Abstract
Microtine rodents display diverse patterns of social organization and behaviors, and thus provide a useful model for studying the effects of the social environment on physiology and behavior. The current study compared the species differences and the effects of oxytocin (OT) on anxiety-like, social affiliation, and social recognition behaviors in female meadow voles (Microtus pennsylvanicus) and prairie voles (M. ochrogaster). Furthermore, cell proliferation and survival in the brains of adult female meadow and prairie voles were compared. We found that female meadow voles displayed a higher level of anxiety-like behavior but lower levels of social affiliation and social recognition compared to female prairie voles. In addition, meadow voles showed lower levels of cell proliferation (measured by Ki67 staining) and cell survival (measured by BrdU staining) in the ventromedial hypothalamus (VMH) and amygdala (AMY), but not the dentate gyrus of the hippocampus (DG), than prairie voles. Interestingly, the numbers of new cells in the VMH and AMY, but not DG, also correlated with anxiety-like, social affiliation, and social recognition behaviors in a brain region-specific manner. Finally, central OT treatment (200 ng/kg, icv) did not lead to changes in behavior or cell proliferation/survival in the brain. Together, these data indicate a potential role of cell proliferation/survival in selected brain areas on different behaviors between vole species with distinct life strategies.
Keywords: anxiety, affiliative behavior, BrdU, Ki67, ventromedial hypothalamus, amygdala, oxytocin
Introduction
Adult neurogenesis, the generation of new neurons from neural stem cells, has been documented primarily in two regions of the adult mammalian brain, the dentate gyrus of the hippocampus (DG) and the subventricular zone (SVZ) (Gross, 2000, Lieberwirth and Wang, 2012). Newly generated cells have also been reported in non-traditional neurogenic brain regions including the neocortex, cerebellum, amygdala (AMY), substantia nigra, striatum, and hypothalamus (Ming and Song, 2005, Martino et al., 2011, Crociara et al., 2013). Although we still know little about the functional relevance of adult neurogenesis (Kempermann et al., 2004), increasing evidence has shown that adult neurogenesis may play an important role in learning and memory (Shors et al., 2001, Dupret et al., 2007), social processing and responding (Feierstein et al., 2010, Lagace et al., 2010, Mak and Weiss, 2010, Oboti et al., 2011), and emotional behavior (Revest et al., 2009, Larsen and Grattan, 2010, Snyder et al., 2011). Studies have indicated that the distinct phases of adult neurogenesis in both traditional and non-traditional neurogenic brain regions are regulated by a variety of endogenous (e.g., neurotransmitters and hormones) and exogenous (e.g., voluntary physical exercise and enriched environment) factors (Fowler et al., 2008, Larsen and Grattan, 2010, Lucassen et al., 2010, Snyder et al., 2011). Recent studies have also shown that social interactions affect adult neurogenesis (Lucassen et al., 2010, Lieberwirth and Wang, 2012). For example, aversive social experience—such as agonistic interactions with dominant and aggressive conspecifics—reduce cell proliferation and survival in the adult brain in a variety of mammalian species (Gould et al., 1997, Westenbroek et al., 2004, Czeh et al., 2007, Thomas et al., 2007, Van Bokhoven et al., 2011, Lieberwirth and Wang, 2012, Pan et al., 2014). Conversely, positive social interactions among conspecifics, such as pheromonal exposure or sociosexual encounters facilitate adult neurogenesis across distinct brain regions (Mak et al., 2007, Ruscio et al., 2008, Furuta and Bridges, 2009, Corona et al., 2011).
Using a comparative approach, striking differences have been found in several types of social behaviors of animals with different life strategies. For example, social species generally show high levels of prosocial behavior among individuals, social affiliation with mates/conspecifics, and bi-parental care for their offspring; whereas non-social species generally exhibit low levels of prosocial behavior and social affiliation, but high levels of aggression (Getz et al., 1981, McGuire and Novak, 1984, Oliveras and Novak, 1986, Bester-Meredith et al., 1999, Xu et al., 2010, Wang et al., 2013). Social behaviors are selected by evolution and are the result of precise adaptations in morphology, connectivity, and chemistry (Insel and Young, 2000). Therefore, such behavioral differences among species with different life strategies may reflect their adaptations to different evolutionary selection pressures and show their potential species differences in the central nervous system.
Oxytocin (OT) is a neurotransmitter that has received substantial attention due to its role in social behaviors. OT is primarily produced in the paraventricular and supraoptic nuclei of the hypothalamus and is involved in a wide variety of processes related to social behavior, including maternal behavior, trust, and pair-bond formation (Carter et al., 2008, Neumann, 2008, Young et al., 2008, Ross and Young, 2009, Anacker and Beery, 2013). Several comparative studies between social and non-social rodent species have shown that species differences in behaviors correlate with the OT system (Young et al., 2008, Anacker and Beery, 2013). For example, the immunoreactive (ir) expression or receptor distribution of OT differs between social and non-social rodent species (Insel and Shapiro, 1992, Beery et al., 2008, Xu et al., 2010, Wang et al., 2013). Moreover, genetic and pharmacological manipulations of the OT system in the brains of social and non-social rodents have shown that OT has differential effects in regulating social behaviors corresponding to the life strategy (Williams et al., 1994, Young et al., 2001, Olazabal and Young, 2006, Ross et al., 2009).
Microtine rodents display diverse social organizations and thus offer an excellent comparative model for studying the effects of the social environment on physiology and behavior (Young and Wang, 2004, Young et al., 2011). For example, prairie voles (Microtus ochrogaster) are a highly affiliative, socially monogamous species and members of the species form long-term bonds after mating (Getz and Hofmann, 1986, Carter and Getz, 1993). Pair-bonded males and females occupy a common nest and guard the territory against unfamiliar conspecifics, and both the male and female provide parental care of their offspring (Wilson, 1982, FitzGerald and Madison, 1983, McGuire and Novak, 1984, Gruder-Adams and Getz, 1985, Getz and Hofmann, 1986, Oliveras and Novak, 1986, Carter and Getz, 1993). In contrast, meadow voles (M. pennsylvanicus) are an asocial, promiscuous species and males and females neither form pair bonds nor share a nest after mating (Getz, 1972, Madison, 1978, Madison, 1980a, Madison, 1980b). In this species, as it is common in other promiscuous mammals, only the mothers provide parental care (Wilson, 1982, McGuire and Novak, 1984, Gruder-Adams and Getz, 1985, Oliveras and Novak, 1986). It has been shown that the distribution patterns and regional densities of OT receptors in the brain differ between prairie and meadow voles (Insel and Shapiro, 1992, Smeltzer et al., 2006). Further, OT appears to play an important role in behaviors associated with social monogamy in prairie voles: central or site-specific (e.g., into the nucleus accumbens) OT treatment facilitates social contact and induces pair bond formation in female prairie voles (Williams et al., 1992, Williams et al., 1994, Insel and Hulihan, 1995, Cho et al., 1999, Liu and Wang, 2003) and these effects are blocked by concurrent administration of an OT receptor antagonist (Cho et al., 1999).
Recent studies have also shown that experimental alterations of the social environment significantly influence the rate of cell proliferation and survival in the vole brain (Smith et al., 2001, Fowler et al., 2002, Ormerod and Galea, 2003, Ormerod et al., 2004, Liu et al., 2007, Ruscio et al., 2008, Lieberwirth et al., 2012, Lieberwirth et al., 2013). For example, in the prairie vole, long-term chronic social isolation decreases the rate of cell proliferation in the DG and medial preoptic area (MPOA) and impairs cell survival in the AMY, DG, and ventromedial nucleus of the hypothalamus (VMH); whereas 48 h of cohabitation with a male results in a significant increase in SVZ cell proliferation in female prairie voles (Smith et al., 2001, Lieberwirth et al., 2012). However, few studies (e.g., Fowler et al., 2005) have examined the relationship between the animal’s sociality and adult neurogenesis. Therefore, in the present study we compared female prairie and meadow voles and examined their differences in behaviors relevant to anxiety-like, affiliation, and social recognition behavior as well as compared cell proliferation and survival in the brain. In addition, because of the reported roles of OT on species-specific behaviors in voles (Young et al., 2011) and on cell proliferation and survival in rats (Leuner et al., 2012), we examined the effects of OT on behaviors and cell proliferation and survival.
Materials and methods
Subjects
Subjects were sexually naïve female meadow and prairie voles that were the offspring of laboratory breeding colonies All voles were weaned at 21 days of age and housed in same sex sibling pairs in plastic cages (12 × 28 × 16 cm) containing cedar chip bedding with food and water ad libitum. It has been well-documented that, unlike traditional rodent species such as rats and mice, female voles are induced ovulators. They do not experience ovarian cycles and can only be induced into estrus by exposure to a conspecific, strange male or male associated cues (Dluzen and Carter, 1979, Cohen-Parsons and Carter, 1987). In our experiments, we used sexually naïve females that were housed in same sex pairs in plastic cages located in the female-only colony rooms for each species. This experimental set-up was designed to prevent any potential effects of gonadal steroid hormones from affecting subject’s behavior and cell proliferation/survival in the brain. The cages were maintained in a 14L:10D photoperiod (lights on at 0700) and at a temperature around 21 °C. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Florida State University.
Experimental design
Female subjects (n = 12 meadow, n = 16 prairie) were injected with a cell cycle marker, 5-bromo-2’-deoxyuridine (BrdU; Sigma: St Louis, MO, USA), intraperitoneally (ip; 100 mg/kg weight) in 0.9 % NaCl and 0.007 N NaOH once per day for 10 consecutive days. On day 11, subjects were implanted with guide cannulae aimed at the lateral ventricle (intracerebroventricular, icv). After 2 days of recovery, subjects were randomly assigned to one of two treatment groups. Subjects received artificial cerebrospinal fluid (CSF, 400 nl; BioFluids Inc, Rockville, MD, USA) without or with OT (200 ng/kg) (Peninsula Laboratory Inc., San Carlos, CA, USA) for seven consecutive days. This dose of OT was chosen due to its demonstrated role in pair bonding behavior in female prairie voles (Williams et al., 1994, Cho et al., 1999). Two weeks after the last CSF/OT administration, subjects underwent testing for anxiety-like behaviors (day 1), social affiliation (day 2), and social recognition (day 3), using previously established methods (Pan et al., 2009, Lieberwirth et al., 2012, Liu et al., 2014). Twenty-four hours following the social recognition test, subjects were deeply anesthetized and perfused. Their brains were processed for BrdU-ir and Ki67-ir staining.
Stereotaxic cannulation and microinjection
Each subject was anesthetized with sodium pentobarbital (1 mg/10 g body weight), and 26-gauge stainless steel guide cannula (Plastics One Inc., Roanoke, VA, USA) was implanted unilaterally, aimed at the lateral ventricle (nose bar at 2.5 mm, 0.6 mm rostral, 1.0 mm lateral, and 2.6 mm ventral to the Bregma). After 2 days of recovery, each subject received microinjections of CSF alone or CSF containing OT (200 ng/kg). Injection was performed using a 33-gauge needle that extended 1 mm below the guide cannula, in an injection volume of 400 nl icv. The needle was connected via PE20 tubing (Plastic One Inc) to a Hamilton syringe that was controlled by a manual injector (Fisher Scientific, Houston, TX, USA). The plunger depression was performed slowly, requiring about 1 min per injection. After completion of the behavioral test, all subjects were killed and cannula placements were verified by histological examination.
Behavioral tests
The open field test was conducted as described in previous studies to evaluate exploratory and anxiety-like behaviors (Pan et al., 2009, Lieberwirth et al., 2012). The plastic apparatus measured 56 × 56 × 20 (H) cm and had a visual line grid that divided the apparatus into 16 squares, each measuring 14 × 14 cm. Each subject was placed into the center of the apparatus. The 10-minute test was video-recorded. Anxiety-like behaviors (latency and frequency of center entries and duration spent in the center) and an index of locomotion (frequency of line crosses) were quantified.
The social affiliation test was performed also using an established method (Pan et al., 2009, Lieberwirth et al., 2012). Briefly, the testing apparatus consisted of two plexiglass cages (13 × 28 × 16 (H) cm) connected by a hollow tube (16 L × 7.5 radius cm). An unfamiliar stimulus female (approximately 70 days of age) was loosely tethered in one chamber, while the other chamber remained empty. Each subject was placed into the empty chamber and then allowed to move freely throughout the apparatus for 60 min. A customized computer program using a series of light beams across the connecting tubes was used to monitor the subject’s movement between the chambers. The frequency of chamber entries (index of locomotion) and the duration subjects spent in each chamber were recorded.
The social recognition test was performed also using an established method (Lieberwirth et al., 2012, Liu et al., 2014). Each subject was put individually into a testing cage (25 × 45 × 20 (H) cm) and allowed to habituate for 10 min. Immediately following habituation, an unfamiliar juvenile female at 30–40 days of age was introduced into the cage for 5 min (trial 1, T1) and then removed and returned to its home cage. After five minutes, the same stimulus animal was reintroduced for another 5 min (trial 2, T2). This process was repeated for a total of three times (T1, T2, and T3). During the fourth period (trial 4, new), an unrelated novel juvenile female was introduced for 5 min. All behavioral interactions were video recorded. The frequency and duration of the subject’s olfactory investigation of the juvenile including sniffing of the anogenital and head regions were quantified.
Brain perfusion and immunohistochemistry
Subjects were deeply anesthetized with sodium pentobarbital (3 mg/kg body weight) and perfused through the ascending aorta with 0.9 % saline followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were harvested, postfixed for 2 h in 4 % paraformaldehyde, and then stored in 30 % sucrose in PBS. All brains were cut into 40 µm coronal sections on a cryostat and stored in 0.1 M PBS with 1 % sodium azide at 4 °C until processed for Ki67 and BrdU immunohistochemistry.
To examine cell proliferation, a set of brain sections at 240 µm intervals was processed for Ki67 immunohistochemistry as previously described (Lieberwirth et al., 2012). The cell proliferation marker Ki67 is a nuclear protein that is expressed by proliferating cells throughout the entire mitotic process (Scholzen and Gerdes, 2000). Brain sections were treated with 10 mM sodium citrate buffer at 90 °C for 10 min, followed by 1 % NaBH4 for 1 h and then 0.5 % hydrogen peroxide in 0.1 M PBS for 1 h. Thereafter, sections were treated in PBS with 0.5 % Triton X-100 (PBT) for 20 min, blocked in 10 % normal goat serum in PBT for 1 h and incubated in rabbit Ki67 polyclonal antibody (1:5000, Vector Laboratories, Inc. Burlingtone, CA, USA) in PBT with 2 % NGS for 48 h at 4 °C and an additional 1 h at room temperature. Sections were then rinsed in 0.1 M PBS and incubated in biotinylated goat-anti-rabbit second antibody (1:300, Vector Laboratories, Inc. Burlingtone, CA, USA) in PBT for 2 h and ABC complex in PBS for 90 min, and staining was revealed with 3’-diainobenzidine (DAB; Sigma).
As BrdU was injected at the beginning of the experiment (i.e., 20–30 days prior to sacrificing), it was used here as a marker of cell survival. A set of brain sections was processed for BrdU immunohistochemistry using an established procedure (Fowler et al., 2002, Lieberwirth et al., 2012). Sections were treated with 2 N HCl for 30 min at 60 °C and with 0.1 M borate buffer at room temperature for 25 min. After rinsing in 0.1 M PBS, sections were incubated in 0.3 % hydrogen peroxide and 10 % methanol in 0.1 M PBS for 15 min following by 10 % normal goat serum in 0.5 % Triton X-100 in 0.1 M PBS (0.5 % PBT) for 1 h. Subsequently, the sections were incubated in rat-anti-BrdU monoclonal antibody (1:5,000; Accurate Chemical, Westbury, NY) in 0.5 % PBT with 2 % normal goat serum at 4 °C for 48 h. Sections were rinsed and incubated in biotinylated goat-anti-rat second antibody (1:300; Vector Laboratories, Inc. Burlingtone, CA, USA) in 0.5 % PBT with 2 % normal goat serum for 2 h. Thereafter, sections were incubated in ABC complex (Vector, Burlingame, CA) in 0.1M PBS for 90 min and BrdU immunoreactivity was revealed using DAB.
All sections were mounted on slides, air-dried, and cover-slipped with Permount. For both Ki67- and BrdU-ir staining, additional brain sections were incubated without the primary antibody. This process resulted in the total absence of specific labeling. In addition, to control for background variability and to standardize the staining, sections from all subjects in each experiment were processed concurrently for each of the markers.
Data quantification and analysis
All behavioral videos were scored by a trained observer blind to the treatment using J-Watcher software (V1.0, Macquarie University and UCLA). Group differences across the behavioral measurements in the open field and social affiliation tests were analyzed by a two-way ANOVA (species × treatment) followed by the Student Newman Keul’s (SNK) post-hoc test. Data from the social recognition test were analyzed by a three-way repeated measure ANOVA with treatment and species as main factors and trials as repeated measures (T1, T2, T3, new), followed by SNK post-hoc test.
All microscope slides were coded to disguise group identity until data quantification was completed. Ki67-ir and BrdU-ir cells were visualized under 40X magnification using a Zeiss Axioskop II microscope. The numbers of Ki67-ir and BrdU-ir cells were counted in the hilus, granular cell layer, and molecular cell layer of the DG [corresponding to Plates 29–32 in Paxinos and Watson (1998)], the VMH [including the dorsomedial, central, and ventrolateral subregions; corresponding to Plates 30–32 in Paxinos and Watson (1998)], and the AMY [including the central, CeA, medial, MeA, and anterior cortical, ACo, nuclei; corresponding to Plates 28–29 in Paxinos and Watson (1998)]. The VMH and AMY were chosen because of their roles in social behaviors (Kling and Brothers, 1992, Wang et al., 1997, Lonstein et al., 1998, Cushing et al., 2003), whereas the DG plays an important role in learning and memory (Jarrard, 1993, Jessberger et al., 2009). For the DG, four brain sections were counted bilaterally per animal. For the VMH and AMY two sections were quantified. For all animals, sections were anatomically matched. Since cells in the VMH were low and no differences were found in the numbers of Ki67-ir and BrdU-ir cells in any of the subregions examined, the cell counts were combined as a total number. The sum of the counted cells across all sections for a given brain area was multiplied by six to obtain an estimate of the total number of Ki67- and BrdU-ir cells (Lieberwirth et al., 2012). Furthermore, the areas of these brain regions were measured by using the NIH IMAGE program. The images were displayed on a computer screen and all regions were traced bilaterally. The sum of the brain areas across all sections for a given brain region was obtained to estimate the total volumes. Group differences in the total volume and the number of Ki67- and BrdU-ir cells in each brain area were analyzed by a two-way ANOVA (species × treatment) followed by SNK post-hoc test. In addition, correlations between the number of the Ki67-ir or BrdU-ir cells in selected brain areas and behaviors were analyzed using Spearmen correlation. All statistical analyses were carried out using the SPSS software package (Version 18). The criterion for significance was set at p < 0.05.
Results
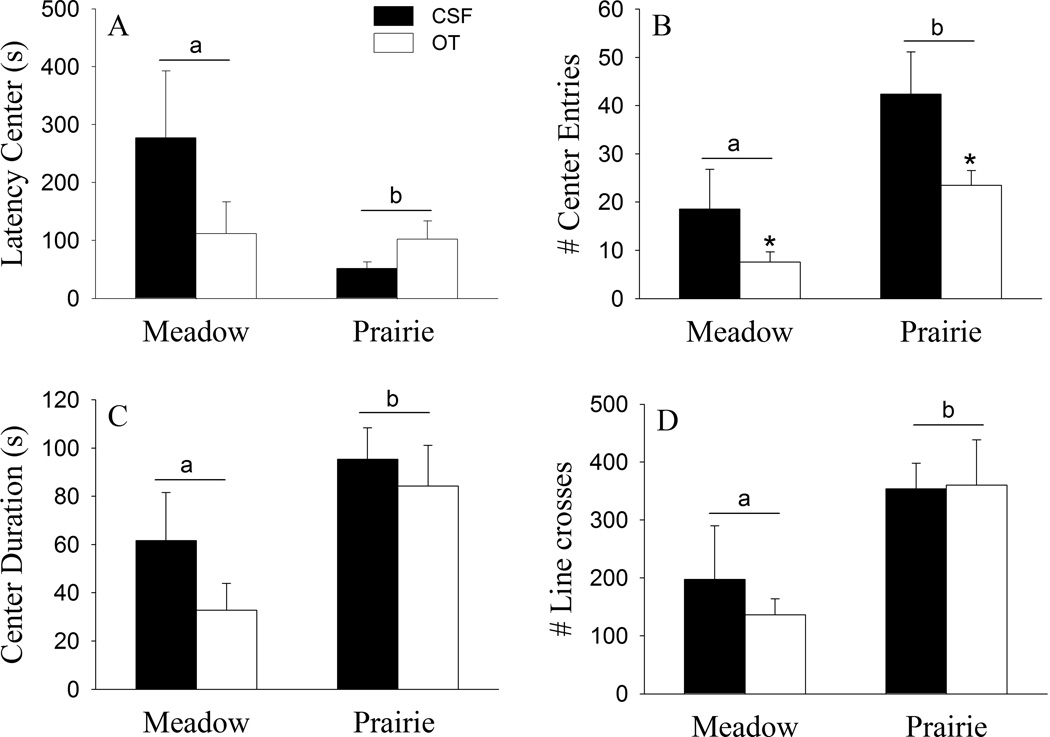
Species differences were found in the open field test (Fig. 1). Female meadow voles showed a longer latency to enter to the center (F(1, 24) = 4.95, p < 0.05; Fig. 1A), fewer entries of the center squares (F(1, 24) = 10.03, p < 0.01; Fig. 1B), and spent less time in the center (F(1, 24) = 7.63 , p < 0.05; Fig. 1C) of the open filed, compared to prairie voles. In addition, female meadow voles also showed a lower level of locomotor activity indicated by the total number of line crossings compared to prairie voles (F(1, 24) = 8.93 , p < 0.01; Fig. 1D). A significant treatment effect was found in center entries: the OT-treated females showed fewer center entries compared to CSF-injected females of both species (F(1, 24) = 5.69, p < 0.05; Fig. 1B). OT treatment did not alter other behaviors in the open field. In addition, no species-by-OT interaction was found in any behavioral measurement.
Fig. 1.
Species differences and the effects of oxytocin (OT) on behaviors in the open field test. Female meadow voles showed a longer latency to enter the center (A), made fewer entries to center squares (B), spent less time in the center (C), and showed a lower level of locomotor activity (D) compared to prairie voles. The OT-treated females showed fewer center entries compared to CSF-injected females (B). OT treatment did not alter other behaviors in the open field. *Indicates significant treatment effects at p < 0.05; while alphabetic letters indicate species differences. Error bars represent standard errors of the mean (SEM).
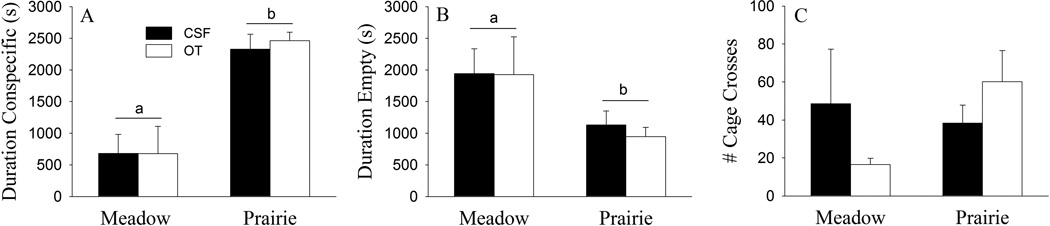
Species differences were also found in the social affiliation test (Fig. 2). Female meadow voles spent less time in the cage containing a conspecific (F(1, 24) = 34.88, p < 0.01; Fig. 2A) and more time in the empty cage (F(1, 24) =5.92, p < 0.05; Fig. 2B) compared to prairie voles. No species difference was found in locomotor activity (Fig. 2C). In addition, no OT effect or species-by-OT interaction was found in these behaviors.
Fig. 2.
Species differences and the effects of oxytocin (OT) on behaviors in the social affiliation test. Female meadow voles spent less time in the cage containing a conspecific (A) and more time in the empty cage (B) compared to prairie voles. No species differences were found in locomotor activity (C). In addition, no treatment effect or species-by-treatment interaction was found in these behaviors. Alphabetic letters indicate species differences. Error bars represent standard errors of the mean (SEM).
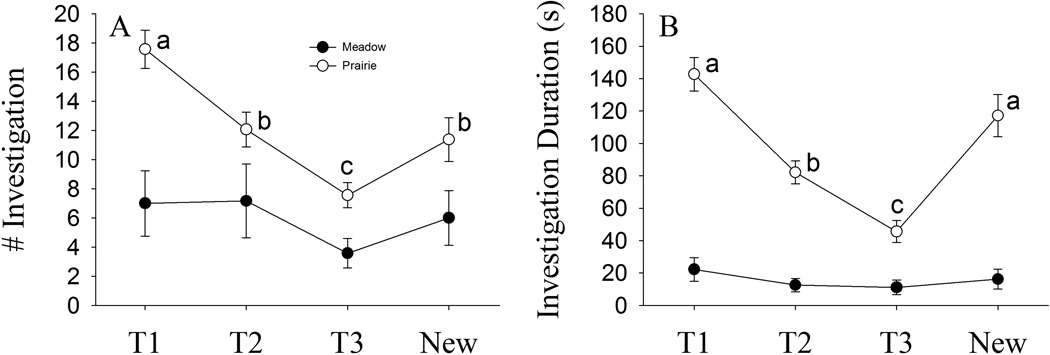
In the social recognition test, there was a significant main effect of trial (F(3, 72) = 9.09, p < 0.01), species (F(1, 24) = 15.40, p < 0.01), trial-by-species-by-treatment interaction (F(3, 72) = 2.89, p < 0.05) on the frequency of olfactory investigation. There was also a significant main effect of trial (F(1.81, 43.53) = 18.04, p < 0.01), species (F(1, 24) = 106.38, p < 0.01) and trial-by-species interaction (F(1.81, 43.53) = 11.58, p < 0.01) on the duration of olfactory investigation. Across trials, female prairie voles showed a characteristic decline in the frequency and duration spent investigating the familiar stimulus animal (T2 compared to T1, T3 compared to T1 and T2, p < 0.01, SNK comparisons) and a significant recovery following the introduction of a novel stimulus animal (p < 0.01, SNK comparisons; Fig. 3A and 3B). Conversely, female meadow voles did not show different frequency and duration over trials. Overall, the frequency and duration of female meadow voles investigating the stimulus animals were significantly lower than that of prairie voles (Fig. 3A and 3B). No treatment effect, trial by OT or species by OT interaction was found on the frequency and duration of olfactory investigation.
Fig. 3.
Species differences on behaviors in the social recognition test. Across trials, female prairie voles showed a characteristic decline in the frequency and duration spent investigating the familiar stimulus conspecific (T2 compared to T1, T3 compared to T1 and T2) and a significant recovery following the introduction of a novel, stimulus conspecific (A and B). Conversely, female meadow voles did not show different frequency and duration over trials. Overall, the levels of frequency and duration of female meadow voles investigating the stimulus conspecific were significantly lower than that of prairie voles (A and B). Alphabetic letters indicate the results of the post hoc test. Error bars represent standard errors of the mean (SEM).
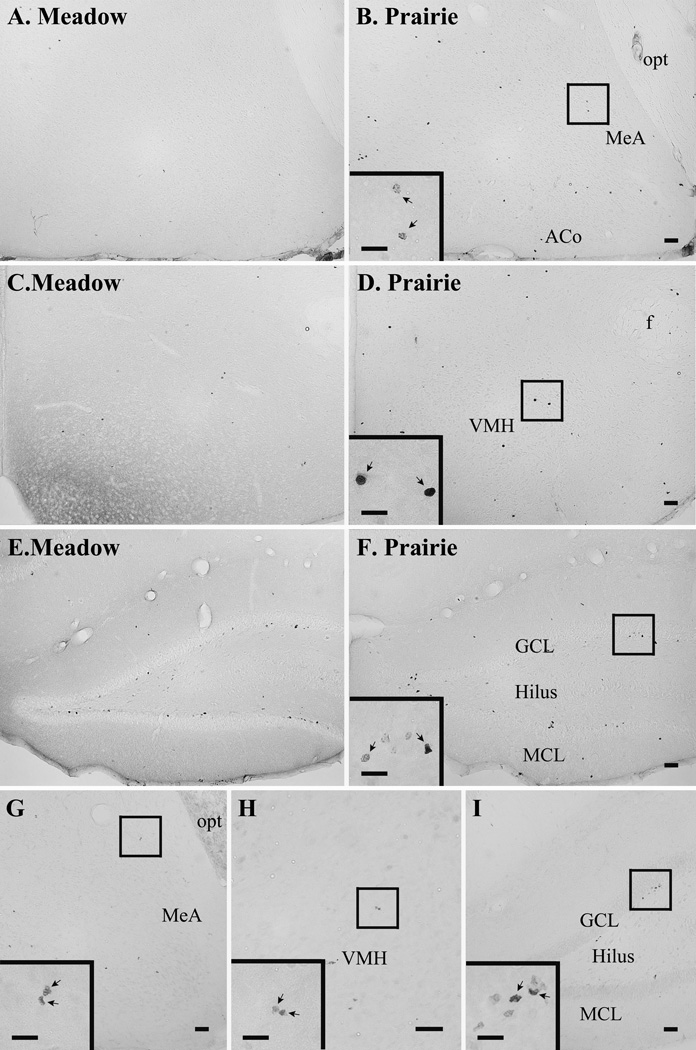
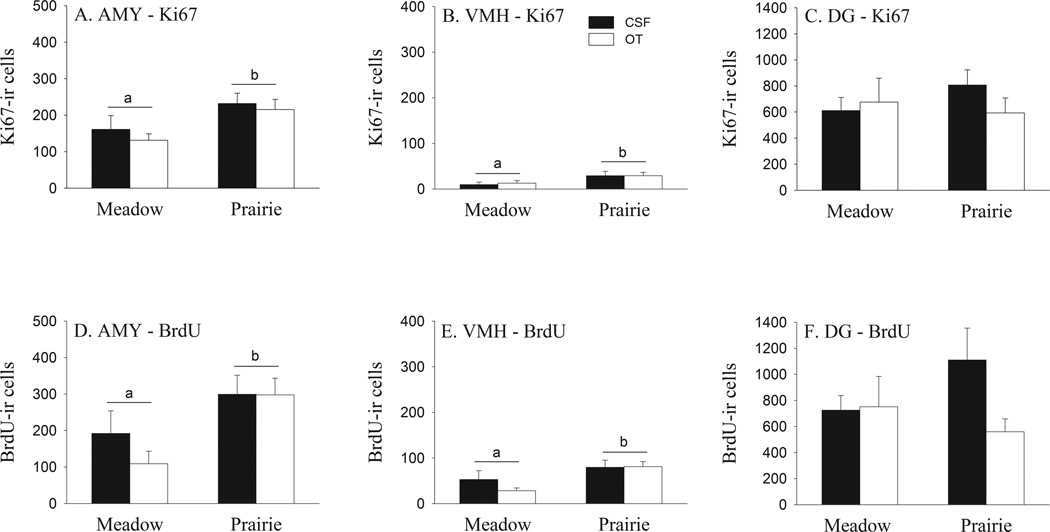
Ki67-ir cells were found in the AMY, VMH, and DG (Fig. 5G–I), and meadow and prairie voles differed in the number of Ki67-ir cells in a brain region-specific manner. For the AMY (F(1, 24) = 7.39, p < 0.05; Fig. 4A) and VMH (F(1, 24) = 5.30, p < 0.05; Fig. 4B), female meadow voles had lower numbers of Ki67-ir cells compared to female prairie voles. This species difference in the AMY was found in the CeA (F(1, 24) = 9.14, p < 0.01), but not ACo and MeA, of the AMY (Table 1). No species differences were found in the number of Ki67-ir cells in the DG (Fig. 4C, Table 2). No significant effects of OT or species-by-OT interaction were found on the number of Ki67-ir cells in any brain regions examined.
Fig. 5.
Composed photomicrographs displaying BrdU-labeled cells in the amygdala (A & B), ventromedial hypothalamus (VMH) (C & D), and dentate gyrus of the hippocampus (DG) (E & F) in the female meadow (A, C & E) and prairie (B, D & F) voles. Ki67-ir cells in the amygdala (G), VMH (H), and DG (I) are also illustrated. ACo: anterior cortical nucleus of the amygdala, GCL: granular cell layer of the DG, MCL: molecular cell layer of the DG, MeA: medial nucleus of the amygdala, and opt: optic tract. Scale bar=100 µm. The insert in Panel B, D, and F illustrates BrdU-ir cells (arrows) and the one in Panel G, H, and I shows Ki67-ir cells (arrows) with scale bar=50µm.
Fig. 4.
Species differences and the effects of oxytocin (OT) on cell proliferation (assessed by Ki67-labeling) and survival (assessed by BrdU-labeling) in the brains of female meadow and prairie voles. Female meadow voles had a lower number of Ki67-ir and BrdU-ir cells in the AMY and VMH, but not in the DG, compared to female prairie voles. No significant effects of OT treatment or species-by-treatment interaction were found on the number of Ki67-ir and BrdU-ir cells in any brain region examined. Alphabetic letters indicate species differences. Error bars represent standard errors of the mean (SEM).
Table 1.
Number of cells labeled for Ki67 or BrdU in the amygdala.
| Brain area | Meadow Vole |
Prairie Vole |
p |
|||||
|---|---|---|---|---|---|---|---|---|
| CSF | OT | CSF | OT | Species | Treatment | S×T | ||
| Ki67 | ||||||||
| ACo | 61.20 ± 10.80† |
48.86± 10.51 |
80.25± 9.55 |
63.00± 8.71 |
ns | ns | ns | |
| CeA | 51.60± 14.02 |
48.86± 7.46 |
82.50± 8.00 |
85.50± 13.59 |
** | ns | ns | |
| MeA | 48.00± 15.76 |
33.43± 6.53 |
69.00± 15.30 |
66.75± 13.34 |
ns | ns | ns | |
| BrdU | ||||||||
| ACo | 51.60± 13.23 |
27.43± 9.70 |
56.25± 12.37 |
48.75± 8.96 |
ns | ns | ns | |
| CeA | 61.20± 30.08 |
39.43± 15.87 |
117.00± 21.00 |
98.25± 19.70 |
* | ns | ns | |
| MeA | 79.20± 22.08 |
42.00± 15.60 |
125.25± 22.80 |
150.75± 25.67 |
** | ns | ns | |
MEAN±SEM;
p<0.05;
p<0.01;
ns: not significantly different.
Table 2.
Number of cells labeled for Ki67 or BrdU in the hippocampus.
| Brain area | Meadow Vole |
Prairie Vole |
p |
|||||
|---|---|---|---|---|---|---|---|---|
| CSF | OT | CSF | OT | Species | Treatment | S×T | ||
| Ki67 | ||||||||
| GCL | 271.20 ± 50.54† |
251.14± 77.07 |
294.75± 62.78 |
199.50± 34.09 |
ns | ns | ns | |
| Hilus | 274.80± 47.07 |
368.57± 100.41 |
393.75± 63.58 |
309.00± 73.19 |
ns | ns | ns | |
| MCL | 64.80± 25.97 |
56.57± 14.39 |
118.50± 15.37 |
84.75± 15.23 |
* | ns | ns | |
| BrdU | ||||||||
| GCL | 378.00 ± 104.04 |
384.00 ± 139.29 |
741.75 ± 187.28 |
295.50 ± 56.45 |
ns | ns | ns | |
| Hilus | 204.00± 51.51 |
127.71± 32.67 |
165.75± 27.40 |
105.75± 30.51 |
ns | ns | ns | |
| MCL | 142.80± 21.75 |
239.14± 131.59 |
203.25± 41.71 |
158.25± 20.31 |
ns | ns | ns | |
MEAN±SEM;
p<0.05;
ns: not significantly different.
Species differences were also found in BrdU-immunoreactivity in the vole brain. Female meadow voles had fewer BrdU-ir cells, compared to female prairie voles, in the AMY (F(1, 24) = 8.98 , p < 0.01; Figs. 4D, 5A and B) and VMH (F(1, 24) = 8.45 , p < 0.01; Figs. 4E, 5C and D). This species difference in the AMY was found in the CeA (F(1, 24) = 7.08, p < 0.05; Table 1) and MeA (F(1, 24) = 11.28, p < 0.01) but not ACo (Table 1). No species differences were found in the number of BrdU-ir cells in the DG (Figs. 4 F, 5E and F, Table 2). No significant effect of OT or species-by-OT interaction were found on the number of BrdU-ir cells in any brain region examined. Finally, no species, treatment and species-by-OT interaction were found on the volume in any brain regions examined.
Correlations between BrdU-ir/Ki67-ir cells and behaviors illustrated several interesting patterns (Table 3). First, the number of adult-proliferated cells in the AMY and VMH that survived for at least 20–30 days (labeled for BrdU-ir) showed significant correlations with anxiety-like behaviors in the open field test. Newly proliferating cells in the AMY (labeled for Ki67-ir) also showed such correlation with anxiety-like behavior. Second, the number of BrdU-ir cells in the AMY and VMH significantly correlated with behaviors associated with social contact or social recognition. Interestingly, the numbers of Ki67-ir cells in the AMY and VMH correlated with behaviors when the subject was first exposed to a novel conspecific (T1 or New). Finally, the number of BrdU-ir or Ki67-ir cells in the DG did not correlate with any behaviors measured.
Table 3.
Correlations between the number of cells labeled for Ki67 or BrdU with behaviors.
| Brain Area |
Open Field |
Social Affiliation |
Social Recognition |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Center Entries |
Center Duration |
Contact Duration |
Cage Crossing |
T1 | T2 | T3 | New | |||
| Ki67 | ||||||||||
| AMY | r | 0.426 | 0.532 | 0.333 | 0.427 | 0.607 | 0.347 | 0.418 | 0.408 | |
| p | 0.024 | 0.004 | 0.084 | 0.024 | 0.001 | 0.070 | 0.027 | 0.031 | ||
| VMH | r | 0.148 | 0.351 | 0.270 | 0.161 | 0.506 | 0.319 | 0.233 | 0.410 | |
| p | 0.454 | 0.067 | 0.165 | 0.414 | 0.006 | 0.098 | 0.233 | 0.030 | ||
| DG | r | 0.123 | 0.049 | 0.064 | 0.059 | 0.276 | 0.241 | 0.193 | 0.132 | |
| p | 0.532 | 0.806 | 0.745 | 0.767 | 0.156 | 0.216 | 0.324 | 0.502 | ||
| BrdU | ||||||||||
| AMY | r | 0.528 | 0.496 | 0.536 | 0.286 | 0.549 | 0.394 | 0.489 | 0.482 | |
| p | 0.004 | 0.007 | 0.003 | 0.140 | 0.002 | 0.038 | 0.008 | 0.009 | ||
| VMH | r | 0.607 | 0.387 | 0.382 | 0.282 | 0.523 | 0.416 | 0.533 | 0.489 | |
| p | 0.001 | 0.042 | 0.045 | 0.146 | 0.004 | 0.028 | 0.004 | 0.008 | ||
| DG | r | 0.185 | 0.301 | 0.065 | 0.037 | 0.239 | 0.046 | 0.095 | 0.110 | |
| p | 0.346 | 0.120 | 0.741 | 0.851 | 0.221 | 0.816 | 0.632 | 0.577 | ||
r = correlation coefficient. The bold values are regions of interest that had significant correlations with p < 0.05.
Discussion
Voles are a group of rodent species that display remarkable different life strategies and thus provide a useful model for studying the effects of social environment on physiological, cognitive, and behavioral functions (Young and Wang, 2004, Young et al., 2011). In the present study, we compared non-social, promiscuous meadow voles with highly social, monogamous prairie voles to examine behaviors and cell proliferation/survival in the brain, and to study the effects of OT on these markers. Our data demonstrate that female meadow voles displayed a higher level of anxiety-like behavior, lower levels of social affiliation and social recognition, as well as lower levels of cell proliferation and survival in a brain region-specific manner, compared to female prairie voles. Interestingly, BrdU-ir or Ki67-ir cells correlated with anxiety-like, social affiliation, and social recognition behaviors in a brain region-specific manner.
The open field test utilizes rodent’s conflicting propensities to explore a novel environment and avoid open areas, and as such has been widely used to assess anxiety in a variety of rodent species including voles (Järbe and Johansson, 1977, Prut and Belzung, 2003, Liu et al., 2014, Clinard et al., 2015). In the present study, a higher level of anxiety in female meadow voles was indicated by a longer latency to enter the center, fewer center entries, and less duration in the center squares of the open field compared to female prairie voles. Meadow voles also showed a lower level of locomotor activity than prairie voles. Previous studies using different rat strains have shown that emotionality and locomotor activity are often related (Gentsch et al., 1981, Courvoisier et al., 1996, Liebsch et al., 1998). In particular, rats bred for high anxiety-related behavior tended to be less active than rats bred for low anxiety-related behavior in the open field (Liebsch et al., 1998). As meadow and prairie voles did not differ in locomotor activity during the social affiliation test, less activity in meadow voles, compared to prairie voles, in the open field test may reflect meadow voles’ high reactivity associated with anxiety.
It has been well-documented that prairie voles are highly affiliative with the conspecifics (Getz et al., 1981, Shapiro and Dewsbury, 1990), whereas meadow voles are non-social and appear to avoid social contact except for mating (Madison, 1978, Madison, 1980a, Madison, 1980b, McGuire and Novak, 1984). Therefore, it is not surprising that, in the present study, meadow voles were less affiliative in the social affiliation test by spending less time in the conspecific cage and more time in the empty cage compared to prairie voles. Furthermore, our data not only replicates the previous finding that prairie voles perform well in the social recognition test (Lieberwirth et al., 2012, Liu et al., 2014), but also illustrate, for the first time, that meadow voles do not perform well in the same test. The social recognition test has been well established (Engelmann and Landgraf, 1994) and has been utilized in a variety of rodent species to evaluate their learning, memory, and individual recognition ability (Engelmann and Landgraf, 1994, Clipperton-Allen et al., 2012, Lieberwirth et al., 2012). Our data show that meadow voles failed to discriminate between the familiar and a novel conspecific and their levels of investigative behaviors were significantly lower than prairie voles. It is unclear whether the social recognition failure of the meadow voles can be attributed to their cognitive processes similar to social anxiety or simply a lack of interest in social interaction. However, giving that meadow voles display social discrimination and memory in the wild (Ferkin et al., 2010) and prefer to live solitary (Madison, 1980a, Madison, 1980b, McGuire and Novak, 1984), it seems to be appropriate to interpret our data as that the meadow voles were simply not interested in taking part in social interactions rather than had deficits in olfaction.
Early studies have shown that social environmental factors can affect adult neurogenesis in a stimulus- and brain region-specific manner (Gheusi et al., 2009, Lieberwirth and Wang, 2012). For example, aversive social experiences, such as exposure to an aggressive and dominant conspecific or social isolation, decrease (Gould et al., 1997, Westenbroek et al., 2004, Czeh et al., 2007, Thomas et al., 2007, Lieberwirth et al., 2012, Pan et al., 2014); whereas positive social interactions, such as exposure to male pheromones, maternal experience, or interactions with a conspecific pup, increase the number of adult-generated cells in the DG and/or the SVZ system (Furuta and Bridges, 2005, Mak et al., 2007, Ruscio et al., 2008, Furuta and Bridges, 2009). Our data extend these findings, showing that vole species with distinct life strategies and sociality differ in the rates of cell proliferation and survival in adult brains in a region-specific manner. Female meadow voles had lower rates of cell proliferation and cell survival in the AMY and VMH, but not in the DG, compared to female prairie voles. As meadow and prairie voles differ in the level of social affiliative behavior (Madison, 1980a, Getz et al., 1981, Carter and Getz, 1993), such species differences in cell proliferation and survival in the present study may be interpreted as effects due to species differences in social interactions with cage mates. It is important to note that female meadow and prairie voles are induced ovulators and only display elevated levels of estrogen following exposure to a conspecific male or male-associated cues (Dluzen and Carter, 1979, Cohen-Parsons and Carter, 1987). Therefore, observed species differences in cell proliferation and survival in the present study are likely not due to fluctuations in circulating estrogen. It should also be noted that in a previous study (Fowler et al., 2005), female meadow voles, in comparison to female prairie voles, had a higher level of cell proliferation, as identified with BrdU, in the ACo and DG, but not in other subnuclei of the AMY or VMH. Discrepancies between the previous and current studies may be due to several notable differences in the methodologies and procedures, among which multiple injections of BrdU over 24 hours was used in the previous study (Fowler et al., 2005), which would have resulted in labeling a heterogeneous population of cells of both newly proliferated cells and cells showing short-term survival (Taupin, 2007), and may not have been a pure measure of cell proliferation unlike in the present study.
It is interesting that in the current study, the number of adult-proliferated cells in the AMY and VMH, but not DG, showed significant correlations with anxiety-like behaviors and behaviors related to social contact and social recognition. As the AMY and VMH have been implicated in variety of behavioral and cognitive functions (Kollack-Walker and Newman, 1995, Lonstein et al., 1998, Cushing et al., 2003, Davis and Marler, 2004, Stowe et al., 2005), these correlation data may indicate the potential roles of newly-proliferated cells in the AMY and VMH in anxiety-like, social affiliation, and social recognition behaviors—an idea similar to the suggested involvement of adult-proliferated cells in the SVZ/olfactory bulb or in the hippocampus in learning and memory (Shors et al., 2001, Dupret et al., 2007), social cognition (Feierstein et al., 2010, Lagace et al., 2010, Mak and Weiss, 2010, Oboti et al., 2011), and emotional behavior (Revest et al., 2009, Larsen and Grattan, 2010, Snyder et al., 2011).
OT in the brain is considered to be anxiolytic (Windle et al., 1997, Neumann, 2008, Guastella et al., 2010) and it regulates social recognition memory (Ferguson et al., 2001, Gur et al., 2014) and a variety of social behaviors, including affiliative behaviors (Neumann, 2008, Young et al., 2008). More recently, OT has also been shown to facilitate cell proliferation and neurogenesis in the DG, and to protect rats from the suppressive effects of stresses on hippocampal plasticity (Leuner et al., 2012). To our surprise, in the present study, although voles were treated with OT at a dose comparable to the doses affecting behaviors and adult neurogenesis in other rodent species (Pedersen et al., 1982, Fahrbach et al., 1984, Leuner et al., 2012), we failed to see any significant effects of OT on most behaviors (except for a subtle effect on center entries in the open field test) and cell proliferation/survival in the brain. This inconsistency may be explained by several factors including species-specific effects of OT and differences in experimental paradigms and procedures. Further, OT effects depend on the dose of application (Bales et al., 2014, Neumann and Slattery, 2015). In mice, for example, chronic (2-week) icv infusion of OT at a dose of 10 ng/h, but not 1 ng/h, induced a robust increase in anxiety-like behavior (Peters et al., 2014). OT facilitates cell proliferation in the rat DG also in a dose-dependent manner (Leuner et al., 2012). Therefore, the caveat in the present study is the use of single dose of OT, which may not be effective to influence behaviors and cell proliferation/survival in the vole brain. A more comprehensive dose response experiment needs to be performed in future studies to evaluate OT effects on anxiety-like and social behaviors as well as on adult neurogenesis in voles.
Conclusion
Data from our current study indicate differences between female prairie and meadow voles in their behaviors as well as cell proliferation and survival in the VMH and AMY, but not DG, in the brain. As the VMH and AMY have been implicated in a variety of behavioral functions (Davis, 1992, Kling and Brothers, 1992, Wang et al., 1997, Lonstein et al., 1998, Cushing et al., 2003), it is reasonable to predict that regional variations in cell proliferation and survival in the vole brains may play important roles in behaviors, which is further supported by our correlation data. It should be mentioned that the phenotypes of the newly-proliferated cells in the brain have already been examined in a previous study in female prairie and meadow voles (Fowler et al., 2005). In the VMH, for example, approximately 26.8% of the BrdU-ir cells co-labeled with a neuronal marker (TuJ1) and 48.6% co-expressed a glial progenitor marker (NG2). In the AMY, 40.5% of BrdU-ir cells co-labeled with TuJ1 and 45.5% co-labeled with NG2. However, female prairie and meadow voles did not differ in the percentages of BrdU-ir cells co-labeled with TuJ1 or NG2 in the AMY and VMH, and estrogen manipulation did not affect the phenotypes of BrdU-ir labeled cells (Fowler et al., 2005). Although these data indicate no species differences in the neuronal and glial phenotypes of newly proliferated cells in the brain of female voles, we still cannot exclude the possibility that OT may affect differentiation of newly proliferated cells in the brain similarly or differently between the two species examined. Clearly, a more comprehensive study needs to be conducted to examine the effects of OT on cell proliferation, survival, and differentiation in the brain as well as on behaviors for conclusive results.
Meadow voles display higher levels of anxiety-like behaviors than prairie voles.
Prairie voles display higher levels of social affiliation and recognition than meadow voles.
Cell proliferation & survival in the VMH and AMY, but not DG, are higher in prairie than meadow voles.
The numbers of new cells in the VMH and AMY correlate with anxiety-like and social behaviors.
Oxytocin treatment was not effective to affect behaviors and cell proliferation & survival.
Acknowledgements
This research was supported by NIH grants MHR01-089852 and MHR01-058616 to ZXW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker AM, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Front Behav Neurosci. 2013;7:185–194. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Clinard CT, Bader LR, Sullivan MA, Cooper MA. Activation of 5-HT2a receptors in the basolateral amygdala promotes defeat-induced anxiety and the acquisition of conditioned defeat in Syrian hamsters. Neuropharmacology. 2015;90:102–112. doi: 10.1016/j.neuropharm.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Lee AW, Reyes A, Devidze N, Phan A, Pfaff DW, Choleris E. Oxytocin, vasopressin and estrogen receptor gene expression in relation to social recognition in female mice. Physiol Behav. 2012;105:915–924. doi: 10.1016/j.physbeh.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiol Behav. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Corona R, Larriva-Sahd J, Paredes RG. Paced-mating increases the number of adult new born cells in the internal cellular (granular) layer of the accessory olfactory bulb. PloS one. 2011;6:e19380. doi: 10.1371/journal.pone.0019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvoisier H, Moisan M-P, Sarrieau A, Hendley ED, Mormède P. Behavioral and neuroendocrine reactivity to stress in the WKHA/WKY inbred rat strains: a multifactorial and genetic analysis. Brain Res. 1996;743:77–85. doi: 10.1016/s0006-8993(96)01023-2. [DOI] [PubMed] [Google Scholar]
- Crociara P, Parolisi R, Conte D, Fumagalli M, Bonfanti L. Cellular and molecular characterization of multipolar Map5-expressing cells: a subset of newly generated, stage-specific parenchymal cells in the mammalian central nervous system. PloS one. 2013;8:e63258. doi: 10.1371/journal.pone.0063258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing BS, Mogekwu N, Le WW, Hoffman GE, Carter CS. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 2003;965:203–211. doi: 10.1016/s0006-8993(02)04199-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacol. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. c-fos Changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Carter CS. Ovarian hormones regulating sexual and social behaviors in female prairie voles, Microtus ochrogaster. Physiol Behav. 1979;23:597–600. doi: 10.1016/0031-9384(79)90063-5. [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy M, Panatier A, Rodríguez JJ, Lamarque S, Lemaire V, Oliet S, Piazza P-V, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18:267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Lazarini F, Wagner S, Gabellec M-M, de Chaumont F, Olivo-Marin J-C, Boussin FD, Lledo P-M, Gheusi G. Disruption of Adult Neurogenesis in the Olfactory Bulb Affects Social Interaction but not Maternal Behavior. Front Behav Neurosci. 2010;4:176–192. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin MH, Ferkin DA, Ferkin BD, Vlautin CT. Olfactory experience affects the response of meadow voles to the opposite-sex scent donor of mixed-sex over-marks. Ethology. 2010;116:821–831. doi: 10.1111/j.1439-0310.2010.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald RW, Madison DM. Social organization of a free-ranging population of pine voles, Microtus pinetorum. Behav Ecol Sociobiol. 1983;13:183–187. [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-α in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Gestation-induced cell proliferation in the rat brain. Dev Brain Res. 2005;156:61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch C, Lichtsteiner M, Feer H. Locomotor activity, defecation score and corticosterone levels during an openfield exposure: A comparison among individually and group-housed rats, and genetically selected rat lines. Physiol Behav. 1981;27:183–186. doi: 10.1016/0031-9384(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Getz L, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: Field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- Getz LL. Social structure and aggressive behavior in a population of Microtus pennsylvanicus. J Mammal. 1972;53:310–317. [Google Scholar]
- Getz LL, Hofmann JE. Social organization in free-living prairie voles, Microtus ochrogaster. Behav Ecol Sociobiol. 1986;18:275–282. [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo P-M. A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Gruder-Adams S, Getz LL. Comparison of the mating system and paternal behavior in Microtus ochrogaster and M. pennsylvanicus. J Mammal. 1985:165–167. [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiat. 2014;76:377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. Neuropeptides and the evolution of social behavior. Curr Opin Neurobiol. 2000;10:784–789. doi: 10.1016/s0959-4388(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Järbe T, Johansson J. Pentobarbital, diazepam and bemegride: their effects on open-field behavior in the gerbil (Meriones unguiculatus) Arch Int Pharmacodyn Ther. 1977;225:88–97. [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Memory. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss; 1992. pp. 353–378. [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Y, Jia X, Liu Y, Wang Z. Fatherhood reduces the survival of adult-generated cells and affects various types of behavior in the prairie vole (Microtus ochrogaster) Eur J Neurosci. 2013;38:3345–3355. doi: 10.1111/ejn.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The Social Environment and Neurogenesis in the Adult Mammalian Brain. Front Hum Neurosci. 2012;6:118–136. doi: 10.3389/fnhum.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94:301–310. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis J, Fowler C, Meredith M, Wang Z. Chemosensory cues affect adult neurogenesis in the amygdala of prairie voles in a sex-specific manner. Soc Behav Neuroendocrinol Abstr. 2007;11:P1. [Google Scholar]
- Liu Y, Lieberwirth C, Jia X, Curtis J, Meredith M, Wang Z. Chemosensory cues affect amygdaloid neurogenesis and alter behaviors in the socially monogamous prairie vole. Eur J Neurosci. 2014;39:1632–1641. doi: 10.1111/ejn.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82:267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czeh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Madison D. An integrated view of the social biology of Microtus pennsylvanicus. Biologist. 1980a;62:20–33. [Google Scholar]
- Madison DM. Movement indicators of reproductive events among female meadow voles as revealed by radiotelemetry. J Mammal. 1978;59:835–843. [Google Scholar]
- Madison DM. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol. 1980b;7:65–71. [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire B, Novak M. A comparison of maternal behaviour in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster) and pine vole (M. pinetorum) Anim Behav. 1984;32:1132–1141. [Google Scholar]
- Ming G-l, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA. Oxytocin in general anxiety and social fear: A translational approach. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Oboti L, Schellino R, Giachino C, Chamero P, Pyrski M, Leinders-Zufall T, Zufall F, Fasolo A, Peretto P. Newborn interneurons in the accessory olfactory bulb promote mate recognition in female mice. Front Neurosci. 2011;5:113–126. doi: 10.3389/fnins.2011.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazabal D, Young L. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Oliveras D, Novak M. A comparison of paternal behaviour in the meadow vole Microtus pennsylvanicus, the pine vole M. pinetorum and the prairie vole M. cchrogaster. Anim Behav. 1986;34:519–526. [Google Scholar]
- Ormerod BK, Galea LAM. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–28. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TTY, Galea LAM. Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience. 2004;128:645–654. doi: 10.1016/j.neuroscience.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li M, Lieberwirth C, Wang Z, Zhang Z. Social defeat and subsequent isolation housing affect behavior as well as cell proliferation and cell survival in the brains of male greater long-tailed hamsters. Neuroscience. 2014;265:226–237. doi: 10.1016/j.neuroscience.2014.01.056. [DOI] [PubMed] [Google Scholar]
- Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. A stereotaxic atlas of the rat brain. New York: Academic; 1998. [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Dewsbury DA. Differences in affiliative behavior, pair bonding, and vaginal cytology in two species of vole (Microtus ochrogaster and M. montanus) J Comp Psychol. 1990;104:268. doi: 10.1037/0735-7036.104.3.268. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2001;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith MT, Pencea V, Wang Z, Luskin MB, Insel TR. Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm Behav. 2001;39:11–21. doi: 10.1006/hbeh.2000.1630. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol Behav. 2005;86:369–378. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen CA, Hoogendijk WJG, Smit AB, Lucassen PJ, Spijker S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33:1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pan Y, Wang Z, Zhang Z. Species Differences in the Immunoreactive Expression of Oxytocin, Vasopressin, Tyrosine Hydroxylase and Estrogen Receptor Alpha in the Brain of Mongolian Gerbils (Meriones unguiculatus) and Chinese Striped Hamsters (Cricetulus barabensis) PLoS One. 2013;8(6):e65807. doi: 10.1371/journal.pone.0065807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–332. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Wilson SC. Parent-young contact in prairie and meadow voles. J Mammal. 1982:300–305. [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Xu L, Pan Y, Young K, Wang Z, Zhang Z. Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton) Neuroscience. 2010;169:1235–1247. doi: 10.1016/j.neuroscience.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Phys C. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]