Abstract
Background
Non-Hodgkin lymphoma (NHL) comprises distinct tumor subtypes. While mortality from NHL overall has changed dramatically in the U.S. over time, little is known about trends for subtypes, because death certificates do not record this information.
Methods
Using data from U.S. States Surveillance, Epidemiology, and End Results (SEER) areas, we assessed NHL mortality rates and mapped NHL deaths to incident NHL cases in SEER cancer registries. This allowed us to evaluate population-level mortality trends attributed to specific NHL subtypes (incidence-based mortality [IBM]). We also describe NHL incidence and survival after NHL diagnosis by calendar year. We used Joinpoint to identify years when IBM and incidence rate trends changed slope.
Results
Overall NHL mortality rates increased during 1975-1997, peaking at 10.9 per 100,000 person-years, then decreased subsequently in 1997-2011. Overall IBM rates mirror this trend during 1990-2011. For B-cell NHL subtypes, IBM rates decreased beginning in the mid-1990s, with yearly declines of -3.0% for diffuse large B-cell lymphoma (DLBCL), -2.7% for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and -5.3% for follicular lymphoma. Incidence rates for these subtypes did not decrease until after 2003. Corresponding five-year cancer-specific survival increased dramatically over time for DLBCL (from 37% to 66%), CLL/SLL (69%-84%), and follicular lymphoma (-69%-82%). IBM for peripheral T-cell lymphoma was flat during 2006-2011, although incidence increased.
Conclusions
Mortality due to three common B-cell NHL subtypes has fallen over time in the U.S.
Impact
This decline reflects better survival after NHL diagnosis, likely from improved therapies, because the decline in NHL incidence occurred later.
Keywords: Incidence-based mortality, Partition mortality trends, Non-Hodgkin lymphoma tumor subtype trends, SEER Registries, Histologic Subtypes
Introduction
Non-Hodgkin lymphoma (NHL) comprises a heterogeneous group of malignancies arising from lymphoid tissue, with varied clinical and biological features (1). The three most common NHL subtypes, accounting for about two-thirds of NHLs, are derived from B-cells: diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), and follicular lymphoma (FL) (2-5). Peripheral T-cell lymphoma (PTCL) is the most common subtype derived from T-cells and accounts for about approximately 50% of T-cell NHLs (2-5).
While incidence trends for distinct NHL subtypes are well described (6-11), little is known about trends in mortality, which reflect the combined effects of NHL incidence and survival after NHL diagnosis. Notably, treatment advances may have improved survival for some but not all NHL subtypes (12-18). US national data on causes of death (CODs) reported on death certificates make it possible to assess trends in mortality for NHL overall. Overall NHL mortality rates showed a steady and significant increase from 1975-1991 at a rate of 2.5% annually, followed by a slower but steady increase in mortality rates during 1991-1997 at a rate of 1.6% annually (5). After reaching a peak in 1997, NHL mortality started to decline with a 3.1% yearly decrease through 2006 and a continuous decrease through 2011 (5). Decomposing this mortality, however, into trends for NHL subtypes is not directly possible because death certificates do not record the subtype.
To provide a resource to address this limitation in mortality data, the US Surveillance, Epidemiology, and End Results (SEER) cancer registry program has linked mortality records to incident cancer cases. As a result, it is possible to calculate “incidence-based mortality” (IBM) rates, which capture population-level mortality attributable to particular tumors reported to SEER registries (19, 20). The numerator for the IBM rate consists of the number of cancer-specific deaths among persons with a particular cancer diagnosis reported to the cancer registry. The denominator for the rate is the person-time in the general population who are at risk at the time of death in the SEER areas. This approach allows for the partition of the general population mortality rate according to characteristics associated with the cancer diagnosis (e.g., tumor subtypes) that are recorded in the SEER registries. The IBM method has been used to assess the impact of screening and treatment on the mortality trends for several different cancer types (19, 21-27).
In this study, we applied IBM methods to SEER data to evaluate population-level mortality trends attributed to DLBCL, CLL/SLL, FL, and PTCL in the US during 1975-2011. We also assessed contributions of NHL incidence and survival after NHL diagnosis to these trends.
Materials and Methods
All analyses used data from 9 US SEER cancer registries (1975-2011) (28) and were restricted to adults (age at least 20 years). We identified cases of DLBCL, CLL/SLL, FL, and PTCL using histology codes (29). PTCL was only assessed beginning in 1992 because of lack of specific coding in earlier years. Cases diagnosed by death certificates or autopsy were excluded. We further restricted analysis to NHLs that were the first or only cancer, to facilitate mapping to cancer-specific deaths in mortality records and also to allow comparison of mortality trends with trends in cancer-specific survival after diagnosis, which are typically derived using first or only cancers. NHL incidence rates were calculated after accounting for reporting delays (30).
CODs were ascertained from death certificates obtained by the National Center for Health Statistics (31). We report mortality rates based on deaths from NHL (see Supplementary Figure 1 legend for specific codes for NHL). For IBM rates, we used linked SEER data on NHL cases to classify these NHL deaths according to NHL histologic subtype. Additionally, because some deaths in NHL patients may have been inaccurately recorded as deaths from other hematologic malignancies (32), we also included in the IBM rates deaths recorded as due to Hodgkin lymphoma, plasma cell neoplasms, or leukemia if they linked to a SEER NHL case (Figure 1 legend). Of the 57,301 total hematologic cancer deaths observed among incident NHL cases in SEER, 42,582 (74.3%) deaths were coded as NHL COD in the death certificate, 14,179 (24.7%) as leukemia COD, 316 (0.6%) as Hodgkin lymphoma COD, and 224 (0.4%) as plasma cell neoplasms COD. With the 2001 WHO classification, CLL is now considered to be synonymous with SLL and is considered part of NHL; therefore, we felt it was especially important to include leukemia CODs to capture deaths related to this subtype of NHL. Indeed, of the 14,179 deaths among NHL cases that were classified as due to leukemia, 9,679 (68.2%) were in CLL/SLL cases, less than 1% were found in DLBCL, FL, or PTCL cases, and the remaining 3,579 (25.2%) were in cases with other NHL subtypes that we did not assess. Also, among the 54,369 deaths during 1975-2011 that had NHL as the COD 7,704 (14.2%) did not link to a SEER NHL diagnosis. These deaths may have been misclassified, or the person may have had NHL diagnosed outside a SEER area or before SEER ascertainment of cancer diagnoses in 1975.
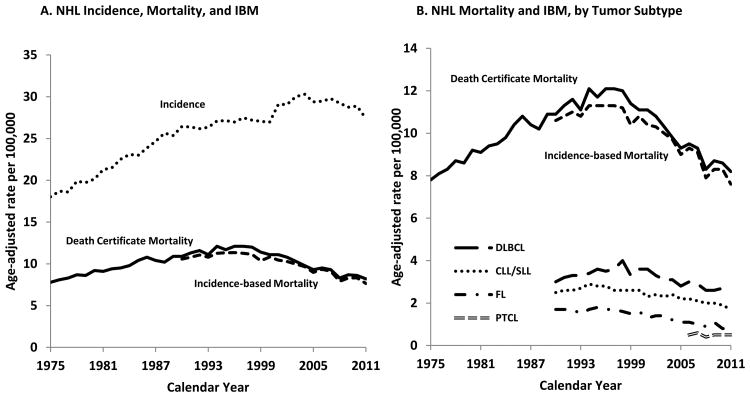
Figure 1. Non-Hodgkin Lymphoma (NHL) Incidence, Death Certificate Mortality, and Incidence-based Mortality (IBM) Rates. SEER-9, 1975-2011.
In panel A, results are shown for NHL death certificate mortality (black solid line), IBM (black dashed line, starting in 1990), and incidence (black dotted line). In panel B, the results for NHL death certificate mortality and IBM are presented again along with the IBM for four NHL subtypes: DLBCL (big dashed line), CLL/SLL (dotted dashed line), FL (dashed followed by dotted line), and PTCL (grey dashed line). Note that the vertical axis differs in panels A and B. Results are shown for SEER-9 registries. See Supplemental Figure 1 legend for a description of these registries and codes for NHL mortality and IBM CODs.
IBM rates are valid for a shorter range of calendar years than death certificate mortality. Since IBM rates are calculated based on the registry incident cases, and some deaths may occur many years after cancer diagnosis, IBM rates are underestimated in the initial years of cancer registration (see Supplementary Figure 1 for more details). Because median survival after NHL diagnosis is more than a decade, some cancer-specific deaths occur late after NHL diagnosis. For this reason, we required 15 years of data on incident cases before each year of mortality data (sometimes known as the “burn-in” period for IBM rates), to ensure capture of almost all deaths from NHL. Thus, we present IBM rates only for 1990-2011 (2006-2011 for PTCL).
We present rates for mortality, IBM, and incidence according to calendar year. We used Joinpoint to characterize piecewise log-linear time calendar trends in the age-standardized rates (33, 34). Incidence trends during 1975-2011 were fitted with up to a maximum of 5 joinpoints, while the PTCL incidence trend during 1992-2011 was fitted with up to 3 joinpoints. Since IBM rates were available for a shorter range of calendar years, these trends were fitted with up to 3 joinpoints (1 joinpoint for PTCL). The resulting trend across each calendar intervals is described by the slope of the line segment (i.e., annual percentage change, APC) (34). We used t-tests to assess whether APCs were statistically different from zero. All statistical tests were two-sided.
Finally, we present estimates of 5-year cancer-specific survival (i.e., the probability that a person with NHL did not die from NHL) according to NHL subtype and diagnosis year. All estimates except those for survival were age-standardized to the 2000 US general population. We have age-adjusted NHL cancer-specific survival using the International Cancer Survival Standard 1 (ICSS 1) (35).
Results
Among people living in US SEER areas during 1975-2011, overall NHL mortality rates increased during 1975-1997, peaking at 10.9 per 100,000 person-years, then decreased subsequently in 1997-2011 (Figure 1A). IBM rates closely mirrored overall NHL mortality rates for the period for which they could be evaluated (1990-2011). When IBM rates were examined for individual NHL subtypes, the IBM rates for each subtype excluding PTCL increased or were flat during the early 1990s and then decreased after approximately 1995, depending on the subtype (Figure 1B). Of note, incidence rates for NHL overall increased during 1975-2002 and then started decreasing, so that the decline in incidence started later than the decline in mortality (Figure 1A).
Based on the IBM rates, DLBCL contributed the most to NHL mortality, followed by CLL/SLL, FL, and PTCL. For example, in 2011, 33% of all NHL deaths were due to DLBCL, 22% to CLL/SLL, 11% to FL, and 7% to PTCL. The remaining 27% of NHL deaths were due to other subtypes combined. The proportion of total NHL incident cases that contributed to NHL deaths over the recent 15 year period (1997-2011) are as follows: 27% DLBCL, 23% CLL/SLL 14% FL, 5% PTCL, and 31% from other subtypes combined (however, we note that the proportion for PTCL is under-estimated because the IBM for this subtype could be assessed only for 2006-2011).
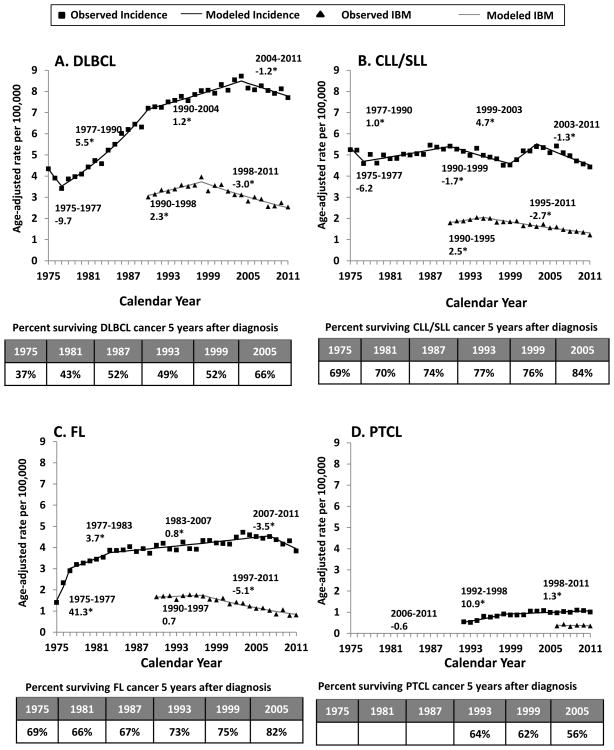
Figure 2 presents results for each NHL subtype separately. For DLBCL (Figure 2A), incidence increased steeply during 1977-1990 and more slowly through 2004, then decreased beginning in 2004. In contrast, IBM increased only until 1998 and subsequently fell by -3.0% per year. Five-year age-standardized cancer-specific survival increased dramatically over time, from 37% for cases diagnosed in 1975 to 66% in 2005.
Figure 2. Non-Hodgkin Lymphoma (NHL) Incidence, Incidence-based Mortality (IBM), and Survival Trends.
Results are shown for DLBCL (panel A), CLL/SLL (panel B), FL (panel C), and PTCL (panel D). For each panel, we show age-adjusted incidence rates in black (observed rates as squares, modeled rates as lines) and age-adjusted IBM rates in black (observed rates as triangles, and modeled rates as lines). Incidence rates were adjusted for reporting delays. The line segments of each curve were selected using the Joinpoint program, and the numbers on the graph indicate the slopes of each line segment denoted as annual percent change (APC). Asterisks (*) are used to denote APCs that are significantly different from zero (p-value <0.05). We also show 5-year age-standardized cancer-specific survival according to year of NHL diagnosis in a table at the bottom of each panel. Results are shown for SEER-9 registries. The following ICD-O-3 histology codes were used to define each of the four main subtypes: DLBCL (9678, 9679, 9680, 9684, 9688, 9712, 9737-9738), CLL/SLL (9670, 9823), FL (9690, 9691, 9695, 9698), and PTCL (9675, 9702, 9705, 9708, 9714, 9716, 9717, 9718, 9709, 9726). See Supplemental Figure 1 legend for a description of these registries and codes for NHL mortality and IBM CODs.
For CLL/SLL (Figure 2B), incidence fluctuated during 1975-2011. In contrast, IBM increased during 1990-1995 and then declined by -2.7% per year. Five-year age-standardized cancer-specific survival increased from 69% to 84% during 1975-2005.
Beginning in 1975, FL incidence increased steeply for three years and gradually until 2007, then declined (Figure 2C). The corresponding IBM trend was flat during 1990-1997 and then declined steeply (-5.1% per year). Five-year age-standardized cancer-specific survival for FL was better than for DLBCL and CLL/SLL, although there was a less clear improvement over calendar time until after 1999 (improving from 75% in 1999 to 82% in 2005).
For PTCL (Figure 2D), incidence increased sharply during 1992-1998 then moderated in slope. In contrast to the three other subtypes, there was no clear trend in IBM, although the range of calendar years was limited and the subtype was much rarer. Five-year age-standardized cancer-specific survival seemed to be decreasing over 1993-2005 (64%-56%), however there is substantial uncertainty in these estimates (data not shown).
Discussion
This is the first study to describe trends in mortality for individual NHL tumor subtypes in the context of changing patterns of incidence and survival. We found that overall mortality rates for NHL increased in the US general population from 1975-1997 and then decreased. Similar patterns in mortality, based on IBM methods, were observed specifically for DLBCL, CLL/SLL, and FL subtypes during the 1990-2011 period, with peaks in IBM noted in 1995-1998. Mortality rates for PTCL remained unchanged over a shorter time period (2006-2011).
Notably, the mortality trends for the three most common NHL subtypes (DLBCL, CLL/SLL, and FL) decreased before there was a decline in incidence, indicating that the reduction in NHL mortality can best be explained by improved survival after NHL diagnosis. We hypothesize that these changes in survival reflect improvements in NHL therapy. One notable improvement was the addition of rituximab to standard chemotherapy (CHOP, consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone) beginning in 1998 (14). With the advent of rituximab, most DLBCL and FL cases have been treated with this initial regimen (14, 17, 18, 36), which has led to improved survival. For example, DLBCL survival increased from 52% to 66% and FL survival increased from 75% to 82% during 1999-2005. In the 1990s, fludarabine and rituximab were also introduced as new agents to treat CLL/SLL (37).
The incidence of DLBCL, CLL/SLL, and FL increased until the mid-2000s before declining. Many factors could have contributed to these incidence trends. Our analyses classify incident NHL using morphology codes abstracted from pathology reports by SEER registries. An overall reliability of 77% for translation of individual codes from Internation Classification of Diseases for Oncology, 2nd edition to 3rd edition, (ICD-O-2) to (ICD-O-3) has been reported by Clarke et al. (38). Moreover, Clarke et al. (2) and Morton et al. (38, 39) reported that when the individual codes were grouped into subtypes, reliability was even greater (>80%) for the major subtypes that we include in our analysis. In a recent study, Proctor et al., (40) reported a similar level of discordance (20%-26%) between expert versus non-expert pathology review of DLBCL, FL, CLL/SLL subtypes. Agreement among pathologists has increased over time (40), but it is unclear how this might have impacted the trends we present. Because we recognized that the classification has evolved for T-cell lymphomas, we conservatively started analyses for the PTCL subtype later (1992+). Also, some known risk factors may have also affected the trends. For example, the human immunodeficiency virus epidemic contributed to the increase in NHL incidence in the 1980s and through the mid-1990s, especially for DLBCL (6, 41, 42). It is unclear whether changes in the prevalence of other NHL risk factors (e.g., cigarette smoking, autoimmune conditions, hepatitis C virus infection, organ transplantation) might explain the incidence trends (43-47).
Our results are population-based and incorporate high quality cancer registry data from the SEER program, which reliably captures and classifies newly diagnosed cancer incident cases in the registry catchment areas. Nonetheless, some challenges of IBM methods should be considered. First, since IBM rates are derived based on deaths that are linked to incident cases from previous years, the follow-up of cancer cases diagnosed in the past is required. The number of years that are required is a function of the pattern of recurrences for the cancer under study, i.e., for cancers with late recurrences more years of follow-up are required to capture the majority of deaths (19). Because patients with NHL can experience late recurrences, we chose to require 15 years of follow-up after an NHL diagnosis, which restricted the range of years for which we could derive IBM rates. Second, cancer patients moving in or out of their registry catchment area can cause mismatches between IBM and death certificate mortality rates (19). Third, IBM methods under-estimate mortality if the cancer registries under-ascertain the incident cancer cases. While SEER registries have high standards for completeness in capturing incident cancers, this is a concern for CLL/SLL, which is increasingly diagnosed and treated in physicians' offices rather than hospital settings (48).
We modified the standard IBM methods slightly to include a range of CODs related to hematologic malignancies, and counted such deaths as long as they linked to an incident NHL case in SEER. This approach, which recognizes that some cancer deaths are miscoded to the wrong type of cancer (32), was especially important given the evolving classification of hematological malignancies over the last two decades. By using broad hematologic death coding, we attributed treatment-related myeloid leukemia deaths to the index NHL diagnosis. Such attribution would be appropriate in these cases, because the NHLs can be considered the indirect cause of the leukemia death, and in any event, these outcomes were likely very rare ( 49,50). Our approach allowed us to assign 69% of all NHL deaths to three common NHL subtypes for the period 1990-2011, and 73% to four subtypes for 2006-2011. Reassuringly, our overall IBM rates closely mirrored the death certificate mortality rates for 1990-2011 (Figure 1A and 1B), although given some of the challenges listed above in mapping cancers to the corresponding deaths, this similarity may be somewhat fortuitous.
We note that data on PTCL were limited. This subtype is uncommon and was reliably identified only beginning in 1992 due to evolving diagnostic methods and classification. Indeed, the steep increase in incidence before 1998 likely reflects the increasing recognition and improved reporting of this subtype. CHOP is the standard therapy for PTCL ( 51) although new drugs (pralatrexate and romidepsin) received approval in 2009 ( 52, 53). Survival is generally described as poor, although our cancer-specific survival estimate of approximately 65% for PTCL was higher than previously suggested ( 54, 55). We find that 5-year cancer-specific survival for the PTCL cases not otherwise specified was 40%, similar to what is reported in the literature (54, 55). Our PTCL classification does include Alk+ T-cell lymphoma, and this also has a low survival rate (5-year survival 52%). The survival for the overall PTCL group is pulled up by cutaneous T-cell lymphoma not otherwise specified, which makes up a major fraction of the cases (28%) and has a relatively good survival (5-year survival 82%). Thus, inclusion of various subtypes of PTCL, especially cutaneous T-cell lymphoma, that have fairly good survival leads to the higher cancer-specific survival estimates for PTCL in our data.
In conclusion, we demonstrate a significant reduction in NHL mortality in the US general population, which was initially due to improved survival after NHL diagnosis for the three most common subtypes (DLBCL, CLL/SLL, and FL), and more recently due to a decline in incidence. Survival benefit from R-CHOP and other novel therapies for treating NHL has been demonstrated in clinical trials, but our study highlights the impact of these therapies at the population level. IBM methods should also be valuable for evaluating mortality trends for other cancer subtypes.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by the National Cancer Institute's Intramural Research Program in the Division of Cancer Epidemiology and Genetics and Surveillance Research Program in the Division of Cancer Control and Population Sciences.
Abbreviations
- IBM
Incidence-based mortality
- NHL
Non-Hodgkin lymphoma
- DLBCL
Diffuse large B-cell lymphoma
- FL
Follicular lymphoma
- CLL/SLL
Chronic lymphocytic leukemia/small lymphocytic lymphoma
- PTCL
Peripheral T-cell lymphoma
- COD
Cause of Death
- SEER
Surveillance, Epidemiology and End Results
- APC
Annual Percent Change
- ICSS 1
International Cancer Survival Standard 1
Footnotes
The authors have no conflict of interest to declare.
Reference List
- 1.Jaffe ESHN, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 2.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton LM, W S, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willett EV, Morton LM, Hartge P, Becker N, Bernstein L, Boffetta P, et al. Non-Hodgkin lymphoma and obesity: A pooled analysis from the InterLymph Consortium. International Journal of Cancer. 2008;122:2062–70. doi: 10.1002/ijc.23344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlader N, Noone Anne-Michelle, Krapcho M. SEER Cancer Statistics Review (CSR) 1975-2011. available from: http://seer.cancer.gov/csr/1975_2011/
- 6.Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, et al. The Epidemic of Non–Hodgkin Lymphoma in the United States: Disentangling the Effect of HIV, 1992–2009. Cancer Epidemiology Biomarkers & Prevention. 2013;22:1069–78. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosetti C, Levi F, Ferlay J, Lucchini F, Negri E, La Vecchia C. Incidence and mortality from non-Hodgkin lymphoma in Europe: The end of an epidemic? International Journal of Cancer. 2008;123:1917–23. doi: 10.1002/ijc.23722. [DOI] [PubMed] [Google Scholar]
- 8.Muller AMS, Ihorst G, Mertelsmann R, Engelhardt M. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Annals of Hematology. 2005;84:1–12. doi: 10.1007/s00277-004-0939-7. [DOI] [PubMed] [Google Scholar]
- 9.Clarke CA. Changing incidence of Kaposi's sarcoma and non-Hodgkin's lymphoma among young men in San Francisco. Aids. 2001;15:1913–5. doi: 10.1097/00002030-200109280-00035. [DOI] [PubMed] [Google Scholar]
- 10.Clarke CA, Glaser SL. Changing incidence of non-Hodgkin lymphomas in the United States. Cancer. 2002;94:2015–23. doi: 10.1002/cncr.10403. [DOI] [PubMed] [Google Scholar]
- 11.Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ. Trends in Kaposi's Sarcoma and Non-Hodgkin's Lymphoma Incidence in the United States From 1973 Through 1998. Journal of the National Cancer Institute. 2002;94:1204–10. doi: 10.1093/jnci/94.16.1204. [DOI] [PubMed] [Google Scholar]
- 12.Monfardini S, Banfi A, Bonadonna G, Rilke F, Milani F, Valagussa P, et al. Improved 5 Year Survival After Combined Radiotherapy-Chemotherapy For Stage-I-II Non-Hodgkins Lymphoma. International Journal of Radiation Oncology Biology Physics. 1980;6:125–34. doi: 10.1016/0360-3016(80)90027-9. [DOI] [PubMed] [Google Scholar]
- 13.Nissen NI, Ersboll J, Hansen HS, Walbomjorgensen S, Pedersenbjergaard J, Hansen MM, et al. A Randomized Study Of Radiotherapy Versus Radiotherapy Plus Chemotherapy In Stage I-II Non-Hodgkins Lymphomas. Cancer. 1983;52:1–7. doi: 10.1002/1097-0142(19830701)52:1<1::aid-cncr2820520102>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 15.Coiffier B. What Treatment For Elderly Patients With Aggressive Lymphoma. Annals of Oncology. 1994;5:873–5. doi: 10.1093/oxfordjournals.annonc.a058722. [DOI] [PubMed] [Google Scholar]
- 16.MacManus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. Journal of Clinical Oncology. 1996;14:1282–90. doi: 10.1200/JCO.1996.14.4.1282. [DOI] [PubMed] [Google Scholar]
- 17.Swenson WT, Wooldridge JE, Lynch CF, Forman-Hoffman VL, Chrischilles E, Link BK. Improved survival of follicular lymphoma patients in the United States. Journal of Clinical Oncology. 2005;23:5019–26. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 18.Horning SJ. Follicular lymphoma, survival, and rituximab: Is it time to declare victory? Journal of Clinical Oncology. 2008;26:4537–8. doi: 10.1200/JCO.2008.16.1398. [DOI] [PubMed] [Google Scholar]
- 19.Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: An application to female breat cancer. Journal of Clinical Epidemiology. 1994;47:1451–61. doi: 10.1016/0895-4356(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 20.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-Based Mortality - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 21.Feuer EJ, Merrill RM, Hankey BF. Cancer Surveillance Series: Interpreting Trends in Prostate Cancer—Part II: Cause of Death Misclassification and the Recent Rise and Fall in Prostate Cancer Mortality. Journal of the National Cancer Institute. 1999;91:1025–32. doi: 10.1093/jnci/91.12.1025. [DOI] [PubMed] [Google Scholar]
- 22.Phipps AI, Scoggins J, Rossing MA, Li CI, Newcomb PA. Temporal Trends in Incidence and Mortality Rates for Colorectal Cancer by Tumor Location: 1975–2007. American Journal of Public Health. 2012;102:1791–7. doi: 10.2105/AJPH.2011.300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner H, Hoffmeister M, Jansen L. Comparisons of colorectal cancer mortality between screening participants and the general population are strongly biased unless an incidence-based mortality approach is used. Journal of Clinical Epidemiology. 2014;67:184–9. doi: 10.1016/j.jclinepi.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast Cancer Mortality Trends in the United States According to Estrogen Receptor Status and Age at Diagnosis. Journal of Clinical Oncology. 2007;25:1683–90. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 25.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins AS, Siegel RL, Jemal A. Racial Disparities in Stage-Specific Colorectal Cancer Mortality Rates From 1985 to 2008. Journal of Clinical Oncology. 2012;30:401–5. doi: 10.1200/JCO.2011.37.5527. [DOI] [PubMed] [Google Scholar]
- 27.Wachtel MS, Nelius T, Haynes AL, Dahlbeck S, de Riese W. PSA screening and deaths from prostate cancer after diagnosis—a population based analysis. The Prostate. 2013;73:1365–9. doi: 10.1002/pros.22680. [DOI] [PubMed] [Google Scholar]
- 28.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 29.Turner JJ, Morton LM, Linet MS, Clarke CA, Kadin ME, Vajdic CM, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116:e90–e98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clegg LX, Feuer EJ, Midthune DN, Fay MP, Hankey BF. Impact of Reporting Delay and Reporting Error on Cancer Incidence Rates and Trends. Journal of the National Cancer Institute. 2002;94:1537–45. doi: 10.1093/jnci/94.20.1537. [DOI] [PubMed] [Google Scholar]
- 31.SEER Cause of Death Recode. Available from: http://seer.cancer.gov/codrecode/
- 32.Howlader N, Ries LAG, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved Estimates of Cancer-Specific Survival Rates From Population-Based Data. Journal of the National Cancer Institute. 2010;102:1584–98. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surveillance Research Program NCI. Joinpoint Regression Program Version 4.1.0. http://surveillance.cancer.gov/joinpoint/
- 34.Kim HJ, F M, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. European Journal of Cancer. 2004;40:2307–16. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 37.CollaborativeGroup CT. Chemotherapeutic Options in Chronic Lymphocytic Leukemia: a Meta-analysis of the Randomized Trials. Journal of the National Cancer Institute. 1999;91:861–8. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 38.Clarke CA, Undurraga DM, Harasty PJ, Glaser SL, Morton LM, Holly EA. Changes in Cancer Registry Coding for Lymphoma Subtypes: Reliability Over Time and Relevance for Surveillance and Study. Cancer Epidemiology Biomarkers & Prevention. 2006;15:630–8. doi: 10.1158/1055-9965.EPI-05-0549. [DOI] [PubMed] [Google Scholar]
- 39.Clarke CA, Glaser SL, Dorfman RF, Bracci PM, Eberle E, Holly EA. Expert Review of Non-Hodgkin's Lymphomas in a Population-Based Cancer Registry: Reliability of Diagnosis and Subtype Classifications. Cancer Epidemiology Biomarkers & Prevention. 2004;13:138–43. doi: 10.1158/1055-9965.epi-03-0250. [DOI] [PubMed] [Google Scholar]
- 40.Proctor IE, McNamara C, Justo MR, Isaacson PG, Ramsay A. Importance of Expert Central Review in the Diagnosis of Lymphoid Malignancies in a Regional Cancer Network. Journal of Clinical Oncology. 2011;29:1431–35. doi: 10.1200/JCO.2010.31.2223. [DOI] [PubMed] [Google Scholar]
- 41.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. International Journal of Cancer. 2008;123:187–94. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 42.Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006;20:1645–54. doi: 10.1097/01.aids.0000238411.75324.59. 10.097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 43.Cerhan JR, Kricker A, Paltiel O, Flowers CR, Wang SS, Monnereau A, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Diffuse Large B-Cell Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs. 2014;2014:15–25. doi: 10.1093/jncimonographs/lgu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linet MS, Vajdic CM, Morton LM, de Roos AJ, Skibola CF, Boffetta P, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Follicular Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs. 2014;2014:26–40. doi: 10.1093/jncimonographs/lgu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, et al. Etiologic Heterogeneity Among Non-Hodgkin Lymphoma Subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs. 2014;2014:130–44. doi: 10.1093/jncimonographs/lgu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang SS, Flowers CR, Kadin ME, Chang ET, Hughes AM, Ansell SM, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Peripheral T-Cell Lymphomas: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs. 2014;2014:66–75. doi: 10.1093/jncimonographs/lgu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slager SL, Benavente Y, Blair A, Vermeulen R, Cerhan JR, Costantini AS, et al. Medical History, Lifestyle, Family History, and Occupational Risk Factors for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. JNCI Monographs. 2014;2014:41–51. doi: 10.1093/jncimonographs/lgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penberthy L, McClish D, Peace S, Gray L, Martin J, Overton S, et al. Hematologic malignancies: an opportunity to fill a gap in cancer surveillance. Cancer Causes Control. 2012;23:1253–64. doi: 10.1007/s10552-012-0003-1. [DOI] [PubMed] [Google Scholar]
- 49.Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett JM, Kaminski MS, Leonard JP, Vose JM, Zelenetz AD, Knox SJ, et al. Assessment of treatment-related myelodysplastic syndromes and acute myeloid leukemia in patients with non-Hodgkin lymphoma treated with tositumomab and iodine I131 tositumomab. 2005 doi: 10.1182/blood-2004-12-4690. [DOI] [PubMed] [Google Scholar]
- 51.Savage KJ. Therapies for Peripheral T-Cell Lymphomas. ASH Education Program Book. 2011;2011:515–24. doi: 10.1182/asheducation-2011.1.515. [DOI] [PubMed] [Google Scholar]
- 52.O'Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. Journal of Clinical Oncology. 2011;29:1182–9. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results From a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. Journal of Clinical Oncology. 2012;30:631–6. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 54.Petrich AM, Helenowski IB, Bryan LJ, Rozell SA, Galamaga R, Nabhan C. Factors predicting survival in peripheral T-cell lymphoma in the USA: a population-based analysis of 8802 patients in the modern era. British Journal of Haematology. 2015;168:708–18. doi: 10.1111/bjh.13202. [DOI] [PubMed] [Google Scholar]
- 55.Chihara D, Ito H, Izutsu K, Hattori M, Nishino Y, Ioka A, et al. Advance and stagnation in the treatment of patients with lymphoma and myeloma: Analysis using population-based cancer registry data in Japan from 1993 to 2006. International Journal of Cancer. 2015:n/a–n/a. doi: 10.1002/ijc.29477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.