Abstract
Tamoxifen-associated mammographic density (MD) reductions are linked to improved breast cancer survival. We evaluated MD at six time points to determine the timing of greatest reduction following tamoxifen initiation. We sampled 40 Kaiser Permanente Northwest ER-positive breast cancer patients from a prior study of MD change, according to tamoxifen use duration and age at diagnosis: <4 years tamoxifen and ≤50 years (N=6) or >50 years (N=10) old; ≥4 years tamoxifen and ≤50 years (N=13) or >50 years (N=11) old. A single reader evaluated percent MD in the contralateral breast on baseline (pre-diagnosis) and five approximately yearly post-diagnostic (T1 to T5) mammograms. Mean MD change was calculated. Interactions with age (≤50, >50 years), tamoxifen duration (<4, ≥4 years), and baseline MD (tertiles) were tested in linear regression models. Overall, the largest MD decline occurred by T1 (mean 4.5%) with little additional decline by T5. Declines differed by tertile of baseline MD (P-interaction<0.01). In the highest tertile, the largest reduction occurred by T1 (mean 14.9%), with an additional reduction of 3.6% by T5. Changes were smaller in the middle and lowest baseline MD tertiles, with cumulative reductions of 3.0% and 0.4% from baseline to T5, respectively. There were no differences by age (P-interaction=0.36) or tamoxifen duration (P-interaction=0.42). Among ER-positive patients treated with tamoxifen and surviving ≥5 years, most of the MD reduction occurred within approximately 12 months of tamoxifen initiation, suggesting that MD measurement at a single time-point following tamoxifen initiation can identify patients with substantial density declines.
Keywords: estrogen receptor-positive breast cancer, tamoxifen, mammographic density, longitudinal trends
Introduction
Mammographic density declines in 30% to 60% of women treated with tamoxifen (1-6). Mean absolute reductions are 5% to 10% after a mean 12 to 18 months (1-6). Such density reductions have been associated with lower risks of breast cancer recurrence (2, 3) and breast cancer-specific death (5, 7). It has been suggested that the density reduction may be a biosensor of tamoxifen effectiveness. Although there are several hypothesized mechanisms (8), including that the reduction is a marker of tamoxifen metabolism, many properties of tamoxifen-associated density declines are unknown. In particular, it is unclear whether additional information might be gained by characterizing the density decline at multiple time points.
Most studies describing density change associated with adjuvant tamoxifen examined one (4, 5, 9, 10) or two (6) post-tamoxifen mammograms, despite the fact that tamoxifen was historically prescribed for five years of use. Two studies (11, 12) evaluated more than two post-tamoxifen mammograms, but neither provided yearly change data that included periods both shortly after and up to five years after tamoxifen initiation. Further, little is known about tamoxifen discontinuation and density decline. 40% to 50% of patients discontinue tamoxifen before completing five years of treatment (13-15), but it is unclear if density returns to baseline levels after early discontinuation.
We conducted an exploratory pilot study of quantitative mammographic density using five successive mammograms following the initiation of tamoxifen treatment in 40 patients with estrogen receptor (ER)-positive breast cancer. Our goal was to clarify patterns of longitudinal mammographic density change among patients treated with tamoxifen, accounting for the potential effects of tamoxifen discontinuation.
Materials and Methods
Population
We previously conducted a study of change in mammographic density after tamoxifen initiation and breast cancer-specific death (5). Briefly, that study included female Kaiser Permanente Northwest (KPNW) health plan members who were diagnosed with localized or regional stage ER-positive breast cancer between 1990 and 2008, aged ≥18 years at diagnosis, and treated with tamoxifen. Vital status was observed through December 31, 2010, and a sample of 97 women who died from breast cancer (cases) and 252 who did not die from breast cancer (controls) were included in analyses.
For the current study, we identified women from the case-control population who were alive ≥5 years after diagnosis (N=305), and selected 40 subjects who had ≥5 post-diagnostic mammograms within strata of tamoxifen use duration and age at diagnosis: <4 years tamoxifen and ≤50 years (N=6) or >50 years (N=10) old; ≥4 years tamoxifen and ≤50 years (N=13) or >50 years (N=11) old. We included uneven numbers in each group to maintain the overall sample size of 40. This study was approved by the National Cancer Institute's Special Studies Institutional Review Board (IRB) and the Kaiser Permanente Northwest IRB.
Mammographic Density
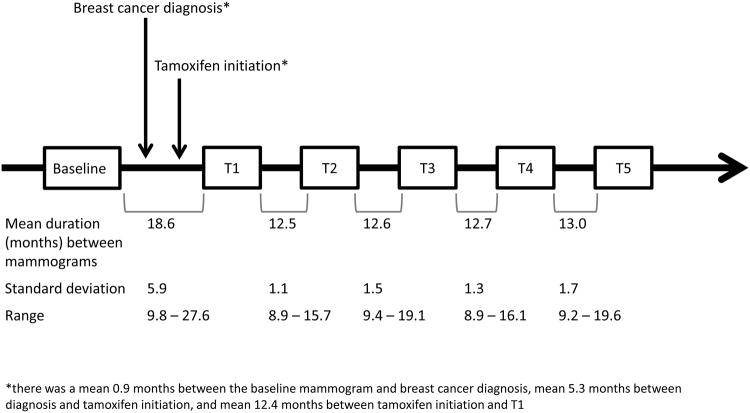
Six film mammograms of the contralateral (unaffected) breast per patient were identified from medical records (Figure 1). The baseline mammogram was a mammogram occurring ≤720 days before diagnosis and before the first tamoxifen prescription. Five follow-up mammograms (T1 to T5) obtained approximately yearly after tamoxifen initiation were also selected. The first follow-up mammogram (T1) must have been >90 days after the first tamoxifen prescription and women must have had tamoxifen prescription coverage within 90 days of the T1 mammogram. One post-tamoxifen mammogram was unavailable at the appropriate time interval for one patient resulting in five mammograms for that patient. Six mammograms each were evaluated for all other patients.
Figure 1.
Mammogram timing. One pre-diagnostic (baseline) and five post-diagnostic (T1-T5) mammograms of the contralateral breast were evaluated among 40 ER-positive breast cancer patients who were treated with tamoxifen. Mammograms were approximately one year apart, with the exception of the first post-diagnostic mammogram (T1) which was approximately 12 months after the initiation of tamoxifen and 18 months after the baseline mammogram.
Craniocaudal views from the selected films were digitized, as described previously (5). Total breast area and dense area were measured by a single reader using computer-assisted thresholding (16). Percent density was calculated as the ratio of dense area to total breast area. All mammograms from the same patient were evaluated within the same session. A reliability study evaluating masked duplicate images conducted within the parent study resulted in intra-class coefficients >95% and coefficients of variation <10% for dense area, total breast area, and percent density (5).
Covariates
Patient characteristics (Supplementary Table S1) were obtained from medical records (5). Body mass index (BMI) was calculated as weight (kg) divided by squared height (m2). Individual prescription records for tamoxifen, antidepressants (selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors), and estrogen or estrogen plus progestin hormone therapy (HT) dispensed between 1986 and 2010 were obtained from KPNW databases. Duration of tamoxifen use was calculated by subtracting the date of the first tamoxifen prescription from the last day of the last tamoxifen prescription prior to discontinuation (indicated by no prescriptions for >1 year).
Statistical Analysis
Mean and standard deviation (SD) percent density were calculated at each time point. Change in percent density was calculated in two ways: as the difference from the previous year and the difference from baseline. Linear regression models were used to examine longitudinal change in density. Generalized estimating equations accounted for correlations among measurements from the same woman (17). Associations between patient characteristics (Supplementary Table S1) and percent density were evaluated by modeling associations with percent density and density change from the previous year. Percent density was square-root transformed and yearly density change was scaled and raised to the 1.75th power to approximate normality. Baseline percent density (tertiles: <15.4%, 15.5-32.1%, >32.1%) was associated with density change from the previous year at P<0.05; thus, we estimated models adjusted for this factor. Other characteristics were not associated with percent density or density change at P<0.05 and were not included in adjusted models. Trend tests were not conducted due to the non-linear pattern of density change over time.
We compared density change according to varying intervals between baseline and T1 mammograms (9-13, >13-18, >18-22, >22 months) using the Kruskal-Wallis test and by testing for an interaction between the baseline to T1 interval and mammogram timing (e.g., T1, T2, etc.) with respect to density change in a linear regression model. We examined the effect of excluding two women who experienced a recurrence either before the T5 mammogram or at an unknown date, as an unmeasured factor could be associated with recurrence and density change.
Interactions with age at diagnosis (≤50, >50 years) and tamoxifen duration (<4, ≥4 years) were estimated by including cross-product terms with indicators for mammogram timing in regression models. We additionally tested for differences related to tamoxifen use at each mammogram by evaluating a model with cross-product terms between mammogram timing and an indicator for a tamoxifen prescription within 60 days of the mammogram of interest. We evaluated interaction between baseline percent density (tertiles) and mammogram timing based on our previously reported association between baseline density and density change after one year of tamoxifen (5). Interaction results from unadjusted and adjusted models were similar; unadjusted P-values are presented.
Analyses were conducted using SAS v9.2 (SAS, Cary, NC) and R (18). Two-sided P-values <0.05 were statistically significant.
Results
Patients were 41 to 77 years old at baseline and used tamoxifen for a mean of 51 months (SD 18). Mean mammographic density at baseline was 26.5% (SD 18.0%) (Table 1). Despite individual variation (Supplementary Figure S1), percent density decreased over time with a cumulative reduction in mean density of 7.2% by T5. The largest reduction was observed at T1; by T3, the yearly mean change was approximately 1% or less. Baseline to T1 density decline was slightly greater among women with >22 months between baseline and T1, but overall, mean change did not differ significantly according to time between baseline and T1 (Kruskal-Wallis P=0.55) or through T5 (P-interaction=0.74; Supplementary Figure S2). Early density reductions were attenuated after adjustment for baseline density (Table 1); results were similar after additional adjustment for time between baseline and T1 mammograms (data not shown). Results were also similar after excluding women with a recurrence (data not shown).
Table 1.
Longitudinal mammographic percent density among ER-positive breast cancer patients treated with tamoxifen (N=40).
| Calculated mean percent density (SD) | Calculated mean differencea in percent density (SD) | Adjusted mean differencea, b in percent density (SE) | |
|---|---|---|---|
|
|
|||
| Baseline | 26.5 (18.0) | ||
| T1 | 22.0 (12.3) | -4.5 (11.4) | -2.0 (1.2) |
| T2 | 20.2 (12.7) | -1.7 (7.6) | 0.0 (1.2) |
| T3 | 19.1 (12.1) | -1.1 (6.9) | 0.5 (1.1) |
| T4 | 18.6 (11.3) | -0.3 (5.8) | 1.1 (0.9) |
| T5 | 19.3 (11.4) | 0.6 (6.7) | 2.1 (1.1) |
Mean difference, compared to previous year
Estimated using linear regression, adjusted for baseline percent density (ordinal tertiles)
SD – standard deviation; SE – standard error
Patterns of density change differed by baseline percent density (P-interaction<0.01; Figure 2). Density declined only among women in the highest tertile of baseline density, where the greatest reduction was observed between baseline and T1 (mean 14.9%) (Supplementary Figure S3). The slight increase in adjusted density change at T5 observed in the overall population was not seen among women in the highest baseline density tertile (Figure 2). There were no differences in density change by tamoxifen use, whether comparing <4 to ≥4 year users (P-interaction=0.42; Supplementary Figure S4) or users to discontinuers at each mammogram (all P-interaction>0.05). Patterns of density change were not statistically different by age (P-interaction=0.36); however, graphical analysis revealed a modest decline among women ≤50 years old that was not evident in women >50 years (Supplementary Figure S5).
Figure 2.
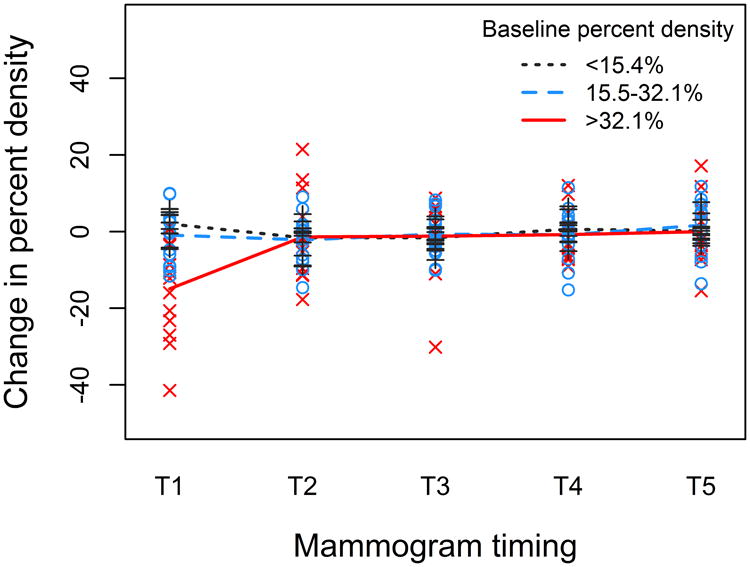
Change in percent density over time is shown stratified by percent density at baseline. Lines represent smoothed mean values of percent density change at each time point (compared to the previous mammogram) and ‘x’, ‘o’, and ‘+’ markers represent values for individual patients. There was a notable reduction in percent density observed at T1 among women in the highest tertile of baseline density (solid red line, ‘x’), but not among women in the middle (dashed blue line, ‘o’) and lowest (dotted black line, ‘+’) tertiles of baseline density. For all subjects, relatively little change in density was observed at T2 to T5.
Discussion
In this pilot study, we examined mammographic density at five time points following tamoxifen initiation and observed that the majority of the density decline occurred a mean 12 months after initiating tamoxifen. There was little additional change in subsequent years, suggesting that a mammogram obtained one year following treatment initiation is sufficient to classify the tamoxifen-associated density response.
Previous studies of this topic are few and have provided conflicting evidence. In the IBIS-I Chemoprevention Trial, density declines among high-risk women treated with tamoxifen appeared to decline monotonically (1), but it is unclear how those results relate to adjuvant use, given that breast cancer patients have higher mammographic densities than cancer-free women (19). Meggiorini et al. (12) evaluated qualitative and semi-quantitative density from six mammograms in breast cancer patients and reported that density declined after the first year and that “variations were stable” throughout follow-up; however, data related to later time points were not presented. In contrast, Konez et al. (11) did not observe a reduction in density when first assessed (two to three years after adjuvant tamoxifen initiation) but did note a reduction after five years. Our results are consistent with aspects reported by Meggiorini et al. and IBIS-I, supporting a model where density declines occur early and persist over time.
Our analysis extends two findings observed previously. First, the cumulative density reduction a mean five years after tamoxifen initiation was greatest among women with the highest baseline density, consistent with the identification of baseline density as a significant predictor of density change in studies that measured change over one to two years (2, 3, 5). Second, the pattern of density change over time did not appear to be influenced by tamoxifen discontinuation, consistent with a previous report that density did not change following tamoxifen cessation after five years of use (11). These data suggest that among women with a reduction, density does not revert to baseline levels once tamoxifen administration ends and that it may not be necessary to have information on long-term tamoxifen use when evaluating the prognostic meaning of density change. It is unclear why density did not continue to decline among women taking tamoxifen for ≥4 years. Research investigating the biological mechanisms involved are necessary to understand the relationship between the pattern of density decline and tamoxifen's effects.
Younger age and chemotherapy receipt showed weak, non-significant associations with larger reductions in density. These associations are consistent with what we observed in the parent case-control study examining change after approximately one year of tamoxfien (5) and with what others reported in similar analyses (1-4, 12, 20).
Strengths of this analysis include the use of quantitative, reproducible density measurements and digitization of all films from a patient during the same session, both of which reduced random variability. We used prospectively-collected medical record data to assess the effects of patient characteristics and treatments. Individual prescription records allowed us to accurately determine periods of tamoxifen use in relation to mammography timing. There were also limitations. Women were selected to have survived at least five years and therefore may not be representative of all ER-positive patients treated with tamoxifen. We lacked information on menopausal status, which is associated with mammographic density (21). We did have information on surgical menopause after diagnosis and found that it was not a predictor of density change, though few women had the procedure during the study period. There was a wide range in time between baseline and T1, although this did not affect the main conclusions regarding patterns of density change. We examined a large number of covariates relative to the number of subjects and some associations we observed may be due to multiple comparisons. Finally, post-hoc calculations show that we had 80% power to detect a minimum difference in density change of 11%, which is greater than the observed differences in density change among the age or tamoxifen duration groups; therefore, conclusions related to statistically significant differences according to these factors should be interpreted with caution.
In summary, we found that density change one year after tamoxifen initiation was representative of density change five years after tamoxifen initiation. Replication of these results in a larger dataset is needed to confirm that assessment of density change one year following tamoxifen initiation is a suitable measure of exposure in studies of density change and tamoxifen response.
Supplementary Material
Acknowledgments
We thank Brenda Rush and Kathy Pearson, of Kaiser Permanente Northwest (Portland, OR) for their invaluable assistance in conducting this study.
Financial support: This research was funded by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, USA (S.J. Nyante, M.E. Sherman, R.M. Pfeiffer, A. Berrington de Gonzalez, L.A. Brinton, R.N. Hoover, and G.L. Gierach) and National Cancer Institute federal funds awarded under contract HHSN261201100441P (E.A. Bowles) and HHSN261200900017C/N02CP-90017 subcontract no. 10-312-0212208 (A. Glass).
List of Abbreviations
- BMI
body mass index
- ER
estrogen receptor
- HT
hormone therapy
- IBIS
International Breast Cancer Intervention Study
- KPNW
Kaiser Permanente Northwest
- PR
progesterone receptor
- SD
standard deviation
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–8. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Han W, Moon HG, Ahn SK, Shin HC, You JM, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012;14:R102. doi: 10.1186/bcr3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko KL, Shin IS, You JY, Jung SY, Ro J, Lee ES. Adjuvant tamoxifen-induced mammographic breast density reduction as a predictor for recurrence in estrogen receptor-positive premenopausal breast cancer patients. Breast Cancer Res Treat. 2013;142:559–67. doi: 10.1007/s10549-013-2726-4. [DOI] [PubMed] [Google Scholar]
- 4.Son HJ, Oh KK. Significance of follow-up mammography in estimating the effect of tamoxifen in breast cancer patients who have undergone surgery. AJR Am J Roentgenol. 1999;173:905–9. doi: 10.2214/ajr.173.4.10511146. [DOI] [PubMed] [Google Scholar]
- 5.Nyante SJ, Sherman ME, Pfeiffer RM, Berrington de Gonzalez A, Brinton LA, Aiello Bowles EJ, et al. Prognostic significance of mammographic density change after initiation of tamoxifen for ER-positive breast cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S, et al. Randomized double-blind 2 × 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27:3749–56. doi: 10.1200/JCO.2008.19.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol. 2013;31:2249–56. doi: 10.1200/JCO.2012.44.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd NF. Tamoxifen, mammographic density, and breast cancer prevention. J Natl Cancer Inst. 2011;103:704–5. doi: 10.1093/jnci/djr115. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson C, Warren R, Bingham SA, Day NE. Mammographic patterns as a predictive biomarker of breast cancer risk: effect of tamoxifen. Cancer Epidemiol Biomarkers Prev. 1999;8:863–6. [PubMed] [Google Scholar]
- 10.Ursin G, Pike MC, Spicer DV, Porrath SA, Reitherman RW. Can mammographic densities predict effects of tamoxifen on the breast? J Natl Cancer Inst. 1996;88:128–9. doi: 10.1093/jnci/88.2.128-a. [DOI] [PubMed] [Google Scholar]
- 11.Konez O, Goyal M, Reaven RE. Can tamoxifen cause a significant mammographic density change in breast parenchyma? Clin Imaging. 2001;25:303–8. doi: 10.1016/s0899-7071(01)00329-1. [DOI] [PubMed] [Google Scholar]
- 12.Meggiorini ML, Labi L, Vestri AR, Porfiri LM, Savelli S, De Felice C. Tamoxifen in women with breast cancer and mammographic density. Eur J Gynaecol Oncol. 2008;29:598–601. [PubMed] [Google Scholar]
- 13.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owusu C, Buist DS, Field TS, Lash TL, Thwin SS, Geiger AM, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–55. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 15.van Herk-Sukel MP, van de Poll-Franse LV, Voogd AC, Nieuwenhuijzen GA, Coebergh JW, Herings RM. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–51. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 16.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal Data Analysis Using Generalized Linear Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 18.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- 19.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 20.Brisson J, Brisson B, Cote G, Maunsell E, Berube S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2000;9:911–5. [PubMed] [Google Scholar]
- 21.Boyd N, Martin L, Stone J, Little L, Minkin S, Yaffe M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol Biomarkers Prev. 2002;11:1048–53. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.