Abstract
Fibrillins constitute the backbone of microfibrils in the extracellular matrix of elastic and non-elastic tissues. Mutations in fibrillins are associated with a wide range of connective tissue disorders, the most common is Marfan syndrome. Microfibrils are on one hand important for structural stability in some tissues. On the other hand, microfibrils are increasingly recognized as critical mediators and drivers of cellular signaling. This review focuses on the signaling mechanisms initiated by fibrillins and microfibrils, which are often dysregulated in fibrillin-associated disorders. Fibrillins regulate the storage and bioavailability of growth factors of the TGF-β superfamily. Cells sense microfibrils through integrins and other receptors. Fibrillins potently regulate pathways of the immune response, inflammation and tissue homeostasis. Emerging evidence show the involvement of microRNAs in disorders caused by fibrillin deficiency. A thorough understanding of fibrillin-mediated cell signaling pathways will provide important new leads for therapeutic approaches of the underlying disorders.
Keywords: Fibrillin, Microfibrils, Cell signaling, Connective tissue disorders, Integrins, Matrix metalloproteinases, MicroRNA
Structure and function of fibrillins
In humans and other non-rodent mammalian species, three highly homologous ~350 kDa extracellular matrix (ECM) proteins, fibrillin-1, −2 and −3 constitute the fibrillin protein family (Hubmacher and Reinhardt 2011). In rodents, the gene for fibrillin-3 is inactive due to chromosomal rearrangements (Corson et al. 2004). Fibrillin-1 is the main form in postnatal life, whereas fibrillin-2 and −3 are primarily expressed during development (Zhang et al. 1995; Corson et al. 2004; Sabatier et al. 2011). Human fibrillin-1, −2 and −3 are encoded by different genes on chromosomes 15, 5 and 19 (Lee et al. 1991; Magenis et al. 1991; Corson et al. 2004), and are highly conserved between species (Robertson et al. 2011; Piha-Gossack et al. 2012).
Domain organization of fibrillins
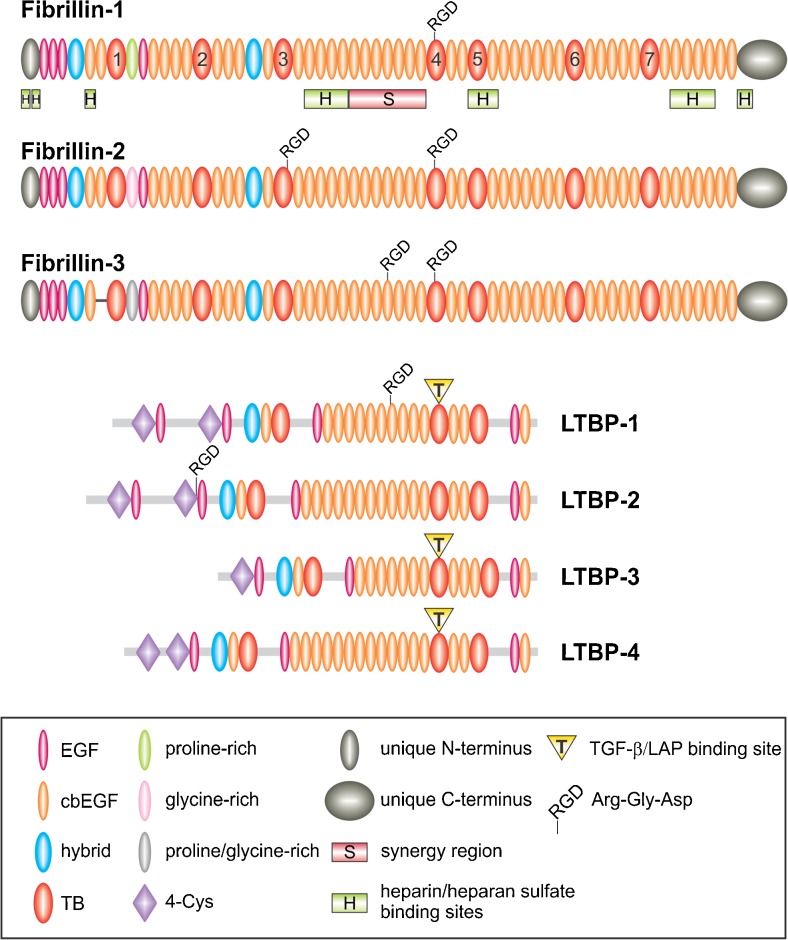
Fibrillins are multi-domain proteins mainly composed of epidermal growth factor-like (EGF) and some other domains (Corson et al. 1993; Pereira et al. 1993) (Fig. 1). EGF domains are present 47 times in fibrillin-1 and −2, and 46 times in fibrillin-3 due to alternative splicing. The majority of these EGF domains, 43 in fibrillin-1 and −2 and 42 in fibrillin-3, contain the calcium binding (cb) consensus sequence D/N-X-D/N-E/Q-Xm-D/N-Xn-Y/F where m and n represent variable numbers of amino acid residues (Rees et al. 1988; Handford et al. 1991; Mayhew et al. 1992). These residues are involved in either the direct ligation of calcium or the stabilization of the calcium binding site. Calcium binding is a key property which provides structural stabilization to the fibrillin proteins (Downing et al. 1996; Reinhardt et al. 1997a; Werner et al. 2000). Together with interdomain hydrophobic packing interactions and short linker regions between individual cbEGF domains, calcium binding contributes to the characteristic rigid rod-like shape of fibrillins (Downing et al. 1996; Reinhardt et al. 1997a). Calcium binding is also functionally important for the protection of fibrillins against proteolysis (Reinhardt et al. 1997b), the control of self-interaction (Lin et al. 2002; Marson et al. 2005), and the interaction with several different ECM components, such as fibulin-2, heparin/heparan sulfate and microfibril-associated glycoprotein-1 (Reinhardt et al. 1996; Tiedemann et al. 2001; Rock et al. 2004). Disulfide bond formation between the six cysteine residues in EGF and cbEGF domains occurs in a C1-C3, C2-C4 and C5-C6 pattern and further stabilizes the protein (Downing et al. 1996; Smallridge et al. 2003). The second most common type of domain in fibrillins is the transforming growth factor-beta-binding protein-like (TB) domain (Corson et al. 1993; Pereira et al. 1993). TB domains occur seven times in fibrillins, and their eight cysteine residues form four disulfide bonds in a C1-C3, C2-C6, C4-C7 and C5-C8 pattern (Yuan et al. 1997; Lee et al. 2004). Fibrillins further contain hybrid (hyb) domains which show sequence similarities with TB domains in their N-terminus and with EGF domains in their C-terminus (Corson et al. 1993; Pereira et al. 1993). Hyb domains occur twice in all mammalian fibrillins and are stabilized by four intradomain disulfide bonds in a C1-C3, C2-C5, C4-C6 and C7-C8 pattern (Jensen et al. 2009). Other protein domains in fibrillins include unique N- and C-terminal domains that are processed by furin convertases (Lönnqvist et al. 1998; Raghunath et al. 1999; Ritty et al. 1999; Kettle et al. 2000; Wallis et al. 2003). A distinguishing feature is a proline-rich domain close to the N-terminus of fibrillin-1, a glycine-rich domain in fibrillin-2, and a domain rich in proline and glycine in fibrillin-3 (Corson et al. 1993; Zhang et al. 1994; Nagase et al. 2001).
Fig. 1.
Schematic of the fibrillin/LTBP superfamily. The domain organization of human fibrillins and LTBPs is shown. The most common type of domain are the cbEGF domains interspersed mainly by TB and hybrid domains. TB domains are numbered in fibrillin-1. The RGD cell binding sites, the synergy region (S) and heparin/heparan sulfate-binding sites (H) are shown for fibrillin-1. The latent small TGF-β/LAP complex covalently interacting with LTBP-1, −3 and −4 is indicated by yellow triangles. Only the longest splice variant is shown for each LTBP isoform and shorter splice variants are omitted for simplicity
In addition to fibrillins, cbEGF domains can be found in many other ECM and serum proteins. TB domains and hyb domains, however, are unique to fibrillins and a related family of ECM proteins, the latent transforming growth factor beta-binding proteins (LTBPs) (Robertson et al. 2015). Fibrillins and LTBPs together constitute the fibrillin/LTBP superfamily (Fig. 1). In humans, four members of the LTBP family are known, LTBP-1, −2, −3 and −4. Similar to fibrillins, LTBPs consist of tandem cbEGF repeats that are interspersed with three TB domains and one hyb domain (Hyytiäinen et al. 2004). LTBPs are important for the sequestration and activation of transforming growth factor-beta (TGF-β)1, −2 and −3 as outlined in detail below.
RGD and heparin/heparan sulfate binding sites
The RGD (arginine-glycine-aspartic acid) cell binding site is a three amino acid motif that occurs in many ECM proteins, including fibrillins (Ruoslahti 1996). It mediates the interaction between ECM proteins and transmembrane receptors, the integrins (see paragraph Integrins). All fibrillins contain an RGD sequence in their TB4 domain (Fig. 1). Fibrillin-2 and −3 have an additional RGD cell binding site in the TB3 or the cbEGF18 domain, respectively (Robertson et al. 2011; Piha-Gossack et al. 2012). X-ray crystallographic structural analysis of the TB4 domain in a cbEGF22-TB4-cbEGF23 recombinant fibrillin-1 fragment demonstrated a tetragonal pyramid shape for this fragment (Lee et al. 2004). The RGD site is located on an exposed flexible loop in the TB4 domain.
Integrins α5β1, αvβ3 and αvβ6 were shown to interact with fibrillin-1 (Pfaff et al. 1996; Sakamoto et al. 1996; Bax et al. 2003; Jovanovic et al. 2007). Fibrillin-1 adhesion and migration on α5β1 is strongly enhanced by an upstream tandem array of cbEGF domains, the synergy site (Bax et al. 2007). This study demonstrated that the strongest cell adhesion to α5β1 integrin was promoted in the presence of seven upstream cbEGF domains, and to αvβ3 in the presence of four upstream domains. This indicates slightly different requirements for these integrins in terms of the complementary synergy site(s) present in fibrillin-1.
In addition, close to the RGD site in the TB4 domain, a downstream heparin/heparan sulfate binding site was identified in TB5-cbEGF25 that stimulates focal adhesion formation (Tiedemann et al. 2001; Ritty et al. 2003; Cain et al. 2005, 2008) (Fig. 1). It is possible that this site can modulate the integrin interaction with fibrillins, but this aspect is not explored. Six other heparin/heparan sulfate binding sites were identified throughout the fibrillin-1 molecule (Tiedemann et al. 2001; Ritty et al. 2003; Cain et al. 2005, 2008; Yadin et al. 2013) (Fig. 1). Some, or possibly all, of them may be relevant for fibrillin-mediated cell signaling. The addition of heparin/heparan sulfate to cultured human skin fibroblasts impaired the formation of a fibrillin network (Tiedemann et al. 2001; Ritty et al. 2003). Moreover, the inhibition of heparan sulfate sulfation or the biosynthesis of heparan sulfate inhibited fibrillin network formation (Trask et al. 2000a; Tiedemann et al. 2001). These data show that heparin/heparan sulfate interactions with fibrillins play a critical role in fibrillin network assembly. Other cell signaling functions of these heparin/heparan sulfate sites in fibrillins require future clarification.
Fibrillin-containing microfibrils
Fibrillins multimerize in the ECM of elastic and non-elastic tissues to form the core of supramolecular structures, the 10 nm in diameter microfibrils (Low 1962). Fibrillin-containing microfibrils (referred to as “microfibrils” throughout this review) provide mechanical stability in some tissues where they typically tether basement membranes to the underlying stroma, for example in superficial regions of the skin, the kidney and the ciliary zonules of the eye (Raviola 1971; Kriz et al. 1990). In elastic tissues, microfibrils provide the key scaffold machinery for the biogenesis of elastic fibers (Wagenseil and Mecham 2007). The importance of fibrillin-1 for the deposition of elastic fibers is highlighted by fibrillin-1 deficient Fbn1−/− mice (Carta et al. 2006). These mice die within two weeks after birth due to impaired lung function and ruptured aortic aneurysms as a result of disorganized elastic fibers in these tissues. Microfibrils also represent the principal units that mediate fibrillin-guided cell signaling as outlined in detail below throughout this review.
Microfibrils are associated with more than 20 other proteins to fulfill their highly specialized role in different tissue contexts (Baldwin et al. 2013). The interacting partners of fibrillins can be divided into functional subgroups: 1) microfibril biogenesis, 2) elastic fiber assembly, 3) proteoglycan interaction, and 4) growth factor regulation. Self-interaction sites, heparin-binding sites and the interaction with fibronectin are important for microfibril biogenesis (Ashworth et al. 1999a; Trask et al. 1999; Tiedemann et al. 2001; Lin et al. 2002; Marson et al. 2005; Kuo et al. 2007; Hubmacher et al. 2008; Sabatier et al. 2009). Crucial fibrillin interaction partners relevant for elastic fiber assembly are, among others, tropoelastin (Trask et al. 2000b; Rock et al. 2004), fibulin-4 and −5, and lysyl oxidase (Freeman et al. 2005; El-Hallous et al. 2007; Kobayashi et al. 2007; Choudhury et al. 2009; Ono et al. 2009). Fibrillin-1 interacting proteoglycans include decorin, versican and perlecan (Trask et al. 2000a; Isogai et al. 2002; Tiedemann et al. 2005). The interaction with growth factors can be either indirect via LTBPs in the case of TGF-βs (Isogai et al. 2003; Hirani et al. 2007; Ono et al. 2009), or direct for bone morphogenetic protein (BMP)-2, −4, −5, −7 and −10 and growth and differentiation factor (GDF)-5 (Gregory et al. 2005; Sengle et al. 2008a, 2011). This aspect will be discussed in detail below.
Fibrillinopathies
Mutations in fibrillin-1 and −2 can lead to a wide variety of heritable autosomal-dominant connective tissue disorders, collectively termed fibrillinopathies. Mutations in fibrillin-1 most commonly cause Marfan syndrome (Dietz et al. 1991), but also other disorders such as stiff skin syndrome (Loeys et al. 2010), acromicric and geleophysic dysplasia (Le Goff et al. 2011), dominant Weill-Marchesani syndrome (Faivre et al. 2003), and dominant ectopia lentis (Tsipouras et al. 1992).
Marfan syndrome affects two to three in 10,000 individuals and manifests in various organ systems, most importantly in large blood vessels, bones and eyes (Pyeritz and Dietz 2002). The clinical symptoms range from long bone overgrowth, scoliosis, dural ectasia and ectopia lentis to life-threatening aortic aneuryms and dissections. Marfan syndrome was initially described in a 5-year old girl in 1896 by Antoine-Bernard Marfan (Marfan 1896). However, the link between Marfan syndrome and mutations in the fibrillin-1 gene (FBN1) on chromosome 15 was not established until 1991 (Kainulainen et al. 1990; Dietz et al. 1991). Until today, more than 3000 mutations have been identified in the fibrillin-1 gene (http://www.umd.be/FBN1) (Collod-Beroud et al. 2003). Most of these mutations cause different forms of Marfan syndrome, ranging from the common classical form to a more severe neonatal form with early disease onset to the progeroid form which is associated with premature aging (Dietz et al. 1991; Kainulainen et al. 1994; Graul-Neumann et al. 2010). Marfan syndrome is characterized by a wide inter- and intra-familial phenotypic variability and severity of the disease, which complicates genotype-phenotype correlations.
Mutations in the RGD-containing TB4 domain of fibrillin-1 cause an autosomal-dominant form of congenital scleroderma, known as stiff skin syndrome (Loeys et al. 2010). These patients present with diffuse skin fibrosis leading to hard, thick skin, which imposes major limitations on joint mobility and causes flexion contractures. The patients also are characterized by short body stature. Electron microscopy of microfibrils from stiff skin syndrome patients displayed accumulation of structurally altered microfibrils containing shorter and denser fibrils in addition to collagen accumulation (Loeys et al. 2010).
Acromicric and geleophysic dysplasias are caused by mutations in the TB5 domain of fibrillin-1 (Le Goff et al. 2011). Stiff joints, thick skin, short stature and extremities characterize both dysplasias. These authors also demonstrated that fibroblasts from these patients form a disorganized and reduced microfibril network. An in-frame deletion of eight amino acid residues in the region of fibrillin-1 that encodes the TB5 domain causes the autosomal dominant form of Weill-Marchesani syndrome (Faivre et al. 2003). Similar to acromicric and geleophysic dysplasias, clinical symptoms include a short stature and joint stiffness. Additionally, those patients often display spherophakia associated with glaucoma.
In summary, mutations within the same gene (FBN1) lead to a range of connective tissue disorders spanning a wide clinical spectrum. The current pressing question in the field is how mutations within FBN1 can cause such a wide spectrum of clinical features. It is likely that these pleotropic disease manifestations are caused by alterations of the fine-tuning of growth factors and other fibrillin-guided cell signaling pathways, as summarized in the following paragraphs.
In addition to mutations in FBN1, mutations in FBN2 give rise to congenital contractural arachnodactyly characterized by multiple joint contractures in the elbows, knees, ankles and fingers as well as a crumpled appearance of the ears (Viljoen 1994). No pathogenic mutation has yet been identified in FBN3, although some studies suggest its involvement in polycystic ovary syndrome (Jordan et al. 2010; Raja-Khan et al. 2010; Hatzirodos et al. 2011).
Growth factor regulation by fibrillins
Matrix sequestration of growth factors is an essential aspect in the regulation of their activity and signaling properties. The interaction of microfibrils and growth factors can be either indirect through LTBPs in case of TGF-β or direct in case of several BMPs. Perturbation of the growth factor matrix sequestration frequently leads to disease pathogenesis.
TGF-β superfamily in relation to fibrillins
The TGF-β superfamily comprises TGF-β1, −2 and −3, BMPs, activins, inhibins and GDFs (Massague 1998). The members of this superfamily include potent cytokines that regulate a variety of processes in development, homeostasis and tissue repair including cellular differentiation, migration, and ECM remodeling.
Synthesis, secretion and activation of TGF-βs
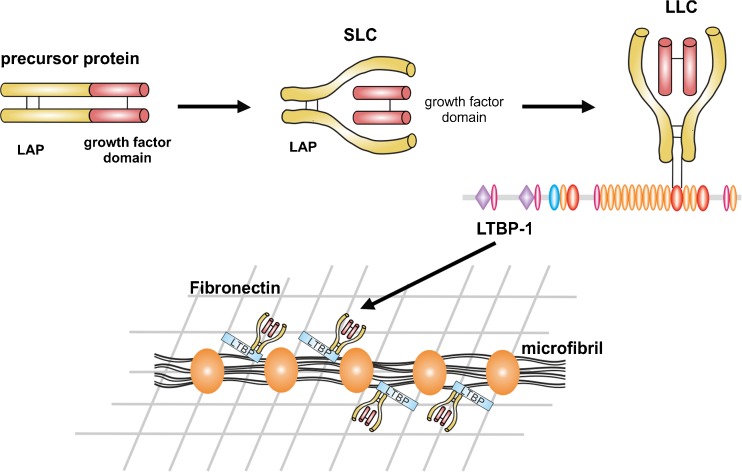
TGF-β1, −2 and −3 are synthesized as 55 kDa precursor proteins composed of the mature growth factor domain at the C-terminus and an N-terminal prodomain, the latency associated peptide (LAP) (Lawrence et al. 1984; Gentry et al. 1988) (Fig. 2). The precursor proteins homodimerize through disulfide bond formation. The homodimers are proteolytically processed by furin-like endoproteases in the trans-Golgi network, forming the small latent complex (SLC) with the LAP dimer non-covalently shielding the active TGF-β dimer (Shi et al. 2011). The SLC covalently interacts with several LTBPs forming the large latent complex (LLC) (Hyytiäinen et al. 2004; Rifkin 2005). LTBP-1 and −3 bind to the LAPs of all three TGF-βs, whereas LTBP-4 only interacts with the LAP of TGF-β1 (Saharinen and Keski-Oja 2000). Most cell types secrete TGF-β as latent complexes (Miyazono et al. 1991; Taipale et al. 1994). LTBP-1 colocalized with fibrillin-1 and fibronectin in cell culture (Taipale et al. 1996; Dallas et al. 2000, 2005; Klingberg et al. 2014). LTBP-1 was also found associated with microfibrils in different tissues, including skin, developing heart and the cardiovascular system, as well as the periosteum of developing bone (Nakajima et al. 1997; Raghunath et al. 1998; Dallas et al. 2000; Isogai et al. 2003). Direct biochemical interactions were shown between fibrillin-1 and the C-termini of LTBP-1, −2 and −4 (Isogai et al. 2003; Hirani et al. 2007; Ono et al. 2009), whereas the N-termini of LTBP-1 and −4 associate with fibronectin (Dallas et al. 2005; Fontana et al. 2005; Kantola et al. 2008). All LTBPs localize to microfibrils in tissues, even LTBP-3 despite the lack of biochemical interaction with fibrillin-1 (Zilberberg et al. 2012). Through this mechanism, inactive TGF-βs are targeted to microfibrils in the ECM, which regulate release and activation of the active growth factors. The formation of the LLC is schematically depicted in Fig. 2.
Fig. 2.
Biosynthesis of TGF-β and targeting to microfibrils. TGF-βs are synthesized as precursor proteins consisting of a growth factor domain at the C-terminus (red) and the LAP at the N-terminus (yellow). Two precursor proteins homodimerize and are proteolytically cleaved to form the SLC where LAP is non-covalently bound to the active TGF-β dimer. The SLC can covalently bind to the penultimate TB domain in LTBP-1, −3 and −4 (shown here for LTBP-1), constituting together the LLC. The C-terminal region of LTBP-1 and −4 interact non-covalently with the N-terminal region of fibrillin-1 present in the core of beaded microfibrils. The N-terminal regions of LTBP-1 and −4 interact with fibronectin. LTBP-3 localizes to microfibrils via a different mechanism. In this way, TGF-βs are targeted to microfibrils, which directly or indirectly regulate their release and activation
In its latent state, TGF-β is not accessible to its receptor and requires release from the ECM either through conformational changes in the SLC or through other mechanisms including proteolytic degradation. The LAPs of TGF-β1 and −3 contain an RGD sequence by which they can interact with αvβ6, αvβ8 or αvβ5 integrins expressed by different cell types (Munger et al. 1999; Mu et al. 2002; Wipff and Hinz 2008). Upon binding of LAPs to cell surface integrins and cell-mediated force transmission, a conformational change is induced in the SLC which leads to the release of active TGF-β (Annes et al. 2004; Wipff et al. 2007; Shi et al. 2011). Matrix metalloproteinase (MMP)-2 and −9 and the serine protease plasmin can either directly target LAPs or alternatively target the LTBPs to induce TGF-β activation (Sato and Rifkin 1989; Yu and Stamenkovic 2000). Interaction between thrombospondin-1 and LAP can also release active TGF-β through a conformational change in LAP (Schultz-Cherry et al. 1995). TGF-β can also be released from the SLC by reactive oxygen species that are generated during inflammation or by irradiation (Barcellos-Hoff et al. 1994). In vitro, TGF-β can be activated by heat or acid treatment, which leads to denaturation of LAP, and in turn to the release of active TGF-β (Lyons et al. 1988).
BMPs in relation to fibrillins
BMPs are also expressed as precursor proteins with a prodomain and a mature C-terminal growth factor. Unlike TGF-β, most BMPs are active when overexpressed as recombinant proteins due to an open armed conformation of the prodomain that allows interaction of the growth factor dimer with its receptor (Mi et al. 2015). BMP-7 is secreted as a stable complex with the growth factor dimer non-covalently associated with its two prodomains (Gregory et al. 2005). Due to the structural similarity to the TGF-β SLC, the prodomain of BMP-7 was tested by these authors for binding to LTBP-1 or fibrillin-1. Interactions were observed between the BMP-7 prodomain or the BMP-7 prodomain/growth factor complex and N-terminal regions of fibrillin-1, whereas the growth factor dimer alone did not interact. It is possible that this interaction sequesters BMP-7 complexes to microfibrils in the ECM. Binding assays with fibrillins were extended to BMP-2, −4, −5, −10 and GDF-5 and −8 using the recombinant prodomains without their respective growth factor dimers (Sengle et al. 2008a, 2011). All prodomains, except the GDF-8 prodomain, interacted with fibrillins. BMPs and GDFs bind to a region between the N-terminus and the proline-rich domain in fibrillin-1. The downstream region between EGF4 and TB3 also interacts with various affinities with GDF‐5 and BMP‐2, −4, and −10. Thus, the N-terminal region of fibrillins appears to act as a high affinity binding site and concentrator for BMP prodomains. However, other mechanisms for targeting some of these growth factors to the ECM exist. For example, the GDF-8 prodomain interacts with a glycosaminoglycan chain at the C-terminus of perlecan, a core basement membrane protein (Sengle et al. 2011). Given that perlecan has been shown to interact directly with fibrillin-1, GDF-8 might be indirectly targeted to microfibrils (Tiedemann et al. 2005).
Unlike the latent TGF-β prodomain/growth factor complex, the BMP-7 prodomain/growth factor complex was able to bind to its receptors and induce BMP signaling in cell culture (Sengle et al. 2008b). The growth factor dimers and the BMP-7 prodomain/growth factor complex did not show any overt differences in their signaling capabilities, and BMP type II receptors could displace the BMP-7 prodomains in solution and bind to the growth factor dimer. In vitro studies showed that capturing the BMP-7 complexes by binding to a fibrillin-2 scaffold impaired BMP signaling (Sengle et al. 2015). This inhibitory effect of fibrillin binding is most likely the result of a conformational change in the prodomain structure, which impedes the interaction of BMP receptors and growth factor. Thus, fibrillins might act as a latency factor for at least some BMPs.
TGF-β and BMP signaling
Active TGF-β family members signal via specific complexes of homodimeric type I and type II transmembrane serine/threonine kinase receptors (Wrana et al. 1992). Upon ligand-induced formation of heterotetrameric receptor complexes, the type II receptor transphosphorylates and activates the type I receptor. The activated type I receptors propagate the growth factor signal by phosphorylating specific Sma and Mad related (SMAD) transcription factors, the receptor-regulated (R-)SMADs (Feng and Derynck 2005). Upon activation, two R-SMADs form heteromeric complexes with the co-SMAD, followed by translocation into the nucleus, where the complex participates in the transcriptional regulation of target genes.
TGF-β can also activate non-canonical signaling through the serine/threonine kinase receptors by activating Ras guanosine triphosphate phosphohydrolases (GTPases) (Derynck and Zhang 2003; Lee et al. 2007; Yamashita et al. 2008). The Ras GTPases phosphorylate and thus activate the mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38. TGF-β can also activate TGF-β-activated kinase (TAK1), which is a MAP kinase kinase kinase activating the MAP kinase cascade. Non-canonical TGF-β signaling can either occur independently or in parallel to canonical signaling via SMADs.
Regulation of TGF-β/BMP activation and signaling in fibrillinopathies
TGF-β and BMP signaling is highly dependent on the cellular and tissue context. The generation of mouse models has significantly extended knowledge of how fibrillin-1 and -2 guide and modulate TGF-β/BMP signaling. Mice heterozygous for a missense mutation in fibrillin-1 (Fbn1C1039G/+) are frequently used as a model for Marfan syndrome (Judge et al. 2004). These mice display aberrant thickening of the aortic media with fragmented and disorganized elastic fibers, and excessive TGF-β activity leads to the formation of aortic aneurysms (Habashi et al. 2006). This study also showed that postnatal administration of TGF-β-neutralizing antibodies improved aortic wall architecture and decreased elastic fiber fragmentation. Fbn1C1039G/+ mice are further characterized by myxomatous changes of the atrioventricular valves, which include a postnatally acquired increase in leaflet length and thickness (Ng et al. 2004). The alterations in mitral valve architecture correlate with excess TGF-β activation and signaling, which could be rescued in vivo by TGF-β antagonism. Fbn1C1039G/+ mice also display architectural abnormalities in the skeletal muscle, which was characterized by decreased muscle fiber sizes and numbers and an increase in the amount of interstitial tissue and fat between muscle fiber bundles (Cohn et al. 2007). These phenotypes also were generally correlated with elevated TGF-β activity as evidenced by the normalization of muscle architecture upon systemic antagonism of TGF-β. Mice that homozygously express a centrally deleted Fbn1 allele at about 10 % of the wild‐type level (Fbn1mgΔ/mgΔ) present in addition to aortic dilation and rupture with lung abnormalities, which manifested as impaired distal alveolar septation (Pereira et al. 1997; Neptune et al. 2003). These mice are again characterized by enhanced TGF-β activation and signaling. Perinatal administration of a TGF-β-neutralizing antibody rescued the failed distal alveolar septation in vivo.
Fbn1C1039G/+ mice show activation of both the canonical TGF-β signaling pathway via SMAD2 and the non-canonical pathway via ERK1/2 (Holm et al. 2011). This study clarified that it is the non-canonical pathway through ERK1/2 that drives aortic aneurysm progression, and hence inhibition of ERK1/2 pathways appears as a potential novel therapeutic strategy. Further evidence for the importance of non-canonical TGF-β signaling comes from studies with vascular smooth muscle cells explanted from the thoracic aortas of Fbn1−/− mice (Carta et al. 2009). These cells displayed abnormal accumulation of TAK1 and the phosphorylated MAPK p38. This study further demonstrated that phosphorylated p38 accumulated earlier than phosphorylated (p-)SMAD2 in the aortic wall of Fbn1−/− mice, and activated signaling via SMAD2. In addition, systemic inhibition of p38 activity lowered SMAD2 phosphorylation.
TGF-β antagonism proved as a valuable therapeutic approach in preclinical mouse models and is also the basis for treatment with losartan, an angiotensin II type 1 (AT1) receptor antagonist. Like beta-blockers, losartan lowers the blood pressure, albeit through a different molecular mechanism. Beta-blockers emerged as standard care for Marfan patients over the years (Shores et al. 1994). In addition to its beneficial effects on blood pressure, losartan was shown to antagonize TGF-β in animal models of chronic renal insufficiency and cardiomyopathy (Lim et al. 2001; Lavoie et al. 2005). The interaction between angiotensin II and AT1 mediates the progression of aortic aneurysms and AT1 blockade abrogated aneurysm progression in Fbn1C1039G/+ mice (Habashi et al. 2011). Loss of AT2 expression, in contrast, accelerates aortic aneurysm formation in these mice. The authors further demonstrate that losartan inhibits TGF-β-mediated activation of ERK1/2 by shunting signaling through the AT2 receptors, which in turn inhibits ERK1/2 activation. This lowers the expression of TGF-β ligands, receptors, and activators as their expression is stimulated through the ERK1/2 pathway.
Postnatal treatment with the beta-blocker propranolol or losartan in comparison demonstrated the advantages of losartan, which fully rescued the abnormalities in the aortic wall and also partially reversed the impaired alveolar septation (Habashi et al. 2006). Administration of propranolol, in contrast, reduced the aortic growth rate, but did not improve the aortic wall architecture. Angiotensin II type 1 receptor blockade through losartan also normalized the architectural abnormalities in the skeletal muscle, similar to TGF-β antagonism (Cohn et al. 2007).
The beneficial effects of losartan for patients with Marfan syndrome was assessed in various clinical trials (Lacro et al. 2007; Detaint et al. 2010). One of the largest recent studies with over 600 children and young adults with Marfan syndrome, however, did not show significantly different beneficial effects of losartan and the beta-blocker atenolol on the aortic growth rate over a period of three years (Lacro et al. 2014). These data exemplify differences that may exist in terms of drug efficiencies between preclinical mouse models and humans.
Deregulation of TGF-β activation and signaling is also observed in other fibrillinopathies. Fibroblasts from patients with acromicric or geleophysic dysplasia displayed enhanced TGF-β signaling (Le Goff et al. 2011). Increased TGF-β signaling was also observed in the dermis of patients with stiff skin syndrome, which can be partly explained by the increased expression of p-SMAD2 and the accumulation of LLCs which causes an elevated concentration of TGF-β in the ECM (Loeys et al. 2010).
It is still not completely understood how mutations in fibrillin-1 lead to enhanced TGF-β activation and signaling, either as a primary or a secondary cause. One explanation is a reduced number of functional microfibrils present in the ECM, another one is the inability of LTBPs to mediate TGF-β sequestration to microfibrils in the disease state. Residues within the hyb1 domain mediate binding of fibrillin-1 to LTBP-1 and LTBP-4 (Ono et al. 2009). This study showed that LTBP-1 and LTBP-4 were not incorporated into microfibrils produced by Fbn1−/− fibroblast cultures, demonstrating that fibrillin-1 is required for correct matrix incorporation of LTBPs. The authors of this study further showed that deletion of the hyb1 domain caused impaired interaction between fibrillin-1 and LTBP-1 and abrogated binding to LTBP-4 in vitro. Homozygous in vivo deletion of the hyb1 domain in Fbn1H1Δ/H1Δ mice did not show any gross phenotype (Charbonneau et al. 2010). Both, homozygous Fbn1H1Δ/H1Δ and heterozygous Fbn1H1Δ/+ mice did not develop aortic aneurysms or dissections and displayed normal assembly of microfibrils. Therefore, it can not only be a compromised interaction between LTBPs and fibrillin-1 that causes the spectrum of diseases associated with fibrillin-1 mutations. Likely, it is an integrated interplay of context specific factors and events that trigger the pathological cascade.
Global deletion of the fibrillin-2 gene (Fbn2−/−) in mice causes a relatively mild phenotype including bilateral syndactyly and temporary joint contractures (Arteaga-Solis et al. 2001). This study showed that fibrillin-2 regulates BMP-7 signaling in the developing autopod. Upon deletion of fibrillin-2, BMP-7 does not localize to the interdigit region, where it normally mediates apoptosis, thus leading to syndactyly. A study analyzing Fbn2−/− null mice on a 129/Sv background described newborn mice with reduced muscle mass, abnormal muscle histology and activated BMP-7 signaling in skeletal muscle. These data indicate that fibrillin-2 mediated BMP signaling is important for muscle differentiation (Sengle et al. 2015).
The crosstalk between TGF-β and BMPs can exemplarily be described in bone remodeling. TGF-β1 stimulates matrix degradation through the induction of osteoclastogenesis (Nistala et al. 2010b). BMPs have an osteoinductive effect during bone maturation, mineralization and homeostasis. Primary cultured osteoblasts from both Fbn1−/− and Fbn2−/− mice showed enhanced activation of TGF-β signaling (Nistala et al. 2010a). Fbn2−/− osteoblasts showed impaired osteoblast maturation and bone formation as a result of enhanced TGF-β activation. Osteoblasts from Fbn1−/− displayed evidence for activated BMP signaling. Osteoblast maturation and mineralization occurred faster despite enhanced TGF-β signaling at similar levels as in Fbn2−/− osteoblasts. The increased availability of normally matrix-bound BMPs enhances osteoinductive BMP signaling and counteracts the anabolic effects of TGF-β signaling.
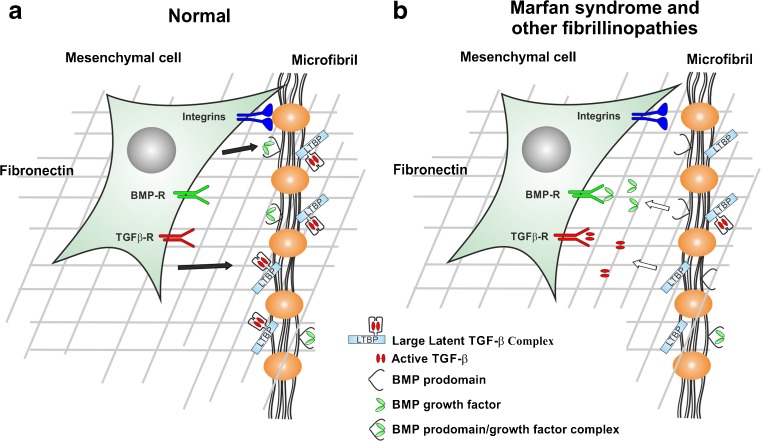
Figure 3 provides an overview of growth factor sequestration by fibrillin microfibrils in normal and in disease state.
Fig. 3.
Microfibrils as growth factor and cell signalling relay stations in normal and pathological situations. a In a normal physiological situation, cell surface located beaded microfibrils interact with smooth muscle cells and fibroblasts through integrins (blue). The TGF-βs LLC and BMP prodomain/growth factor complexes are secreted by cells and deposited onto microfibrils (black arrows). b In pathological situations, the release regulation of active TGF-β and BMP growth factors from microfibrils is disturbed. Mutations in fibrillin-1 lead to enhanced release of TGF-β, and potentially BMPs. The growth factors can interact with their respective cell surface receptors and trigger intracellular signaling events (white arrows). The interaction of integrins with microfibrils might also be dysregulated in pathological situations. Cells, microfibrils and growth factors are not drawn to scale
Other growth factors relevant to fibrillins
Wnts (Wingless-type mouse mammary tumor virus integration site family) are important growth factors required for cellular differentiation, tissue morphogenesis and homeostasis with critical roles in cardiac development and differentiation, angiogenesis, cardiac hypertrophy, cardiac failure, and aging (Rao and Kuhl 2010). The Wnt signaling pathway is still largely unstudied in the context of its regulation by the ECM and in regard to its involvement in fibrillinopathies. Wnts propagate signaling through the stabilization of β-catenin, which is otherwise targeted for destruction. β-catenin can then mediate the regulation of gene expression in the nucleus with other transcription factors. Wnt signaling has been shown to play a role in skin fibrosis where it crosstalks with fibrillin. The tight skin (Tsk) mouse model displays certain features that are overlapping with stiff skin syndrome. It is caused by a large in-frame duplication of exons 17–40 in fibrillin-1, thus resulting in a larger ~420 kDa fibrillin-1 protein (Siracusa et al. 1996; Saito et al. 1999). Heterozygous Fbn1Tsk/+ mice are characterized by tight skin and accumulation of microfibrils in the loose connective tissue (Green et al. 1976). mRNA microarray analysis of the Tsk mouse skin showed increased levels of several genes integrated with the Wnt signaling pathway, including Wnt2, -9a, -10b, and −11, inhibitors such as secreted frizzled-related protein 2 and −4, as well as Wnt-induced secreted protein 2 (Bayle et al. 2008). Furthermore, the authors demonstrated that Wnt3a increased fibrillin matrix formation in vitro, which further indicates that Wnts contribute to increased microfibril accumulation in the skin of Fbn1Tsk/+ mice.
The CCN family is a group of six secreted proteins that are named after three of its members: cysteine-rich protein 61 (CYR61, also called CCN1), connective tissue growth factor (CTGF, also called CCN2) and nephroblastoma overexpressed protein (NOV, also called CCN3) (Leask and Abraham 2006). CCNs are generally induced by growth factors and cytokines and are overexpressed in pathological conditions such as fibrosis. The CCNs act as adaptor molecules connecting the ECM with the cell surface. It has been shown that CCN3 plays a role in skin matrix remodeling of Fbn1Tsk/+ mice (Lemaire et al. 2010). These authors showed that TGF-β and Wnt worked synergistically to stimulate fibrillin matrix assembly and CCN3 expression in the skin. CCN3 overexpression markedly impaired fibrillin assembly, as it blocked the effects of both cytokines. Fibrillin matrix in turn upregulated CCN3 expression, and thus provided a counter-regulatory mechanism to the stimulating effects of TGF-β and Wnt on fibrillin assembly.
Cellular sensing of fibrillin microfibrils
Fibrillin microfibrils are sensed by transmembrane receptors which transduce signals to the interior of the cells that influence cell shape, gene expression and function. Integrins and potentially other receptors mediate those effects.
Integrins
Numerous ECM proteins contain an RGD sequence, which mediates cell-matrix interactions by binding to integrins. In addition, many other amino acid sequences in extracellular ligands can mediate interaction with integrins. Each integrin heterodimer consists of an α and a β single-pass transmembrane protein that associate non-covalently (Hynes 2002). The interaction between RGD cell binding sites and integrins mediates cell attachment, focal adhesion formation and integrin signaling. This interaction is essential for cells to sense and react to their extracellular microenvironment. Important intracellular components of this signaling complex are talin, Src-family kinases, focal adhesion kinase, vinculin and paxillin (Harburger and Calderwood 2009). Talin is a structural adaptor that links integrins directly to the cytoskeleton. Scaffolding adaptors (e.g., paxillin, vinculin) form bridges between focal adhesion proteins. Focal adhesion kinas and Src-family kinases are catalytic adaptors that propagate signal transduction.
Relevant integrins that mediate cell attachment to fibrillin-1 are α5β1, αvβ3 and αvβ6 (Pfaff et al. 1996; Sakamoto et al. 1996; Bax et al. 2003; Jovanovic et al. 2007). Mutations in the TB4 domain of fibrillin-1 that harbors the RGD sequence lead to stiff skin syndrome characterized by diffuse skin fibrosis as outlined in section Fibrillinopathies (Loeys et al. 2010). Interestingly, no mutation that directly affects the RGD cell binding site is listed in the fibrillin-1 mutation database (http://www.umd.be/FBN1) (Collod-Beroud et al. 2003). All known mutations that cause stiff skin syndrome are located across the entire TB4 domain in close vicinity of the RGD sequence. In vitro analyses of two stiff skin syndrome mutations, p.W1570C and p.C1564S, displayed significant loss of both, cell attachment and spreading, suggesting that the fibrillin-1 interaction with integrins α5β1 and/or αvβ3 was affected. However, keratinocytes expressing integrin αvβ6 attached and spread normally on mutated fibrillin-1. Moreover, studies with cultured dermal fibroblasts from patients with stiff skin syndrome display reduced amounts of active focal adhesion kinase. This underlines the importance of integrin ligation and the resulting downstream signaling. In vivo studies showed that heterozygous mice with an RGD to RGE substitution in fibrillin-1 (Fbn1RGE/+) phenocopied the diffuse skin fibrosis observed in patients with stiff skin syndrome (Gerber et al. 2013). The mice further mimicked the typical autoantibody production and dermal infiltration of pro-inflammatory immune cells including plasmacytoid dendritic cells, T helper cells and plasma cells. Homozygous inactivation of the RGD sequence in mice (Fbn1RGE/RGE) resulted in early embryonic lethality. The in vivo studies of heterozygous Fbn1RGE/+ mice further emphasize the importance of correct integrin ligation (Gerber et al. 2013). In this study, integrin-modulating therapies mimicking the integrin-matrix ligand interaction, which is impaired by the mutation, normalized the skin architecture and dermal infiltration of proinflammatory immune cells.
Other transmembrane receptors
The AT1 receptor is another possible cellular sensor. It can be activated by mechanical stress, independent of angiotensin II, in cardiac hypertrophy (Zou et al. 2004). Mice homozygous for a hypomorphic Fbn1 allele (Fbn1mgR/mgR) present with dilated cardiomyopathy (Cook et al. 2014). This study further showed that crossing these mice with AT1-deficient mice restored normal cardiac size and function, which was also normalized upon treatment with losartan, but not with TGF-β-neutralizing antibodies. This suggests that the activation of TGF-β signaling does not account for cardiomyopathy, but rather the abnormal muscle mechanosignaling via AT1.
Pericellular heparan-sulfate-containing proteoglycans (e.g., syndecans) might also be important in sensing the fibrillin matrix. The importance of the interaction between heparan sulfate and the TB5 domain in fibrillin-1 is highlighted by the disruption of heparin binding in acromicric and geleophysic dysplasias caused by mutations in the TB5 domain (Cain et al. 2012). However, the full spectrum of functional contributions of how cells sense their microenvironment needs to be clarified.
Fibrillin-associated ECM remodeling and inflammation
Regulation of matrix metalloproteinases
MMPs are a large family of endopeptidases, which are involved in degradation and remodeling of the ECM (Sekhon 2010). Several lines of evidence demonstrate that the regulation of MMPs is involved in the pathogenesis of Marfan syndrome and other fibrillinopathies. Several MMPs, including MMP-1, -2, −3 and −9, were present at increased concentrations in aortic specimens obtained from Marfan patients (Segura et al. 1998; Ikonomidis et al. 2006). Additionally, these aortic specimens displayed fibrillin fragmentation (Fleischer et al. 1997), and it has been shown that fibrillin is susceptible to proteolysis by several MMPs (Ashworth et al. 1999b; Hindson et al. 1999; Kirschner et al. 2011). Increased MMP expression was also found in lens specimens from individuals affected with Marfan syndrome (Sachdev et al. 2002).
Fibrillin-1 fragments that contain the RGD cell binding site were capable of inducing MMP-1 and −3 expression at the mRNA and protein level in human skin fibroblasts, whereas MMP-2 and −9 levels remained unaltered (Booms et al. 2005). Furthermore, this study also showed that fibrillin-1 fragments without the RGD cell binding site did not cause an increase in MMP-1 expression, but in MMP-3 expression. This indicates that both, RGD-dependent and independent mechanism exist for fibrillin-1 to regulate the expression of MMPs. One RGD-independent sequence that mediates MMP expression is the XGXXPG elastin-binding protein (EBP) consensus sequence present three times in fibrillin-1 (Brassart et al. 2001). The EBP is one of three subunits of the elastin-laminin receptor which has been implicated in different cell signaling processes by binding to XGXXPG motifs (Mecham et al. 1989). It acts as a mechanotransducer in vascular smooth muscle cells (Spofford and Chilian 2001), and it induces proliferation of arterial smooth muscle cells (Mochizuki et al. 2002). A fibrillin-1 fragment containing a XGXXPG (EGFEPG) sequence in cbEGF33 upregulated the MMP-1 protein production in skin fibroblasts (Booms et al. 2006). This fragment was also capable of inducing monocyte chemotaxis as outlined in the section below Inflammatory infiltration of immune cells.
In aortic aneurysms of Fbn1C1039G/+ mice, increasing MMP-2 and −9 mRNA and protein expression was detected between three to six months of age (Chung et al. 2007). Altered MMP expression was accompanied by elastic fiber degradation and deterioration of mechanical properties and aortic contraction. Treatment with doxycycline, a broad-spectrum inhibitor of all MMPs, significantly improved life expectancy in Fbn1mgR/mgR mice (Xiong et al. 2008). Doxycycline reduced elastic fiber degradation by decreasing MMP-2 and −9 levels. Similar results were obtained for Fbn1C1039G/+ mice. Whereas aneurysm formation was prevented upon doxycycline treatment in Fbn1C1039G/+ mice, there was still evidence of mild aneurysm formation after administration of atenolol (Chung et al. 2008). In this study, doxycyline improved elastic fiber integrity, normalized vasomotor function and reduced TGF-β activation. After establishment of aortic aneurysms at four months of age, neither doxycycline nor losartan treatment completely restored aortic wall integrity and function in Fbn1C1039G/+ mice (Yang et al. 2010). The combination of doxycycline and losartan in this study led to a better outcome compared to single-drug treatment, as it improved elastic fiber organization, decreased MMP-2, −9 and TGF-β expression and normalized aortic mechanical properties. In another study, losartan and doxycycline were found to be equally effective in delaying, but not preventing aneurysm formation and rupture (Xiong et al. 2012). These authors further demonstrated that MMP-2 plays a role in the release of active TGF-β from the LLC, which then results in downstream signaling via ERK1/2. A combination therapy with losartan and doxycycline might prove more effective than a single-drug therapy, as these compounds additively reduce ERK1/2 signaling, and thus delay aneurysm progression and rupture.
In summary, these findings illustrate that proteolytic degradation, which generates fibrillin-1 fragments, triggers a vicious cycle. The generated fibrillin-1 fragments upregulate MMP expression, which in turn causes more proteolytic degradation of fibrillin, eventually impairing the sequestration of growth factors on microfibrils.
Inflammatory infiltration of immune cells
Monocyte infiltration in the medial layer of the aorta can be observed as early as eight weeks of age in Fbn1mgR/mgR mice (Pereira et al. 1999). The inflammatory infiltration is followed by adventitial inflammation, elastolysis of the media and a fibroproliferative response. Macrophages in inflammatory foci originate from blood monocytes that are attracted by a plethora of chemotactic factors (Brömme et al. 1993). There are also some ECM-derived components harboring a XGXXPG sequence, that mediate chemotaxis of fibroblasts and monocytes by binding to the EBP present on the surface of monocytes (Senior et al. 1984).
In line with the finding that aortic extracts from Fbn1mgR/mgR mice induced macrophage chemotaxis, recombinant fibrillin-1 fragments containing a XGXXPG EBP recognition sequence acted as chemotactic stimuli for macrophages (Guo et al. 2006). This response was significantly reduced by pretreatment with monoclonal antibodies directed against an EBP recognition sequence VGVAPG, or by a mutation of the EBP sequence in fibrillin-1 fragments. These results show the involvement of the EBP sequence in the stimulation of macrophage chemotaxis in Fbn1mgR/mgR mice. Macrophage infiltration was also observed in aortic specimens obtained from individuals with Marfan syndrome (He et al. 2008). Treatment of Fbn1mgR/mgR mice with a monoclonal antibody directed against EBP recognition sequence XGXXPG in fibrillin-1 and elastin rescued elastin degradation and reduced macrophage infiltration in the aorta (Guo et al. 2013). Moreover, the treatment had beneficial effects on MMP expression and TGF-β signaling. The upregulation of MMP-2 and −9 was inhibited, and the upregulation of p-SMAD2, TGF-β1 and LTBP-1 was decreased. In addition, monoclonal antibody treatment prevented the development of pulmonary emphysema in this study.
The proinflammatory cytokine interleukin (IL)-6 plays a key role in vascular inflammation. Hypomorphic Fbn1mgR/mgR mice displayed significantly enhanced IL-6 signaling, which contributed to the aortic ECM degeneration and increased activity of MMP-9 (Ju et al. 2014). However, IL-6 deficiency delayed aneurysm formation, but did not prevent rupture or improve the survival time. This indicates the involvement of other pathways in the early stages of disease progression.
MicroRNA-dependent regulation in relation to fibrillins
MicroRNAs have been identified during the past decade as important regulators of gene expression (Bartel 2004). They represent single-stranded short non-coding RNAs that are about 21–23 nucleotides in length. MicroRNAs regulate gene expression on the mRNA level by binding directly to the 3′ untranslated regions of specific target mRNAs. This results in the repression of mRNA translation and thus protein expression.
Overall, the relationship between microRNAs, ECM and disease pathogenesis is very little explored. However, emerging evidence demonstrates that microRNAs are important regulators of the ECM as it relates to fibrillinopathies. MicroRNAs regulate the expression of critical extracellular proteins including fibrillin, elastin, fibronectin and CTGF (van Rooij et al. 2008; Duisters et al. 2009; Shan et al. 2009), as well as vascular tissue integrity (Fish et al. 2008). MicroRNA-dependent regulation further controls smooth muscle fate and plasticity (Cordes et al. 2009), contractility (Boettger et al. 2009), and cytoskeletal dynamics (Xin et al. 2009). Moreover, microRNAs can integrate with TGF-β signaling in vessel stabilization (Climent et al. 2015).
Specific targets for members of the miR-29 family include fibrillin-1, tropoelastin and several collagens (van Rooij et al. 2008). Overexpression of miR-29a/b/c mimics repressed, whereas miR-29 inhibitors increased elastin mRNA and protein levels in cell culture (Zhang et al. 2012). Interestingly, transfection of smooth muscle cells obtained from a patient with elastin deficiency (supravalvular aortic stenosis) with an inhibitor for miR-29a increased tropoelastin mRNA and protein expression levels. It was shown that miR-29b regulates aortic wall apoptosis and ECM abnormalities with significantly increased levels in Fbn1C1039G/+ mice compared to control animals (Merk et al. 2012). Increased apoptosis was found in the aorta of the Fbn1C1039G/+ mice as evidenced by increased caspase-3 activity and decreased levels of anti-apoptotic proteins, Mcl-1 and Bcl-2. This study also showed evidence for an involvement of the nuclear factor (NF)-κB pathway. NF-κB is known to repress miR-29b and is itself suppressed by TGF-β. As expected, decreased activation of NF-κB was observed as a result of enhanced TGF-β signaling, and administration of an NF-κB inhibitor increased miR-29b levels. There was an effective reduction in miR-29b levels upon TGF-β blockade or losartan administration. Direct miR-29b blockade by antisense oligonucleotides significantly ameliorated the outcome by preventing early aneurysm development, aortic wall apoptosis, and ECM deficiencies. miR-29b was also shown as a critical player in abdominal aortic aneurysm development (Maegdefessel et al. 2012). The authors showed in this study that aortic aneurysm development in mouse models was accompanied by decreased aortic expression of miR-29b, correlating with increased expression of miR-29b targets including tropoelastin. miR-29b inhibition led to a significant reduction of the abdominal aneurysms.
microRNAs were also shown to be deregulated in systemic sclerosis, a disorder similar to stiff skin syndrome with unclear etiology. Fibroblasts from patients with systemic sclerosis showed decreased levels of miR-29 in vitro (Gerber et al. 2013). miR-29 is known to be repressed by TGF-β and inhibits the expression of multiple ECM components and suppresses fibrosis (van Rooij et al. 2008; Maurer et al. 2010). Integrin-modulating therapies and TGF-β antagonism restored miR-29 levels (Gerber et al. 2013).
In conclusion, these emerging results indicate the need to explore microRNA pathways in relation to fibrillinopathies. They may hold future potential for the design of novel therapies.
Conclusions
In summary, evidence accumulate demonstrating that fibrillin-containing microfibrils represent key relay stations for the transmission of extracellular information into cellular signaling and function. Microfibrils store growth factors including TGF-β and several BMPs and regulate their bioavailability and activity. Microfibrils directly or indirectly interact with cell surface receptors to sense normal and pathologically altered ECM. The integrity of microfibrils and associated elastic fibers determine expression of matrix degrading proteases, recruitment of inflammatory cells, and regulation of microRNAs. Loss of microfibril integrity and abundance leads to a number of connective tissue diseases through defects in cellular signaling mechanisms. The challenge for the next years is to understand and integrate all cell signaling functions mediated by fibrillin-containing microfibrils, which will ultimately aid in the design of novel preventative and therapeutic strategies for the associated fibrillinopathies.
Acknowledgments
We are grateful for the financial support of the Canadian Institutes of Health Research (MOP-106494), the Canada Research Chair program, the Quebec Network of Oral and Bone Health Research, and the German Studienstiftung des deutschen Volkes.
Abbreviations
- AT1/2
Angiotensin II type 1/2 receptor
- BMP
Bone morphogenetic protein
- (cb)EGF domain
(calcium binding) epidermal growth factor-like domain
- EBP
Elastin-binding protein
- ECM
Extracellular matrix
- ERK1/2
Extracellular signal-regulated kinase 1/2
- GDF
Growth and differentiation factor
- GTPase
Guanosine triphosphate phosphohydrolase
- Hyb domain
Hybrid domain
- IL
Interleukin
- JNK
C-Jun N-terminal kinase
- LAP
Latency associated peptide
- LLC
Large latent complex
- LTBP
Latent transforming growth factor beta-binding protein
- MAPK
Mitogen-activated protein kinase
- MicroRNA
Micro ribonucleic acid
- MMP
Matrix metalloproteinase
- NF-κB
Nuclear factor-κB
- p-
phosphorylated
- SMAD
Sma and Mad related
- SLC
Small latent complex
- TAK
Transforming growth factor-beta-activated kinase
- TB domain
Transforming growth factor-beta-binding protein-like domain
- TGF-β
Transforming growth factor-beta
- Wnt
Wingless-type mouse mammary tumor virus integration site family
References
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai LY, Ramirez F. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Kelly V, Wilson R, Shuttleworth CA, Kielty CM. Fibrillin assembly: dimer formation mediated by amino-terminal sequences. J Cell Sci. 1999;112:3549–3558. doi: 10.1242/jcs.112.20.3549. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999;340:171–181. [PMC free article] [PubMed] [Google Scholar]
- Baldwin AK, Simpson A, Steer R, Cain SA, Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2013;15 doi: 10.1017/erm.2013.9. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–899. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bax DV, Bernard SE, Lomas A, Morgan A, Humphries J, Shuttleworth A, Humphries MJ, Kielty CM. Cell adhesion to fibrillin-1 molecules and microfibrils is mediated by alpha5 beta1 and alphav beta3 integrins. J Biol Chem. 2003;278:34605–34616. doi: 10.1074/jbc.M303159200. [DOI] [PubMed] [Google Scholar]
- Bax DV, Mahalingam Y, Cain S, Mellody K, Freeman L, Younger K, Shuttleworth CA, Humphries MJ, Couchman JR, Kielty CM. Cell adhesion to fibrillin-1: identification of an Arg-Gly-Asp-dependent synergy region and a heparin-binding site that regulates focal adhesion formation. J Cell Sci. 2007;120:1383–1392. doi: 10.1242/jcs.003954. [DOI] [PubMed] [Google Scholar]
- Bayle J, Fitch J, Jacobsen K, Kumar R, Lafyatis R, Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Investig Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booms P, Pregla R, Ney A, Barthel F, Reinhardt DP, Pletschacher A, Mundlos S, Robinson PN. RGD-containing fibrillin-1 fragments upregulate matrix metalloproteinase expression in cell culture: a potential factor in the pathogenesis of the Marfan syndrome. Hum Genet. 2005;116:51–61. doi: 10.1007/s00439-004-1194-7. [DOI] [PubMed] [Google Scholar]
- Booms P, Ney A, Barthel F, Moroy G, Counsell D, Gille C, Guo G, Pregla R, Mundlos S, Alix AJ, Robinson PN. A fibrillin-1-fragment containing the elastin-binding-protein GxxPG consensus sequence upregulates matrix metalloproteinase-1: biochemical and computational analysis. J Mol Cell Cardiol. 2006;40:234–246. doi: 10.1016/j.yjmcc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Brassart B, Fuchs P, Huet E, Alix AJ, Wallach J, Tamburro AM, Delacoux F, Haye B, Emonard H, Hornebeck W, Debelle L. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J Biol Chem. 2001;276:5222–5227. doi: 10.1074/jbc.M003642200. [DOI] [PubMed] [Google Scholar]
- Brömme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet T. Functional expression of human cathepsin S in Saccharomyces cerevisiae. J Biol Chem. 1993;268:4832–4838. [PubMed] [Google Scholar]
- Cain SA, Baldock C, Gallagher J, Morgan A, Bax DV, Weiss AS, Shuttleworth CA, Kielty CM. Fibrillin-1 interactions with heparin: Implications for microfibril and elastic fibre assembly. J Biol Chem. 2005;280:30526–30537. doi: 10.1074/jbc.M501390200. [DOI] [PubMed] [Google Scholar]
- Cain SA, Baldwin AK, Mahalingam Y, Raynal B, Jowitt TA, Shuttleworth CA, Couchman JR, Kielty CM. Heparan sulfate regulates fibrillin-1 N- and C-terminal interactions. J Biol Chem. 2008;283:27017–27027. doi: 10.1074/jbc.M803373200. [DOI] [PubMed] [Google Scholar]
- Cain SA, McGovern A, Baldwin AK, Baldock C, Kielty CM. Fibrillin-1 mutations causing Weill-Marchesani syndrome and acromicric and geleophysic dysplasias disrupt heparan sulfate interactions. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J Biol Chem. 2009;284:5630–5636. doi: 10.1074/jbc.M806962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau NL, Carlson EJ, Tufa S, Sengle G, Manalo EC, Carlberg VM, Ramirez F, Keene DR, Sakai LY. In vivo studies of mutant fibrillin-1 microfibrils. J Biol Chem. 2010;285:24943–24955. doi: 10.1074/jbc.M110.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov AJ, Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM. Differential regulation of elastic fiber formation by fibulins −4 and −5. J Biol Chem. 2009;284:24553–24567. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AWY, Au Yeung K, Sandor GGS, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and −9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007;101:512–522. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- Chung AWY, Yang HHC, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and-9. Circ Res. 2008;102:E73–E85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L. TGFbeta triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res. 2015;116(11):1753–1764. doi: 10.1161/CIRCRESAHA.116.305178. [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collod-Beroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, Holman K, Kaitila I, Loeys B, Matyas G, Nuytinck L, Peltonen L, Rantamaki T, Robinson P, Steinmann B, Junien C, Beroud C, Boileau C. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat. 2003;22:199–208. doi: 10.1002/humu.10249. [DOI] [PubMed] [Google Scholar]
- Cook JR, Carta L, Benard L, Chemaly ER, Chiu E, Rao SK, Hampton TG, Yurchenco P, Costa KD, Hajjar RJ, Ramirez F. Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome 168. J Clin Invest. 2014;124:1329–1339. doi: 10.1172/JCI71059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson GM, Chalberg SC, Dietz HC, Charbonneau NL, Sakai LY. Fibrillin binds calcium and is coded by cDNAs that reveal a multidomain structure and alternatively spliced exons at the 5′ end. Genomics. 1993;17:476–484. doi: 10.1006/geno.1993.1350. [DOI] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY. Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics. 2004;83:461–472. doi: 10.1016/j.ygeno.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Keene DR, Bruder SP, Saharinen J, Sakai LY, Mundy GR, Bonewald LF. Role of the latent transforming growth factor beta binding protein 1 in fibrillin-containing microfibrils in bone cells in vitro and in vivo. J Bone Miner Res. 2000;15:68–81. doi: 10.1359/jbmr.2000.15.1.68. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Detaint D, Aegerter P, Tubach F, Hoffman I, Plauchu H, Dulac Y, Faivre LO, Delrue MA, Collignon P, Odent S, Tchitchinadze M, Bouffard C, Arnoult F, Gautier M, Boileau C, Jondeau G. Rationale and design of a randomized clinical trial (Marfan Sartan) of angiotensin II receptor blocker therapy versus placebo in individuals with Marfan syndrome. Arch Cardiovasc Dis. 2010;103:317–325. doi: 10.1016/j.acvd.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Downing AK, Knott V, Werner JM, Cardy CM, Campbell ID, Handford PA. Solution structure of a pair of calcium-binding epidermal growth factor-like domains: implications for the Marfan syndrome and other genetic disorders. Cell. 1996;85:597–605. doi: 10.1016/s0092-8674(00)81259-3. [DOI] [PubMed] [Google Scholar]
- Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. [DOI] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Bätge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adapter function to tropoelastin. J Biol Chem. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J Med Genet. 2003;40:34–36. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in TGF-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer KJ, Nousari HC, Anhalt GJ, Stone CD, Laschinger JC. Immunohistochemical abnormalities of fibrillin in cardiovascular tissues in Marfan’s syndrome. Ann Thorac Surg. 1997;63:1012–1017. doi: 10.1016/s0003-4975(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol Cell Biol. 1988;8:4162–4168. doi: 10.1128/mcb.8.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber EE, Gallo EM, Fontana SC, Davis EC, Wigley FM, Huso DL, Dietz HC. Integrin-modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature. 2013;503:126–130. doi: 10.1038/nature12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graul-Neumann LM, Kienitz T, Robinson PN, Baasanjav S, Karow B, Gillessen-Kaesbach G, Fahsold R, Schmidt H, Hoffmann K, Passarge E. Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am J Med Genet A. 2010;152A:2749–2755. doi: 10.1002/ajmg.a.33690. [DOI] [PubMed] [Google Scholar]
- Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976;82:493–512. [PMC free article] [PubMed] [Google Scholar]
- Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bächinger HP, Sakai LY. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem. 2005;280:27970–27980. doi: 10.1074/jbc.M504270200. [DOI] [PubMed] [Google Scholar]
- Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, Hecht J, Mundlos S, Robinson PN. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. doi: 10.1161/CIRCULATIONAHA.105.601674. [DOI] [PubMed] [Google Scholar]
- Guo G, Munoz-Garcia B, Ott CE, Grunhagen J, Mousa SA, Pletschacher A, von Kodolitsch Y, Knaus P, Robinson PN. Antagonism of GxxPG fragments ameliorates manifestations of aortic disease in Marfan syndrome mice. Hum Mol Genet. 2013;22:433–443. doi: 10.1093/hmg/dds439. [DOI] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991;351:164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzirodos N, Bayne RA, Irving-Rodgers HF, Hummitzsch K, Sabatier L, Lee S, Bonner W, Gibson MA, Rainey WE, Carr BR, Mason HD, Reinhardt DP, Anderson RA, Rodgers RJ. Linkage of regulators of TGF-beta activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Guo DC, Sun W, Papke CL, Duraisamy S, Estrera AL, Safi HJ, Ahn C, Buja LM, Arnett FC, Zhang J, Geng YJ, Milewicz DM. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg. 2008;136:922–929. doi: 10.1016/j.jtcvs.2007.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson VJ, Ashworth JL, Rock MJ, Cunliffe S, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: identification of amino- and carboxy-terminal cleavage sites. FEBS Lett. 1999;452:195–198. doi: 10.1016/s0014-5793(99)00623-7. [DOI] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–223. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubmacher D, Reinhardt DP. Microfibrils and fibrillin. In: Mecham RP, editor. Biology of extracellular matrix. New York: Springer; 2011. pp. 233–265. [Google Scholar]
- Hubmacher D, El-Hallous E, Nelea V, Kaartinen MT, Lee ER, Reinhardt DP. Biogenesis of extracellular microfibrils: Multimerization of the fibrillin-1 C-terminus into bead-like structures enables self-assembly. Proc Natl Acad Sci U S A. 2008;105:6548–6553. doi: 10.1073/pnas.0706335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hyytiäinen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, Zeeshan A, Bavaria JE, Gorman JH, Spinale FG, Gorman RC. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365–I370. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem. 2002;277:4565–4572. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Jensen SA, Iqbal S, Lowe ED, Redfield C, Handford PA. Structure and interdomain interactions of a hybrid domain: a disulphide-rich module of the fibrillin/LTBP superfamily of matrix proteins. Structure. 2009;17:759–768. doi: 10.1016/j.str.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CD, Bohling SD, Charbonneau NL, Sakai LY. Fibrillins in human adult ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J Histochem Cytochem. 2010;58:903–915. doi: 10.1369/jhc.2010.956615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic J, Takagi J, Choulier L, Abrescia NG, Stuart DI, van der Merwe PA, Mardon HJ, Handford PA. αVß6 is a novel receptor for human fibrillin-1: comparative studies of molecular determinants underlying integrin-RGD affinity and specificity. J Biol Chem. 2007;282:6743–6751. doi: 10.1074/jbc.M607008200. [DOI] [PubMed] [Google Scholar]
- Ju X, Ijaz T, Sun H, Lejeune W, Vargas G, Shilagard T, Recinos A, 3rd, Milewicz DM, Brasier AR, Tilton RG. IL-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgR/mgR mouse model of severe Marfan syndrome. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainulainen K, Pulkkinen L, Savolainen A, Kaitila I, Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990;323:935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Kainulainen K, Karttunen L, Puhakka L, Sakai LY, Peltonen L. Mutations in the fibrillin gene responsible for dominant ectopia lentis and neonatal Marfan syndrome. Nat Genet. 1994;6:64–69. doi: 10.1038/ng0194-64. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kettle S, Card CM, Hutchinson S, Sykes B, Handford PA. Characterisation of fibrillin-1 cDNA clones in a human fibroblast cell line that assembles microfibrils. Int J Biochem Cell Biol. 2000;32:201–214. doi: 10.1016/s1357-2725(99)00120-x. [DOI] [PubMed] [Google Scholar]
- Kirschner R, Hubmacher D, Iyengar G, Kaur J, Fagotto-Kaufmann C, Brömme D, Bartels R, Reinhardt DP. Classical and neonatal Marfan syndrome mutations in fibrillin-1 cause differential protease susceptibilities and protein function. J Biol Chem. 2011;286:32810–32823. doi: 10.1074/jbc.M111.221804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol. 2014;207:283–297. doi: 10.1083/jcb.201402006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bachinger HP, Hanisch FG, Markova D, Tsuda T, Timpl R, Chu ML, Sasaki T. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–11816. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- Kriz W, Elger M, Lemley K, Sakai T. Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int Suppl. 1990;30:S2–S9. [PubMed] [Google Scholar]
- Kuo CL, Isogai Z, Keene DR, Hazeki N, Ono RN, Sengle G, Bächinger HP, Sakai LY. Effects of fibrillin-1 degradation on microfibril ultrastructure. J Biol Chem. 2007;282:4007–4020. doi: 10.1074/jbc.M606370200. [DOI] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Wruck LM, Bradley TJ, Colan SD, Devereux RB, Klein GL, Li JS, Minich LL, Paridon SM, Pearson GD, Printz BF, Pyeritz RE, Radojewski E, Roman MJ, Saul JP, Stylianou MP, Mahony L. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am Heart J. 2007;154:624–631. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, Pearson GD, Selamet Tierney ES, Levine JC, Atz AM, Benson DW, Braverman AC, Chen S, De Backer J, Gelb BD, Grossfeld PD, Klein GL, Lai WW, Liou A, Loeys BL, Markham LW, Olson AK, Paridon SM, Pemberton VL, Pierpont ME, Pyeritz RE, Radojewski E, Roman MJ, Sharkey AM, Stylianou MP, Wechsler SB, Young LT, Mahony L. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–2071. doi: 10.1056/NEJMoa1404731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P, Robitaille G, Agharazii M, Ledbetter S, Lebel M, Lariviere R. Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23:1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- Lawrence DA, Pircher R, Kryceve-Martinerie C, Jullien P. Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol. 1984;121:184–188. doi: 10.1002/jcp.1041210123. [DOI] [PubMed] [Google Scholar]
- Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PO, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Megarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFß binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Lee B, Godfrey M, Vitale E, Hori H, Mattei MG, Sarfarazi M, Tsipouras P, Ramirez F, Hollister DW. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991;352:330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Lee SS, Knott V, Jovanovic J, Harlos K, Grimes JM, Choulier L, Mardon HJ, Stuart DI, Handford PA. Structure of the integrin binding fragment from fibrillin-1 gives new insights into microfibril organization. Structure. 2004;12:717–729. doi: 10.1016/j.str.2004.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire R, Farina G, Bayle J, Dimarzio M, Pendergrass SA, Milano A, Perbal B, Whitfield ML, Lafyatis R. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J Investig Dermatol. 2010;130:1514–1523. doi: 10.1038/jid.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Tiedemann K, Vollbrandt T, Peters H, Bätge B, Brinckmann J, Reinhardt DP. Homo- and heterotypic fibrillin-1 and −2 interactions constitute the basis for the assembly of microfibrils. J Biol Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, Chillakuri CR, Macaya D, Coucke PJ, De Paepe A, Judge DP, Wigley F, Davis EC, Mardon HJ, Handford P, Keene DR, Sakai LY, Dietz HC. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist L, Reinhardt DP, Sakai LY, Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998;7:2039–2044. doi: 10.1093/hmg/7.13.2039. [DOI] [PubMed] [Google Scholar]
- Low FN. Microfibrils: fine filamentous components of the tissue space. Anat Rec. 1962;142:131–137. doi: 10.1002/ar.1091420205. [DOI] [PubMed] [Google Scholar]
- Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenis RE, Maslen CL, Smith L, Allen L, Sakai LY. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics. 1991;11:346–351. doi: 10.1016/0888-7543(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Marfan AB. Un cas de déformation congénitale des quatre membres plus prononcée aux extrémites charactérisée par l’allongement des os avec uncertain degré d’amincissement. Bull Mem Soc Med Hop Paris. 1896;13:220–226. [Google Scholar]
- Marson A, Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Shuttleworth CA, Baldock C, Kielty CM. Homotypic fibrillin-1 interactions in microfibril assembly. J Biol Chem. 2005;280:5013–5021. doi: 10.1074/jbc.M409029200. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- Mayhew M, Handford P, Baron M, Tse AG, Campbell ID, Brownlee GG. Ligand requirements for Ca2+ binding to EGF-like domains. Protein Eng. 1992;5:489–494. doi: 10.1093/protein/5.6.489. [DOI] [PubMed] [Google Scholar]
- Mecham RP, Hinek A, Entwistle R, Wrenn DS, Griffin GL, Senior RM. Elastin binds to a multifunctional 67-kilodalton peripheral membrane protein. Biochemistry. 1989;28:3716–3722. doi: 10.1021/bi00435a014. [DOI] [PubMed] [Google Scholar]
- Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res. 2012;110:312–324. doi: 10.1161/CIRCRESAHA.111.253740. [DOI] [PubMed] [Google Scholar]
- Mi LZ, Brown CT, Gao Y, Tian Y, Le VQ, Walz T, Springer TA. Structure of bone morphogenetic protein 9 procomplex. Proc Natl Acad Sci U S A. 2015;112:3710–3715. doi: 10.1073/pnas.1501303112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-ß1-binding protein in the assembly and secretion of TGF-ß1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki S, Brassart B, Hinek A. Signaling pathways transduced through the elastin receptor facilitate proliferation of arterial smooth muscle cells. J Biol Chem. 2002;277:44854–44863. doi: 10.1074/jbc.M205630200. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XXII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2001;8:85–95. doi: 10.1093/dnares/8.2.85. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol. 1997;136:193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest. 2004;114:1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Carta L, Ono RN, Sengle G, Arteaga-Solis E, Levasseur R, Ducy P, Sakai LY, Karsenty G, Ramirez F. Fibrillin-1 and −2 differentially modulate endogenous TGF-beta and BMP bioavailability during bone formation. J Cell Biol. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala H, Lee-Arteaga S, Smaldone S, Siciliano G, Ramirez F. Extracellular microfibrils modulate osteoblast-supported osteoclastogenesis by restricting TGF beta stimulation of RANKL production. J Biol Chem. 2010;285:34126–34133. doi: 10.1074/jbc.M110.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, Chu ML, Sakai LY. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, D’Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Hum Mol Genet. 1993;2:961–968. doi: 10.1093/hmg/2.7.961. [DOI] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono RN, Reinhardt DP, Sakai LY, Jensen-Biery N, Bunton T, Dietz HC, Ramirez F. Targeting of fibrillin-1 recapitulates the vascular phenotype of Marfan syndrome in the mouse. Nat Genet. 1997;17:218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96:3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M, Reinhardt DP, Sakai LY, Timpl R. Cell adhesion and integrin binding to recombinant human fibrillin-1. FEBS Lett. 1996;384:247–250. doi: 10.1016/0014-5793(96)00325-0. [DOI] [PubMed] [Google Scholar]
- Piha-Gossack A, Sossin WS, Reinhardt DP. The evolution of extracellular fibrillins and their functional domains. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyeritz R, Dietz H. Marfan syndrome and other microfibrillar disorders. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. New York: Wiley-Liss, Inc.; 2002. pp. 585–626. [Google Scholar]
- Raghunath M, Unsöld C, Kubitscheck U, Bruckner-Tuderman L, Peters R, Meuli M. The cutaneous microfibrillar apparatus contains latent transforming growth factor-beta binding protein-1 (LTBP-1) and is a repository for latent TGF-beta1. J Investig Dermatol. 1998;111:559–564. doi: 10.1046/j.1523-1747.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschödrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112:1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Raja-Khan N, Kunselman AR, Demers LM, Ewens KG, Spielman RS, Legro RS. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94:2916–2919. doi: 10.1016/j.fertnstert.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. Investig Ophthalmol. 1971;10:851–869. [PubMed] [Google Scholar]
- Rees DJG, Jones IM, Handford PA, Walter SJ, Esnouf MP, Smith KJ, Brownlee GG. The role of ß-hydroxyaspartate and adjacent carboxylate residues in the first EGF domain of human factor IX. EMBO J. 1988;7:2053–2061. doi: 10.1002/j.1460-2075.1988.tb03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt DP, Sasaki T, Dzamba BJ, Keene DR, Chu ML, Göhring W, Timpl R, Sakai LY. Fibrillin-1 and fibulin-2 interact and are colocalized in some tissues. J Biol Chem. 1996;271:19489–19496. doi: 10.1074/jbc.271.32.19489. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Mechling DE, Boswell BA, Keene DR, Sakai LY, Bächinger HP. Calcium determines the shape of fibrillin. J Biol Chem. 1997;272:7368–7373. doi: 10.1074/jbc.272.11.7368. [DOI] [PubMed] [Google Scholar]
- Reinhardt DP, Ono RN, Sakai LY. Calcium stabilizes fibrillin-1 against proteolytic degradation. J Biol Chem. 1997;272:1231–1236. doi: 10.1074/jbc.272.2.1231. [DOI] [PubMed] [Google Scholar]
- Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Broekelmann T, Tisdale C, Milewicz DM, Mecham RP. Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem. 1999;274:8933–8940. doi: 10.1074/jbc.274.13.8933. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Broekelmann TJ, Werneck CC, Mecham RP. Fibrillin-1 and −2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem J. 2003;375:425–432. doi: 10.1042/BJ20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I, Jensen S, Handford P. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem J. 2011;433:263–276. doi: 10.1042/BJ20101320. [DOI] [PubMed] [Google Scholar]
- Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB (2015) Latent TGF-beta-binding proteins. Matrix Biol [DOI] [PMC free article] [PubMed]
- Rock MJ, Cain SA, Freeman LJ, Morgan A, Mellody K, Marson A, Shuttleworth CA, Weiss AS, Kielty CM. Molecular basis of elastic fiber formation. Critical interactions and a tropoelastin-fibrillin-1 cross-link. J Biol Chem. 2004;279:23748–23758. doi: 10.1074/jbc.M400212200. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier L, Miosge N, Hubmacher D, Lin G, Davis EC, Reinhardt DP. Fibrillin-3 expression in human development. Matrix Biol. 2011;30:43–52. doi: 10.1016/j.matbio.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev NH, Di Girolamo N, McCluskey PJ, Jennings AV, McGuinness R, Wakefield D, Coroneo MT. Lens dislocation in Marfan syndrome: potential role of matrix metalloproteinases in fibrillin degradation. Arch Ophthalmol. 2002;120:833–835. [PubMed] [Google Scholar]
- Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nishimura H, Brumeanu TD, Casares S, Stan AC, Honjo T, Bona CA. Characterization of mutated protein encoded by partially duplicated fibrillin-1 gene in tight skin (TSK) mice. Mol Immunol. 1999;36:169–176. doi: 10.1016/s0161-5890(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Broekelmann T, Cheresh DA, Ramirez F, Rosenbloom J, Mecham RP. Cell-type specific recognition of RGD- and non-RGD-containing cell binding domains in fibrillin-1. J Biol Chem. 1996;271:4916–4922. [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA, Jr, Willerson JT, Ferrans VJ. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan’s syndrome. Circulation. 1998;98:II331–II337. [PubMed] [Google Scholar]
- Sekhon BS. Matrix metalloproteinases - an overview. J Res Rep Biol. 2010;2010:1–20. [Google Scholar]
- Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bachinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283:13874–13888. doi: 10.1074/jbc.M707820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Lyons KM, Bachinger HP, Sakai LY. A new model for growth factor activation: type II receptors compete with the prodomain for BMP-7. J Mol Biol. 2008;381:1025–1039. doi: 10.1016/j.jmb.2008.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Ono RN, Sasaki T, Sakai LY. Prodomains of transforming growth factor beta (TGFbeta) superfamily members specify different functions: extracellular matrix interactions and growth factor bioavailability. J Biol Chem. 2011;286:5087–5099. doi: 10.1074/jbc.M110.188615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Carlberg V, Tufa SF, Charbonneau NL, Smaldone S, Carlson EJ, Ramirez F, Keene DR, Sakai LY. Abnormal activation of BMP signaling causes myopathy in Fbn2 null mice. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J Cell Biol. 1984;99:870–874. doi: 10.1083/jcb.99.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, Zhang Y, Xuan JW, Yee SP, Siragam V, Yang BB. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol. 2009;11:1031–1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan’s syndrome. N Engl J Med. 1994;330:1335–1341. doi: 10.1056/NEJM199405123301902. [DOI] [PubMed] [Google Scholar]
- Siracusa LD, McGrath R, Ma Q, Moskow JJ, Manne J, Christner PJ, Buchberg AM, Jimenez SA. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res. 1996;6:300–313. doi: 10.1101/gr.6.4.300. [DOI] [PubMed] [Google Scholar]
- Smallridge RS, Whiteman P, Werner JM, Campbell ID, Handford PA, Downing AK. Solution structure and dynamics of a calcium binding epidermal growth factor-like domain pair from the neonatal region of human fibrillin-1. J Biol Chem. 2003;278:12199–12206. doi: 10.1074/jbc.M208266200. [DOI] [PubMed] [Google Scholar]
- Spofford CM, Chilian WM. The elastin-laminin receptor functions as a mechanotransducer in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2001;280:H1354–H1360. doi: 10.1152/ajpheart.2001.280.3.H1354. [DOI] [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-ß1 associates to fibroblast extracellular matrix via latent TGF-ß binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Saharinen J, Hedman K, Keski-Oja J. Latent transforming growth factor-beta 1 and its binding protein are components of extracellular matrix microfibrils. J Histochem Cytochem. 1996;44:875–889. doi: 10.1177/44.8.8756760. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Bätge B, Müller PK, Reinhardt DP. Interactions of fibrillin-1 with heparin/heparan sulfate: Implications for microfibrillar assembly. J Biol Chem. 2001;276:36035–36042. doi: 10.1074/jbc.M104985200. [DOI] [PubMed] [Google Scholar]
- Tiedemann K, Sasaki T, Gustafsson E, Göhring W, Bätge B, Notbohm H, Timpl R, Wedel T, Schlötzer-Schrehardt U, Reinhardt DP. Microfibrils at basement membrane zones interact with perlecan via fibrillin-1. J Biol Chem. 2005;280:11404–11412. doi: 10.1074/jbc.M409882200. [DOI] [PubMed] [Google Scholar]
- Trask TM, Ritty TM, Broekelmann T, Tisdale C, Mecham RP. N-terminal domains of fibrillin 1 and fibrillin 2 direct the formation of homodimers: a possible first step in microfibril assembly. Biochem J. 1999;340:693–701. [PMC free article] [PubMed] [Google Scholar]
- Trask BC, Trask TM, Broekelmann T, Mecham RP. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol Biol Cell. 2000;11:1499–1507. doi: 10.1091/mbc.11.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem. 2000;275:24400–24406. doi: 10.1074/jbc.M003665200. [DOI] [PubMed] [Google Scholar]
- Tsipouras P, Del Mastro R, Sarfarazi M, Lee B, Vitale E, Child AH, Godfrey M, Devereux RB, Hewett D, Steinmann B, Viljoen D, Sykes BC, Kilpatrick M, Ramirez F, The International Marfan Syndrome Collaborative S Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contractural arachnodactyly to the fibrillin genes on chromosomes 15 and 5. N Engl J Med. 1992;326:905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen D. Congenital contractural arachnodactyly. J Med Genet. 1994;31:640–643. doi: 10.1136/jmg.31.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–240. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Wallis DD, Putnam EA, Cretoiu JS, Carmical SG, Cao SN, Thomas G, Milewicz DM. Profibrillin-1 maturation by human dermal fibroblasts: proteolytic processing and molecular chaperones. J Cell Biochem. 2003;90:641–652. doi: 10.1002/jcb.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JM, Knott V, Handford PA, Campbell ID, Downing AK. Backbone dynamics of a cbEGF domain pair in the presence of calcium. J Mol Biol. 2000;296:1065–1078. doi: 10.1006/jmbi.1999.3513. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–172. doi: 10.1016/j.jvs.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res. 2012;110:e92–e101. doi: 10.1161/CIRCRESAHA.112.268268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadin DA, Robertson IB, McNaught-Davis J, Evans P, Stoddart D, Handford PA, Jensen SA, Redfield C. Structure of the fibrillin-1 N-terminal domains suggests that heparan sulfate regulates the early stages of microfibril assembly. Structure. 2013;21:1743–1756. doi: 10.1016/j.str.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J Thorac Cardiovasc Surg. 2010;140:305–312. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Downing AK, Knott V, Handford PA. Solution structure of the transforming growth factor ß-binding protein-like module, a domain associated with matrix fibrils. EMBO J. 1997;16:6659–6666. doi: 10.1093/emboj/16.22.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Apfelroth SD, Hu W, Davis EC, Sanguineti C, Bonadio J, Mecham RP, Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994;124:855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hu W, Ramirez F. Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol. 1995;129:1165–1176. doi: 10.1083/jcb.129.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Huang A, Ferruzzi J, Mecham RP, Starcher BC, Tellides G, Humphrey JD, Giordano FJ, Niklason LE, Sessa WC. Inhibition of microRNA-29 enhances elastin levels in cells haploinsufficient for elastin and in bioengineered vessels--brief report. Arterioscler Thromb Vasc Biol. 2012;32:756–759. doi: 10.1161/ATVBAHA.111.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Courousse T, Sakai LY, Rifkin DB. Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol. 2012;227:3828–3836. doi: 10.1002/jcp.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]