Abstract
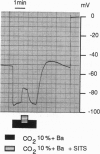
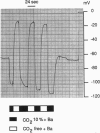
Electrogenic cotransport of Na+ with HCO3- has been reported in numerous tissues. It has always been shown with a net transfer of negative charge, but in some situations achieves net outward transport of both species with a stoichiometry of at least three HCO3- ions per Na+ ion (3:1), and in other situations achieves net inward transport of both species and has a stoichiometry of at most two HCO3- ions per Na+ ion (2:1). This suggests either that there may be more than one protein responsible for Na(+)-HCO3- cotransport in different tissues or that if there is a single protein, its stoichiometry may differ depending on the orientation of net transport. The present study, using conventional or double-barreled ion-selective microelectrodes to follow basolateral membrane potential and intracellular pH or Na+ activity in Necturus proximal convoluted tubule in vivo, shows that the orientation of the basolateral Na(+)-HCO3- cotransporter can be reversed upon switching from a perfusate simulating normal acid-base conditions to one that imposes peritubular isohydric hypercapnia. Moreover, accompanying the reversal of orientation is a change of apparent stoichiometry from 3:1 to 2:1. Given that the observed change of orientation and accompanying change of apparent stoichiometry occur within seconds and in the same preparation, these results suggest that a single transport protein is responsible for both types of behavior.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos T., Planelles G. Cell and luminal activities of chloride, potassium, sodium and protons in the late distal tubule of Necturus kidney. J Physiol. 1987 Dec;393:73–89. doi: 10.1113/jphysiol.1987.sp016811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol. 1989;51:419–441. doi: 10.1146/annurev.ph.51.030189.002223. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983 Jan;81(1):53–94. doi: 10.1085/jgp.81.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. The electrogenic Na/HCO3 cotransporter. Kidney Int. 1989 Sep;36(3):392–402. doi: 10.1038/ki.1989.208. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Hogan E., Russell J. M. pH-sensitive activation of the intracellular-pH regulation system in squid axons by ATP-gamma-S. Nature. 1988 Mar 17;332(6161):262–265. doi: 10.1038/332262a0. [DOI] [PubMed] [Google Scholar]
- Dart C., Vaughan-Jones R. D. Na(+)-HCO3- symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Schlue W. R. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1989 Apr;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geibel J., Giebisch G., Boron W. F. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Adorante J. S., Miller S. S., Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989 Jul;94(1):125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T. J., Matthes H., Keller S. K., Wiederholt M. Electrical properties of sodium bicarbonate symport in kidney epithelial cells (BSC-1). Am J Physiol. 1986 Dec;251(6 Pt 2):F954–F968. doi: 10.1152/ajprenal.1986.251.6.F954. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Schwartz P., Schill B. S., Langner B., Lepple A. P., Keller S. K., Wiederholt M. Kinetic properties of the sodium bicarbonate (carbonate) symport in monkey kidney epithelial cells (BSC-1). Interactions between Na+, HCO-3, and pH. J Biol Chem. 1986 Aug 15;261(23):10673–10679. [PubMed] [Google Scholar]
- Laprade R., Lapointe J. Y., Breton S., Duplain M., Cardinal J. Intracellular potassium activity in mammalian proximal tubule: effect of perturbations in transepithelial sodium transport. J Membr Biol. 1991 May;121(3):249–259. doi: 10.1007/BF01951558. [DOI] [PubMed] [Google Scholar]
- Lopes A. G., Siebens A. W., Giebisch G., Boron W. F. Electrogenic Na/HCO3 cotransport across basolateral membrane of isolated perfused Necturus proximal tubule. Am J Physiol. 1987 Aug;253(2 Pt 2):F340–F350. doi: 10.1152/ajprenal.1987.253.2.F340. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Cohen B., Guggino W. B., Giebisch G. Electrical effects of potassium and bicarbonate on proximal tubule cells of Necturus. J Membr Biol. 1984;79(2):145–152. doi: 10.1007/BF01872118. [DOI] [PubMed] [Google Scholar]
- Morgunov N., Boulpaep E. L. Electrochemical analysis of renal Na+-glucose cotransport in salamander proximal tubules. Am J Physiol. 1987 Jan;252(1 Pt 2):F154–F169. doi: 10.1152/ajprenal.1987.252.1.F154. [DOI] [PubMed] [Google Scholar]
- NISHI S., SOEDA H. HYPERPOLARIZATION OF A NEURONE MEMBRANE BY BARIUM. Nature. 1964 Nov 21;204:761–764. doi: 10.1038/204761a0. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Kersting U., Hunter M. Cytoplasmic pH determines K+ conductance in fused renal epithelial cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8345–8349. doi: 10.1073/pnas.85.21.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles G., Anagnostopoulos T. Basolateral electrogenic Na/HCO3 symport in the amphibian distal tubule. Pflugers Arch. 1991 Feb;417(6):582–590. doi: 10.1007/BF00372955. [DOI] [PubMed] [Google Scholar]
- Planelles G., Teulon J., Anagnostopoulos T. The effects of barium on the electrical properties of the basolateral membrane in proximal tubule. Naunyn Schmiedebergs Arch Pharmacol. 1981 Dec;318(2):135–141. doi: 10.1007/BF00508838. [DOI] [PubMed] [Google Scholar]
- Reeves R. B. Temperature-induced changes in blood acid-base status: pH and PCO2 in a binary buffer. J Appl Physiol. 1976 May;40(5):752–761. doi: 10.1152/jappl.1976.40.5.752. [DOI] [PubMed] [Google Scholar]
- Ruiz O. S., Arruda J. A. Regulation of the renal Na-HCO3 cotransporter by cAMP and Ca-dependent protein kinases. Am J Physiol. 1992 Apr;262(4 Pt 2):F560–F565. doi: 10.1152/ajprenal.1992.262.4.F560. [DOI] [PubMed] [Google Scholar]
- Sardet C., Counillon L., Franchi A., Pouysségur J. Growth factors induce phosphorylation of the Na+/H+ antiporter, glycoprotein of 110 kD. Science. 1990 Feb 9;247(4943):723–726. doi: 10.1126/science.2154036. [DOI] [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M., Hattabaugh Y. J., Bizal G. L. pH sensitivity of the Na+:HCO3- cotransporter in basolateral membrane vesicles isolated from rabbit kidney cortex. J Biol Chem. 1992 Sep 15;267(26):18349–18355. [PubMed] [Google Scholar]
- Stahl F., Lepple-Wienhues A., Kuppinger M., Tamm E., Wiederholt M. Electrogenic sodium-bicarbonate cotransport in human ciliary muscle cells. Am J Physiol. 1992 Feb;262(2 Pt 1):C427–C435. doi: 10.1152/ajpcell.1992.262.2.C427. [DOI] [PubMed] [Google Scholar]
- Strazzabosco M., Mennone A., Boyer J. L. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991 May;87(5):1503–1512. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A., Grossman E. B., Lombardi M., Hebert S. C. Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl- to Na+:K+:2Cl- cotransport in mouse medullary thick ascending limb. J Membr Biol. 1991 Feb;120(1):83–94. doi: 10.1007/BF01868594. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- la Cour M. Kinetic properties and Na+ dependence of rheogenic Na(+)-HCO3- co-transport in frog retinal pigment epithelium. J Physiol. 1991 Aug;439:59–72. doi: 10.1113/jphysiol.1991.sp018656. [DOI] [PMC free article] [PubMed] [Google Scholar]