Abstract
Background
Glaucoma is an optic neuropathy that leads to vision loss and blindness. It is the second most common cause of irreversible blindness worldwide. The main treatment for glaucoma aims to reduce intraocular pressure (IOP) in order to slow or prevent further vision loss. IOP can be lowered with medications, and laser or incisional surgeries. Trabeculectomy is the most common incisional surgical procedure to treat glaucoma. Device‐modified trabeculectomy is intended to improve drainage of the aqueous humor to lower IOP. Trabeculectomy‐modifying devices include Ex‐PRESS, Ologen, amniotic membrane, expanded polytetrafluoroethylene (E‐PTFE) membrane, Gelfilm and others. However, the effectiveness and safety of these devices are uncertain.
Objectives
To assess the relative effectiveness, primarily with respect to IOP control and safety, of the use of different devices as adjuncts to trabeculectomy compared with standard trabeculectomy in eyes with glaucoma.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2014, Issue 12), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to December 2014), EMBASE (January 1980 to December 2014), PubMed (1948 to December 2014), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to December 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on 22 December 2014.
Selection criteria
We included randomized controlled trials comparing devices used during trabeculectomy with trabeculectomy alone. We also included studies where antimetabolites were used in either or both treatment groups.
Data collection and analysis
We used standard procedures expected by Cochrane.
Main results
We found 33 studies that met our inclusion criteria, of which 30 were published as full‐length journal articles and three as conference abstracts. Only five studies have been registered. The 33 studies included a total of 1542 participants with glaucoma, and compared five types of devices implanted during trabeculectomy versus trabeculectomy alone. Five studies reported the use of Ex‐PRESS (386 participants), eight studies reported the use of Ologen (327 participants), 18 studies reported the use of amniotic membrane (726 participants), one study reported the use of E‐PTFE (60 participants), and one study reported the use of Gelfilm (43 participants). These studies were conducted in North America, South America, Europe, Asia, and the Middle East. Planned participant follow‐up periods ranged from three months to five years. The studies were reported poorly which limited our ability to judge risk of bias for many domains. Only two studies explicitly masked outcome assessment so, we rated 31 studies at high risk of detection bias.
Low‐quality evidence from three studies showed that use of Ex‐PRESS compared with trabeculectomy alone may be associated with a slightly lower IOP at one year (mean difference (MD) ‐1.58 mm Hg, 95% confidence interval (CI) ‐2.74 to ‐0.42; 165 eyes). Cataract surgery and hyphema may be less frequent in the Ex‐PRESS group than in the trabeculectomy‐alone group (cataract surgery: risk ratio (RR) 0.32, 95% CI 0.14 to 0.74, 3 studies, low‐quality evidence; hyphema: RR 0.33, 95% CI 0.12 to 0.94, 4 studies, low‐quality evidence). The effect of whether Ex‐PRESS prevents hypotony was uncertain (RR 0.92, 95% CI 0.63 to 1.33, 2 studies, very low‐quality evidence). All these studies received funding from the device manufacturer.
Very low‐quality evidence from five studies suggests that use of Ologen compared with trabeculectomy alone is associated with slightly higher IOP at one year (MD 1.40 mm Hg, 95% CI ‐0.57 to 3.38; 177 eyes). The effect of Ologen on preventing hypotony was uncertain (RR 0.75, 95% CI 0.47 to 1.19, 5 studies, very low‐quality evidence). Differences between the two treatment groups for other reported complications also were inconclusive.
Low‐quality evidence from nine studies suggests that use of amniotic membrane with trabeculectomy may be associated with lower IOP at one year compared with trabeculectomy alone (MD ‐3.92 mm Hg, 95% CI ‐5.41 to ‐2.42; 356 eyes). Low‐quality evidence showed that use of amniotic membrane may prevent adverse events and complications, such as hypotony (RR 0.40, 95% CI 0.17 to 0.94, 5 studies, low‐quality evidence).
The report from the only E‐PTFE study (60 eyes) showed no important differences for postoperative IOP at one year (MD ‐0.44 mm Hg, 95% CI ‐1.76 to 0.88) between the trabeculectomy + E‐PTFE versus the trabeculectomy‐alone groups. Hypotony was the only postoperative complication observed less frequently in the E‐PTFE group compared to the trabeculectomy‐alone group (RR 0.29, 95% CI 0.11 to 0.77).
The one Gelfilm study reported uncertainty in the difference in IOP and complication rates between the two groups at one year; no further data were provided in the study report.
Authors' conclusions
Overall, the use of devices with standard trabeculectomy may help with greater IOP reduction at one‐year follow‐up than trabeculectomy alone; however, due to potential biases and imprecision in effect estimates, the quality of evidence is low. When we examined outcomes within subgroups based on the type of device used, our findings suggested that the use of an Ex‐PRESS device or an amniotic membrane as an adjunct to trabeculectomy may be slightly more effective in reducing IOP at one year after surgery compared with trabeculectomy alone. The evidence that these devices are as safe as trabeculectomy alone is unclear. Due to various limitations in the design and conduct of the included studies, the applicability of this evidence synthesis to other populations or settings is uncertain. Further research is needed to determine the effectiveness and safety of other devices and in subgroup populations, such as people with different types of glaucoma, of various races and ethnicity, and with different lens types (e.g. phakic, pseudophakic).
Plain language summary
Device‐modified trabeculectomy for glaucoma
Review Question
We reviewed the evidence about the effectiveness and safety of the use of devices in a standard glaucoma surgery (trabeculectomy) for the treatment of glaucoma.
Background
Glaucoma is a disease of the optic nerve that leads to vision loss and blindness. It is the second leading cause of worldwide blindness, and the blindness caused by glaucoma is permanent. Treatment for glaucoma aims to reduce pressure in the eye (IOP), which helps to slow down or prevent further vision loss from glaucoma. Eye pressure can be lowered with medications, laser therapy, or surgery. Trabeculectomy, the most common standard surgical procedure for the treatment of glaucoma, can be modified by using aids or devices during the surgery. Current studies have reported using various devices such as Ex‐PRESS, Ologen, amniotic membrane, expanded polytetrafluoroethylene (E‐PTFE) membrane, Gelfilm, gold shunt, T‐flux, etc.
Study Characteristics
We found 33 studies that met our inclusion criteria. These studies included a total of 1542 glaucoma participants and compared five types of devices implanted during trabeculectomy versus trabeculectomy alone. Five studies reported the use of Ex‐PRESS (386 participants), eight studies reported the use of Ologen (327 participants), 18 studies reported the use of amniotic membrane (726 participants), one study reported the use of E‐PTFE (60 participants), and one study reported the use of Gelfilm (43 participants). These studies were conducted in North America, South America, Europe, Asia, and the Middle East. Planned participant follow‐up periods ranged from three months to five years.
Key Results
Three studies found that the use of the Ex‐PRESS shunt during trabeculectomy may slightly reduce eye pressure by about 1.6 mm Hg more than trabeculectomy alone. Another study did not find any difference in eye pressure at one year between trabeculectomy combined with Ex‐PRESS versus trabeculectomy alone. Five studies did not find any important difference between trabeculectomy and Ologen compared to trabeculectomy alone. Nine studies found that the use of amniotic membrane during trabeculectomy may reduce IOP by about 4 mm Hg more than trabeculectomy alone at one‐year follow‐up. We did not find important differences for postoperative eye pressure at one year between trabeculectomy + E‐PTFE and trabeculectomy alone. We did not find enough data regarding the evidence for the use of Gelfilm. It is uncertain whether these devices are as safe as trabeculectomy alone. The evidence is current to 22 December 2014.
Quality of the Evidence
The overall quality of the included studies varied by the type of device studied. Specifically, the quality was very low for Ex‐PRESS studies, very low for Ologen studies, low for amniotic membrane studies, and unclear for other devices. Due to the various flaws in study design and incomplete reporting, the data need to be interpreted with caution, particularly for the amniotic membrane studies.
Summary of findings
Background
Description of the condition
Glaucoma is an optic neuropathy that leads to vision loss and blindness (Foster 2002). Among the many known and unknown factors that contribute to the damage to the optic nerve, elevated intraocular pressure (IOP) is the only modifiable risk factor (Coleman 2012). Normally, the IOP is maintained in balance when the rate of aqueous production by the ciliary body is equal to the rate of its outflow from the posterior to the anterior chamber through the trabecular meshwork and the canal of Schlemm in the anterior chamber angle (Small 1986). When excess aqueous humor is produced or when part or all of the drainage system of aqueous humor is blocked, the result is an increase in IOP, which has been shown to be associated with progressive glaucomatous optic nerve damage (Pan 2011; Turkoski 2012).
There are several types of glaucoma, of which open‐angle glaucoma (OAG) and angle‐closure glaucoma (ACG) are two major types.
Epidemiology
The World Health Organization (WHO) has estimated that glaucoma is the second most frequent cause of blindness worldwide (Quigley 2011). It has been estimated that there were 60.5 million people with OAG and ACG in 2010, and the number will increase globally to 79.6 million by 2020. The most common type of glaucoma is OAG, accounting for 74% of glaucoma cases worldwide. ACG is less common. Women comprise 55% of OAG cases, 70% of ACG cases, and 59% of all glaucoma cases. Asians represent 47% of people who have glaucoma and 87% of those with ACG (Quigley 2006).
Symptoms and diagnosis
OAG is often asymptomatic initially. There is no pain and those affected tend not to notice the loss of visual field until central vision is affected in the later stage of the disease; by then optic nerve damage is often already severe (Boland 2008; Quigley 2011; Small 1986). The symptoms for ACG vary. It may occur suddenly without warning or gradually with progressive deterioration; patients may have signs and symptoms including severe pain and red eye, decreased or cloudy vision, nausea, vomiting, and bradycardia (Boland 2008; Douglas 1975; Small 1986). Clinical examinations for diagnosing glaucoma include, but are not limited to, tonometry, gonioscopy, optic nerve imaging, visual acuity measurement, and visual field assessment.
Description of the intervention
Trabeculectomy, first introduced by John Cairns in 1968 and then modified by Watson in 1972, remains the gold standard and is the most common incisional surgical procedure for the treatment of glaucoma (Cairns 1968; Watson 1972; Watson 1981). It includes lifting the conjunctiva and dissecting a partial thickness scleral flap and then making a perforating scleral entrance into the anterior chamber to allow aqueous humor drainage. Trabeculectomy may also include removing part of the eye's trabecular meshwork and adjacent structures. This procedure lowers IOP by allowing aqueous fluid to percolate into the subconjunctival space through the scleral hole or the cut ends of the trabecular meshwork into the subconjunctival space. Over the years trabeculectomy has been modified in various ways, including the use of 5‐fluorouracil (5‐FU) (Green 2014) and mitomycin C (MMC) (Wilkins 2005), and creation of a fornix‐based rather than the traditional limbus‐based conjunctival flap. Most recently, the modifications have included the use of adjunctive devices with standard trabeculectomy. Surgeons may use a tube without a reservoir (for example, Ex‐PRESS) or a space‐holder or reservoir to enhance aqueous humor outflow or to modify healing (for example, Ologen) and to promote continued drainage from the anterior chamber through a standard partial thickness scleral flap used in a standard trabeculectomy. Another technique, a device composed of both a silicone tube and an explant plate, called an aqueous shunt, is also a surgical option to treat glaucoma, but this technique is not within the scope of this review (Minckler 2006).
How the intervention might work
We consider in this review adjunctive devices used with trabeculectomy to lower IOP, with and without concomitant use of antimetabolites. The devices used with standard trabeculectomy are intended to maintain drainage of aqueous humor, and may be used with or without antimetabolites to maintain patency of the bleb (a bubble‐like blister of conjunctiva to facilitate drainage).
Tube implants
1. Ex‐PRESS mini glaucoma implant
The Ex‐PRESS implant is a miniature stainless steel shunt developed as an adjunct to trabeculectomy to promote continued aqueous drainage. The device is implanted under a partial thickness scleral flap to allow aqueous humor to flow from the anterior chamber to the subconjunctival space; implantation leads to the formation of a thin‐walled filtration bleb, similar to the bleb formed during standard trabeculectomy. Investigators who have conducted retrospective studies and randomized controlled trials have reported that the Ex‐PRESS shunt provides IOP control that is similar to or better than that provided by standard trabeculectomy (Dahan 2012; De Jong 2009; Francis 2011; Gallego‐Pinazo 2009; Maris 2007). They have also reported that the Ex‐PRESS shunt results in fewer complications, fewer postoperative surgical interventions, and less need for glaucoma medications. The device is manufactured by Alcon (a Novartis company).
2. Silicon tube implant
Jordan 2006 reported the use of a silicon tube implant for suprachoroidal drainage during a standard trabeculectomy. The tube was inserted at a junction connecting the anterior chamber and the suprachoroidal space. These investigators concluded that the tube was an effective surgical adjunct to treat intractable glaucoma. However, Jordan 2006 was not a randomized controlled trial, and the effectiveness and safety of silicon tube implants are unclear. The device is produced by a variety of manufacturers.
Antimetabolites and biodegradable implant
Wound healing and scar formation are the main sequelae that limit IOP lowering after standard trabeculectomy. They may lead to fibrosis of the bleb and obstruction of the drainage fistula, and finally cause bleb failure (Skuta 1987). Antifibrotic agents, such as 5‐FU and MMC, have been demonstrated to be effective in delaying wound healing and thus improving the success rate of trabeculectomy (Azuara‐Blanco 1998; Fraser 2004). Therefore, 5‐FU and MMC have become widely used as adjuncts to glaucoma‐filtering surgery. Although 5‐FU and MMC improve the success of trabeculectomy, concern persists about the complications associated with antimetabolites, for example bleb‐related infections, bleb leaks, and bleb dysesthesia (defined as burning, foreign body sensation, tearing, pain, or ocular discomfort in an eye with a filtering bleb) (Bell 1997; Jampel 1992; Lama 2003). Biodegradable implants have therefore been developed for use with trabeculectomy to maintain drainage while avoiding the use or reducing the amount of antimetabolite used by the surgeon.
1. Ologen implant
The Ologen implant is a plate‐shaped, tissue bioengineered, biodegradable porous collagen‐glycosaminoglycan matrix used during trabeculectomy. The device randomly reorganizes the regeneration of myofibroblasts, fibroblasts, and the secreted extracellular matrix, and consequently reduces postoperative scar formation (Dietlein 2008; Tseng 1999). Investigators who have conducted studies in animal models and several randomized clinical trials have reported that Ologen implanted in the subconjunctival space provides an alternative method for controlling the wound‐healing process and avoids the complications associated with the use of antifibrotic agents (Chen 2006; Cillino 2011; Hsu 2008; Papaconstantinou 2010; Rosentreter 2010). The device is manufactured by PRO Top & Mediking Co. Ltd and its subsidiaries: Body organ biomedical Corps, Optous, Aeon Astron B.V., OculusGen Biomedical.
2. Amniotic membrane
Human amniotic membrane is an antifibrotic, anti‐inflammatory agent. It was known for its beneficial effect in preventing subconjunctival fibrosis in glaucoma filtering surgery by suppressing transforming growth factor ß, as this signaling system is a strategy for preventing scarring during wound healing (Ricci 2013). A Cochrane review also found amniotic membrane to be useful for treating acute ocular surface burns (Clare 2012). Although recent animal and human studies on amniotic membrane have been promising, it remains unclear whether these membranes can provide a potential alternative tissue to conjunctiva in the construction of filtration blebs during trabeculectomy (Barton 2001; Eliezer 2006; Ji 2013; Khairy 2015; Sheha 2008; Stavrakas 2012). The device is produced by a variety of manufacturers.
Other implants
Other devices have been developed and studied for non‐penetrating glaucoma surgeries.
1. Expanded polytetrafluoroethylene (E‐PTFE) membrane implant
E‐PTFE is an expanded polytetrafluoroethylene implant made up of solid nodes interconnected by a thin fibril matrix. Its use has been reported in several animal studies and human trials in the form of either a membrane patch or an implant placed beneath the partial thickness scleral flap (Bae 1988; Cillino 2008; Jacob 2001) or combined with a silicone tube shunt (Choi 2003; Kim 2003). The device is manufactured by GORE Inc., under the brand names Gore‐Tex® and PRECLUDE®.
2. Gelfilm (absorbable gelatin film) implant
Gelfilm is a sterile film derived from denaturated collagen. When moistened, the film is about 0.075 mm thick and can be cut to fit the eye during surgery. The film is absorbed completely within one to six months, so that no additional surgery is required to remove the implant. Gelfilm is thought to prevent adhesion of ocular structures, which may be helpful in preventing closure of drainage passages created by trabeculectomy. The device is manufactured by Pfizer.
3. SOLX Gold Shunt
The SOLX Gold Shunt is a new biocompatible device made of pure gold (24 carat) that reduces IOP by using the eye's natural pressure difference (www.solx.com/content/solx‐gold‐shunt). The device is inserted into the anterior chamber through a special corneal or scleral incision, with the posterior end left in the suprachoroidal space. The device is currently approved for use in Canada and a few European countries, and is under evaluation in a multicenter clinical trial in the United States (clinicaltrials.gov; NCT01282346). The device is manufactured by SOLZ Inc.
Why it is important to do this review
The purpose of this review is to compare the effectiveness and safety of device‐modified trabeculectomy procedures versus standard trabeculectomy in the surgical treatment of glaucoma with or without the use of antifibrotic agents. Device‐modified trabeculectomy is a relatively new procedure; many studies have not had sample sizes sufficiently large to provide reliable evidence to assess the effectiveness and safety of the procedure. It is therefore important to examine the evidence from multiple completed studies. When meta‐analysis of outcomes is appropriate, pooling across studies should increase the power and yield valuable information. Authors of a few recently published articles have addressed the effectiveness and safety of the Ex‐PRESS and Ologen devices (Chen 2014; He 2014; Wang 2013a). However, a comprehensive, rigorous systematic review in this area is warranted.
Objectives
To assess the relative effectiveness, primarily with respect to IOP control and safety, of the use of different devices as adjuncts to trabeculectomy compared with standard trabeculectomy in eyes with glaucoma.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials in this review.
Types of participants
We include trials in which the participants were 18 or more years of age and had been diagnosed with glaucoma. We include trials in which participants had any type of glaucoma (for example, primary open‐angle glaucoma, angle‐closure glaucoma, pigmentary glaucoma, exfoliation glaucoma, and secondary glaucoma such as neovascular glaucoma) except pediatric and congenital glaucoma. There were no restrictions with regard to participant gender, ethnicity, comorbidity, use of adjunctive medication, lens status (phakic, aphakic, or pseudophakic), or number of participants enrolled in an individual trial. We excluded studies in which all participants were less than 18 years of age, as pediatric glaucoma and congenital glaucoma were not the purpose of this review. Surgical interventions for primary congenital glaucoma are evaluated in another Cochrane review (Ghate 2015).
Types of interventions
We include trials that compared, with or without the use of antimetabolites, device‐modified trabeculectomy versus standard trabeculectomy. The devices we intended to assess include the Ex‐PRESS, silicone tube implant, Ologen, amniotic membrane, expanded polytetrafluoroethylene (E‐PTFE), Gelfilm, and SOLX Gold Shunt, which are all popular devices used in glaucoma surgeries and can be deployed under a standard trabeculectomy flap. In future updates of this review, we will include other new devices used in a trabeculectomy. We planned to make the following comparisons:
Trabeculectomy + any of the above devices versus trabeculectomy alone;
Trabeculectomy + any device + antimetabolites (MMC, 5‐FU, or both) versus trabeculectomy + antimetabolites (MMC, 5‐FU, or both);
Trabeculectomy + Ologen device versus trabeculectomy + antimetabolites (MMC, 5‐FU, or both).
There are two pairs of comparisons that we did not plan to include, as these are already covered in other Cochrane reviews:
MMC with 5‐FU on the outcome of standard trabeculectomy (Clarke 2006);
Fornix‐based (the modification) versus traditional limbus‐based trabeculectomy (Al‐Haddad 2015).
In addition, we excluded studies in which trabeculectomy combined with cataract surgery were performed or were permitted, as this was outside the scope of this review.
Types of outcome measures
Primary outcomes
The primary outcomes for comparison of treatments of this review included:
Change in IOP, measured as a mean decrease from baseline (immediate preoperative IOP) at one year after the intervention when IOP had been measured using Goldmann tonometry, TonoPen, or another standard device. When change in IOP was not available, and when baseline IOP distributions were similar in the two surgery groups, we compared postoperative IOP as a surrogate to estimate the effect of device‐modified trabeculectomy.
We planned to report IOP fluctuation, assessed as a standard deviation or range of IOPs before or one year after the procedure, whenever such data were available.
Secondary outcomes
The secondary outcomes for comparison of treatments included:
Mean change in IOP from baseline, measured on day one, during weeks one to 12, at four to six months, seven to 12 months, and thereafter as available throughout follow‐up. When change in IOP was not available, and when baseline IOPs were similar between treatment groups, we used postoperative IOP to estimate the relative effect of device‐modified trabeculectomy in the meta‐analyses.
Best‐corrected visual acuity (BCVA), measured using a Snellen chart or Snellen equivalent and assessed at one year postintervention. We analyzed BCVA data as a continuous outcome in the meta‐analyses.
Visual field change, measured in units of mean deviation or mean defect (the average point‐wise difference between a given test result and the normal age‐matched reference value) at one year postintervention.
Quality of life, measured by the National Eye Institute Visual Function Questionnaire (NEI VFQ) or any other validated instrument at one year postintervention.
Frequency of the following complications: loss of vision of more than two lines, IOP < 5 mm Hg (hypotony), surgical revision within three months and one year after surgery, endophthalmitis or blebitis, retinal detachment, corneal transplant, cataract extraction (among phakic eyes), choroidal hemorrhage, and others as reported in the included studies.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2014 Issue 12), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to December 2014), EMBASE (January 1980 to December 2014), PubMed (1948 to December 2014), Latin American and Caribbean Literature on Health Sciences (LILACS) (January 1982 to December 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not impose any date, language, or publication status restrictions in the electronic search for trials. All electronic databases were all searched on 22 December 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), PubMed (Appendix 4), LILACS (Appendix 5), mRCT (Appendix 6), ClinicalTrials.gov (Appendix 7), and ICTRP (Appendix 8).
Searching other resources
We searched the references listed in reports from included studies to identify additional relevant studies, without restriction regarding language or date of publication.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts of all reports identified through the electronic and manual searches. We first classified all titles and abstracts as 'definitely relevant', 'unsure', or 'definitely not relevant'. We then adjudicated discrepancies through discussion and retrieved full‐text reports for those classified as 'definitely relevant' or 'unsure' by both review authors. By review of full‐text reports, we independently assessed eligibility and classified each study as 'include', 'unsure', or 'exclude'. For studies labeled as 'unsure' at this stage, we requested further information from study investigators. When they did not respond within two weeks, we used the information available. We resolved disagreements by discussion between the two review authors. When resolution was not possible, we consulted a third review author. All publications from studies that met the inclusion criteria then underwent assessment of risk of bias and data extraction. We recorded the reasons for exclusion of studies classified as 'exclude' in the 'Characteristics of excluded studies' tables. For reports not published in English or Chinese, we planned to use Google Translate to screen titles and abstracts and to ask translators to translate or assess reports for full‐text screening. However, all reports relevant to this review were published in English or Chinese languages.
Data extraction and management
Two review authors independently extracted data regarding study design and methods, participant characteristics, and the primary and secondary outcomes, and recorded the information onto paper data collection forms developed in collaboration with Cochrane Eyes and Vision. Whenever there were discrepancies between review authors, we reached consensus by discussion. When we could not reach a consensus, we consulted a third review author who made the final decision. We contacted study investigators to obtain missing information and to elucidate unclear reporting. When they did not respond within two weeks, we used the information available. One review author entered data into Review Manager 5 (RevMan 2014) and a second review author verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed each included study for risks of bias as part of the data extraction process. We based our judgments on the tools for assessing risk of bias set forth in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We judged each study with respect to the following risk of bias domains:
Selection bias (sequence generation and allocation concealment before randomization);
Performance bias (masking of participants and personnel);
Detection bias (masking of outcome assessors);
Attrition bias (incomplete outcome data);
Reporting bias (selective outcome reporting);
Other potential sources of bias (e.g., funding source).
We assessed each trial for each risk of bias criterion as being at high, at low , or at unclear risk of bias (lack of information or uncertainty over the potential for bias).
Measures of treatment effect
Dichotomous outcomes
We analyzed dichotomous outcomes, such as complications, using summary risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous outcomes
We estimated the difference between continuous outcomes, such as mean change (or mean) IOP and BCVA, as the mean difference (MD) with 95% CIs for IOP. We planned to analyze IOP fluctuations, visual field changes, and quality‐of‐life scores as continuous outcomes, but such data were not available.
Unit of analysis issues
The unit of analysis was the eye that had glaucoma surgery. We recorded whether studies used a parallel‐group design or a paired‐eye design, and whether the study used matched‐analysis when a paired‐eye design was used. When both eyes of all or some participants were allocated to the same intervention group, we recorded the information as available and did not estimate or impute intra‐person correlations for individual outcomes.
More than half of the studies (18/33) were parallel‐group designs and included only one eye per participant. Both eyes of some participants were included in another 13 parallel‐group trials; an average of 22% of participants across these 13 trials contributed both eyes to the analysis. Two trials were paired‐eye designs in which each participant had one eye in each intervention group. None of the 13 studies that included more than one eye per participant accounted for intra‐person correlation.
Dealing with missing data
We contacted study investigators to request missing data or to clarify unclearly reported data or information, including but not limited to information about study methods, effect estimates, and standard deviations of effect estimates. When study investigators did not respond within two weeks or after three attempts to contact them, we used the available information. We did not impute data for this review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity among included trials by examining variations in the trial designs and methods, characteristics of the trial participants, variations in interventions, and lengths of follow‐up. We assessed statistical heterogeneity among the reported treatment effect estimates of included trials by examining the overlap of the 95% CIs on estimates from individual trials in forest plots and I² values (Higgins 2003). We considered poor overlap in the 95% CIs and an I² above 50% as indications of substantial statistical heterogeneity.
Assessment of reporting biases
We investigated whether our review was subject to reporting biases. For selective reporting bias, we compared outcomes specified in trial protocols or trial register records with outcomes reported in published full‐text articles. When no trial protocol or trial register record was available, we examined whether outcomes specified in the Methods section were reported in the Results section of the same published report. We did not use funnel plots to examine signs of asymmetry due to the limited number of studies included in the same meta‐analysis.
Data synthesis
We determined whether data synthesis in meta‐analyses was appropriate based on evidence of heterogeneity. When we considered that there was substantial heterogeneity, we presented results in a narrative summary. In the absence of clinical and methodological heterogeneity across studies, and when the I² statistic was less than 50% (indicating no substantial statistical heterogeneity), when the I² statistic was greater than 50% but all studies favored the same intervention, or when the I² statistic was greater than 50% but no study showed a clinical difference between groups, we combined study results using a random‐effects meta‐analysis model. When the number of included studies was small (three or fewer), we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analysis for comparisons of outcomes with use of individual devices and surgeries including adjuvant antimetabolites (e.g. MMC) in one or both the intervention groups. We were not able to carry out the following planned subgroup analyses as the included studies did not stratify participants based on 1) the status of the lens (i.e. eyes that possessed their natural lens (phakic), eyes without the crystalline lens (aphakic, cataract extraction), or eyes with an intraocular lens implanted that replaced the eye's natural lens (pseudophakic)); 2) ethnicity; 3) baseline IOP; or 4) type of glaucoma.
Sensitivity analysis
We were not able to conduct sensitivity analyses to assess the influence on effect estimates of excluding studies at a high risk of reporting bias, as studies at a high risk of reporting bias were grouped with respect to interventions compared. We had also planned to conduct a sensitivity analysis after excluding industry‐funded studies; however, funding information was not always available, so we did not have enough information to conduct such analyses.
Summary of findings
We reported risk ratios and measures of effect in a ‘Summary of findings’ table, showing our judgment of the quality of the evidence by outcome using the GRADE system (Guyatt 2011).
Our prespecified outcome measures were:
Change in IOP, measured as a mean decrease from baseline (immediate preoperative IOP) at one year after the intervention, when IOP had been measured using Goldmann tonometry, TonoPen, or another standard device. When change in IOP was not available, and when baseline IOP distributions were similar in the two surgery groups, we compared postoperative IOPs as a surrogate to estimate the effect of device‐modified trabeculectomy.
We planned to report IOP fluctuation, assessed as a standard deviation or range of IOPs before or one year after the procedure, whenever such data were available.
Mean change in IOP from baseline, measured on day one, during weeks one to 12, at four to six months, seven to 12 months, and thereafter as available throughout follow‐up. When change in IOP was not available, and when baseline IOPs were similar between treatment groups, we used postoperative IOP to estimate the relative effect of device‐modified trabeculectomy in the meta‐analyses.
Best‐corrected visual acuity (BCVA), measured using a Snellen chart or Snellen equivalent and assessed at one year postintervention. We analyzed BCVA data as a continuous outcome in the meta‐analyses.
Visual field change, measured in units of mean deviation or mean defect (the average point‐wise difference between a given test result and the normal age‐matched reference value) at one year postintervention.
Quality of life, measured by the National Eye Institute Visual Function Questionnaire (NEI VFQ) or any other validated instrument at one year postintervention.
Frequency of the following complications: loss of vision of more than two lines, IOP < 5 mm Hg (hypotony), surgical revision within three months and one year after surgery, endophthalmitis or blebitis, retinal detachment, corneal transplant, cataract extraction (among phakic eyes), choroidal hemorrhage, and others as reported in the included studies.
Results
Description of studies
Results of the search
The electronic searches as of 22 December 2014 yielded 4957 titles and abstracts and 533 records from trial registers (Figure 1). After the Trials Search Co‐ordinator removed 1565 duplicate records, there remained 3509 unique titles and abstracts and 416 unique records from trial registers. Two review authors (XW and RK) independently reviewed these 3925 records and through review of titles and abstracts identified a systematic review written in Chinese that included another 14 Chinese articles that we had not identified in our searches (Gao 2013). We therefore screened a total of 3939 records and assessed 3878 as 'not relevant', leaving 61 reports for full‐text review. Two review authors (XW and RK) independently reviewed these 61 full‐text reports, and found 39 reports of 33 unique studies that were eligible for this review. These included 30 studies published as full‐length journal articles and three studies reported only as conference abstracts (Birt 1998; Bruno 2008; De Jong 2005). Additionally, five trials were ongoing and we recorded the study characteristics in the 'Characteristics of ongoing studies' tables. We excluded the remaining 17 reports from 17 studies.
1.
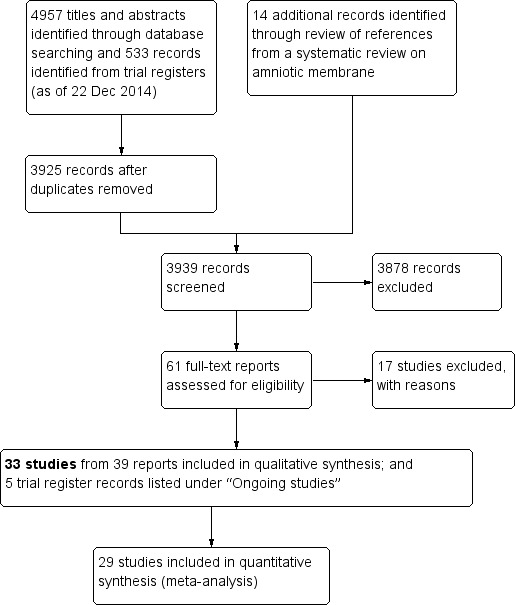
Study flow diagram.
Included studies
The following is a summary of the characteristics of the 33 included studies. Twenty‐one studies were published in English and 12 studies that had assessed amniotic membrane were published in Chinese. Details of each trial are presented in the 'Characteristics of included studies' tables. We also summarized the basic study characteristics in Table 4.
1. Summary of included studies.
| Device | Study ID | Study design | Country | Participant diagnosis | Interventions | Total number of participants randomized | Total number of eyes randomized | Total number of eyes analyzed | Longest follow‐up period (months) |
| Ex‐PRESS | Dahan 2012 | RCT, paired‐eye design | South Africa | POAG | Trab + MMC Trab + MMC + Ex‐PRESS |
15 | 30 | 30 | 12 |
| De Jong 2005 (abstract) | RCT, parallel‐group design | The Netherlands | OAG | Trab + Ex‐PRESS under a scleral flap Trab Trab + Ex‐PRESS under conjunctiva |
109 | 120 | N/A | 6 | |
| De Jong 2009 | RCT, parallel‐group design | The Netherlands | OAG | Trab Trab + Ex‐PRESS |
78 | 78 | 78 | 60 | |
| Netland 2014 | RCT, parallel‐group design | USA | OAG | Trab + MMC Trab + MMC + Ex‐PRESS |
120 | 120 | 114 | 24 | |
| Wagschal 2013 | RCT, parallel‐group design | Canada | OAG, uncontrolled IOP | Trab + MMC Trab + MMC + Ex‐PRESS |
64 | 64 | 60 | 12 | |
| Subtotal for Ex‐PRESS | 386 | 412 | N/A | Range 6 to 60 months | |||||
| Ologen | Cillino 2011 | RCT, parallel‐group design | Italy | POAG, PEXG, uncontrolled IOP | Trab + MMC Trab + Ologen |
40 | 40 | 40 | 24 |
| Maheshwari 2012 | RCT, parallel‐group design | India | OAG | Trab + MMC Trab + Ologen |
40 | 40 | 40 | 12 | |
| Marey 2013 | RCT, parallel‐group design | Egypt | POAG, ACG, PEXG, uveitic glaucoma, uncontrolled pseudophakic glaucoma | Trab + MMC Trab + Ologen |
60 | 60 | 60 | 12 | |
| Mitra 2012 | RCT, parallel‐group design | India | Uncontrolled OAG | Trab + MMC Trab + Ologen |
64 | 64 | N/A | 6 | |
| Papaconstantinou 2010 | RCT, parallel‐group design | Greece | glaucoma | Trab Trab + Ologen |
40 | 40 | 40 | 6 | |
| Rosentreter 2010 | RCT, parallel‐group design | Germany | OAG, uncontrolled IOP | Trab + MMC Trab + Ologen |
20 | 20 | 20 | 12 | |
| Rosentreter 2014 | RCT, parallel‐group design | Germany | OAG, uncontrolled IOP | Trab + MMC Trab + Ologen |
30 | 30 | 30 | 12 | |
| Senthil 2013 | RCT, parallel‐group design | India | Uncontrolled POAG or PACG | Trab + MMC Trab + Ologen |
33 | 39 | 32 | 24 | |
| Subtotal for Ologen | 327 | 333 | N/A | Range 6 to 24 month | |||||
| Amniotic membrane | Bruno 2008 (abstract) | RCT, parallel‐group design | N/A | OAG and failed surgery | Trab + MMC Trab + MMC + AMT |
19 | N/A | N/A | 6 |
| Cai 2012 | RCT, parallel‐group design | China | Refractory glaucoma | Trab Trab + AMT |
40 | 48 | 48 | 12 | |
| Cho 2013 | RCT, parallel‐group design | China | POAG, ACG, uncontrolled IOP | Trab + MMC Trab + AMT |
47 | 52 | 39 | 3 | |
| Eliezer 2006 | RCT, parallel‐group design | Brazil | POAG | Trab Trab + AMT |
32 | 32 | N/A | 12 | |
| Huang 2007 | RCT, parallel‐group design | China | PACG | Trab Trab + AMT |
50 | 63 | N/A | 6 | |
| Ji 2013 | RCT, paired‐eye design | China | Uncontrolled glaucoma | Trab Trab + AMT |
17 | 34 | N/A | 24 | |
| Khairy 2015 | RCT, parallel‐group design | Egypt | OAG | Trab + MMC Trab + AMT |
52 | 52 | 52 | 24 | |
| Li 2010 | RCT, parallel‐group design | China | Refractory glaucoma | Trab + MMC Trab + MMC + AMT |
50 | 62 | N/A | 12 | |
| Liu 2009 | RCT, parallel‐group design | China | Refractory glaucoma | Trab + MMC Trab + MMC + AMT |
35 | 35 | 32 | 12 | |
| Ren 2009 | RCT | China | ACG | Trab Trab + AMT |
30 | 36 | N/A | 12 | |
| Sheha 2008 | RCT, parallel‐group design | Saudi Arabia | Refractory glaucoma | Trab + MMC Trab + MMC + AMT |
37 | 37 | 30 | 12 | |
| Stavrakas 2012 | RCT | Greece | POAG, uncontrolled IOP | Trab Trab + AMT |
50 | 59 | 52 | 24 | |
| Wang 2008 | RCT, parallel‐group design | China | glaucoma | Trab Trab + AMT |
40 | 40 | N/A | 12 | |
| Wang 2009 | RCT | China | Refractory glaucoma | Trab Trab + MMC Trab + MMC + AMT |
38 | 44 | N/A | 12 | |
| Yan 2004 | RCT | China | PACG | Trab Trab + AMT |
52 | 63 | 63 | 6 | |
| Yang 2004 | RCT, parallel‐group design | China | glaucoma | Trab Trab + AMT |
70 | 70 | N/A | 6 | |
| Zhang 2009 | RCT | China | ACG | Trab Trab + AMT |
39 | 52 | N/A | 6 | |
| Zheng 2005 | RCT | China | POAG, PACG | Trab + MMC Trab + AMT |
28 | 48 | N/A | 12 | |
| Subtotal for amniotic membrane | 726 | N/A | N/A | Range 3 to 24 months | |||||
| E‐PTFE | Cillino 2008 | RCT, parallel‐group design | Italy | POAG, PEXG, uncontrolled IOP | Trab Trab + MMC Trab + E‐PTFE Trab + MMC + E‐PTFE |
60 | 60 | 60 | 24 |
| Subtotal for E‐PTFE | 60 | 60 | 60 | 24 | |||||
| Gelfilm | Birt 1998 (abstract) | RCT, parallel‐group design | Not reported | Not reported | Trab Trab + Gelfilm Trab + MMC Trab + MMC + Gelfilm |
43 | N/A | N/A | 12 |
| Subtotal for Gelfilm | 43 | N/A | N/A | 12 | |||||
| Total for all included studies | 1542 | N/A | N/A | Range 3 months to 5 years | |||||
ACG: angle‐closure glaucoma AMT: amniotic membrane E‐PTFE: expanded polytetrafluoroethylene IOP: intraocular pressure MMC: mitomycin C N/A: not applicable OAG: open‐angle glaucoma PACG: primary angle‐closure glaucoma PEXG: pseudoexfoliation glaucoma POAG: primary open‐angle glaucoma RCT: randomized controlled trial
Types of participants
The 33 studies included 1542 participants and had follow‐up periods ranging from three months to five years after surgery. The studies represented adults of both genders. One study did not specify the participants' diagnoses (Birt 1998), but all other studies included participants with different types of glaucoma, including primary open‐angle glaucoma, angle‐closure glaucoma, uveitic glaucoma, pseudophakic glaucoma, pseudoexfoliation glaucoma, refractory glaucoma, and uncontrolled intraocular pressure (IOP). None of the studies stratified participants by type of glaucoma, race, or lens type. These studies were conducted in North America, South America, Europe, Asia, and the Middle East.
Types of interventions
The 33 studies, reported in 36 full‐text articles and three conference abstracts, assessed five different devices used with standard trabeculectomy: Ex‐PRESS, Ologen, amniotic membrane, expanded polytetrafluoroethylene (E‐PTFE), and Gelfilm. No trials assessed silicon tube implant or SOLX Gold Shunt.
Five studies assessed the use of trabeculectomy with Ex‐PRESS compared with trabeculectomy alone (Dahan 2012; De Jong 2005; De Jong 2009; Netland 2014; Wagschal 2013). Five studies enrolled a total of 412 eyes of 386 participants. Four of the five studies were two‐arm studies that compared trabeculectomy alone versus trabulectomy and Ex‐PRESS, with mitomycin C (MMC) applied to both groups. One study was a three‐arm trial: (1) Ex‐PRESS implanted under scleral flap with standard trabeculectomy, (2) Ex‐PRESS implanted under conjunctiva with standard trabeculectomy, and (3) trabeculectomy alone (De Jong 2005).
Eight studies assessed the use of Ologen (Cillino 2011; Maheshwari 2012; Marey 2013; Mitra 2012; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). They enrolled a total of 333 eyes of 327 participants. While seven of the eight studies compared trabeculectomy + MMC with trabeculectomy + Ologen implant, one study compared trabeculectomy versus trabeculectomy + Ologen implant without any use of MMC or 5‐fluorouracil (5‐FU) (Papaconstantinou 2010).
Eighteen studies, reported in 17 full‐text articles and one conference abstract, assessed the use of amniotic membrane compared with trabeculectomy, with or without the use of MMC (Bruno 2008; Cai 2012; Cho 2013; Eliezer 2006; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Ren 2009; Sheha 2008; Stavrakas 2012; Wang 2008; Wang 2009; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005). Findings from 13 of these studies were published in Chinese and accounted for 647 eyes of 536 participants, more than 70% of all 726 participants in the 18 studies.
One study assessed the use of E‐PTFE and compared four intervention groups: 1) trabeculectomy; 2) trabeculectomy + E‐PTFE; 3) trabeculectomy + MMC; and 4) trabeculectomy + E‐PTFE + MMC (Cillino 2008). The study included 60 eyes of 60 glaucoma participants who had primary open‐angle glaucoma, pseudoexfoliation glaucoma or uncontrolled IOP; 15 eyes of 15 participants were assigned to each of the four surgery groups. The study was conducted in Italy and all participants were followed for 24 months.
One study, which was reported only in a conference abstract, evaluated Gelfilm and MMC using a two‐by‐two factorial design, and compared outcomes among four intervention groups (Birt 1998). The study included 43 participants (number of eyes not specified): 14 participants in the trabeculectomy group, 11 in the trabeculectomy + Gelfilm group, seven in the trabeculectomy + MMC group, and 11 in the trabeculectomy + Gelfilm + MMC group. All participants completed one year of follow‐up.
Types of outcomes
All studies considered IOP control as their main outcome; however, the method in which IOP outcomes were reported differed among studies. None of the studies reported the data as change of IOP from baseline (although two studies reported individual participant data so we were able to calculate the mean change in IOP from baseline). All studies reported postoperative IOP at certain time points, and one study did not report any quantitative data but provided a descriptive summary only.
Thirteen studies reported visual acuity outcomes at different time points; one study reported visual field outcomes; and all studies reported postoperative complications. None of the studies reported IOP fluctuation or quality‐of‐life outcomes.
Funding sources
Of the 33 studies, the funding source of seven studies had been reported: six studies were supported by industry (Dahan 2012; De Jong 2009; Netland 2014; Rosentreter 2010; Rosentreter 2014; Wagschal 2013) and one study reported receiving no funding (Senthil 2013). Reports from the other 26 studies did not disclose information about sources of funding.
Excluded studies
We excluded 17 studies and listed the reasons for exclusion in the 'Characteristics of excluded studies' tables. We excluded nine studies due to insufficient information for confirmation of randomized controlled trial (RCT) status, three studies due to their inclusion of children under 18 years of age and failure to report outcomes for adult participants separately, two studies for not being RCTs, one study due to insufficient follow‐up time, one study with no device included in the comparison group, and one study was only available as a registered trial in clinicaltrials.gov with no indication of having enrolled participants.
Risk of bias in included studies
Figure 2 shows a summary of the 'Risk of bias in included studies' assessments. All but one study had a high risk of detection bias. Most studies either had missing or inadequate information in study reports to assess the risk of selection bias. A description for each domain is summarized below.
2.
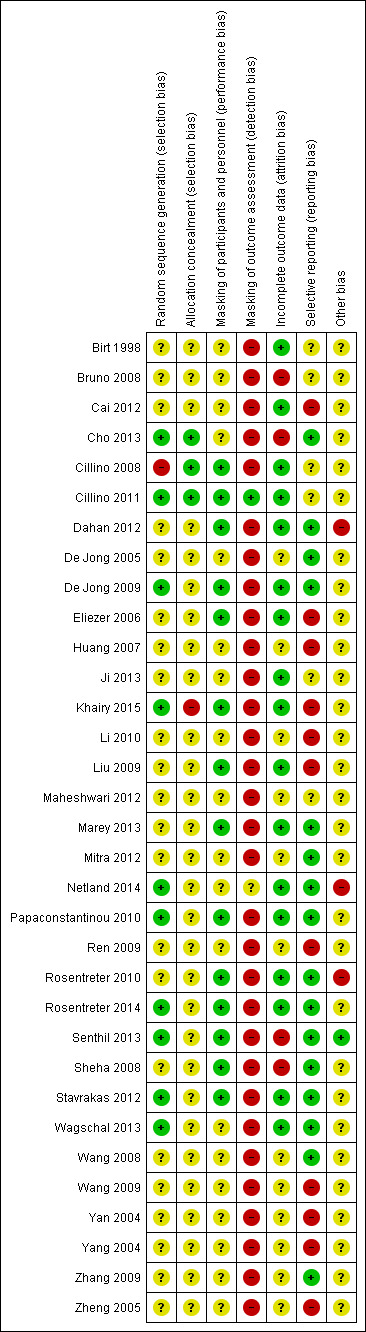
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 33 studies, 10 specified adequate methods of randomization and we assessed them as being at low risk of bias: three out of five studies for Ex‐PRESS (De Jong 2009; Netland 2014; Wagschal 2013), four out of eight studies for Ologen (Cillino 2011; Papaconstantinou 2010; Rosentreter 2014; Senthil 2013), three out of 18 studies for amniotic membrane (Cho 2013; Khairy 2015; Stavrakas 2012). The one study that evaluated E‐PTFE did not use a completely random allocation method (medical chart number), so we judged it to be at high risk of bias (Cillino 2008). All other 22 studies did not specify methods for random sequence generation, so we judged them to be at unclear risk of bias.
Of the 33 studies, only three performed proper allocation concealment and we judged them to be at low risk of bias: one out of eight studies for Ologen, one out of 18 studies for amniotic membrane, and one study for E‐PTFE (Cho 2013; Cillino 2008; Cillino 2011). We judged Khairy 2015 to be at high risk of bias as the investigators labeled the envelope used to allocate the treatment. The other 29 studies did not specify the method for allocation concealment, so we judged them to be at unclear risk of bias.
Masking (performance bias and detection bias)
Authors of reports from 14 of the 33 studies noted masking of participants: two of five studies for Ex‐PRESS (Dahan 2012; De Jong 2009), six of eight studies for Ologen (Cillino 2011; Marey 2013; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013), five of 18 studies for amniotic membrane (Eliezer 2006; Khairy 2015; Liu 2009; Sheha 2008; Stavrakas 2012), and one study for E‐PTFE (Cillino 2008). The remaining 19 studies did not report whether participants were masked and we judged them to be at unclear risk of performance bias. Because masking of surgeons is logistically difficult and because trabeculectomy is a standardized procedure (all 14 studies described the surgical procedures in detail), we did not consider the lack of masking of surgeons to be an important modifiable source of bias.
Investigators of one of the 33 studies reported masking of outcome assessors (Cillino 2011) and one study used a special protocol to minimize bias (Netland 2014); we judged these two studies to be at low and unclear risk of detection bias, respectively. None of the other 31 studies specified masking of outcome assessors. Due to the easy detection of devices when examining the eye, unmasked outcome assessors could tend to anticipate and thus report favorable change in IOP among participants with the implant or alternatively, among participants who received the surgery the outcome assessor preferred; we therefore judged these studies to be at high risk of detection bias.
Incomplete outcome data
Of the 33 studies, investigators of 17 studies reported few or no losses to follow‐up, resulting in our assessment of them to be at low risk of attrition bias: four of six trials of Ex‐PRESS (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013), five of eight studies for Ologen (Cillino 2011; Marey 2013; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014), six of 18 studies for amniotic membrane (Cai 2012; Eliezer 2006; Ji 2013; Khairy 2015; Liu 2009; Stavrakas 2012), one study for E‐PTFE (Cillino 2008), and one study for Gelfilm (Birt 1998). Four studies had a relatively large percentage of losses to follow‐up (> 10%) and we assessed the risk of attrition bias to be high for these studies (Bruno 2008; Cho 2013; Senthil 2013; Sheha 2008). We assessed the remaining 12 studies at unclear risk of attrition bias as they did not report the number of losses to follow‐up; nine of the 12 studies were amniotic membrane studies.
Selective reporting
We judged 16 of 33 studies as being at low risk of reporting bias as they had 1) clinical trial registry records and reported all outcomes listed in the registry (Dahan 2012; Netland 2014; Rosentreter 2010; Rosentreter 2014; Wagschal 2013), or 2) reported all outcome measures defined in the Methods section of the full‐text reports (Cho 2013; De Jong 2005; De Jong 2009; Marey 2013; Mitra 2012; Papaconstantinou 2010; Senthil 2013; Sheha 2008; Stavrakas 2012; Wang 2008; Zhang 2009). We judged six studies to have unclear risk of bias because some outcomes that were measured were not reported in primary result papers, but we presume they will be reported in future publications from the trials (Bruno 2008; Cillino 2008; Cillino 2011; Ji 2013; Maheshwari 2012). We rated the remaining 11 studies at high risk of bias, as the outcome measures were not defined in the Methods section of the full text and no protocol or trial registration was publicly available.
Studies judged to be at low risk of reporting bias by devices: all studies for Ex‐PRESS, six of eight studies for Ologen, and five of 18 studies for amniotic membrane (Cho 2013; Sheha 2008; Stavrakas 2012; Wang 2008; Zhang 2009). Studies judged to be at unclear risk of reporting bias by devices: one of eight studies for Ologen, two of 18 studies for amniotic membrane, the only study that evaluated E‐PTFE (Bruno 2008; Cillino 2008; Cillino 2011; Ji 2013; Maheshwari 2012). The study for Gelfilm was at a high risk of reporting bias (Birt 1998), as were 11 of 18 studies for amniotic membrane (Cai 2012; Eliezer 2006; Huang 2007; Khairy 2015; Li 2010; Liu 2009; Ren 2009; Wang 2009; Yan 2004; Yang 2004; Zheng 2005).
Other potential sources of bias
We judged one out of 33 included studies to be at low risk of having other potential sources of bias. We judged three studies to be at high risk because one did not recruit an adequate number of participants that met the prespecified power requirements of the study (Rosentreter 2010), and two studies received funding from the manufacturer of the device (Dahan 2012; Netland 2014). We judged the remaining studies to be at unclear risk of bias, as methodological details were reported insufficiently to render a judgment of low or high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Summary of findings for Ex‐PRESS.
| Ex‐PRESS implanted during trabeculectomy compared with trabeculectomy alone for people with open‐angle glaucoma | ||||||
|
Patient or population: people with open‐angle glaucoma Settings: ophthalmic surgery Intervention: Ex‐PRESS implanted during trabeculectomy Comparison: trabeculectomy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Eyes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| trabeculectomy alone | Ex‐PRESS | |||||
| Postoperative mean IOP at 1 year | The mean IOP in the trabeculectomy‐alone groups was 13.9 mm Hg, ranged from 11.6 to 16.4 mm Hg |
The mean IOP in the Ex‐PRESS groups was 1.58 lower (2.74 lower to 0.42 lower) | ‐ | 165 (3 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
| Postoperative mean logMAR BCVA at 1 year | The mean logMAR BCVA in the trabeculectomy‐alone groups was 0.59, ranged from 0.43 to 0.80 | The mean logMAR BCVA in the Ex‐PRESS groups was 0.15 lower (0.40 lower to 0.10 higher) | ‐ | 90 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Complication as defined in protocol‐ Hypotony Follow‐up: ranged from 1 to 5 years |
565 per 1000 |
520 per 1000 (356 to 751) |
RR 0.92 (0.63 to 1.33) | 94 (2 studies) |
⊕⊝⊝⊝ very low1,2,3 | ‐ |
| Other complications reported by included studies | ||||||
|
Shallow/flat anterior chamber Follow‐up: ranged from 1 to 5 years |
150 per 1000 | 108 per 1000 (60 to 198) | RR 0.72 (0.40 to 1.32) | 294 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Bleb leakage Follow‐up: ranged from 1 to 5 years |
48 per 1000 | 60 per 1000 (24 to 154) | RR 1.26 (0.50 to 3.20) | 294 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Hyphema Follow‐up: ranged from 1 to 5 years |
82 per 1000 | 27 per 1000 (10 to 77) | RR 0.33 (0.12 to 0.94) | 294 (4 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
|
Cataract surgery Follow‐up: ranged from 1 to 5 years |
152 per 1000 | 49 per 1000 (21 to 112) | RR 0.32 (0.14 to 0.74) | 264 (3 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; IOP: intraocular pressure; logMAR: logarithm of the minimum angle of resolution; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for limitations in the design and implementation of available studies suggesting high likelihood of bias (‐1); high likelihood that studies did not mask outcome assessors. 2Downgraded for high probability of reporting bias (‐1). 3Downgraded for imprecision (‐1): wide confidence intervals.
Summary of findings 2. Summary of findings for Ologen.
| Ologen implanted during trabeculectomy compared with trabeculectomy alone for people with glaucoma | ||||||
|
Patient or population: people with glaucoma, including open‐angle, angle‐closure, and uncontrolled IOP Settings: ophthalmic surgery Intervention: Ologen implanted during trabeculectomy Comparison: trabeculectomy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Eyes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| trabeculectomy alone | Ologen | |||||
| Postoperative mean IOP at 1 year | The mean IOP in the trabeculectomy‐alone groups was 15.2 mm Hg, ranged from 11 to 19.3 mm Hg. |
The mean IOP in the Ologen groups was 1.40 higher (0.57 lower to 3.38 higher) | ‐ | 177 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | Analyzed using the generic inverse method |
| Postoperative mean logMAR BCVA at 1 year | See comment. | See comment. | ‐ | ‐ | ‐ |
Senthil 2013 reported BCVA for 32 eyes at 6 weeks post‐surgery: MD ‐0.24 logMAR, 95% CI ‐0.58 to 0.10 |
|
Complication as defined in protocol‐ Hypotony Follow‐up: ranged from 6 to 24 months |
223 per 1000 | 167 per 1000 (105 to 265) | RR 0.75 (0.47 to 1.19) | 233 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
| Other complications reported by included studies | ||||||
|
Shallow anterior chamber Follow‐up: ranged from 6 to 24 months |
90 per 1000 | 71 per 1000 (29 to 174) | RR 0.79 (0.32 to 1.93) | 213 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Bleb leakage Follow‐up: ranged from 6 to 24 months |
138 per 1000 | 117 per 1000 (46 to 304) | RR 0.85 (0.33 to 2.20) | 129 (4 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Hyphema Follow‐up: ranged from 6 to 24 months |
78 per 1000 | 114 per 1000 (40 to 327) | RR 1.46 (0.51 to 4.19) | 229 (6 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Surgical revision Follow‐up: ranged from 6 to 24 months |
40 per 1000 |
68 per 1000 (15 to 305) |
RR 1.70 (0.38 to 7.63) | 150 (4 studies) |
⊕⊝⊝⊝ very low1,2,3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; IOP: intraocular pressure; logMAR: logarithm of the minimum angle of resolution; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for limitations in the design and implementation of available studies suggesting high likelihood of bias (‐1); high likelihood that studies did not mask outcome assessors. 2Downgraded for high probability of reporting bias (‐1). 3Downgraded for imprecision (‐1): wide confidence intervals.
Summary of findings 3. Summary of findings for amniotic membrane.
| Amniotic membrane implanted during trabeculectomy compared with trabeculectomy alone for people with glaucoma | ||||||
|
Patient or population: people with glaucoma, including open‐angle, angle‐closure, uncontrolled IOP, and refractive glaucoma Settings: ophthalmic surgery Intervention: Amniotic membrane implanted during trabeculectomy Comparison: trabeculectomy alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Eyes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| trabeculectomy alone | amniotic membrane | |||||
| Postoperative mean IOP at 1 year | The mean IOP in the trabeculectomy alone groups was 17.6 mm Hg, ranged from 15.1 to 19.8 mm Hg. |
The mean IOP in the Ologen groups was 3.92lower (5.41 lower to 2.42 lower) | ‐ | 356 (9 studies) | ⊕⊕⊝⊝ low1,2 | |
| Postoperative mean logMAR BCVA at 1 year | See comment. | See comment. | Only 1 study reported this outcome; the amniotic membrane group had statistically significantly better BCVA than the trabeculectomy group, but no data for between‐group difference were provided | |||
|
Complications ‐ Hypotony Follow‐up: ranged from 3 to 24 months |
206 per 1000 | 82 per 1000 (35 to 193) | RR 0.40 (0.17 to 0.94) | 205 (5 studies) | ⊕⊕⊝⊝ low1,3 | ‐ |
| Other complications reported by included studies | ||||||
|
Complications ‐ Shallow anterior chamber Follow‐up: ranged from 3 to 24 months |
240 per 1000 | 113 per 1000 (72 to 175) | RR 0.47 (0.30 to 0.73) | 632 (13 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
|
Complications ‐ Bleb leakage Follow‐up: ranged from 3 to 24 months |
327 per 1000 | 91 per 1000 (32 to 258) | RR 0.28 (0.10 to 0.79) | 98 (2 studies) | ⊕⊕⊝⊝ low1,2 | ‐ |
|
Complications ‐ Hyphema Follow‐up: ranged from 3 to 24 months |
91 per 1000 | 39 per 1000 (12 to 122) | RR 0.43 (0.14 to 1.34) | 235 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 | ‐ |
|
Complications ‐ Surgical revision Follow‐up: ranged from 3 to 24 months |
See comment. | See comment. | ‐ | ‐ | None of the studies reported this outcome. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best corrected visual acuity; CI: Confidence interval; IOP: intraocular pressure; logMAR: Logarithm of the Minimum Angle of Resolution; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for limitations in the design and implementation of available studies suggesting high likelihood of bias (‐1); high likelihood that studies did not mask outcome assessors. 2Downgraded for high probability of reporting bias (‐1). 3Downgraded for imprecision (‐1): wide confidence intervals.
Trabeculectomy + Ex‐PRESS versus trabeculectomy
Five studies assessed the use of Ex‐PRESS (Dahan 2012; De Jong 2005; De Jong 2009; Netland 2014; Wagschal 2013). Four of five studies reported a sample size calculation: Dahan 2012 had a power of 96% to detect a 2.0 mm Hg intraocular pressure (IOP) difference between groups; De Jong 2009 had a power of 80% to detect a 32% between‐group difference in IOP; and both Netland 2014 and Wagschal 2013 had power of 80% to detect a 2.0 mm Hg IOP difference between groups. De Jong 2005 did not report a power or sample size calculation. The overall risk of bias for these studies was high or unclear, especially with respect to detection bias and potential conflicts of interest. Four of five studies reported receiving some funding or resource support from the device manufacturer (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013) and one study did not reported information on potential conflicts of interest (De Jong 2005).
Intraocular pressure (IOP)
Four studies reported postoperative IOP at follow‐up time points (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). Dahan 2012 reported IOP data at last follow‐up time point and presented a figure with IOP reduction over time. The study encompassed 30 eyes of 15 participants at one year, 20 eyes of 10 participants at two years, and 14 eyes of seven participants at 30 months (last follow‐up). Upon our request, the study investigators shared their original data, so we were able to calculate the mean change in IOP from baseline to one‐year follow‐up and postoperative IOP at various follow‐up time points (months 6, 12, and 24).
At one year, only Dahan 2012 provided data on the mean change in mean IOP for 30 eyes of 15 participants from baseline to one‐year follow‐up. We calculated the mean change in IOP from baseline to one‐year follow‐up. It is uncertain whether use of Ex‐PRESS (MD ‐14.07, SD 8.50) leads to IOP reduction at one year (MD ‐14.73, SD 12.60) because the quality of the evidence is very low (MD 0.67, 95% CI ‐7.52 to 8.86).
At one year, three studies comprising 165 eyes reported mean IOP by treatment group (Dahan 2012; De Jong 2009; Wagschal 2013). We rated the low‐quality evidence at high risk of detection and publication bias. We found that the use of Ex‐PRESS may lead to a slightly improved IOP reduction at one year (Analysis 1.1; Figure 3; MD ‐1.58 mm Hg, 95% CI ‐2.74 to ‐0.42; I² = 0%). A fourth study did not provide quantitative data, but reported that there was no between‐group difference in IOP reduction at one year (Netland 2014).
1.1. Analysis.
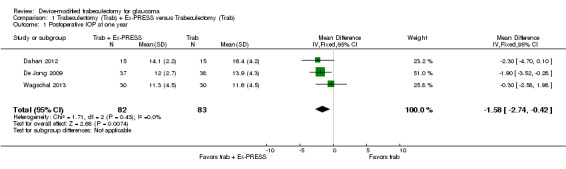
Comparison 1 Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab), Outcome 1 Postoperative IOP at one year.
3.
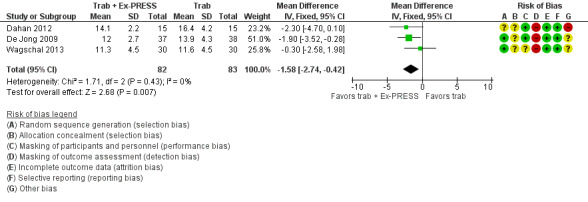
Forest plot of comparison: 2 Trabeculectomy + Ex‐PRESS versus Trabeculectomy, outcome: Postoperative IOP at one year.
At six months, three studies comprising 205 eyes reported mean IOP (Dahan 2012; Netland 2014; Wagschal 2013). It is unclear whether the use of Ex‐PRESS leads to IOP reduction at six months because the quality of the evidence is very low (Analysis 1.2; MD 0.18, 95% CI ‐0.90 to 1.26). The I² was 75%, indicating inconsistency in the effect of treatments among studies.
1.2. Analysis.
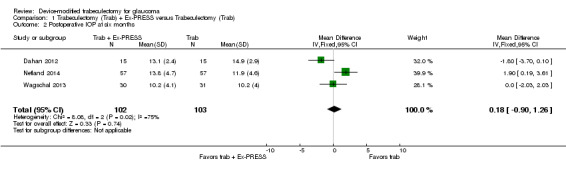
Comparison 1 Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab), Outcome 2 Postoperative IOP at six months.
At two years, three studies comprising 212 eyes reported IOP outcomes (Dahan 2012; De Jong 2009; Netland 2014). The low‐quality evidence shows Ex‐PRESS may slightly improve IOP reduction at two years (Analysis 1.3; MD ‐1.45 mm Hg, 95% CI ‐2.52 to ‐0.37).
1.3. Analysis.
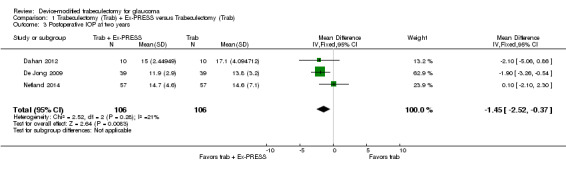
Comparison 1 Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab), Outcome 3 Postoperative IOP at two years.
Best‐corrected visual acuity (BCVA) at one year postintervention
We combined Wagschal 2013 and Dahan 2012 in a meta‐analysis for logMAR BCVA at one year. We judged the very low‐quality evidence to be at high risk of detection and publication bias. It is unclear whether Ex‐PRESS prevents loss in BCVA because the quality of the evidence is very low (Analysis 1.4; MD ‐0.15, 95% CI ‐0.40 to 0.10; 90 eyes).
1.4. Analysis.
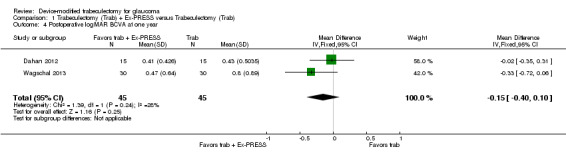
Comparison 1 Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab), Outcome 4 Postoperative logMAR BCVA at one year.
Wagschal 2013 reported logMAR BCVA at one year, but Dahan 2012 did not publish quantitative data for this outcome. The authors of Dahan 2012 provided us with original data from which we calculated the postoperative mean logMAR BCVA to be 0.41 ± 0.11 (mean ± SE) for the Ex‐PRESS group and 0.43 ± 1.33 (mean ± SE) for the trabeculectomy group at one year.
Although De Jong 2009 also assessed visual acuity preoperatively and at each follow‐up visit, quantitative data were not reported. They reported that visual acuity remained equivalent in the majority of participants, with no significant difference between the groups at one year. Netland 2014 did not report on this outcome.
IOP fluctuation, visual field, and quality of life
No study reported these outcomes.
Complications
All four full‐text studies reported complications in a total of 294 eyes during their respective follow‐up visits. The four studies had different lengths of follow‐up periods, ranging from one to two years. We considered the reported postoperative complications as occurring within the first year after surgery. These results should be interpreted with caution, due to possible differences in length of follow‐up. We conducted a meta‐analysis using the proportion of participants with each complication in each group (Analysis 1.5). De Jong 2005 did not report any complications.
1.5. Analysis.
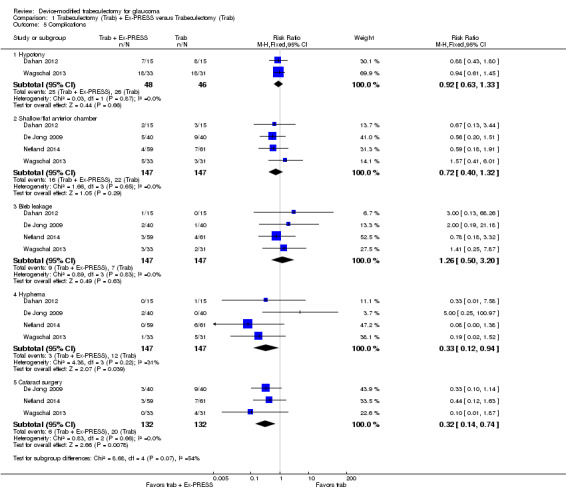
Comparison 1 Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab), Outcome 5 Complications.
Loss of vision of more than two lines
Wagschal 2013 reported loss of vision more than two lines of BCVA within and after the first six months follow‐up. Within the first six months follow‐up period, there were seven of 30 eyes in the trabeculectomy group and 11 of 31 eyes in the Ex‐PRESS group with loss of vision more than two lines of BCVA (RR 0.66, 95% CI 0.29 to 1.47).
IOP < 5 mm Hg (hypotony)
Two studies comprising 94 eyes reported this complication (Dahan 2012; Wagschal 2013). We judged the very low‐quality evidence to be at high risk of detection and publication bias. It is unclear whether Ex‐PRESS prevents hypotony because the quality of the evidence is very low (Analysis 1.5.1; RR 0.92, 95% CI 0.63 to 1.33).
None of the included studies reported these complications: Surgical revision within three months and one year after surgery, endophthalmitis or blebitis, retinal detachment, corneal transplant, cataract extraction (among phakic eyes), choroidal hemorrhage.
Others as reported from the included studies
Other complications not included in the protocol were: shallow/flat anterior chamber, hyphema, and needling.
Shallow/flat anterior chamber
Four studies comprising 294 eyes reported this complication (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). We rated the very low‐quality evidence at high risk of detection and publication bias. It is unclear whether Ex‐PRESS prevents shallow/flat anterior chamber because the quality of the evidence is very low (Analysis 1.5.2; RR 0.72, 95% CI 0.40 to 1.32).
Bleb leakage
Four studies comprising 294 eyes reported this complication (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). It is unclear whether Ex‐PRESS prevents bleb leakage because the quality of the evidence is very low (Analysis 1.5.3; RR 1.26, 95% CI 0.50 to 3.20).
Hyphema
Four studies comprising 294 eyes reported this complication (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). The low‐quality evidence shows that Ex‐PRESS may prevent hyphema (Analysis 1.5.4; RR 0.33, 95% CI 0.12 to 0.94).
Cataract surgery
Three studies comprising 264 eyes reported this complication (De Jong 2009; Netland 2014; Wagschal 2013). The low‐quality evidence shows that Ex‐PRESS may prevent cataract surgery (Analysis 1.5.5; RR 0.32, 95% CI 0.14 to 0.74).
Needling
Only one study covering 80 eyes reported this complication (De Jong 2009). There were nine of 40 eyes in the trabeculectomy group and three of 40 eyes in the Ex‐PRESS group that required needling. The low‐quality evidence shows that the use of Ex‐PRESS may prevent needling (RR 0.33, 95% CI 0.10 to 1.14).
Trabeculectomy + Ologen versus trabeculectomy
Eight studies assessed the use of Ologen during trabeculectomy (Cillino 2011; Maheshwari 2012; Marey 2013; Mitra 2012; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). Only three studies reported sample size calculations. Cillino 2011 reported a power of 90% to detect a 3 mm Hg IOP difference between groups; Rosentreter 2014 reported a power of 50% to detect a 2.73 mm Hg IOP difference between groups and a power of 95% to detect a difference of 5.03 mm Hg in IOP. The sample size calculation originally performed for Rosentreter 2010 targeted 40 participants, but the desired power was not reported. Only 20 participants enrolled. We judged the very low‐quality evidence on IOP and BCVA outcomes at all follow‐up time points to be at high risk of detection and publication bias.
Intraocular pressure (IOP)
Overall, the effect of Ologen on IOP compared to trabeculectomy alone is uncertain (Analysis 2.1). None of the eight studies reported mean change in IOP from baseline. However, two of eight studies reported individual participant data, so we were able to calculate the mean change in IOP from baseline to all follow‐up time points between the trabeculectomy‐alone group and the Ologen group (Rosentreter 2010; Rosentreter 2014). It is uncertain whether Ologen led to IOP reduction because the quality of the evidence is very low at every follow‐up time point (Figure 4; Analysis 2.1.1; MD ‐0.32 mm Hg, 95% CI ‐5.88 to 5.24). Six of eight studies reported postoperative IOP at certain time points instead, and these six studies had comparable baseline IOP values between groups, except for Maheshwari 2012. For the six studies without reporting of change of IOPs, we analyzed the postoperative IOP at certain time points between groups as a surrogate for mean difference in change of IOP from baseline between groups.
2.1. Analysis.
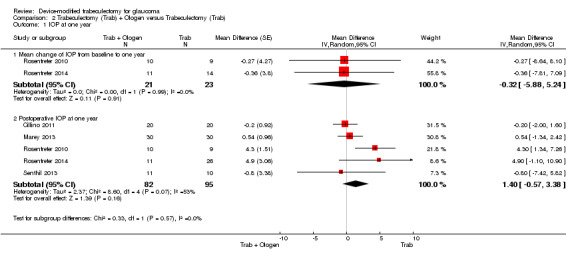
Comparison 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), Outcome 1 IOP at one year.
4.
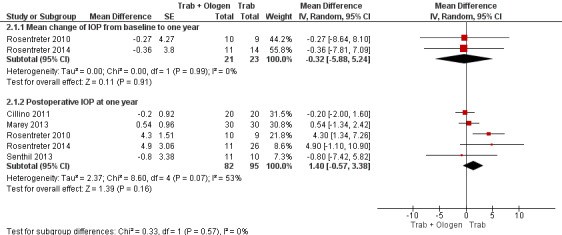
Forest plot of comparison: 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), outcome: 2.1 IOP at one year.
At one year, six studies comprising 217 eyes reported IOP outcomes (Cillino 2011; Maheshwari 2012; Marey 2013; Rosentreter 2010; Rosentreter 2014; Senthil 2013). We excluded Maheshwari 2012 from the pooled analysis as the baseline IOP in Maheshwari 2012 differed between the two groups. At one year, they reported a mean IOP of 15.6 mm Hg, and a 43% reduction in IOP from baseline in the Ologen group, and a mean IOP of 10.5 mm Hg, and a 50% reduction in IOP in the trabeculectomy‐alone group. However, the two groups were not comparable as the baseline IOP in the Ologen group was about 30% greater than in the trabeculectomy‐alone group. It is unclear whether Ologen improves IOP reduction at one year because the quality of the evidence is very low (Analysis 2.1; MD 1.40 mm Hg, 95% CI ‐0.57 to 3.38; 5 studies). The I² was not substantial (I² = 0%) and no study showed a clinical difference between groups, so we combined the data.
At the first day after surgery, five studies comprising 162 eyes reported IOP outcomes (Cillino 2011; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether Ologen improves IOP reduction because the quality of the evidence is very low (Analysis 2.2; MD 0.51, 95% CI ‐1.95 to 2.97). Although there was substantial statistical heterogeneity (I² = 55%), mostly due to Cillino 2011, we combined the data as there was a high degree of overlap among the confidence intervals of studies.
2.2. Analysis.
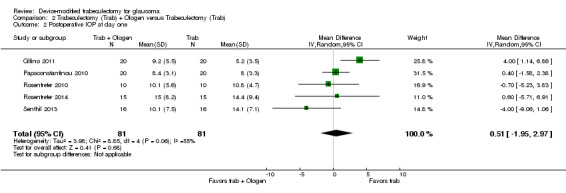
Comparison 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), Outcome 2 Postoperative IOP at day one.
At six months, seven studies comprising 282 eyes reported on IOP outcomes (Cillino 2011; Marey 2013; Mitra 2012; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether Ologen improves IOP reduction because the quality of the evidence is very low (Analysis 2.3.1; MD ‐1.24 mm Hg, 95% CI ‐6.23 to 3.76; I² = 0%; Rosentreter 2010; Rosentreter 2014). Similarly, at six months follow‐up, we found that it is unclear whether Ologen improves IOP reduction because the quality of the evidence is very low (Analysis 2.3.2; MD 0.43, 95% CI ‐0.97 to 1.84). Although there was substantial heterogeneity (I² = 53%), no study showed a clinical difference between groups, so we combined the data.
2.3. Analysis.
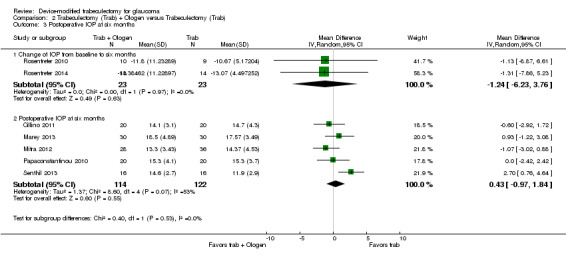
Comparison 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), Outcome 3 Postoperative IOP at six months.
At two years, the longest length of follow‐up in two studies comprising 55 eyes (Cillino 2011; Senthil 2013), it is unclear whether Ologen improves IOP reduction because the quality of the evidence is very low (Analysis 2.4; MD 0.20 mm Hg, 95% CI ‐1.29 to 1.69).
2.4. Analysis.
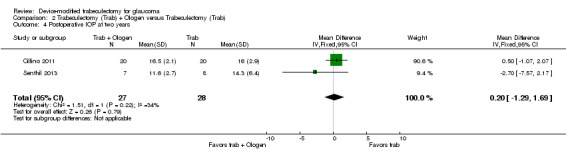
Comparison 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), Outcome 4 Postoperative IOP at two years.
Best‐corrected visual acuity (BCVA)
Two studies comprising 51 eyes reported BCVA data at different time points. None of the studies reported BCVA at one year after surgery.
Senthil 2013 reported BCVA for 32 eyes at six weeks post‐surgery. We judged the very low‐quality evidence to be at high risk of detection and attrition bias. The BCVA for the Ologen group was 0.44 ± 0.66 (mean ± SD) and for the trabeculectomy‐alone group was 0.20 ± 0.24 (mean ± SD), and showed it is uncertain whether use of Ologen may prevent loss in BCVA (MD ‐0.24 logMAR, 95% CI ‐0.58 to 0.10). The estimated mean difference is equivalent to more than two lines on a logMAR chart; however, the small number of participants yielded a wide confidence interval.
Rosentreter 2010 reported that in all 19 eyes observed, the visual acuity remained stable over the one‐year follow‐up (no data provided).
Visual field
Only one study reported on this outcome (Rosentreter 2010). It reported that the visual field remained stable over one‐year follow‐up among all 19 participants but did not provide any data.
IOP fluctuation and quality of life
No study reported these outcomes.
Complications
All eight studies reported complications in 333 eyes of 327 participants during their respective follow‐up visits, with the exception of Maheshwari 2012, who reported no complications. Although these studies had different lengths of follow‐up, ranging from six months to two years, we assumed that most postoperative complications occurred within the first six months after the surgery, so we combined the data in the meta‐analyses. These results should be interpreted with caution, due to our assumption that the complications all occurred within the six‐month window. Other complications not included in the protocol were: bleb leakage, hyphema, choroidal detachment, shallow anterior chamber, Tenon's cysts, anterior chamber reaction, positive Seidel test, needling, and flat anterior chamber. We judged the very low‐quality evidence to be at high risk of detection and publication bias, and are therefore uncertain if there is a difference between the two groups for each complication (Analysis 2.5).
2.5. Analysis.
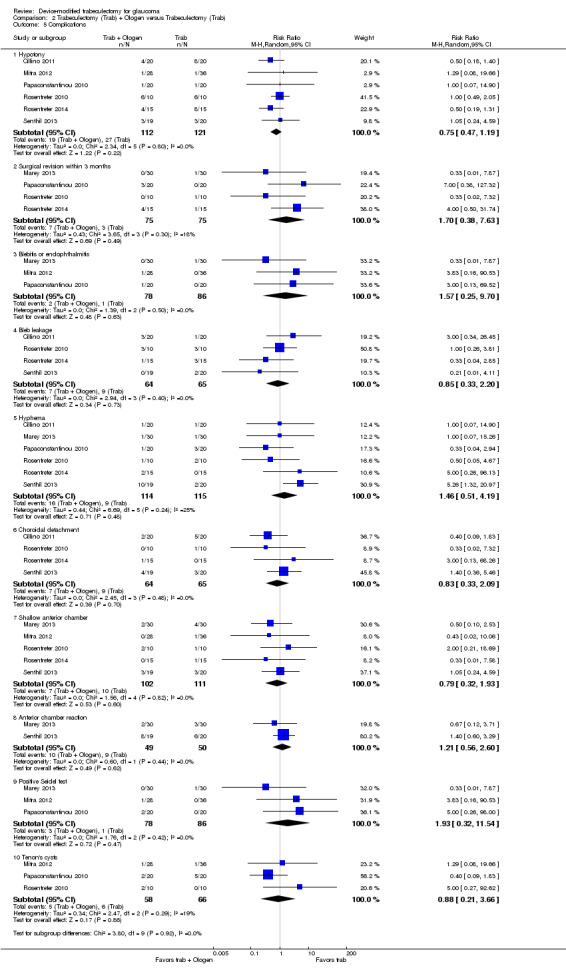
Comparison 2 Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab), Outcome 5 Complications.
Loss of vision of more than two lines
None of the included studies reported this complication.
IOP < 5 mm Hg (hypotony)
Six studies including 233 eyes reported this complication (Cillino 2011; Mitra 2012; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether use of Ologen prevents hypotony because the quality of the evidence is very low (Analysis 2.5.1; RR 0.75, 95% CI 0.47 to 1.19).
Surgical revision within three months and one year after surgery
Four studies including 150 eyes reported this complication (Marey 2013; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014). It is unclear whether use of Ologen prevents surgical revision within three months and one year after surgery, because the quality of the evidence is very low (Analysis 2.5.2; RR 1.70, 95% CI 0.38 to 7.63).
Endophthalmitis or blebitis
Three studies including 164 eyes reported this complication (Marey 2013; Mitra 2012; Papaconstantinou 2010). It is unclear whether use of Ologen prevents endophthalmitis or blebitis because the quality of the evidence is very low (Analysis 2.5.3; RR 1.57, 95% CI 0.25 to 9.70).
None of the included studies reported these complications: retinal detachment, corneal transplant, cataract extraction (among phakic eyes), and choroidal hemorrhage.
Others as reported from the included studies
Bleb leakage
Four studies including 129 eyes reported this complication (Cillino 2011; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether use of Ologen prevents bleb leakage because the quality of the evidence is very low (Analysis 2.5.4; RR 0.85, 95% CI 0.33 to 2.20).
Hyphema
Six studies including 229 eyes reported this complication (Cillino 2011; Marey 2013; Papaconstantinou 2010; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether use of Ologen prevents hyphema because the quality of the evidence is very low (Analysis 2.5.5; RR 1.46, 95% CI 0.51 to 4.19).
Choroidal detachment
Four studies including 129 eyes reported this complication (Cillino 2011; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether use of Ologen prevents choroidal detachment because the quality of the evidence is very low (Analysis 2.5.6; RR 0.83, 95% CI 0.33 to 2.09).
Shallow anterior chamber
Five studies including 213 eyes reported this complication (Marey 2013; Mitra 2012; Rosentreter 2010; Rosentreter 2014; Senthil 2013). It is unclear whether use of Ologen prevents shallow anterior chamber because the quality of the evidence is very low (Analysis 2.5.7; RR 0.79, 95% CI 0.32 to 1.93).
Anterior chamber reaction
Two studies including 99 eyes reported this complication (Marey 2013; Senthil 2013). It is unclear whether use of Ologen prevents anterior chamber reaction because the quality of the evidence is very low (Analysis 2.5.8; RR 1.21, 95% CI 0.56 to 2.60)
Positive Seidel test
Three studies including 164 eyes reported this complication (Marey 2013; Mitra 2012; Papaconstantinou 2010). It is unclear whether use of Ologen prevents a positive Seidel test because the quality of the evidence is very low (Analysis 2.5.9; RR 1.93, 95% CI 0.32 to 11.54)
Tenon's cysts
Three studies including 124 eyes reported this complication (Mitra 2012; Papaconstantinou 2010; Rosentreter 2010). It is unclear whether use of Ologen prevents Tenon's cysts because the quality of the evidence is very low (Analysis 2.5.10; RR 0.88, 95% CI 0.21 to 3.66)
Flat anterior chamber
Only one study covering 40 eyes reported this complication (Papaconstantinou 2010). There was one of 20 eyes in the trabeculectomy‐alone group and two of 20 eyes in the Ex‐PRESS group with flat anterior chamber, and it is unclear whether use of Ologen prevents flat anterior chamber because the quality of the evidence is very low (RR 2.00, 95% CI 0.20 to 20.33)
Trabeculectomy + AMT (amniotic membrane) versus trabeculectomy
We identified 18 studies that compared the use of amniotic membrane (AMT) during trabeculectomy versus standard trabeculectomy (Bruno 2008; Cai 2012; Cho 2013; Eliezer 2006; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Ren 2009; Sheha 2008; Stavrakas 2012; Wang 2008; Wang 2009; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005). Only one study reported a sample size calculation, where a power of 80% was achieved with a sample size of 15 participants (Sheha 2008). However, the minimum detectable difference was not specified. In consideration of potential losses to follow‐up, the study recruited 37 participants.
Intraocular pressure (IOP)
None of the 18 included studies reported change in IOP from baseline; instead, they all reported postoperative IOP at follow‐up time points. Caution must be used in interpreting the evidence from each time point, as a lower mean IOP favoring the amniotic membrane group could be attributable to eyes in the amniotic membrane group having a lower baseline IOP. Futhermore, we judged most studies to be at high risk of detection and reporting bias.
Among the 18 studies, only seven accounted for participants lost to follow‐up, whereby 316 of 346 eyes were assessed at their respective last follow‐up visits. For the remaining 11 studies we used the eyes randomized in data analyses (see Table 4 for losses to follow‐up). Numbers of eyes below refers to those randomized, when numbers of eyes analyzed were not reported. Wang 2009 had three groups, including trabeculectomy, trabeculectomy + MMC, and trabeculectomy + amniotic membrane + MMC. We only analyzed the latter two groups, as they both included MMC.
Of the 18 studies, one was a conference abstract that did not give any IOP data in the results (Bruno 2008). However, it concluded that smaller IOP changes were associated with larger avascular blebs. Another study reported median IOP values and the inter‐quartile range from 52 eyes (Stavrakas 2012). The median IOP at 24 months was 15.5 mm Hg for the amniotic membrane group and 16 mm Hg for the trabeculectomy‐alone group. It did not report a median difference, but reported that there was no difference between the two groups. The remaining 16 studies provided postoperative IOP findings at various time points; we summarize data from those 16 studies below.
At one year, nine studies comprising 356 eyes provided postoperative IOP data (Cai 2012; Eliezer 2006; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Ren 2009; Sheha 2008; Wang 2009). The low‐quality evidence showed that the use of amniotic membrane may slightly improves IOP reduction (Analysis 3.1; Figure 5; MD ‐3.92 mm Hg, 95% CI ‐5.41 to ‐2.42; I² = 84%). We stratified these studies on whether MMC was applied to both groups or to neither group or to the trabeculectomy‐alone group, and found no differences from the overall results. Because Khairy 2015 is the only study that does not have comparable groups (MMC was added to one group only), we excluded that study in a sensitivity analysis, and found the pooled mean IOP in the amniotic membrane group to be about 4 mm Hg lower than in the trabeculectomy‐alone group (MD ‐4.38, 95% CI ‐5.77 to ‐2.98).
3.1. Analysis.
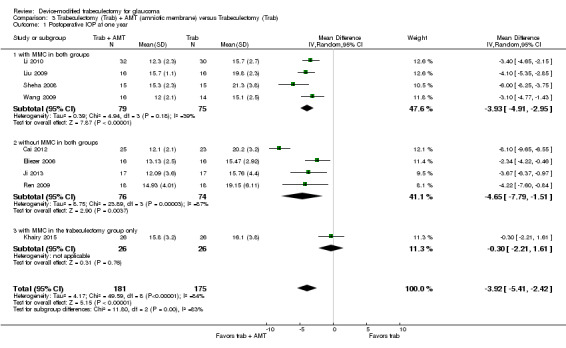
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 1 Postoperative IOP at one year.
5.
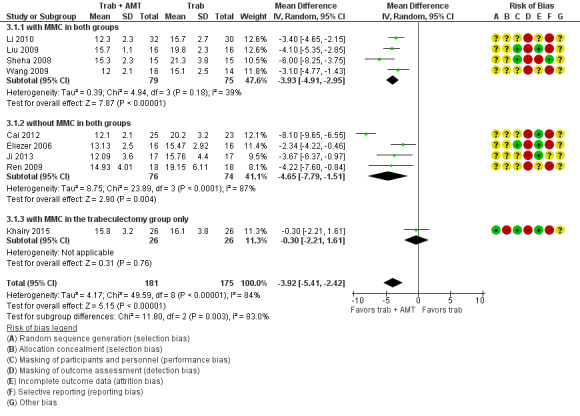
Forest plot of comparison: 4 Trabeculectomy + AMT (amniotic membrane) versus Trabeculectomy, outcome: 4.1 Postoperative IOP at one year.
At one day, eight studies comprising 405 eyes provided postoperative IOP data (Huang 2007; Khairy 2015; Liu 2009; Sheha 2008; Wang 2008; Yan 2004; Yang 2004; Zheng 2005). We did not report the pooled data as the results of different studies were inconsistent in the direction of effect and there was substantial heterogeneity (I² = 91%). There was also substantial statistical heterogeneity for two subgroups: 'with MMC in both groups' (I² = 75%) and 'without MMC in both groups' (I² = 95%). The subgroup 'with MMC in the trabeculectomy group' had no substantial heterogeneity (I² = 0%) and each study showed that It remains uncertain if there is a difference in mean IOP comparing the AMT and trabeculectomy‐alone groups (Analysis 3.2).
3.2. Analysis.
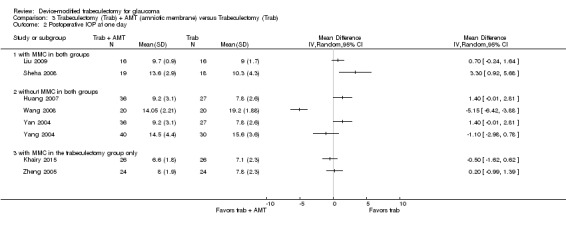
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 2 Postoperative IOP at one day.
At one week, 13 studies comprising 625 eyes provided postoperative IOP data (Cai 2012; Cho 2013; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Sheha 2008; Wang 2008; Wang 2009; Yan 2004; Yang 2004; Zheng 2005). We did not combine the data as results of different studies were inconsistent in the direction of effect and there was substantial heterogeneous (I² = 89%). There was also substantial statistical heterogeneity for two subgroups: 'with MMC in both groups' (I² = 96%) and 'without MMC in both groups' (I² = 87%) . The subgroup 'with MMC in the trabeculectomy‐only group' had no substantial statistical heterogeneity (I² = 48%) and each study showed that there was no difference in mean IOP comparing the AMT and trabeculectomy‐alone groups (Analysis 3.3).
3.3. Analysis.
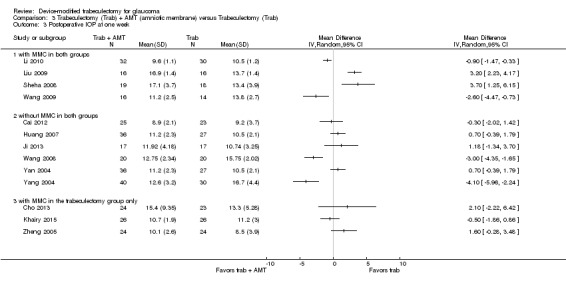
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 3 Postoperative IOP at one week.
At one month, 13 studies comprising 646 eyes provided postoperative IOP data (Cai 2012;Cho 2013; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Sheha 2008; Wang 2008; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005), and suggests that the use of amniotic membrane may lead to little or no difference in IOP‐lowering effect (Analysis 3.4; MD ‐1.05 mm Hg, 95% CI ‐1.96 to ‐0.13). We stratified these studies on whether MMC was applied to both or neither or to the trabeculectomy‐alone group and found the results to be the same as the overall results. Although there was overall substantial statistical heterogeneity (I² = 83%), we combined the data as the direction of effect was consistent in favoring the amniotic membrane group.
3.4. Analysis.
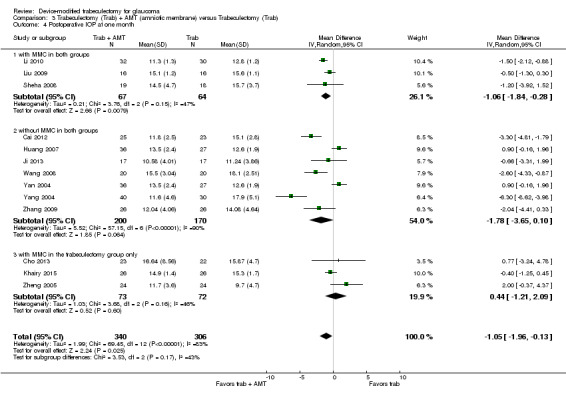
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 4 Postoperative IOP at one month.
At three months, 11 studies comprising 551 eyes provided postoperative IOP data (Cho 2013; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Sheha 2008; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005), and showed the use of amniotic membrane may slightly improve IOP reduction compared to the trabeculectomy‐alone group (Analysis 3.5; MD ‐2.23 mm Hg, 95% CI ‐2.93 to ‐1.53) . We stratified these studies on whether MMC was applied to both or neither or to the trabeculectomy‐alone group and found no differences from the overall results. As with one‐year outcomes, Khairy 2015 and Zheng 2005 were the only studies that included MMC in the trabeculectomy‐only group. We excluded these two studies in a sensitivity analysis, which showed that IOP in the amniotic membrane group was 2.54 mm Hg lower than in the trabeculectomy‐only group at three months (MD ‐2.54, 95% CI ‐3.27 to ‐1.81). Although there was substantial statistical heterogeneity (I² = 62%), we combined the data as the direction of effect was consistent in favoring the amniotic membrane group. It also is important to note that there was a lot of missing data for these studies due to underreporting of numbers of eyes analyzed at different time points, and the results were likely to be biased.
3.5. Analysis.
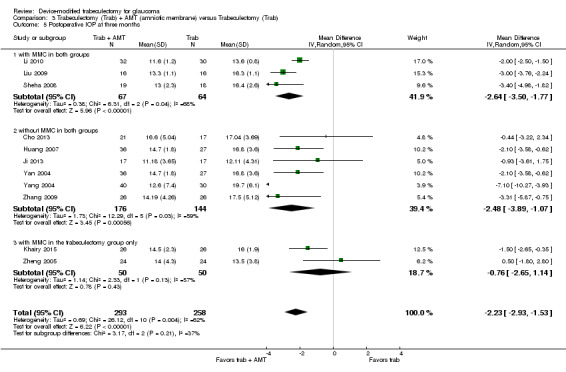
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 5 Postoperative IOP at three months.
At six months, 13 studies comprising 613 eyes provided postoperative mean IOP data (Huang 2007; Ji 2013; Khairy 2015; Li 2010; Liu 2009; Ren 2009; Sheha 2008; Wang 2008; Wang 2009; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005), and showed the use of amniotic membrane may slightly improve IOP reduction compared to the trabeculectomy‐alone group (Analysis 3.6; MD ‐2.50 mm Hg, 95% CI ‐3.34 to ‐1.67). We stratified these studies on whether MMC was applied to both or neither or to the trabeculectomy‐alone group and the results still show an IOP‐lowering effect favoring the amniotic membrane group. Although there was overall substantial statistical heterogeneity (I² = 74%), we combined the data as the direction of effect was consistent in favoring the amniotic membrane group.
3.6. Analysis.
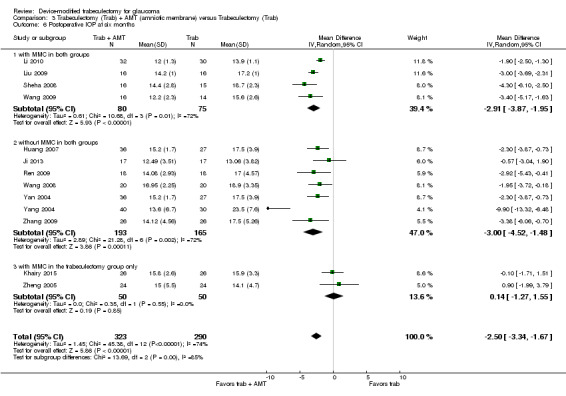
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 6 Postoperative IOP at six months.
At two years, two studies comprising 86 eyes provided postoperative mean IOP data (Analysis 3.7; Ji 2013; Khairy 2015). Although Khairy 2015 showed no difference between the two groups, the study only applied MMC in the trabeculectomy‐alone group and the groups were not comparable to Ji 2013 (which did not apply MMC to both groups). Ji 2013 reported that IOP in the amniotic membrane group was 2.96 mm Hg lower than the trabeculectomy‐alone group (MD ‐2.96, 95% CI ‐5.52 to ‐0.40). Due to the substantial statistical heterogeneity (I² = 64%), methodological heterogeneity, and high risk of bias, we did not combine these two studies.
3.7. Analysis.
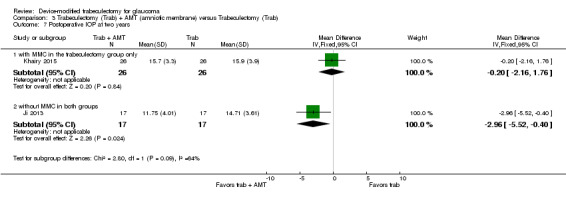
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 7 Postoperative IOP at two years.
Best‐corrected visual acuity (BCVA)
Only one study comprising 48 eyes reported BCVA at one year and reported that the amniotic membrane group had better BCVA than the trabeculectomy‐alone group, but no additional data were provided (Cai 2012).
IOP fluctuation, visual field and quality of life
No study reported on these outcomes.
Complications
All 17 studies reported in full‐length articles provided data on complications in 840 eyes during postoperative follow‐up. We compared proportions of participants with each complication between groups in meta‐analyses. Complications reported included loss of vision, hypotony, shallow anterior chamber, hyphema, bleb leakage, encapsulated blebs, choroidal detachment, anterior chamber reaction, and flat anterior chamber. Complications were less frequent in the amniotic membrane group than in the trabeculectomy‐alone group (Analysis 3.8), except for anterior chamber reaction (Li 2010).
3.8. Analysis.
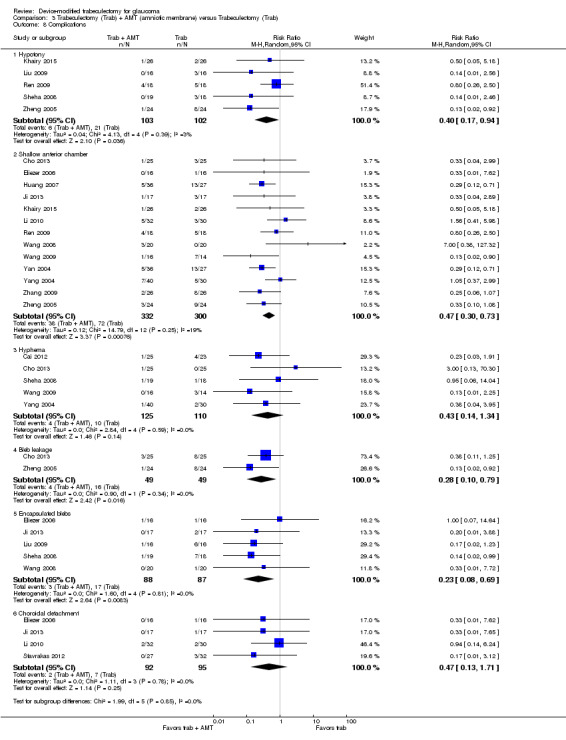
Comparison 3 Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab), Outcome 8 Complications.
Loss of vision of more than two lines
None of the included studies reported this complication.
IOP < 5 mm Hg (hypotony)
Five studies including 205 eyes reported this complication (Khairy 2015; Liu 2009; Ren 2009; Sheha 2008; Zheng 2005). The low‐quality evidence showed use of amniotic membrane may prevent hypotony (Analysis 3.8.1; RR 0.40, 95% CI 0.17 to 0.94).
None of the included studies reported these complications: surgical revision within three months and one year after surgery, endophthalmitis or blebitis, retinal detachment, corneal transplant, cataract extraction (among phakic eyes), and choroidal hemorrhage.
Others as reported from the included studies
Shallow anterior chamber
Thirteen studies including 632 eyes reported this complication (Cho 2013; Eliezer 2006; Huang 2007; Ji 2013; Khairy 2015; Li 2010; Ren 2009; Wang 2008; Wang 2009; Yan 2004; Yang 2004; Zhang 2009; Zheng 2005). The low‐quality evidence showed that use of amniotic membrane may prevent shallow anterior chamber (Analysis 3.8.2; RR 0.47, 95% CI 0.30 to 0.73).
Hyphema
Five studies including 235 eyes reported this complication (Cai 2012; Cho 2013; Sheha 2008; Wang 2009; Yang 2004). It is unclear whether use of amniotic membrane prevents hyphema because the quality of the evidence is very low (Analysis 3.8.3; RR 0.43, 95% CI 0.14 to 1.34).
Bleb leakage
Two studies including 98 eyes reported this complication (Cho 2013; Zheng 2005). The low‐quality evidence showed use of amniotic membrane may prevent bleb leakage (Analysis 3.8.4; RR 0.28, 95% CI 0.10 to 0.79).
Encapsulated blebs
Five studies including 175 eyes reported this complication (Eliezer 2006; Ji 2013; Liu 2009; Sheha 2008; Wang 2008). The low‐quality evidence showed use of amniotic membrane may prevent encapsulated blebs (Analysis 3.8.5; RR 0.23, 95% CI 0.08 to 0.69).
Choroidal detachment
Four studies including 187 eyes reported this complication (Eliezer 2006; Ji 2013; Li 2010; Stavrakas 2012). The low‐quality evidence showed use of amniotic membrane may prevent choroidal detachment (Analysis 3.8.6; RR 0.47, 95% CI 0.13 to 1.71).
Anterior chamber reaction
Only one study comprising 62 eyes reported this complication (Li 2010). There were 21 of 30 eyes in the trabeculectomy‐alone group and 25 of 32 eyes in the AMT group with flat anterior chamber, but there was uncertainty if the relative risk of flat anterior chamber favored the AMT or the trabeculectomy‐alone group, as the result was statistically non‐significant (RR 1.12, 95% CI 0.83 to 1.50).
Flat anterior chamber
Only one study comprising 37 eyes reported this complication (Sheha 2008). There were two of 18 eyes in the trabeculectomy‐alone group and none of 19 eyes in the AMT group with flat anterior chamber, but there was uncertainty if the relative risk of flat anterior chamber favored the AMT or the trabeculectomy‐alone group, as the result was statistically non‐significant (RR 0.19, 95% CI 0.01 to 3.71).
Trabeculectomy + expanded polytetrafluoroethylene (E‐PTFE) versus trabeculectomy
One study assessed the use of E‐PTFE (Cillino 2008). Because all participants completed 24 months of follow‐up, all 60 eyes of 60 participants contributed to all of the following outcomes measured. The study performed sample size calculations, based on a power of 90% or greater to detect at least a 3 mm Hg IOP difference among groups. Cillino 2008 did not report on IOP fluctuation, BCVA, visual field, or quality of life.
Intraocular pressure (IOP)
The study did not report change in IOP from baseline; instead, it reported postoperative IOP measured at one day, one week, and 1, 3, 6, 9, 12, 18, and 24 months. It also reported that baseline IOPs among the four intervention groups did not show any statistically significant differences. We therefore compared the postoperative IOP among groups as a surrogate for change of IOP from baseline between groups. At all follow‐up time points, it remains uncertain if there is a difference between the intervention groups. Specifically, at one year, the low‐quality evidence showed that use of E‐PTFE may lead to little or no difference in lower mean IOP compared to use of trabeculectomy alone (MD ‐0.44 mm Hg, 95% CI ‐1.76 to 0.88), and it remains uncertain if there is a between‐group difference with or without the use of MMC (Analysis 4.1; Figure 6; with MMC: MD 0.10, 95% CI ‐1.58 to 1.78; without MMC: MD ‐1.30, 95% CI ‐3.42 to 0.82).
4.1. Analysis.
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 1 Postoperative IOP at one year.
6.
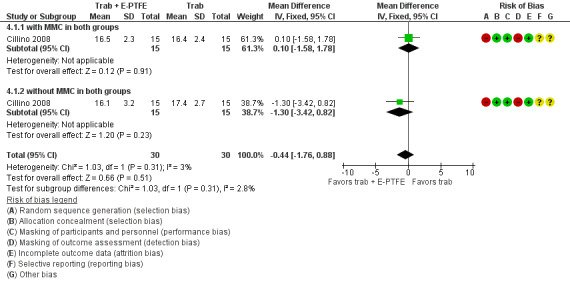
Forest plot of comparison: 1 Trabeculectomy + E‐PTFE versus Trabeculectomy, outcome: 1.1 Postoperative IOP at one year.
IOP fluctuation, BCVA, visual field, and quality of life
Cillino 2008 did not report on IOP fluctuation, BCVA, visual field, or quality of life.
Complications
The study assessed complications at 24 months, including other complications: hyphema, inflammation, shallow anterior chamber, flat anterior chamber, choroidal detachment, and cystic/avascular bleb.
Loss of vision of more than two lines
The included study did not report this complication.
IOP < 5 mm Hg (hypotony)
There were 14 of 30 eyes in the trabeculectomy‐alone group and four of 30 eyes in the E‐PTFE group with hypotony. We judged the very low‐quality evidence to be at high risk of selection and detection bias. It is unclear whether use of E‐PTFE prevents hypotony because the quality of the evidence is very low (Analysis 4.2; RR 0.29, 95% CI 0.11 to 0.77). Futhermore, after stratifying by the presence or absence of MMC, the 95% CIs went from being narrow to being wide, and the results changed from possibly different to little or no difference. In the E‐PTFE group with MMC, three of 15 eyes had hypotony, compared to the trabeculectomy‐alone group (seven of 15 eyes), leaving it unclear whether the use of E‐PTFE prevents hypotony, because the quality of the evidence is very low (Analysis 4.2.1; RR 0.43, 95% CI 0.14 to 1.35). In the E‐PTFE group without MMC, one of 15 eyes had hypotony, compared to the trabeculectomy‐alone group (seven of 15 eyes), leaving it unclear whether the use of E‐PTFE prevents hypotony because the quality of the evidence is very low (Analysis 4.2.2; RR 0.14, 95% CI 0.02 to 1.02).
4.2. Analysis.
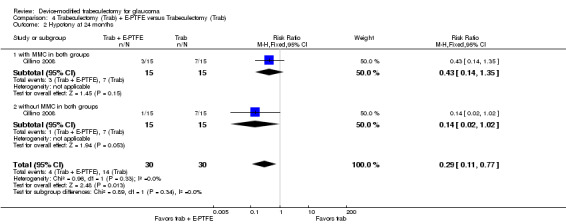
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 2 Hypotony at 24 months.
The included study did not report these complications: surgical revision within three months and one year after surgery, endophthalmitis or blebitis, retinal detachment, corneal transplant, cataract extraction (among phakic eyes), and choroidal hemorrhage.
Others as reported from the included studies
Hyphema
There were eight of 30 eyes in the E‐PTFE group with hyphema and 10 of 30 eyes in the trabeculectomy‐alone group, but it was uncertain whether the E‐PTFE group was favored over the trabeculectomy‐alone group (Analysis 4.3; RR 0.80, 95% CI 0.37 to 1.74). There was uncertainty after stratifying by the presence or absence of MMC. In the E‐PTFE group with MMC, four of 15 participants had hyphema compared to the trabeculectomy‐alone group (five of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.3.1; RR 0.80, 95% CI 0.27 to 2.41). In the E‐PTFE group without MMC, four of 15 eyes had hyphema, compared to the trabeculectomy‐alone group (five of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.3.2; RR 0.80, 95% CI 0.27 to 2.41).
4.3. Analysis.
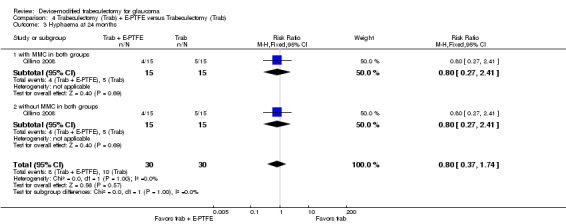
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 3 Hyphaema at 24 months.
Inflammation
There were seven of 30 eyes in the E‐PTFE group with inflammation and five of 30 eyes in the trabeculectomy‐alone group, but it was uncertain whether the E‐PTFE group was favored over the trabeculectomy‐alone group (Analysis 4.4; RR 1.40, 95% CI 0.50 to 3.91). There was uncertainty after stratifying by the presence or absence of MMC. In the E‐PTFE group with MMC, three of 15 eyes had inflammation compared to the trabeculectomy‐alone group (three of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.4.1; RR 1.33, 95% CI 0.36 to 4.97). In the E‐PTFE group without MMC, four of 15 eyes had inflammation compared to the trabeculectomy‐alone group (two of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.4.2; RR 1.50, 95% CI 0.29 to 7.73).
4.4. Analysis.
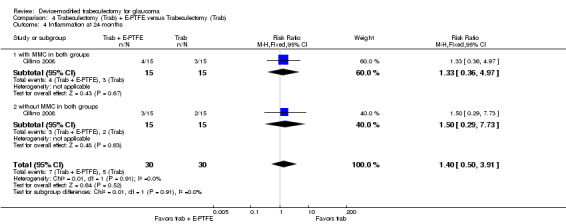
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 4 Inflammation at 24 months.
Shallow anterior chamber
There were four of 30 eyes in the E‐PTFE group with shallow anterior chamber and nine of 30 eyes in the trabeculectomy‐alone group, but there was uncertainty whether the E‐PTFE group was favored over the trabeculectomy‐alone group (Analysis 4.5; RR 0.44, 95% CI 0.15 to 1.29). There was uncertainty after stratifying by the presence or absence of MMC. In the E‐PTFE group with MMC, two of 15 eyes had shallow anterior chamber, compared to the trabeculectomy‐alone group (four of 15 eyes) but there was uncertainty and the result was not statistically significant (Analysis 4.5.1; RR 0.50, 95% CI 0.11 to 2.33). In the E‐PTFE group without MMC, two of 15 eyes had shallow anterior chamber compared to the trabeculectomy‐alone group (five of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.5.2; RR 0.40, 95% CI 0.09 to 1.75).
4.5. Analysis.
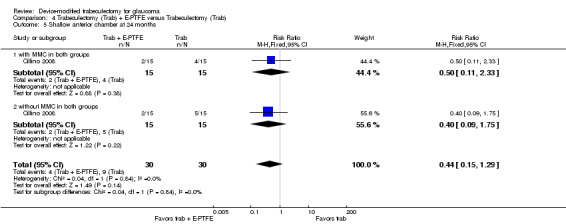
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 5 Shallow anterior chamber at 24 months.
Flat anterior chamber
There were one of 30 eyes in the E‐PTFE group with flat anterior chamber and two of 30 eyes in the trabeculectomy‐alone group, but there was uncertainty whether the E‐PTFE group was favored over the trabeculectomy‐alone group (Analysis 4.6; RR 0.60, 95% CI 0.08 to 4.28). There was uncertainty after stratifying by the presence or absence of MMC. In the E‐PTFE group with MMC, one of 15 eyes had flat anterior chamber, compared to the trabeculectomy‐alone group (one of 15 eyes) but there was uncertainty and the result was not statistically significant (RR 1.00, 95% CI 0.07 to 14.55). In the E‐PTFE group without MMC, zero of 15 eyes had flat anterior chamber compared to the trabeculectomy‐alone group (one of 15 eyes), but there was uncertainty and the result was not statistically significant (RR 0.33, 95% CI 0.01 to 7.58).
4.6. Analysis.
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 6 Flat anterior chamber at 24 months.
Choroidal detachment
There were three of 30 eyes in the E‐PTFE group with choroidal detachment and seven of 30 eyes in the trabeculectomy‐alone group, but there was uncertainty whether the E‐PTFE group was favored over the trabeculectomy‐alone group (Analysis 4.7; RR 0.43, 95% CI 0.12 to 1.51). There was uncertainty after stratifying by the presence or absence of MMC. In the E‐PTFE group with MMC, one of 15 eyes had choroidal detachment compared to the trabeculectomy‐alone group (four of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.7.1; RR 0.25, 95% CI 0.03 to 1.98). In the E‐PTFE group without MMC, two of 15 eyes had choroidal detachment compared to the trabeculectomy‐alone group (three of 15 eyes), but there was uncertainty and the result was not statistically significant (Analysis 4.7.2; RR 0.67, 95% CI 0.13 to 3.44).
4.7. Analysis.
Comparison 4 Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab), Outcome 7 Choroidal detachment at 24 months.
Cystic/avascular bleb
We could not compare findings with and without MMC as there were no eyes with cystic/avascular bleb in the without‐MMC group. In the E‐PTFE group with MMC, three of 15 eyes had cystic/avascular bleb compared to the trabeculectomy‐alone group (three of 15 eyes), but there was uncertainty and the result was not statistically significant (RR 1.00, 95% CI 0.24 to 4.18).
Trabeculectomy + Gelfilm versus trabeculectomy
No quantitative data were reported from the only study of Gelfilm (43 eyes) (Birt 1998). The study investigators concluded that at one month, six months, and one year after surgery there was uncertainty about the differences among the four intervention groups for postoperative IOP or complication rates. This study was reported only in a conference abstract and we were unable to find other information.
Discussion
Summary of main results
Ex‐PRESS
Ex‐PRESS may slightly lower IOP based on data from three trials (Dahan 2012; De Jong 2009; Wagschal 2013), which were funded by the device manufacturer and assessed at high risk of detection bias. It is uncertain if Ex‐PRESS prevents loss of visual acuity or if the complication rates are similar between the two groups. For best‐corrected visual acuity (BCVA), all four studies reported wide 95% confidence intervals alluding to uncertainty in the treatment effect difference between the two groups at one year (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). We conducted meta‐analyses for complications for four studies in 294 eyes. They appeared to be similar between the two groups, except that the Ex‐PRESS group may prevent postoperative interventions compared with trabeculectomy alone (for example, needling, cataract surgery, repeat trabeculectomy, etc.).
Ologen
It is uncertain whether the use of Ologen lowers IOP, prevents loss of visual acuity, or prevents complications. Very low‐quality evidence from two studies in 44 eyes contributed to the primary outcome of this review, and showed that it is uncertain whether the use of Ologen decreases the postoperative mean IOP from baseline to one year, at one day, six months, and two years compared with trabeculectomy alone (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4). The evidence was supported by varying number of studies and eyes at each follow‐up time point: five studies in 177 eyes at one year; five studies in 162 eyes at one day; seven studies in 236 eyes at six months; two studies in 55 eyes at two years (Analysis 2.2; Analysis 2.3; Analysis 2.4).
Evidence from two studies in 51 eyes contributed data to show no statistically significant difference in change in BCVA at one year and six weeks of follow‐up, respectively (Rosentreter 2010; Senthil 2013). One study reported on visual field changes, but did not find any changes over time (Rosentreter 2010). We did not find any overall statistical difference between the two treatment groups for any complications, although hypotony appeared to be more frequent in the trabeculectomy‐alone group.
Amniotic membrane
None of the included studies reported the primary outcome of this review of change in IOP from baseline to one year; instead, they all reported postoperative IOP at certain time points. Caution should be used in interpreting the evidence from nine studies in 356 eyes, showing that the use of amniotic membrane may slightly lower the mean IOP at one year follow‐up, as eyes in the amniotic membrane group could have had lower baseline mean IOP than in the trabeculectomy‐alone group (Analysis 3.1). Similar results were shown at one month, three months and six months (Analysis 3.4; Analysis 3.5; Analysis 3.6). Six studies reported BCVA (no meta‐analysis) at one year or six months, but the findings were inconsistent. The amniotic membrane group appeared to have a lower frequency of complications than the trabeculectomy‐alone group, although the time points assessed were inconsistent or unclear across studies.
Expanded polytetrafluoroethylene (E‐PTFE)
One study (Cillino 2008) reported the use of E‐PTFE during trabeculectomy, and found no statistically significant difference between the groups with E‐PTFE with or without MMC and standard trabeculectomy in IOP control at all follow‐up time points up to two years. In the E‐PTFE group, the complication (hypotony) rates were no different in the subgroups with and without MMC at 24 months, although the pooled result favored the E‐PTFE group (Analysis 4.2).
Overall completeness and applicability of evidence
Of the 33 included studies, 16 reported numbers of antiglaucoma medications used during the postoperative follow‐up periods; within these, 11 reported IOP by 'complete success' (defined as IOP reached < 21 mm Hg without use of medication), 'qualified success' (defined as IOP reached < 21 mm Hg with use of medication), or 'failure' (defined at IOP > 22 mm Hg at follow‐up).
Ex‐PRESS
Four studies were powered to detect a between‐group difference between Ex‐PRESS and controls (Dahan 2012; De Jong 2009; Netland 2014; Wagschal 2013). The studies were conducted in South Africa, the Netherlands, USA, and Canada respectively. They included a mix of white, African‐American, Asian, and Indian participants, and all with a mean age of around 65 years. Three studies could be meta‐analyzed for efficacy of Ex‐PRESS. One study reported that the Ex‐PRESS device was associated with a one‐year overall cost of USD 956 greater than trabeculectomy (Wagschal 2013). Patients' choices could be affected by the effectiveness, safety, and cost of the intervention.
Since all five studies included participants with open‐angle glaucoma, the Ex‐PRESS results are most applicable to people with open‐angle glaucoma. The effectiveness and safety in people with other types of glaucoma remain uncertain.
Ologen
Only one study was adequately powered to detect a between‐group difference (Cillino 2011). The other seven studies were either inconclusive or did not find any statistically significant difference for postoperative IOP reduction. The findings from the meta‐analysis remain inconclusive and should be interpreted with caution.
Ologen implant is an alternative method for controlling the wound‐healing process similar to antifibrotic agents. Among the eight Ologen trials, only one (Papaconstantinou 2010) did not use MMC in the standard trabeculectomy group.
All or some of the participants in all eight studies had open‐angle glaucoma; two studies included some participants with angle‐closure glaucoma (Marey 2013; Senthil 2013), and one study did not specify the glaucoma type (Papaconstantinou 2010). None of the studies stratified participants by type of glaucoma.
Amniotic membrane
Only one study reported power and sample size calculations (Sheha 2008). In addition, a majority of the studies did not report losses to follow‐up and did not report details of study design (i.e. randomization, allocation concealment, and masking). Due to the poor quality of most of the studies, concerns still remain as to whether IOP is reduced when amniotic membrane is used compared with standard trabeculectomy, and how safe it is.
Three studies used MMC in the trabeculectomy group only and not in the amniotic membrane group (Cho 2013; Khairy 2015; Zheng 2005).
Of the 18 studies, three included only participants with open‐angle glaucoma (Eliezer 2006; Khairy 2015; Stavrakas 2012), four studies included only participants with angle‐closure glaucoma (Huang 2007; Ren 2009; Yan 2004; Zhang 2009), two studies included open‐angle and angle‐closure glaucoma (Cho 2013; Zheng 2005), seven studies included participants with refractory glaucoma (Cai 2012; Ji 2013; Li 2010; Liu 2009; Sheha 2008; Wang 2009; Yang 2004), and two studies did not specify the glaucoma type (Birt 1998; Wang 2008).
Expanded polytetrafluoroethylene (E‐PTFE)
Cillino 2008 enrolled the number of participants needed to be adequately powered to detect a between‐group difference. The study reported the number of antiglaucoma medications as an outcome, so the efficacy of E‐PTFE to control IOP might be partially attributed to antiglaucoma medication and not to E‐PTFE alone.
Quality of the evidence
Overall, the agreement in IOP measurement across the included studies was high, as the width of the 95% CI for postoperative IOP at one year ranged from 2 to 7 mm Hg for each device. Similarly, the evidence showed a small and consistent effect size, with mean differences ranging from ‐4 to 2 mm Hg.
Most of the trials were at high risk of detection bias for lack of masking outcome assessors. Further, the Ex‐PRESS studies had potential conflicts of interest for receiving funding support from the device manufacturer. Overall, we graded the quality of the evidence as low or very low for most outcomes due to potential risks of bias and imprecision for many adverse events.
Potential biases in the review process
We conducted comprehensive electronic searches for studies with no imposed date or language restrictions to minimize potential biases in the study selection process. We followed standard Cochrane review methodology.
Agreements and disagreements with other studies or reviews
Trabeculectomy + Ex‐PRESS versus trabeculectomy
Our meta‐analyses of three trials found that the use of Ex‐PRESS may lead to IOP reduction compared with trabeculectomy alone at one year and two years. Our review also showed there is uncertainty whether the risk of complications differs between the Ex‐PRESS and trabeculectomy‐alone group.
A retrospective comparative series by Maris 2007 of 100 eyes, Good 2011 of 70 eyes, and Moisseiev 2015 of 200 eyes did not find a significant difference between Ex‐PRESS and trabeculectomy in lowering IOP. A retrospective review by Marzette 2011 of 153 eyes showed a lower risk of postoperative hypotony and a greater but statistically nonsignificant decrease in IOP with Ex‐PRESS.
A systematic review by Wang 2013a concluded that Ex‐PRESS has the same effectiveness in IOP reduction compared to trabeculectomy alone, with significantly fewer events of hypotony and hyphema compared to trabeculectomy alone. However, these pooled results were from a mix of RCTs, prospective non‐RCTs, and retrospective studies, which limits the reliability of their inference.
A recent meta‐analysis by Chen 2014 found no statistically significant reduction in IOP between Ex‐PRESS and trabeculectomy alone, and a significantly lower frequency of hyphema with Ex‐PRESS. The other complications were not statistically significantly different between the two groups. However, this review was flawed in that it mixed the different follow‐up periods from different studies for IOP control (e.g. six months and one year) in one meta‐analysis. Also, one included study was a subset of another (both were references from Wagschal 2013), and its meta‐analyses of complications included both studies, thereby double‐counting the data.
Trabeculectomy + Ologen versus trabeculectomy
Our meta‐analyses of five trials showed that there is uncertainty if there is a difference in IOP postoperatively and at one year. This is contrary to Narayanaswamy 2012, which conducted a non‐randomized prospective comparative study of 33 participants and found a greater decrease in IOP and a lower incidence of bleb needling procedures in the trabeculectomy and MMC group than in the trabeculectomy and Ologen group. However, our results are in agreement with a systematic review by He 2014 of seven RCTs (227 eyes), which found no statistically significant difference between the two groups except at one and 12 months, in which the amount of IOP reduction was less in the Ologen group. However, the review is limited because it included a non‐randomized trial (Nilforushan 2010), despite being restricted to RCTs only.
Our meta‐analysis of complications in all studies showed that there is uncertainty whether there is a difference between the two groups. Similarly, the authors of He 2014 reported comparable rates of adverse events in the Ologen and trabeculectomy‐alone groups.
Trabeculectomy + amniotic membrane versus trabeculectomy
Our meta‐analysis of nine trials found a slight decrease in IOP in the amniotic membrane group compared to the trabeculectomy‐alone group. This is similar to an animal model study, that also showed a greater decrease in IOP (Barton 2001).
Expanded polytetrafluoroethylene (E‐PTFE)
A retrospective study enrolling 43 eyes with refractory glaucoma found that an E‐PTFE membrane safely reduced IOP in 65% of the eyes (Kim 2003). The mean follow‐up period was 32.9 months. However, in the remaining 35% of the eyes, surgical revisions were needed to control IOP or treat complications.
Other devices
We did not find any randomized controlled trials (RCTs) for other devices used in a standard trabeculectomy procedure, but a few case series without comparators have shown some benefit of using them. Dahan 2011 conducted a trial on the use of T‐flux in trabeculectomy for neovascular glaucoma. The trial showed that modified trabeculectomy augmented by MMC and T‐flux adequately controlled IOP in neovascular participants after a mean follow‐up of 32 ± 12 months. However, surgical revisions may be required to maintain an IOP ≤ 21 mm Hg. Two trials reported the use of gold micro shunt (GMS). One study with 38 participants found that GMS can significantly decrease IOP, but two‐thirds of participants required medications to achieve adequate IOP control (Melamed 2009). Another study with 55 participants with uncontrolled refractory glaucoma reported that GMS can achieve adequate IOP control in about 67.3% of eyes with relatively few complications (Figus 2011). The follow‐up was two years in this study. Devices inserted into the suprachoroidal space are at high risk for fibrosis and failure of the procedure (Agnifili 2012).
Comparison between different devices
There was no RCT directly comparing the use of any two devices. However, we found one retrospective review that compared Ologen versus MMC in participants implanted with Ex‐PRESS (Johnson 2014). The study included 99 eyes of 85 participants who had undergone trabeculectomy with implantation of an Ex‐PRESS shunt, and had applied MMC in 50 eyes of 48 participants and used Ologen in 49 eyes of 37 participants. The investigators found no significant difference between the two groups in IOP reduction or rates of complications.
Authors' conclusions
Implications for practice.
Our findings suggest that the use of devices with a standard trabeculectomy may help with slightly greater intraocular pressure (IOP) reduction at one‐year follow‐up than trabeculectomy alone; however, conclusions for each type of device are limited due to methodological concerns for bias and poor reporting of outcomes. Currently, these devices add extra costs to insurance companies and patients compared with those incurred for a trabeculectomy alone. Whether the IOP reduction that can be achieved with these devices is sufficient to outweigh these additional costs will need to be determined for each individual patient. Since it is frequently reported that a 1 mm Hg reduction in IOP can be associated with a 10% decrease in the risk of glaucomatous progression, the additional IOP reduction that may be obtained at one‐year follow‐up may be valuable in selected populations (Heijl 2002).
Implications for research.
Low‐quality evidence warrants better quality trials to determine the comparative effectiveness of all devices included in this review. These studies are limited and the applicability of the evidence to other population or settings remains unclear. More research is therefore needed to generate evidence for or against devices such as Ex‐PRESS, Ologen, amniotic membrane, Gelfilm, E‐PTFE, gold micro shunt, and T‐flux.
In the absence of definitive evidence, we need more trials of better quality for most comparisons and outcomes. These should account for losses to follow‐up at each follow‐up time point measured. They should also account for the correlation of outcomes between two eyes when applicable. They also need to consider the appropriate use of adjunctive agents, such as mitomycin C in both groups to ensure comparability. It would be helpful for future trials to specify the types of glaucomas, and also to consider stratifying participants by type of glaucoma, and perhaps by lens type, when the sample size is met. We need further research to evaluate the effectiveness and safety of each intervention on patients with different types of glaucoma. Data reporting needs to improve to include reporting of differences between groups to allow more robust inferences when applicable. Future trials should also report on the elements of trial quality identified above and ensure consistency between protocols and published studies.
Acknowledgements
We acknowledge Lori Rosman, Trials Search Co‐ordinator for the US Satellite of Cochrane Eyes and Vision (CEV), for developing the search strategy and executing the electronic searches. We thank Sueko M Ng and Dongyu Zhang for their help with screening titles/abstracts and data extraction for the Chinese studies. We thank Andrew Law for his edits and revision of the main text of the review. We also acknowledge the CEV editorial team and the peer reviewers Barbara Hawkins, Kuang Hu, and Brian Song for their insightful comments during the preparation of this review. We thank Guy Ben Simon and his group for sharing their trial data (Dahan 2012).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Trabeculectomy] explode all trees #2 MeSH descriptor: [Glaucoma] explode all trees and with qualifiers: [Surgery ‐ SU] #3 MeSH descriptor: [Trabecular Meshwork] explode all trees and with qualifiers: [Surgery ‐ SU] #4 MeSH descriptor: [Filtering Surgery] explode all trees #5 Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectom* #6 (Glaucoma* near/5 (surg* or filter* or filtrate*)) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Glaucoma Drainage Implants] explode all trees #9 (modif* near/5 (Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectom*)) #10 MeSH descriptor: [Polytetrafluoroethylene] explode all trees #11 (Polytef or Politef or "E PTFE" or EPTFE or PTFE or TFE or FEP or SOLX or polytetrafluoroethylen* or polytetrafluorethylen* or polytetrafluoroethen* or Fluoroflex or Fluoroplast or Ftoroplast or Halon or Polyfene or Tetron or Tarflen or "GORE TEX" or Goretex or gortex or Teflon or Fluon or Ex‐press or ologen or Baerveldt or Krupin or Ahmed or Molteno or ExPress or collagen matrix or collagen‐GAG or collagen‐glycosaminoglycan copolymer matrix) #12 Device* or implant* or shunt* or valve* or tube* #13 #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Fluorouracil] explode all trees #15 5FU or 5‐FU or Fluorouracil* or Fluoruracil* or 5‐HU or Adrucil or Carac or Efudix or Fluoro Uracile or Fluoro‐Uracile or Efudex or Fluoroplex or Flurodex or Fluracedyl or Haemato‐fu or Neofluor or Onkofluor or Ribofluor or 5‐Fluorouracil #16 MeSH descriptor: [Mitomycin] explode all trees #17 Mitomycin* or NSC‐26980 or NSC 26980 or NSC26980 or Mutamycin or Ametycine or Mitocin‐C or MitocinC or mytomycin* or mitomicin* or mytomicin* or MMC #18 MeSH descriptor: [Mitomycins] explode all trees #19 #18 from 1966 to 1991 #20 MeSH descriptor: [Antimetabolites] explode all trees #21 MeSH descriptor: [Antimetabolites, Antineoplastic] explode all trees #22 MeSH descriptor: [Nucleic Acid Synthesis Inhibitors] explode all trees #23 Antimetabolite* or anti‐metabolite* #24 Antifibrotic* or anti‐fibrotic* #25 #14 or #15 or #16 or #17 or #19 or #20 or #21 or #22 or #23 or #24 #26 #7 and (#13 or #25)
Appendix 2. MEDLINE (OvidSP) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Trabeculectomy/ 13. exp Glaucoma/su [Surgery] 14. exp Trabecular Meshwork/su [Surgery] 15. (Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectomy).tw. 16. (Glaucoma$ adj5 (surg$ or filter$ or filtrat$)).tw. 17. exp filtering surgery/ 18. 12 or 13 or 14 or 15 or 16 or 17 19. exp Glaucoma Drainage Implants/ 20. (modif* adj5 (Trabeculectom* or Trabeculoplast* or Trabeculotom* or Goniotom* or Microtrabeculectomy)).tw. 21. exp Polytetrafluoroethylene/ 22. (Polytef or Politef or "E PTFE" or EPTFE or PTFE or TFE or FEP or SOLX or polytetrafluoroethylen* or polytetrafluorethylen* or polytetrafluoroethen* or Fluoroflex or Fluoroplast or Ftoroplast or Halon or Polyfene or Tetron or Tarflen or "GORE TEX" or Goretex or gortex or Teflon or Fluon or Ex‐press or ologen or Baerveldt or Krupin or Ahmed or Molteno or ExPress or collagen matrix or collagen‐GAG or collagen‐glycosaminoglycan copolymer matrix).tw. 23. (Device* or implant* or shunt* or valve* or tube*).tw. 24. 19 or 20 or 21 or 22 or 23 25. exp Fluorouracil/ 26. (5FU or 5‐FU or Fluorouracil* or Fluoruracil* or 5‐HU or Adrucil or Carac or Efudix or Fluoro Uracile or Fluoro‐Uracile or Efudex or Fluoroplex or Flurodex or Fluracedyl or Haemato‐fu or Neofluor or Onkofluor or Ribofluor or 5‐Fluorouracil).tw. 27. exp Mitomycin/ 28. (Mitomycin* or NSC‐26980 or NSC 26980 or NSC26980 or Mutamycin or Ametycine or Mitocin‐C or MitocinC or mytomycin* or mitomicin* or mytomicin* or MMC).tw. 29. exp Mitomycins/ 30. limit 29 to yr="1966 ‐ 1991" 31. antimetabolites/ 32. Antimetabolites, Antineoplastic/ 33. Nucleic Acid Synthesis Inhibitors/ 34. (Antimetabolite* or anti‐metabolite*).tw. 35. (Antifibrotic* or anti‐fibrotic*).tw. 36. 25 or 26 or 27 or 28 or 30 or 31 or 32 or 33 or 34 or 35 37. 11 and 18 and (24 or 36) The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE.com search strategy
1. 'randomized controlled trial'/exp 2. 'randomization'/exp 3. 'double blind procedure'/exp 4. 'single blind procedure'/exp 5. random*:ab,ti 6. 1 OR 2 OR 3 OR 4 OR 5 7. 'animal'/exp OR 'animal experiment'/exp 8. 'human'/exp 9. 7 AND 8 10. 7 NOT 9 11. 6 NOT 10 12. 'clinical trial'/exp 13. (clin* NEAR/3 trial*):ab,ti 14. ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti 15. 'placebo'/exp 16. placebo*:ab,ti 17. random*:ab,ti 18. 'experimental design'/exp 19. 'crossover procedure'/exp 20. 'control group'/exp 21. 'latin square design'/exp 22. 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 23. 22 NOT 10 24. 23 NOT 11 25. 'comparative study'/exp 26. 'evaluation'/exp 27. 'prospective study'/exp 28. control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti 29. 25 OR 26 OR 27 OR 28 30. 29 NOT 10 31. 30 NOT (11 OR 23) 32. 11 OR 24 OR 31 33. 'trabeculectomy'/exp 34. 'trabeculoplasty'/exp 35. 'trabeculotomy'/exp 36. trabeculectom*:ab,ti OR trabeculoplast*:ab,ti OR trabeculotom*:ab,ti OR goniotom*:ab,ti OR microtrabeculectom*:ab,ti 37. 'glaucoma surgery'/de 38. 'trabecular meshwork'/exp 39. (glaucoma* NEAR/5 (surg* OR filter* OR filtrate*)):ab,ti 40. 'filtering operation'/de 41. 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 OR 40 42. 'glaucoma drainage implant'/exp 43. (modif* NEAR/5 (trabeculectom* OR trabeculoplast* OR trabeculotom* OR goniotom* OR microtrabeculectom*)):ab,ti 44. 'politef'/exp 45. (Polytef or Politef or 'E PTFE' or EPTFE or PTFE or TFE or FEP or SOLX or polytetrafluoroethylen* or polytetrafluorethylen* or polytetrafluoroethen* or Fluoroflex or Fluoroplast or Ftoroplast or Halon or Polyfene or Tetron or Tarflen or 'GORE TEX' or Goretex or gortex or Teflon or Fluon or Ex‐press or ologen or Baerveldt or Krupin or Ahmed or Molteno or ExPress or 'collagen matrix' or 'collagen‐GAG' or 'collagen‐glycosaminoglycan copolymer matrix'):ab,ti 46. device*:ab,ti OR implant*:ab,ti OR shunt*:ab,ti OR valve*:ab,ti OR tube*:ab,ti 47. 42 OR 43 OR 44 OR 45 48. 'fluorouracil'/exp 49. 5fu:ab,ti OR '5 fu':ab,ti OR fluorouracil*:ab,ti OR fluoruracil*:ab,ti OR '5 hu':ab,ti OR adrucil:ab,ti OR carac:ab,ti OR efudix:ab,ti OR fluoro:ab,ti AND uracile:ab,ti OR 'fluoro uracile':ab,ti OR efudex:ab,ti OR fluoroplex:ab,ti OR flurodex:ab,ti OR fluracedyl:ab,ti OR 'haemato fu':ab,ti OR neofluor:ab,ti OR onkofluor:ab,ti OR ribofluor:ab,ti OR '5 fluorouracil':ab,ti OR '5 fluoro 2':ab,ti OR '4 pyrimidinedione':ab,ti OR '5 fu':ab,ti OR accusite:ab,ti OR 'actino hermal':ab,ti OR effluderm:ab,ti OR efurix:ab,ti OR f6627:ab,ti OR fivoflu:ab,ti OR fluoroblastin:ab,ti OR fluouracil:ab,ti OR fluoxan:ab,ti OR fluracil:ab,ti OR fluracilium:ab,ti OR fluril:ab,ti OR 'fluro uracil':ab,ti OR fluroblastin:ab,ti OR ifacil:ab,ti OR 'nsc 18913':ab,ti OR 'nsc 19893':ab,ti OR 'nsc18913':ab,ti OR nsc19893:ab,ti OR 'oncofu':ab,ti OR 'ro 2‐9757':ab,ti OR 'ro 2 9757':ab,ti OR 'ro2‐9757':ab,ti OR 'ro2 9757':ab,ti OR uflahex:ab,ti OR utoral:ab,ti OR verrumal:ab,ti OR '51 21 8':ab,ti 50. 'mitomycin'/exp 51. mitomycin*:ab,ti OR 'nsc 26980':ab,ti OR nsc:ab,ti AND 26980:ab,ti OR nsc26980:ab,ti OR mutamycin:ab,ti OR ametycine:ab,ti OR 'mitocin c':ab,ti OR mitocinc:ab,ti OR mytomycin*:ab,ti OR mitomicin*:ab,ti OR mytomicin*:ab,ti OR mmc:ab,ti OR datisan:ab,ti OR metomit:ab,ti OR mitocyna:ab,ti OR mitosol:ab,ti OR mixandex:ab,ti OR mytocine:ab,ti OR mytozytrex:ab,ti OR vetio:ab,ti OR '1404 00 8':ab,ti 52. 'antimetabolite'/de 53. 'antineoplastic antimetabolite'/de 54. 'nucleic acid synthesis inhibitor'/de 55. antimetabolite*:ab,ti OR (anti NEAR/1 metabolite*):ab,ti 56. antifibrotic*:ab,ti OR (anti NEAR/1 fibrotic*):ab,ti 57. 48 OR 49 OR 50 OR 51 OR 52 OR 53 OR 54 OR 55 OR 56 58. 32 AND 41 AND (47 OR 57)
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 (Trabeculectom*[tiab] OR Trabeculoplast*[tiab] OR Trabeculotom*[tiab] OR Goniotom*[tiab] OR Microtrabeculectomy[tiab]) NOT MEDLINE[sb] #3 (Glaucoma*[tiab] AND (surge*[tiab] OR surgi*[tiab] OR filter*[tiab] OR filtrate*[tiab])) NOT MEDLINE[sb] #4 #2 OR #3 #5 (modif*[tiab] AND (Trabeculectom*[tiab] OR Trabeculoplast*[tiab] OR Trabeculotom*[tiab] OR Goniotom*[tiab] OR Microtrabeculectomy[tiab])) NOT MEDLINE[sb] #6 (Polytef[tiab] OR Politef[tiab] OR "E PTFE"[tiab] OR EPTFE[tiab] OR PTFE[tiab] OR TFE[tiab] OR FEP[tiab] OR SOLX[tiab] OR polytetrafluoroethylen*[tiab] OR polytetrafluorethylen*[tiab] OR polytetrafluoroethen*[tiab] OR Fluoroflex[tiab] OR Fluoroplast[tiab] OR Ftoroplast[tiab] OR Halon[tiab] OR Polyfene[tiab] OR Tetron[tiab] OR Tarflen[tiab] OR "GORE TEX"[tiab] OR Goretex[tiab] OR gortex[tiab] OR Teflon[tiab] OR Fluon[tiab] OR Ex‐press[tiab] OR ologen[tiab] OR Baerveldt[tiab] OR Krupin[tiab] OR Ahmed[tiab] OR Molteno[tiab] OR ExPress[tiab] OR collagen matrix[tiab] OR collagen‐GAG[tiab] OR collagen‐glycosaminoglycan copolymer matrix[tiab]) NOT MEDLINE[sb] #7 (Device*[tiab] OR implant*[tiab] OR shunt*[tiab] OR valve*[tiab] OR tube[tiab] OR tubes[tiab]) NOT MEDLINE[sb] #8 (5FU[tiab] OR 5‐FU[tiab] OR Fluorouracil*[tiab] OR Fluoruracil*[tiab] OR 5‐HU[tiab] OR Adrucil[tiab] OR Carac[tiab] OR Efudix[tiab] OR Fluoro Uracile[tiab] OR Fluoro‐Uracile[tiab] OR Efudex[tiab] OR Fluoroplex[tiab] OR Flurodex[tiab] OR Fluracedyl[tiab] OR Haemato‐fu[tiab] OR Neofluor[tiab] OR Onkofluor[tiab] OR Ribofluor[tiab] OR 5‐Fluorouracil[tiab]) NOT MEDLINE[sb] #9 (Mitomycin*[tiab] OR NSC‐26980[tiab] OR NSC 26980[tiab] OR NSC26980[tiab] OR Mutamycin[tiab] OR Ametycine[tiab] OR Mitocin‐C[tiab] OR MitocinC[tiab] OR mytomycin*[tiab] OR mitomicin*[tiab] OR mytomicin*[tiab] OR MMC[tiab]) NOT MEDLINE[sb] #10 (Antimetabolite*[tiab] OR anti‐metabolite*[tiab]) NOT MEDLINE[sb] #11 (Antifibrotic*[tiab] OR anti‐fibrotic*[tiab]) NOT MEDLINE[sb] #12 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 #1 AND #4 AND #12
Appendix 5. LILACS Controlled Trials search strategy
(Trabeculectom$ or Trabeculoplast$ or Trabeculotom$ or Goniotom$ or Microtrabeculectom$ or "trabecular meshwork" or "filtering surgery" or glaucoma$) AND (Polytef or Politef or "E PTFE"or EPTFE or PTFE or TFE or FEP or SOLX or polytetrafluoroethylen$ or polytetrafluorethylen$ or polytetrafluoroethen$ or Fluoroflex or Fluoroplast or Ftoroplast or Halon or Polyfene or Tetron or Tarflen or "GORE TEX" or Goretex or gortex or Teflon or Fluon or Ex‐press or ologen or Baerveldt or Krupin or Ahmed or Molteno or ExPress or "collagen matrix" or "collagen‐GAG" or "collagen‐glycosaminoglycan copolymer matrix" or Device$ or implant$ or shunt$ or valve$ or tube$ or (modif$ and Trabeculectom$ or Trabeculoplast$ or Trabeculotom$ or Goniotom$ or Microtrabeculectom$) or Fluorouracil$ or 5FU or 5‐FU or Fluoruracil$ or 5‐HU or Adrucil or Carac or Efudix or Fluoro Uracile or Fluoro‐Uracile or Efudex or Fluoroplex or Flurodex or Fluracedyl or Haemato‐fu or Neofluor or Onkofluor or Ribofluor or 5‐Fluorouracil or Mitomycin$ or NSC‐26980 or NSC 26980 or NSC26980 or Mutamycin or Ametycine or Mitocin‐C or MitocinC or mytomycin$ or mitomicin$ or mytomicin$ or MMC or Antimetabolite$ or anti‐metabolite$ or Antifibrotic$ or anti‐fibrotic$)
Appendix 6. metaRegister of Controlled Trials search strategy
(Trabeculectomy OR (glaucoma surgery)) AND (device OR implant OR implants OR shunt OR valve OR tube OR 5FU OR 5‐FU OR Fluorouracil OR Fluoruracil OR Fluoro Uracile OR 5‐Fluorouracil OR Mitomycin OR MMC OR Antimetabolite OR Antimetabolites OR Antifibrotic)
Appendix 7. ClinicalTrials.gov search strategy
(search terms) Trabeculectomy OR Trabeculoplasty OR Trabeculotomy OR Goniotomy OR Microtrabeculectomy OR glaucoma
(intervention) Device OR implant OR implants OR shunt OR valve OR tube OR Fluorouracil OR 5‐ Fluorouracil OR 5‐FU OR Fluoruracil OR Mitomycin OR mytomycin OR mitomicin OR mytomicin OR MMC OR Antimetabolite OR Antifibrotic
Appendix 8. ICTRP search strategy
(condition) Trabeculectomy OR Trabeculoplasty OR Trabeculotomy OR Goniotomy OR Microtrabeculectomy OR Goniotomy OR Microtrabeculectomy OR glaucoma (intervention) Device OR implant OR implants OR shunt OR valve OR tube OR Fluorouracil OR 5‐ Fluorouracil OR 5‐FU OR Fluoruracil OR Mitomycin OR mytomycin OR mitomicin OR mytomicin OR MMC OR Antimetabolite OR Antifibrotic
Data and analyses
Comparison 1. Trabeculectomy (Trab) + Ex‐PRESS versus Trabeculectomy (Trab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative IOP at one year | 3 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.74, ‐0.42] |
| 2 Postoperative IOP at six months | 3 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.90, 1.26] |
| 3 Postoperative IOP at two years | 3 | 212 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.52, ‐0.37] |
| 4 Postoperative logMAR BCVA at one year | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 5 Complications | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Hypotony | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.63, 1.33] |
| 5.2 Shallow/flat anterior chamber | 4 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.40, 1.32] |
| 5.3 Bleb leakage | 4 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.50, 3.20] |
| 5.4 Hyphema | 4 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.12, 0.94] |
| 5.5 Cataract surgery | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.74] |
Comparison 2. Trabeculectomy (Trab) + Ologen versus Trabeculectomy (Trab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 IOP at one year | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Mean change of IOP from baseline to one year | 2 | 44 | Mean Difference (Random, 95% CI) | ‐0.32 [‐5.88, 5.24] |
| 1.2 Postoperative IOP at one year | 5 | 177 | Mean Difference (Random, 95% CI) | 1.40 [‐0.57, 3.38] |
| 2 Postoperative IOP at day one | 5 | 162 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐1.95, 2.97] |
| 3 Postoperative IOP at six months | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Change of IOP from baseline to six months | 2 | 46 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐6.23, 3.76] |
| 3.2 Postoperative IOP at six months | 5 | 236 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.97, 1.84] |
| 4 Postoperative IOP at two years | 2 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.29, 1.69] |
| 5 Complications | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Hypotony | 6 | 233 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.47, 1.19] |
| 5.2 Surgical revision within 3 months | 4 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.38, 7.63] |
| 5.3 Blebitis or endophthalmitis | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.25, 9.70] |
| 5.4 Bleb leakage | 4 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.33, 2.20] |
| 5.5 Hyphema | 6 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.51, 4.19] |
| 5.6 Choroidal detachment | 4 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.09] |
| 5.7 Shallow anterior chamber | 5 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.32, 1.93] |
| 5.8 Anterior chamber reaction | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.56, 2.60] |
| 5.9 Positive Seidel test | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.32, 11.54] |
| 5.10 Tenon's cysts | 3 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.21, 3.66] |
Comparison 3. Trabeculectomy (Trab) + AMT (amniotic membrane) versus Trabeculectomy (Trab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative IOP at one year | 9 | 356 | Mean Difference (IV, Random, 95% CI) | ‐3.92 [‐5.41, ‐2.42] |
| 1.1 with MMC in both groups | 4 | 154 | Mean Difference (IV, Random, 95% CI) | ‐3.93 [‐4.91, ‐2.95] |
| 1.2 without MMC in both groups | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐7.79, ‐1.51] |
| 1.3 with MMC in the trabeculectomy group only | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.21, 1.61] |
| 2 Postoperative IOP at one day | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 with MMC in both groups | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 without MMC in both groups | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 with MMC in the trabeculectomy group only | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Postoperative IOP at one week | 13 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 with MMC in both groups | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 without MMC in both groups | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 with MMC in the trabeculectomy group only | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Postoperative IOP at one month | 13 | 646 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.96, ‐0.13] |
| 4.1 with MMC in both groups | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐1.84, ‐0.28] |
| 4.2 without MMC in both groups | 7 | 370 | Mean Difference (IV, Random, 95% CI) | ‐1.78 [‐3.65, 0.10] |
| 4.3 with MMC in the trabeculectomy group only | 3 | 145 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐1.21, 2.09] |
| 5 Postoperative IOP at three months | 11 | 551 | Mean Difference (IV, Random, 95% CI) | ‐2.23 [‐2.93, ‐1.53] |
| 5.1 with MMC in both groups | 3 | 131 | Mean Difference (IV, Random, 95% CI) | ‐2.64 [‐3.50, ‐1.77] |
| 5.2 without MMC in both groups | 6 | 320 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐3.89, ‐1.07] |
| 5.3 with MMC in the trabeculectomy group only | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.65, 1.14] |
| 6 Postoperative IOP at six months | 13 | 613 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐3.34, ‐1.67] |
| 6.1 with MMC in both groups | 4 | 155 | Mean Difference (IV, Random, 95% CI) | ‐2.91 [‐3.87, ‐1.95] |
| 6.2 without MMC in both groups | 7 | 358 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐4.52, ‐1.48] |
| 6.3 with MMC in the trabeculectomy group only | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐1.27, 1.55] |
| 7 Postoperative IOP at two years | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 with MMC in the trabeculectomy group only | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.16, 1.76] |
| 7.2 without MMC in both groups | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐5.52, ‐0.40] |
| 8 Complications | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Hypotony | 5 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.17, 0.94] |
| 8.2 Shallow anterior chamber | 13 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.73] |
| 8.3 Hyphema | 5 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.34] |
| 8.4 Bleb leakage | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.79] |
| 8.5 Encapsulated blebs | 5 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.08, 0.69] |
| 8.6 Choroidal detachment | 4 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.13, 1.71] |
Comparison 4. Trabeculectomy (Trab) + E‐PTFE versus Trabeculectomy (Trab).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative IOP at one year | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐1.76, 0.88] |
| 1.1 with MMC in both groups | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.58, 1.78] |
| 1.2 without MMC in both groups | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐3.42, 0.82] |
| 2 Hypotony at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.11, 0.77] |
| 2.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.14, 1.35] |
| 2.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.02] |
| 3 Hyphaema at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.37, 1.74] |
| 3.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.27, 2.41] |
| 3.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.27, 2.41] |
| 4 Inflammation at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.50, 3.91] |
| 4.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.36, 4.97] |
| 4.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.73] |
| 5 Shallow anterior chamber at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.15, 1.29] |
| 5.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.33] |
| 5.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.75] |
| 6 Flat anterior chamber at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.28] |
| 6.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.55] |
| 6.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 7 Choroidal detachment at 24 months | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.51] |
| 7.1 with MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.98] |
| 7.2 without MMC in both groups | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birt 1998.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 43 eyes of 43 participants total; 11 participants in trabeculectomy + MMC + Gelfilm® (Pfizer) group; 7 participants in trabeculectomy + MMC group; 11 participants in trabeculectomy + Gelfilm® (Pfizer) group; 14 participants in trabeculectomy group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 43 participants total; 11 participants in trabeculectomy + MMC + Gelfilm® (Pfizer) group; 7 participants in trabeculectomy + MMC group; 11 participants in trabeculectomy + Gelfilm® (Pfizer) group; 14 participants in trabeculectomy group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: not reported
Mean age: not reported
Gender: not reported
Inclusion criteria: not reported
Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + Gelfilm® (Pfizer)
Intervention 2: trabeculectomy + MMC
Intervention 3: trabeculectomy + Gelfilm® (Pfizer)
Intervention 4: trabeculectomy Length of follow‐up: Planned: not reported Actual: 1 year |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: postoperative IOP, use of postoperative 5‐FU, and complications Intervals at which outcomes assessed: 1 year |
|
| Notes |
Publication type: published abstract Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Attempted to contact author, but unable to find contact information in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | Outcome assessors not masked as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed 1‐year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available and the authors did not define which outcomes and complications they were going to report |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest The abstract did not have enough information |
Bruno 2008.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 19 eyes of 19 participants total; not reported by intervention group Exclusions after randomization: 2 participants not analyzed due to death or protocol deviation Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 17 participants total; 9 participants in trabeculectomy + MMC + AMT group; 8 participants in trabeculectomy + MMC group How were missing data handled?: 2 participants excluded from analysis Power calculation: not reported |
|
| Participants |
Country: not reported
Mean age: 72 years; not reported by intervention group
Gender: 7/19 (37%) men and 12/19 (63%) women; not reported by intervention group
Inclusion criteria: OAG at high risk for filtration failure; previous failed surgery
Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + AMT
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: target IOP, complications, association between positional IOP change (IOP supine‐IOP sitting) and bleb morphology, and comparing positional IOP changes in successful versus failed trabs Intervals at which outcomes assessed: 2 and 6 months |
|
| Notes |
Publication type: published abstract Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Attempted to contact author, but unable to find contact information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater than 10% of participants were excluded from the analysis |
| Selective reporting (reporting bias) | Unclear risk | Unclear as only published abstract was available; protocol not published. Authors did publish outcome results as defined in the abstract |
| Other bias | Unclear risk | Did not report source of support and conflict of interest The abstract did not have enough information |
Cai 2012.
| Methods |
Study design: parallel‐group randomized controlled trial (8 participants both eyes included) Number randomized: 48 eyes of 40 participants total; 25 eyes in trabeculectomy + AMT group; 23 eyes in trabeculectomy group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: eye Number analyzed: 48 eyes of 40 participants total; 25 eyes in trabeculectomy + AMT group; 23 eyes in trabeculectomy group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: China Mean age: 52 years (range 22 ‐ 75 years); not reported by intervention group Gender: 30/40 (75%) men and 10/40 (25%) women; not reported by intervention group Inclusion criteria: refractory glaucoma treatment Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcome, as reported: IOP, filtering bleb, visual acuity, complications Intervals at which outcomes assessed: IOP at 1 week, 1 month and 1 year; filtering bleb and visual acuity at 1 year; complications and anterior chamber condition at 1 week |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: February 2010 to December 2011 Subgroup analyses: none reported Publication language: Chinese Attempted to contact authors to clarify methods to assess risk of bias, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From the published report we can see all data were analyzed |
| Selective reporting (reporting bias) | High risk | Protocol was not available and outcomes were not defined |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest No other potential bias identified |
Cho 2013.
| Methods |
Study design: parallel‐group randomized controlled trial (5 participants both eyes included) Number randomized: 52 eyes of 47 participants total; 26 eyes of 25 participants in trabeculectomy + AMT group; 26 eyes of 22 participants in trabeculectomy + MMC group Exclusions after randomization: none reported Losses to follow‐up: 13 eyes total; 4 eyes in trabeculectomy + AMT group; 9 eyes in trabeculectomy + MMC group Unit of analysis: eye Number analyzed: 39 eyes total; 22 eyes in trabeculectomy + AMT group; 17 eyes in trabeculectomy + MMC group How were missing data handled?: 13 eyes with missing data excluded from analysis Power calculation: not reported |
|
| Participants |
Country: China Mean age: 64 years; 63.9 years for trabeculectomy + AMT group; 64.0 years for trabeculectomy + MMC group Gender: 22/52 (42%) men and 30/52 (58%) women; 8/26 (31%) men and 18/26 (69%) women in trabeculectomy + AMT group; 14/26 (54%) men and 12/26 (46%) women in trabeculectomy + MMC group Inclusion criteria: PACG, chronic ACG, or POAG; IOP uncontrolled after medical or laser treatment; age between 18 and 80 years; signed consent form Exclusion criteria: history of diabetes, leukemia, AIDS, uncontrolled blood pressure, atherosclerosis, immunological disease, Alzheimer disease, disseminated sclerosis, previous trabeculectomy, or other ongoing ocular disease Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 3 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: IOP, anterior chamber depth, filtering bleb shape, operative efficacy, and complications (specified) Intervals at which outcomes assessed: non‐contact IOP at 1 week, 2 weeks, 1 month, and 3 months after surgery; filtering bleb and complications at 1 week, 2 weeks, 1 month, and 3 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: October 2009 to October 2011 Subgroup analyses: none reported Publication language: Chinese Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors used “random number tables” for sequence generation |
| Allocation concealment (selection bias) | Low risk | The authors used “opaque envelopes” for allocation concealment |
| Masking of participants and personnel (performance bias) | Unclear risk | Method used to mask participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be easily seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/26 participants (35%) in trabeculectomy + MMC and 4/22 (18%) in trabeculectomy + AMT were lost to follow‐up at 3 months |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. The study used t‐test for 2 independent samples, while they included both eyes of some participants but did not account for intra‐person correlation. No other sources of bias identified |
Cillino 2008.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 60 eyes of 60 participants; 15 eyes of 15 participants in each of the 4 groups Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 60 participants total; 15 participants in each of the 4 groups How were missing data handled?: no missing data Power calculation: a power of 90% or greater to detect at least a 3 mm Hg IOP difference among groups |
|
| Participants |
Country: Italy
Mean age: 68 years; 65.3 years for trabeculectomy + MMC + E‐PTFE group; 68.1 years for trabeculectomy + MMC group; 67.2 years for trabeculectomy + E‐PTFE group; 71.1 years for trabeculectomy group; Gender: 24/45 (53%) men and 21/45 (47%) women; 6/15 (40%) men and 9/15 (60%) women in trabeculectomy + MMC + E‐PTFE; 9/15 (60%) men and 6/15 (40%) women in trabeculectomy + MMC; 7/15 (47%) men and 8/15 (53%) women in trabeculectomy + E‐PTFE; 8/15 (53%) men and 7/15 (47%) women in trabeculectomy Inclusion criteria: “age 18 or older, diagnosis of POAG or pseudoexfoliative glaucoma (PEXG), and inadequate IOP control (IOP > 21 mm Hg) or progressive visual field deterioration on maximum‐tolerated medical therapy, availability, willingness and sufficient cognitive awareness to comply with examination procedures” Exclusion criteria: “concurrent participation during the last 30 days in any other clinical trial, use of systemic or ocular medications that may affect vision, acute or chronic disease or illness that would increase the operative risk or confound the outcomes of the study (e.g. immunodeficiency, connective tissue disease, diabetes, etc.), uncontrolled systemic or ocular disease other than glaucoma, clinically significant cataract where combined surgery was indicated, history of ocular trauma or prior ocular surgery.” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + E‐PTFE (GORE PRECLUDE®) Intervention 2: trabeculectomy + MMC Intervention 3: trabeculectomy + E‐PTFE (GORE PRECLUDE®) Intervention 4: trabeculectomy Length of follow‐up: Planned: 24 months Actual: 24 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: IOP, complete success (with medication ≤ 21 and without medication 17 mm Hg), complications, number of antiglaucoma medications, and visual field testing by Humphrey visual field Intervals at which outcomes assessed: 24 hours, 7 days, 2 weeks, 3 weeks, and 1, 2, 3, 6, 12, 18, and 24 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: September 2003 to August 2004 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Randomization was determined just before surgery. Sixty participants – 15 for each surgical technique – were randomly assigned based on a surgical chart number to undergo a trabeculectomy (T) (which served as surgical control group), a trabeculectomy with mitomycin‐C (TMMC), a trabeculectomy with GORE PRECLUDE pericardial implant (TG) or a trabeculectomy with mitomycin‐C and GORE PRECLUDE pericardial implant (TGMMC).” The method of sequence generation was not done completely randomly, thus we assessed it at high risk |
| Allocation concealment (selection bias) | Low risk | “Randomization was determined just before surgery by sealed‐envelope technique.” |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed and differences in the surgical protocol of the 2 groups were minimized, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be easily seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All participants completed the 24 month follow‐up period.” |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. In the Methods section, the study mentioned testing of visual field by Humphrey visual field, however, the Result section did not report this outcome |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest |
Cillino 2011.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 40 eyes of 40 participants total; 20 eyes of 20 participants in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 40 participants total; 20 participants in each group How were missing data handled?: no missing data Power calculation: a power of 90% to detect 3 mm Hg IOP difference between groups |
|
| Participants |
Country: Italy
Mean age: 64.5 years; 63.2 years for trabeculectomy + Ologen® group; 65.8 years for trabeculectomy + MMC group Gender: 23/40 (58%) men and 17/40 (42%) women; 12/20 (60%) men and 8/20 (40%) women in trabeculectomy + Ologen® group; 11/20 (55%) men and 9/20 (45%) women in trabeculectomy + MMC group Inclusion criteria: “age 18 or older, diagnosis of POAG or pseudoexfoliative glaucoma (PEXG), and inadequate IOP control (IOP > 21 mm Hg) or progressive visual field deterioration on maximum‐tolerated medical therapy” Exclusion criteria: “normal‐tension glaucoma, use of systemic or ocular medications that might affect vision, acute or chronic disease that could confound the outcomes of the study (e.g., immunodeficiency, connective tissue disease, and diabetes), clinically significant cataract where combined surgery was indicated, and history of ocular trauma or prior ocular surgery” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (Aeon Astron Europe BV, Leiden, The Netherlands) Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 24 months Actual: 24 months |
|
| Outcomes |
Primary outcomes, as defined: IOP and surgical success
“IOP was the primary outcome measure” and 3 different IOP target levels were considered: ≤ 21, ≤ 17, and ≤ 15 mm Hg. "Complete success" defined as a target endpoint IOP without antiglaucomatous medications, while "qualified success" defined as a target endpoint IOP regardless of medications Secondary outcomes, as defined: bleb evaluation, number of glaucoma medications, frequency of postoperative adjunctive procedures, and complications Intervals at which outcomes assessed: 24 hours, 7 days, 2 weeks, and 1, 2, 3, 6, 12, 18, and 24 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: "The authors declare no conflict of interest." Trial registry: not registered Study period: January 2008 to December 2008 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was determined just before surgery by sealed‐envelope technique based on their surgical chart number. The sequence of random allocation was generated by pulling 40 standard sized pieces of paper out of a hat by the trial statistician (AC). Twenty pieces of paper were marked with letter A, and 20 with letter B. Each piece of paper was sequentially placed into 40 sealed, opaque envelopes by the trial statistician. The sealed envelopes were numbered 1 to 40 and given to the surgeon (SC). Patients were numbered randomly from 1 to 40 based on a surgical chart number related to the baseline testing session and intervention period." |
| Allocation concealment (selection bias) | Low risk | Same support for judgment listed above |
| Masking of participants and personnel (performance bias) | Low risk | Given that trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, a strict surgical protocol was followed, thus minimizing risk |
| Masking of outcome assessment (detection bias) | Low risk | “The clinical data collecting and measurement of outcome variables were performed by skilled personnel (ophthalmologists and optometrists) masked to randomization and who had not been directly involved in patient surgery.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All patients completed the 24‐month follow‐up period.” |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. In the Methods section, the study stated that number of glaucoma medications and frequency of postoperative adjunctive procedures was measured, but in the results, they were not reported |
| Other bias | Unclear risk | Information regarding funding source was not reported. No other sources of bias identified |
Dahan 2012.
| Methods |
Study design: paired‐eye randomized controlled trial
Number randomized: 30 eyes of 15 participants total; each participant had 1 eye in each intervention group Exclusions after randomization: none reported Losses to follow‐up: none up to 1 year after surgery; 1 participant died at 13 months and 2 were subsequently lost to follow‐up Unit of analysis: eye Number analyzed: 30 eyes of 15 participants total; each participant had one eye in each intervention group How were missing data handled?: no missing data at 1 year Power calculation: a power of 96% to detect a 2 mm Hg IOP difference between groups |
|
| Participants |
Country: South Africa
Mean age: 65 years; not reported by intervention group
Gender: 10/15 (67%) men and 5/15 (33%) women; not reported by intervention group
Inclusion criteria: “at least 18 years of age and presented with medically uncontrolled POAG requiring bilateral incisional surgery for IOP reduction. Patients with prior cataract operation or failed filtration surgery in either eye were eligible if surgery took place at least 3 months prior to enrolment.”
Exclusion criteria: “any form of glaucoma other than POAG; history of active uveitis; and any ocular abnormality that would preclude accurate IOP assessment.” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + Ex‐PRESS X200
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: a minimum of 1 year Actual: all participants followed at least 1 year; the longest follow‐up visit for a participant was 30 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: IOP, visual acuity, number of medicines for IOP control, complications Intervals at which outcomes assessed: 1 day, 7 days, and 1, 3, 6, 9, 12, 18, 24, and 30 months after surgery |
|
| Notes |
Publication type: published article Funding sources: “the study was supported by a financial grant from Alcon Laboratories” Disclosures of interest: "E Dahan is a paid consultant in Alcon Laboratories. GJ Ben Simon and A Lafuma has no financial or proprietary interest in any of the drugs or materials mentioned in this study. A Lafuma is employed by CEMKAEVAL, a company that provides services in statistical analyses and epidemiology." Trial registry:NCT00698438 (clinicaltrials.gov) Study period: not reported Subgroup analyses: none reported Publication language: English Study authors contacted and outcome data shared (IOP reduction, number of medications, and mean IOP at 1 day, 7 days, and 1, 3, 6, 12, 18, 24, and 30 months follow‐up) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation of contralateral operations was achieved by opening an envelope in which the procedure (trabeculectomy or Ex‐PRESS implantation) that would be applied to the first eye was stated, thereby determining the procedure in the other eye.” The method of randomization is not described and thus its adequacy cannot be judged |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that “After sub‐tenonian local anaesthesia, surgery was performed by one experienced surgeon (ED), for consistency, using a standardized technique for both procedures”, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | The study mentioned: “It was not possible to mask the surgical technique as trabeculectomy is easily differentiated from Ex‐PRESS implantation during postoperative follow‐ups. However, this limitation is overcome by the fact that all patients were followed up concurrently by their referring ophthalmologists from the first month postoperatively till completion of the study.” Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All 15 patients were followed‐up for 1 year after surgery. One patient died 13 months after surgery and two patients were subsequently lost to follow‐up. All data available for these patients (i.e., up to 1 year) are included in the analyses.” |
| Selective reporting (reporting bias) | Low risk | The study was registered in www.ClinicalTrials.gov. All defined outcomes in www.ClinicalTrials.gov were reported in full text. Complete and qualified success was reported and defined using IOP |
| Other bias | High risk | Received industry monetary support from device manufacturer; “ED is a paid consultant to Alcon Laboratories.” “The study was supported by a financial grant from Alcon Laboratories.” To consider intra‐person correlation between eyes, the analysis used Wilcoxon matched‐pairs t‐test to compare pre‐operative and final IOP values No other sources of bias identified |
De Jong 2005.
| Methods |
Study design: parallel‐group randomized controlled trial (11 participants both eyes included) Number randomized: 120 eyes of 109 participants; not reported by intervention group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: Netherlands
Mean age: overall not reported; 61.8 years for trabeculectomy + Ex‐PRESS R50® implanted under a scleral flap group; 61.8 years for trabeculectomy + Ex‐PRESS R50® implanted under the conjunctiva group; 68.7 years for trabeculectomy group; Gender: not reported Inclusion criteria: OAG; medical treatment failure, indicated for glaucoma surgery Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ex‐PRESS R50® implanted under a scleral flap
Intervention 2: trabeculectomy + Ex‐PRESS R50® implanted under the conjunctiva Intervention 3: trabeculectomy Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: success rate (defined as % IOP reduction and medication reduction), IOP, and use of IOP‐lowering medications Intervals at which outcomes assessed: 6 months after surgery |
|
| Notes |
Publication type: published abstract Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Attempted to contact author, but unable to find contact information in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Method use to mask participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be easily seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. Not enough information from the abstract |
De Jong 2009.
| Methods |
Study design: parallel‐group randomized controlled trial (2 participants both eyes included)
Number randomized: 80 eyes of 78 participants total; 40 eyes in each group Exclusions after randomization: none reported Losses to follow‐up: 5 eyes total at 1 year; 3 eyes in trabeculectomy + Ex‐PRESS group; 2 eyes in trabeculectomy group Unit of analysis: eye Number analyzed: 75 eyes total; 37 eyes in trabeculectomy + Ex‐PRESS group; 38 eyes in trabeculectomy group How were missing data handled?: 5 eyes with missing data excluded from analysis Power calculation: a power of 80% to detect 32% between‐group difference in IOP |
|
| Participants |
Country: Netherlands
Mean age: 66 years; 62.3 years for trabeculectomy + Ex‐PRESS group; 68.9 years for trabeculectomy group Gender: 46/80 (58%) men and 34/80 (42%) women; 19/40 (48%) men and 21/40 (52%) women in trabeculectomy + Ex‐PRESS group; 27/40 (68%) men and 13/40 (32%) women in trabeculectomy group Inclusion criteria: “over 18 years old, and had a diagnosis of open‐angle glaucoma that could not be controlled with maximal‐tolerated medical therapy” Exclusion criteria: “any other ocular disease or previous ocular surgery other than cataract extraction” Equivalence of baseline characteristics: not reported |
|
| Interventions |
Intervention 1: trabeculectomy + Ex‐PRESS (Optonol Ltd., Neve Ilan, Israel)
Intervention 2: trabeculectomy Length of follow‐up: Planned: 5 years Actual: mean of 262 weeks for Ex‐PRESS group and 266 weeks for the trabeculectomy group |
|
| Outcomes |
Primary outcome, as defined: complete success (final IOP > 4 mm Hg and ≤ 18 mm Hg without antiglaucoma medication) and overall success (final IOP > 4 mm Hg and ≤ 18 mm Hg with or without medications) Secondary outcomes, as defined: IOP, postoperative medication use, surgical failure (IOP > 18 mm Hg or the requirement for further glaucoma surgery), stringent target (final IOP > 4 mm Hg and ≤ 15 mm Hg), complications, and visual acuity Intervals at which outcomes assessed: 1 day, 1 week, 1, 3, and 6 months, and 1, 2, 3, 4, and 5 years after surgery |
|
| Notes |
Publication type: published article Funding sources: Alcon Management SA, Geneva, Switzerland Disclosures of interest: "L. de J. has no proprietary interest in any of the products mentioned here." Trial registry: not registered Study period: October 2003 to November 2004 Subgroup analyses: none reported Publication language: English Authors contacted to retrieve number of participants lost to follow‐up at 2 to 5 years, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The participants were assigned randomly to receive either Ex‐PRESS implantation under a scleral flap (Group A), or trabeculectomy (Group B) in the study eye, according to a computer‐generated randomization list.” “Randomization was determined before surgery according to a block randomization sequence prepared by SAS (version 9.1; SAS Institute Inc., Cary, NC, USA).” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed and differences in the surgical protocol of the 2 groups were minimized, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | “Secondly, the evaluator was not blinded to the procedure used in each case; however, it is difficult to carry out truly blinded evaluation as the type of surgery used is usually visible to the assessor.” But how they controlled the risk was not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There are 2 articles related to this study, as reported in 2009, the number analyzed was 40 eyes per treatment group (no loss to follow‐up); however, as reported in 2011, the number analyzed was 38 eyes per treatment group. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Total industry support but not other source of potential bias identified. “There were no significant differences between the two groups except for age; the Ex‐PRESS group (Group A) included significantly younger patients compared with the trabeculectomy group (Group B).” Age‐adjusted values are reported in de Jong 2011 and do not significantly change the results |
Eliezer 2006.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 32 eyes of 32 participants total; 16 eyes of 16 participants in each group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: individual (1 eye per participant) Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: Brazil
Mean age: 68 years; 68.3 years for trabeculectomy + AMT group; 67.6 years for trabeculectomy group Gender: 19/32 (59%) men and 13/32 (41%) women; 8/16 (50%) men and 8/16 (50%) women in trabeculectomy + AMT group; 11/16 (69%) men and 5/16 (31%) women in trabeculectomy group Inclusion criteria: POAG Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT
Intervention 2: trabeculectomy Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: IOP, number of glaucoma medications, visual acuity, and appearance of bleb Intervals at which outcomes assessed: 1 day, 1 week, and 1, 2, 3, 4, 5, 6 and 12 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: August 2001 to August 2003 Subgroup analyses: none reported Publication language: English Authors contacted for the number of participants lost to follow‐up at 12 months, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Thirty‐two white patients with primary open‐angle glaucoma (POAG) scheduled for glaucoma filtration surgery at the Glaucoma Service of the “Santa Casa de São Paulo” from August 2001 to August 2003 were randomly assigned to two groups.” The method of sequence generation is not described, thus its adequacy cannot be judged |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed, differences in the surgical protocol of the 2 groups were minimized, and the same 2 surgeons performed all surgeries, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | Not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “At the end of a 12‐month follow‐up period, in the study group two of 31 eyes (6.25%) exhibited flat, vascularized bleb, 14 eyes (45.16%) had elevated but not avascular blebs and nine eyes (56.25%) showed thin, avascular blebs.” This suggests that 31 of the 32 randomized participants completed the full 12‐month follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Complications not defined, but reported |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. Baseline IOP values not reported. |
Huang 2007.
| Methods |
Study design: parallel‐group randomized controlled trial (13 participants both eyes included) Number randomized: 63 eyes of 50 participants total; 36 eyes of 25 participants in the trabeculectomy + AMT group; 27 eyes of 25 participants in the trabeculectomy group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: not reported (range 46 to 68 years) Gender: 23/50 (46%) men and 27/50 (54%) women; by intervention group not reported Inclusion criteria: not reported (included participants were diagnosed with PACG) Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: depth of anterior chamber, filtering bleb, surgical success, postoperative IOP, and visual acuity Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, and 1, 3, and 6 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: "the authors declare no conflict of interest" Trial registry: not registered Study period: participants recruited from January 2010 and January 2011 Subgroup analyses: none reported Publication language: Chinese Authors contacted for the number of participants lost to follow‐up, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Complications were not defined, but reported |
| Other bias | Unclear risk | Did not report source of funding. This article provided nothing on study design; we contacted the authors but did not receive a response |
Ji 2013.
| Methods |
Study design: paired‐eye randomized controlled trial Number randomized: 34 eyes of 17 participants total; each participant had 1 eye in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: eye Number analyzed: 34 eyes of 17 participants total; each participant had 1 eye in each group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: China Mean age: 61.6 years; not reported by intervention group Gender: 10/17 (59%) men and 7/17 (41%) women; not reported by intervention group Inclusion criteria: at least 18 years of age; medically uncontrolled glaucoma requiring bilateral surgery for IOP reduction Exclusion criteria: history of active uveitis; any ocular abnormality that would influence accurate IOP assessment; refractory glaucoma and secondary glaucoma Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: 24 months Actual: 24 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcome, as defined: BCVA, IOP, complications, anti‐glaucoma drug usage, surgical success (IOP < 21 mm Hg without glaucoma medication), and morphology of the filtering bleb Intervals at which outcomes assessed: 1, 3, and 7 days and 1, 3, 6, 12, 18, and 24 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Author not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomisation of surgical procedures was achieved by opening an envelope in which the procedure (trabeculectomy or with AMT) that would be performed to the first eye was stated, thereby determining the procedure in the other eye.”, but no sequence generation method was reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Surgeons cannot be masked. Whether participants were masked was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. In the Methods section, the study mentioned measuring best‐corrected visual acuity; however, the Results section did not report this outcome |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. No other sources of bias was identified |
Khairy 2015.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 52 eyes of 52 participants total; 26 eyes of 26 participants in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 52 eyes of 52 participants total; 26 eyes of 26 participants in each group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: Egypt Mean age: 67.9 years; by intervention not reported Gender: 47/78 (60%) men and 31/78 (40%) women; 15/26 (58%) men and 11/26 (42%) women in trabeculectomy + AMT group; 32/52 (62%) men and 20/52 (38%) women in trabeculectomy + MMC group Inclusion criteria: age > 60 years; diagnosed clinically with bilateral OAG with IOP > 21; clinical evidence of glaucomatous optic disc cupping or visual field loss; BCVA of 6/36 or better in each eye; central corneal thickness measuring < 590 mm; open anterior chamber angle on gonioscopy; willing to attend the eye clinic at the timing required by the study design Exclusion criteria: other forms of OAG (pseudo‐exfoliation syndrome, pigment dispersion syndrome, etc.); history of chronic eye diseases; previous glaucoma surgical procedure; previous ocular laser or surgical treatment; history of systemic medical condition or medications that could affect the optic nerve or visual field Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 2 years Actual: 2 years |
|
| Outcomes |
Primary outcome, as defined: surgical success (IOP < 22 mm Hg or lowered 20% without medication) Secondary outcomes: bleb morphology Intervals at which outcomes assessed: 1 day, 7 days, 1, 3, and 6 months, 1 year, and 2 year after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: "authors declare no conflict of interest" Trial registry: not registered Study period: January 2010 to January 2011 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization process was carried out using 4 opaque envelops in 2 containers. One contained 2 envelops labelled AMT and MMC, and the other contained 2 envelops with the names of 2 patients listed for glaucoma surgery on that day. The 2 patients were randomized to one of the procedures by asking an independent person to choose 1 envelop from each container.” |
| Allocation concealment (selection bias) | High risk | Allocation not concealed because envelopes marked. See above. |
| Masking of participants and personnel (performance bias) | Low risk | Participants were masked; surgeons cannot be masked and this was not likely to cause any bias |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “There was no loss of follow‐up at any point during the 2 years of follow‐up for either group.” |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Complications not defined, but reported |
| Other bias | Unclear risk | Did not report source of funding and no conflict of interest. No other sources of bias identified |
Li 2010.
| Methods |
Study design: parallel‐group randomized controlled trial (12 participants both eyes included) Number randomized: 62 eyes of 50 participants total; 32 eyes in the trabeculectomy + MMC + AMT group; 30 eyes in the trabeculectomy + MMC group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: not reported (range 24 to 72 years); not reported by intervention group Gender: 28/50 (56%) men and 22/50 (44%) women; not reported by intervention group Inclusion criteria: refractory glaucoma Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, surgical success, postoperative IOP, visual acuity, and complications Intervals at which outcomes assessed: 1 week and 1, 3, 6, and 12 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted for the number of participants lost to follow‐up at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Visual acuity, and complications reported in the Results section were not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest This article provided nothing on study design; we contacted the authors but did not receive a response |
Liu 2009.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 35 eyes of 35 participants total; not reported by intervention group Exclusions after randomization: none reported Losses to follow‐up: 3 participants (abstract stated no losses to follow‐up, but main text reported 32 participants had follow‐up) Unit of analysis: individual (1 eye per participant) Number analyzed: 32 participants total; 16 participants in trabeculectomy + MMC + AMT group; 16 participants in trabeculectomy + MMC group How were missing data handled?: 3 participants excluded from analysis Power calculation: not reported |
|
| Participants |
Country: China Age: not reported Gender: not reported Inclusion criteria: refractory glaucoma Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: postoperative IOP, number of antiglaucoma medications, bleb morphology, and complications Intervals at which outcomes assessed: 1 day, 1 week, and 1, 3, 6, 9, and 12 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted for the number of participants lost to follow‐up at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | The study mentioned "double blinded". Because surgeons could not be masked, participants should have been masked |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/35 participants were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Number of antiglaucoma medication and complications reported in the Results section were not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. The study included 1 eye for each participant. No other potential bias identified |
Maheshwari 2012.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 40 eyes of 40 participants total; 20 eyes of 20 participants in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 40 eyes of 40 participants total; 20 eyes of 20 participants in each group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: India Age: not reported Gender: not reported Inclusion criteria: requiring glaucoma surgery for uncontrolled IOP in POAG Exclusion criteria: ACG, post‐traumatic,uveitic, neovascular, or dysgenetic glaucoma; allergy to collagen, preliminary conjunctival damage (trauma, vitreo–retinal surgery, previous glaucoma surgery, and other); under 18 years of age Equivalence of baseline characteristics: no; at 1 year, a mean IOP of 15.6 mm Hg in the Ologen group was with 43% reduction and a mean IOP of 10.5 mm Hg in the trabeculectomy group was with 50% group; based on this information, the baseline IOP in Ologen group seemed to be about 30% more than the trabeculectomy group |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen (brand not reported) Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcome, as reported: visual acuity, IOP measurement, filtering bleb, and complications Intervals at which outcomes assessed: 1 day and 1 year after the surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | Not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available. Visual acuity and filtering bleb were defined in the Methods section, but the results were not reported |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. The baseline IOP seemed not equivalent between the 2 groups. At 1 year, a mean IOP of 15.6 mm Hg in the Ologen group was with 43% reduction and a mean IOP of 10.5 mm Hg in the trabeculectomy group was with 50% group; based on this information, the baseline IOP in the Ologen group seemed to be about 30% more than the trabeculectomy group |
Marey 2013.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 60 eyes of 60 participants total; 30 eyes of 30 participants in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 60 eyes of 60 participants total; 30 eyes of 30 participants in each group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: Egypt
Mean age: 50 years; 50.2 years for trabeculectomy + Ologen® group; 49.7 years for trabeculectomy + MMC group Gender: 35/60 (%) men and 25/60 (%) women; 18/30 (%) men and 12/30 (%) women in trabeculectomy + Ologen® group; 17/30 (%) men and 13/30 (%) women in trabeculectomy + MMC group Inclusion criteria: “POAG, chronic angle‐closure glaucoma (CACG), uveitic glaucoma, pseudoexfoliation glaucoma (PEXG), and pseudophakic glaucoma not controlled medically (more than 21 mm Hg despite medications)” Exclusion criteria: “neovascular glaucoma, age < 18, and previous ocular surgery or laser procedures” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (Aeon Astron Group B.V. Leiden, The Netherlands)
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcome, as reported: IOP, bleb status, complications, absolute success (IOP ≤ 21 mm Hg without topical medication), and qualified success (IOP ≤ 21 mm Hg with topical medication) Intervals at which outcomes assessed: 1, 3, 6, 9, and 12 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: "no competing financial interests exist" Trial registry: not registered Study period: February 2009 to January 2011 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were randomly enrolled in 2 groups.” The method of sequence generation is not described, thus its adequacy cannot be judged |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | According to the Cochrane Handbook (8.11.1): “Blinding can be impossible for at least some people (e.g. most patients receiving surgery). However, such studies can take other measures to reduce the risk of bias, such as treating patients according to a strict protocol to reduce the risk of differential behaviours by patients and healthcare providers". As masking of participants and personnel is not feasible in the case of surgical interventions, examination of the surgical protocol reveals a fairly strict surgical protocol, as the “surgical steps were the same as group I except for application of MMC” |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “This is a prospective randomized controlled study, which included 60 eyes of 60 patients who received ophthalmologic service at the Menoufia University Hospital during the period of February 2009 to January 2011.” Data are available for all participants who were randomized. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of funding. No other sources of bias identified |
Mitra 2012.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 64 eyes of 64 participants total; 28 participants in trabeculectomy + Ologen® group; 36 participants in trabeculectomy + MMC group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: individual (1 eye per participant) Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: India Mean age: 62 years; 61.2 years for trabeculectomy + Ologen® group; 62.4 years for trabeculectomy + MMC group Gender: 38/64 (59%) men and 26/64 (41%) women; 22/36 (%) men and 14/36 (%) women in trabeculectomy + Ologen® group; 16/28 (%) men and 12/28 (%) women in trabeculectomy + MMC group Inclusion criteria: age 18 years or over; uncontrolled OAG; willing to sign informed consent; able and willing to complete post‐operative follow‐up requirements Exclusion criteria: inflammatory eye diseases; ACG; single functional eye; previous conjunctival surgery; known allergic reactions to ingredients of Ologen Collagen Matrix; excessive myopia (axial length > 27 mm or more than ‐10 diopters); previous vitrectomy eye surgery; unconsenting Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (brand not reported) Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 6 months Actual: 6 months |
|
| Outcomes |
Primary outcome, as defined: IOP
Secondary outcomes, as defined: number of glaucoma medication and complications Intervals at which outcomes assessed: 1 week and 1, 3, and 6 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. Complete and qualified success was reported, but IOP cut‐offs used were not reported |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. No other sources of bias identified |
Netland 2014.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 120 eyes of 120 participants total; 59 participants in trabeculectomy + MMC + Ex‐PRESS® group; 61 participants in trabeculectomy + MMC group Exclusions after randomization: 1 participant randomized to receive treatment but was withdrawn prior to surgery because of thin sclera Losses to follow‐up: 6 participants total; 2 participants in trabeculectomy + MMC + Ex‐PRESS® group; 4 participants in the trabeculectomy + MMC group Unit of analysis: individual (1 eye per participant) Number analyzed: 114 participants total; 57 participants in trabeculectomy + MMC + Ex‐PRESS® group; 57 participants in trabeculectomy + MMC group How were missing data handled?: 6 participants excluded from analysis Power calculation: a power of 80% to detect a 2 mm Hg IOP difference between groups with a sample size of 60 participants in each group |
|
| Participants |
Country: USA Mean age: 69 years; 69.4 years for trabeculectomy + MMC + Ex‐PRESS® group; 67.8 years for trabeculectomy + MMC group Gender: 32/59 (54%) men and 27/59 (46%) women in trabeculectomy + MMC + Ex‐PRESS® group; 33/61 (54%) men and 28/61 (46%) women in trabeculectomy + MMC group Inclusion criteria: older than 18 years of age; diagnosed with OAG (including POAG, PEXG, and pigmentary glaucoma); previously treated with ocular hypotensive medications; candidate for glaucoma surgery with intraoperative MMC; IOP ≥ 18 mm Hg Exclusion criteria: ACG, normal tension glaucoma, or neovascular glaucoma; history of previous incisional glaucoma surgery, penetrating keratoplasty, extracapsular cataract extraction; visually significant cataract planned for extraction at the time of filtering surgery or within 12 months thereafter; any significant ocular disease or history in the operated eye other than glaucoma and cataract; ocular pathology that could interfere with accurate IOP measurements; vitreous present in the anterior chamber for which vitrectomy is anticipated; participation in any other concurrent ophthalmic clinical trial Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + Ex‐PRESS® (Alco Laboratories, Fort Worth, Texas, USA) Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 2 years Actual: 2 years |
|
| Outcomes |
Primary outcomes, as defined: IOP, medication reduction, and surgical success (5 mm Hg ≤ IOP ≤ 18 mm Hg)
Secondary outcomes, as defined: visual acuity, complications, and IOP at week 2 follow‐up Intervals at which outcomes assessed: 1 day, 7 days, and 1, 3, 6, 12, 18, and 24 months |
|
| Notes |
Publication type: published article Funding sources: "research support for this investigator‐initiated trial was obtained from Optonol Ltd. (Neve Ilan, Israel) and Alcon Laboratories, Inc. (Fort Worth, TX)” Disclosures of interest: several co‐authors received research support, consulting fees, and speaker honoraria from industries, but no company wrote or influenced the writing of the manuscript Trial registry: NCT00444080 (www.ClinicalTrials.gov) Study period: not reported Subgroup analyses: none reported Publication language: English Authors contacted for 1‐year IOP and visual acuity data, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed separately for each study site. Each subject was assigned a 3‐digit identifying number, and all subjects were randomized using a computer‐based random‐number generator to undergo treatment with EX‐PRESS glaucoma filtration implant under scleral flap or trabeculectomy” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | No‐one was masked in this study. We are uncertain whether this has introduced bias |
| Masking of outcome assessment (detection bias) | Unclear risk | The outcome assessors were not masked. However, the authors mentioned that “we did provide standardized methods for measurement of IOP and documentation of other clinical findings, which may reduce, to some degree, the potential for bias” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study had small percent of participants lost to follow‐up (6/120) and with approximately even numbers of participants lost in the 2 groups |
| Selective reporting (reporting bias) | Low risk | The study was registered in www.ClinicalTrials.gov. All defined outcomes in www.ClinicalTrials.gov were reported in full text |
| Other bias | High risk | Received funding from manufacturer of device. No other sources of bias identified |
Papaconstantinou 2010.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 40 eyes of 40 participants total; 20 eyes of 20 participants in each group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: individual (1 eye per participant) Number analyzed: 40 participants total; 20 participants in each group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: Greece
Mean age: 66 years; 61.3 years for trabeculectomy + Ologen® group; 70.9 years for trabeculectomy group Gender: 23/40 (%) men and 17/40 (%) women; 11/20 (55%) men and 9/20 (45%) women in trabeculectomy + Ologen® group; 12/20 (60%) men and 8/20 (40%) women in trabeculectomy group Inclusion criteria: requiring glaucoma surgery for IOP control Exclusion criteria: “neovascular glaucoma, age < 18 years, previous surgical interventions or laser procedures and patients unable or unwilling to be followed up for an extended period postoperatively” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (OculusGen Biomedical Inc. Taipei, Taiwan)
Intervention 2: trabeculectomy
Length of follow‐up: Planned: 6 months Actual: 6 months |
|
| Outcomes |
Primary outcomes, as defined: absolute success (≤ 21 mm Hg without glaucoma medication) and qualified success (≤ 21 mm Hg with or without glaucoma medication)
Secondary outcomes, as defined: number of postoperative medications used, complications Intervals at which outcomes assessed: 1, 7, and 15 days and 1, 2, 3, 4, 5, and 6 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Forty eyes of 40 patients were enrolled in the study and divided randomly into two groups using the random number table; 20 were assigned to receive OloGen implant on top of the scleral flap subconjunctivally (study group) and 20 were assigned to undergo trabeculectomy without any implant (control group).” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict surgical protocol was followed, with the only difference in the 2 groups being that “The OloGen implant was placed on top of the scleral flap under the conjunctiva in the implant group”, and that the surgeries were all performed by the same surgeon, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | This was not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “None of the patients refused to enrol and none were lost to follow‐up during the course of the study.” |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined primary and secondary outcomes were reported |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. Limited duration of follow‐up (6 months). 5‐FU was used as adjuvant therapy for encapsulated blebs (2 participants in study group (trabeculectomy with Ologen implant) and 5 participants in control group (trabeculectomy)). |
Ren 2009.
| Methods |
Study design: parallel‐group randomized controlled trial (6 participants both eyes included) Number randomized: 36 eyes of 30 participants total; 18 eyes in each group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: 63.6 years; not reported by intervention group Gender: 13/30 (43%) men and 17/30 (57%) women; not reported by intervention group Inclusion criteria: ACG Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: visual acuity, anterior chamber, filtering bleb, postoperative IOP, and facility of outflow Intervals at which outcomes assessed: 1 week and 1, 3, 6, and 12 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Complications were not defined as an outcome, but were reported |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
Rosentreter 2010.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 20 eyes of 20 participants total; 10 eyes of 10 participants in each group Exclusions after randomization: none reported Losses to follow‐up: 1 participant died 8 months after surgery in trabeculectomy + MMC group Unit of analysis: individual (1 eye per participant) Number analyzed: 19 participants total; 10 participants in trabeculectomy + Ologen® group; 9 participants in trabeculectomy + MMC group How were missing data handled?: 1 participant with missing data excluded from analysis Power calculation: a power calculation was originally performed for a group of 40 participants, but power of detection was not reported and the study only recruited 20 participants (required sample size not met) |
|
| Participants |
Country: Germany
Mean age: 62.8 years; not reported by intervention group
Gender: 8/20 (40%) men and 12/20 (60%) women; not reported by intervention group
Inclusion criteria: “primary or secondary open‐angle glaucoma with uncontrolled IOP while receiving maximal tolerable anti‐glaucomatous therapy"
Exclusion criteria: “angle‐closure glaucoma, post‐traumatic, uveitic, neovascular, or dysgenetic glaucoma were not considered for this study. Participants with an allergy to collagen, preliminary conjunctival damage (trauma, vitreo–retinal surgery, previous glaucoma surgery, and other) or those < 18 years of age were excluded from the study.” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (Aeon Astron Europe BV, Leiden, The Netherlands)
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes |
Primary outcomes, as defined: IOP, number of glaucoma medication, filtering bleb Secondary outcomes, as defined: visual acuity, visual field, and complications Intervals at which outcomes assessed: 1, 7, and 14 days and 1, 2, 3, 6, and 12 months (except visual field examination only done at 6 and 12 months) |
|
| Notes |
Publication type: published article Funding sources: “PJ Dahlhausen & Co. GmbH (Emil‐Hoffmann‐Str. 53, 50996 Cologne, Germany) supported the study” Disclosures of interest: “the sponsor had no role in the design or conduct of this research” Trial registry: ClinicalTrials.gov: NCT00538590 Study period: not reported Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “We initiated a randomised, prospective trial with two study groups undergoing penetrating anti‐glaucomatous surgery (trabeculectomy)" "Randomisation was performed by an individual not involved in the study according to the Consort Guidelines description.” However, the method of sequence generation is not described and thus adequacy cannot be judged |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed and differences in the surgical protocol of the 2 groups were minimized, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Twenty (10/10) patients, 12 women and 8 men, began the study, and it was completed by 19 patients. One patient from the MMC group died 8 months after surgery.” |
| Selective reporting (reporting bias) | Low risk | The study was registered in www.ClinicalTrials.gov. All defined outcomes in www.ClinicalTrials.gov were reported in full text. |
| Other bias | High risk | Total industry support and other source(s) of potential bias identified. “PJ Dahlhausen & Co. GmbH (Emil‐Hoffmann‐Str. 53, 50996 Cologne, Germany) supported the study.” Although the authors state that “The sponsor had no role in the design or conduct of this research”, the risk of bias from an industry‐funded study is unclear. The authors did not specify how power of detection was affected; “the plan was to include a consecutive series of 40 patients (20 to each group).” “After the first 20 patients, an interim analysis was prearranged.” “After interim analysis, the study was aborted because of the significantly lower IOP and significantly higher complete success rate in the MMC group after 1 year (P = 0.01).” |
Rosentreter 2014.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 30 eyes of 30 participants total; 15 eyes of 15 participants in each group Exclusions after randomization: none reported Losses to follow‐up: 5 participants total; 4 participants in trabeculectomy + Ologen® group; 1 participant in trabeculectomy + MMC group Unit of analysis: individual (1 eye per participant) Number analyzed: 25 participants total; 14 participants in trabeculectomy + Ologen® group; 11 participants in trabeculectomy + MMC group How were missing data handled?: 5 participants excluded from analysis Power calculation: a power of 50% to detect 2.73 mm Hg IOP difference between groups and a power of 95% for a difference of 5.03 mm Hg in IOP |
|
| Participants |
Country: Germany
Mean age: 66.4 years; not reported by intervention group
Gender: 13/30 (43%) men and 17/30 (57%) women; not reported by intervention group
Inclusion criteria: “primary or secondary open‐angle glaucoma with uncontrolled IOP while receiving maximal tolerable anti‐glaucomatous therapy"
Exclusion criteria: “angle‐closure glaucoma, posttraumatic, uveitic, neovascular, or dysgenetic glaucoma were not considered for this study. Participants with an allergy to collagen, preliminary conjunctival damage (due to trauma, vitreoretinal surgery, previous glaucoma surgery or other causes) as well as those younger than 18 years of age were excluded from the study.” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (Version 2; Aeon Astron Europe BV, the Netherlands)
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes |
Primary outcomes, as defined: IOP, number of glaucoma medications used, bleb morphology Secondary outcomes, as defined: visual acuity, visual field, absolute success ("IOP of 18 mm Hg or lower and an additional reduction of 20% or more in IOP compared to the preoperative IOP, without any additional glaucoma surgery, but with topical medication of a maximum medication score of 2 allowed"), and complications Intervals at which outcomes assessed: 1 day, 7 days, and 1, 3, 6, and 12 months (except visual field at 12 months only) |
|
| Notes |
Publication type: published article Funding sources: “PJ Dahlhausen & Co. GmbH (Cologne, Germany) supported the study” Disclosures of interest: "no authors have any financial/conflicting interests to disclose" Trial registry: ClinicalTrials.gov: NCT01174420 Study period: not reported Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by a person not involved in the study by drawing lots." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed and differences in the surgical protocol of the 2 groups were minimized, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25/30 eyes completed 1‐year follow‐up of the study |
| Selective reporting (reporting bias) | Low risk | The study was registered in www.ClinicalTrials.gov. All defined outcomes in www.ClinicalTrials.gov were reported in full text |
| Other bias | Unclear risk | Total industry support but not other source of potential bias identified. |
Senthil 2013.
| Methods |
Study design: parallel‐group randomized controlled trial (6 participants both eyes included)
Number randomized: 39 eyes of 33 participants total; 19 eyes in trabeculectomy with Ologen® group; 20 eyes in trabeculectomy with MMC group Exclusions after randomization: none reported Losses to follow‐up: 7 eyes of 5 participants had less than 6 months of follow‐up Unit of analysis: eye Number analyzed: 32 eyes of 28 participants total; 16 eyes of 14 participants in each group How were missing data handled?: 7 eyes of 5 participants excluded from analysis Power calculation: not reported |
|
| Participants |
Country: India
Mean age: 46 years; 48 years for trabeculectomy + Ologen® group; 45 years for trabeculectomy + MMC group Gender: 20/39 (51%) men and 19/39 (49%) women; 9/19 (47%) men and 10/19 (53%) women in trabeculectomy + Ologen® group; 11/20 (55%) men and 9/20 (45%) women in trabeculectomy + MMC group Inclusion criteria: ≥ 18 years of age; medically uncontrolled POAG or PACG with no previous intraocular surgery (POAG defined as the presence of IOP > 21 mm Hg, open anterior chamber angle on gonioscopy, glaucomatous optic disc damage on clinical examination, and corresponding glaucomatous visual field defects; PACG defined as the presence of an occludable angle on gonioscopy (posterior trabecular meshwork not seen in at least 180° of the total circumference of the angle in primary position), glaucomatous optic disc damage, and corresponding glaucomatous visual field defects) Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + Ologen® (OculusGen Biomedical Inc. Taipei, Taiwan)
Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 2 years Actual: 2 years |
|
| Outcomes |
Primary outcomes, as defined: success, defined as, “complete success if an IOP was > 5 and ≤ 21 mm Hg without any glaucoma medications or re‐surgery. Qualified success was defined as IOP ≤ 21 mm Hg with or without anti‐glaucoma medications. Failure was defined as IOP ≥ 22 mm Hg despite medications or ≤ 5 mm Hg (on 2 or more examinations) with hypotony maculopathy or if an additional procedure like needling or repeat trabeculectomy was required to control the IOP or if there was loss of light perception”, assessed at 6, 12, 18, and 24 months
Secondary outcomes, as defined: success ("achieving IOP ≤ 18 mm Hg and ≤ 15 mm Hg in both the groups without anti‐glaucoma medications”), complications, and visual acuity Intervals at which outcomes assessed: 1 day, 7 days, and 1, 3, 6, 12, 18, and 24 months |
|
| Notes |
Publication type: published article Funding sources: “source of support: nil" Disclosures of interest: "none declared" Trial registry: not registered Study period: May 2007 to December 2008 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was done using a permuted block randomization. Block size of 4 was determined, and 2 eyes of group 1 and 2 eyes of group 2 were randomly allocated into each block.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed and differences in the surgical protocol of the 2 groups were minimized, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all data were available for all participants who were randomized, given that “7 eyes of 5 participants, who had less than 6 months of follow‐up, were excluded from the outcome analysis, but were included in the analysis of complications.” In addition, “The number of eyes lost to follow‐up at 2 years was close to 50%” |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All primary and secondary outcomes were reported |
| Other bias | Low risk | No industry support reported and no other source of potential bias identified. No other sources of bias identified |
Sheha 2008.
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized: 37 eyes of 37 participants total; 19 participants in trabeculectomy + MMC + AMT group; 18 participants in the trabeculectomy + MMC group Exclusions after randomization: none reported Losses to follow‐up: 7 participants total at 12 months; 4 participants in trabeculectomy + MMC + AMT group; 3 participants in trabeculectomy + MMC group Unit of analysis: individual (1 eye per participant) Number analyzed: 30 participants total; 15 participants in each group How were missing data handled?: 7 participants excluded from analysis Power calculation: a power of 80% achieved with a sample size of 15 participants; the minimum detectable difference was not specified |
|
| Participants |
Country: Saudi Arabia
Mean age: 57 years; 57.6 years for trabeculectomy + MMC + AMT group; 56.6 years for trabeculectomy + MMC group Gender: 24/37 (65%) men and 13/37 (35%) women; 13/19 (68%) men and 6/19 (32%) in trabeculectomy + MMC + AMT group; 11/18 (61%) men and 7/18 (39%) women in trabeculectomy + MMC group Inclusion criteria: not reported (the study population was described as follows: “This prospective, randomized study included 37 eyes with refractory glaucoma at such high risks as neovascular, pseudophakic, and prior failure.” “All patients had previously failed trabeculectomy with MMC once or twice. Only 1 eye of each patient was included. A number of risk factors were randomized such as race, and type of refractory glaucoma, which was subdivided into 3 subgroups including phakic open angle glaucoma, pseudophakic glaucoma, and neovascular glaucoma. All eyes with phakic open angle glaucoma had 2 previously failed trabeculectomies with MMC; all eyes with pseudophakic and neovascular glaucomas had 1 previously failed trabeculectomy with MMC.”) Exclusion criteria: not reported Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: IOP, bleb characteristics, complications, and number of antiglaucoma medications, complete success ("IOP of 21mm Hg or less without antiglaucoma medications"), qualified success ("defined as having an IOP of 21mm Hg or less with or without antiglaucoma medications"), and failure ("IOP was controlled by additional surgeries such as needling, but not suture lysis, or if chronic hypotony, defined as an IOP less than 6 mm Hg after 3 months, occurred") Intervals at which outcomes assessed: 1 day, 1 week, and 1, 3, 6, 9, and 12 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: “no author has a financial or proprietary interest in any material or method mentioned” Trial registry: not registered Study period: April 2004 to August 2005 Subgroup analyses: none reported Publication language: English Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed, differences in the surgical protocol of the 2 groups were minimized, and the same surgeon performed all surgeries, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 4 participants lost from the trabeculectomy + MMC + AMT group and 3 from the trabeculectomy + MMC group; the reasons for loss to follow‐up and how it was accounted for are not addressed |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of funding. No other sources of bias identified |
Stavrakas 2012.
| Methods |
Study design: parallel‐group randomized controlled trial (9 participants both eyes included)
Number randomized: 59 eyes of 50 participants total; 32 eyes in trabeculectomy + AMT group; 27 eyes in trabeculectomy group Exclusions after randomization: none reported Losses to follow‐up: 7 eyes total at 6 months; 5 eyes in trabeculectomy + AMT group; 2 eyes in the trabeculectomy group Unit of analysis: eye Number analyzed: 52 eyes total; 30 eyes in trabeculectomy + AMT group; 22 eyes in trabeculectomy group How were missing data handled?: 7 eyes excluded from analysis Power calculation: not reported |
|
| Participants |
Country: Greece
Mean age: not reported; median age 70.0 (63.0, 80.0) years in trabeculectomy + AMT group; median age 71.5 (67.0, 76.0) years in trabeculectomy group Gender: 35/59 (59%) men and 24/59 (41%) women; 19/27 (70%) men and 8/27(30%) women in trabeculectomy + AMT group; 16/32 (50%) men and 16/32 (50%) women in trabeculectomy group Inclusion criteria: “presence of POAG and at least one of the following: unsatisfactory target intraocular pressure (IOP) control with topical antiglaucoma treatment; optic nerve damage progression on two consecutive visual field tests and increase of cup‐to‐disk ratio in a period of 24 months; allergy to topical agents; or poor compliance” Exclusion criteria: “any other type of glaucoma; only‐eye patients; past or present anterior segment pathology coexistence (apart from cataract); and previously failed glaucoma filtration surgery” Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT
Intervention 2: trabeculectomy Length of follow‐up: Planned: 24 months Actual: 24 months |
|
| Outcomes |
Primary outcomes, as defined: IOP, and functionality and morphology of the bleb Secondary outcomes, as defined: visual acuity, reduction of antiglaucoma medications, and complications Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, 1 month, 2 months, and every 6 months thereafter for a minimum of 2 years after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: “none of the authors has any financial interest in any of the materials or the methods mentioned in the study” Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: English Authors contacted for mean IOP and standard deviations, etc., but they were not able to provide data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was not specified in the full‐text. The email response from the author suggest that participants were randomized using simple randomization (flipping a coin, etc.). However, simple randomization could result in uneven number of participants in each group, thus investigators could set the ratio of participant assigned to the AMT group or trabeculectomy group to keep the groups even. "it was the method of simple randomisation with monitoring during the process in order to ensure relatively even numbers. At the end, the numbers were not even due to financial restrictions regarding the amniotic membranes." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Low risk | Because trabeculectomy is a surgical procedure with informed consent, masking of the participants and personnel becomes impossible. However, given that a strict and standardized surgical protocol was followed, differences in the surgical protocol of the 2 groups were minimized, and the same surgeon performed all surgeries, the risk of performance bias is comparably low for a surgical procedure |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Eyes lost from follow‐up have been included in the analysis until they stopped attending the scheduled postoperative visit and then censored from the study (right censoring).” |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of funding. No other sources of bias identified |
Wagschal 2013.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 64 eyes of 64 participants total; 33 participants in trabeculectomy + MMC + Ex‐PRESS® P50 group; 31 participants in trabeculectomy + MMC group Exclusions after randomization: none reported Losses to follow‐up: 4 participants total at 1 year; 3 participants in trabeculectomy + MMC + Ex‐PRESS® P50 group; 1 participant in trabeculectomy + MMC group Unit of analysis: individual (1 eye per participant) Number analyzed: 60 participants total; 30 participants in each group How were missing data handled?: 4 participants excluded from analysis Power calculation: a power of 80% to detect a 2 mm Hg IOP difference with a sample size of 52 eyes |
|
| Participants |
Country: Canada Mean age: overall not reported; 61.9 years for trabeculectomy + MMC + Ex‐PRESS® P50 group; 65.9 years for trabeculectomy + MMC group Gender: 41/64 (64%) men and 23/64 (36%) women overall; not reported by intervention group Inclusion criteria: “participants with open‐angle glaucoma between 18 and 85 years of age with uncontrolled intraocular pressure (IOP) on maximum tolerated medication and trabeculectomy as the planned surgical procedure” Exclusion criteria: “previous ocular incisional surgery (with the exception of clear cornea cataract surgery or 1 trabeculectomy), history of uveitis, unwilling or unable to give consent, unwilling to accept randomization, or unable to return for scheduled protocol visits” Equivalence of baseline characteristics: yes; baseline IOP and VA are comparable |
|
| Interventions |
Intervention 1: trabeculectomy + MMC + Ex‐PRESS® P50 (Alcon Canada Inc. Mississauga, ON, Canada) Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: 1 year Actual: 1 year |
|
| Outcomes |
Primary outcome, as defined: IOP, complete success ("IOP between 5 and 18 mm Hg and a 20% reduction from baseline without medication and qualified success was defined as above with hypotensive medications"), failure ("reoperation for glaucoma or loss of light perception") Secondary outcomes, as defined: visual acuity, surgery time, glaucoma medication usage, IOP, bleb morphology, Seidel test, additional procedures, complications, and potential risk factors for vision loss Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, and 1, 2, 3, and 6 months after surgery |
|
| Notes |
Publication type: published article Funding sources: “some Ex‐PRESS shunts were provided at no cost by Imed and Alcon Canada” Disclosures of interest: “Y.M.B. has received speaking honoraria from Alcon Canada. The remaining authors declare no conflict of interest.” Trial registry: NCT01263561 (www.ClinicalTrials.gov) Study period: May 2009 to July 2011 Subgroup analyses: subgroup of 43 participants randomly chosen for cost‐effectiveness analysis Publication language: English Authors contacted for randomization method, reasons for lost to follow‐up, etc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | As this was not provided in the published report, we contacted the study investigator, and received the following response: “randomization was done by drawing a piece of paper with procedure name from a bag” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | By contacting the study investigators, we found participants were not masked, but whether this would introduce bias is uncertain as current evidence did not show 1 procedure significantly better than the other |
| Masking of outcome assessment (detection bias) | High risk | By contacting the study investigators, we found the outcome assessors were not masked. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study had a low percentage of participants lost to follow‐up (4 out of 64), and not much difference in numbers in the 2 groups (lost 3 versus 1). Also, exclusions were because of death (3 participants) and 1 participant not adhering to the assigned procedure |
| Selective reporting (reporting bias) | Low risk | The study was registered in www.ClinicalTrials.gov. All defined outcomes in www.ClinicalTrials.gov were reported in full text |
| Other bias | Unclear risk | Partial industry support and other source(s) of potential bias |
Wang 2008.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 40 eyes of 40 participants total; 20 eyes of 20 participants in each group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: individual (1 eye per participant) Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: overall not reported; 54 years for the trabeculectomy + AMT group; 56 years for the trabeculectomy group Gender: 11/40 (28%) men and 29/40 (72%) women overall; not reported by intervention group Inclusion criteria: glaucoma Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: 12 months Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, surgical success, postoperative IOP, adverse events, and visual acuity Intervals at which outcomes assessed: 1, 2, and 3 days, 1, 2, and 3 weeks, and 1, 2, and 6 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted in regards to number with missing data at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Method use to mask participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes defined were reported |
| Other bias | Unclear risk | Did not report source of support or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
Wang 2009.
| Methods |
Study design: parallel group randomized controlled trial (6 participants both eyes included) Number randomized: 44 eyes of 38 participants total; 16 eyes in trabeculectomy + AMT + MMC group; 14 eyes in trabeculectomy + MMC group; 14 eyes in trabeculectomy group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: mean age not reported; age range 19 to 75 years; not reported by intervention group Gender: 24 men and 14 women; not reported by intervention group Inclusion criteria: refractory glaucoma Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT + MMC Intervention 2: trabeculectomy + MMC Intervention 3: trabeculectomy Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, postoperative IOP, and adverse events Intervals at which outcomes assessed: 1 week, 2 weeks, and 1, 6, and 12 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted in regards to number with missing data at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | Not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Filtering blebs and complications reported in the Results section were not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response. |
Yan 2004.
| Methods |
Study design: parallel‐group randomized controlled trial (11 participants both eyes included) Number randomized: 63 eyes of 52 participants total; 36 eyes of 28 participants in trabeculectomy + AMT group; 27 eyes of 24 participants in trabeculectomy group Exclusions after randomization: none reported Losses to follow‐up: none reported Unit of analysis: eye Number analyzed: 63 eyes of 52 participants total; 36 eyes of 28 participants in trabeculectomy + AMT group; 27 eyes of 24 participants in trabeculectomy group How were missing data handled?: no missing data Power calculation: not reported |
|
| Participants |
Country: China Mean age: mean age not reported; age range 45 to 76 years; not reported by intervention group Gender: 25/52 (48%) men and 27/52 (52%) women overall; not reported by intervention group Inclusion criteria: PACG Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: depth of anterior chamber, IOP, visual acuity, filtering bleb, surgical success, complications Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks, and 1, 3, and 6 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Surgical success and visual acuity were reported in the Results section, but were not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
Yang 2004.
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized: 70 eyes of 70 participants total; 40 participants in trabeculectomy + AMT group; 30 participants in trabeculectomy group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: individual (1 eye per participant) Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: mean age not reported; age range 20 to 72 years; not reported by intervention group Gender: 42/70 (60%) men and 28/70 (40%) women overall; not reported by intervention group Inclusion criteria: glaucoma Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, surgical success, postoperative IOP, visual acuity, and complications Intervals at which outcomes assessed: 1 day, 1 week, and 1, 3, and 6 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted in regards to number with missing data at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | Not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Surgical success reported in the Results section were not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
Zhang 2009.
| Methods |
Study design: parallel‐group randomized controlled trial (13 participants both eyes included) Number randomized: 52 eyes of 39 participants total; 26 eyes in each group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Mean age: mean age not reported; age range 40 to 70 years; not reported by intervention group Gender: 18/39 (46%) men and 21/39 (54%) women overall; not reported by intervention group Inclusion criteria: ACG Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy Length of follow‐up: Planned: not reported Actual: 6 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, surgical success, postoperative IOP, visual acuity, and complications Intervals at which outcomes assessed: 1, 3, and 6 months after surgery |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted in regards to number with missing data at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | It is not possible to mask outcome assessors, as devices can be seen during eye examination |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | Low risk | Protocol was not available. All defined outcomes were reported |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
Zheng 2005.
| Methods |
Study design: parallel‐group randomized controlled trial (20 participants both eyes included) Number randomized: 48 eyes of 28 participants total; 24 eyes in each group Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis: eye Number analyzed: not reported How were missing data handled?: not reported Power calculation: not reported |
|
| Participants |
Country: China Age: not reported Gender: not reported Inclusion criteria: not reported; all participants were diagnosed primary angle‐closure glaucoma or primary open‐angle glaucoma Exclusion criteria: not specified Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: trabeculectomy + AMT Intervention 2: trabeculectomy + MMC Length of follow‐up: Planned: not reported Actual: 12 months |
|
| Outcomes | Primary and secondary outcomes not distinguished Outcomes, as reported: filtering bleb, surgical success, IOP, visual acuity, and complications Intervals at which outcomes assessed: 1 day, 1 week, and 1, 3, and 6 months |
|
| Notes |
Publication type: published article Funding sources: not reported Disclosures of interest: not reported Trial registry: not registered Study period: not reported Subgroup analyses: none reported Publication language: Chinese Authors contacted in regards to number with missing data at different follow‐up time points, but no response received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported |
| Masking of outcome assessment (detection bias) | High risk | Not reported. Although it is not possible to mask outcome assessors, devices can be easily seen during examination of the eye |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants with missing or incomplete outcome assessment was not reported |
| Selective reporting (reporting bias) | High risk | Protocol was not available. Surgical success reported in the Results section was not mentioned in the Methods section |
| Other bias | Unclear risk | Did not report source of funding or conflict of interest. This article provided nothing on study design; we contacted the authors but did not receive a response |
5‐FU: 5‐fluorouracil ACG: angle‐closure glaucoma BCVA: best‐corrected visual acuity AMT: amniotic membrane E‐PTFE: expanded polytetrafluoroethylene IOP: intraocular pressure MMC: mitomycin C OAG: open‐angle glaucoma PACG: primary angle‐closure glaucoma PEXG: pseudoexfoliation glaucoma POAG: primary open‐angle glaucoma VA: visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cao 2004 | This trial was published in Chinese and identified from a meta‐analysis published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data at specific time points clearly reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Dahan 2011 | This trial evaluated augmented trabeculectomy with T‐flux, but there was no comparison group |
| Dai 2011 | This RCT compared standard trabeculectomy versus trabeculectomy + MMC; no device was included |
| Gao 2002 | This trial was published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data in the scope of this review reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Ha 2008 | The study included participants from 12 to 74 years of age and did not separately report outcomes for adult participants |
| Jin 2006 | This trial was published in Chinese and identified from a meta‐analysis published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data at specific time points clearly reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Lau 2008 | This study was reported as an abstract only and we were unable to identify a corresponding full‐text publication. The study did not specify whether it used a randomized method to allocate glaucoma patients into Ex‐PRESS or standard trabeculectomy groups. We contacted the author but did not receive any response. We excluded the study as it was unclear whether the study was a RCT |
| Li 2003 | This trial was published in Chinese and identified from a meta‐analysis published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data at specific time points clearly reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Li 2008 | This trial was published in Chinese and identified from a meta‐analysis published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified). In addition, the study authors did not report outcomes by the 2 intervention groups, but according to different types of glaucoma; thus, the results would not contribute to treatment effects even if randomization was employed correctly. We excluded the study as it was unclear whether the study was a RCT |
| Liu 2003 | The study included participants from 12 to 75 years of age and did not separately report outcomes for adult participants |
| Liu 2004 | This study compared amniotic membrane and trabeculectomy, but was not described as randomized. We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Mahdy 2010 | This study included pediatric participants 1 to 13 years of age |
| NCT00472810 | Only available as a record in clinicaltrials.gov and there is no indication that participants were enrolled into the study |
| NCT00597181 | This trial was identified from ClinicalTrials.gov and has been terminated. We contacted the trial investigators for reasons for termination and preliminary data if applicable. The investigator responded that this study was funded by the company who developed the Ex‐PRESS shunt and had introduced it to the US market. However, the study had to be terminated because soon after beginning enrolment, the company elected to stop funding the study. The study investigators provided us with preliminary data for 15 participants, but lacked data for numbers of eyes allocated to each group (Ex‐PRESS and standard trabeculectomy), so we were unable to analyze the data. In addition, the study only followed participants for 2 months, which does not satisfy our primary and secondary outcomes of interest |
| Sugiyama 2011 | This non‐randomized study compared Ex‐PRESS and trabeculectomy. We contacted the study investigator regarding assignment to treatment groups and found that patients themselves selected the procedures performed |
| Wang 2010 | This trial was published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data in the scope of this review reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
| Zhang 2008 | This trial was published in Chinese. The methodological quality was very low (randomization method not specified, lost to follow‐up not specified, no outcome data in the scope of this review reported). We tried to contact the study investigator, but were unsuccessful in getting a response. We excluded the study as it was unclear whether the study was a RCT |
IOP: intraocular pressure MMC: mitomycin C RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
JPRN‐UMIN000008391.
| Trial name or title | Comparison between Ex‐PRESS shunt surgery and trabeculectomy for refractory glaucoma |
| Methods |
Study design: "interventional, randomized, parallel, open label clinical trial" Number randomized: 40 participants total (number of eyes unknown); not reported by intervention group Unit of analysis: not reported Power calculation: not reported |
| Participants |
Country: Japan Age: the study required participants to be 20 years and older Gender (percent): both genders are eligible Inclusion Criteria: "Patients with exfoliation glaucoma or neovascular glaucoma who require intraocular pressure reduction by glaucoma surgery." Exclusion criteria: "Patients who does not agree with the informed consent." |
| Interventions |
Intervention 1: Ex‐PRESS Intervention 2: trabeculectomy Length of follow‐up: planned: not reported |
| Outcomes |
Primary outcome, as defined in study reports: IOP and adverse events
Secondary outcomes, as defined in study reports: not reported
Adverse events reported: not reported Intervals at which outcomes assessed: not reported |
| Starting date | 1 July 2012 |
| Contact information | Masaki Tanito: tanito‐oph@umin.ac.jp Enya 89‐1, Izumo Japan Shimane University Faculty of Medicine |
| Notes |
Funding sources: not reported Last updated: 3 June 2014 This study is currently recruiting participants. |
JPRN‐UMIN000008981.
| Trial name or title | Prospective comparative study of the Ex‐PRESS mini glaucoma shunt with standard trabeculectomy. |
| Methods |
Study design: "interventional, randomized, parallel, open label clinical trial" Number randomized: 200 participants (number of eyes not reported) total; not reported by intervention group Unit of analysis: not reported Power calculation: not reported |
| Participants |
Country: Japan Inclusion criteria: open‐angle glaucoma; participants to be 20 years and older Exclusion Criteria: angle‐closure glaucoma; uveitis |
| Interventions |
Intervention 1: trabeculectomy + Ex‐PRESS mini shunt implantation Intervention 2: trabeculectomy Length of follow‐up: planned: not reported |
| Outcomes |
Primary outcome, as defined in study reports: IOP reduction
Secondary outcomes, as defined in study reports: not reported
Adverse events reported: not reported Intervals at which outcomes assessed: not reported |
| Starting date | 1 October 2012 |
| Contact information | Hideki Mochizuki: mochizuki‐h@hiroshima‐u.ac.jp 1‐2‐3 Kasumi Minamiku Hiroshima, Japan Hiroshima University Dept. of Ophthalmology |
| Notes |
Funding source: self funding Last updated: 3 June 2014 This study is currently recruiting participants. |
NCT00449098.
| Trial name or title | Ologen (OculusGen)‐Glaucoma MMC control trial in India |
| Methods |
Study design: "interventional, randomized, parallel, open label clinical trial" Number randomized: 40 participants (number of eyes not reported) total; not reported by intervention group Unit of analysis: not reported Power calculation: not reported |
| Participants |
Country: India Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention 1: trabeculectomy + OculusGen Biodegradable Collagen Matrix Implant Intervention 2: trabeculectomy + MMC Length of follow‐up: planned: 180 days |
| Outcomes |
Primary outcome, as defined in study reports: reduction of IOP at 180 days
Secondary outcomes, as defined in study reports: incidence of complications and adverse events at 180 days
Adverse events reported: this was planned Intervals at which outcomes assessed: "there will be 7 post‐operative and follow‐up visits within 6 months of surgery: postoperative days 1, 7, 14, 30, 60, 90 and 180" |
| Starting date | January 2007 |
| Contact information | Rajul S Parikh, MD rajulparikh@lvpei.org L. V. Prasad Eye Institute, India |
| Notes |
Funding source: not reported Last updated: October 6, 2011 This study is currently recruiting participants. |
NCT00524758.
| Trial name or title | Oculusgen (Ologen) Glaucoma MMC control in Estonia |
| Methods |
Study design: "interventional, randomized, parallel, open label clinical trial" Number randomized: 20 participants (number of eyes not reported) total; not reported by intervention group Unit of analysis: not reported Power calculation: not reported |
| Participants |
Country: Estonia Inclusion criteria: "maximum anti glaucoma medication failed" Exclusion Criteria: "age less than 18, pregnant women, hemodialysis patient" |
| Interventions |
Intervention 1: trabeculectomy + Ologen Intervention 2: trabeculectomy + MMC Length of follow‐up: planned: 180 days |
| Outcomes |
Primary outcome, as defined in study reports: IOP < 21 mm Hg without anti‐glaucoma medication at 180 days
Secondary outcomes, as defined in study reports: IOP < 21 mm Hg or IOP drops more than 30% with anti‐glaucoma medication at 180 days
Adverse events reported: not reported Intervals at which outcomes assessed: not reported |
| Starting date | July 2007 |
| Contact information | Kuldar Kaljurand, MD +372 737 6189 kristel.mikkor@ut.ee |
| Notes |
Funding source: not reported Last updated: October 24, 2011 This study is currently recruiting participants. |
NCT01440751.
| Trial name or title | Comparative study of the safety and effectiveness of Ologen Collagen Matrix versus mitomycin‐C in glaucoma filtering surgery |
| Methods |
Study design: "interventional, randomized, parallel, open label clinical trial", multi‐center trial Number randomized: 128 participants (number of eyes not reported) total; not reported by intervention group Unit of analysis: not reported Power calculation: not reported |
| Participants |
Country: India Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Intervention 1: Ologen Collagen Matrix in trabeculectomy Intervention 2: MMC in trabeculectomy Length of follow‐up: planned: 24 months |
| Outcomes |
Primary outcome, as defined in study reports: "Intraocular pressure (IOP) reduction at postoperative up to 24 months; 'Complete success' is considered for IOP less than 21 mm Hg (inclusive) with no glaucoma medications and with more than 20% reduction (inclusive) from baseline IOP. Definition of success rate is calculated in percentage by the number of complete success patients over the total sample size. 'Qualified success' that meets the postoperative IOP requirements with postoperative glaucoma medications and 'Failure' of meeting the IOP requirements are the other efficacy parameters. In the specified time frame, patients will also visit for record at day 1, 7, 14, 30, 90, 180 days, 12, 18, and 24 months." Secondary outcomes, as defined in study reports: "Postoperative complications and appearances at postoperative up to 24 months. Inspections of hyphema, severe anterior chamber reaction, hypotony, superchoroidal hemorrhage, flat anterior chamber, endophthalmitis, choroidal detachment, wound or bleb leak. Visual acuity, bleb appearance, and anterior chamber inflammation." Adverse events reported: this was planned Intervals at which outcomes assessed: day 1, 7, 14, 30, 90, 180 days, 12, 18, and 24 months |
| Starting date | February 2010 |
| Contact information | Robert Ritch, MD 212‐673‐5140 ritchmd@earthlink.net |
| Notes |
Funding sources: not reported Last updated: December 24, 2013 This study is ongoing, but not recruiting participants. |
IOP: intraocular pressure MMC: mitomycin C
Differences between protocol and review
1. We planned to assess mean change of IOP from baseline between the two groups. However, as in most cases mean change of IOP was not reported in individual studies, we used postoperative IOP as a surrogate. This may result in some bias because of the differences in baseline IOPs in participants in a few studies.
2. For amniotic membrane studies, losses to follow‐up were not reported in a majority of studies (11/18), and we used the number randomized in the analyses. This may bias the analyses as those participants lost to follow‐up did not contribute to the outcome measurements.
3. For the Ologen studies, one study compared trabeculectomy without MMC versus trabeculectomy and Ologen (Papaconstantinou 2010). The other seven studies were included in the meta‐analysis to give a broader view, but the lack of the use of MMC in one study may have reduced the success rate of the trabeculectomy‐alone group in comparison to the trabeculectomy‐with‐Ologen group. This may have made the trabeculectomy‐with‐Ologen group appear better at IOP reduction than it is.
4. For amniotic membrane studies, three studies only used MMC in the trabeculectomy group but not in the amniotic membrane group (Cho 2013; Khairy 2015; Zheng 2005). Although both MMC and amniotic membrane affect wound healing, whether amniotic membrane can substitute for MMC is unknown. To ensure comparability, MMC should either have been used in both groups, or should not have been used at all. The impact of this on the outcome is unknown.
5. For complications reported for each study, the time of measurement may be different. We combined them in the analyses, based on the assumption that most complications occur within a short time after the surgery. These data should be interpreted with caution.
Contributions of authors
XW conceived and designed the review, screened the search results, assessed trial quality, extracted and entered data, analyzed data and wrote the main text of the review.
RK assessed trial quality, extracted data, participated in the adjudication process, co‐authored the Agreements and disagreements with other studies or reviews section, and helped edit and revise the main text of the review.
AC conceived and designed the review, advised on data interpretations and provided substantial comments on the content of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
Xue Wang was supported by the Cochrane Eyes and Vision Group US Project, funded by the National Eye Institute Grant 1 U01 EY020522
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
AC reports grants unrelated to this work from the National Eye Institute, National Institutes of Health, US and the Agency for Healthcare Research and Quality (AHRQ), US. The AHRQ Comparativeness Effectiveness of glaucoma treatment trial is an observational study on the management of glaucoma patients with additional medications, laser trabeculoplasty, or incisional surgery. Modified trabeculectomies are one of the types of incisional surgeries allowed in the trial if the treating physician and patient choose that method to lower eye pressures. Since the study is observational, AC has no role in which treatment modality is chosen.
XW: None known.
RK: None known.
New
References
References to studies included in this review
Birt 1998 {unpublished data only}
- Birt CM. Gelfilm® as a wound healing modulator in filtration surgery: a pilot study. Investigative Ophthalmology and Visual Science 1998; Vol. 39:ARVO E‐abstract 3202.
Bruno 2008 {unpublished data only}
- Bruno CA, Radenbaugh PA, Trzcinka A, Kim DS, John DA, Lutz D, et al. Effect of amniotic membrane on trabeculectomy outcome in a prospective, randomized pilot study of patients at high risk for filtration failure. American Glaucoma Society. 2008:86.
Cai 2012 {published data only}
- Cai JS, Li FZ, Liao YJ, Gu ZH. Modified trabeculectomy combined with amniotic membrane implantation for treatment of refractory glaucoma [改良双瓣型小梁切除联合羊膜移植治疗难治性青光眼]. International Eye Science 2012;12(5):928‐30. [Google Scholar]
Cho 2013 {published data only}
- Cho YL, Huang P, Zhang C. Randomized‐controlled trial of anti‐scarring effectiveness on filtrating surgery combined with amniotic membrane [羊膜在小梁切除术中抗瘢痕作用的随机对照研究]. Zhonghua Shiyan Yanke Zazhi [Chinese Journal of Experimental Ophthalmology] 2013;31(3):265‐9. [Google Scholar]
Cillino 2008 {published data only}
- Cillino S, Zeppa L, Pace F, Casuccio A, Morreale D, Bocchetta F, et al. E‐PTFE (Gore‐Tex) implant with or without low‐dosage mitomycin‐C as an adjuvant in penetrating glaucoma surgery: 2 year randomized clinical trial. Acta Ophthalmologica 2008;86(3):314‐21. [DOI] [PubMed] [Google Scholar]
Cillino 2011 {published data only}
- Cillino S, Pace FD, Cillino G, Casuccio A. Biodegradable collagen matrix implant vs mitomycin‐C as an adjuvant in trabeculectomy: a 24‐months, randomized clinical trial. Eye 2011;25(12):1598‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahan 2012 {published data only}
- Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex‐PRESS implantation in fellow eyes of the same patient: a prospective, randomised study. Eye 2012;26(5):703‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Jong 2005 {published data only}
- Jong LA. Ex‐PRESS® tiva in patients with open angle glaucoma. A prospective comparison randomized 3‐arms study. Investigative Ophthalmology and Visual Science 2005; Vol. 46:ARVO E‐abstract 68.
De Jong 2009 {published data only}
- Jong L, Lafuma A, Aguade AS, Berdeaux G. Five‐year extension of a clinical trial comparing the Ex‐PRESS glaucoma filtration device and trabeculectomy in primary open‐angle glaucoma. Clinical Ophthalmology 2011;5:527‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong L, Lafuma A, Aguade AS, Clement O, Berdeaux G. Cost‐effectiveness of the Ex‐PRESS glaucoma filtration device in the Netherlands. Value in Health 2011;14(7):A250. [Google Scholar]
- Jong LA. Ex‐PRESS minishunt under scleral flap compared to standard trabeculectomy. Investigative Ophthalmology and Visual Science 2006; Vol. 47:ARVO E‐abstract 3544.
- Jong LA. The Ex‐PRESS glaucoma shunt versus trabeculectomy in open‐angle glaucoma: a prospective randomized study. Advances in Therapy 2009;26(3):336‐45. [DOI] [PubMed] [Google Scholar]
Eliezer 2006 {published data only}
- Eliezer RN, Kasahara N, Caixeta‐Umbelino C, Pinheiro RK, Mandia C Jr, Malta RF. Use of amniotic membrane in trabeculectomy for the treatment of glaucoma: a pilot study. Arquivos Brasileiros de Oftalmologia 2006;69(3):309‐12. [DOI] [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang H. Trabeculectomy with use of amnion transplantation in glaucoma [羊膜移植小梁切除术的应用]. Chinese Journal of Prime Medicine and Pharmacology 2007;14(5):729‐30. [Google Scholar]
Ji 2013 {published data only}
- Ji QS, Qi B, Liu L, Lao W, Yang ZH, Wang GF, et al. Comparison of trabeculectomy and trabeculectomy with amniotic membrane transplantation in the same patient with bilateral glaucoma. International Journal of Ophthalmology 2013;6(4):448‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khairy 2015 {published data only}
- Khairy HA, Elsawy MF. Trabeculectomy with mitomycin‐C versus trabeculectomy with amniotic membrane transplant: a medium‐term randomized, controlled trial. Journal of Glaucoma 2015;24(7):556‐9. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li L, Wang XM, Cui GD, Jiang H, Liu CY. Clinical study of bio‐amnion implantation used in combined trabeculectomy for refractory glaucoma [生物羊膜在难治性青光眼复合式小梁切除术中应用的临床观察]. International Journal of Ophthalmology 2010;10(10):1897‐9. [Google Scholar]
Liu 2009 {published data only}
- Liu H, Nie Q, Chen X, Zhu Y, Li X, Lu Y, et al. Clinical research of amniotic membrane transplantation in complex trabeculectomy for the treatment of refractory glaucoma [羊膜移植在复合式小梁切除术治疗难治性青光眼的临床研究]. Journal of China Medical University 2009;38(8):615‐7. [Google Scholar]
Maheshwari 2012 {published data only}
- Maheshwari D, Gupta A, Ramakrishnan R. Comparative study of MMC augmented trabeculectomy vs an Ologen implant in open angle glaucoma. Glaucoma‐II Free Papers 2012:363‐5.
Marey 2013 {published data only}
- Marey HM, Mandour SS, Ellakwa AF. Subscleral trabeculectomy with mitomycin‐C versus Ologen for treatment of glaucoma. Journal of Ocular Pharmacology and Therapeutics 2013;29(3):330‐4. [DOI] [PubMed] [Google Scholar]
Mitra 2012 {published data only}
- Mitra A, Krishnan R, Kadar MA, Chaudhury D. To compare the outcome, complications and management of complications of trabeculectomy with Ologen implant versus trabeculectomy with MMC. Glaucoma‐II Free Papers 2012:330‐5.
Netland 2014 {published data only}
- Netland PA, Sarkisian SR Jr, Moster MR, Ahmed II, Condon G, Salim S, et al. Randomized, prospective, comparative trial of Ex‐PRESS glaucoma filtration device versus trabeculectomy (XVT study). American Journal of Ophthalmology 2014;157(2):433‐40. [DOI] [PubMed] [Google Scholar]
Papaconstantinou 2010 {published data only}
- Papaconstantinou D, Georgalas I, Karmiris E, Diagourtas A, Koutsandrea C, Ladas I, et al. Trabeculectomy with OloGen versus trabeculectomy for the treatment of glaucoma: a pilot study. Acta Ophthalmologica 2010;88(1):80‐5. [DOI] [PubMed] [Google Scholar]
Ren 2009 {published data only}
- Ren Y. Clinical study of bio‐amnion implantation combined with trabeculectomy in angle‐closure glaucoma [小梁切除联合生物羊膜植入术治疗闭角型青光眼的临床观察]. Journal of Clinical Ophthalmology [Lin Chuang Yan Ke Za Zhi] 2009;17(3):220‐2. [Google Scholar]
Rosentreter 2010 {published data only}
- Rosentreter A, Schild AM, Jordan JF, Krieglstein GK, Dietlein TS. A prospective randomised trial of trabeculectomy using mitomycin C vs an Ologen implant in open angle glaucoma. Eye 2010;24(9):1449‐57. [DOI] [PubMed] [Google Scholar]
Rosentreter 2014 {published data only}
- Rosentreter A, Gaki S, Cursiefen C, Dietlein TS. Trabeculectomy using mitomycin C versus an atelocollagen implant: clinical results of a randomized trial and histopathologic findings. Ophthalmologica 2014;231(3):133‐40. [DOI] [PubMed] [Google Scholar]
Senthil 2013 {published data only}
- Senthil S, Rao HL, Babu JG, Mandal AK, Garudadri CS. Comparison of outcomes of trabeculectomy with mitomycin C vs. Ologen implant in primary glaucoma. Indian Journal of Ophthalmology 2013;61(7):338‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheha 2008 {published data only}
- Sheha H, Kheirkhah A, Taha H. Amniotic membrane transplantation in trabeculectomy with mitomycin C for refractory glaucoma. Journal of Glaucoma 2008;17(4):303‐7. [DOI] [PubMed] [Google Scholar]
Stavrakas 2012 {published data only}
- Stavrakas P, Georgopoulos G, Milia M, Papaconstantinou D, Bafa M, Stavrakas E, et al. The use of amniotic membrane in trabeculectomy for the treatment of primary open‐angle glaucoma: a prospective study. Clinical Ophthalmology 2012;6:205‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagschal 2013 {published data only}
- Beltran‐Agullo L, Trope GE, Jin Y, Wagschal LD, Jinapriya D, Buys YM. Comparison of visual recovery following Ex‐PRESS versus trabeculectomy: results of a prospective randomized controlled trial. Journal of Glaucoma 2015;24(3):181‐6. [DOI] [PubMed] [Google Scholar]
- Patel H, Wagschal LD, Jinapriya D, Trope G, Buys Y. Prospective randomized study comparing Ex‐PRESS miniature glaucoma device to trabeculectomy. Clinical and Experimental Ophthalmology. 2011; Vol. 39:25.
- Patel HY, Wagschal LD, Trope GE, Buyes YM. Economic analysis of the Ex‐PRESS miniature glaucoma device versus trabeculectomy. Journal of Glaucoma 2014;23(6):385‐90. [DOI] [PubMed] [Google Scholar]
- Wagschal LD, Trope GE, Jinapriya D, Jin YP, Buys YM. Prospective randomized study comparing Ex‐PRESS to trabeculectomy: 1‐year results. Journal of Glaucoma 2013 Nov 16 [Epub ahead of print]. [DOI] [PubMed]
Wang 2008 {unpublished data only}
- Wang M. Clinical trial of trabeculectomy with finished‐product amniotic membrane transplantation [成品生物羊膜在青光眼小梁切除术中应用的临床研究]. [doctoral thesis] 2008.
Wang 2009 {published data only}
- Wang Z, Shen J, Zhang S. Trabeculectomy with mitomycin C and amniotic membrane transplantation and adjustable suture for refractory glaucoma [小梁切除术联合MMC及生物羊膜移植可调整缝线治疗难治性青光眼的临床观察]. Journal of Otolaryngolophthalogy and Ophthalmology Shandong University 2009;23(6):67‐70. [Google Scholar]
Yan 2004 {published data only}
- Yan 2004. Trabeculectomy with use of amnion and releasable sutures for sclera flap in glaucoma [可调整缝线联合羊膜移植在小梁切除术中的应用]. Medical Journal of Wuhan University 2004;25(6):728‐30, 738. [Google Scholar]
Yang 2004 {published data only}
- Yang J, Sun L, Liu S, Peng H, Wu S. A clinical applicative study of trabeculectomy with amniotic membrane transplanted [小梁切除术联合羊膜植入术的临床应用研究]. Chinese Journal of Ophthalmology and Otorhinolaryngology 2004;4(4):228‐9. [Google Scholar]
Zhang 2009 {published data only}
- Zhang Y, Cui W. Clinical efficacy of trabeculectomy combined with sub‐flap implantation with amniotic membrane preserved in glycerin for angle closure glaucoma [小梁切除联合双瓣下甘油保存羊膜植入术治疗闭角型青光眼的临床观察]. Inner Mongolia Medical Journal 2009;41(3):473‐6. [Google Scholar]
Zheng 2005 {published data only}
- Zheng K, Huang Z, Zou H, Li H, Huang Y, Xie M. The comparison study of glaucoma trabeculectomy applying amniotic membrane or mitomycin C [青光眼小梁切除术中应用羊膜与丝裂霉素C的对比研究]. Yan Ke Xue Bao [Eye science] 2005;21(2):84‐7, 91. [PubMed] [Google Scholar]
References to studies excluded from this review
Cao 2004 {published data only}
- Cao Z, Chen S, Ye J. Trabeculectomy with amniotic membrane for acute angle closure glaucoma [小梁切除联合羊膜移植治疗急性闭角型青光眼疗效分析]. Journal of Chinese Physician [Zhong Guo Yi Shi Za Zhi] 2004;6:1531‐32. [Google Scholar]
Dahan 2011 {published data only}
- Dahan E, Ben Simon GJ. An augmented trabeculectomy for neovascular glaucoma. Ophthalmic Surgery, Lasers and Imaging 2011;42(3):196‐201. [DOI] [PubMed] [Google Scholar]
Dai 2011 {published data only}
- Dai SH. Clinical observation of modified trabeculectomy for glaucoma. International Journal of Ophthalmology 2011;11(7):1278‐9. [Google Scholar]
Gao 2002 {published data only}
- Gao W, Chui W, Liu SY, Chen XH, Zhang GR, He Y. A clinical comparative study of human amniotic membrane with mitomycin C applied in glaucoma trabeculectomy [人羊膜与丝裂霉素C用于青光眼小梁切除术的临床对比研究]. Chinese Journal of Practical Ophthalmology 2002;20(8):623‐5. [Google Scholar]
Ha 2008 {published data only}
- Ha SP, Fan WY, Yang QL. A comparative study between biotic amniotic membrane and mitomycin C applied in refractory glaucoma trabeculectomy [难治性青光眼小梁切除术中应用生物羊膜与丝裂霉素C的对比研究]. International Journal of Ophthalmology 2008;8(1):158‐60. [Google Scholar]
Jin 2006 {published data only}
- Jin Y, Shi X, Qian Y. Case series of trabeculectomy with amniotic membrane [穿透性小梁切除联合生物羊膜移植的临床观察]. Chinese Journal of Ophthalmology and Otorhinolaryngology 2006;6:244. [Google Scholar]
Lau 2008 {published data only}
- Lau G, Young S, Lehrer R. Efficacy of trabeculectomy with and without the Ex‐PRESS shunt. American Academy of Optometry. 2008.
Li 2003 {published data only}
- Li Y, Liu G, Zhao J, Xu X. Case series of amniotic membrane filtration surgery for glaucoma [羊膜用于青光眼滤过术的临床研究]. Journal of Clinical Ophthalmology [Lin Chuang Yan Ke Za Zhi] 2003;11(3):213‐5. [Google Scholar]
Li 2008 {published data only}
- Li X. Trabeculectomy with amniotic membrane for glaucoma [小梁切除联合羊膜移植术治疗青光眼]. Chinese Journal of Ophthalmology and Otorhinolaryngology 2008;8:172‐3. [Google Scholar]
Liu 2003 {published data only}
- Liu Z, Pan Y, Liu X. The clinical observation of filtering surgery with amniotic membrane implant for treatment of glaucoma [滤过手术联合羊膜植入物治疗青光眼的临床观察]. Acta Acdemic Medical Journal in Weifang [Wei Fang Yi Xue Yuan Xue Bao] 2003;25(3):175‐7. [Google Scholar]
Liu 2004 {published data only}
- Liu X, Wang L, Huang X. Clinical trial of trabeculectomy with amniotic membrane transplantation [羊膜在小梁切除术中应用的临床研究]. Journal of Sun Yat‐Sen University (Medical Sciences) 2004;25(5):462‐6. [Google Scholar]
Mahdy 2010 {published data only}
- Mahdy RA, Nada WM, Almasalamy SM, Anany HA, Almasary AM. A freeze‐dried (lyophilized) amniotic membrane transplantation with mitomycin C and trabeculectomy for pediatric glaucoma. Cutaneous and Ocular Toxicology 2010;29(3):164‐70. [DOI] [PubMed] [Google Scholar]
NCT00472810 {unpublished data only}
- NCT00472810. Ologen (OculusGen)‐Glaucoma MMC control in Pakistan. clinicaltrials.gov/ct2/show/NCT00472810 (accessed 22 February 2015).
NCT00597181 {unpublished data only}
- NCT00597181. A clinical study comparing the inflammatory response of the Ex‐PRESS mini shunt to trabeculectomy (Optonol). clinicaltrials.gov/ct2/show/NCT00597181 (accessed 22 February 2015).
Sugiyama 2011 {published data only}
- Sugiyama T, Shibata M, Kojima S, Ueki M, Ikeda T. The first report on intermediate‐term outcome of Ex‐PRESS glaucoma filtration device implanted under scleral flap in Japanese patients. Clinical Ophthalmology 2011;5:1063‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang JY, Zhang Q, Lu M. Changes of tear film functions in trabeculectomy with human amniotic membrane or mitomycin C [两种复合式小梁切除术对泪膜的影响]. International Journal of Ophthalmology 2010;10(8):1550‐1. [Google Scholar]
Zhang 2008 {published data only}
- Zhang J, Wang YW, Chen B, Li RX. Clinical study of bio‐amnion implantation in conjunctive flap used in glaucoma trabeculectomy [球结膜瓣下植入生物羊膜在难治性青光眼滤过手术中的应用]. International Journal of Ophthalmology 2008;8(6):1248‐9. [Google Scholar]
References to ongoing studies
JPRN‐UMIN000008391 {unpublished data only}
- JPRN‐UMIN000008391. Comparison between Ex‐PRESS shunt surgery and trabeculectomy for refractory glaucoma. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000008391 (accessed 22 February 2015).
JPRN‐UMIN000008981 {unpublished data only}
- JPRN‐UMIN000008981. Prospective comparative study of the Ex‐PRESS mini glaucoma shunt with standard trabeculectomy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000008981 (accessed 22 February 2015).
NCT00449098 {unpublished data only}
- NCT00449098. Ologen (OculusGen)‐glaucoma MMC control trial in India. clinicaltrials.gov/ct2/show/NCT00449098 (accessed 22 February 2015).
NCT00524758 {unpublished data only}
- NCT00524758. Oculusgen (Ologen) glaucoma MMC control in Estonia. clinicaltrials.gov/ct2/show/NCT00524758 (accessed 22 February 2015).
NCT01440751 {unpublished data only}
- NCT01440751. Comparative study of the safety and effectiveness of Ologen collagen matrix versus mitomycin‐C in glaucoma filtering surgery. clinicaltrials.gov/ct2/show/NCT01440751 (accessed 22 February 2015).
Additional references
Agnifili 2012
- Agnifili L, Costagliola C, Figus M, Iezzi G, Piattelli A, Carpineto P, et al. Histological findings of failed gold micro shunts in primary open‐angle glaucoma. Graefe’s Archive for Clinical and Experimental Ophthalmology 2012;250(1):143‐9. [DOI] [PubMed] [Google Scholar]
Al‐Haddad 2015
- Al‐Haddad C, Abdulaal M, Al‐Moujahed A, Ervin AM. Fornix‐based versus limbal‐based conjunctival trabeculectomy flaps for glaucoma. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD009380.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Azuara‐Blanco 1998
- Azuara‐Blanco A, Katz LJ. Dysfunctional filtering blebs. Survey of Ophthalmology 1998;43(2):93–126. [DOI] [PubMed] [Google Scholar]
Bae 1988
- Bae HB, Kim CS, Ahn BH. A membranous drainage implant in glaucoma filtering surgery: animal trial. Korean Journal of Ophthalmology 1988;2(2):49‐56. [DOI] [PubMed] [Google Scholar]
Barton 2001
- Barton K, Budenz DL, Khaw PT, Tseng SC. Glaucoma filtration surgery using amniotic membrane transplantation. Investigative Ophthalmology and Visual Science 2001;42(8):1762‐8. [PubMed] [Google Scholar]
Bell 1997
- Bell RW, Habib NE, O'Brien C. Long‐term results and complications after trabeculectomy with a single per‐operative application of 5‐fluorouracil. Eye 1997;11(Pt 5):663‐71. [DOI] [PubMed] [Google Scholar]
Boland 2008
- Boland MV, Zhang L, Broman AT, Jampel HD, Quigley HA. Comparison of optic nerve head topography and visual field in eyes with open‐angle and angle‐closure glaucoma. Ophthalmology 2008;115(2):239‐45. [DOI] [PubMed] [Google Scholar]
Cairns 1968
- Cairns JE. Trabeculectomy. Preliminary report of a new method. American Journal of Ophthalmology 1968;66(4):673‐9. [PubMed] [Google Scholar]
Chen 2006
- Chen HS, Ritch R, Krupin T, Hsu WC. Control of filtering bleb structure through tissue bioengineering: an animal model. Investigative Ophthalmology and Visual Science 2006;47(12):5310‐4. [DOI] [PubMed] [Google Scholar]
Chen 2014
- Chen G, Li W, Jiang F, Mao S, Tong Y. Ex‐PRESS implantation versus trabeculectomy in open‐angle glaucoma: a meta‐analysis of randomized controlled clinical trials. PLoS One 2014;9(1):e86045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2003
- Choi YJ, Kim CS, Ahn BH. A comparison of the clinical effect between e‐PTFE membrane‐tube implant and Ahmed glaucoma valve implant for the treatment of refractory glaucoma. Korean Journal of Ophthalmology 2003;17(2):106‐13. [DOI] [PubMed] [Google Scholar]
Clare 2012
- Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009379.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2006
- Clarke JCK, Schlottmann PG. Mitomycin C versus 5‐Fluorouracil for wound healing in glaucoma surgery. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coleman 2012
- Coleman AL. Advances in glaucoma treatment and management: surgery. Investigative Ophthalmology and Visual Science 2012;58(5):2491‐4. [DOI] [PubMed] [Google Scholar]
Dietlein 2008
- Dietlein TS, Jordan J, Lueke C, Krieglstein GK. Modern concepts in antiglaucomatous implant surgery. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(12):1653‐64. [DOI] [PubMed] [Google Scholar]
Douglas 1975
- Douglas GR, Drance SM, Schulzer M. The visual field and nerve head in angle‐closure glaucoma. A comparison of the effects of acute and chronic angle closure. Archives of Ophthalmology 1975;93(6):409‐11. [DOI] [PubMed] [Google Scholar]
Figus 2011
- Figus M, Lazzeri S, Fogagnolo P, Lester M, Martinelli P, Nardi M. Supraciliary shunt in refractory glaucoma. British Journal of Ophthalmology 2011;95(11):1537‐41. [DOI] [PubMed] [Google Scholar]
Foster 2002
- Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. British Journal of Ophthalmology 2002;86(2):238‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2011
- Francis BA, Singh K, Lin SC, Hodapp E, Jampel HD, Samples JR, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011;118(7):1466‐80. [DOI] [PubMed] [Google Scholar]
Fraser 2004
- Fraser S. Trabeculectomy and antimetabolites. British Journal of Ophthalmology 2004;88(7):855‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallego‐Pinazo 2009
- Gallego‐Pinazo R, Lopez‐Sanchez E, Marin‐Montiel J. Postoperative outcomes after combined glaucoma surgery. Comparison of Ex‐PRESS miniature implant with standard trabeculectomy. Archivos de la Sociedad Espanola de Oftalmologia 2009;84(6):293‐8. [DOI] [PubMed] [Google Scholar]
Gao 2013
- Gao Y, Wu YJ, Zeng R, Li WS. A meta‐analysis of operative effectiveness of trabeculectomy combined with amniotic membrane implant for glaucoma. Chinese Journal of Experimental Ophthalmology [Zhonghua Shiyan Yanke Zazhi] 2013;31(3):275‐81. [Google Scholar]
Ghate 2015
- Ghate D, Wang X. Surgical interventions for primary congenital glaucoma. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008213.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Good 2011
- Good TJ, Kahook MY. Assessment of bleb morphologic features and postoperative outcomes after Ex‐PRESS drainage device implantation versus trabeculectomy. American Journal of Ophthalmology 2011;151(3):507‐13. [DOI] [PubMed] [Google Scholar]
Green 2014
- Green E, Wilkins M, Bunce C, Wormald R. 5‐Fluorouracil for glaucoma surgery. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD001132.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
He 2014
- He M, Wang W, Zhang X, Huang W. Ologen implant versus mitomycin C for trabeculectomy: a systematic review and meta‐analysis. PLoS One 2014;9(1):e85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heijl 2002
- Heijl A, Leske MC, Bengtsson B, Hyman L, Begtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Archieves of Ophthalmology 2002;120(10):1268‐79. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane‐handbook.org.
Hsu 2008
- Hsu WC, Ritch R, Krupin T, Chen HS. Tissue bioengineering for surgical bleb defects: an animal study. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(5):709‐17. [DOI] [PubMed] [Google Scholar]
Jacob 2001
- Jacob T, LaCour OJ, Burgoyne CF, LaFleur PK, Duzman E. Expanded polytetrafluoroethylene reinforcement material in glaucoma drain surgery. Journal of Glaucoma 2001;10(2):115‐20. [DOI] [PubMed] [Google Scholar]
Jampel 1992
- Jampel HD, Pasquale LR, Dibernardo C. Hypotony maculopathy following trabeculectomy with mitomycin C. Archives of Ophthalmology 1992;110(8):1049‐50. [DOI] [PubMed] [Google Scholar]
Johnson 2014
- Johnson MS, Sarkisian SR Jr. Using a collagen matrix implant (Ologen) versus mitomycin‐C as a wound healing modulator in trabeculectomy with the Ex‐PRESS mini glaucoma device: a 12‐month retrospective review. Journal of Glaucoma 2014;23(9):649‐52. [DOI] [PubMed] [Google Scholar]
Jordan 2006
- Jordan JF, Engels BF, Dinslage S, Dietlein TS, Ayertey HD, Roters S, et al. A novel approach to suprachoroidal drainage for the surgical treatment of intractable glaucoma. Journal of Glaucoma 2006;15(3):200‐5. [DOI] [PubMed] [Google Scholar]
Kim 2003
- Kim C, Kim Y, Choi S, Lee S, Ahn B. Clinical experience of e‐PTFE membrane implant surgery for refractory glaucoma. British Journal of Ophthalmology 2003;87(1):63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lama 2003
- Lama PJ, Fechtner RD. Antifibrotics and wound healing in glaucoma surgery. Survey of Ophthalmology 2003;48(3):314‐46. [DOI] [PubMed] [Google Scholar]
Maris 2007
- Maris PJ Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex‐PRESS miniature glaucoma device implanted under scleral flap. Journal of Glaucoma 2007;16(1):14‐9. [DOI] [PubMed] [Google Scholar]
Marzette 2011
- Marzette L, Herndon LW. A comparison of the Ex‐PRESS™ mini glaucoma shunt with standard trabeculectomy in the surgical treatment of glaucoma. Ophthalmic Surgery, Lasers and Imaging 2011;42(6):453‐9. [DOI] [PubMed] [Google Scholar]
Melamed 2009
- Melamed S, Ben Simon GJ, Goldenfeld M, Simon G. Efficacy and safety of gold micro shunt implantation to the supraciliary space in patients with glaucoma. Archives of Ophthalmology 2009;127(3):264‐9. [DOI] [PubMed] [Google Scholar]
Minckler 2006
- Minckler D, Vedula SS, Li T, Mathew M, Ayyala R, Francis B. Aqueous shunts for glaucoma. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004918.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moisseiev 2015
- Moisseiev E, Zunz E, Tzur R, Kurtz S, Shemesh G. Standard trabeculectomy and Ex‐PRESS miniature glaucoma shunt: a comparative study and literature review. Journal of Glaucoma 2015;24(6):410‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Narayanaswamy 2012
- Narayanaswamy A, Perera SA, Htoon HM, Hoh ST, Seah SK, Wong TT, et al. Efficacy and safety of collagen matrix implants in phacotrabeculectomy and comparison with mitomycin‐C augmented phacotrabeculectomy at 1 year. Clinical and Experimental Ophthalmology 2013;41(6):552‐60. [DOI] [PubMed] [Google Scholar]
Nilforushan 2010
- Nilforushan N, Yadgari M, Falavarjani KG, Afshar AE. Evaluation of subconjunctival oculusgen implantation as an adjunct to trabeculectomy. Iranian Journal of Ophthalmology 2010;22:55‐62. [Google Scholar]
Pan 2011
- Pan Y, Varma P. Natural history of glaucoma. Indian Journal of Ophthalmology 2011;59(Suppl 1):S19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2006
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology 2006;90(3):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quigley 2011
- Quigley HA. Glaucoma. Lancet 2011;377(9774):1367‐77. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci 2013
- Ricci E, Vanosi G, Lindenmair A, Hennerbichler S, Peterbauer‐Scherb A, Wolbank S, et al. Anti‐fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell Tissue Bank 2013;14(3):475‐88. [DOI] [PubMed] [Google Scholar]
Skuta 1987
- Skuta GL, Parrish RK 2nd. Wound healing in glaucoma filtering surgery. Survey of Ophthalmology 1987;32(3):149–70. [DOI] [PubMed] [Google Scholar]
Small 1986
- Small RG. Ophthalmology Notes. Library of Congress Cataloging‐in‐Publication Data, 1986. [Google Scholar]
Tseng 1999
- Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor‐beta isoforms, TGF‐beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. Journal Cell Physiology 1999;179(3):325‐35. [DOI] [PubMed] [Google Scholar]
Turkoski 2012
- Turkoski BB. Glaucoma and glaucoma medications. Orthopaedic Nursing 2012;31(1):37‐41. [DOI] [PubMed] [Google Scholar]
Wang 2013a
- Wang W, Zhou M, Huang W, Zhang X. Ex‐PRESS implantation versus trabeculectomy in uncontrolled glaucoma: a meta‐analysis. PLoS One 2013;8(5):e63591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1972
- Watson PG. Surgery of the glaucomas. British Journal of Ophthalmology 1972;56(3):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1981
- Watson PG. Trabeculectomy. Developments in Ophthalmology 1981;1:61‐70. [PubMed] [Google Scholar]
Wilkins 2005
- Wilkins M, Indar A, Wormald R. Intraoperative mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002897.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wang 2013b
- Wang X, Wang R, Coleman A. Device modified trabeculectomy for glaucoma. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010472] [DOI] [PMC free article] [PubMed] [Google Scholar]


