive findings in clinical trials that examine the effects of a soy diet on various disease states.
Genistein is the most frequently studied isoflavone due to its presence in diverse dietary sources and an increasing number of reports that suggest genistein and other isoflavones have biological activity that may modulate disease progression [24–27] (reviewed in [28]). Specifically, there is evidence that genistein and other isoflavones possess cancer preventative and/or therapeutic activity, and can improve cardiovascular, bone and post-menopausal health [25, 29–34]. Much of this research has been supported by epidemiological findings that demonstrate health benefits conferred to Asian populations that consume a soy rich diet as compared to populations that consume a western diet [26, 27]. Nevertheless, previous studies also suggest that genistein and other isoflavones may be adverse to human health by promoting cancer progression and/or interfering with reproductive development early in life [35–38]. Additional work is needed to address the controversy as to whether these natural compounds have a negative impact on the health of humans clinically.
Many of the biological responses produced by isoflavones are believed to be mediated by cellular proteins including the estrogen receptor and tyrosine kinase receptors. In fact, due to the early discovery of isoflavones as estrogen receptor agonists [39] and modulators of reproductive function [40], these compounds have been termed phytoestrogens. The phenolic ring structure of isoflavones, allow the compounds to bind to estrogen receptors [39] and initiate transcription of estrogen receptor-responsive genes. Typically, isoflavones bind with greater affinity to the estrogen receptor β than estrogen receptor α [41]. Moreover, in 1987, Akiyama et al. reported that isoflavones can inhibit tyrosine kinase enzymes in A431 epidermoid cancer cells, including the epidermal growth factor receptor (genistein > prunetin >biochanin A >daidzein=genistin) [12]. Since then, a number of reports have confirmed these findings in vitro as well as in vivo [24, 42–45] and suggest that by targeting these molecular pathways often involved in cancer, isoflavones may possibly be combined with chemotherapeutic regimens to improve the efficacy of cancer treatment.
In addition to estrogen and tyrosine kinase receptors, isoflavones also interact with the breast cancer resistance protein (BCRP) efflux transporter, which is involved in the disposition of xenobiotics and endogenous compounds. These compounds are substrates of BCRP as well as inhibitors of its function. Further, many of the isoflavones modulate chemoresistance and in vivo pharmacokinetics of other BCRP substrates that are administered concomitantly.
BCRP
BCRP is a member of the ATP-binding cassette (ABC) superfamily of transmembrane transporters. Localized to the apical plasma membrane of cells, BCRP actively transports xenobiotic and endogenous substrates out of the cell with energy derived from the hydrolysis of ATP. In the 1990’s, BCRP was discovered in multiple laboratories using cell lines that conferred resistance to mitoxantrone, and other xenobiotics, and that lacked the previously characterized multidrug resistance proteins, multidrug resistance protein 1 (MDR1) and the multidrug resistance-associated protein 1 (MRP1) [46–48]. The discovery of the new transporter in a number of models including a breast cancer cell line (MCF-7/AdrVp) [46], human placenta [47], and mitoxantrone-selected human colon carcinoma cells (S1-M1-80) [48], resulted in the concurrent publication of multiple names for the same transporter: BCRP, “ABC transporter highly expressed in the placenta” (ABCP), and the “mitoxantrone resistance (MXR) transporter”. Subsequently, the human gene nomenclature committee officially designated the transcript as the second member of the G subfamily of the ABC superfamily of transporters, ABCG2 [49].
Since its discovery in cancer cells, BCRP has been found to play an important role in mediating the disposition of substrates in normal human tissues thereby preventing chemical accumulation and subsequent toxicity [50]. BCRP is expressed in the epithelium of tissues such as the intestines, liver, and kidney, which are involved in the pharmacokinetics of chemicals. Localization of BCRP to the apical or luminal surface favors active transport of substrates into the intestinal lumen, bile duct, and proximal tubule lumen, respectively [50]. In addition to playing a role in excretion, BCRP aids in the formation of blood-organ barriers as it is expressed in syncytiotrophoblasts of the placenta, capillary endothelial cells of the blood-brain barrier, and luminal capillary endothelial cells of the blood-testis barrier [50–52]. BCRP mRNA and protein are expressed most abundantly in the human placenta, earning its name as “the placental transporter” [47].
In vitro, in vivo, and in situ model systems have been employed to detect BCRP substrates and inhibitors including several drugs, dietary components, and endogenous compounds (Table 1). While previous review articles have summarized the interactions of flavonoids with ABC transporters [53, 54], this review article provides an up-to-date summary of the evidence for the interaction of isoflavones specifically with BCRP. This includes a discussion of isoflavones as BCRP substrates (Table 2, 3, 4), inhibitors of BCRP transport activity (Table 5), and regulators of BCRP expression (Table 6) with commentary on the implications of these interactions.
Table 1.
Substrates and Inhibitors of BCRP/Bcrp.
| Substrates | References |
|---|---|
| Carcinogens | |
| Aflatoxin B1 | [55] |
| 2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) | [56] |
| Chemotherapy drugs | |
| Etoposide | [57] |
| Imatinib | [58] |
| Mitoxantrone | [46–48] |
| Methotrexate | [59] |
| Topotecan | [60] |
| SN-38 | [61] |
| Endogenous compounds | |
| Estrone-3-sulfate | [62] |
| Folic acid | [63] |
| Protoporphyrin IX | [64] |
| Uric acid | [65] |
| Flavonoids | |
| Biochanin A-glucuronide | [66] |
| Biochanin A-sulfate | [67] |
| Daidzein | [68, 69] |
| Daidzein-sulfate | [70] |
| Genistein | [69, 71] |
| Genistein-glucuronide | [70, 72, 73] |
| Genistein-sulfate | [70, 72, 73] |
| Quercetin | [74] |
| Fluorescent dyes | |
| BODIPY-Prazosin | [75] |
| Hoechst 33342 | [76] |
| Pheophorbide a | [60, 77] |
| Rhodamine 123 | [75] |
| Other pharmaceutical drugs | |
| Cimetidine | [56] |
| Ciprofloxacin | [78] |
| Danofloxacin | [79] |
| Enrofloxacin | [80] |
| Glyburide | [81] |
| Nitrofurantoin | [82] |
| Prazosin | [83] |
| Inhibitors | |
| Chemotherapy drugs | |
| Gefitinib | [84] |
| Imatinib | [85] |
| Fungal toxins | |
| FTC | [48] |
| Ko143 | [86] |
| Flavonoids | |
| Biochanin A | [87, 88] |
| Daidzein | [87, 89] |
| Formonetin | [90] |
| Genistein | [87, 89, 90] |
| Glycitein | [90] |
| Endogenous compounds | |
| 17β-estradiol | [91] |
| Estrone | [91] |
| Pharmaceutical drugs | |
| Cyclosporin A | [92] |
| Omeprazole | [93] |
| Other | |
| GF120918 | [94] |
Table 2.
Summary of in vitro and in vivo experiments describing genistein as a substrate of BCRP/Bcrp.
| Experiment Type | Model | Genistein Concentration | Detection Method | Time | BCRP Inhibitor | References |
|---|---|---|---|---|---|---|
| In Vitro | ||||||
|
| ||||||
| Cellular uptake | K562/BCRP cells | 30 nM (3H) | LSC | 4 h | - | [71] |
| Trancellular transport | LLC-PK1/BCRP cells | 30 nM (3H) | LSC | 4 h | 3 μM FTC | [71] |
| MDCK/BCRP cells | 3 μM | LC/MS | 4 h | - | [69] | |
| Caco-2 cells | 100 nM (14C) | LSC | 1 h | 1 μM FTC | [95] | |
| Caco-2 cells | 50 μM | HPLC-ECD | 50 min | Estrone-3-sulfate | [96] | |
| MDCK/Bcrp | 3 μM | LC/MS | 4 h | - | [69] | |
| MDCK/Bcrp | 1–3 μM | LC/MS | 3 h | - | [97] | |
|
| ||||||
| In Vivo Pharmacokinetics | ||||||
|
| ||||||
| Oral (p.o.) | ||||||
|
| ||||||
| Plasma | FVB Bcrp −/− mice | 8.1 mg/kg | LC/MS | 4 h | - | [69] |
| FVB Bcrp −/− mice | 2–20 mg/kg | UPLC/MS/MS | 24 h | - | [73] | |
|
| ||||||
| Intravenous (i.v.) infusion | ||||||
|
| ||||||
| Brain | FVB Bcrp −/− mice | 1.4 mg/kg/h | LC/MS | 2 h | - | [69] |
| FVB Bcrp −/− mice | 0.8 mg/kg initial, 4.3 mg/kg/h | LC-MS/MS | 2 h | - | [97] | |
| Testis | FVB Bcrp −/− mice | 1.4 mg/kg/h | LC/MS | 2 h | - | [69] |
| Epididymis | FVB Bcrp −/− mice | 1.4 mg/kg/h | LC/MS | 2 h | - | [69] |
| Placenta | Pregnant FVB Bcrp −/− mice | 1.4 mg/kg/h | LC/MS | 2 h | - | [69] |
| Fetal Brain | Pregnant FVB Bcrp −/− mice | 1.4 mg/kg/h | LC/MS | 2 h | - | [69] |
Table 3.
Summary of in vitro, in situ, and in vivo experiments describing genistein-sulfate as a substrate of BCRP/Bcrp.
| Experiment type | Model | Genistein concentration | Detection Method | Time | BCRP Inhibitor | References |
|---|---|---|---|---|---|---|
| In Vitro | ||||||
|
| ||||||
| Transcellular transport | Caco-2 | 2–10 μM | UPLC-MS/MS | 4 h | 5 μM Ko143 | [73] |
|
| ||||||
| Vesicle uptake | Sf9-BCRP vesicles | Chow (Amount unknown; mouse urine) | HPLC/MS | 40 s | - | [98] |
| Sf9-BCRP vesicles | 35S-genistein-sulfate | LSC | 40 s | - | [98] | |
|
| ||||||
| In Situ Intestinal Perfusion | ||||||
|
| ||||||
| SI and colon excretion | FVB Bcrp −/− mice | 10 μM | UPLC-DAD-MS/MS | 120 min | - | [72] |
| Biliary excretion | FVB Bcrp −/− mice | 10 μM | UPLC-MS/MS | 2.5 h | - | [73] |
|
| ||||||
| In Vivo Pharmacokinetics | ||||||
|
| ||||||
| Oral (p.o.) | ||||||
|
| ||||||
| Plasma | FVB Bcrp −/− mice | 50 mg/kg | HPLC-DAD-MS/MS | 3 h | - | [70] |
| FVB Bcrp −/− mice | 2–20 mg/kg | UPLC-MS/MS | 24 h | - | [73] | |
| FVB Bcrp −/− mice | Chow (Amount unknown) | HPLC/MS | - | - | [98] | |
|
| ||||||
| Intraperitoneal (i.p.) | ||||||
|
| ||||||
| Plasma | FVB Bcrp −/− mice | 20 mg/kg | UPLC/MS/MS | 24 h | - | [73] |
Table 4.
Summary of in vitro, in situ, and in vivo experiments describing genistein-glucuronide as a substrate of BCRP/Bcrp.
| Experiment type | Model | Genistein concentration | Detection Method | Time | BCRP Inhibitor | References |
|---|---|---|---|---|---|---|
| In Vitro | ||||||
|
| ||||||
| Cellular uptake | HeLa/UGT1A9 cells | 10 μM | UPLC-MS/MS | 120 min | 5–10 μM Ko143 | [99] |
|
| ||||||
| Transcellular transport | Caco-2 | 2–10 μM | UPLC-MS/MS | 4 h | 5 μM Ko143 | [73] |
|
| ||||||
| In situ Intestinal perfusion | ||||||
|
| ||||||
| SI Excretion | FVB Bcrp −/− mice | 10 μM | UPLC-DAD-MS/MS | 120 min | - | [72] |
|
| ||||||
| In Vivo Pharmacokinetics | ||||||
|
| ||||||
| Oral (p.o.) | ||||||
|
| ||||||
| Plasma | FVB Bcrp −/− mice | 50 mg/kg | HPLC-DAD-MS/MS | 3 h | - | [70] |
| FVB Bcrp −/− mice | 2–20 mg/kg | UPLC/MS/MS | 24 h | - | [73] | |
|
| ||||||
| Intraperitoneal (i.p.) | ||||||
|
| ||||||
| Plasma | FVB Bcrp −/− mice | 20 mg/kg | UPLC/MS/MS | 24 h | - | [73] |
Table 5.
Summary of in vitro, in situ, and in vivo experiments describing genistein as an inhibitor of BCRP/Bcrp function.
| Experiment type | Model | Genistein concentration | Detection method | Time | BCRP Substrate | References |
|---|---|---|---|---|---|---|
| In Vitro | ||||||
|
| ||||||
| Chemo-resistance | MCF-7/MX100 cells | 10–50 μM | Sulforhodamine B assay | 48 h | 0–1000 μM Mitoxantrone | [87, 100] |
| K562/BCRP cells | 3 μM | Cell counter | 5 days | SN-38 | [71] | |
| K562/BCRP cells | NR | Cell counter | 5 days | SN-38 | [101] | |
|
| ||||||
| Cellular uptake | MCF-7/MX100 cells | 50 μM | Flow cytometry | 30 min | 3 μM Mitoxantrone | [87, 100] |
| NCI-H460/MX20 cells | 50 μM | Flow cytometry | 30 min | 3 μM Mitoxantrone | [87] | |
|
| ||||||
| Vesicle uptake | Sf9-BCRP vesicles | 10–100 μM | LSC | 2 min | 100 μM 3H-Mitoxantrone | [90] |
|
| ||||||
| ATPase activity | Lactating cow mammary gland plasma membranes | 10 μM | Colorimetric detection of Pi | 40 min | 50 μM Mitoxantrone | [80] |
|
| ||||||
| In Vivo | ||||||
|
| ||||||
| Oral (p.o.) | ||||||
|
| ||||||
| Plasma | Lactating sheep | 0.8 mg/kg | HPLC | 24 h | Enrofloxacin | [80] |
Table 6.
Summary of experiments describing the regulation of BCRP by genistein.
Evidence for Isoflavones as Substrates of BCRP
In Vitro
Transport of Genistein
Genistein was first described as a substrate for BCRP-mediated transport in 2004 (Table 2). Imai et al. demonstrated a significant decrease in the accumulation of 3H-genistein in human K562 myelogenous leukemia cells that overexpressed the human BCRP/ABCG2 gene (K562/BCRP cells) compared to the parent cells that lacked BCRP (K562 cells) [71]. In addition, the transcellular transport of genistein was assessed using pig kidney epithelial cells (LLC-PK1 cells) that overexpressed the human BCRP/ABCG2 gene (LLC/BCRP cells). When grown in monolayers, BCRP protein localizes to the apical membrane of LLC-PK1 cells [62, 105]. For these experiments, cells are grown on a thin filter in between two chambers that recapitulate the polarization of cells (apical and basolateral) typically seen in vivo. These studies revealed that genistein is selectively transported by BCRP in the basolateral-to-apical (BL-to-AP) direction as compared to the apical-to-basolateral (AP-to-BL) direction [71]. The preference for BL-to-AP transport was abolished by the BCRP specific inhibitor, fumitremorgin C (FTC, 3 μM), further confirming a critical role for BCRP in the transcellular transfer of genistein [71]. Since this initial report, a number of studies have characterized the bidirectional transport of genistein in other BCRP-overexpressing (i.e., Madin-Darby canine kidney II; MDCK/BCRP) and endogenously expressing (i.e., Caco-2) cell lines [69, 95, 96]. Similar to the LLC/BCRP cells, the BL-to-AP transport of genistein (as quantified by LC/MS) was favored in the MDCK/BCRP cells with a efflux ratio that was 2-fold higher in the overexpressing cells as compared to the empty vector cells that lacked BCRP protein [69]. In such bidirectional permeability assays, an efflux ratio ≥ 2 indicates active efflux in the BL-to-AP direction as the value is calculated by dividing the apparent permeability (Papp) of a compound in the BL-to-AP direction by the Papp of the compound in the AP-to-BL direction (Papp BL-AP/Papp AP-BL) [95, 106]. The human colorectal adenocarcinoma cell line, Caco-2 is an in vitro model of intestinal transport. In culture, Caco-2 cells form monolayers and the BCRP protein localizes to the apical, or microvillous membrane, similar to its known trafficking in tissues [107–109]. In Caco-2 monolayers, the BCRP specific inhibitor, FTC (1 μM) decreased the efflux ratio of genistein by 3-fold, confirming that genistein is actively transported by BCRP in the BL-to-AP direction [95]. BCRP-mediated transport of genistein in Caco-2 cells was confirmed in another study which demonstrated that the BCRP inhibitor, estrone-3-sulfate increased the permeability of genistein by 40% in these cells [96]..
Additional studies indicate that genistein is also transported by the mouse isoform of the Bcrp protein overexpressed in MDCK/Bcrp cells as the efflux ratio was approximately 2-fold higher than the cells harboring the control expression vector in two studies [69, 97]. The similar ability of the mouse and human Bcrp/BCRP proteins to transport genistein in vitro, suggests that the mouse is an appropriate model for studying the in vivo transport of genistein by Bcrp/BCRP. This is in line with other compounds, such as cimetidine and aflatoxin B1, which are transported by both the human and mouse BCRP/Bcrp orthologs [55, 56].
Transport of Daidzein
Similar to genistein, daidzein was first suggested to be a substrate of BCRP in 2004 using the ATPase assay [68]. Briefly, the ATPase assay uses plasma membranes isolated from cells or tissues that express BCRP and detects the ability of a compound to stimulate ATP hydrolysis as an indirect measure of substrate transport. In membranes isolated from the bacteria L. Lactis that overexpressed the human BCRP gene, daidzein (3–50 μM) stimulated the baseline ATP-hydrolysis relative to control membranes. Direct transport of daidzein by BCRP was verified in monolayers of MDCK/BCRP and Caco-2 cells [69, 96]. In the MDCK/BCRP cells, the efflux ratio was 3-fold greater than the empty vector cells [69] while the BCRP inhibitor, estrone-3-sulfate increased the relative permeation (apical-to-basolateral transport) of daidzein by 80% compared to vehicle-treated control Caco-2 cells [96]. In addition to interacting with human BCRP, daidzein was also transported in MDCK cells by mouse Bcrp (MDCK/Bcrp), which was evidenced by an approximately 2-fold greater efflux ratio than the empty vector cells in two separate studies [69, 97].
Transport of Isoflavone Conjugates
Approximately 70–90% of an orally administered dose of isoflavones undergo phase II conjugation by glucuronidation and sulfation in the intestines and liver [13, 14, 110]. Phase-II conjugates of genistein are also transported by BCRP in Caco-2 cells as demonstrated by the reduced BL-to-AP transport of G-7-G, G-4′-G, G-7-S, and G-4′-S in the presence of the BCRP inhibitor Ko143 (5 μM) [73]. To better understand the transport of genistein-phase II metabolites by BCRP, human cervical carcinoma HeLa cells that endogenously expressed BCRP were genetically-engineered to overexpress the glucuronidation enzyme, UDP-glucuronosyltransferase (UGT) 1A9 [99]. Following a 2 h incubation with genistein (10 μM) and Ko143 (5 and 10 μM), these cells demonstrated reduced elimination of genistein-glucuronide and increased intracellular accumulation of the phase-II metabolite suggesting that genistein-glucuronide is transported by BCRP in the genetically-engineered HeLa cells [99]. Despite having a moderate and statistically significant effect on the overall glucuronidation rate in these cells, the authors still suggested that Ko143 did not reduce BCRP transport of genistein-glucuronide by decreasing the in situ formation of genistein-glucuronide [99]. To more thoroughly interpret these results, future experiments should address the ability of Ko143 to inhibit UGT function.
In addition to cells that overexpress the BCRP gene, there are alternative in vitro assays for identifying BCRP substrates that focus on plasma membrane transport. One common model are membrane vesicles isolated from Spodoptera frugiperda (Sf9) insect cells that overexpress human BCRP and are configured in an inverted (or inside-out) orientation. Because of this orientation, one can measure intravesicular concentrations of BCRP substrates directly in the presence of ATP. Using Sf9-BCRP vesicles, van de Wetering and Sapthu (2012) demonstrated a high rate of ATP-dependent transport of genistein-sulfate and daidzein-sulfate collected from the urine of Bcrp −/− mice (FVB) [98]. The mice were not dosed with the isoflavones per se, but rather consumed a normal rodent diet (AM-II) which contained isoflavones at low levels. This experimental approach allowed for the in vivo generation of isoflavone metabolites at physiologically relevant concentrations. BCRP-dependent transport of six isoflavone secondary metabolites (genistein-sulfate I, genistein-sulfate II, daidzein-4′-sulfate, daidzein-7-sulfate, glycitein-sulfate, and formononetin-sulfate) was confirmed using the inverted membrane vesicle system and 35S-labeled isoflavone metabolites [98]. These studies suggested that BCRP is particularly important in the transport of sulfated isoflavone conjugates.
An and Morris (2011) demonstrated that biochanin A-sulfate, not biochanin A, was selectively transported in the BL-to-AP direction in MDCK/Bcrp cells as compared to the AP-to-BL direction [67]. The BCRP specific inhibitor, FTC did not increase the accumulation of biochanin A in MCF-7/MX100 cells overexpressing BCRP, further confirming that the aglycone, biochanin A was not a substrate for BCRP-mediated transport [67]. In this same experiment, the contribution of human BCRP to the transport of biochanin A phase II metabolites could not be identified as low concentrations of phase II metabolites were detected intracellularly [67]. The authors suspect this was due to the low expression of phase II metabolizing enzymes in the MCF-7 cells [67]. Moreover, following the addition of biochanin A to the apical and basolateral chambers, genistein was detected in the medium of MDCK/BCRP as a phase I metabolite (oxidative demethylation) of biochanin A [67]. Similar to studies that added genistein to the medium, An and Morris (2011) confirmed that genistein is transported to a greater extent in the BL-to-AP direction compared to the empty vector cells. Another study found that after adding biochanin A to the apical chamber of a Caco-2 polarized monolayer, the AP-to-BL and BL-to-AP transport of biochanin A-glucuronide was reduced by the BCRP inhibitor dipyridamole (10 uM) [66]. The authors suggested that the abolished BL-to-AP transport is a result of reduced BCRP activity by dipyridamole [66], however the authors acknowledged that dipyridamole is also an inhibitor of MDR1 function [111]. Because MDR1 also localizes to the apical membrane of Caco-2 cells [112], this transporter cannot be ruled out as playing a role in biochanin A-glucuronide transport. Furthermore, dipyridamole is an inhibitor of MRP1 that is localized to the basolateral membrane of Caco-2 cells [113]. This indicates that inhibition of MRP1 transport may be responsible for the reduced AP-to-BL transport of biochanin A-glucuronide in Caco-2 cells. Interestingly, formononetin-glucuronide transport was not altered with the addition of dipyridamole in a similar experiment [66].
In Vivo
Bioavailability of Individual Isoflavones
Pharmacokinetic studies using Bcrp knockout (Bcrp −/−) mice have been conducted to assess the in vivo contribution of Bcrp to the disposition of various compounds including isoflavones and isoflavone metabolites. A number of studies have emphasized the ability of intestinal and hepatic Bcrp to transport isoflavones because the relevant route of exposure in the human population is oral ingestion. In two studies, oral (p.o.) administration of genistein [69, 73] or daidzein [69] resulted in significantly greater plasma concentrations of the respective isoflavone over time (or area-under-the-curve AUC) in Bcrp −/− mice on a FVB background strain as compared to wild-type mice. These data suggested that Bcrp was responsible for limiting the oral bioavailability of genistein and daidzein [69, 73]. In a similar study performed by Alvarez et al. (50 mg/kg, 3 h) [70], the plasma AUC values for the aglycones, genistein and daidzein, alone in the Bcrp−/− mice were elevated by 1.5- and 2-fold, respectively, which was not significantly greater than the wild-type values, unlike the other two studies which noted statistical significance [69, 73]. Alvarez et al. reported that the plasma AUC values for total genistein or daidzein (aglycone and metabolites) were significantly greater (up to 9-times) in Bcrp −/− mice compared to wild-type mice and proposed that the difference was due primarily to the increased concentration of phase II metabolites in the plasma of Bcrp −/− mice [70]. For example, the plasma daidzein-sulfate concentration was significantly increased 6-fold in the Bcrp −/− mice compared to wild-type mice [70]. Also, daidzein-glucuronide was detected in Bcrp −/− mice while the metabolite was undetectable in wild-types [70]. In two different studies, systemic genistein-sulfate concentrations were elevated up to 26- and 7-fold in Bcrp −/− mice administered 20 mg/kg genistein [73] and 50 mg/kg genistein [70], respectively, as compared to wild-type mice. In the same studies, genistein-glucuronide concentrations were elevated up to 16- and 8-fold in Bcrp −/− mice following administration of 20 mg/kg [73] and 50 mg/kg genistein [70], respectively. This evidence is in line with an intestinal in situ perfusion study performed by Zhu et al. which demonstrated that the excretion of genistein-sulfate and genistein-glucuronide was significantly decreased in the small intestines of Bcrp −/− mice compared to wild-type mice, suggesting that genistein conjugates are substrates for Bcrp-mediated transport [72].
The exact mechanism by which isoflavones and their phase II metabolites occur at high concentrations in the plasma of Bcrp −/− mice is a topic of great controversy [114]. Alvarez et al. offered an explanation for the increased plasma concentration of only the phase II metabolites by suggesting that decreased excretion of the aglycones due to the lack of intestinal Bcrp in Bcrp −/− mice increased exposure of the isoflavones to phase II metabolizing enzymes in the intestine and subsequently enhanced plasma concentrations of isoflavone metabolites [70]. Enokizono et al. suggested that because glucuronidation is the primary metabolic pathway contributing to the elimination of gensitein [13, 110], and there is no difference in the intestinal rate of glucuronidation between wild-type and Bcrp−/− mice [115], that increased intestinal absorption of genistein is the primary cause of elevated plasma genistein concentrations (rather than altered glucuronide formation) in Bcrp−/− mice [69]. Yang et al. countered this assertation and demonstrated that there was no difference in the intestinal absorption of genistein using in situ perfusion of wild-type and Bcrp−/− mouse intestines and human Caco-2 cells in the presence and absence of Bcrp specific inhibitor, Ko143 [73]. Instead, Yang et al. suggested that in Bcrp −/− mice, genistein conjugates were increasingly excreted into the blood rather than into the intestinal lumen and bile duct (due to loss of Bcrp function) and that any elevated genistein concentration in the plasma was likely due to the hydrolysis of genistein-glucuronides conjugates back to the aglycone form, in the blood [73]. The multidrug resistance-associated protein 3 (MRP3) is expressed on the basolateral membrane of the rat intestine [116] and Yang et al. suggested that this transporter may be a candidate for the active transport of genistein conjugates into the portal blood [117], providing a possible mechanistic explanation for increased plasma concentrations of isoflavone phase II metabolites in Bcrp −/− mice [73]. It is important to note that a number of experimental methods varied between studies and may have contributed to the differences in the isoflavone (aglyone and metabolite) plasma profiles; these included the time period (i.e., 3 h vs 4 h vs 24 h) and/or isoflavone dose (i.e., 50 mg/kg vs 8.1 mg/kg vs 20 mg/kg).
In another study, Bcrp −/− knockout mice consumed a normal chow diet containing trace amounts of isoflavones, and exhibited significantly greater concentrations of daidzein-sulfate and genistein-sulfate in the plasma of male and female mice, respectively, compared to the wild-types [98]. A study of this nature suggests that BCRP/Bcrp may play a role in the disposition of isoflavones at a wide range of concentrations, however these findings must be interpreted with caution as the exact dose of isoflavone that each mouse received was unknown.
Bioavailability of Isoflavone Mixtures
To recapitulate a more relevant dietary exposure to isoflavones mixtures, Alvarez et al. also examined the plasma profiles of the individual compounds following the intragastric co-administration of genistein (25 mg/kg) and daidzein (25 mg/kg) [70]. Interestingly, the findings of this experiment were similar to the results of studies in which mice were treated with individual isoflavones [70]. Notably, the AUC values for both aglycones, genistein and daidzein, were increased 1.6- and 2-fold in the Bcrp−/− mice but still not significantly different from wild-type mice [70]. Further, the total isoflavones and phase II metabolites were significantly greater in Bcrp−/− than wild-type mice [70]. In the animals dosed with a mixture of isoflavones, the phase II metabolite concentrations were approximately equal to the concentrations of the same metabolites in animals treated with individual isoflavones [70].
Intestinal Metabolism and Transport of Isoflavones
To better understand the role of intestinal Bcrp in the disposition of isoflavones, Enokizono et al. [69] and Yang et al. [73] examined plasma profiles of isoflavones following alternate routes of exposure. Following a continuous intravenous (i.v.) infusion of genistein (i.v., 1.4 mg/kg/h, 2h) in the right jugular vein, or an intraperitoneal (i.p.) administration (20 mg/kg, 24 h), there was no difference in the plasma concentration of genistein between the Bcrp −/− and wild-type mice suggesting that Bcrp in the intestine plays a primary role in altering plasma genistein concentrations [69, 73]. The plasma profiles of the genistein secondary metabolites in the i.p. dosing study were similar to the oral dosing studies in that the AUC values of G-7-G, G-4′-S, and G-7-S were significantly greater in Bcrp −/− mice compared to wild-type mice [73]. Interestingly, in the study performed by Enokizono et al., i.v. administration of daidzein (1.4 mg/kg/h, 2 h) resulted in a significantly increased plasma concentration of the aglycone in the Bcrp−/− mice as compared to the wild-type mice, but to a lesser extent than the p.o. dose [69]. The authors note that the increased plasma concentration of daidzein following i.v. infusion was unexpected, but suggest that “impaired urinary excretion” of daidzein may be a contributing factor since about 10% of daidzein is eliminated via the urine in rats administered an oral dose of daidzein (100 mg/kg) [69, 118].
Biliary Transport of Isoflavones and Conjugates
In the study performed by Yang et al., genistein-sulfate (G-7-S and G-4′-S) bile levels were significantly reduced by 93% compared to wild-type mice following a 2.5 h in situ intestinal perfusion experiment [73]. Interestingly, there were no changes in the parent or genistein-glucuronide concentrations between genotypes suggesting that hepatic Bcrp selectively transports genistein-sulfate metabolites. Another study examined the role of Bcrp in the hepatic excretion of daidzein-sulfate in Bcrp −/− mice fed a normal rodent diet with trace amounts of isoflavones [98]. Daidzein-sulfate was detected in the bile of male and female Bcrp −/− mice at significantly lower concentrations (~60%) than the wild-types.
Distribution of Isoflavones
The transport of isoflavones by Bcrp in other organs has also been investigated in mice. Tissues with low basal expression of Bcrp, such as the stomach, have preferential isoflavone accumulation further supporting the contention that Bcrp limits isoflavone accumulation in an organ-specific manner [69, 97, 119]. In wild-type mice, the Bcrp protein is localized to the capillary endothelial cells of the blood brain barrier [120], endothelial cells within the testis [69, 121], and the epithelial (head) and endothelial cells (body) of the epididymis. Two studies examined the brain concentrations of genistein and daidzein following a 120 minute i.v. infusion of genistein or daidzein into the right jugular vein of adult FVB wild-type and Bcrp −/− mice [69, 97]. Both studies found that the brain concentrations of the two isoflavones were increased significantly in Bcrp −/− mice compared to wild-type mice, demonstrating that Bcrp limits the distribution of genistein and daidzein to the brain. In the same study by Enokizono et al. [69], testis concentrations of genistein and daidzein were significantly increased by 200% and 400%, respectively, in Bcrp −/− mice compared to wild-type mice suggesting that Bcrp limits accumulation of isoflavones in this reproductive organ. Further, epididymal concentrations of genistein were increased 150% in Bcrp −/− mice compared to wild-type mice. No difference was observed in ovarian concentrations of genistein between Bcrp −/− and wild-type mice [69] even though Bcrp mRNA is detectable in mouse ovaries [121, 122]. Bcrp protein, however is not consistently expressed throughout the ovary but when present, it is localized to the small capillaries of the ovary endothelium [121]. Dankers et al. suggested that the varying protein expression throughout the organ may be a result of “the dynamic environment of the estrous cycle, where there is a constant regeneration and degradation of capillaries” [121]. Constantly changing protein expression may result in unclear findings in the tissue distribution of Bcrp substrates. Future studies should control for rodent estrous cycle stage to examine the accumulation of isoflavones in the ovary.
In the placenta, the BCRP protein localizes to the apical membrane of syncytiotrophoblasts which are in direct contact with the maternal circulation [50]. Transporter function and localization in this important organ of pregnancy suggest that BCRP is responsible for protecting the fetus from exposure to harmful chemicals that may be present in the maternal circulation. The mouse orthologue, Bcrp, in the placenta limited fetal exposure to Bcrp substrates including topotecan [60] and is therefore a useful model for studying the in vivo function of placental Bcrp/BCRP. To evaluate the role of placental Bcrp in the in utero distribution of genistein, pregnant FVB wild-type and Bcrp −/− mice were infused via the right jugular vein with genistein (1.25 mM) for 120 min and there was no difference in the plasma concentration between the wild-type and Bcrp −/− dams [69]. Interestingly, Bcrp −/− fetuses accumulated significantly more (1.6-fold) genistein than the wild-type fetuses [69]. Further, significantly greater concentrations of genistein were detected in the brains of the Bcrp −/− fetuses [69] than the wild-type fetal brains, demonstrating the importance of Bcrp in protecting against genistein penetration of the developing blood-brain barrier. Of note, there is evidence that isoflavones such as genistein alter the epigenome of developing mice [123, 124]. Taken together, future research should focus on risk factors that may reduce BCRP activity in the placenta and impair the development of offspring following in utero exposure to isoflavones.
Evidence for Isoflavones as Inhibitors of BCRP Function
Recent research has begun to explore the ability of isoflavones to alter the pharmacokinetics or pharmacodynamics of chemicals that are transported by BCRP/Bcrp. This may be beneficial in situations where isoflavones enhance the efficacy of a particular treatment, such as a chemotherapeutic drug. However, negative outcomes such as an increase in the toxicity or a reduction in the efficacy of the drug may also occur as a result of BCRP inhibition by isoflavones. To date, a variety of in vitro and in vivo experimental models have been used to detect isoflavone-inhibitors of BCRP/Bcrp function and to better understand the potential implications of concomitantly consuming isoflavones (i.e., a soy rich diet) with drugs that are transported by BCRP.
Altered Chemoresistance
Many chemotherapy drugs, including mitoxantrone, SN-38 (the active metabolite of irinotecan), topotecan, and methotrexate are substrates of BCRP (Table 1). These drugs are routinely used to screen for potential inhibitors of BCRP function indirectly by assessing the cytotoxic effect of the chemotherapy drug on cell growth in the presence of the potential inhibitor. In the presence of BCRP inhibitors, cells that express the BCRP protein cannot effectively remove the cytotoxic chemical which leads to a potentiation of the chemotherapeutic response. Human breast cancer and lung cancer cells expressing BCRP, MCF-7/MX100 and NCI-H460/MX20, respectively, are commonly used as models for measuring BCRP activity. MCF-7 and NCI-H460 cells that express BCRP at high levels are selected for using 100 nM or 20 nM mitoxantrone, respectively, prior to experiments [87]. Forty-eight hour exposure with genistein (10–50 μM) or biochanin A (5–50 μM) reversed mitoxantrone resistance in MCF-7/MX100 cells in a concentration-dependent manner using the sulforhodamine B assay to assess cell density [87, 100]. Furthermore, genistein inhibited the cell growth of human leukemia cells that overexpressed the BCRP transporter (K562/BCRP) in the presence of SN-38 compared to cells cultured without isoflavones [71, 101]. Daidzein inhibited the cell growth of K562/BCRP cells in the presence of SN-38 but to a lesser extent than genistein [101]. Genistein (10 μM) alone did not reduce the cell viability of MCF-7 breast cancer cells following 72 h incubation [125], whereas 48 h incubation of K562 leukemia cells with genistein (10–100 μM) modestly reduced cell viability by about 15% [126]. The inconsistency of these findings may arise from innate differences in the protective mechanisms of each cell type. For example, moderate levels of ABC transporters (MDR1 and BCRP) [125] are expressed in the MCF-7 cells and may participate in the removal of genistein or other toxic metabolites thereby protecting the cells from potential toxic effects of the compounds. Additionally, two different methods were used to detect cell viability. Zhang et al. utilized a more sensitive and direct measure of cell viability, trypan blue, which quantified the number of viable cells [126], while Pick et al. drew conclusions based on the ATP assay which indirectly indicates the amount of ATP generated which was positively correlated with cell viability [125].
Probe Substrates
Well-characterized and easily detected BCRP substrates (i.e., fluorescent and radiolabeled compounds) are commonly used as probe substrates for detecting inhibitors of BCRP-mediated transport. Using whole cell model systems, BCRP function is assessed by determining the intracellular accumulation of a probe substrate in the absence and presence of an inhibitor. Membrane-based assays including inverted plasma membrane vesicles and ATPase assays are isolated subcellular systems that are used to better characterize transporter kinetics and stimulation of transporter ATPase activity by a substrate in the presence of an inhibitor.
Cell-Based Assays
In addition to being a chemotherapy drug, mitoxantrone also has fluorescent properties and intracellular accumulation of the drug can be quantified by flow cytometry. A 30-minute co-incubation of mitoxantrone with genistein, daidzein, or biochanin A in MCF-7/MX100 and NCI-H460/MX20 cells, significantly increased the accumulation of mitoxantrone up to 4-fold compared to the control cells treated with mitoxantrone alone [87, 100]. In addition, intracellular topotecan fluorescence increased in K562/BCRP cells in the presence of genistein (30 and 100 μM) relative to the parent K562 cells [71]. Pick et al. described genistein as an enhancer of fluorescent Hoechst 33342 accumulation in MDCK/BCRP and MCF-7/MX cells with IC50 values for BCRP efflux of 6.9 μM and 8.8 μM, respectively, in each cell type [125]. Daidzein significantly increased the accumulation of mitoxantrone in MCF-7/MX cells and K562/BCRP cells up to 3-fold which was similar to the fluorescence intensity detected in the respective parental cells [68]. In the same cell types, daidzein increased the accumulation of the fluorescent derivative of the BCRP substrate and blood pressure medication, prazosin (BODIPY-prazosin) to levels comparable to that in the parental cell line [68].
Similar to their interaction with human BCRP, the mouse Bcrp protein is also sensitive to isoflavone-mediated inhibition. Biochanin A (2.5–25 μM) significantly increased the intracellular fluorescence of mitoxantrone up to 4 and 2.5-fold in MDCK cells that expressed mouse or human Bcrp/BCRP, respectively [88]. In addition, the BL-to-AP transport of mitoxantrone was attenuated by biochanin A (5 μM) in MDCK/Bcrp cells [88]. Together, genistein and daidzein (100 μM each) reduced the BL-to-AP transport of BCRP substrates, nitrofurantoin and danofloxacin, in MDCK/BCRP and MDCK/Bcrp cells [89, 127], respectively. Even further, a combination of multiple flavonoids including biochanin A and genistein (apigenin, biochanin A, chrysin, genistein kaempferol, 1 μM total flavonoid) increased the accumulation of mitoxantrone in MCF-7/MX100 cells to a greater extent (EC50: 0.23 ± 0.08 μM) than each individual flavonoid (i.e., genistein EC50: 14.9 ± 2.69 μM). These data suggest that low concentrations of various isoflavones or flavonoids may have an additive or synergistic effect on the inhibition of BCRP transport [100].
Membrane-Based Assays
Inhibitors of BCRP function can be detected using BCRP inverted plasma membrane vesicles. In this assay, inhibitors are recognized by a significant decrease in the vesicular accumulation of a BCRP probe substrate compared to vesicles without the inhibitor. In BCRP vesicles, there was a trend for aglycones (genistein> biochanin A> glycitein> formononetin> daidzein) to reduce the ATP-dependent transport of the BCRP substrate, 3H-methotrexate (100 μM) to a greater extent than their respective glucosides (biochanin A-glucoside, sissotrin> genistin> formononetin-glucoside, ononin> glycitin>daidzin) as detected by liquid scintillation counting [90]. In general, because the glucosides are quickly converted to the aglycone forms in vivo, the former finding holds more bearing on the potential for BCRP inhibition in vivo. Importantly, the concentration of genistein that inhibits BCRP function in inverted vesicles, is within the range of plasma concentrations of genistein (1–3 μM, n=12 healthy, human volunteers) that may be found circulating following dietary consumption of soy products [128]. Interestingly, soybean extract (1 mg/ml) reduced the uptake of 3H-methotrexate in BCRP-overexpressing vesicles to the same extent as BCRP inhibitor, FTC (5 μM) suggesting that the multiple compounds present in a soy diet, including genistein, daidzein, glycitein and their glucosides, collectively reduce BCRP function [90].
Another plasma membrane-based method used to detect functional inhibitors of BCRP-mediated transport is the ATPase assay. ATP hydrolysis is measured as an indirect indicator of substrate transport and is reduced in the presence of a BCRP-specific functional inhibitor [129]. In the mammary gland, BCRP is expressed on the apical membrane of alveolar epithelial cells and actively concentrates substrates in the milk of lactating animals (mouse and cow) and humans [130]. In plasma membranes isolated from the mammary gland of a lactating cow, genistein (10 μM) reduced the Bcrp ATPase activity stimulated by mitoxantrone [80], suggesting that genistein may alter the transport of drugs into the milk of lactating animals and potentially humans.
Altered Pharmacokinetics of Other Drugs
The ability of dietary constituents, including isoflavones, to alter the in vivo pharmacokinetics and pharmacodynamics of a drug is important to consider since their sale and consumption are unregulated. This is especially of concern when a drug that is a BCRP substrate is administered during lactation. Inhibition of chemical transfer into the milk may provide benefits or deficits to the offspring resulting in protection of the infant from the toxic effects of the transferred drug, or reduced transfer of antiviral medications intended to limit the infant’s exposure to HIV, respectively. Enrofloxacin is an antibacterial drug that has been used in veterinary medicine to treat gram-negative and gram-positive bacterial infections in ruminants such as cows and sheep. Its metabolite, ciprofloxacin, is also efficacious in vivo and is used in humans for the treatment of bacterial infections. Importantly, ciprofloxacin is recognized by the American Academy of Pediatrics as a maternal medication usually compatible with breastfeeding [131]. Both the parent enrofloxacin compound and its metabolite ciprofloxacin are recognized substrates of BCRP [78, 80]. To understand the effect of genistein on the milk concentrations of concomitantly administered enrofloxacin in sheep, 12 lactating sheep were infused with enrofloxacin either with or without genistein via the left jugular vein [80]. There was no change in the plasma concentration of enrofloxacin (area under the curve, AUC or maximal concentration, Cmax) between the sheep that received genistein and those that did not. However, genistein decreased the enrofloxacin milk content which was demonstrated by a 1.5-fold reduction in the enrofloxacin milk AUC value [80]. These data suggest that in dairy animals, genistein may reduce milk concentrations of enrofloxacin and shorten the time in which the animals are withdrawn from producing usable milk. Further, in humans genistein may protect the developing infant from unnecessary exposure to ciprofloxacin.
Because genistein and daidzein are often found together in nature, Perez et al. [127, 132] examined the effect of a combined isoflavone exposure on the distribution of BCRP substrates danofloxacin and nitrofurantoin into the milk of lactating sheep. Danofloxacin is an antibiotic used in veterinary medicine to treat a broad range of bacterial infections. Nitrofurantoin is an antibiotic that is often prescribed for the treatment of urinary tract infections in humans and animals. In particular, nitrofurantoin is prescribed to pregnant [133] and lactating women as it is recognized by the American Academy of Pediatrics as a maternal medication typically compatible with breast feeding [131]. The compounds concentrate in the milk of lactating animals (danofloxacin and nitrofurantoin) [82, 134] and/or women (nitrofurantoin) [135]. In the nitrofurantoin experiment, sheep exposed to genistein and daidzein via the standard diet or exogenous dosing with the isoflavones (10 mg/kg genistein + 10 mg/kg daidzein) by oral gavage, experienced a significant decrease in the milk AUC of nitrofurantoin [132]. Additionally, the nitrofurantoin-treated control animals experienced higher plasma AUC and Cmax nitrofurantoin as compared to those that were exposed to isoflavones [132]. Because this is not in line with the Bcrp-inhibition paradigm, the authors suggest that the isoflavone treated sheep may have experienced a reduced plasma concentration of nitrofurantoin due to decreased absorption or enhanced elimination of nitrofurantoin. Unfortunately, they do not offer mechanistic evidence to explain the reduced systemic exposure to the drug. In the danofloxacin experiment, lactating sheep received a 15-day soy supplemented diet that significantly reduced the milk AUC and Cmax of danofloxacin compared to the sheep that received regular forage without isoflavones [127]. Interestingly, the sheep that received an oral dose of genistein and daidzein (10 mg/kg genistein + 10 mg/kg daidzein) did not demonstrate a similar decrease in milk concentrations of danofloxacin suggesting that the form in which the compounds are administered (i.e., liquid vs. solid, aglycone vs. glycone) or presence of other compounds in the diet may determine its effect on the pharmacokinetics of other chemicals [127]. The authors list the various constituents of the soy diet but did not mention the amount of genistein, daidzein, or other isoflavones present in each diet [127].
Bcrp −/− mice can be used to elucidate the functional role of BCRP/Bcrp in mediating potential nutrient-drug interactions in an in vivo situation. In the absence of isoflavones, nitrofurantoin was confirmed as a BCRP substrate due to increased plasma AUC of nitrofurantoin in Bcrp −/− mice compared to wild-type mice [82],[89]. While treatment of wild-type mice with genistein and daidzein had no effect on the plasma AUC of nitrofurantoin over a 2 hr period compared to vehicle-treated controls, higher plasma nitrofurantoin levels were in fact observed at 30 min [89]. At that same time point, genistein and daidzein significantly reduced the nitrofurantoin concentrations in the milk and bile of wild-type mice as compared to the vehicle-treated mice [89]. This study suggests that examination of early time points may be necessary to observe changes in drug pharmacokinetics brought on by isoflavones. These data combined with those in the sheep indicate that a soy diet may reduce the potential for infant exposure to nitrofurantoin in milk.
Only one study has investigated whether biochanin A can alter the pharmacokinetics of other BCRP/Bcrp substrates [88]. Male wild-type ND4 Swiss Webster mice were administered an intravenous dose of mitoxantrone (5 mg/kg) 5 minutes following an intravenous dose of biochanin A (10 mg/kg) [88]. Biochanin A actually enhanced the mitoxantrone elimination from the kidney and spleen as there was a trend for those organs to accumulate less mitoxantrone than the vehicle-treated mice, though this was not statistically significant [88]. These findings were inconsistent with the in vitro results of this same study that found biochanin A to be an inhibitor of the Bcrp-mediated transport of mitoxantrone, indicating that there may be some disconnect between in vitro and in vivo models for measuring inhibition of BCRP function. Future research should examine the effects of isoflavones on the pharmacodynamics of a drug, such as measuring the contribution of the isoflavones to the exacerbation of a toxic side effect. Such evidence may provide the impetus for improving the current guidelines for prescribing drugs that are BCRP substrates.
The Regulation of BCRP by Isoflavones
Genistein interacts with a variety of cellular proteins that modulate gene expression and/or protein localization such as the estrogen receptor, aryl hydrocarbon receptor, and the epidermal growth factor receptor [12, 39, 41, 42, 136]. Because BCRP expression is regulated by the aryl hydrocarbon receptor [136], Ebert et al. examined the ability of genistein (1–50 μM) to alter BCRP mRNA expression in Caco-2 cells and described no effect compared to the vehicle-treated cells following a 48 h incubation [102]. This finding was confirmed when Arias et al. reported no change in BCRP protein expression in Caco-2 cells following 48 h exposure to genistein (0.1–10 μM) [103]. Further, a 5-day exposure to genistein (3 and 10 μM) did not alter the BCRP protein expression in K562 cells that expressed BCRP [71]. Conversely, genistein (15 μM, 24 h) reduced the mRNA expression of BCRP by approximately 70% in gastric cancer (MGC-803) cells [104]. Taken together, the effect of genistein and possibly other isoflavones on BCRP regulation is likely tissue-specific. Further investigation of the effect of isoflavones on BCRP expression in other cell lines and tissue types that rely on BCRP function for protection against xenobiotic accumulation, such as the placenta and kidneys, is required to fully understand the role of isoflavones in the regulation of BCRP expression and ultimately function.
Conclusions
Isoflavones interact with the BCRP efflux transporter through a variety of ways including direct transport, chemical antagonism, and altered transporter expression. Evidence for the selective removal of isoflavones from target organs which express BCRP/Bcrp [69, 73, 97], suggests that the transporter plays an important role in the in vivo disposition of isoflavones. Therefore the potential effects of isoflavones on human health may be influenced by the efficiency of BCRP function. In addition to transporting parent isoflavones, BCRP also transports sulfated and glucuronidated isoflavone metabolites [70, 73, 98, 99] which, too, possess some biological activity [137]. Furthermore, sulfatase and glucuronidase deconjugating enzymes are present in the liver, intestines, blood, and fetus [16, 73, 138, 139] and may convert the metabolites back to the parent compound. Because of these hydrolytic reactions, the ability of BCRP to transport both parent isoflavones and their metabolites is important. Due to the potential for isoflavones to disrupt the endocrine system, future research should focus on understanding the role of BCRP/Bcrp in reproductive organs and how disruption of transport may lead to inappropriate exposure of isoflavones to target organs and developing tissues.
As a result of being directly transported by BCRP, isoflavones may competitively inhibit the transport of other chemicals including pharmaceuticals which depend on BCRP for appropriate distribution, metabolism, and elimination. In addition to competitive inhibition, evidence suggests that isoflavones may also non-competitively inhibit BCRP-mediated transport as there are more isoflavone and flavonoid compounds that inhibit BCRP function than those that are directly transported [71, 90, 101]. Further examination of the molecular interactions of isoflavones with ABC transporters and other proteins that contain a nucleotide binding domain, reveal that the compounds may disrupt BCRP function through interference with ATP hydrolysis via association with the BCRP nucleotide binding domain [140] (reviewed in [141]). It is important to note that affinity of a compound for a particular site within the BCRP protein will determine the nature and extent of the chemical-transporter interaction. A high-resolution 3D structure of the human BCRP protein is not yet available, though working towards completing this task will help to elucidate the complexities of BCRP-isoflavone interactions.
According to self-reported surveys, the average consumption of total isoflavones ranges between 2 and 50 mg/day and is vastly different depending upon ethnicity and geographic location [142–144]. Consumption of a soy food diet in this range resulted in total plasma isoflavone concentrations of approximately 19–500 nM. However, in a pharmacokinetic study which enlisted 12 healthy volunteers to consume soy food (96 mg/day) with 3 meals every day for 7 days, led to a total plasma isoflavone concentration of about 5 μM on day 7 [128]. These data suggest that the isoflavone concentrations which inhibited BCRP function in vitro (low μM range [87, 88, 90, 125]) may be applicable to a population that consumes soy regularly. To recapitulate a soy diet, a few investigations examined the effect of multiple isoflavones or flavonoids on BCRP function [70, 90, 100]. These preliminary studies revealed the compounds may have additive or synergistic effects, supporting the need for future studies to address the effect of isoflavone mixtures on BCRP function. This is of particular importance because the population which consumes soy is rapidly increasing, particularly in the United States. Since 1996, soy food sales in the United States dramatically increased from 1 billion to about 5.2 billion dollars spent per year by 2011 according to the Soy Foods Association of North America [145], which is likely a result of multiple reports suggesting the health benefits of soy consumption. Furthermore, there has been a steady increase in the overall prescription of drugs in the United States over the past 10 years [146]. Taken together with the findings that suggest that isoflavones alter the pharmacokinetics of drugs which are substrates for BCRP, the risk for a nutrient-drug interaction mediated by BCRP is conceivable and warrants further investigation to help improve the guidance for the prescription of drugs that are BCRP substrates.
Acknowledgments
Kristin Bircsak and Lauren Aleksunes contributed to the writing of this manuscript.
Abbreviations
- −/−
knockout
- ABC
ATP-binding cassette
- ABCP
ABC transporter highly expressed in the placenta
- AP-to-BL
apical-to-basolateral
- AUC
area under the curve
- BCRP/Bcrp
human/mouse breast cancer resistance protein
- BL-to-AP
basolateral-to-apical
- Cmax
maximal concentration
- FTC
fumitremorgin C
- G-7-G
genistein-7-glucuronide
- G-4′-G
genistein-4′-glucuronide
- G-7-S
genistein-7-sulfate
- G-4′-S
genistein-4′-sulfate
- HPLC-DAD-MS/MS
high performance liquid chromatography diode array detection tandem mass spectrometry
- HPLC-ECD
high performance liquid chromatography electrochemical detection
- i.p
intraperitoneal
- i.v
intravenous
- LC/MS
liquid chromatography/mass spectrometry
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- LSC
liquid scintillation counting
- MDCK
Madin-Darby canine kidney II
- MDR1
multidrug resistance protein 1
- MRP
multidrug resistance-associated protein
- MXR
mitoxantrone resistance transporter
- p.o
oral
- Sf9
Spodoptera frugiperda
- SULT
sulfotransferase
- UGT
UDP-glucuronosyltransferase
- UPLC-DAD-MS/MS
ultra-performance liquid chromatography diode array detection tandem mass spectrometry
- UPLC-MS/MS
ultra-performance liquid chromatography tandem mass spectrometry
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest. This work was supported by the National Institutes of Environmental Health Sciences (Grants ES020522, ES005022, ES007148, ES021800, DK093903), a component of the National Institutes of Health. Kristin Bircsak is supported by predoctoral fellowships from the American Foundation for Pharmaceutical Education and Pharmaceutical Research and Manufacturers of America.
References
- 1.U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. Nutrient Data Laboratory; 2008. [Accessed August 13, 2013]. Home Page: http://www.ars.usda.gov/nutrientdata/isoflav. [Google Scholar]
- 2.Rolfe BG. Flavones and isoflavones as inducing substances of legume nodulation. BioFactors. 1988;1:3–10. [PubMed] [Google Scholar]
- 3.Burdette CQ, Marcus RK. Determination of isoflavone content in soy, red clover, and kudzu dietary supplement materials by liquid chromatography-particle beam/electron ionization mass spectrometry. J AOAC Int. 2013;96:925–32. doi: 10.5740/jaoacint.12-431. [DOI] [PubMed] [Google Scholar]
- 4.Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and a new internal standard. Am J Clin Nutr. 1998;68:1474S–1479S. doi: 10.1093/ajcn/68.6.1474S. [DOI] [PubMed] [Google Scholar]
- 6.Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr. 2005;135:48–52. doi: 10.1093/jn/135.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436:71–5. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 8.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 9.Murota K, Shimizu S, Miyamoto S, Izumi T, Obata A, Kikuchi M, Terao J. Unique uptake and transport of isoflavone aglycones by human intestinal caco-2 cells: comparison of isoflavonoids and flavonoids. J Nutr. 2002;132:1956–61. doi: 10.1093/jn/132.7.1956. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–35. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 11.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 13.Doerge DR, Chang HC, Churchwell MI, Holder CL. Analysis of soy isoflavone conjugation in vitro and in human blood using liquid chromatography-mass spectrometry. Drug Metab Dispos. 2000;28:298–307. [PubMed] [Google Scholar]
- 14.Pritchett LE, Atherton KM, Mutch E, Ford D. Glucuronidation of the soyabean isoflavones genistein and daidzein by human liver is related to levels of UGT1A1 and UGT1A9 activity and alters isoflavone response in the MCF-7 human breast cancer cell line. J Nutr Biochem. 2008;19:739–45. doi: 10.1016/j.jnutbio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–94. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 16.Sfakianos J, Coward L, Kirk M, Barnes S. Intestinal uptake and biliary excretion of the isoflavone genistein in rats. J Nutr. 1997;127:1260–8. doi: 10.1093/jn/127.7.1260. [DOI] [PubMed] [Google Scholar]
- 17.Kulling SE, Honig DM, Metzler M. Oxidative metabolism of the soy isoflavones daidzein and genistein in humans in vitro and in vivo. J Agric Food Chem. 2001;49:3024–33. doi: 10.1021/jf0012695. [DOI] [PubMed] [Google Scholar]
- 18.Tolleson WH, Doerge DR, Churchwell MI, Marques MM, Roberts DW. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J Agric Food Chem. 2002;50:4783–90. doi: 10.1021/jf025549r. [DOI] [PubMed] [Google Scholar]
- 19.Schroder C, Matthies A, Engst W, Blaut M, Braune A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl Environ Microbiol. 2013;79:3494–502. doi: 10.1128/AEM.03693-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedlund TE, Johannes WU, Miller GJ. Soy isoflavonoid equol modulates the growth of benign and malignant prostatic epithelial cells in vitro. The Prostate. 2003;54:68–78. doi: 10.1002/pros.10137. [DOI] [PubMed] [Google Scholar]
- 21.Ingram D, Sanders K, Kolybaba M, Lopez D. Case-control study of phyto-oestrogens and breast cancer. Lancet. 1997;350:990–4. doi: 10.1016/S0140-6736(97)01339-1. [DOI] [PubMed] [Google Scholar]
- 22.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. Proc Soc Exp Biol Med. 1995;208:40–3. doi: 10.3181/00379727-208-43829. [DOI] [PubMed] [Google Scholar]
- 23.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Eltoum IE, Lamartiniere CA. Genistein alters growth factor signaling in transgenic prostate model (TRAMP) Mol Cell Endocrinol. 2004;219:171–80. doi: 10.1016/j.mce.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Bitto A, Burnett BP, Polito F, Marini H, Levy RM, Armbruster MA, Minutoli L, Di Stefano V, Irrera N, Antoci S, Granese R, Squadrito F, Altavilla D. Effects of genistein aglycone in osteoporotic, ovariectomized rats: a comparison with alendronate, raloxifene and oestradiol. Br J Pharmacol. 2008;155:896–905. doi: 10.1038/bjp.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. British journal of cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–63. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer letters. 2008;269:226–42. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Constantinou AI, White BE, Tonetti D, Yang Y, Liang W, Li W, van Breemen RB. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur J Cancer. 2005;41:647–54. doi: 10.1016/j.ejca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Chen YM, Ho SC, Lam SS, Ho SS, Woo JL. Soy isoflavones have a favorable effect on bone loss in Chinese postmenopausal women with lower bone mass: a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4740–7. doi: 10.1210/jc.2003-030290. [DOI] [PubMed] [Google Scholar]
- 31.Strom SS, Yamamura Y, Duphorne CM, Spitz MR, Babaian RJ, Pillow PC, Hursting SD. Phytoestrogen intake and prostate cancer: a case-control study using a new database. Nutr Cancer. 1999;33:20–5. doi: 10.1080/01635589909514743. [DOI] [PubMed] [Google Scholar]
- 32.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, Fontana J, Chinni S, Davis J, Forman J, Wood DP, Kucuk O. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–7. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 33.Squadrito F, Marini H, Bitto A, Altavilla D, Polito F, Adamo EB, D’Anna R, Arcoraci V, Burnett BP, Minutoli L, Di Benedetto A, Di Vieste G, Cucinotta D, de Gregorio C, Russo S, Corrado F, Saitta A, Irace C, Corrao S, Licata G. Genistein in the metabolic syndrome: results of a randomized clinical trial. J Clin Endocrinol Metab. 2013;98:3366–74. doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- 34.Clarkson TB, HUW, Allmen TI, Aso T, Barnes S, Basaria SS, Brinton RD, MCJ, Frankenfeld CL, Gallagher JC, Gold EB, Hodis HN, Ishimi Y, Kim H, Kroenberg F, Landgren BM, Maki PM, Messina MJ, Setchell KD, Shu XO, Weaver CM, Wong WW, Goldstein SR, Pace DT, Shifren JL, Clarkson TB, Contestabile E, Gass ML, Kagan R, Maki PM, Manson JE, Schnatz PF, Sievert LL, Stuenkel CA, Warren MP, Schiff I, Utian WH. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010) Menopause. 2011;18:732–53. doi: 10.1097/gme.0b013e31821fc8e0. [DOI] [PubMed] [Google Scholar]
- 35.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–62. doi: 10.1093/jn/131.11.2957. [DOI] [PubMed] [Google Scholar]
- 36.Allred CD, Ju YH, Allred KF, Chang J, Helferich WG. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis. 2001;22:1667–73. doi: 10.1093/carcin/22.10.1667. [DOI] [PubMed] [Google Scholar]
- 37.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–8. [PubMed] [Google Scholar]
- 38.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 39.Martin PM, Horwitz KB, Ryan DS, McGuire WL. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978;103:1860–7. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- 40.Barrett JF, JG, Lamond DR. Reproductive performance of Merino ewes grazing red clover (Trifolium pratense L.), improved pasture, or native pasture. Australian Journal of Agricultural Research. 1965;16:189–200. [Google Scholar]
- 41.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 42.Yan GR, Xiao CL, He GW, Yin XF, Chen NP, Cao Y, He QY. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics. 2010;10:976–86. doi: 10.1002/pmic.200900662. [DOI] [PubMed] [Google Scholar]
- 43.Wegner CC, Zhou X, Ding ZM, Kuo MT, Carson DD. Tyrosine kinase inhibition decreases Muc-1 expression in mouse epithelial cells. J Cell Physiol. 1997;170:200–8. doi: 10.1002/(SICI)1097-4652(199702)170:2<200::AID-JCP12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Sakla MS, Shenouda NS, Ansell PJ, Macdonald RS, Lubahn DB. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine. 2007;32:69–78. doi: 10.1007/s12020-007-9006-1. [DOI] [PubMed] [Google Scholar]
- 45.Aptel HB, Burnay MM, Rossier MF, Capponi AM. The role of tyrosine kinases in capacitative calcium influx-mediated aldosterone production in bovine adrenal zona glomerulosa cells. J Endocrinol. 1999;163:131–8. doi: 10.1677/joe.0.1630131. [DOI] [PubMed] [Google Scholar]
- 46.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Research. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 48.Rabindran SK, He H, Singh M, Brown E, Collins KI, Annable T, Greenberger LM. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Research. 1998;58:5850–8. [PubMed] [Google Scholar]
- 49.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 50.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg ACLM, Schinkel AH, van de Vijver MJ, Scheper RJ, Schellens JHM. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Research. 2001;61:3458–3464. [PubMed] [Google Scholar]
- 51.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–63. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 52.Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–70. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–30. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Alvarez AI, Real R, Perez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- 55.van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis. 2006;27:123–130. doi: 10.1093/carcin/bgi176. [DOI] [PubMed] [Google Scholar]
- 56.Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312:144–52. doi: 10.1124/jpet.104.073916. [DOI] [PubMed] [Google Scholar]
- 57.Allen JD, Van Dort SC, Buitelaar M, van Tellingen O, Schinkel AH. Mouse breast cancer resistance protein (Bcrp1/Abcg2) mediates etoposide resistance and transport, but etoposide oral availability is limited primarily by P-glycoprotein. Cancer Research. 2003;63:1339–44. [PubMed] [Google Scholar]
- 58.Burger H, van Tol H, Boersma AWM, Brok M, Wiemer EAC, Stoter G, Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 59.Volk EL, Farley KM, Wu Y, Li F, Robey RW, Schneider E. Overexpression of wild-type breast cancer resistance protein mediates methotrexate resistance. Cancer Research. 2002;62:5035–40. [PubMed] [Google Scholar]
- 60.Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci US A. 2002;99:15649–54. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brangi M, Litman T, Ciotti M, Nishiyama K, Kohlhagen G, Takimoto C, Robey R, Pommier Y, Fojo T, Bates SE. Camptothecin resistance: role of the ATP-binding cassette (ABC), mitoxantrone-resistance half-transporter (MXR), and potential for glucuronidation in MXR-expressing cells. Cancer Research. 1999;59:5938–46. [PubMed] [Google Scholar]
- 62.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64:610–8. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 63.Chen ZS, Robey RW, Belinsky MG, Shchaveleva I, Ren XQ, Sugimoto Y, Ross DD, Bates SE, Kruh GD. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Research. 2003;63:4048–54. [PubMed] [Google Scholar]
- 64.Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 65.Nakayama A, Matsuo H, Takada T, Ichida K, Nakamura T, Ikebuchi Y, Ito K, Hosoya T, Kanai Y, Suzuki H, Shinomiya N. ABCG2 is a high-capacity urate transporter and its genetic impairment increases serum uric acid levels in humans. Nucleosides, Nucleotides and Nucleic Acids. 2011;30:1091–1097. doi: 10.1080/15257770.2011.633953. [DOI] [PubMed] [Google Scholar]
- 66.Wang SW, Chen Y, Joseph T, Hu M. Variable isoflavone content of red clover products affects intestinal disposition of biochanin A, formononetin, genistein, and daidzein. J Altern Complement Med. 2008;14:287–97. doi: 10.1089/acm.2007.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.An G, Morris ME. The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2) Biopharm Drug Dispos. 2011;32:446–57. doi: 10.1002/bdd.772. [DOI] [PubMed] [Google Scholar]
- 68.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem Biophys Res Commun. 2004;317:269–75. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 69.Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–75. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomas-Barberan F, Espin JC. Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice. Drug Metab Dispos. 2011;39:2008–12. doi: 10.1124/dmd.111.040881. [DOI] [PubMed] [Google Scholar]
- 71.Imai Y, Tsukahara S, Asada S, Sugimoto Y. Phytoestrogens/Flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Research. 2004;64:4346–4352. doi: 10.1158/0008-5472.CAN-04-0078. [DOI] [PubMed] [Google Scholar]
- 72.Zhu W, Xu H, Wang SW, Hu M. Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. AAPS J. 2010;12:525–36. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang Z, Zhu W, Gao S, Yin T, Jiang W, Hu M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:1883–93. doi: 10.1124/dmd.111.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sesink AL, Arts IC, de Boer VC, Breedveld P, Schellens JH, Hollman PC, Russel FG. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol Pharmacol. 2005;67:1999–2006. doi: 10.1124/mol.104.009753. [DOI] [PubMed] [Google Scholar]
- 75.Litman T, Brangi M, Hudson E, Fetsch P, Abati A, Ross DD, Miyake K, Resau JH, Bates SE. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) J Cell Sci. 2000;113(Pt 11):2011–21. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 76.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–12. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 77.Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Research. 2004;64:1242–6. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 78.Haslam IS, Wright JA, O’Reilly DA, Sherlock DJ, Coleman T, Simmons NL. Intestinal ciprofloxacin efflux: the role of breast cancer resistance protein (ABCG2) Drug Metab Dispos. 2011;39:2321–8. doi: 10.1124/dmd.111.038323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Real R, Egido E, Perez M, Gonzalez-Lobato L, Barrera B, Prieto JG, Alvarez AI, Merino G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: interaction with ivermectin. J Vet Pharmacol Ther. 2011;34:313–21. doi: 10.1111/j.1365-2885.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 80.Pulido MM, Molina AJ, Merino G, Mendoza G, Prieto JG, Alvarez AI. Interaction of enrofloxacin with breast cancer resistance protein (BCRP/ABCG2): influence of flavonoids and role in milk secretion in sheep. J Vet Pharmacol Ther. 2006;29:279–87. doi: 10.1111/j.1365-2885.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 81.Gedeon C, Behravan J, Koren G, Piquette-Miller M. Transport of glyburide by placental ABC transporters: implications in fetal drug exposure. Placenta. 2006;27:1096–102. doi: 10.1016/j.placenta.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–64. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- 83.Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos. 2009;37:946–55. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]
- 84.Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, Sugimoto Y. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Mol Cancer Ther. 2004;3:1119–25. [PubMed] [Google Scholar]
- 85.Houghton PJ, Germain GS, Harwood FC, Schuetz JD, Stewart CF, Buchdunger E, Traxler P. Imatinib mesylate is a potent inhibitor of the ABCG2 (BCRP) transporter and reverses resistance to topotecan and SN-38 in vitro. Cancer Research. 2004;64:2333–7. doi: 10.1158/0008-5472.can-03-3344. [DOI] [PubMed] [Google Scholar]
- 86.Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002;1:417–25. [PubMed] [Google Scholar]
- 87.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–16. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 88.An G, Morris ME. Effects of the isoflavonoid biochanin A on the transport of mitoxantrone in vitro and in vivo. Biopharm Drug Dispos. 2010;31:340–50. doi: 10.1002/bdd.717. [DOI] [PubMed] [Google Scholar]
- 89.Merino G, Perez M, Real R, Egido E, Prieto J, Alvarez A. In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: a comparative study in Bcrp1 (−/−) mice. Pharmaceutical Research. 2010;27:2098–2105. doi: 10.1007/s11095-010-0208-5. [DOI] [PubMed] [Google Scholar]
- 90.Tamaki H, Satoh H, Hori S, Ohtani H, Sawada Y. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metabolism and Pharmacokinetics. 2010;25:170–179. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 91.Imai Y, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Estrone and 17beta-estradiol reverse breast cancer resistance protein-mediated multidrug resistance. Jpn J Cancer Res. 2002;93:231–5. doi: 10.1111/j.1349-7006.2002.tb02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gupta A, Dai Y, Vethanayagam RR, Hebert MF, Thummel KE, Unadkat JD, Ross DD, Mao Q. Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006;58:374–83. doi: 10.1007/s00280-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 93.Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, Schinkel AH, van Tellingen O, Borst P, Schellens JH. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Research. 2004;64:5804–11. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 94.de Bruin M, Miyake K, Litman T, Robey R, Bates SE. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer letters. 1999;146:117–26. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 95.Mease K, Sane R, Podila L, Taub ME. Differential selectivity of efflux transporter inhibitors in Caco-2 and MDCK-MDR1 monolayers: a strategy to assess the interaction of a new chemical entity with P-gp, BCRP, and MRP2. J Pharm Sci. 2012;101:1888–97. doi: 10.1002/jps.23069. [DOI] [PubMed] [Google Scholar]
- 96.Kobayashi S, Shinohara M, Nagai T, Konishi Y. Transport mechanisms for soy isoflavones and microbial metabolites dihydrogenistein and dihydrodaidzein across monolayers and membranes. Biosci Biotechnol Biochem. 2013;77:2210–7. doi: 10.1271/bbb.130404. [DOI] [PubMed] [Google Scholar]
- 97.Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J Pharmacol Exp Ther. 2011;339:935–44. doi: 10.1124/jpet.111.180398. [DOI] [PubMed] [Google Scholar]
- 98.van de Wetering K, Sapthu S. ABCG2 functions as a general phytoestrogen sulfate transporter in vivo. FASEB J. 2012;26:4014–24. doi: 10.1096/fj.12-210039. [DOI] [PubMed] [Google Scholar]
- 99.Jiang W, Xu B, Wu B, Yu R, Hu M. UDP-glucuronosyltransferase (UGT) 1A9-overexpressing HeLa cells is an appropriate tool to delineate the kinetic interplay between breast cancer resistance protein (BRCP) and UGT and to rapidly identify the glucuronide substrates of BCRP. Drug Metab Dispos. 2012;40:336–45. doi: 10.1124/dmd.111.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang S, Yang X, Morris M. Combined effects of multiple flavonoids on breast cancer resistance protein (ABCG2)-mediated transport. Pharmaceutical Research. 2004;21:1263–1273. doi: 10.1023/b:pham.0000033015.84146.4c. [DOI] [PubMed] [Google Scholar]
- 101.Katayama K, Masuyama K, Yoshioka S, Hasegawa H, Mitsuhashi J, Sugimoto Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: transporter specificity and structure-activity relationship. Cancer Chemother Pharmacol. 2007;60:789–97. doi: 10.1007/s00280-007-0426-7. [DOI] [PubMed] [Google Scholar]
- 102.Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007;96:227–36. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 103.Arias A, Rigalli JP, Villanueva SS, Ruiz ML, Luquita MG, Perdomo VG, Vore M, Catania VA, Mottino AD. Regulation of expression and activity of multidrug resistance proteins MRP2 and MDR1 by estrogenic compounds in Caco-2 cells. Role in prevention of xenobiotic-induced cytotoxicity. Toxicology. 2014;320:46–55. doi: 10.1016/j.tox.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Huang W, Wan C, Luo Q, Huang Z, Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci. 2014;15:3432–43. doi: 10.3390/ijms15033432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perantoni A, Berman JJ. Properties of Wilms’ tumor line (TuWi) and pig kidney line (LLC-PK1) typical of normal kidney tubular epithelium. In vitro. 1979;15:446–54. doi: 10.1007/BF02618414. [DOI] [PubMed] [Google Scholar]
- 106.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro P-glycoprotein assays in drug discovery. J Pharmacol Exp Ther. 2001;299:620–8. [PubMed] [Google Scholar]
- 107.Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Research. 1988;48:1936–42. [PubMed] [Google Scholar]
- 108.Xia CQ, Liu N, Yang D, Miwa G, Gan LS. Expression, localization, and functional characteristics of breast cancer resistance protein in Caco-2 cells. Drug Metab Dispos. 2005;33:637–43. doi: 10.1124/dmd.104.003442. [DOI] [PubMed] [Google Scholar]
- 109.Englund G, Rorsman F, Ronnblom A, Karlbom U, Lazorova L, Grasjo J, Kindmark A, Artursson P. Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci. 2006;29:269–77. doi: 10.1016/j.ejps.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Hendrich S, Murphy PA. Glucuronides are the main isoflavone metabolites in women. J Nutr. 2003;133:399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]
- 111.Vaidya SS, Walsh SW, Gerk PM. Formation and efflux of ATP-binding cassette transporter substrate 2,4-dinitrophenyl-S-glutathione from cultured human term placental villous tissue fragments. Mol Pharm. 2009;6:1689–702. doi: 10.1021/mp900019z. [DOI] [PubMed] [Google Scholar]
- 112.Belliard AM, Lacour B, Farinotti R, Leroy C. Effect of tumor necrosis factor-alpha and interferon-gamma on intestinal P-glycoprotein expression, activity, and localization in Caco-2 cells. J Pharm Sci. 2004;93:1524–36. doi: 10.1002/jps.20072. [DOI] [PubMed] [Google Scholar]
- 113.Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH. Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther. 2004;311:476–84. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- 114.Alvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomas-Barberan F, Espin JC. Reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:2219–20. doi: 10.1124/dmd.112.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Enokizono J, Kusuhara H, Sugiyama Y. Regional expression and activity of breast cancer resistance protein (Bcrp/Abcg2) in mouse intestine: overlapping distribution with sulfotransferases. Drug Metab Dispos. 2007;35:922–8. doi: 10.1124/dmd.106.011239. [DOI] [PubMed] [Google Scholar]
- 116.Rost D, Mahner S, Sugiyama Y, Stremmel W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282:G720–6. doi: 10.1152/ajpgi.00318.2001. [DOI] [PubMed] [Google Scholar]
- 117.van de Wetering K, Feddema W, Helms JB, Brouwers JF, Borst P. Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology. 2009;137:1725–35. doi: 10.1053/j.gastro.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 118.Bayer T, Colnot T, Dekant W. Disposition and biotransformation of the estrogenic isoflavone daidzein in rats. Toxicol Sci. 2001;62:205–11. doi: 10.1093/toxsci/62.2.205. [DOI] [PubMed] [Google Scholar]
- 119.Zhou S, Hu Y, Zhang B, Teng Z, Gan H, Yang Z, Wang Q, Huan M, Mei Q. Dose-dependent absorption, metabolism, and excretion of genistein in rats. J Agric Food Chem. 2008;56:8354–9. doi: 10.1021/jf801051d. [DOI] [PubMed] [Google Scholar]
- 120.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Research. 2004;64:3296–301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]
- 121.Dankers AC, Sweep FC, Pertijs JC, Verweij V, van den Heuvel JJ, Koenderink JB, Russel FG, Masereeuw R. Localization of breast cancer resistance protein (Bcrp) in endocrine organs and inhibition of its transport activity by steroid hormones. Cell Tissue Res. 2012;349:551–63. doi: 10.1007/s00441-012-1417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–7. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 123.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vanhees K, Coort S, Ruijters EJ, Godschalk RW, van Schooten FJ, Barjesteh van Waalwijk van Doorn-Khosrovani S. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- 125.Pick A, Muller H, Mayer R, Haenisch B, Pajeva IK, Weigt M, Bonisch H, Muller CE, Wiese M. Structure-activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP) Bioorg Med Chem. 2011;19:2090–102. doi: 10.1016/j.bmc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 126.Zhang SJ, Sun D, Hao JB, Wei YF, Yin LF, Liu X. The effect of dietary soyabean isoflavones on photodynamic therapy in K562 leukemia cells. J Photochem Photobiol B. 2012;110:28–33. doi: 10.1016/j.jphotobiol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 127.Perez M, Otero JA, Barrera B, Prieto JG, Merino G, Alvarez AI. Inhibition of ABCG2/BCRP transporter by soy isoflavones genistein and daidzein: effect on plasma and milk levels of danofloxacin in sheep. Vet J. 2013;196:203–8. doi: 10.1016/j.tvjl.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 128.Gardner CD, Chatterjee LM, Franke AA. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem. 2009;20:227–34. doi: 10.1016/j.jnutbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glavinas H, Kis E, Pal A, Kovacs R, Jani M, Vagi E, Molnar E, Bansaghi S, Kele Z, Janaky T, Bathori G, von Richter O, Koomen GJ, Krajcsi P. ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug Metab Dispos. 2007;35:1533–42. doi: 10.1124/dmd.106.014605. [DOI] [PubMed] [Google Scholar]
- 130.Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, Schinkel AH. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–9. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- 131.American Academy of Pediatrics: Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–89. [PubMed] [Google Scholar]
- 132.Perez M, Real R, Mendoza G, Merino G, Prieto JG, Alvarez AI. Milk secretion of nitrofurantoin, as a specific BCRP/ABCG2 substrate, in assaf sheep: modulation by isoflavones1. J Vet Pharmacol Ther. 2009;32:498–502. doi: 10.1111/j.1365-2885.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 133.Health Protection Agency. [Accessed July 19, 2014];Management of infection guidance for primary care for consultation and local adaption. 2013 ( http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/PrimaryCareGuidance/)
- 134.Escudero E, Carceles CM, Fernandez-varon E, Marin P, Benchaoui H. Pharmacokinetics of danofloxacin 18% in lactating sheep and goats. J Vet Pharmacol Ther. 2007;30:572–7. doi: 10.1111/j.1365-2885.2007.00898.x. [DOI] [PubMed] [Google Scholar]
- 135.Gerk PM, Kuhn RJ, Desai NS, McNamara PJ. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001;21:669–75. doi: 10.1592/phco.21.7.669.34574. [DOI] [PubMed] [Google Scholar]
- 136.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–82. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J Nutr. 1999;129:399–405. doi: 10.1093/jn/129.2.399. [DOI] [PubMed] [Google Scholar]
- 138.O’Leary KA, Day AJ, Needs PW, Sly WS, O’Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–6. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 139.Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118:1196–203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Randak C, Auerswald EA, Assfalg-Machleidt I, Reenstra WW, Machleidt W. Inhibition of ATPase, GTPase and adenylate kinase activities of the second nucleotide-binding fold of the cystic fibrosis transmembrane conductance regulator by genistein. Biochem J. 1999;340(Pt 1):227–35. [PMC free article] [PubMed] [Google Scholar]
- 141.Di Pietro A, Conseil G, Perez-Victoria JM, Dayan G, Baubichon-Cortay H, Trompier D, Steinfels E, Jault JM, de Wet H, Maitrejean M, Comte G, Boumendjel A, Mariotte AM, Dumontet C, McIntosh DB, Goffeau A, Castanys S, Gamarro F, Barron D. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell Mol Life Sci. 2002;59:307–22. doi: 10.1007/s00018-002-8424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uehar M, Arai Y, Watanabe S, Adlercreutz H. Comparison of plasma and urinary phytoestrogens in Japanese and Finnish women by time-resolved fluoroimmunoassay. BioFactors. 2000;12:217–25. doi: 10.1002/biof.5520120134. [DOI] [PubMed] [Google Scholar]
- 143.Frankenfeld CL, Patterson RE, Horner NK, Neuhouser ML, Skor HE, Kalhorn TF, Howald WN, Lampe JW. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am J Clin Nutr. 2003;77:674–80. doi: 10.1093/ajcn/77.3.674. [DOI] [PubMed] [Google Scholar]
- 144.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 145.Soy Foods Association of North America. [Accessed August 13, 2014];Soy Products: Sales and Trends. ( http://www.soyfoods.org/soy-products/sales-and-trends)
- 146.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: US prescription drug data for 2007–2008. Hyattsville, MD: National Center for Health Statistics; 2010. pp. 1–8. NCHS data brief, no 42. [PubMed] [Google Scholar]