Abstract
Many immunostimulants act as vaccine adjuvants via activation of the innate immune system, although in many cases it is unclear which specific molecules contribute to the stimulatory activity. QS-21 is a defined, highly purified, and soluble saponin adjuvant currently used in licensed and exploratory vaccines, including vaccines against malaria, cancer, and HIV-1. However, little is known about the mechanisms of cellular activation induced by QS-21. We observed QS-21 to elicit caspase-1-dependent IL-1β and IL-18 release in antigen-presenting cells such as macrophages and dendritic cells when co-stimulated with the TLR4-agonist adjuvant monophosphoryl lipid A. Furthermore, our data suggest that the ASC-NLRP3 inflammasome is responsible for QS-21-induced IL-1β/IL-18 release. At higher concentrations, QS-21 induced macrophage and dendritic cell death in a caspase-1-, ASC-, and NLRP3-independent manner, whereas the presence of cholesterol rescued cell viability. A nanoparticulate adjuvant that contains QS-21 as part of a heterogeneous mixture of saponins also induced IL-1β in an NLRP3-dependent manner. Interestingly, despite the role NLRP3 plays for cellular activation in vitro, NLRP3-deficient mice immunized with HIV-1 gp120 and QS-21 showed significantly higher levels of Th1 and Th2 antigen-specific T cell responses and increased IgG1 and IgG2c compared with wild type controls. Thus, we have identified QS-21 as a nonparticulate single molecular saponin that activates the NLRP3 inflammasome, but this signaling pathway may contribute to decreased antigen-specific responses in vivo.
Keywords: caspase 1 (CASP1), human immunodeficiency virus (HIV), inflammasome, NLRP3, saponin, Toll-like receptor 4 (TLR4), vaccine, monophosphoryl lipid A, adjuvants*
Introduction
Because many protein antigens do not elicit strong immune responses on their own, vaccines often contain stimulatory adjuvants that enhance cell-mediated and humoral immune responses to help confer stronger protection. However, despite widespread use, there is little known regarding the pathways affected by many adjuvants. A better understanding of the mechanisms involved in adjuvant-generated protection can assist in the design of better vaccines against infections that currently lack effective immunization.
Adjuvants activate an innate immune response, which in turn determines the strength and quality of the adaptive immune response. This response is first mediated by activation of antigen-presenting cells (APCs) such as dendritic cells and macrophages. Engagement of pattern recognition receptors, such as extracellular, membrane-bound Toll-like receptors (TLRs)2 and cytosolic inflammasome-stimulating Nod-like receptors (NLRs) by their ligands elicits an inflammatory milieu and can eventually lead to a honed adaptive immune response.
The NLR inflammasomes are multiprotein complexes that upon activation license the proteolytic processing of the zymogen pro-caspase-1 into mature caspase-1 (1). caspase-1 can then activate pro-forms of the inflammatory cytokines IL-1β and IL-18 into mature proteins, which are then secreted through unknown pathways. IL-1β and IL-18 are potent pro-inflammatory cytokines that can, for instance, promote T helper 17 cell maturation or drive IFN-γ-mediated Th1 responses, respectively. Thus, inflammasome signaling has the potential to direct the development of T-helper subsets (2). The NLRP3 inflammasome is described to have a library of ligands including pathogen-derived and environmental stimuli such as alum crystals (3). Alum, an aluminum hydroxide and magnesium hydroxide adjuvant licensed for use in some human vaccines, specifically activates NLRP3-driven immune responses (4, 5). Further, some candidate adjuvants can directly stimulate TLRs. For example, CpG DNA oligonucleotides activate TLR9 (6), and monophosphoryl lipid A (MPLA), a detoxified synthetic form of bacterial LPS stimulates TLR4 (7, 8).
Alum is among the most widely used vaccine adjuvant despite being a poor inducer of cell-mediated immunity (9, 10). In vitro, alum and other inflammasome activators require priming with other stimulatory molecules such as LPS to up-regulate pro-IL-1β and NLRP3 expression (4, 5, 11). The inflammasome signaling of alum is well described to be NLRP3-dependent, although the in vivo role of NLRP3 in mediating adjuvant effects after immunizations with alum remains controversial (12). Studies have described a reduction in antibody and cell-mediated responses in NLRP3-deficient animals (13, 14), whereas others demonstrated reductions in antibody only (5), and again others described no phenotypes in NLRP3-driven antibody or cell-mediated responses (15, 16). Similarly, experiments utilizing a biodegradable microparticulate adjuvant indicated that NLRP3 played no role in enhancing antibody responses, but antigen-induced cell-mediated responses were impaired in the absence of NLRP3 (17). However, an effect of NLRP3 is not universal when using all types of particulate vaccine adjuvants (18).
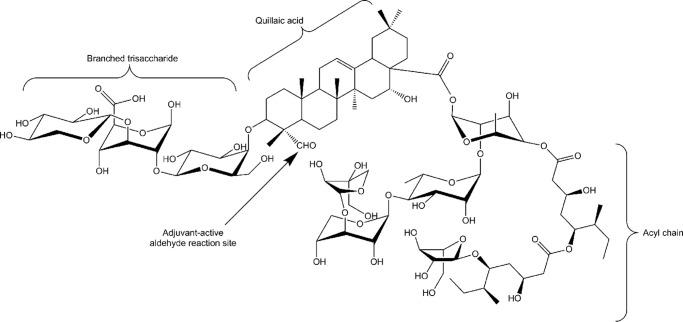
Another group of clinically relevant adjuvants are saponins derived from the bark of the South American soapbark tree, Quillaja saponaria. Quillaja saponins are triterpene glycosides with most containing the triterpene base, quillaic acid. Structural differences in glycosylation or acylation patterns distinguish the saponins from one another and can affect their biological activities. Quil A® is an enriched mixture of soluble Quallija-derived saponins that was found to stimulate humoral and cell-mediated immunity (19) and has been used as an adjuvant in veterinary vaccines (20). In addition, Quil A® can be combined with cholesterol and phospholipids to form a 40-nm particulate antigen delivery system first described by Morein et al. (21). These more complex, particulate saponins, such as ISCOMATRIXTM and Matrix-MTM are highly immunogenic and are being tested in human vaccines (22, 23). Nonparticulated Quil A® consists of more than 20 structurally diverse saponins (24), with 10 containing adjuvant activity. Of the 10, QS-21 (Fig. 1) was described as having robust adjuvant activity with toxicity only observed at high doses in mice. QS-21 is found in the “fraction C” of Quillaja saponins (25) and is a component of all complex Quillaja-containing adjuvants. Synthetic versions of QS-21 and structural variants have been developed that also act as adjuvants (26).
FIGURE 1.
QS-21 structure. Structure of QS-21 showing three key structural domains and the aldehyde site that has been identified as an adjuvant-active site.
QS-21 elicits a robust antibody and cell-mediated immune response and is a potent activator of Th1 and CD8 T cells (27–29). The adjuvant-active sites on QS-21 have been identified as the triterpene aldehyde group (30) (Fig. 1), and deacylation diminished its ability to generate a Th1 response but was less critical for a Th2 response (31). Because QS-21 activates a broad adaptive immune response, it is not surprising that it is a candidate adjuvant for many current trial vaccines such as HIV-1 (32, 33), cancer (34, 35), hepatitis B (36), malaria (37–42), and others. The malaria RTS,S vaccine targets the predominant circumsporozoite protein expressed during the infectious stage of the parasite as an antigen, in conjunction with a combination of QS-21 and MPLA as a liposome (AS01) or emulsion (AS02), adjuvant systems designed by GlaxoSmithKline. The recently licensed MosquirixTM RTS,S malaria vaccine contains QS-21 in AS01. The combination of MPLA and QS-21 further boosts the innate immune response, and results from a phase 3 trial indicate significant protection in vaccinated children (37).
Despite the apparent success of QS-21 as a vaccine adjuvant, the mechanism of action is largely unknown. It has been described to stimulate the innate immune response in mice by increasing natural killer cell activity and in vitro by propagating the release of inflammatory cytokines (43). In addition, soluble and particulate adjuvants that contain heterogeneous mixtures of Quillaja saponins, including QS-21, have been shown to release IL-1β in murine cells in a manner influenced by NLRP3 (5, 44, 45).
Here we show that QS-21, in combination with MPLA, activates the NLRP3 inflammasome in mouse APCs (dendritic cells and macrophages), thus identifying QS-21 as a prominent inflammasome-inducing component of Quillaja-based saponin adjuvants, because only the QS-21-containing saponins appear to activate NLRP3. Interestingly, immunization of NLRP3-deficient mice with QS-21/HIV-1 gp120 caused higher antigen-specific T cell and antibody responses compared with wild type mice, suggesting that NLRP3 may have a dampening effect on the antigen-specific responses mediated by QS-21-adjuvanted vaccines in this experimental setting.
Experimental Procedures
Mice and Reagents
All of the mice were maintained in accordance with the Institutional Animal Care and Use Committee at the University of Massachusetts Medical School (Worcester). C57Bl/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice deficient in TLR4 (TLR4−/−),MyD88 (Myd88−/−), and TRIF (Trif−/−) were from S. Akira, and mice deficient in caspase-1/11 (Casp1/11−/−) were from M. Starnbach. Mice deficient in ASC (Pycard−/−), NLRP3 (Nlrp3−/−), and NLRC4 (Nlrc4−/−) were generated by Millennium Pharmaceuticals, RIP3-deficient mice (Rip3−/−) were generated by V. Dixit (Genentech, South San Francisco, CA) (46), and RIP3/caspase-8-double deficient mice (Rip3−/−xCasp8−/−) (47) were generated by W. J. Kaiser and E. S. Mocarski and provided by K. Fitzgerald (University of Massachusetts Medical School). Mice deficient in GBP5 (Gbp5−/−) were as reported (48). Clinical grade QS-21 was from Antigenics Inc. (Woburn, MA), and synthetic monophosphoryl lipid A (MPLA) derived from Salmonella minnesota R595 structure was purchased from Avanti Polar Lipids (Alabaster, AL). Alum (Imject alum) was purchased from Thermo Scientific and Ab-ISCO-100® was purchased from Novavax AB (formerly Isconova AB, Uppsala, Sweden) Ab-ISCO-100® is the research equivalent of the clinical grade Matrix-MTM from Novavax and is composed of purified saponin fractions A and C (49, 50). QS-21 belongs to fraction C (25). The concentration of Ab-ISCO-100® used in this study is defined as the saponin concentration within the particles. Quil A was from Accurate Chemical & Scientific Corporation (Westbury, NY), and VET-SAP® was from Desert Kings (San Diego, CA). Cytochlasin D, bafilomycin A, poly(dA-dT), nigericin, cholesterol (SyntheChol), and Escherichia coli LPS (repurified in our lab (51, 52)) was from Sigma. Digoxin was from the University of Massachusetts Pharmacy and used at 5 μg/ml. Caspase-1 (YVAD) and cathepsin B inhibitors were from Calbiochem. Sapindoside A, hedaracoside C, and β-escin, all at 5 μg/ml, were generously provided by Su Chiang (Institute of Chemistry and Cell Biology-Longwood Screening Facility, Harvard Medical School).
Immunizations
C57Bl/6 and NLRP3-deficient mice were immunized intramuscularly with saline, 5 μg of QS-21 or QS-21 with 2.5 μg of highly purified, codon-optimized gp120 protein previously used in clinical studies from primary HIV-1 isolate B produced in CHO cell lines by Advanced Bioscience Laboratories (Kensington, MD) as previously described (53). Immunizations were given at 0 and 4 weeks. One week following the second immunization, mice were euthanized, and injections sites were excised and homogenized in GentleMACS M tubes with phosphate-buffered saline containing protease inhibition mixture (Roche Applied Science). Homogenates were centrifuged at 4 °C, and IL-1β in supernatants was measured by ELISA. Other assays were performed as described below. Immunization groups consisted of five animals per group and were repeated twice.
Growth and Stimulation of Cells
Bone marrow-derived dendritic cells were generated by culturing bone marrow cells for 8–9 days in medium supplemented with 20 ng/ml of GM-CSF. Immortalized mouse macrophages were generated using J2 virus. Cells were plated overnight in 96-well plates at 1 × 105 cells per well for ELISA or in 12 wells plates at 1–2 × 106 cells per well for Western blot. The next day cells were primed with LPS (10 ng/ml) or MPLA (5 μg/ml or 500 ng/ml depending on the batch) for 3 h prior to a 6-h incubation with poly(dA-dT) or ISCOM (5 μg/ml unless otherwise indicated) or a 1-h incubation with nigericin. When used, the inhibitors cytochlasin D (5, 1.7 μm), bafilomycin A (250, 83 μm), caspase-1 (Z-YVAD-FMK, 20 μm), or cathepsin B (Ca-074-me, 20 μm) were added 1 h prior to the start of stimulations. To induce maximal guanylate binding protein 5 (Gbp5) protein levels, wild type and GBP5-deficient cells were treated with 100 units/ml of IFN-γ for 14 h prior to stimulation. Salmonella enterica serovar Typhimurium (strain SL1344, provided by Mary O'Riordan) was grown overnight at 37 °C, diluted 1:4 the next day and cultured for 3 h more. After washing, the bacteria were resuspended in culture medium, and cells were infected at a multiplicity of infection (MOI) of 1 bacterium per cell for 3 h before addition of 40 μg/ml of gentamycin followed by 3 h more of stimulation. Yersinia pestis (strain KIM5) and Yersinia enterocolitica (strain 8081) were grown at 26 °C overnight, diluted 1:4 the next day and cultured for 1 h at 26 °C followed by 2 h at 37 °C (54). After washing, the bacteria were resuspended in culture medium, and cells were infected at a MOI of 10 for 3 h before addition of 40 μg/ml of gentamycin followed by 3 h more of stimulation. For alum (125 μg/ml) and QS-21 (2 μg/ml, well below the critical micelle concentration by ∼20-fold (30)) stimulations, MPLA was added at the same time, and the cells were stimulated for 6 h. Culture supernatants and cell lysates were immediately frozen. Cytokines (IL-6, IL-1β, and IL-18) were measured by ELISA according to the manufacturer's instructions using kits or antibodies from R&D.
Western Blot
Protein extracts from cell supernatants were precipitated by methanol-chloroform extraction, and protein from cell lysates were generated by lysing adherent cells with 1× radioimmune precipitation assay buffer (Boston BioProducts) containing 1 mm sodium orthovanadate, 1 mm PMSF, and cOmplete Protease Inhibitor Mixture (Roche Applied Science). Immunoblot analysis was performed as previously described (4, 52) with antibodies to mouse caspase-1 p20 (clone 5B10; eBioscience), IL-1β (catalogue no. AF-40-NA; R&D Systems), and β-actin (BD Pharmingen).
Measurement of Cell Viability
Cells were treated as previously described for stimulations except they were cultured in serum-free X-Vivo15 (Lonza) medium. Cell viability was quantified by measuring calcein AM (Invitrogen) uptake or lactate dehydrogenase (LDH; Promega) release according to the manufacturer's instructions. For calcein AM uptake, samples were normalized to untreated cells. For LDH release, the percentage of cell death was calculated as (sample LDH − background LDH)/(total LDH − background LDH) × 100%, where background LDH was determined for untreated cells, and total LDH was determined for cells lysed with lysis buffer.
Serum Antibody Responses
Serum gp120-specific antibody end point titers were evaluated by ELISA. MaxiSorpTM microtiter plates (Thermo Scientific, Rochester, NY) were coated with 1 μg/ml of HIV-gp140 (Immune Technology, New York, NY) overnight and blocked with 1% BSA (Sigma) in saline for 1 h. Plates were incubated with mouse sera for 2 h, and total IgG, IgG1, IgG2b, IgG2c, and IgG3 were detected with biotinylated antibodies (Southern Biotech, Birmingham, AL) followed by HRP-conjugated streptavidin (R&D Systems, Minneapolis, MN). Serum titers were determined as the highest dilution of immune serum producing values (A450 nm) greater than or equal to the average plus three times the standard deviation of preimmune serum at the lowest dilution.
T-cell Stimulation Assay (ELISpot)
ELISpot assays were performed mainly as described (55, 56). Spleens were collected and homogenized to obtain single cell suspensions 7 days following the final immunization. Splenocytes were filtered through a 40-μm mesh, and red blood cells were lysed with RBC lysis buffer (Sigma). Cells were seeded in triplicate into precoated ELISpot plates (Millipore, Bedford, MA) at 2.5 × 105/well in RPMI 1640 (Lonza, Basel, Switzerland) with 10% heat-inactivated FBS (HyClone, Logan, UT), 1% ciprofloxacin, and 50 μm 2-mercaptoethanol (Sigma). Antigen-specific stimulation was performed with truncated peptide pools (G pool (peptides 8840–8853) or V3 pool (8836–8844), National Institutes of Health AIDS Research and Reference Reagent Program) derived from clade B consensus Env peptide pool at 2 μg/ml as previously described (57). Control wells contained either media alone (mock) or 20 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin (Sigma). Stimulations were carried out for 18–20 h at 37 °C in 5% CO2 and IFNγ, IL-2, IL-4 (Mabtech, Mariemont, OH), and IL-6 (BD Biosciences, San Diego, CA) ELISpot assays were conducted per the manufacturer's directions. Cytokine spots were visualized using a CTL Imager and counted with ImmunospotTM software (Cellular Technology Ltd., Shaker Heights, OH).
Statistics
The results are presented as means ± S.E. All experiments were repeated at least two times. Statistical analysis was performed with GraphPad Prism 6.02. To evaluate the differences between two groups, the two-tailed t test was used. Alternatively, one-way analysis of variance with Tukey's post-test was used.
Results
QS-21 Induces Caspase-1-dependent IL-1β Secretion
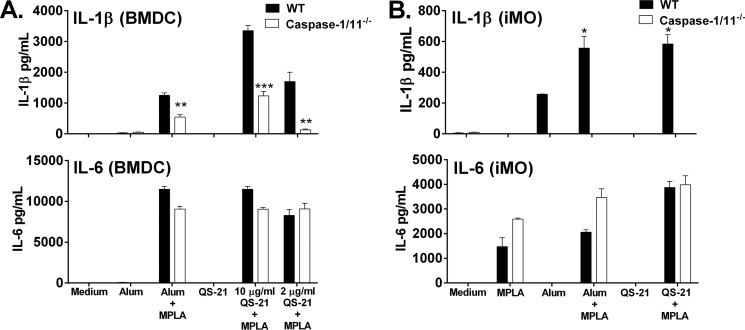
To assess the stimulatory ability of QS-21, we stimulated mouse dendritic cells and macrophages with increasing concentrations of QS-21 and analyzed cytokine secretion. The pro-inflammatory cytokines IL-6 and IL-1β were not detected in the supernatant of bone marrow-derived dendritic cells (BMDCs) or immortalized mouse macrophages (macrophages) stimulated with purified QS-21 alone (Fig. 2, A and B). However, QS-21 (2 μg/ml) in the presence of the TLR4 stimulatory adjuvant MPLA induced release of IL-1β, which was dependent on caspase-1/11, indicating that QS-21 activates the inflammasome (Fig. 2, A and B). The activation of caspase-1 directly indicates the activation of the inflammasome. The levels of IL-1β in the supernatant after QS-21 stimulation were higher than those seen by alum, a known inflammasome activator (4, 5). In contrast, supernatant concentrations of IL-6, induced by MPLA alone, were not negatively affected by the absence of caspase-1/11 for any of the stimuli tested.
FIGURE 2.
QS-21 induces caspase-1/11-dependent IL-1β secretion. Murine BMDCs (A) or macrophages (B) from WT or caspase-1/11-deficient mice were stimulated for 6 h with alum (100 μg/ml) or QS-21 (10 or 2 μg/ml) with or without MPLA (5 μg/ml). IL-6 and IL-1β were measured in culture supernatants by ELISA. The data are triplicates ± S.E. and are representative of two or more independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 indicate a significant difference detected between WT and caspase-1/11 cells determined by Student's t test.
QS-21-MPLA Induced IL-1β Secretion Requires TLR4
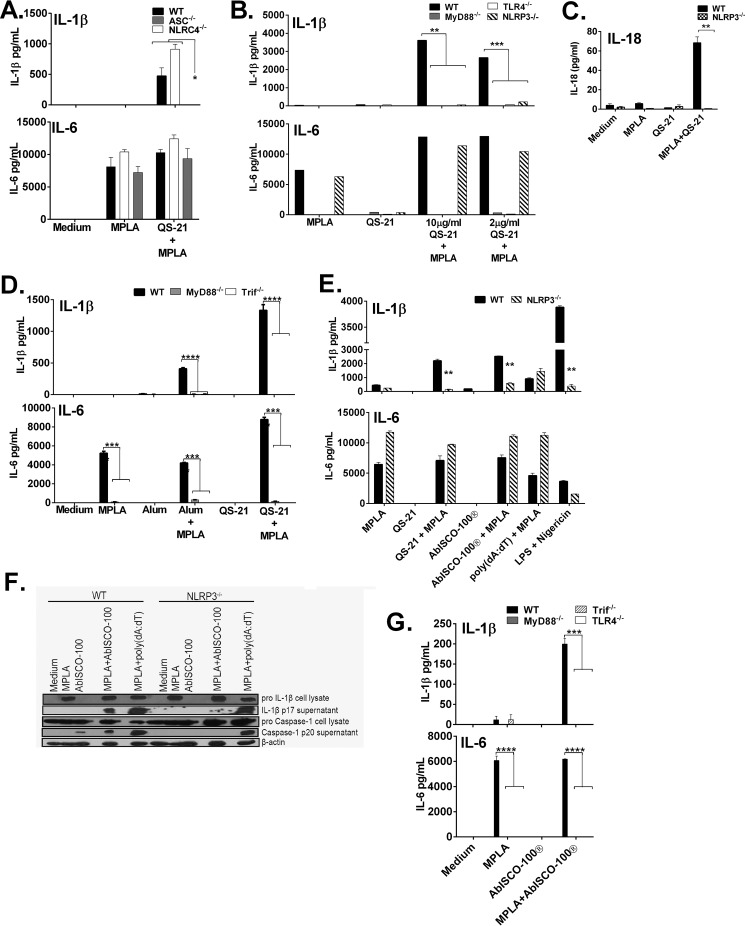
To determine the mechanism through which QS-21 induced caspase-1-dependent IL-1β release, WT, NLRC4/IPAF-deficient, and ASC-deficient BMDCs were stimulated with MPLA in the presence or absence of QS-21. IL-1β release was comparable between WT and NLRC4-deficient cells stimulated with QS-21 and MPLA but was completely abolished in cells lacking the inflammasome adaptor protein ASC (Fig. 3A), which some inflammasomes require to activate caspase-1. Production of IL-6 was high in all stimulated cells (Fig. 3A).
FIGURE 3.
QS-21-MPLA induced IL-1β secretion requires TLR signaling and activates the NLRP3 inflammasome. A–G, murine BMDCs (A–C) or macrophages (D–G) from WT or ASC-, NLRC4-, TLR4-, MyD88-, Trif-, GBP5-, or NLRP3-deficient mice were stimulated for 6 h with the indicated stimulants with or without MPLA (5 μg/ml); LPS was at 10 ng/ml, AbISCO-100® was 5 μg/ml, and QS-21 was at 2 μg/ml unless otherwise indicated. IL-18 (C), IL-6 (A, B, D, E, and G), and IL-1β (A, B, D, E, and G) were measured in culture supernatants by ELISA. The data are triplicates ± S.E. and are representative of two or more independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 indicate a significant difference detected between WT and deficient cells determined by Student's t test (B–E and G) or by analysis of variance followed by Tukey's multiple comparisons test (A). F, Western blot indicated cleaved caspase-1 p20 and IL-1β p17 and full-length caspase-1 p45 and β-actin p 45 as a loading control.
Because QS-21 did not induce IL-1β in the absence of MPLA, this suggested that TLR4 signaling is necessary for IL-1β release induced by QS-21. Indeed, BMDCs from TLR4 and MyD88-deficient mice exhibited no IL-6 in response to MPLA or QS-21/MPLA, but cells from NLRP3-deficient mice produced IL-6 at levels similar to WT cells (Fig. 3B). However, MPLA and QS-21 induced IL-1β and IL-18 production in WT but not in NLRP3-deficient cells (Fig. 3, B and C). Macrophages lacking the TLR4 adaptor proteins MyD88 or TRIF were refractory to IL-6 and IL-1β production by MPLA alone and in combination with alum and QS-21 (Fig. 3D).
We wanted to compare APC stimulation induced by purified QS-21 to the mixed Quillaja saponin-containing particulate adjuvant, AbISCO-100® (22), which includes QS-21. This type of complex belongs to a group of nanoparticle adjuvants such as ISCOMATRIXTM and NovaMatrixTM all composed of saponins, cholesterol and phospholipids (22). They are proposed to be less toxic than pure QS-21 while maintaining the ability to induce antigen-specific antibody titers and directed T cell responses (58, 59). Further, similar saponin particles are known to induce IL-1β production both in vivo and in vitro (44, 45).
NLRP3-deficient macrophages had significantly reduced IL-1β after stimulation with MPLA/QS-21 and MPLA/AbISCO-100® (Fig. 3E). Nigericin, known to drive NLRP3-dependent responses (60) also had reduced IL-1β similar to QS-21 and AbISCO-100® in the presence of MPLA or LPS (Fig. 3E). Wild type and NLRP3-deficient macrophages stimulated with poly(dA-dT), an AIM2 inflammasome ligand, had a similar IL-1β response (Fig. 3E). Interestingly, the IL-1β response in the wild type cells was similar whether stimulated with QS-21 or AbISCO-100®. IL-6 responses were unchanged between WT and NLRP3-deficient cells under all conditions (Fig. 3E). The IL-1β generated by AbISCO-100® was mature cytokine and also induced cleavage of caspase-1 that was dependent on NLRP3 (Fig. 3F). Similar to cytokine production seen with QS-21, TLR4 and the adapter molecules MyD88 and Trif are essential for IL-6 and IL-1β secretion by macrophages when stimulated with MPLA/AbISCO-100® (Fig. 3G).
QS-21-induced Cell Death under Serum-free Conditions Occurs Independent of Caspase-1/11 and Is Rescued by the Addition of Exogenous Cholesterol
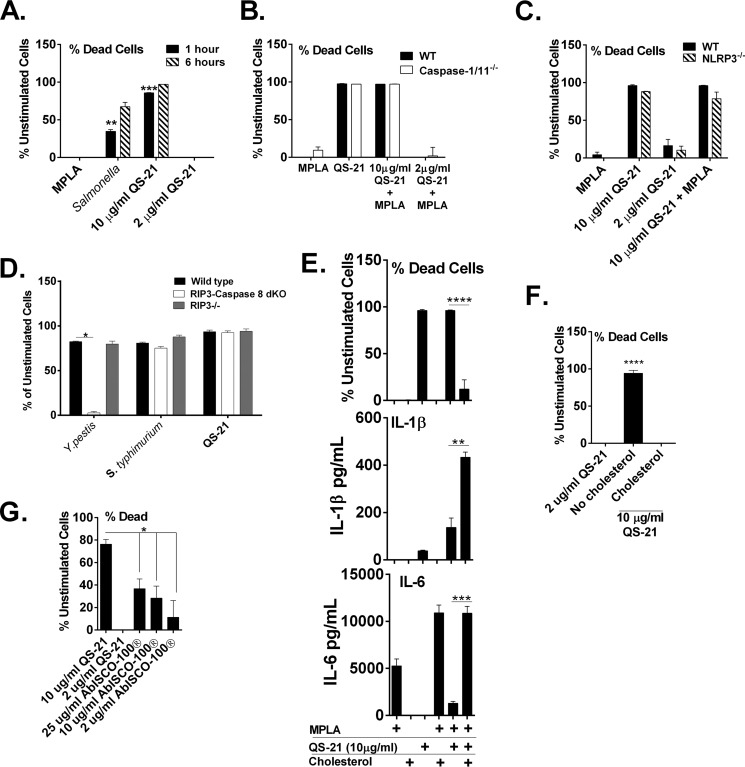
The cytotoxicity of QS-21 in vitro is well documented and results in hemolysis of red blood cells (24), though the cytotoxic effect of QS-21 on APCs is not well characterized. Under serum-free conditions, rapid and nearly 100% cell death occurs after 1 h in macrophages treated with a high concentration of QS-21 (10 μg/ml) (Fig. 4A). However, a lower concentration, as used for IL-1β and IL-18 induction (Figs. 2 and 3) does not result in increased macrophage cytotoxicity at either time point tested, uncoupling IL-1β release and cell death for this stimulant. Infection with Salmonella, known for activating NLRC4-dependent pyroptosis, results in 40% cell death after 1 h postinfection and increases almost 2-fold by 6 h.
FIGURE 4.
QS-21-induced cell death under serum-free conditions is independent of caspase-1 and rescued by the addition of exogenous cholesterol. Murine BMDCs (B) or macrophages (A and C–G) from WT or ASC-, caspase-1/11-, NLRP3-, RIP3-, or RIP3/caspase-8-deficient mice were stimulated for 1 (A) or 6 h (A–G) with the indicated stimulants with or without MPLA (5 μg/ml). In A and D, S. enterica serovar Typhimurium used a MOI of 1 bacterium per cell. In E, QS-21 was used at 10 μg/ml. In F, cholesterol was added at 30 μg/m l. Cell viability was determined with calcein AM (A–C and E–G) or LDH (D) and is represented as the percentage of dead cells relative to unstimulated cells and a positive control. IL-6 and IL-1β (E) were measured in culture supernatants by ELISA. Experiments were performed in serum-free medium. The data are triplicates ± S.E. and are representative of two or more independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 indicate a significant difference determined by Student's t test.
Pyroptosis is a form of programmed cell death dependent on the activation of caspase-1 or caspase-11(61). Because caspase-1 activation is a direct result of inflammasome formation, we wanted to determine whether QS-21 was driving pyroptosis. Cytotoxicity of wild type and caspase-1/11-deficient BMDCs incubated with a high concentration of QS-21 with and without MPLA were unchanged, indicating that caspase-1/11-dependent pyroptosis is not occurring (Fig. 4B). Similarly, QS-21-driven cytotoxicity was unchanged in NLRP3-deficient BMDCs compared with wild type cells (Fig. 4, B and C). Taken together, this further confirms that QS-21-induced cell death is independent of inflammasome activation, unlike the production of IL-1β. QS-21 at the lower combination incubated with MPLA did not result in cell death even in the absence of serum (Fig. 4B). Caspase-8-dependent apoptosis or RIP3-dependent necroptosis are unlikely routes of cell death induced by QS-21 because BMDMs from mice deficient in both RIP3 and caspase-8 (47) had unchanged levels of cytotoxicity after incubation with QS-21 (Fig. 4D). Y. pestis-induced apoptosis was abrogated in these cells (Fig. 4D) (54). S. enterica serovar Typhimurium, shown to drive macrophage cytotoxicity via NLRC4- and caspase-1-dependent pyroptosis (54), also generated cell death independently of caspase-8 and RIP3 (Fig. 4D).
Although details of the mechanism of QS-21-driven cytotoxicity are mostly unclear, it could favor interactions between the saponin and membrane cholesterol, promoting membrane pore formation or cell lysis via its acyl component, because deacyl-saponins show no toxicity (62). Furthermore, particulate saponins containing cholesterol appear to have lower toxic effects (22). To determine whether the cytotoxicity toward dendritic cells observed at high concentrations of QS-21 could be rescued by the addition of cholesterol, exogenous soluble cholesterol was added to the serum-free culture medium in the presence of the uniformly cell-toxic dose of QS-21 (10 μg/ml) and MPLA. The addition of cholesterol (well below concentrations that could lead to crystal formation) inhibited cell death, and IL-1β was increased in parallel. This may be due to increased cell survival and not through activation of NLRP3 via cholesterol, because MPLA and cholesterol together did not induce IL-1β, as has been shown for crystallized cholesterol (Fig. 4E) (63). Cell death is often associated with IL-1β release and inflammasome activation (61); however, in this case, cholesterol-inhibited cell death and increased cell death were not associated with increased IL-1β production. Thus, these experiments suggest that cell death and IL-1β release may be uncoupled in the case of QS-21-stimulation of macrophages; these events appear to occur independently of each other. The addition of cholesterol in the presence of QS-21 and in the absence of MPLA also rescued cell death to levels seen with 2 μg/ml of QS-21 (Fig. 4F).
Comparison of the cellular toxicity of AbISCO-100® with QS-21 showed a dose-dependent induction of cell death with AbISCO-100®, but even at 25 μg/ml of the mixed saponins, cell death was 2-fold lower compared with 10 μg/ml of QS-21 (Fig. 4G), potentially reflecting both the presence of cholesterol (Fig. 4E) in the nanoparticles and the reduced actual amount of cytotoxic saponins on a weight basis.
Lysosomal Acidification and Caspase-1 Activity Are Required for QS-21-induced IL-1β Secretion
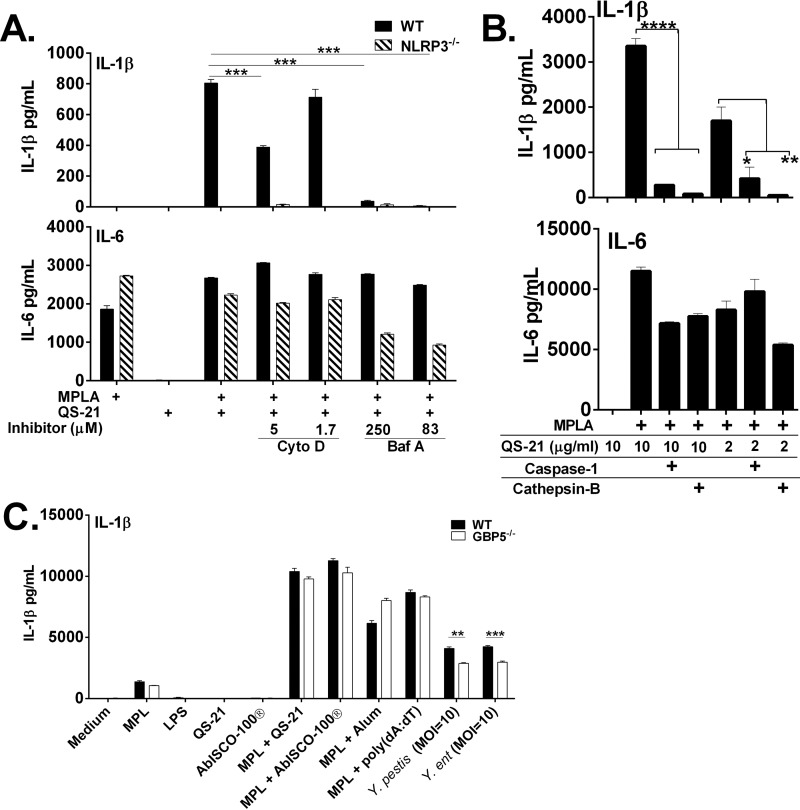
How QS-21 is internalized and how uptake may impact cellular functions are poorly understood. Stimuli such as aluminum salts, monosodium uric acid crystals, silica, and PLG microparticles are phagocytosed and cause disruption of the lysosome, resulting in activation of NLRP3 (4, 17, 64, 65). Pretreatment of WT or NLRP3-deficient macrophages with cytochlasin D, an inhibitor of actin filament assembly, or bafilomycin A, an inhibitor of lysosomal acidification, has little effect on IL-6 in either cell type (Fig. 5A). However, there was only a partial reduction of IL-1β production when cells were pretreated with cytochlasin D prior to the addition of QS-21 and MPLA. In contrast, bafilomycin A had nearly complete inhibition of IL-1β, indicating that lysosomal acidification may be important for mediating inflammasome activation by QS-21. IL-1β was not detected in NLRP3-deficient cells with any stimuli.
FIGURE 5.
Phagocytosis, lysosomal acidification, and caspase-1 activity are required for QS-21-induced IL-1β secretion. Murine macrophages from WT or GBP5- or NLRP3-deficient mice were stimulated for 6 h with the indicated stimulants (A–C). Cytochlasin D (5 μm) and bafilomycin A (83 μm) inhibitors (A) or 20 μm caspase-1 and cathepsin B inhibitors (B) were added 1 h before the cells were stimulated. QS-21 was used at 2 μg/ml unless otherwise indicated. In C, cells were treated with 100 units/ml of IFN-γ for 14 h prior to stimulation to induce maximal levels of Gbp5; Yersinia species were used at an MOI of 10 bacteria per cell and S. enterica serovar Typhimurium was used at an MOI of 1. IL-1β and IL-6 were measured in culture supernatants by ELISA. The data are triplicates ± S.E. and are representative of two or more independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001 indicate a significant difference determined by Student's t test.
Cathepsin B is a lysosomal protease that has been implicated in causing activation of NLRP3 when it is released during lysosomal disruption (66). Pretreatment of wild type BMDCs with a caspase-1 inhibitor or cathepsin B marginally affected IL-6 in stimulated cells stimulated with QS-21 and MPLA (Fig. 5B). IL-1β was significantly decreased by both cathepsin B and caspase-1 inhibitors at least 10-fold with both doses of QS-21(10 and 2 μg/ml) and MPLA.
Pathogenic bacteria and soluble agents have been shown to activate NLRP3 through Gbp5 (guanylate binding protein 5) (48). GBP5-deficient BMDMs showed a moderate reduction in IL-1β production induced upon infection with Y. pestis and Y. enterocolitica bacteria, but no decrease was seen with saponin NLRP3 activators tested, indicating that QS-21 does not activate NLRP3 via Gbp5 (Fig. 5C). This may fit the bifurcating profile reported earlier in which Gbp5-deficient BMDMs had normal NLRP3 responses to other adjuvants such as alum but compromised activities elicited by bacterial stimuli (48).
Other QS-21-containing Saponins Activate IL-1β Production through the NLRP3 Inflammasome
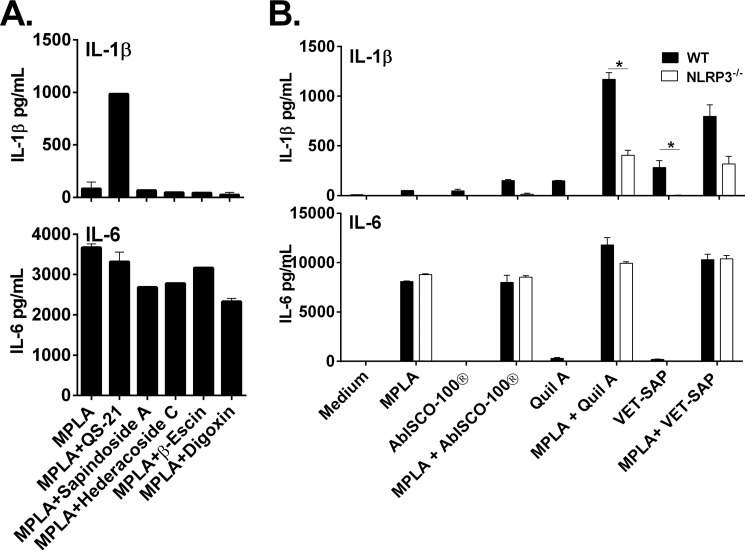
To determine whether inflammasome activation is a general phenomenon elicited by saponins or whether it is specific to QS-21, we tested the ability of non-QS-21 members of the saponin compound family to produce IL-1β. Macrophages were treated with QS-21, digoxin, sapindoside A, hedaracoside C, or β-escin in combination with MPLA. Of the four other saponins tested, only QS-21 induced IL-1β release (Fig. 6A), indicating that inflammasome activation may be specific to Quillaja-containing saponins. IL-6 release was similar for all stimulants (Fig. 6A).
FIGURE 6.
Other QS-21-containing saponins activate IL-1β secretion through NLRP3. Murine macrophages from WT or NLRP3-deficient mice were stimulated for 6 h with the indicated stimulants (5 μg/ml for all except 2 μg/ml for QS-21) in the presence or absence of MPLA (5 μg/ml) (A and B). IL-1β and IL-6 were measured in culture supernatants by ELISA. The data are triplicates ± S.E. and are representative of two or more independent experiments. *, p < 0.05 indicates a significant difference determined by Student's t test.
Furthermore, we tested the more heterogenous semi-fractionated Quillaja-containing saponin used in veterinary vaccines, Quil-A® and VET-SAP® for their ability to activate the NLRP3 inflammasome. Both compounds were able to produce NLRP3-dependent IL-1β, whereas IL-6 was unaffected (Fig. 6B).
NLRP3 Dampens Antigen-specific Responses to a HIV-1 gp120/QS-21 Combination in Vivo
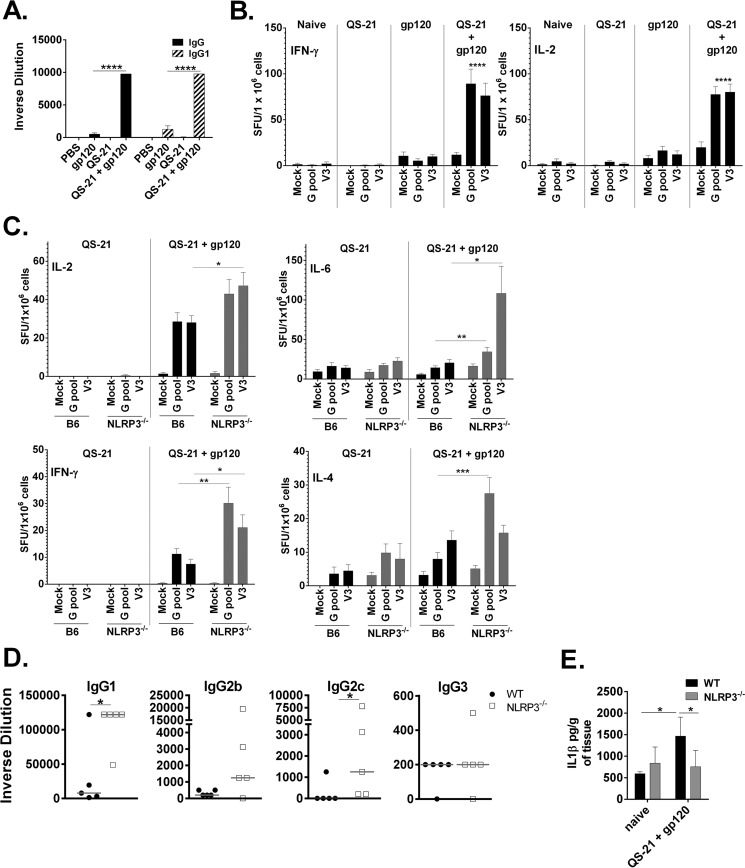
Several adjuvants are proposed to trigger NLRP3-dependent inflammasome activation, but the role of NLRP3 in antigen-specific responses in vivo is debated (67, 68). The role of NLRP3 may be impacted by variations in antigen, vaccine formulation, and immunization scheme. QS-21 clearly activates the NLRP3 inflammasome in vitro, but the importance of NLRP3 for eliciting its adjuvanticity in vivo is unknown. To address this, wild type and NLRP3-deficient mice were immunized two times at 4-week intervals with QS-21 in the absence or presence of highly purified, GMP grade, clinically relevant HIV-1 gp120 antigen. Seven days after the second immunization, splenocytes were collected and stimulated with gp120 peptides for evaluation of T-cell mediated responses. In addition, production of gp120-specific antibodies were measured in serum from vaccinated animals. In a separate experiment, wild type mice were given the same immunization regimen and immunized with gp120 alone, QS-21 alone or the combination of gp120 with QS-21 to demonstrate the effectiveness of QS-21 as an adjuvant. End point serum IgG and IgG1 were significantly increased in mice that received QS-21 and gp120 compared with mice immunized with protein only (Fig. 7A). Additionally, antigen-specific T-cell-mediated IFN-γ and IL-2 were significantly more robust compared with mock-immunized mice (Fig. 7B), demonstrating that QS-21 is an effective adjuvant.
FIGURE 7.
NLRP3 suppresses antigen-specific responses by a QS-21 adjuvanted vaccine in vivo. A and B, WT mice were immunized with QS-21, gp120, or a combination of QS-21/gp120 two times, with the second immunization 4 weeks after the first. C–E, WT and NLRP3-deficient mice were immunized with QS-21 with or without HIV gp120 two times, with the second immunization 4 weeks after the first. Seven days after the second immunization (B and C), IL-2, IFN-γ (C), IL-6, and IL-4 ELISpots were performed on splenocytes and anti-gp120 ELISAs were performed on serum (A and D). For ELISpots, splenocytes were seeded into 96-well plates in triplicate and stimulated for 18 h with twogp120-specific peptide pools (G pool or V3) or media alone (Mock). Cytokine spots were quantified using CTL software; the data are triplicates ± S.E. E, the muscle at the injection site was removed and homogenized, and the supernatants were analyzed by ELISA for IL-1β. Values were normalized to the total amount of tissue removed from each animal. *, p < 0.05; **, p < 0.01; ***, p < 0.001 indicate a significant difference determined by Student's t test. The data represent one of three experiments with five mice per group.
Interestingly, NLRP3-deficient mice had increased antigen-induced IL-2, IL-6, IL-4, and IFN-γ compared with wild type mice (Fig. 7C), indicating that both the Th1 and Th2 T cell responses were enhanced in the absence of NLRP3. Similarly, the terminal antigen-specific humoral IgG1 and IgG2c antibody responses were significantly increased in the NLRP3-deficient mice, whereas IgG2b and IgG3 were largely unchanged in NLRP3-deficient mice (Fig. 7D). Mice immunized with QS-21and gp120 had low but detectable levels of IL-1β at the injection site in a manner dependent upon NLRP3 (Fig. 7E).
Discussion
Complex and heterogeneous saponin-containing adjuvants have previously been suggested to trigger IL-1β and IL-18 release, but it has been unclear which saponin components contribute to the cytokine release. Here we show that the defined saponin molecule QS-21 triggers IL-1β and IL-18 release in an NLRP3-Asc-caspase-1-dependent manner. However, we observed that signaling via NLRP3 actually decreased vaccine effects in vivo to the HIV-1 antigen gp120 envelope protein in the presence of QS-21.
It has been hypothesized that the particulate structure of ISCOMs mediates some of the effects on IL-1β release via NLRP3, because many particulates do indeed trigger inflammasome activation via NLRP3. In this study, we identify a chemically defined saponin that is able to trigger inflammasome activation. QS-21-triggered NLRP3 responses are likely not due to particulate formation because the amount of QS-21 used here is ∼20-fold lower than the critical micelle concentration (30). QS-21 should be present in all current saponin containing vaccines, hence the inflammasome-inducing action of the heterogeneous saponins may be due to QS-21 and similar compounds (26, 69).
Innate immune cells sense a number of cellular danger signals via NLRP3, and cell death can be a consequence of NLRP3-triggered inflammasome responses. NLRP3 inflammasome activation can induce caspase-1-dependent pyroptosis, but in the absence of caspase-1 signaling, QS-21 still triggered cell death. In addition, cell death occurred independently of caspase-8-induced apoptosis because RIP3/caspase-8 double-deficient cells also died when treated with QS-21 (Fig. 3D).
The toxicity of QS-21 and saponin-containing compounds is typically determined by injecting them into mice or measuring hemolysis in vitro (24, 26, 30, 70). Hemolysis likely occurs because of the affinity of saponins for membrane cholesterols, which is the basis for the design of the cholesterol-phospholipid-saponin particle complexes (22). In this study, we show that death of antigen presenting cells induced by higher concentrations of QS-21 was completely abrogated with the addition of exogenous soluble cholesterol. This result implies that at least one method by which QS-21 initiates cell death is through binding to cell membrane-associated cholesterol leading to increased cell permeability through pore formation and ultimately rupturing of the cell and that binding to cholesterol in ISCOM-based particles decreases cytotoxicity toward macrophages and dendritic cells. This process has previously been observed with crude preparations of saponins (71) and is the basis for routine use of these saponins to permeabilize cells for assays such as intracellular cytokine staining.
Alum has also been shown to have cytotoxic effects. Cell death results in the release of host DNA into the cytoplasm and has been suggested to influence its adjuvanticity through triggering STING (stimulator of IFN gene)-dependent DNA sensing mechanisms (72). Because QS-21 induces cell death independent of its inflammasome activating ability, similar mechanisms are also possible for Quillaja-derived saponin adjuvants. The presence of endogenous cellular cholesterol in vivo may help to dampen any cytotoxic effects of QS-21, because a typical dose used in humans in vivo (50 μg) (32) is high compared with our studies (2–10 μg/ml in vitro, 5 μg in vivo in mice). On the other hand, some limited cytotoxicity may contribute to release of DAMPs such as HMGB1, nucleic acids, or other components that could contribute to a priming signal. Therefore, it is possible that QS-21-induced cell death may impact adjuvant effects.
Our data suggested that NLRP3 does not mediate adjuvant effects of QS-21 in vivo, using a clinically relevant vaccine antigen, HIV-1 gp120 (Fig. 6). In fact, antigen-specific T cell responses and IgG1 and IgG2c are increased in NLRP3-deficient mice compared with wild type mice, suggesting that NLRP3-mediated signals may impair vaccine effects. Interestingly, several synthetic variants of QS-21 have been generated (26). It is possible that certain structural components may be responsible for the NLRP3 activation. If it is shown that the impairment of the effect of QS-21-containing vaccines by NLRP3 is a general feature, then the generation of compounds with reduced NLRP3 activating abilities may be desirable. We cannot, however, exclude the possibility that by using a different immunization route, regimen, and/or QS-21-containing vaccine formulation that we may have seen an altered outcome of the experiment. The presence of MPLA or other TLR stimulating adjuvants co-formulated with QS-21 may also modulate signaling pathways in vivo. Of note, the role of NLRP3 activation by alum in vaccinations is controversial because there are several reports with contrasting results (5, 13, 15, 16). However, there was much variation between the studies when comparing immunization routes (intraperitoneal, subcutaneous, intranasal, or intramuscular) and the frequency, dose, and time between immunizations and type of antigens used (i.e. model antigens such as OVA versus bacterial/viral protein antigens).
The combination of QS-21 with gp120 is an efficient pairing that induces a robust humoral and cell-mediated immune response (32, 53, 56). These studies were undertaken to investigate the response to QS-21 to evaluate how the innate responses to the adjuvant can direct downstream adaptive responses. It is unclear whether the same response would be seen using a combination adjuvant such as GSK AS01 or AS02, which include MPLA, because this additional component and special formulations (liposomes for AS01 and emulsions for AS02) were not present in our in vivo studies. However, it is conceivable that AS01 can signal through NLRP3 in vivo because IL-1β was detected in the muscle and draining lymph node upon immunization (73).
Although MPLA was required to prime for eliciting IL-1β in vitro, QS-21 alone lead to detection of IL-1β at the injection site in a manner dependent on NLRP3 (Fig. 7E). This is perhaps partly a reflection on the ability of some inflammasome stimulators to induce IL-1β release in vivo without the strong priming of signal 1 required in mouse macrophages and dendritic cells in vitro (17). As previously stated, QS-21 may have cytotoxic effects in vivo allowing for release of DAMPs. It is worth mentioning that the dose of QS-21 used here is 10-fold lower than that used in human studies, and QS-21 has been associated with reactogenicity at the injection site (32). In addition, QS-21 could be contributing to antigen internalization and cross-presentation by antigen-presenting cells contributing to the immune response shown here (44).
Even though QS-21 activates NLRP3 in vitro in a manner similar to alum, there are clear differences in vivo. It has been proposed that the more complex saponin-containing adjuvant ISCOMATRIXTM activated the NLRP3-ASC-caspase-1 inflammasome in vitro, but NLRP3 was dispensable in the in vivo mouse immunizations using the model antigen OVA, whereas IL-18 was crucial for a portion of the innate and adaptive responses (45). We have not tested the effects of the absence of IL-1β or IL-18 on adjuvant effects in the present study, but this is an issue that warrants further evaluation. Some adjuvants contain both MPLA and QS-21 as mentioned above, whereas other vaccines contain antigen and QS-21 alone (74). Potentially, the impact of NLRP3 may vary depending on the specific formulation of the vaccine, such as in ISCOMATRIXTM nanoparticles containing cholesterol and phospholipids or emulsions or liposomes used in the AS series of GSK adjuvants (75). Another variable is whether QS-21 is given in the presence or absence of another stimulant of innate immunity, such as MPLA, alum, lipoproteins, poly(IC), TLR7/8 stimulators, or CpG DNA. It is thus difficult to predict how our results may be applied to all vaccines containing QS-21 and immunostimulatory saponins, and the impact of NLRP3 in vaccinations with each vaccine/adjuvant combination and specific immunization scheme would need to be tested.
The combination of QS-21 and HIV-1 gp120 used here reveal that the activation of NLRP3 in vivo decreased vaccine effects. The mechanism of how NLRP3 may inhibit antigen-specific responses is unclear at this time. A study by Kool et al. (14) showed an increased IgG2c antibody response in NLRP3-deficient mice immunized with alum/OVA, whereas IgG1 remained unchanged, and IgE was significantly decreased in the NLRP3-deficient mice. It is possible that in the absence of NLRP3, activation of other innate sensing pathways through release of host DNA through cell death and other cellular danger signals may be altered, and NLRP3 may influence other signaling pathways. In light of these observations and the discrepancies seen in studies using alum, it is critical to understand the exact responses for specific vaccine formulations to better design future vaccines. In some cases, it may be premature to make broader conclusions based on the use of in vitro studies or model antigen systems. As mentioned above, novel synthetic variants of QS-21 have been generated (26). It would be interesting to test how these new molecules interact with inflammasome signaling. We predict that it may be possible to design new saponin-based adjuvants that have reduced ability to induce less desirable aspects of adjuvant effects while promoting the induction of specific immunity. The result may be new promising adjuvants with less unwanted side effects. The investigation of innate immunity signaling pathways associated with adjuvant activity for clinically relevant adjuvant/antigen combinations should reveal information that will be valuable when designing new and improved schemes for vaccine protection against a variety of diseases.
Author Contributions
Experiments were performed by R. M.-R. with help from G. I. V., K. P., D. W., K. W., and R. B. C., after discussions with E. L., S. W., and S. L. E. L. conceived the study with input from R. M.-R. and S. L. Funding was obtained by E. L. and S. L. J. D. M. and J. D. C. provided legs from GBP5-deficient mice and contributed to interpretation of experiments in Fig 4B. The paper was written by R. M.-R. and E. L. with contributions from the other authors. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Gail Germain, Anna Cerny, and Kelly Army for animal husbandry and Gerald A. Beltz for reading the manuscript.
This study was supported by National Institutes of Health Grants 5 U19 AI082676 and 5 PO1 AI082274; in part by National Institutes of Health Grants AI07538, AI117706, and AI057588 through the American Recovery and Reinvestment Act (to E. L.); National Institutes of Health Grant AI095213 (to G. I. V.); funds from the Norwegian Cancer Society; and Research Council of Norway Center of Excellence Funding Scheme Project 223255/F50. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TLR
- Toll-like receptor
- NLR
- Nod-like receptor
- MPLA
- monophosphoryl lipid A
- FMK
- fluoromethyl ketone
- MOI
- multiplicity of infection
- LDH
- lactate dehydrogenase
- BMDC
- bone marrow-derived dendritic cell.
References
- 1.Davis B. K., Wen H., and Ting J. P. (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M., Wang H., Chen W., and Meng G. (2011) Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 11, 549–554 [DOI] [PubMed] [Google Scholar]
- 3.Tschopp J., and Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 [DOI] [PubMed] [Google Scholar]
- 4.Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., and Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H., Willingham S. B., Ting J. P., and Re F. (2008) Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 181, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weeratna R. D., Makinen S. R., McCluskie M. J., and Davis H. L. (2005) TLR agonists as vaccine adjuvants: comparison of CpG ODN and Resiquimod (R-848). Vaccine 23, 5263–5270 [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki A., and Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin M., Michalek S. M., and Katz J. (2003) Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 71, 2498–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oleszycka E., and Lavelle E. C. (2014) Immunomodulatory properties of the vaccine adjuvant alum. Curr. Opin. Immunol. 28, 1–5 [DOI] [PubMed] [Google Scholar]
- 10.Didierlaurent A. M., Morel S., Lockman L., Giannini S. L., Bisteau M., Carlsen H., Kielland A., Vosters O., Vanderheyde N., Schiavetti F., Larocque D., Van Mechelen M., and Garçon N. (2009) AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183, 6186–6197 [DOI] [PubMed] [Google Scholar]
- 11.Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., and Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spreafico R., Ricciardi-Castagnoli P., and Mortellaro A. (2010) The controversial relationship between NLRP3, alum, danger signals and the next-generation adjuvants. Eur. J. Immunol. 40, 638–642 [DOI] [PubMed] [Google Scholar]
- 13.Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., and Flavell R. A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., and Tschopp J. (2008) Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 [DOI] [PubMed] [Google Scholar]
- 15.Franchi L., and Núñez G. (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKee A. S., Munks M. W., MacLeod M. K., Fleenor C. J., Van Rooijen N., Kappler J. W., and Marrack P. (2009) Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp F. A., Ruane D., Claass B., Creagh E., Harris J., Malyala P., Singh M., O'Hagan D. T., Pétrilli V., Tschopp J., O'Neill L. A., and Lavelle E. C. (2009) Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc. Natl. Acad. Sci. U.S.A. 106, 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann S., Burkert K., Kemp R., Rades T., Rod Dunbar P., and Hook S. (2014) Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol. Cell Biol. 92, 535–542 [DOI] [PubMed] [Google Scholar]
- 19.Campbell J. B., and Peerbaye Y. A. (1992) Saponin. Res. Immunol. 143, 526–530 [DOI] [PubMed] [Google Scholar]
- 20.Dalsgaard K. (1974) Saponin adjuvants. 3. Isolation of a substance from Quillaja saponaria Molina with adjuvant activity in foot-and-mouth disease vaccines. Arch. Gesamte Virusforsch. 44, 243–254 [PubMed] [Google Scholar]
- 21.Morein B., Sundquist B., Höglund S., Dalsgaard K., and Osterhaus A. (1984) Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 308, 457–460 [DOI] [PubMed] [Google Scholar]
- 22.Sun H. X., Xie Y., and Ye Y. P. (2009) ISCOMs and ISCOMATRIX. Vaccine 27, 4388–4401 [DOI] [PubMed] [Google Scholar]
- 23.Magnusson S. E., Reimer J. M., Karlsson K. H., Lilja L., Bengtsson K. L., and Stertman L. (2013) Immune enhancing properties of the novel Matrix-M adjuvant leads to potentiated immune responses to an influenza vaccine in mice. Vaccine 31, 1725–1733 [DOI] [PubMed] [Google Scholar]
- 24.Kensil C. R., Patel U., Lennick M., and Marciani D. (1991) Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146, 431–437 [PubMed] [Google Scholar]
- 25.Bengtsson K. L. (2013) Matrix M adjuvant technology. In Novel Immune Potentiators and Delivery Technologies for Next Generation Vaccines (Singh M., ed) pp. 309–320, Springer, Cambridge, MA [Google Scholar]
- 26.Fernández-Tejada A., Chea E. K., George C., Pillarsetty N., Gardner J. R., Livingston P. O., Ragupathi G., Lewis J. S., Tan D. S., and Gin D. Y. (2014) Development of a minimal saponin vaccine adjuvant based on QS-21. Nat. Chem. 6, 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashala O., Amador R., Valero M. V., Moreno A., Barbosa A., Nickel B., Daubenberger C. A., Guzman F., Pluschke G., and Patarroyo M. E. (2002) Safety, tolerability and immunogenicity of new formulations of the Plasmodium falciparum malaria peptide vaccine SPf66 combined with the immunological adjuvant QS-21. Vaccine 20, 2263–2277 [DOI] [PubMed] [Google Scholar]
- 28.Kensil C. R. (1996) Saponins as vaccine adjuvants. Crit. Rev. Ther. Drug Carrier Syst. 13, 1–55 [PubMed] [Google Scholar]
- 29.Kim S. K., Ragupathi G., Musselli C., Choi S. J., Park Y. S., and Livingston P. O. (1999) Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine 18, 597–603 [DOI] [PubMed] [Google Scholar]
- 30.Soltysik S., Wu J. Y., Recchia J., Wheeler D. A., Newman M. J., Coughlin R. T., and Kensil C. R. (1995) Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 13, 1403–1410 [DOI] [PubMed] [Google Scholar]
- 31.Liu G., Anderson C., Scaltreto H., Barbon J., and Kensil C. R. (2002) QS-21 structure/function studies: effect of acylation on adjuvant activity. Vaccine 20, 2808–2815 [DOI] [PubMed] [Google Scholar]
- 32.Kennedy J. S., Co M., Green S., Longtine K., Longtine J., O'Neill M. A., Adams J. P., Rothman A. L., Yu Q., Johnson-Leva R., Pal R., Wang S., Lu S., and Markham P. (2008) The safety and tolerability of an HIV-1 DNA prime-protein boost vaccine (DP6–001) in healthy adult volunteers. Vaccine 26, 4420–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans T. G., McElrath M. J., Matthews T., Montefiori D., Weinhold K., Wolff M., Keefer M. C., Kallas E. G., Corey L., Gorse G. J., Belshe R., Graham B. S., Spearman P. W., Schwartz D., Mulligan M. J., Goepfert P., Fast P., Berman P., Powell M., Francis D., and NIAID AIDS Vaccine Evaluation Group (2001) QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine 19, 2080–2091 [DOI] [PubMed] [Google Scholar]
- 34.Gin D. Y., and Slovin S. F. (2011) Enhancing immunogenicity of cancer vaccines: QS-21 as an immune adjuvant. Curr. Drug Ther. 6, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krug L. M., Ragupathi G., Hood C., George C., Hong F., Shen R., Abrey L., Jennings H. J., Kris M. G., and Livingston P. O. (2012) Immunization with N-propionyl polysialic acid-KLH conjugate in patients with small cell lung cancer is safe and induces IgM antibodies reactive with SCLC cells and bactericidal against group B meningococci. Cancer Immunol. Immunother. 61, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandepapelière P., Horsmans Y., Moris P., Van Mechelen M., Janssens M., Koutsoukos M., Van Belle P., Clement F., Hanon E., Wettendorff M., Garçon N., and Leroux-Roels G. (2008) Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 26, 1375–1386 [DOI] [PubMed] [Google Scholar]
- 37.RTS,S Clinical Trials Partnership (2015) Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olotu A., Fegan G., Wambua J., Nyangweso G., Awuondo K. O., Leach A., Lievens M., Leboulleux D., Njuguna P., Peshu N., Marsh K., and Bejon P. (2013) Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 368, 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RTS,S Clinical Trials Partnership, Agnandji S. T., Lell B., Fernandes J. F., Abossolo B. P., Methogo B. G., Kabwende A. L., Adegnika A. A., Mordmuller B., Issifou S., Kremsner P. G., Sacarlal J., Aide P., Lanaspa M., Aponte J. J., et al. (2012) A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bejon P., White M. T., Olotu A., Bojang K., Lusingu J. P., Salim N., Otsyula N. N., Agnandji S. T., Asante K. P., Owusu-Agyei S., Abdulla S., and Ghani A. C. (2013) Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect. Dis. 13, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agnandji S. T., Lell B., Soulanoudjingar S. S., Fernandes J. F., Abossolo B. P., Conzelmann C., Methogo B. G., Doucka Y., Flamen A., Mordmüller B., Issifou S., Kremsner P. G., Sacarlal J., Aide P., Lanaspa M., et al. (2011) First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365, 1863–1875 [DOI] [PubMed] [Google Scholar]
- 42.Garcon N., and Van Mechelen M. (2011) Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 10, 471–486 [DOI] [PubMed] [Google Scholar]
- 43.Kensil C. R., Mo A. X., and Truneh A. (2004) Current vaccine adjuvants: an overview of a diverse class. Front. Biosci. 9, 2972–2988 [DOI] [PubMed] [Google Scholar]
- 44.Duewell P., Kisser U., Heckelsmiller K., Hoves S., Stoitzner P., Koernig S., Morelli A. B., Clausen B. E., Dauer M., Eigler A., Anz D., Bourquin C., Maraskovsky E., Endres S., and Schnurr M. (2011) ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J. Immunol. 187, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson N. S., Duewell P., Yang B., Li Y., Marsters S., Koernig S., Latz E., Maraskovsky E., Morelli A. B., Schnurr M., and Ashkenazi A. (2014) Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J. Immunol. 192, 3259–3268 [DOI] [PubMed] [Google Scholar]
- 46.Newton K., Sun X., and Dixit V. M. (2004) Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser W. J., Upton J. W., Long A. B., Livingston-Rosanoff D., Daley-Bauer L. P., Hakem R., Caspary T., and Mocarski E. S. (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shenoy A. R., Wellington D. A., Kumar P., Kassa H., Booth C. J., Cresswell P., and MacMicking J. D. (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 [DOI] [PubMed] [Google Scholar]
- 49.Lövgren Bengtsson K., Morein B., and Osterhaus A. D. (2011) ISCOM technology-based Matrix M adjuvant: success in future vaccines relies on formulation. Expert Rev. Vaccines 10, 401–403 [DOI] [PubMed] [Google Scholar]
- 50.Bengtsson K. L., Karlsson K. H., Magnusson S. E., Reimer J. M., and Stertman L. (2013) Matrix-M adjuvant: enhancing immune responses by “setting the stage” for the antigen. Expert Rev. Vaccines 12, 821–823 [DOI] [PubMed] [Google Scholar]
- 51.Montminy S. W., Khan N., McGrath S., Walkowicz M. J., Sharp F., Conlon J. E., Fukase K., Kusumoto S., Sweet C., Miyake K., Akira S., Cotter R. J., Goguen J. D., and Lien E. (2006) Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7, 1066–1073 [DOI] [PubMed] [Google Scholar]
- 52.Vladimer G. I., Weng D., Paquette S. W., Vanaja S. K., Rathinam V. A., Aune M. H., Conlon J. E., Burbage J. J., Proulx M. K., Liu Q., Reed G., Mecsas J. C., Iwakura Y., Bertin J., Goguen J. D., Fitzgerald K. A., and Lien E. (2012) The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buglione-Corbett R., Pouliot K., Marty-Roix R., West K., Wang S., Lien E., and Lu S. (2013) Serum cytokine profiles associated with specific adjuvants used in a DNA prime-protein boost vaccination strategy. PLoS One 8, e74820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weng D., Marty-Roix R., Ganesan S., Proulx M. K., Vladimer G. I., Kaiser W. J., Mocarski E. S., Pouliot K., Chan F. K., Kelliher M. A., Harris P. A., Bertin J., Gough P. J., Shayakhmetov D. M., Goguen J. D., Fitzgerald K. A., Silverman N., and Lien E. (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. U.S.A. 111, 7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buglione-Corbett R., Pouliot K., Marty-Roix R., Li W., West K., Wang S., Morelli A. B., Lien E., and Lu S. (2014) Reduced MyD88 dependency of ISCOMATRIX adjuvant in a DNA prime-protein boost HIV vaccine. Hum. Vaccin. Immunother. 10, 1078–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pouliot K., Buglione-Corbett R., Marty-Roix R., Montminy-Paquette S., West K., Wang S., Lu S., and Lien E. (2014) Contribution of TLR4 and MyD88 for adjuvant monophosphoryl lipid A (MPLA) activity in a DNA prime-protein boost HIV-1 vaccine. Vaccine 32, 5049–5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown L. E., White D. O., Agius C., Kemp B. E., Yatzakis N., Poumbourios P., McPhee D. A., and Jackson D. C. (1995) Synthetic peptides representing sequences within gp41 of HIV as immunogens for murine T- and B-cell responses. Arch. Virol 140, 635–654 [DOI] [PubMed] [Google Scholar]
- 58.Morelli A. B., Becher D., Koernig S., Silva A., Drane D., and Maraskovsky E. (2012) ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 61, 935–943 [DOI] [PubMed] [Google Scholar]
- 59.McKenzie A., Watt M., and Gittleson C. (2010) ISCOMATRIX() vaccines: safety in human clinical studies. Hum. Vaccin. 6, pii: 10754 [DOI] [PubMed] [Google Scholar]
- 60.Elinav E., Strowig T., Henao-Mejia J., and Flavell R. A. (2011) Regulation of the antimicrobial response by NLR proteins. Immunity 34, 665–679 [DOI] [PubMed] [Google Scholar]
- 61.Lamkanfi M., and Dixit V. M. (2014) Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 [DOI] [PubMed] [Google Scholar]
- 62.Oliveira-Freitas E., Casas C. P., Borja-Cabrera G. P., Santos F. N., Nico D., Souza L. O., Tinoco L. W., da Silva B. P., Palatnik M., Parente J. P., and Palatnik-de-Sousa C. B. (2006) Acylated and deacylated saponins of Quillaja saponaria mixture as adjuvants for the FML-vaccine against visceral leishmaniasis. Vaccine 24, 3909–3920 [DOI] [PubMed] [Google Scholar]
- 63.Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nuñez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., and Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B. T., and Tschopp J. (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinon F., Pétrilli V., Mayor A., Tardivel A., and Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 66.Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., and Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu D., Rhebergen A. M., and Eisenbarth S. C. (2013) Licensing adaptive immunity by NOD-like receptors. Front Immunol. 4, 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambrecht B. N., Kool M., Willart M. A., and Hammad H. (2009) Mechanism of action of clinically approved adjuvants. Curr. Opin. Immunol. 21, 23–29 [DOI] [PubMed] [Google Scholar]
- 69.Chea E. K., Fernández-Tejada A., Damani P., Adams M. M., Gardner J. R., Livingston P. O., Ragupathi G., and Gin D. Y. (2012) Synthesis and preclinical evaluation of QS-21 variants leading to simplified vaccine adjuvants and mechanistic probes. J. Am. Chem. Soc. 134, 13448–13457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beck Z., Matyas G. R., and Alving C. R.. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim. Biophys. Acta 1848, 775–780 [DOI] [PubMed] [Google Scholar]
- 71.Bangham A. D., Horne R. W., Glauert A. M., Dingle J. T., and Lucy J. A. (1962) Action of saponin on biological cell membranes. Nature 196, 952–955 [DOI] [PubMed] [Google Scholar]
- 72.McKee A. S., Burchill M. A., Munks M. W., Jin L., Kappler J. W., Friedman R. S., Jacobelli J., and Marrack P. (2013) Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 110, E1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Didierlaurent A. M., Collignon C., Bourguignon P., Wouters S., Fierens K., Fochesato M., Dendouga N., Langlet C., Malissen B., Lambrecht B. N., Garçon N., Van Mechelen M., and Morel S. (2014) Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 193, 1920–1930 [DOI] [PubMed] [Google Scholar]
- 74.Ragupathi G., Gardner J. R., Livingston P. O., and Gin D. Y. (2011) Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines 10, 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garçon N., Chomez P., and Van Mechelen M. (2007) GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6, 723–739 [DOI] [PubMed] [Google Scholar]