Abstract
Purpose: Pre-eclampsia (PE) is associated with intravascular inflammation and endothelial dysfunction. Interestingly, endocan plays a predominant role in the vascular inflammation and is considered as a biomarker of endothelial dysfunction. The aim of this study was to explore whether the endocan levels in serum and placenta were different between pregnant women with PE and the normal pregnancies. Methods: Total 22 patients, including 10 normal pregnant women and 12 patients with PE, were included in this study. Immunohistochemistry was used to evaluate the location of endocan. Then, the mRNA and protein levels of endocan in placenta were detected using qRT-PCR and western blotting. Serum endocan concentration was measured by ELISA. Results: Endocan protein was present in the human placenta, and the mRNA and protein levels of placenta tissues were elevated (P < 0.05) in the normal pregnancy with third trimester than those with first trimester. Furthermore, the expression of endocan mRNA and protein were increased in the placenta tissues of PE compared with in the normal pregnancy (P < 0.05); however, the endocan concentration of maternal serum did not have significant differences. Conclusion: Endocan may play a role in the progression of pregnancy and has a potential to be a new marker for the detective of PE.
Keywords: Endocan, pre-eclampsia, vascular inflammation, endothelial dysfunction
Introduction
Pre-eclampsia (PE), a pregnancy-specific syndrome, is characterized by new-onset hypertension and proteinuria after 20 weeks of gestation [1]. It still remains to be one of the leading causes of preterm birth [2], as well as results in death of approximately 63000 maternal and 500000 infant annually [3]. In view of considerable maternal and fetal morbidity and mortality caused by PE, it is urgent to search for specific and early diagnosis criteria for this pregnancy syndrome.
Although the exact causes of this disease are still unknown, innumerable researches have shown that several processes have been involved in this pathophysiology [4]. It is well recognized that pre-eclampsia is associated with impaired trophoblast invasion and poor placentation during early pregnancy [5], as well as the subsequent oxidative stress, angiogenic imbalance, intravascular inflammation and endothelial dysfunction [6,7]. These processes increase the peripheral vascular resistance, thereby leading to maternal hypertension and proteinuria [8]. During normal pregnancy, a controlled mild maternal inflammation is observed, while PE can induce the adverse pregnancy outcomes through the entire inflammatory network of the circulation [9]. What’ s more, the releasing of soluble factors from the ischemic placenta into maternal plasma plays a crucial role in endothelial dysfunction, which also known as the prominent feature of PE [10].
Endocan, previously known as endothelial cell-specific molecule-1(ESM-1), is a novel soluble dermatan sulphate proteoglycan primarily secreted by endothelial cells of the kidneys, lung and gastrointestinal tract of humans [11]. Clinical and experimental data suggest that endocan plays a central role in the regulation of many major processes such as inflammatory disorders [12], cell adhesion, and endothelial dysfunction [13]. High levels of endocan may be related to the promotion of systemic inflammation by mediating the circulating lymphocytes into inflammatory sites [14]. Previous studies have shown high levels of endocan in hypertention and cancers such as human non-small cell lung cancer, colorectal cancer and hepatocellular carcinoma [15-18]. In addition, the endocan level of serum is related to the severity of sepsis, and may represent a novel marker of endothelial dysfunction [19]. Recently, Hentschke et al. [20] and Adekola et al. [21] have found that the endocan level of serum is elevated in patients with PE; however, Yuksel et al. [22] suggests that serum endocan concentrations are not significantly different between normal pregnant women and pregnant women with PE. Therefore, the endocan level of serum in PE is controversial.
In the current study, we determined the differences of placental endocan expression and the maternal serum endocan level between the pregnant women with PE and normal pregnancy, aimed to further investigate the expression and location of endocan in patients with PE.
Materials and methods
Patients
Total 22 patients, including 10 normal pregnant women and 12 patients with PE, were included in this study. PE was defined as new onset of hypertension with systolic of 140 mmHg and/or diastolic blood pressure of 90 mmHg after 20 weeks of gestation that measured at least two occasions, 4 h to 1 week apart and consistent proteinuria (300 mg in a 24-h urine collection, or containing 1 + protein by dipstick) according to the guidelines of the US National Institutes of Health [23]. The collection of placental samples and blood samples were approved by the Scientific and Ethical Committee of the Shanghai First Maternity and Infant Hospital affiliated with Tongji University. All of the samples were collected with a written informed consent provided by the participants.
Sample collection
Placental tissues were immediately (< 30 min) obtained from normal pregnancy (38.90 ± 0.11 weeks, n = 10) and PE (38.26 ± 0.40 weeks, n = 12) patients after delivery via caesarean section, respectively, and then fixed in 4% paraformaldehyde solution for immunohistochemical assessment. The chorionic villi samples in the first trimester of pregnancy (6.91 ± 0.27 weeks, n = 10) were dissected and obtained immediately after vacuum aspiration, then washed in sterile PBS. Small pieces (about 0.5 cm3) were cut from the part placentas or villis of the fetal under the aseptic conditions, and then briefly washed in sterile PBS to remove maternal blood contamination. All samples were frozen after delivery within 15 min and stored in liquid nitrogen for western blotting and quantitative real-time PCR (qRT-PCR) analysis. Patient characteristics are shown in Table 1.
Table 1.
Characteristics of normal pregnant women and patients with pre-eclampsia (PE)
| Normal (n = 10) | PE (n = 12) | |
|---|---|---|
| Patient age (year) | 30.20 ± 0.59 | 29.75 ± 1.11 |
| Gestation age (week) | 38.90 ± 0.11 | 38.26 ± 0.40 |
| Systolic pressure (mmHg) | 100.70 ± 0.70 | 151.58 ± 4.10** |
| Diastolic pressure (mmHg) | 70.80 ± 0.80 | 97.33 ± 3.38** |
| Proteinuria | - | +-++++** |
| Fetal weights (g) | 3349.60 ± 70.12 | 3191.67 ± 234.90 |
All the data are expressed as mean ± S.E.M.
P < 0.01.
Maternal blood samples from normal pregnancy (39.14 ± 0.39 weeks, n = 9) and the PE patients (38.51 ± 0.48 weeks, n = 9) were collected and then centrifuged at 1,500 × g for 10 min at 4°C. The supernatant serum samples were transferred to clean 1.5 mL Eppendorf tubes and stored at -80°C for enzyme-linked immuno sorbent assay (ELISA) analysis. Patient characteristics are shown in Table 2.
Table 2.
Characteristics of normal pregnant women and patients with pre-eclampsia (PE) who provided serum
| Normal (n = 9) | PE (n = 9) | |
|---|---|---|
| Patient age (year) | 29.22 ± 1.27 | 28.56 ± 0.87 |
| Gestation age (week) | 39.14 ± 0.39 | 38.51 ± 0.48 |
| Systolic pressure (mmHg) | 108.22 ± 2.63 | 139.78 ± 4.36** |
| Diastolic pressure (mmHg) | 69.67 ± 0.62 | 95.44 ± 3.12** |
| Proteinuria | - | +-++++** |
| Fetal weights (g) | 3208.57 ± 100.05 | 3338.89 ± 169.82 |
All the data are expressed as mean ± S.E.M.
P < 0.01.
qRT-PCR
Tissue RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the Total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. Complementary DNA (cDNA) was obtained by reverse transcription of high-quality RNA using the PrimeScript RT reagent kit (TaKaRa, Dalian, China). The mRNA levels of endocan and β-actin were evaluated using SYBR Green Premix Ex Taq (TaKaRa, Dalian, China) on an ABI Prism 7000 Sequence Detection System (Life Technology). The primer sequences were as follows: endocan: forward primer 5’-CAGGCATGGATGGCATGAAG-3’, and reverse primer 5’-CTGACTGGCAGTTGCAGGTCTC-3’; β-actin: forward 5’-CCAACCGCGAGAAGATGA-3’ and reverse 5’-CCAGAGGCGTACAGGGATAG-3’. The PCR program was: 95°C for 30 min, 40 cycles of 95°C for 15 s and 56°C for 20 s. To confirm the amplification specificity, the PCR productions were subjected to a melting curve analysis. Relative mRNA level of endocan were normalized to the control β-actin mRNA and analyzed by the comparative threshold (Ct) cycle method (2-ΔΔCT).
Western blotting
Placental tissues were homogenized and then lysed by sonication in lysis buffer (50 mM HEPES, 0.1 M NaCl, 10 mM EDTA, 4 mM sodium pyrophosphate, 10 mM sodium fluoride, 2 mM sodium orthovanadate (pH 7.5); 1 mM phenylmethylsulfonylfluoride, 1% Triton X-100, 5 μg/mL leupeptin, 5 μg/mL aprotinin). Supernatant were acquired after centrifugation for 20 min at 12,000 × g at 4°C. Subsequently, the extracted protein concentration was detected by the BCA Protein Assay Kit (Thermo Scientific, Hudson, NH, USA). Protein sample was mixed with loading buffer and boiled for 10 min. Total 60 μg protein was separated on 10% SDS-PAGE gel per well, and then transferred to polyvinylidenedifluoride membranes (Roche Diagnostics, Indianapolis, IN, USA). After blocked with 5% defatted milk at room temperature for 1 h, the membranes were incubated overnight at 4°C with goat anti-human endocan polyclonal antibody (1:1000; R&D Systems, Minneapolis, MN, USA) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10000; Abmart, shanghai, China) monoclonal antibody, respectively. GAPDH was served as an endogenous loading control. Next, the membranes were washed for three times with TBST, then incubated with rabbit anti-goat IgG (H+L)-HRP (1:5000, Abmart) for 2 h at 25°C, respectively. Ultimately, proteins were visualized using enhanced chemiluminescence reagents (Thermo Scientific), and the relative expression of endocan protein was analyzed by densitometry using the Image-J imaging analysis software (NIH, Bethesda, MD).
Immunohistochemistry (IHC)
Placental tissue was sliced paraffin-embedded tissue into 5 μm-thick sections. Next, sections were mounted on poly-L-lysine-coated slides and then deparaffinized in xylene and dehydrated with gradient ethanol. Antigen retrieval was carried out in 10 mM citrate buffer (pH 6.0) buffer for 10 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min at room temperature. After blocked with 1% horse serum albumin for 20 min at room temperature, the sections were incubated with a goat-anti-human endocan polyclonal antibody (1:100; R&D Systems) overnight at 4°C and then incubated with rabbit-anti-goat secondary antibody (1:200, R&D Systems) at 37°C for 30 min. The sections were stained with DAB for 5 min and re-stained with hematoxylin for 2 min. The primary antibody was replaced by PBS in the negative controls.
ELISA
Maternal endocan concentration in serum was determined using a Human Endocan/ESM-1 DIY ELISA Kit (LIK-1101, Lunginnov, Lille, France) according to the manufacturer’s instructions. Briefly, a 96-well microplate was coated with 100 μL capture antibody (2 μg⁄mL) against ESM-1, and incubated overnight at 4°C. Then 100 μL serially diluted ESM-1 standard solution and samples diluted two fold in dilution buffer were applied to wells in duplicate and incubated for 1 h at room temperature. Next, 100 μL detection antibody was added into per well for 1 h at room temperature. After washing with phosphate buffer solution, 100 μL diluted HRP-conjugated strepatvidin was applied to each well for 30 min. Subsequently, tetramethylbenzidine (TMB) solution was added to the wells, and the reaction was stopped with 2 M H2SO4 solution. Samples were incubated with internal standard working solution at room temperature for 1 h and followed by several cleanup steps. The absorbance was read at 630 nm using a microplate reader (MULTISKAN MK3, Thermo Scientific). The concentrations of Endocan were calculated based on the standard curve.
Statistical analysis
All data were expressed as the mean ± S.E.M and analyzed using the SPSS 20.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). An unpaired Student’s t-test was used to analyze the difference of endocan mRNA and protein levels between the placental tissues of first trimester and full term pregnancy as well as the normal pregnancy and PE patients, and their plasma endocan concentrations. A P-value < 0.05 was considered to be statistically significant.
Results
Endocan level was elevated with the progression of pregnancy in the human placenta
Firstly, we detected whether endocan expressed in placental tissues. IHC results showed positive brownish staining for endocan in the vascular endothelial cell of placenta tissues of normal pregnancy and PE patients (Figure 1A), suggested that endocan localized in the human placentas. Then, the qRT-PCR and western blotting were used to detect the mRNA and protein levels of endocan in placenta tissues of normal pregnancy. No significant difference was found in maternal age of first trimester and third trimester. The results revealed that the mRNA and protein levels of endocan were significantly higher (P < 0.05) in the normal pregnancy with third trimester than those with first trimester (Figure 1B, 1C). These results indicated that the expression of endocan in placenta tissue was increasing with the progression of pregnancy.
Figure 1.
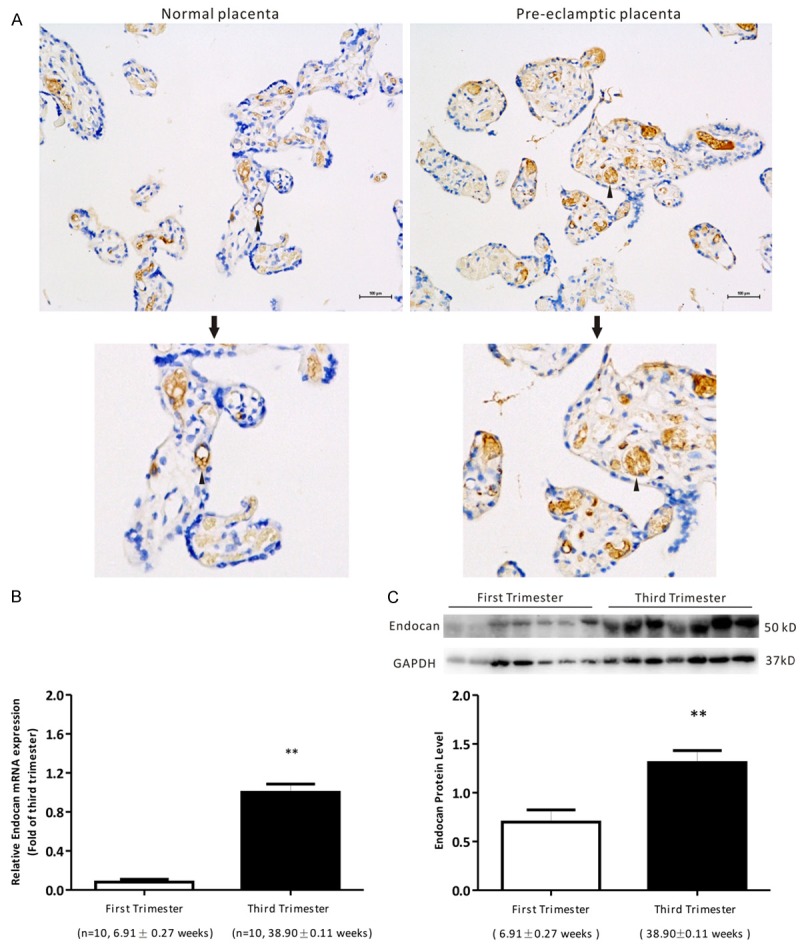
The localization and expression of endocan in pregnant women. A. Immunohistochemical revealed that endocan located in the vascular endothelial cell of normal placenta and pre-eclamptic placentas. Brownish color indicates positive staining for endocan. Arrows, vascular endothelial cell. Bars = 100 μm. B. qRT-PCR showed the mRNA levels of endocan in the placenta tissues of normal pregnant women with the first trimester (n = 10) and the third trimester (n = 10). Data were normalized to β-actin and shown as mean ± S.E.M. C. Western blotting showed the protein expression of endocan in the placenta tissues of normal pregnant women with the first trimester (n =7) and the third trimester (n = 7). Data were normalized to GAPDH and shown as mean ± S.E.M. **P < 0.01 versus first trimester.
Endocan expression was increased in the placenta tissues of patients with PE
Demographic and clinical characteristics of patients from the normal pregnancy and PE are shown in Table 1. Patient ages, gestation ages and fetal weights were similar between the enrolled groups. In addition, clinical characteristics including systolic pressure, diastolic pressure and proteinuria content were higher in patients with PE than that in patients with normal pregnancy. An increased expression of endocan mRNA and protein were observed in the placenta tissues of PE compared with in the normal pregnancy (Figure 2A, 2B, P < 0.05), indicating that endocan expressions were increased in patients with PE.
Figure 2.
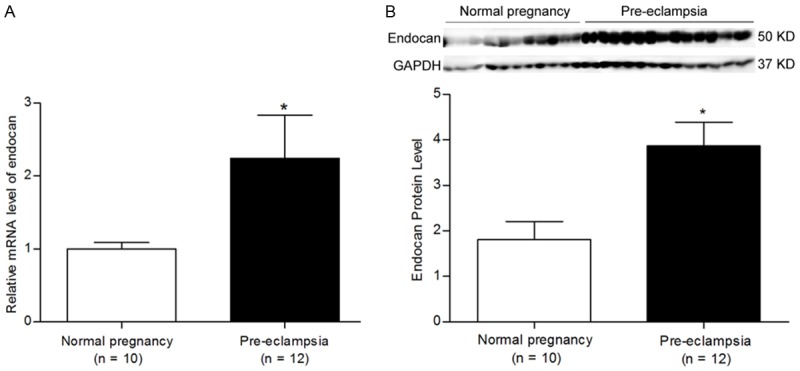
The endocan mRNA and protein expressions of normal pregnancy and pre-eclampsia women. A. qRT-PCR showed the mRNA levels of endocan in the placenta tissues of the normal (n = 10) and the pre-eclamptic (n = 12) pregnancies. Data were normalized to β-actin and shown as mean ± S.E.M. B. Western blotting showed the protein expression of endocan in the placenta tissues of the normal (n = 10) and the pre-eclamptic (n = 12) pregnancies. Data were normalized to GAPDH and shown as mean ± S.E.M. *P < 0.05 versus normal pregnancy.
The content of endocan was similar in maternal serum between patients with normal pregnancy and PE
Furthermore, we evaluated whether the increased endocan expression in placenta tissues of PE would be apparent in serum. Characteristics of patients from the normal pregnancy and PE who provided serum samples are shown in Table 2. Patient ages and gestation ages were similar between the enrolled groups. ELISA analysis showed that the mean concentration of endocan was slightly higher in the serum of patients with PE (1.68 ± 0.95 ng/mL) than normal pregnancies (0.84 ± 0.24 ng/mL), but no significant difference was found (Figure 3).
Figure 3.
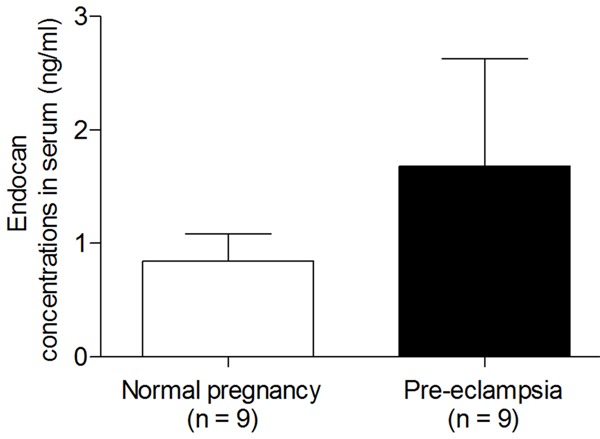
ELISA analysis showed the median serum endocan concentration of patients with normal pregnancy and pre-eclampsia. Data are expressed as means ± S.E.M.
Discussion
In this study, we firstly demonstrated that endocan localized in the human placentas and placenta endocan levels were elevated with the progression of pregnancy, suggesting its important roles in the placenta growth. We also found a significant increase in placenta endocan level of patients with PE compared with the normotensive full term controls. However, in contract to our hypothesis, we found maternal serum endocan concentration was slightly higher in PE but with no significance.
Endothelial dysfunction and inflammation had been proved to be related to the pathophysiology of PE [24]. Angiogenesis and arterial remolding were necessary for the appropriate development of placental vascular network [25]. Endocan might be related to the vascular development by inducing inflammation and endothelium dysfunction [26]. Tumor necrosis factor-α (TNF-α), a regulator of maternal dysfunction, had been proved to stimulate the production of endocan in vitro experiments [27]. In addition, previous study had demonstrated that patients with PE had higher vascular endothelial growth factor receptor (VEGFR) than normal pregnancies [28]. Importantly, VEGF, a key angiogenic factor, could be released by placenta and then induce the expression of endocan [29,30]. It was well known that a mild inflammatory response could be observed with the progression of pregnancy in the healthy pregnant woman [31], which might explain our results that placenta endocan levels were elevated during normal pregnancy. Moreover, our study showed that endocan expression was increased in the placenta tissues of patients with PE. All these results indicated that endocan might play an important role in the development of placental and be associated with the pathophysiology of PE.
Till now, only few studies reported endocan expression in PE serum and placentas. The first research was designed by Adekola et al. [21], they found that the median plasma endocan concentration in patients with PE was significantly higher than that of women with uncomplicated pregnancies; however, subgroup analysis performed among patients with different severities of PE (mild versus severe PE) revealed that the median plasma endocan level did not significantly differ. Recently, Yuksel et al. [22] suggested that there was no significant difference in maternal plasma endocan levels between the PE and uncomplicated pregnancies, which was in line with our results of serum. The possible reasons for the differences of these researches are as follows: i) the race of pregnant women are not the same, the research conducted by Adekola et al. contains several races, including African American, Caucasian, Hispanic and others; ii) The proportions of severe and mild or early-onset and late-onset PE patients in these studies are different; iii) There is a significant difference in the sample size of the three studies.
In conclusion, our study provided new evidence that endocan levels were increased during the normal pregnancy. Also, endocan may be a new marker for the detective of PE. However, to obtain a more confirmed results, it is necessary to extend sample size and divide them into different subgroups according to the severity extent of PE in the further study.
Disclosure of conflict of interest
None.
References
- 1.Brennan LJ, Morton JS, Davidge ST. Vascular dysfunction in preeclampsia. Microcirculation. 2014;21:4–14. doi: 10.1111/micc.12079. [DOI] [PubMed] [Google Scholar]
- 2.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Naderi M, Yaghootkar H, Tara F, Tavakkol Afshari J, Farid Hosseini R, Ghayour Mobarhan M, Shapouri Moghadam A, Mirteimouri M, Tara SM. Tumor necrosis factor-alpha polymorphism at position -238 in preeclampsia. Iran Red Crescent Med J. 2014;16:e11195. doi: 10.5812/ircmj.11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrin Met. 2010;21:529–536. doi: 10.1016/j.tem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Sepulveda A, Espana-Perrot PP, Norwitz ER, Illanes SE. Metabolic Pathways Involved in 2-Methoxyestradiol Synthesis and Their Role in Preeclampsia. Reprod Sci. 2013;20:1020–1029. doi: 10.1177/1933719113477483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Eiland E, Nzerue C, Faulkner M. Preeclampsia 2012. J Pregnancy. 2012;2012:586578. doi: 10.1155/2012/586578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechard D, Meignin V, Scherpereel A, Oudin S, Kervoaze G, Bertheau P, Janin A, Tonnel A, Lassalle P. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37:417–425. doi: 10.1159/000025758. [DOI] [PubMed] [Google Scholar]
- 12.Pawlak K, Mysliwiec M, Pawlak D. Endocan -- the new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular disease. Clin Biochem. 2015;48:425–30. doi: 10.1016/j.clinbiochem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Aparci M, Isilak Z, Uz O, Yalcin M, Kucuk U. Endocan and Endothelial Dysfunction. Angiology. 2015;66:488–9. doi: 10.1177/0003319714568791. [DOI] [PubMed] [Google Scholar]
- 14.Bechard D, Gentina T, Delehedde M, Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P, Jude B, Janin A, Fernig DG, Tonnel AB, Lassalle P. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem. 2001;276:48341–48349. doi: 10.1074/jbc.M108395200. [DOI] [PubMed] [Google Scholar]
- 15.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Kurtoglu E, Demir M, Celik T, Turker T, Iyisoy A. Endocan-A Novel Inflammatory Indicator in Newly Diagnosed Patients With Hypertension: A Pilot Study. Angiology. 2014;65:773–7. doi: 10.1177/0003319713513492. [DOI] [PubMed] [Google Scholar]
- 16.Grigoriu BD, Depontieu F, Scherpereel A, Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB, Lassalle P. Endocan expression and relationship with survival in human non-small cell lung cancer. Clin Cancer Res. 2006;12:4575–4582. doi: 10.1158/1078-0432.CCR-06-0185. [DOI] [PubMed] [Google Scholar]
- 17.Zuo L, Zhang SM, Hu RL, Zhu HQ, Zhou Q, Gui SY, Wu Q, Wang Y. Correlation between expression and differentiation of endocan in colorectal cancer. World J Gastroenterol. 2008;14:4562–4568. doi: 10.3748/wjg.14.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang GW, Tao YM, Ding X. Endocan expression correlated with poor survival in human hepatocellular carcinoma. Dig Dis Sci. 2009;54:389–394. doi: 10.1007/s10620-008-0346-3. [DOI] [PubMed] [Google Scholar]
- 19.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers. 2011;16(Suppl 1):S11–21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 20.Hentschke MR, Lucas LS, Mistry HD, Pinheiro da Costa BE, Poli-de-Figueiredo CE. Endocan-1 concentrations in maternal and fetal plasma and placentae in pre-eclampsia in the third trimester of pregnancy. Cytokine. 2015;74:152–156. doi: 10.1016/j.cyto.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Adekola H, Romero R, Chaemsaithong P, Korzeniewski SJ, Dong Z, Yeo L, Hassan SS, Chaiworapongsa T. Endocan, a putative endothelial cell marker, is elevated in preeclampsia, decreased in acute pyelonephritis, and unchanged in other obstetrical syndromes. J Matern Fetal Neonatal Med. 2015;28:1621–1632. doi: 10.3109/14767058.2014.964676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuksel MA, Tuten A, Oncul M, Acikgoz AS, Yuksel IT, Toprak MS, Ekmekci H, Ekmekci OB, Madazli R. Serum endocan concentration in women with pre-eclampsia. Archives of gynecology and obstetrics. 2015;292:69–73. doi: 10.1007/s00404-014-3605-x. [DOI] [PubMed] [Google Scholar]
- 23.Obstetricians ACo and Gynecologists. Diagnosis and management of preeclampsia and eclampsia. ACOG Practice Bulletin. 2002;33:159–167. [Google Scholar]
- 24.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RD, De Long NE, Wang RC, Yazdi FT, Holloway AC, Raha S. Angiogenesis in the placenta: the role of reactive oxygen species signaling. Biomed Res Int. 2015;2015:814543. doi: 10.1155/2015/814543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bechard D, Meignin V, ronique e, Scherpereel A, Oudin S, Kervoaze G, Bertheau P, Janin A, Tonnel AB, Lassalle P. Characterization of the secreted form of endothelial-cell-specific molecule 1 by specific monoclonal antibodies. J Vasc Res. 2000;37:417–425. doi: 10.1159/000025758. [DOI] [PubMed] [Google Scholar]
- 27.Delehedde M, Devenyns L, Maurage CA, Vivès RR. Endocan in cancers: a lesson from a circulating dermatan sulfate proteoglycan. Int J Cell Biol. 2013;2013:705027. doi: 10.1155/2013/705027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 29.Shin JW, Huggenberger R, Detmar M. Transcriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesis. Blood. 2008;112:2318–2326. doi: 10.1182/blood-2008-05-156331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, Westholm JO, Komorowski J, Lassalle P, Cross MJ. Endocan is a VEGFA and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res. 2007;313:1285–1294. doi: 10.1016/j.yexcr.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy–an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]


