Abstract
Background
Patients hoping to preserve their fertility receive conservative treatment with high-dose medroxyprogesterone acetate (MPA) for well-differentiated endometrioid adenocarcinoma (EC) or atypical endometrial hyperplasia (AEH) . Such treatment generally involves frequent intrauterine operations, including dilation and curettage (D&C) and endometrial biopsy (EMB), which could result in endometritis, endometrial thinning, or intrauterine adhesion. In turn, any of these outcomes could adversely affect implantation and pregnancy development. The current study thus aimed to identify factors that might affect pregnancy following conservative treatment by MPA.
Methods
We compared a pregnancy group (45 patients) with a non-pregnancy group (53 patients) of MPA-treated patients to evaluate the factors affecting clinical pregnancy establishment. We undertook a multivariate logistic regression analysis based on factors shown by univariate analysis to be significantly different between the groups. Univariate analysis identified number of D&C, endometrial thickness, duration of MPA administration, age of pregnancy permission (the age at which a patient was first allowed to attempt pregnancy after disappearance of the lesion), period of disappearance of lesions, and recurrence as independent variables.
Results
The odds ratios (95 % confidence interval) of multivariate analysis for disease recurrence, endometrial thickness during ovulation, and age of pregnancy permission were 0.283 (0.102–0.785), 1.677 (1.251–2.248), and 0.889 (0.792–0.998), respectively. There was no significant difference in the other independent variables between groups.
Conclusions
We identified three factors considered to affect pregnancy establishment following conservative treatment with MPA: recurrence, endometrial thickness during ovulation, and the age of the pregnancy permission. Introduction of infertility treatment including assisted reproductive technology (ART) soon after achieving tumor disappearance by MPA would therefore be beneficial for patients with disease recurrence, thin endometrium, or a higher age of pregnancy permission.
Electronic supplementary material
The online version of this article (doi:10.1186/s12958-015-0136-7) contains supplementary material, which is available to authorized users.
Keywords: High-dose medroxyprogesterone acetate, MPA, Fertility-preserving therapy, Well-differentiated endometrial cancer, Atypical endometrial hyperplasia, Infertility treatment, Assisted reproductive technology
Background
Endometrial cancer (EC) is typically a disease of postmenopausal women, but approximately 5.5 % of cases occur in women younger than 40 years [1]. Of note, a trend for increasing prevalence of EC in younger patients has recently emerged [2], with cases in women younger than 40 years increasing from 45 to over 157 in a million during a 20-year period in Japan [3]. Atypical endometrial hyperplasia (AEH) is a precancerous lesion, but 29 % of such cases progress to EC within several years [4]. The standard treatment of EC/AEH includes a total hysterectomy with bilateral oophorectomy resulting in total loss of fertility [5]; however, with the recent trend for delayed marriage, many women who have been diagnosed with EC/AEH do not accept the standard treatment. Instead, conservative treatment using medroxyprogesterone acetate (MPA) is increasingly used as an effective fertility-preserving therapy for early stage EC and AEH without myometrial invasion or extra-uterine spread.
MPA confers a progesterone receptor-mediated anti-tumor effect, inhibition against estrogen action [6], and inhibition of angiogenesis not mediated via progesterone receptors [7]. MPA may also reduce the number of glandular cells and decidualization of the stroma [6, 8]. A disadvantage of the conservative treatment with MPA is the need for frequent intrauterine operations including dilation and curettage (D&C) and endometrial biopsy (EMB) that can cause endometritis, endometrial thinning, and intra-uterine adhesion. These potential effects of MPA raise concerns about associated adverse effects on uterine implantation, as does the high tumor relapse rate associated with such conservative treatment. Therefore, patients treated by MPA are advised to try pregnancy establishment early after the treatment.
Pregnancy is commonly reported following conservative treatment [9–13]; however, there are no reports on the factors that contribute to pregnancy in such patients. In the current study, we compared a pregnancy group with a non-pregnancy group to elucidate variables significant in establishing pregnancy after conservative treatment for EC/AEH.
Methods
The institutional review board of Keio University School of Medicine approved the current retrospective study (approved number # 20110237), which comprised patients diagnosed with EC stage IA (G1) or AEH at the Keio University Hospital from January 1998 to December 2012. Four patients diagnosed as EC stage IA (G2) and who clearly understood the significant risks of disease recurrence and progression elected to receive conservative treatment. After careful examination using transvaginal ultrasonography, magnetic resonance imaging, and hysteroscopy to rule out myometrial or cervical invasion, and, if needed, computed tomography to confirm that the patient had neither distant metastasis nor double cancer, endometrial curettage was performed to determine the initial pathological diagnosis and to remove lesions. Informed consent was obtained from all patients regarding the risk of disease progression, in undergoing conservative treatment with MPA (600 mg/day) instead of the standard therapy of total hysterectomy with bilateral salpingo-oophorectomy. We almost performed conservative treatment according to the same protocol (Additional file 1: Figure S1). An endometrial biopsy was performed once a month up to three months after initiating MPA administration, and D&C was performed at 4 months using a metallic curette. In cases with residual lesions, MPA administration was continued, and D&C was performed every 2 months for up to 12 months to monitor any progression of the lesion. In cases with no residual lesions, MPA was discontinued and an attempt of pregnancy was permitted, but EMB was concurrently performed every 3–4 months. Patients who did not wish to conceive immediately underwent Holmstrom therapy, whereby MPA (15 mg/day) was administered orally for 12 days after 14 days from the start of withdrawal bleeding.
Study end-points
Subjects were divided into a pregnancy group and a non-pregnancy group, and data were collected regarding number of D&C, duration of MPA administration, and endometrial thickness during ovulation. We then analyzed the data to determine the pregnancy-affecting factors.
Exclusion criteria
All patients undergoing conservative treatment (n = 174) did not necessarily wish to conceive immediately; therefore, subjects were limited to those wishing to conceive following therapy. We excluded women aged ≥ 42 years at the time of the initial treatment (n = 11), women without a partner (n = 44), and women with no fertile period or for whom detailed medical records could not be obtained immediately following conservative treatment (n = 17). The remaining 98 women were divided into a pregnancy group (n = 45) and a non-pregnancy group (n = 53).
Definitions
In the present study, pregnancy was defined as a serum level of human chorionic gonadotropin ≥ 25 mIU/mL or observation of a gestational sac on a transvaginal ultrasound scan. An abnormal menstrual cycle was defined as a menstrual cycle not lasting 25–38 days. Endometrial thickness is reported to reach its minimum on day 4 of the menstrual cycle, then increase by 1 mm/day to a plateau on day 9 [14]. Therefore, endometrial thickness during ovulation was measured in the sagittal plane by transvaginal ultrasound scan when the follicle diameter was observed to be ≥ 16 mm. The age of pregnancy permission was defined as the age at which the patient was first allowed to attempt pregnancy after disappearance of the lesion. Periods of disappearance of lesions were defined as from the start of the administration till diagnosis of the disappearance of lesions. Follow up was defined as the period from the start of MPA administration to the final medical examination. Possible intrauterine adhesion was investigated in 81 cases in which hysteroscopy was performed during MPA therapy and in which a detailed intrauterine examination was performed during treatment by transcervical resection. A good-quality embryo was morphologically defined by a Veeck classification ≥ grade 2 on day 2/3 [15] or a Gardner classification ≥ 3BB on day 5 after oocyte pick up [16].
Statistical analysis
Shapiro-Wilk tests for all data showed a non-normal distribution. Mann–Whitney U tests were used for statistical analysis, while nominal scale comparisons were performed using the Pearson Chi-squared test (see Footnotes a and b in Table 1). Multicollinearity was examined using the Spearman rank correlation coefficient to create a correlation matrix; it was confirmed that no combination of the independent variables demonstrated a correlation coefficient with an absolute value ≥ 0.9. Multivariate analysis was performed in the pregnancy and non-pregnancy groups using endometrial thickness, number of D&C, duration of MPA administration, age of pregnancy permission, and recurrence as independent variables, using a logistic regression to determine the likelihood ratio. Model Chi-squared test results with P < 0.05 were considered to be statistically significant. Analyses were conducted using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
Patient characteristics of the pregnancy group (n = 45) and the non-pregnancy group (n = 53) are summarized in Table 1. The average age of the first pregnancy was 34.8 ± 3.9 years. There were no significant differences between the groups in age of initial treatment, nulliparity, histological type/grade, body mass index, PCO on ultrasonography, irregular menstrual cycle, or use of ovarian stimulation.
Table 1.
Patient characteristics
| Total | Pregnancy | Non-pregnancy | P | |
|---|---|---|---|---|
| Patients (n) | 98 | 45 | 53 | - |
| Age of initial treatment (years) | 33.8 ± 4.4 | 32.8 ± 4.6 | 34.6 ± 4.1 | 0.051a |
| Pregnancy age (years) | - | 34.8 ± 3.9 | - | - |
| Nulliparity (%) | 87 (88.8) | 42 (83.3) | 52 (92.9) | 0.088a |
| Histological type/grade | 0.408b | |||
| AEH | 37 | 15 | 22 | |
| G1/G2 | 61 | 30 | 31 | |
| BMI (kg/m2) | 21.9 ± 4.7 | 21.5 ± 4.9 | 22.3 ± 4.5 | 0.264a |
| PCO on ultrasonography (%) | 29 (29.6) | 16 (35.6) | 13 (24.5) | 0.233a |
| Irregular menstrual cycle (%) | 54 (55.1) | 24 (53.3) | 30 (56.6) | 0.116a |
| Use of ovarian stimulation (%) | 58 (59.2) | 30 (66.7) | 28 (52.8) | 0.165a |
Note : Values listed as mean ± standard deviation
aPregnancy versus Non-pregnancy (Mann–Whitney U test)
bPregnancy versus Non-pregnancy (Chi-squared test)
Infertility treatments after conservative treatment
Table 2 shows that 68.4 % of the pregnancies resulted from infertility treatment including timing treatment, controlled ovarian stimulation, IUI, and in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) (22.8 %), but 71.9 % of them were achieved by natural insemination. With respect to the non-pregnancy group, 84.9 % of the subjects also had the aid of infertility treatment, and 37.7 % of them underwent IVF.
Table 2.
Infertility treatment rates in the pregnancy and the non-pregnancy groups
| Pregnancy | Non-pregnancy | |
|---|---|---|
| Natural insemination (%) | 41 (71.9) | 28 (52.8) |
| No infertility treatment (%) | 18 (31.6) | 8 (15.1) |
| Timing treatment (%) | 8 (14.0) | 8 (15.1) |
| Clomifene Citrate-timing treatment (%) | 8 (14.0) | 6 (11.3) |
| HMG-timing treatment (%) | 7 (12.3) | 6 (11.3) |
| IUI (%) | 3 (5.3) | 5 (9.5) |
| IVF/ICSI (%) | 13 (22.8) | 20 (37.7) |
| Total | 57 (100) | 53 (100) |
Matters relating to conservative treatment
The duration of MPA administration was significantly shorter in the pregnancy group at 277.5 ± 167.0 days than in the non-pregnancy group at 431.5 ± 342.5 days, as was the time to disappearance of lesions (136.2 ± 133.8 days vs. 187.0 ± 147.7) (Table 3). The age of pregnancy permission and the number of D&C procedures performed were also both significantly lower in the pregnancy group than in the non-pregnancy group (33.9 ± 4.4 years vs. 36.0 ± 4.8 years; 4.18 ± 2.34 vs. 5.66 ± 3.77, respectively). Endometrial thickness during ovulation was significantly higher in the pregnancy group at 8.56 ± 1.87 mm than in the non-pregnancy group at 6.70 ± 1.87 mm.
Table 3.
Matters relating to conservative treatment
| Total | Pregnancy | Non-pregnancy | P* | |
|---|---|---|---|---|
| Age of pregnancy permission | 35.0 ± 4.7 | 33.9 ± 4.4 | 36.0 ± 4.8 | 0.023 |
| Number of D&C (times) | 4.98 ± 3.27 | 4.18 ± 2.34 | 5.66 ± 3.77 | 0.049 |
| Endometrial thickness during ovulation (mm) | 7.50 ± 2.08 | 8.56 ± 1.87 | 6.70 ± 1.87 | <0.001 |
| Duration of MPA administration (days) | 357.7 ± 285.4 | 277.5 ± 167.0 | 431.5 ± 342.5 | 0.010 |
| Periods of disappearance of lesions (days) | 164.0 ± 143.1 | 136.2 ± 133.8 | 187.0 ± 147.7 | 0.042 |
| Periods from the last MPA administration to menstruation or starting date of the pregnancy (days) | 314.0 ± 392.8 | ー | ー | |
| Infertile period after conservative treatment (days) | 1146 ± 1024 | 1206 ± 887 | 1096 ± 1131 | 0.116 |
| Follow up (days) | 2032 ± 1266 | 2027 ± 1115 | 2037 ± 1389 | 0.592 |
| Recurrence (%) | 61 (62.2) | 23 (51.1) | 38 (71.7) | 0.036 |
| Intrauterine adhesion (%) | 20/81 (24.7) | 5/29 (17.2) | 15/52 (28.8) | 0.191 |
Note : Values listed as mean ± standard deviation
*Pregnancy versus Non-pregnancy (Mann–Whitney U test)
Univariate analysis showed significant differences between the pregnancy and the non-pregnancy groups in number of D&C, endometrial thickness, duration of MPA administration, age of pregnancy permission, and recurrence. Thus, these five factors were used as independent variables for the multivariate logistic regression analysis (Table 4), which identified recurrence (odds ratio 0.283; 95 % CI 0.10–0.785), endometrial thickness during ovulation (1.677; 1.251–2.248), and age of pregnancy permission (0.889; 0.792–0.998) as significant factors affecting pregnancy outcomes.
Table 4.
Logistic regression analysis
| P | Odd ratio | 95 % CI | |
|---|---|---|---|
| Endometrial thickness | 0.001 | 1.677 | 1.251–2.248 |
| Recurrence | 0.015 | 0.283 | 0.102–0.785 |
| Age of pregnancy permission | 0.046 | 0.889 | 0.792–0.998 |
| Duration of MPA administration | 0.065 | ||
| Number of D&C | 0.407 | ||
| Periods of disappearance of lesions | 0.358 |
Number of D&C as a potential confounder of endometrial thickness
Although few studies have reported on the role of D&C in the etiology of thin endometrium [17], the relationship between these variables remains controversial. In the current study, regression analysis of the correlation between ultrasonographically measured endometrial thickness during 365 ovulation cycles (included the same person) and the number of D&C procedures (1–10) for all the subjects yielded an absolute value of 0.4, indicating a correlation (Fig. 1). In the regression analysis, the regression equation was significant (P < 0.01) according to an analysis of variance (ANOVA) table, as was the regression coefficient (P < 0.01). Curvilinear regression demonstrated that endometrial thickness during ovulation decreases as the number of D&C procedures increase.
Fig. 1.
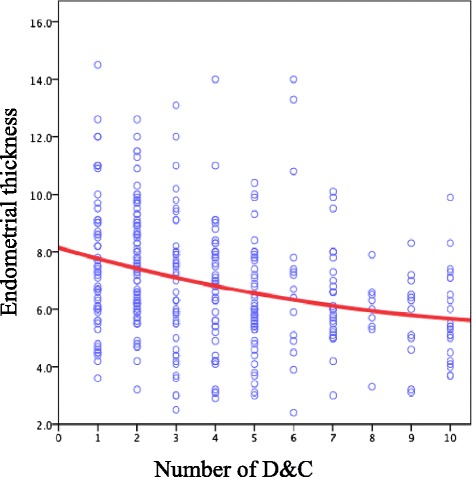
Regression analysis of the correlation between ultrasonographically measured endometrial thickness during 365 ovulation cycles (included the same person) and the number of D&C procedures (1–10) for all the subjects yielded an absolute value of 0.4, indicating a correlation. The regression equation was significant (P < 0.01) according to an analysis of variance (ANOVA) table, as was the regression coefficient (P < 0.01). Curvilinear regression demonstrated that endometrial thickness during ovulation decreases as the number of D&C procedures increase
Discussion
In the current retrospective study, the EC/AEH patients who had undergone conservative treatment with MPA were divided into pregnancy and non-pregnancy groups, which were compared to determine factors in conservative treatment contributing to pregnancy after achieving complete response. Based on the multivariate multiple regression analysis, disease recurrence, endometrial thickness during ovulation, and the age of pregnancy permission were significantly associated with pregnancy establishment. Although a significant difference in the age of pregnancy permission was observed, with an OR of 0.889, its effect on pregnancy was considered small. Thus, we concluded that recurrence and endometrial thickness, with ORs of 0.283 and 1.677, respectively, have an important effect on pregnancy establishment among the factors examined in the study. In general, sufficient endometrial thickness is important for pregnancy establishment [18–25], and when endometrial thickness is ≤ 7 mm, the functional layer is thin or absent, resulting in implantation of the embryo near the spinal artery. High blood flow impedance of radial arteries impairs the growth of the glandular epithelium and results in decreased vascular endothelial growth factor (VEGF) levels in the endometrium. Low VEGF and low blood flow lead to a “thin” endometrium, which is related to impaired endometrial receptivity [26]. These previous data thus support the result of our multivariate multiple regression analysis that endometrial thickness is an important factor for pregnancy outcomes after conservative treatment for EC/AEH. Therefore, it is necessary to minimize damage to normal endometrium. To this end, Fujimoto et al. [27, 28] suggested that hysteroscopic resection of early stage EC combined with hormonal therapy might be more effective than endometrial curettage for eliminating lesions in such patients hoping to preserve their fertility. Such an approach would minimize a specimen, but does not increase the risk of peritoneal dissemination [29].
We further studied if the number of D&C procedures is a potential confounder of thin endometrium. Endometrial thickness was measured in 365 cycles of ovulation following conservative treatment and regression analysis consequently showed that endometrial thickness during ovulation decreases as the number of D&C increases (P < 0.01). Furthermore, repeated D&C procedures might increase intrauterine adhesion [30]. In the current study, the frequency of intrauterine adhesions after D&C in the pregnancy group was not significantly different from that in the non-pregnancy group by univariate analysis. Although nearly all of the intrauterine adhesions that we observed were mild, film-like adhesions not likely to affect pregnancy, a future extensive analysis of more cases is needed to accurately determine the number of D&C likely to significantly impair fertility. On the other hand, MPA administration causes histological changes in the endometrium, including a decreased gland-to-stroma ratio, reduction in the number of glandular cells, decidualization of the stroma, and decreased mitosis [6, 8]. These effects may cause atrophy and thinning of the endometrium, and considerable time is likely needed for the tissue’s functional recovery after MPA termination. Randall et al. [31] reported that a median duration of 9 months is required after progestin therapy for the lesions to improve, and herein, we showed that the period from MPA termination till the beginning of the final menstrual cycle before pregnancy was 314.0 ± 392.8 days. Accordingly, the duration of MPA administration also might be a potential confounder of endometrial thinning.
Conservative treatment needs to provide a promising option for EC/AEH patients who wish to preserve fertility. However, there is a considerable risk of lesion recurrence and disease progression with such therapies. Yamazawa et al. [32] reported a recurrence rate of approximately 35 %, while Ushijima and Chiva et al. [33, 34] reported that tumors recur in 20–47.9 months. Furthermore, the current study found that 61 of 98 (62.2 %) patients experienced recurrence during the follow-up period (2189 ± 1346 days) from the start of MPA administration to the final medical examination. Therefore, pregnancy should be promptly attempted following complete response to conservative treatment in EC/AEH patients. Furthermore, it is known that the infertility rate is higher among EC/AEH patients than among the general population, due to an increased prevalence of anovulatory cycles or polycystic ovaries. Based on these discussion points, infertility treatment is strongly recommended after achieving a complete response to conservative treatment, to enhance the chance of pregnancy. All patients tried to conceive by natural conception after receiving MPA, and those who could not easily fall pregnant proceeded to IUI or IVF. The current study indeed demonstrated many cases of pregnancy occurring early after the cessation of treatment through natural methods (no infertility treatment: 18 cases, timed sexual intercourse: 8 cases) and the use of fertility agents for ovarian stimulation (clomiphene citrate: 8 cases, HMG: 7 cases). In addition, but importantly, Ichinose et al. [35] reported that the use of fertility drugs did not increase the recurrence of EC/AEH.
ART has been reported as a useful alternative for EC/AEH patients after conservative treatment [36]; however, in the current study, only 13 out of 52 pregnancies required the aid of ART, and only 2 of the 15 patients who received ART at our hospitals in the study were successful in achieving pregnancy (Additional file 2: Table S1 and 3: Table S2). These patients who became pregnant were aged 32 and 34 years, and did not have thin endometrium. The other 13 non-pregnant patients were at least 37 years old, and 9 were older than 40 years. Since morphologically good embryos were transferred in the majority of cases based on the reproductive outcomes achieved in another clinic, we deduced that failed IVF was most likely attributable to an endometrial factor and not embryo quality. At an advanced age, endometrial thinning resulted from MPA therapy and D&C, as well as maternal age, could contribute to poor pregnancy outcomes with ART. Thus, this study could not provide any evidence on the effectiveness of ART for EC/AEH patients after conservative treatment. Nonetheless, the age of pregnancy permission and recurrence were also shown by the multivariate regression analysis as a significant factor for pregnancy outcomes after conservative treatment. A higher age of pregnancy permission and a prolonged period of medical treatment due to recurrence may be accompanied by ovarian aging and hypofunction. Considering the possibility of recurrence, early referral to an infertility treatment specialist and proactive recommendation of ART should be advised for EC/AEH patients who have already experienced recurrence, have a long period till remission, or have a higher age of pregnancy permission and/or thin endometrium.
Conclusions
Tumor recurrence, endometrial thickness during ovulation, and the age of pregnancy permission were found to affect pregnancy establishment following conservative treatment. A prompt introduction of infertility treatment including ART following conservative treatment should be advised for EC/AEH patients with thin endometrium, a higher age of pregnancy permission or recurrence, and a long period till remission.
Acknowledgements
The authors thank Dr. Kouichi Akaboshi (Sugimura Lady’s Clinic), Dr. Yoshio Anko (Anko ladies Clinic), Dr. Takuya Ayabe (Seto Hospital), Dr. Pei Chokan (Toyo Suzuran Clinic), Dr. Kazuto Fukuba (Ota Memorial Hospital), Dr. Noboru Inagaki (Saint Women’s Clinic), Dr. Seiji Kitamura (Ogikubo Hospital), Dr. Shuichi Kitamura (Towakou Ladies Clinic), Dr. Junichi Kobayashi (Kanagawa Ladies Clinic), Dr. Takahisa Oda (Ichikawa ART Clinic), Dr. Chikahiro Oka (Tokyo HART Clinic), Dr. Yukari Sumi (Toranomon Women’s Clinic), Dr. Kiyoshi Takamatsu (Ichikawa General Hospital, Tokyo Dental College), Dr. Yuji Takehara (Keiai Clinic), Dr. Yudai Tanaka (Medical Park Shonan), Dr. Susumu Tokuoka (Tokuoka Ladies Clinic) and Dr. Atsumi Yoshida (Kiba park Clinic) for their assistance in data collections. The authors would like to extend sincere thanks to the patients who participated in the current study. The authors acknowledge the assistance of Inter-Biotech (http://www.inter-biotech.com)/ with the English language editing of this manuscript.
Funding
This work was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (Kiban-C-23592413 to O.I. and T.H., Kiban-B-25293345 to T.H. and Kiban-C-25462612 to N.S).
Additional files
Our conservative treatment protocol. We generally performed conservative treatment according to the same protocol. An endometrial biopsy was performed once a month up to three months after initiating MPA administration and D&C was performed at 4 months. In cases with residual lesions, MPA administration was continued, and D&C was performed every 2 months for up to 12 months. In cases with no residual lesions, MPA was discontinued and an attempt at pregnancy was permitted, while EMB was concurrently performed every 3–4 months. (PPTX 70 kb)
Baseline characteristics for IVF cases in Keio University. (PPTX 63 kb)
Reproductive outcomes. (PPTX 68 kb)
Footnotes
Competing interests
All authors declare that they have no conflict of interest that would prejudice the impartiality of the scientific work.
Authors’ contributions
OI, TH, WY, and NS: study design and execution, analysis and interpretation, manuscript preparation, and critical discussion; SO and TT: analysis of data and critical discussion; AH, KB, NK, MT and DA: assisted with design, data analysis, interpretation, and critical discussion. All the authors examined the data and approved the final manuscript.
Contributor Information
Osamu Inoue, Email: oo_inoue@yahoo.co.jp.
Toshio Hamatani, Phone: +81-3-3353-1211, Email: toshiohamatani@z3.keio.jp.
Nobuyuki Susumu, Email: susumu35@a6.keio.jp.
Wataru Yamagami, Email: gami@z8.keio.jp.
Seiji Ogawa, Email: cfy00450@nyc.odn.ne.jp.
Takashi Takemoto, Email: ta_ta10_24@yahoo.co.jp.
Akira Hirasawa, Email: hir-aki@z8.keio.jp.
Kouji Banno, Email: kbanno@z7.keio.jp.
Naoaki Kuji, Email: naoaki@tokyo-med.ac.jp.
Mamoru Tanaka, Email: mtanaka@a6.keio.jp.
Daisuke Aoki, Email: aoki@z7.keio.jp.
References
- 1.Aoki D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J Obstet Gynaecol Res. 2014;40:338–48. doi: 10.1111/jog.12360. [DOI] [PubMed] [Google Scholar]
- 2.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83(2):388–393. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 3.Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H. Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44(4):388–396. doi: 10.1093/jjco/hyu003. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56(2):403–412. doi: 10.1002/1097-0142(19850715)56:2<403::AID-CNCR2820560233>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2015;4. NCCN guidelines http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 6.Deligdisch L. Hormonal Pathology of the Endometrium. Mod Pathol. 2000;13(3):285–294. doi: 10.1038/modpathol.3880050. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mentrikoski MJ, Shah AA, Hanley KZ, Atkins KA. Assessing Endometrial Hyperplasia and Carcinoma Treated With Progestin Therapy. Am J Clin Pathol. 2012;138(4):524–534. doi: 10.1309/AJCPM2TSDDF1MHBZ. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95(1):133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Park H, Seok JM, Yoon BS, Seong SJ, Kim JY, Shim JY, et al. Effectiveness of high-dose progestin and long-term outcomes in young women with early-stage, well-differentiated endometrioid adenocarcinoma of uterine endometrim. Arch Gynecol Obstet. 2012;285(2):473–478. doi: 10.1007/s00404-011-1959-x. [DOI] [PubMed] [Google Scholar]
- 11.Han AR, Kwon YS, Kim DY, Kim JH, Kim YM, Kim YT, et al. Pregnancy Outcomes Using Assisted Reproductive Technology After Fertility-Preserving Therapy in Patients With Endometrial Adenocarcinoma or Atypical Complex Hyperplasia. Int J Gynecol Cancer. 2009;19(1):147–151. doi: 10.1111/IGC.0b013e31819960ba. [DOI] [PubMed] [Google Scholar]
- 12.Lowe MP, Bender D, Sood AK, Davis W, Syrop CH, Sorosky JI. Two successful pregnancies after conservative treatment of endometrial cancer and assisted reproduction. Fertil Steril. 2002;77(1):188–189. doi: 10.1016/S0015-0282(01)02937-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim MK, Yoon BS, Park H, Seong SJ, Chung HH, Kim JW, et al. Conservative Treatment With Medroxyprogesterone Acetate Plus Levonorgestrel Intrauterine System for Early-Stage Endometrial Cancer in Young Women. Int J Gynecol Cancer. 2011;21(4):673–677. doi: 10.1111/IGC.0b013e3181fd9a06. [DOI] [PubMed] [Google Scholar]
- 14.Jason G, Bromer MD, Tamir S, Aldad BA, Hugh S, Taylor MD. Defining the proliferative phase endometrial defect. Fertil Steril. 2009;91(3):698–704. doi: 10.1016/j.fertnstert.2007.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veeck LL. Oocyte Assessment and Biological Performance. Ann NY Acad Sci. 1988;541:259–295. doi: 10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single balastocyst transfer. Fertil Steril. 2000;73(6):1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 17.Shufaro Y, Simon A, Laufer N, Fatum M. Thin unresponsive endometrium--a possible complication of surgical curettage compromising ART outcome. J Assist Reprod Genet. 2008;25(8):421–425. doi: 10.1007/s10815-008-9245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18(11):2337–2341. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Zhang Q, Li Y. 2012. Reprod Biol Endocrinol. 2012;28(10):100. doi: 10.1186/1477-7827-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Chen CH, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83(2):336–340. doi: 10.1016/j.fertnstert.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Esmailzadeh S, Faramarzi M. Endometrial thickness and pregnancy outcome after intrauterine insemination. Fertil Steril. 2007;88(2):432–437. doi: 10.1016/j.fertnstert.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 22.McWilliams GD, Frattarelli JL. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil Steril. 2007;88(1):74–81. doi: 10.1016/j.fertnstert.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 23.Detti L, Yelian FD, Kruger ML, Diamond MP, Rode A, Mitwally MFM. Endometrial thickness is related to miscarriage rate, but not the estradiol concentration, in cycles down-regulated with gonadotropin-releasing hormone antagonist. Fertil Steril. 2008;89(4):998–1001. doi: 10.1016/j.fertnstert.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Lédée-Bataille N, Olivennes F, Lefaix JL, Chaouat G, Frydman R, Delanian S. Combined treatment by pentoxifylline and tocopherol for recipient women with a thin endometrium enrolled in an oocyte donation programme. Human Repro. 2002;17(5):1249–1253. doi: 10.1093/humrep/17.5.1249. [DOI] [PubMed] [Google Scholar]
- 25.Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril. 2010;94(6):2030–2036. doi: 10.1016/j.fertnstert.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miwa I, Tamura H, Takasaki A, Yamagata Y, Shimamura K, Sugino N. Pathophysiologic features of “thin” endometrium. Fertil Steril. 2009;91(4):998–1004. doi: 10.1016/j.fertnstert.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto A, Ichinose M, Harada M, Hirata T, Osuga Y, Fujii T. The outcome of infertility treatment in patients undergoing assisted reproductive technology after conservative therapy for endometrial cancer. J Assist Reprod Genet. 2014;31(9):1189–1194. doi: 10.1007/s10815-014-0297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010;93:1286–9. doi: 10.1016/j.fertnstert.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Chang YN, Zhang Y, Wang YJ, Wang LP, Duan H. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957–961. doi: 10.1016/j.fertnstert.2011.07.1146. [DOI] [PubMed] [Google Scholar]
- 30.Friedler S, Margalioth EJ, Kafka I, Yaffe H. Incidence of post abortion intra-uterine adhesions evaluated by hysteroscopy: a prospective study. Hum Reprod. 1993;8(3):442–444. doi: 10.1093/oxfordjournals.humrep.a138068. [DOI] [PubMed] [Google Scholar]
- 31.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well- differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90(3):434–440. doi: 10.1016/S0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 32.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikra H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22(7):1953–1958. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 33.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25(19):2798–803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 34.Chiva L, Lapuente F, González-Cortijo L, Carballo N, García JF, Rojo A, et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111(2 Suppl):S101–4. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 35.Ichinose M, Fujimoto A, Osuga Y, Minaguchi T, Kawana K, Yano T, et al. The influence of infertility treatment on the prognosis of endometrial cancer and atypical complex endometrial hyperplasia. Int J Gynecol Cancer. 2013;23(2):288–293. doi: 10.1097/IGC.0b013e31827c18a1. [DOI] [PubMed] [Google Scholar]
- 36.Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, et al. Pregnancy Outcomes After Fertility-Sparing Management in Young Women With Early Endometrial. Obstet Gynecol. 2013;121(1):136–142. doi: 10.1097/AOG.0b013e31827a0643. [DOI] [PubMed] [Google Scholar]


