Abstract
Campylobacter jejuni is the most prevalent cause of bacterial gastroenteritis worldwide. Polyphosphate kinases 1 and 2 (PPK1 and PPK2) regulate several cellular processes, including the biosynthesis of the bacterial cell wall. Despite their importance, whether PPK1 and PPK2 modulate the composition of C. jejuni outer membrane constituents (OMCs) and consequently impact its interaction with host cells remains unknown. Our comparative analysis between C. jejuni wild type, Δppk1, and Δppk2 strains showed qualitative and quantitative differences in the total OMC composition among these strains. Importantly, these OMC variations observed on the C. jejuni polyphosphate kinase mutants are directly related to their capacity to invade, survive, and alter the immune response of intestinal epithelial cells in vitro. Specifically, sub-fractionation of the C. jejuni OMC indicated that OMC proteins are uniquely associated with bacterial invasion, whereas C. jejuni OMC proteins, lipids, and lipoglycans are all associated with C. jejuni intracellular survival. This study provides new insights regarding the function of polyphosphate kinases and their role in C. jejuni infection.
Keywords: Campylobacter jejuni, invasion, outer membrane constituents, poly P kinases, survival
Introduction
Campylobacter jejuni is a foodborne pathogen responsible for causing gastroenteritis in humans worldwide. Campylobacteriosis cases in developing countries are underestimated because of the lack of routine surveillance programs.1 According to the disability-adjusted life year, approximately 7.5 million people are affected with Campylobacter worldwide.2
The C. jejuni cell envelope is mainly composed of a capsular polysaccharide (CPS), lipooligosaccharides, and proteins with or without N- or O-linked glycosylation.3 The C. jejuni cell envelope is thought to play an important role in immune evasion or host immune system resistance, as well as in determining the capacity of C. jejuni invasion of epithelial cells.4,5,6,7 However, to date, the exact mechanism of how C. jejuni modulates the host immune system to successfully colonize the host epithelia is poorly understood.
Inorganic polyphosphate (poly P) is a linear polymer of orthophosphate residues that serves as an energy source and modulates several key cellular processes in bacteria.8,9,10 Poly P also contributes to several important functions such as DNA entry through membrane channels, capsule composition, resistance to various stresses including nutritional and antibiotic stresses, DNA replication fidelity, growth, motility, biofilm formation, quorum sensing, bacterial signaling, stationary-phase survival, invasion and intracellular survival, and host colonization.10,11,12,13,14 Recent studies have shown that poly P is important for adaptation, resistance to stress, and cellular homeostasis in C. jejuni.14 However, the mechanisms through which poly P and its cognate enzymes impact C. jejuni pathophysiology remain largely unknown. The poly P levels in the cells are regulated mainly by poly P kinases and exopolyphosphatases (PPXs). C. jejuni has two poly P kinases: poly P kinase 1 (PPK1), which catalyzes the synthesis of poly P from adenosine triphosphate (ATP), and poly P kinase 2 (PPK2), which hydrolyzes poly P to generate guanosine triphosphate (GTP), a molecule known to have important roles in cell signaling and DNA, RNA, protein, and polysaccharide synthesis.10,12,14,15,16 In addition, PPXs degrade poly P into a smaller branch of inorganic phosphate.17
Previous studies have shown that ppk1 contributes to C. jejuni pathogenesis and affects its tolerance to specific stresses and its stringent response.14,15 Furthermore, it was shown that the deletion of ppk1 results in the decreased capacity for intracellular survival of C. jejuni in epithelial cells in vitro and a dose-dependent defect in colonization of chickens. The deletion of ppk2 also results in impaired growth of C. jejuni under osmotic, nutrient, and antimicrobial stresses, as well as decreased intracellular survival in a human intestine epithelial cell line and decreased capacity for colonization of chickens in vivo.16 Additionally, the deletion of ppk2 results in a significant decrease in poly P-dependent GTP synthesis. Reduced GTP levels can impact several cellular processes, including the composition of glycoconjugates such as glycosylated proteins.18 C. jejuni possesses both N- or O-linked glycosylation; defects in glycosylation can impact host colonization and cell invasion.19
Taken together, these data suggest that poly P metabolism will have significant effects on the fitness of C. jejuni. However, how poly P and its cognate enzymes contribute to the pathogenesis of C. jejuni remains unclear. Here, we investigated whether PPK1 and PPK2 participate in shaping the C. jejuni outer membrane constituents (OMCs) and how OMC alterations in C. jejuni may contribute to its infection of human epithelial cells in vitro. Our data indicate that PPK1 and PPK2 regulate the C. jejuni OMC composition, whereby variations in OMC proteins play a role in the capacity of C. jejuni to modulate both invasion of and intracellular survival within the host.
Materials and methods
Reagents
All chemical reagents used in this study were of high-grade purity from Sigma-Aldrich, St. Louis, MO, USA, unless otherwise specified. Endotoxin-free sterile water (Baxter Healthcare Corporation, Chicago, IL, USA) was used for the dialysis of all C. jejuni OMC fractions. Dulbecco's phosphate-buffered saline (DPBS) without CaCl2 and MgCl2 (Invitrogen, Grand Island, NY, USA) was used in all experiments. A bicinchoninic acid (BCA) Kit (Pierce, Rockford, IL, USA) was used for protein estimation, and a human CXCL8/IL-8 ELISA Kit was used for IL-8 quantification (R&D Systems, Minneapolis, MN, USA).
Bacterial growth and OMC extraction
C. jejuni strains 81–176 (wild type, WT), Δppk1, and Δppk2 were used in this study.15,16 WT, Δppk1, and Δppk2 strains were grown on Mueller Hinton (MH) agar (Becton Dickinson and Company, Sparks, MD, USA) under microaerobic conditions (5% O2, 10% CO2, 85% N2); kanamycin was added to a final concentration of 50 µg/mL when appropriate, and the cultures incubated for 24–48 h at 42 °C. For OMC extraction, 5 g (wet weight) of C. jejuni WT and mutants were harvested, suspended in 50 mL of 0.1 M NaCl, and gently stirred for 48 h at 4 °C. These mixtures were centrifuged at 27 000g, and the supernatant containing the OMC was filtered using a 0.2 µm membrane filter (Corninig Inc., Corning, NY, USA) lyophilized, and suspended in 10 mL of endotoxin-free sterile water. The samples were then dialyzed for 72 h (three times with water changes every 12 h) using a molecular mass cut-off membrane of 500 Da (Spectrum Labs, Rancho Dominguez, CA, USA). Dialyzed samples were lyophilized, reconstituted in endotoxin free sterile water, and normalized by weight to 60 mg. The protein concentration was estimated using a BCA Kit. Samples (10 µg) were analyzed by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS—PAGE) and stained with periodic acid-Schiff staining and silver nitrate staining to visualize glycosylations.20
Fractionation of C. jejuni OMCs
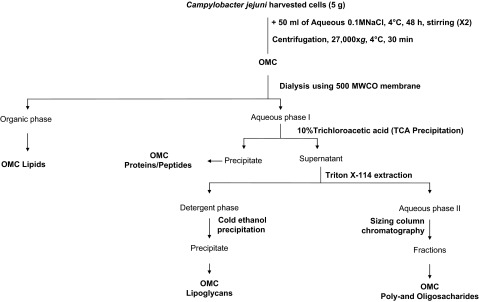
OMC total lipid fractions were obtained as previously described21 (Figure 1). Briefly, the OMC from each strain was delipidated by sequential organic solvent extractions using (i) chloroform/methanol (2:1, v/v); (ii) chloroform/methanol (1:2, v/v); and (iii) chloroform/methanol/water (10:10:3, v/v/v). Each extraction was performed by gentle shaking for 18 h at 37 °C, with centrifugation at 27 000g for 10 min between extractions. All organic extracts were combined to obtain the total lipid extract, and then the extracts were dried at room temperature and kept at −20 °C until use.
Figure 1.
C. jejuni OMC extraction and fractionation. C. jejuni WT, Δppk1, and Δppk2 OMCs were fractionated into total lipids, proteins, and poly-and oligo-saccharides, as indicated in the flowchart and as described in detail in the Materials and Methods section. Fractions were normalized by weight before each extraction. The obtained fractions were analyzed for their contribution to invasion by and intracellular survival of C. jejuni in INT-407 cells.
Delipidated OMCs from each strain was further treated with 10% trichloroacetic acid and incubated overnight at 4 °C to precipitate the total protein from the OMCs (Figure 1). The precipitate containing the OMC protein fraction was washed twice with cold acetone and dried, the total protein concentration was estimated using a BCA Kit. Samples were then aliquoted and stored at −80 °C until use.
After protein removal, the supernatant containing the lipoglycan (OMC LPG) and oligo-/poly-saccharide fractions (OMC O/P) was further extracted three times with 8% Triton X-114 (detergent) for 2 h at 4 °C, as previously described22 (Figure 1). This extract was further incubated in a water bath at 50 °C for 30 min to obtain a bipartition into an aqueous phase (top layer, containing OMC oligo-/poly-saccharides) and a detergent phase (bottom layer, containing OMC lipoglycans). OMC lipoglycans extracted in the detergent phase were further precipitated by adding nine volumes of 95% cold ethanol at −20 °C for 12 h. This new precipitate (OMC lipoglycans) was washed several times with cold ethanol, dialyzed, lyophilized, aliquoted, and stored at −20 °C until use. The remaining aqueous phase after detergent extraction (containing OMC oligo-/poly-saccharides) was dialyzed, lyophilized, aliquoted, and stored at −20 °C until use.
Sugar and fatty acid analyses
Neutral sugars and fatty acid methyl esters in the total extracted OMC sample from each strain were analyzed by gas chromatography/mass spectrometry (GC/MS, Trace GC/MS ultra, Thermo Quest, Austin, TX, USA) using appropriate internal standards as previously described.21,22,23 Briefly, extracted OMC (normalized by 10 μg of protein content) from each strain was hydrolyzed with 2 M trifluoroacetic acid in water at 120 °C for 2 h. Using scyllo-inositol as the internal standard, hydrolyzed samples were reduced with sodium borodeuteride and acetylated using acetate anhydride at 120 °C for 1 h. The resulting alditol acetates were analyzed using a GC-MS (Thermo DSQII Trace coupled with GC Ultra) fitted with a Rtx-5MS column (30 m × 0.25 mm with 5 m of Integra-Guard, Restek, Bellefonte, PA, USA) with an initial temperature of 150 °C for 3 min, increased to 200 °C at 2 °C/min then to 250 °C at 40 °C/min and held for 4 min. The peak areas of the individually separated alditol acetates were used for relative quantification. Experiments were performed twice in duplicate.
Mass spectrometry analysis of the C. jejuni protein fraction
Three replicates of C. jejuni WT, Δppk1, and Δppk2 OMC total protein (10 µg protein per replicate) were trypsin digested and analyzed by capillary liquid chromatography nanospray ionization tandem mass spectrometry (capLC-NSI/MS/MS). Briefly, global protein identification was performed on a Thermo Finnigan LTQ Orbitrap mass spectrometer equipped with a microspray source (Michrom Bioresources, Inc., Auburn, CA, USA) using a µ-Precolumn Cartridge (Dionex, Sunnyvale, CA, USA) in tandem with an UltiMateTM 3000 HPLC system from LC-Packing A Dionex Co. Mobile phases A and B consisting of 50 mM acetic acid in water and 100% acetonitrile, respectively, were used with a flow rate at 2 µL/min. Mobile phase B was increased from 2% to 5% over 3 min, followed by an increase from 5% to 30% over 60 min, and then from 30% to 90% over 20 min. MS/MS data were acquired with a spray voltage of 2.2 kV and a capillary temperature of 175 °C. The full scan resolution was set at 60 000 to achieve high mass accuracy MS determination. The collision-induced dissociation fragmentation energy was set to 35%. Dynamic exclusion was enabled with a repeat count of 1 within 18 s, a mass list size limit of 500, an exclusion duration of 10 s, and a low mass width and high mass width of 30 ppm. Sequence information from the MS/MS data was processed using Mascot Daemon by Matrix Science version 2.3.2 (Boston, MA, USA) against the NCBI nr ‘C. jejuni'database (vr. 20133017, 44572 sequences). A decoy database was also searched to determine the false discovery rate (FDR), and peptides were filtered according to this FDR. The significance threshold was set at P < 0.05.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed as previously described.24,25 Briefly, C. jejuni bacterial pellets were exposed to a fixative containing 3% glutaraldehyde/1% paraformaldehyde in 0.1 M potassium phosphate buffer (PB), pH 7.2. Cells were fixed for 2 h at room temperature and then embedded in 0.6% agarose; fixation was continued overnight at 4 °C with fresh fixative. After three washes with excess PB, samples were post-fixed in 1% osmium tetroxide with 1% uranyl acetate in PB for 1 h. The samples were subsequently washed three times with distilled water, dehydrated in graded ethanol, and ethanol-propylene oxide series and embedded in EM Bed812 resin following the manufacturer's instructions (Electron Microscopy Sciences, Hatfield, PA, USA). Ultrathin sections (70 nm) were prepared using a Leica EM UC6 ultra-microtome. After staining with 3% aqueous uranyl acetate for 20 min, followed by Reynolds' lead citrate for 10 min, sections were viewed using a Hitachi H-7500 transmission electron microscope at 80 kV at the Molecular and Cellular Imaging Center (http://www.oardc.ohio-state.edu/mcic), and images were recorded with an Optronics QuantiFire S99835 (SIA) digital camera.
Cell culture assays
Cell culture studies using INT-407 cells (human embryonic intestine, ATCC CCL 6; http://www.atcc.org/products/all/CCL-6.aspx) were approved by The Institutional Biosafety Committee, The Ohio State University, under the protocol number 2007R0009AR4. For cell culture, INT-407 cells were grown in Dulbecco's minimal essential medium (DMEM) supplemented with 4 mM L-glutamine, 4.5 g/L L-glucose, and 10% fetal bovine serum (Thermo Scientific, South Logan, UT, USA) at 37 °C in a 5% CO2 humidified incubator. Cells on monolayers were treated with trypsin (1% trypsin, Gibco, Grand Island, NY, USA), suspended in 24 well tissue culture plates and incubated until confluent monolayers were obtained. To assess the number of INT-407 cells prior to infection, two extra wells were seeded, and the average cell number per well was determined by staining the cells with trypan blue and counting the cells with a hemocytometer under a microscope. For infection, ∼2 × 106 cells per/well were used. Antibiotics were not used for culturing the INT-407 cells in this study.
Invasion and survival assays
Different fractions from C. jejuni WT, Δppk1, and Δppk2 were individually suspended in DMEM (150 µg/mL of the total OMC, 150 µg/mL of OMC protein fractions, 75 µg/mL of OMC lipid fractions, 75 µg/mL of OMC oligo-/poly-saccharides fractions, and 50 µg/mL of OMC lipoglycan fractions) and sterilized by filtration using 0.22 µm membrane filters (Millipore, Billerica, MA, USA). INT-407 cells (∼2 × 106 cells) were pre-incubated with the C. jejuni total OMC and OMC fractions (1 mL/well in a 24 well tissue culture plate) at 37 °C for 1 h, followed by a challenge with mid-log phase-grown WT C. jejuni (∼2 × 108 cells at a multiplicity of infection of 100). The bacterial numbers were determined by measuring the OD600 and by standard plating and determining the colony-forming units (CFU) after a serial dilution. Following infection with C. jejuni, plates were centrifuged at 1000g for 3 min at room temperature and further incubated for 3 h at 37 °C, 5% CO2. Infected monolayers were rinsed three times with DPBS and treated with 150 µg/mL of gentamicin for 2 h. Infected monolayers were then washed three times with DPBS and lysed with 0.1% of Triton X-100, and a 100 µL aliquot from each well was serially diluted 10-fold in DPBS and plated on MH agar in duplicate to determine the CFUs.
For the survival assay, following the invasion and after treating the infected cell monolayers with gentamicin, monolayers were rinsed twice with DPBS and incubated for an additional 24 h with 10 µg/mL of gentamicin in complete DMEM. Following incubation, infected cells were processed to assess CFUs, as described above for the invasion assay. The invasion and survival results are expressed as the mean ± SEM of experiments performed in triplicate and were repeated at least three times on different days. INT-407 cells that were not pre-exposed to the C. jejuni total OMC or OMC fractions but that were challenged with WT C. jejuni were used as a positive control for the invasion and survival assays. INT-407 cells only exposed to the C. jejuni total OMC but not infected with C. jejuni were used as a negative control.
Assay for IL-8 secretion
Supernatants from the survival assays were collected to evaluate IL-8 release by INT-407 cells after exposure to the total OMC and OMC fractions.26 IL-8 production was determined using a human CXCL8/IL-8 ELISA Kit (R&D Systems) using standard curves following the manufacturer's instructions. INT-407 cells were exposed to either C. jejuni (MOI 100:1) for 3 h, to phorbol 12-myristate 13-acetate (PMA) for 1 h as a positive control or to medium alone as a baseline; the three treatments were compared.
Statistical analyses
For all experiments, the data are presented as the mean ± SEM of a minimum of n = 2 performed at least in duplicate. Statistical analyses of data generated in this study were performed by one-way analysis of variance (ANOVA) using the Dunnett post-test in GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Poly P kinases influence OMC composition in C. jejuni
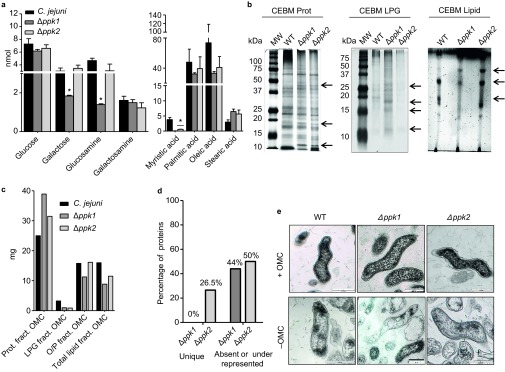
C. jejuni strains 81–176 (WT), Δppk1, and Δppk2 were used in this study; compared to isogenic WT 81-176, these mutants have been previously shown to have no growth defect when grown in MH media.15,16 Neutral sugar analysis of the C. jejuni total OMC revealed that poly P kinases may modulate the sugar content in C. jejuni WT and mutant OMCs. As depicted in Figure 2a, a significant decrease (P < 0.05) in galactose and glucosamine was observed only in Δppk1. In contrast, the levels of glucose and galactosamine were not altered among all strains studied. The C. jejuni total OMC fatty acid profile also displayed variations among the strains studied; lower levels of myristic (14:0), palmitic (16:0), and oleic (18:0) acids were detected in the OMCs of the poly P mutants; however, only myristic acid levels were significantly less (P < 0.05) compared to the WT (Figure 2a). SDS–PAGE and thin-layer chromatography analyses of the total OMCs of poly P mutants also showed some qualitative and quantitative protein (Prot), lipoglycan (LPG), and lipid alterations compared to the WT (Figure 2b). Fractionation of the total OMC into proteins, lipids, lipoglycans, and oligo-/poly-saccharide fractions verified the observed quantitative differences (Figure 2c). Moreover, our LC/MS-MS analysis revealed qualitative differences in the total protein profiles present in the OMC protein fraction among all studied strains (Figure 2d, Table 1); compared to WT, Δppk1, and Δppk2 showed 44% and 50% of proteins either absent or underrepresented, respectively, whereas 26.5% of the proteins were unique to Δppk2. In comparison to WT, underrepresented proteins in the Δppk2 were γ-glutamyltransferase Ggt, SodB, PorA, and flagellins, whereas proteins such as TolB, biotin sulfoxide reductase, nitrate reductase NapA, thiol:disulfide interchange protein DsbA, FdhA, and tungstate ABC transporter were overrepresented in the Δppk2. In addition, the Δppk2 completely lacked the cation ABC transporter, major antigenic peptide PEB4, PebA, methyl accepting chemotaxis protein, and KatA proteins. The Δppk1 mutant OMC also showed several proteins such as Ggt, biotin sulfoxide reductase, PEB4, high affinity branched-chain amino acid ABC transporter, methyl-accepting chemotaxis protein, PorA, and flagellins, that were either absent or underrepresented (Table 1).
Figure 2.
(A) Neutral sugar and fatty acid profile from the total OMC of C. jejuni WT, Δppk1, and Δppk2. Results are presented as the mean ± SEM sugar amount detected by GC and GC/MS based on 100 µg of protein. Each value is the mean of two separate experiments performed in duplicate on different days. Asterisks (*) indicate the significant difference in the sugar content compared with C. jejuni WT (one-way ANOVA, Dunnett's post-test, *P < 0.05). (B) Visualization of alterations in the OMC components produced by the deletion of poly P kinases. C. jejuni protein OMC fraction was analyzed in 12% SDS–PAGE and visualized by periodic acid-Schiff staining and silver nitrate staining. The C. jejuni lipoglycan fraction was analyzed in 15% SDS–PAGE and visualized by periodic acid-Schiff staining and silver nitrate staining. The C. jejuni lipid OMC fraction was analyzed by TLC using chloroform-ethanol-water-triethylamine (35:35:7:35, v/v/v/v) and visualized by charring with 10% concentrated H2SO4 in ethanol at 120°C. Arrows represent the differences in quantity and/or absence of bands between mutants compared with WT. (C) Quantitative analysis of the C. jejuni WT, Δppk1, and Δppk2 total OMC material fractions. Samples were normalized by weight to 60 mg of dry OMC content. The graph represents the weight in milligrams of one biological sample extraction. (D) The percentage of unique and absent or underrepresented proteins present in the OMC of C. jejuni Δppk1 and Δppk2 compared with WT. Samples were analyzed using capLC-NSI/MS/MS. Sequence information was processed using Mascot Daemon, Matrix version 2.3.2 using C. jejuni database. The percentage of unique and absent or underrepresented proteins was calculated based on the overall number of proteins divided by the total number of proteins identified by capLC-NSI/MS/MS. (E) TEM images of C. jejuni WT, Δppk1, and Δppk2 before (+OMC) and after treatment (−OMC) with 0.1 M NaCl. Black lines correspond to a scale bar 200 nm.
Table 1. Identification of proteins presented in the C. jejuni protein OMC fractions by capLC-NSI/MS/MS.
| Locus tag/gene | Protein name | Molecular mass (kDa) | WT | Δppk1 | Δppk2 |
|---|---|---|---|---|---|
| CJJ81176_0067; ggt | Gamma-glutamyltransferase | 60 | 10 | 0 | 2 |
| CJJ81176_0075 | Cytochrome c family protein | 39 | 6 | 0 | 3 |
| CJJ81176_0080; flgD | Flagellar basal body rod modification protein | 31 | 0 | 1 | 5 |
| CJJ81176_0097; fliY | Flagellar motor switch protein FliY | 30 | 0 | 0 | 3 |
| CJJ81176_0147; tolB | Translocation protein TolB | 45 | 3 | 0 | 6 |
| CJJ81176_0179 | Cation ABC transporter, periplasmic cation-binding protein | 35 | 2 | 10 | 0 |
| CJJ81176_0205; sodB | Superoxide dismutase, Fe | 25 | 17 | 26 | 7 |
| CJJ81176_0211 | Iron ABC transporter, periplasmic iron-binding protein | 37 | 93 | 135 | 93 |
| CJJ81176_0291 | Biotin sulfoxide reductase | 93 | 1 | 0 | 8 |
| CJJ81176_0325; modA | Molybdenum ABC transporter, periplasmic molybdenum-binding protein | 24 | 3 | 2 | 3 |
| CJJ81176_0354; ndk | Nucleoside diphosphate kinase | 15 | 1 | 5 | 0 |
| CJJ81176_0382; ccpA-2 | Cytochrome C551 peroxidase | 37 | 0 | 0 | 3 |
| CJJ81176_0624 | Major antigenic peptide PEB4 | 30 | 68 | 34 | 0 |
| CJJ81176_0642 | Phosphate ABC transporter, periplasmic phosphate-binding protein, putative | 36 | 0 | 0 | 6 |
| CJJ81176_0801; napA | Nitrate reductase catalytic subunit | 105 | 4 | 12 | 65 |
| CJJ81176_0836 | Amino acid-binding protein | 29 | 16 | 2 | 0 |
| CJJ81176_0883 | Thiol:disulfide interchange protein DsbA, putative | 26 | 0 | 0 | 12 |
| CJJ81176_0928; pebA | Bifunctional adhesin/ABC transporter aspartate/glutamate-binding protein | 28 | 135 | 122 | 0 |
| CJJ81176_0974 | Putative lipoprotein | 16 | 4 | 1 | 0 |
| CJJ81176_1002; jlpA | Surface-exposed lipoprotein | 42 | 5 | 0 | 4 |
| CJJ81176_1016 | Hypothetical protein | 21 | 0 | 0 | 24 |
| CJJ81176_1037 | High affinity branched-chain amino acid ABC transporter, periplasmic amino acid-binding protein | 40 | 0 | 0 | 22 |
| CJJ81176_1038 | High affinity branched-chain amino acid ABC transporter, periplasmic amino acid-binding protein | 81 | 46 | 20 | 0 |
| CJJ81176_1128; tlp8 | Methyl-accepting chemotaxis protein | 48 | 20 | 11 | 0 |
| CJJ81176_1242; htrA | Protease DO | 51 | 2 | 2 | 4 |
| CJJ81176_1275; porA | Major outer membrane protein | 46 | 83 | 15 | 67 |
| CJJ81176_1308; accB | Acetyl-CoA carboxylase, biotin carboxyl carrier protein | 16 | 0 | 2 | 4 |
| CJJ81176_1338 | Flagellin | 60 | 156 | 0 | 105 |
| CJJ81176_1339 | Flagellin | 60 | 215 | 40 | 157 |
| CJJ81176_1387; katA | Catalase | 50 | 9 | 11 | 0 |
| CJJ81176_1503; fdhA | Formate dehydrogenase, alpha subunit, selenocysteine-containing | 104 | 0 | 0 | 6 |
| CJJ81176_1525 | Tungstate ABC transporter, periplasmic tungstate-binding protein, putative | 30 | 0 | 0 | 39 |
| CJJ81176_1604 | Hemin ABC transporter, periplasmic hemin-binding protein, putative | 29 | 8 | 8 | 0 |
| CJJ81176_1650 | Hypothetical protein | 19 | 0 | 0 | 20 |
Numbers in each strain represent the relative protein abundance (based on spectral counts) from three replicates of 10 µg of outer material analyzed. Only proteins having spectral counts of more than two in at least one of the strains analyzed are included.
Using TEM, we further corroborated that OMC extraction does not alter the cell wall integrity of the C. jejuni strains studied (Figure 2e). Importantly, our viability studies assessing the ability of C. jejuni strains to grow on plates before and after 0.1 NaCl (0.58%) extractions showed no differences in viability when cultured in MH agar at 42 °C (Supplementary Figure S1). There are many studies showing that treatment of Gram-negative bacteria with 0.1 M NaCl removes cell envelope-bound material that maintains the integrity of the cell wall. In this regard, studies have shown that C. jejuni grown in a salt buffered medium at 37 °C shows no killing;27 only increasing the NaCl concentration in this salt buffered medium to 2%, which is 3.5-fold higher than the 0.58% used in this study, was lethal.27 Another study examining 44 different Gram-negative and Gram-positive bacteria established that these strains are able to grow without any deficiency after being exposed to 0.1 M NaCl.28 In addition, another study demonstrated that extraction of whole cells of Escherichia coli and Salmonella typhimurium with a 10-fold higher concentration (1 M NaCl) released OMC (outer material) without causing cell lysis.29
OMC modifications on ppk1 and ppk2 mutants mediate C. jejuni invasion and survival in INT-407 cells
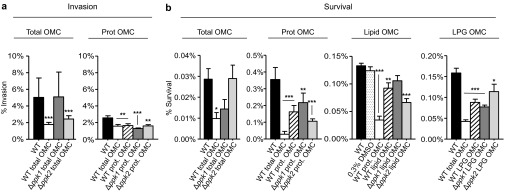
To assess whether differences in the total OMC composition of ppk mutants alter the invasion of INT-407 cells by the C. jejuni WT strain, INT-407 cells were exposed to total OMC extracts from WT, Δppk1, or Δppk2 prior to challenge with C. jejuni WT. Our rationale was that components present in the WT OMC will block the invasion by WT C. jejuni. Alternatively, if the total OMC from Δppk1 or Δppk2 lacks an essential component that C. jejuni WT uses to invade cells, then pre-exposure of INT-407 cells to the Δppk1 or Δppk2 total OMC will not affect the invasion of INT-407 by the C. jejuni WT strain. It is possible that the incubation of the total OMC for 1 h might damage the host cells, making them permeable to gentamicin and thereby leading to less viable bacterial recovery at the end of the assay. Hence, we performed trypan blue staining at the end of the 1 h incubation to test the viability of the host cells after exposure to OMC. The results indicated that neither WT nor mutant OMC had an effect on the host cell viability (Supplementary Figure S2). As expected, our results revealed that the total OMC from WT were capable of significantly decreasing the invasion by C. jejuni WT by ∼300% (Figure 3a, white bar, ***P < 0.001) compared to the infected cells that were not pre-exposed to the WT total OMC (Figure 3a, black bar). However, when INT-407 cells were exposed to either the Δppk1 total OMC (Figure 3a, dark gray bar) or the Δppk2 total OMC (Figure 3a, light gray bar), no significant effects were observed on the invasion capacity of C. jejuni WT. These results imply that Δppk1 and Δppk2 lack component(s) on their OMC that participate in the invasion of the bacterium into human intestinal epithelial cells.
Figure 3.
(A) Effect of the total OMC (150 µg/mL) and protein OMC (150 µg/mL) from WT, Δppk1, and Δppk2 on C. jejuni invasion of INT-407 cells. (B) Effect of the protein (150 µg/mL), lipid (75 µg/mL), and lipoglycan (50 µg/mL) OMC fractions from WT, Δppk1, and Δppk2 on C. jejuni survival within INT-407 cells. The total OMC was also tested with each fraction as a control using the same concentration that was used for each fraction. Results are presented as the mean ± SEM of the number of bacteria recovered after cell lysis. Each value is the mean of at least two separate experiments performed in triplicate on different days. Asterisks (*) indicate the significant difference compared to the unexposed infected INT-407 cells (one way ANOVA, ***P < 0. 001, **P < 0.01, *P < 0.05).
Next, we assessed which OMC fraction (lipid, protein, lipoglycans, and/or oligo-/poly-saccharides) was responsible for the decreased capacity of invasion by C. jejuni observed in the INT-407 cells pre-exposed to the total OMC derived from the WT strain (Figure 3a, Prot OMC). Only when INT-407 cells were exposed to the OMC protein fraction from any of the strains studied was a significant decrease 120% in bacterial invasion observed compared with the unexposed infected cells (Figure 3a, Prot OMC, **P < 0.01, ***P < 0.001). In contrast to the total OMC fraction, pre-exposure to the Prot OMC fraction of Δppk1 and Δppk2 resulted in decreased invasion of INT-407 cells by WT C. jejuni. This phenomenon could be due to interference or masking of the proteins involved in invasion by other components of Δppk1 or Δppk2 OMC such as lipoglycans or proteins present in the total OMC fraction and/or also to a difference in the relative abundance of functional protein component(s) present in the Prot OMC fraction compared to the total OMC of the Δppk1 and Δppk2 mutants. These data suggest that the OMC protein fraction from WT, Δppk1, and Δppk2 mediate C. jejuni invasion into INT-407 cells. No significant differences were found in bacterial invasion when exposed to the lipid, lipoglycan, or oligo-/poly-saccharides fractions of any of the strains studied (Supplementary Figure S3a).
The role of poly P kinases in C. jejuni intracellular survival was also determined after 24 h of incubation with gentamicin to kill extracellularly attached bacteria. Here, we hypothesized that the lack of certain OMC components of mutants (but present in WT) that are key mediators of host cell receptor recognition and downstream signaling events might compromise the intracellular survival ability of WT C. jejuni in host cells when exposed to WT OMC but not when exposed to mutants' OMC. Pre-exposure of INT-407 cells to the C. jejuni WT total OMC fraction resulted in a 230% reduction in intracellular survival (*P <0.05) compared to the INT-407 cells that were not pre-exposed to the C. jejuni WT total OMC (Figure 3b). Similarly, pre-exposure to the OMC protein fractions from WT, Δppk1, and Δppk2 resulted in a significant decrease in bacterial intracellular survival (Figure 3b). Conversely, only pre-exposure to the OMC lipid fraction from Δppk2 or the OMC lipoglycan fraction from WT, Δppk1, and Δppk2 showed a significant reduction in the % of intracellular bacteria counts (***P < 0.001, **P < 0.01, *P < 0.05; Figure 3b). No significant differences were found in bacterial survival when exposed to the oligo-/poly-saccharides fraction of any of the strains studied (Supplementary Figure S3b).
OMC modifications on ppk1 and ppk2 mutants modulate IL-8 production in INT-407 cells
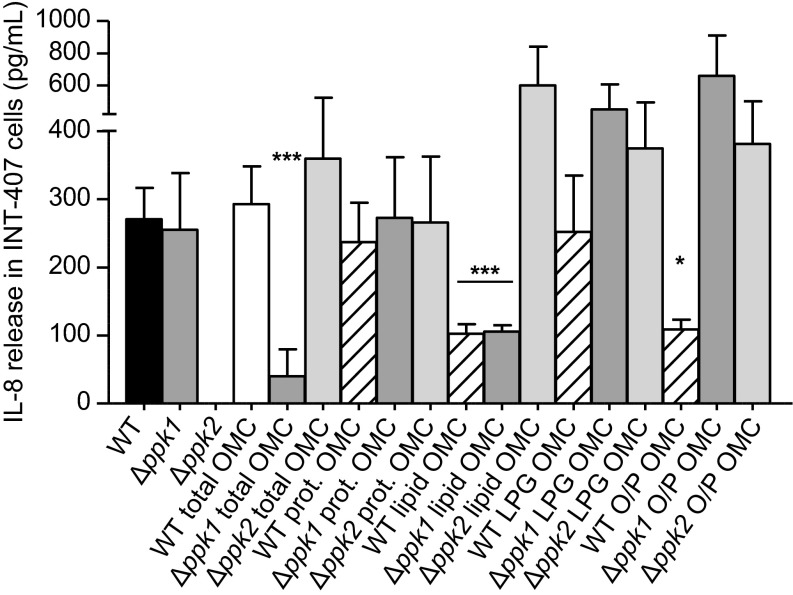
We next evaluated the IL-8 release by intestinal epithelial cells in the presence of the total OMC and its fractions (proteins, lipids, lipoglycans, and oligo-/poly-saccharides) from WT, Δppk1, and Δppk2. It has been previously reported that exposure of INT-407 cells with C. jejuni for 24 h induces IL-8 production;26 hence, in this study, INT-407 cells infected with live C. jejuni were used as a positive control. IL-8 production was observed at different levels between the total OMC and each of the OMC fractions studied (Figure 4). Conversely, IL-8 production was similar between the strains for any given OMC fraction, with the exception of the Δppk1 total OMC and lipid OMC fractions, as well as the WT OMC oligo-/poly-saccharide and lipid OMC fractions, where lower levels of IL-8 production were observed (***P < 0.001, *P < 0.05; Figure 4). Surprisingly, IL-8 secretion was not induced by the Δppk2 strain (Figure 4).
Figure 4.
IL-8 release by INT-407 cells during C. jejuni intracellular survival after exposure to the OMC and the OMC fractions. INT-407 cells were incubated with C. jejuni OMC fractions for 1 h, infected with C. jejuni WT, and incubated for an additional 24 h. IL-8 release was assessed using ELISA. The results are presented as the mean ± SEM of the quantity of IL-8 released by INT-407 cells detected by ELISA. Each value is the mean of two separate experiments performed in triplicate (one way ANOVA, ***P <0.001, *P <0.05).
Overall, these results support the concept that poly P kinases play an important role in the modulation of C. jejuni virulence by altering its OMC composition, leading to the modulation of IL-8 secretion by INT-407 cells.
Discussion
C. jejuni possesses a highly variable OMC composition, including molecules such as lipids, oligo- and poly-saccharides, and lipoproteins besides mechanisms of N-glycosylation in the periplasm that affects C. jejuni periplasmic and surface proteins and O-glycosylation mechanisms of the flagellin, which are critical for structural integrity and the pathogen–host interaction.30,31,32,33 Poly P kinases have been shown to be associated with C. jejuni virulence.14,15,16 However, there is limited information about how poly P kinases interfere with the mechanisms of regulation associated with C. jejuni outer structures, including the OMC. Here, we provide insights about the contribution of poly P kinases (PPK1 and PPK2) in shaping the composition of the C. jejuni OMC and how these modifications play a role in invasion and survival, as well as in modulating the secretion of IL-8. Here, we purified and confirmed the existence of variability in sugar and fatty acid composition. Overall, the variability in the amounts of OMC proteins, lipids, and carbohydrates among WT, Δppk1, and Δppk2 strains indicated the important role of poly P kinases in the plasticity of the C. jejuni OMC composition.
Many virulence factors have been suggested to be associated with the invasion and survival in C. jejuni in different cell lines in vitro.34 Some of these virulence factors, such as LOS, CPS, flagellins, chemotactic proteins, O- and N-linked protein glycosylation systems and lipoproteins, have been linked to the mechanism regarding the invasion of epithelial cells in vitro.30,35,36,37 Thus, it is not surprising that there are complex interactions occurring between C. jejuni and host cells, involving several molecular structures from its cell envelope. Moreover, studies on the function of poly P kinases revealed the importance of these enzymes as regulators for metabolic processes, including biosynthesis of the bacterial cell envelope.38 Specifically, compared to WT, the C. jejuni ppk2 mutant is defective in poly P-dependent GTP generation and also displays a significant increase in poly P-dependent ATP generation, suggesting that PPK2 plays a role in maintaining the intracellular nucleotide pool.16 Because polysaccharides are synthesized via guanosine 5′-diphosphate -linked sugars as intermediates, the need for nucleoside triphosphates (NTPs), particularly GTP, to generate glycoconjugates is substantial.18 Because PPK2 affects GTP and the NTP pools, PPK2 might also impact glycosylation profiles in C. jejuni. For example, several proteins that were absent in ppk2 mutant OMC (methyl-accepting chemotaxis protein -type signal transduction protein, major antigenic peptide PEB-cell binding factor, etc.) were found to carry potential N-linked glycosylated sites, as predicted using GLYCOPP V1.0 (http://www.imtech.res.in/raghava/glycopp/submit.html). These findings support our study and suggest that PPK1 and PPK2 are involved in varying the OMC composition of C. jejuni, thus defining their role in virulence.
Our results identified the existence of C. jejuni OMC components that play a major role in invasion and survival, which we speculate are directly involved in host cell receptor recognition and in initiating the signaling events that lead to invasion of and survival within the intestinal epithelium. In this regard, both Δppk1 and Δppk2 OMCs provide us with a unique tool to identify what specific components within the C. jejuni WT OMC fraction mediate invasion and, importantly, which host cell receptors and signaling mechanisms are involved. In this regard, to assess the association of poly P kinases of C. jejuni with virulence, we saturated cell receptors related to invasion and/or intracellular survival processes by persistent incubation of INT-407 cells with the C. jejuni total OMC and OMC fractions prior to infection. Our rationale was that components present in the WT OMC would block invasion or alter intracellular signaling events by WT C. jejuni; however, if OMC from the ppk mutants lacks a component that C. jejuni uses to invade and survive in cells, then pre-exposure to the total OMC from the ppk mutants will affect the invasion and survival. Our results indicated that poly P kinases have an impact on the total OMC, altering invasion and intracellular survival in C. jejuni. We further defined the OMC protein and lipoglycan fractions as capable of interacting with the receptors used by C. jejuni to invade human intestinal epithelial cells, thus implying their role in receptor recognition. In this context, previous studies have described the association of several proteins (PEB1 (a major C. jejuni cell adhesion molecule), including lipoproteins such as JlpA (a C. jejuni adhesion promoting bacterial interaction with host cells) and CPS, with adhesion and invasion processes in C. jejuni.39,40 Our proteomic results concur with above studies and that found the presence of PEB-cell binding factors (PebA and PEB4) in the C. jejuni WT and Δppk1 total OMC but not in the Δppk2 total OMC39 (Table 1). Similarly, JlpA was present in C. jejuni WT and Δppk2 but not in the Δppk1 total OMC. In addition, several proteins were absent in Δppk1 or Δppk2 OMC compared to WT OMC (i.e. flagellins, Ggt, biotin sulfoxide reductase, catalase), whereas some proteins were over- or underrepresented in these mutants (i.e. MOMP, DsbA periplasmic iron and amino acid binding proteins). The lack of CJJ81176_1128 (encoding a methyl chemotaxis protein, tlp8) results in decreased invasion and intracellular survival.41 In our study, the CJJ81176_1128 was either absent or underrepresented in both Δppk mutants. Similarly, SodB was underrepresented in the Δppk2 OMC; the sodB mutant of 81-176 has been shown to have a significant defect in the invasion of INT407 cells.42 These data further suggest a participating role for the poly P kinases in determining the C. jejuni OMC protein distribution and/or content and thereby modulating invasion by and intracellular survival of C. jejuni.
The role of OMC lipids in bacterial virulence has been studied in Mycobacterium tuberculosis and Francisella novicida, among other pathogens.43,44,45 Likewise, C. jejuni OMC contains lipid-containing components such as lipoglycans, which play important roles in binding, adhesion, and invasion of human epithelial cells in vitro.35,44 Our results using OMC lipid and lipoglycan fractions from C. jejuni WT and poly P kinases' mutants indicate that missing OMC lipoglycans and lipids from ppk1 and ppk2 OMC, respectively, are involved in C. jejuni survival. This outcome suggests the importance of poly P kinases as virulence factors associated with C. jejuni intracellular survival. Incubation of INT-407 cells with live C. jejuni also leads to the release of IL-8, and its production is directly proportional to the invasive ability of C. jejuni strains.26 Our data indicate that INT-407 cells incubated with the C. jejuni WT and Δppk1 strains lead to IL-8 secretion, whereas there was no detectable production of IL-8 when cells were exposed to the Δppk2 strain. In this context, C. jejuni secretes virulence factors, such as outer membrane vesicles and Campylobacter invasion antigens proteins associated with host cell signaling events that promote epithelial cell invasion, inflammatory response stimulation, and intracellular survival.46,47 According to our results, this discrepancy in the lack of IL-8 stimulation by the Δppk2 strain in INT-407 cells could be explained by (i) deletion of Δppk2 altered the OMC protein composition and disposition (Table 1), such that host cell receptors are not able to effectively recognize OMC proteins and thus fail to induce the secretion of IL-8, and/or (ii) that an essential protein for C. jejuni host cell recognition and subsequent stimulation of the immune response is missing or in low abundance in the Δppk2 OMC.
Conversely, studies using sub-fractions of the C. jejuni cell envelope extracted by sonication and cell envelope sub-fractions inactivated with formalin have demonstrated the inability of these sub-fractions to stimulate IL-8 in INT-407 cells, suggesting that INT-407 cells require live C. jejuni 81-176 WT to release IL-8.26 However, our results suggest that the C. jejuni total OMC and some of the OMC fractions studied are capable of inducing the secretion of IL-8. This finding may be because the OMC was obtained solely by the disruption of non-covalent interactions using 0.1 M NaCl. Another plausible explanation is that in our studies, host cells are exposed to individual OMC fractions; thus, we are enriching for a specific pool of C. jejuni OMC components. This notion may explain some of the differences observed in invasion, survival, and IL-8 production between the total OMC and a particular OMC fraction.
In summary, this study adds to the known function of poly P kinases in C. jejuni virulence, providing the basis for further investigations in determining which specific OMC components are responsible for invasion, intracellular survival, and immune response generation. In addition, future studies are required to identify the host cell receptor(s) involved in these processes and to elucidate the possible trafficking and signaling pathways that these receptors can activate. Furthermore, in vivo assays will be required to evaluate the implications of OMC and OMC fractions in C. jejuni pathogenesis to further establish the basis of drug development targeting C. jejuni PPK1 and PPK2.
Acknowledgments
We thank the Mass Spectrometry and Proteomics facility within the Campus Chemical Instrument Center at The Ohio State University for their technical assistance and the Molecular and Cellular Imaging Center at The Ohio State University at the Ohio Agricultural Research and Development Center (OARDC) for their technical support in the electron microscopy experiments. This study was supported by the OARDC Research Enhancement Competitive Grants Program to Gireesh Rajashekara. Gireesh Rajashekara's laboratory is supported by the federal funds appropriated to the OARDC of The Ohio State University and the Agriculture and Food Research Initiative (grant# 2012-68003-19679), US Department of Agriculture. Jordi B Torrelles was partially supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases AI093570 and internal funds provided by the Department of Microbial Infection and Immunity, The Ohio State University.
Footnotes
Supplementary information of this article can be found on the Emerging Microbes and Infections's website: http://www.nature.com/emi.
Supplementary Information
References
- 1Coker A, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. Human campylobacteriosis in developing countries. Emerg Infect Dis 2002; 8: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2World Health Organization. The global view of campylobacteriosis, report of an expert consultation. Utrecht: WHO, 2012. Available at http://www.who.int/foodsafety/publications/foodborne_disease/global_view_campylobacterosis/en/ (accessed 17 March 2015). [Google Scholar]
- 3Young NM, Brisson JR, Kelly J et al. Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium, Campylobacter jejuni. J Biol Chem 2002; 277: 42530–42539. [DOI] [PubMed] [Google Scholar]
- 4Zilbauer M, Dorrell N, Elmi A et al. A major role for intestinal epithelial nucleotide oligomerization domain 1 (NOD1) in eliciting host bactericidal immune responses to Campylobacter jejuni. Cell Microbiol 2007; 9: 2404–2416. [DOI] [PubMed] [Google Scholar]
- 5Samuelson DR, Eucker TP, Bell JA, Dybas L, Mansfield LS, Konkel ME. The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease. Cell Commun Signal 2013; 11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Guerry P, Szymanski CM. Campylobacter sugars sticking out. Trends Microbiol 2008; 16: 428–435. [DOI] [PubMed] [Google Scholar]
- 7Maue AC, Mohawk KL, Giles DK et al. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 2013; 81: 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Jahid IK, Silva AJ, Benitez JA. Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl Environ Microbiol 2006; 72: 7043–7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Chuang YM, Belchis DA, Karakousis PC. The polyphosphate kinase gene ppk2 is required for Mycobacterium tuberculosis inorganic polyphosphate regulation and virulence. MBio 2013; 4: e00039–e00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Rao NN, Gomez-Garcia MR, Kornberg A. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 2009; 78: 605–647. [DOI] [PubMed] [Google Scholar]
- 11Castuma CE, Huang R, Kornberg A et al. Inorganic polyphosphates in the acquisition of competence in Escherichia coli. J Biol Chem 1995; 270: 12980–12983. [DOI] [PubMed] [Google Scholar]
- 12Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol 1995; 177: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Singh R, Singh M, Arora G, Kumar S, Tiwari P, Kidwai S. Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J Bacteriol 2013; 195: 2839–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Candon HL, Allan BJ, Fraley CD, Gaynor EC. Polyphosphate kinase 1 is a pathogenesis determinant in Campylobacter jejuni. J Bacteriol 2007; 189: 8099–8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Gangaiah D, Kassem II, Liu Z, Rajashekara G. Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl Environ Microbiol 2009; 75: 7838–7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16Gangaiah D, Liu Z, Arcos J et al. Polyphosphate kinase 2: a novel determinant of stress responses and pathogenesis in Campylobacter jejuni. PLoS One 2010; 5: e12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Malde A, Gangaiah D, Chandrashekhar K, Pina-Mimbela R, Torrelles JB, Rajashekara G. Functional characterization of exopolyphosphatase/guanosine pentaphosphate phosphohydrolase (PPX/GPPA) of Campylobacter jejuni. Virulence 2014; 5: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol Microbiol 1998; 28: 875–882. [DOI] [PubMed] [Google Scholar]
- 19Karlyshev AV, Everest P, Linton D, Cawthraw S, Newell DG, Wren BW. The Campylobacter jejuni general glycosylation system is important for attachment to human epithelial cells and in the colonization of chicks. Microbiology 2004; 150: 1957–1964. [DOI] [PubMed] [Google Scholar]
- 20Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 1982; 119: 115–119. [DOI] [PubMed] [Google Scholar]
- 21Yang L, Sinha T, Carlson TK, Keiser TL, Torrelles JB, Schlesinger LS. Changes in the major cell envelope components of Mycobacterium tuberculosis during in vitro growth. Glycobiology 2013; 23: 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Shi L, Torrelles JB, Chatterjee D. Lipoglycans of Mycobacterium tuberculosis: isolation, purification, and characterization. Methods Mol Biol 2009; 465: 23–45. [DOI] [PubMed] [Google Scholar]
- 23Torrelles JB, Khoo KH, Sieling PA et al. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem 2004; 279: 41227–41239. [DOI] [PubMed] [Google Scholar]
- 24Fraley CD, Rashid MH, Lee SS et al. A polyphosphate kinase 1 (ppk1) mutant of Pseudomonas aeruginosa exhibits multiple ultrastructural and functional defects. Proc Natl Acad Sci USA 2007; 104: 3526–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Ogawa N, Tzeng CM, Fraley CD et al. Inorganic polyphosphate in Vibrio cholerae: genetic, biochemical, and physiologic features. J Bacteriol 2000; 182: 6687–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Hickey TE, Baqar S, Bourgeois AL, Ewing CP, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT-407 cells. Infect Immun 1999; 67: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Palumbo SA. Heat injury and repair in Campylobacter jejuni. Appl Environ Microbiol 1984; 48: 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28Roessler M, Sewald X, Muller V. Chloride dependence of growth in bacteria. FEMS Microbiol Lett 2003; 225: 161–165. [DOI] [PubMed] [Google Scholar]
- 29Koski P, Vaara M. Polyamines as constituents of the outer membranes of Escherichia coli and Salmonella typhimurium. J Bacteriol 1991; 173: 3695–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol 2001; 40: 769–777. [DOI] [PubMed] [Google Scholar]
- 31Jerome JP, Bell JA, Plovanich-Jones AE, Barrick JE, Brown CT, Mansfield LS. Standing genetic variation in contingency loci drives the rapid adaptation of Campylobacter jejuni to a novel host. Plos One 2011; 6: e16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Alemka A, Nothaft H, Zheng J, Szymanski CM. N-glycosylation of Campylobacter jejuni surface proteins promotes bacterial fitness. Infect Immun 2013; 81: 1674–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Guerry P, Szymanski CM, Linton D et al. Phase variation of Campylobacter jejuni 81-176 lipooligosaccharide affects ganglioside mimicry and invasiveness in vitro. Infect Immun 2002; 70: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34O Croinin T, Backert S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Infect Microbiol 2012; 2: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 2007; 5: 665–679. [DOI] [PubMed] [Google Scholar]
- 36Bachtiar BM, Coloe PJ, Fry BN. Knockout mutagenesis of the kpsE gene of Campylobacter jejuni 81116 and its involvement in bacterium-host interactions. FEMS Immunol Med Microbiol 2007; 49: 149–154. [DOI] [PubMed] [Google Scholar]
- 37Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol 2012; 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Rashid MH, Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 2000; 97: 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Pei Z, Blaser MJ. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J Biol Chem 1993; 268: 18717–18725. [PubMed] [Google Scholar]
- 40Jin S, Joe A, Lynett J, Hani EK, Sherman P, Chan VL. JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol 2001; 39: 1225–1236. [DOI] [PubMed] [Google Scholar]
- 41Chandrashekhar K, Gangaiah D, Pina-Mimbela R, Kassem II, Jeon BH, Rajashekara G. Transducer like proteins of Campylobacter jejuni 81-176: role in chemotaxis and colonization of the chicken gastrointestinal tract. Front Cell Infect Microbiol 2015; 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Pesci EC, Cottle DL, Pickett CL. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun 1994; 62: 2687–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Ehrt S, Schnappinger D. Mycobacterium tuberculosis virulence: lipids inside and out. Nat Med 2007; 13: 284–285. [DOI] [PubMed] [Google Scholar]
- 44Louwen R, Heikema A, van Belkum A et al. The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect Immun 2008; 76: 4431–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Kanistanon D, Powell DA, Hajjar AM et al. Role of Francisella lipid A phosphate modification in virulence and long-term protective immune responses. Infect Immun 2012; 80: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Elmi A, Watson E, Sandu P et al. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun 2012; 80: 4089–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47Samuelson DR, Eucker TP, Bell JA, Dybas L, Mansfield LS, Konkel ME. The Campylobacter jejuni CiaD effector protein activates MAP kinase signaling pathways and is required for the development of disease. Cell Commun Signal 2013; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.