Abstract
In the mammalian heart, multiple types of K+ channels contribute to the control of cardiac electrical and mechanical functioning through the regulation of resting membrane potentials, action potential waveforms and refractoriness. There are similarly vast arrays of K+ channel pore-forming and accessory subunits that contribute to the generation of functional myocardial K+ channel diversity. Maladaptive remodeling of K+ channels associated with cardiac and systemic diseases results in impaired repolarization and increased propensity for arrhythmias. Here, we review the diverse transcriptional, post-transcriptional, post-translational and epigenetic mechanisms contributing to regulating the expression, distribution and remodeling of cardiac K+ channels under physiological and pathological conditions.
Keywords: myocardial excitability, arrhythmias, microRNAs, transcription factors, long non-coding RNAs, cardiac hypertrophy, heart failure, diabetes
Introduction
Cardiac action potentials are generated by the coordinated activation and inactivation of ion channels conducting depolarizing, inward (Na+ and Ca2+) and repolarizing, outward (K+) currents (Figure 1) [1]. While only a few Na+ and Ca2+ channels account for cardiomyocyte depolarization, multiple types of voltage-gated (Kv) and non-voltage-gated inwardly rectifying (Kir) K+ channels contribute to repolarization, determining action potential amplitudes, durations and waveforms (Figure 1; Table 1) [1]. Myocardial Kv and Kir channels are differentially expressed, resulting in regional- and cell type-specific differences in excitability and action potential waveforms (Figure 1). The expression, distribution, and functioning of Kv and Kir channels are altered in a variety of cardiac and systemic diseases, leading to abnormal myocardial repolarization and increased propensity for life-threatening arrhythmias. A large number of Kv and Kir channel pore-forming (α) and accessory (β) subunits have been identified and the roles of many of these subunits in the generation of native myocardial Kv and Kir channels (Table 1) have been defined [1]. In addition, two pore domain (K2P) K+ channels [2] and small conductance (SK) [3] Ca2+-activated K+ channels have been shown to be expressed and demonstrated to play roles in cardiac electrophysiological functioning. Here, the factors contributing to the diverse properties and functional roles of myocardial K+ channels are reviewed, and the molecular mechanisms contributing to the physiological regulation and pathological remodeling of myocardial K+ channels are discussed.
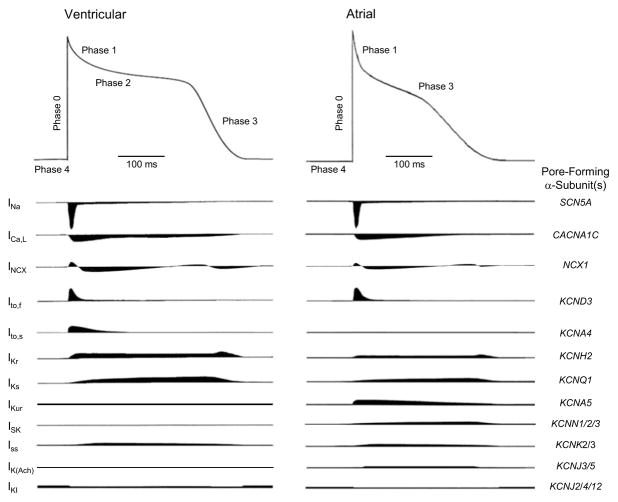
Figure 1. Schematics of action potential waveforms and underlying ionic currents in human ventricular (left) and atrial (right) myocytes.
Note that relative inward (downward) and outward (upward) current densities and waveforms, estimated from voltage-clamp data and modeling studies, in non-diseased ventricular and atrial are shown. The voltage-gated inward Na+ (Nav) and Ca2+ (Cav) currents in human atrial and ventricular myocytes are similar. In contrast, there are multiple types of outward K+ currents, particularly Kv currents, contributing to atrial and ventricular action potential repolarization. In addition, the time- and voltage-dependent properties of the various Kv currents are distinct. Differences in the densities and in the detailed time- and voltage-dependent of the repolarizing Kv and Kir channels contribute to differences in the waveforms of atrial and ventricular action potentials. The genes encoding the pore-forming (α) subunits underlying the various cardiac ion channels are also indicated (on the right).
Table 1.
Potassium Conductances in the Mammalian Myocardium
| Channel Type | Current Name | Activation | Gating | Function | Pharmacology1 | α Subunit Gene | Chromosomal Location | Auxiliary Subunits |
|---|---|---|---|---|---|---|---|---|
| Kv | Ito,f | fast | Voltage | Plateau Potential, Repolarization | mM 4-AP HaTX HpTX Ba2+ |
KCND3 | 1p13.3 | KChIP2, DPP6/10 (minK, MiRP2/3) |
| Ito,s | fast | Voltage | Plateau Potential, Repolarization | μ4-AP | KCNA4 | 11p14 | ?? | |
| IKr | fast | Voltage | Plateau Potential, Repolarization | E-4031 Dofetilide |
KCNH2 | 7q36.1 | minK, MiRP1/2 | |
| IKs | slow | Voltage | Repolarization | NE-10064 NE-10133 |
KCNQ1 | 11p15.5 | minK | |
| IKur | fast | Voltage | Repolarization | μM 4-AP | KCNA5 | 12p13 | SAP97 | |
| IK,slow22 | slow | Voltage | Repolarization | mM TEA | Kcnb1 | 2H3 | Amigo? | |
| K2P | Iss2 | – | H+, Fatty Acids, Anesthetics | Resting potential, Repolarization, Diastolic Potential | mM TEA A1899 |
Kcnk2/3 | 1q41, 2p23 | ?? |
| IKp | – | ?? | Resting Potential, Repolarization | Ba2+ | ?? | ?? | ?? | |
| KCa | ISK | slow | Ca2+-Calmodulin | Repolarization | Apamin | KCNN1/2/3 | 19p13.1, 5q22.2,1q21.3 | ?? |
| KNa | INaK | fast | Na+ | ??3 | Quinidine Clofilium |
KCNT1/2 | 9q34.3, 1q31.3 | ?? |
| Kir | IKI | – | Spermines Mg2+ | Resting Potential, Diastolic Potential | Ba2+ | KCNJ2/4/12 | 17q24.1, 17p11.2 22q13.1 | ?? |
| IK(Ach) | – | Ach | Resting Potential, Diastolic Potential | Tertiapin-Q | KCNJ3/5 | 2q24.1, 11q24 | ?? | |
| IK(ATP) | – | ATP ADP |
??3 | SURs | KCNJ8/11 | 12p11.23, 11p15.1 | SUR1/2 |
4-AP=4-aminopyridine; HaTX=hanatoxin; HpTX=heteropodatoxin; TEA=tetraethylammonium; SURs=sulfonylureas;
Current found in rodent, not clear if also present in other species, including human.
Suggested to function to hyperpolarize membrane potential with ischemia or metabolic stress.
Molecular Determinants of Myocardial K+ Channel Diversity
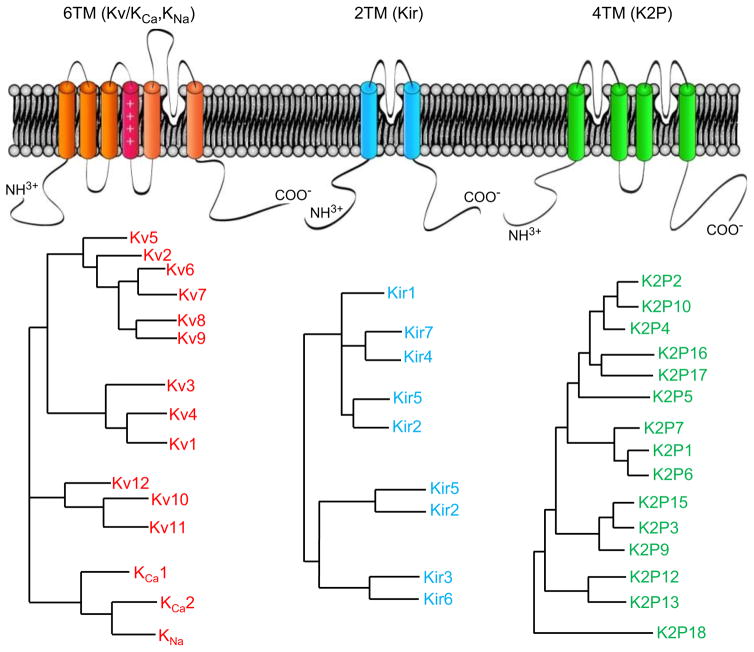
Although the hyperconserved (GYGD) sequence that underlies K+ selectivity is a common feature of all K+ channels, the activation, inactivation, and regulatory mechanisms vary markedly among different types of K+ channels. The common ancestor of prokaryotic and eukaryotic K+ channels formed a primitive channel structure with two transmembrane domains (TM) [4], which has evolved into over 100 different K+ channel pore-forming (α) subunit genes (Figure 2) through extensive gene duplication and divergence; more than 40 K+ channel α subunit genes are expressed in the heart [1]. There are three types of K+ channel α subunits: (1) the six transmembrane-domain (6-TM) family, which includes Kv and SK channels; (2) the two-transmembrane-domain (2-TM) Kir channels; and, (3) the four-transmembrane-domain (4-TM) K2P channels (Figure 2). Some K+ channel α subunit genes undergo alternative splicing [5] and different K+ channel α subunits in the same subfamily can, in principle, heteromultimerize, thereby increasing the potential for functional K+ channel diversity.
Figure 2. Pore-forming subunits of cardiac Kv, Kir and K2P channels.
(A) Schematics illustrate the membrane topologies of Kv, Kir and K2P pore-forming (α) subunits. (B) Phylogentic dendrograms of K+ channel α subunits of the Kv (KCA), Kir and K2P subfamilies.
In addition to the pore-forming α subunits, multiple types of cytosolic and transmembrane K+ channel accessory subunits, including Kvβ subunits, minK and minK-related proteins (MiRPs), K+ channel interacting proteins (KChIPs), K+ channel associated protein (KChAP) and the membrane-associated guanylate kinase homologs (MAGUK proteins), have been identified [1]. The various K+ channel accessory subunits contribute to regulating trafficking, membrane anchoring, organization and biophysical properties of assembled, functional K+ channel complexes.
Myocardial K+ Channel Regulation
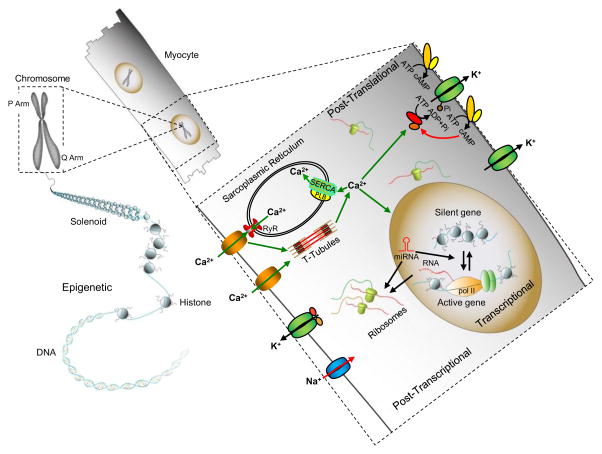
Multiple mechanisms contribute to the regulation of K+ channel expression and functioning in cardiomyocytes (Figure 3).
Figure 3. Multiple mechanisms contribute to the regulation of myocardial K+ channel expression, localization and properties.
Myocardial K+ channel expression and function are regulated by transcriptional, post-transcriptional, post-translational and epigenetic mechanisms. Various transcription factors and signaling pathways are involved in regulating the temporal and spatial expression of K+ channels during cardiac development and in response to cardiac injury or illness. Alternative splicing, RNA editing and K+ channel-targeting miRNAs contribute to the post-transcriptional regulation of K+ channels. Post-translational modifications, including phosphorylation, sumoylation, palmitoylation and glycosylation also contribute to the dynamic regulation of K+ channel trafficking and functioning. Myocardial Ca2+ homeostasis also impacts K+ channel expression and properties by modulating Ca2+-sensitive transcriptional programs and Ca2+-dependent enzymes, including protein kinases and phosphatases. The membrane lipid environment also modulates myocardial K+ channel functions. Although less well studied, it is increasingly clear that epigenetic mechanisms also contribute to the regulation of cardiac K+ channel gene expression.
Transcriptional Regulation of Myocardial K+ Channels
Transcriptional mechanisms control the temporal and spatial expression of cardiac K+ channels during development and in response to cardiac damage or disease. The fast component of the Kv4-encoded transient outward current, Ito,f, for example, is expressed at higher densities in epicardial than in endocardial myocytes, resulting in the transmural repolarization gradient that is critical for normal cardiac electrical and contractile and function [6]. The transmural Ito,f gradient has also been suggested to underlie the ST-segment elevation of the Brugada type ECG pattern [7]. In the mouse, the homeodomain transcription factor Irx5, which is expressed in a gradient opposite that of Kv4.2, represses Kv4.2 mRNA expression by recruiting the cardiac transcriptional repressor mBop [8]. Deletion of Irx5 results in the selective increase in Kv4.2 (and Ito,f) in endocardial myocytes, flattening the transmural repolarization gradient [8].
The densities of the transient outward current, Ito,f, and of the delayed rectifier currents, IKs and IKr, are higher in right (RV), compared with left (LV), ventricles, contributing to differences in action potential durations in RV (shorter) and LV (longer) myocytes [9]. The expression levels of the transcripts encoding the IKs and IKr channel α subunit proteins, KvLQT1 and HERG1, respectively, are also higher in RV than in LV [10]. It has been reported that the transcription factor Sp1 modulates the activities of the KCNQ1 (which encodes KvLQT1) and KCNH2 (which encodes HERG1) promoters, suggesting that the differential expression of Sp1 (RV>LV) may account for the interventricular gradients of KvLQT1 and HERG1 protein expression [10, 11].
The expression of IK,ATP also exhibits atrioventricular and interventricular gradients: the Kir 6.1 and SUR1A mRNAs are 4- to 12-fold higher in the atria than in ventricle, and SUR2B mRNA is 6-fold higher in RV than in LV [12]. The forkhead transcription factors FoxO1, FOXO3 and FOXF2 modulate the expression levels of IK,ATP channel subunits, contributing to the observed regional differences. These transcription factors also coordinate the expression of genes encoding IK,ATP channel subunits and genes involved in glycolysis and β-oxidation [12]. The transcriptional activity of Kcnj2, which encodes inwardly rectifying IK1 channel subunit Kir2.1, is regulated transcription factors Sp1, Sp3 and NF-Y [13]. Importantly, several transcriptional regulators, including T-box-containing transcriptional repressors (Tbx3, Tbx5 and Tbx18) and Notch, play critical roles in programming specific cardiac cell types. For example, Tbx18 guides the formation of sinus node head from mesenchymal precursors, followed by Tbx3-mediated pacemaker gene programming [14]. In addition, Tbx5 cooperates with Nkx2.5 to modulate the expression of Id2, a transcription factor that promotes ventricular conduction system differentiation [15]. Myocardial Notch signaling, on the other hand, promotes the differentiation of chamber cardiac progenitors into specialized conduction system-like cells [16].
In addition to transcription factors, multiple signaling pathways have been implicated in the transcriptional control of K+ channel expression. Phosphoinositide 3-kinase alpha (PI3Kα) signaling activation, for instance, has been shown to upregulate multiple cardiac K+ channel transcriptionally through an Akt-independent mechanism [17]. The calcium-activated phosphatase, calcineurin, increases the transcriptional activity of Kcnd2 (Kv4.2) and Ito,f density in neonatal rat ventricular myocytes by activating nuclear factors of activated T-cells (NFATs) [18]. In vivo overexpression of calcineurin in the mouse heart, however, downregulates Ito,f and IK,slow, which can be reversed by the treatment of the calcineurin inhibitor, cyclosporin A [19].
Post-Transcriptional Regulation of Myocardial K+ Channels
Following synthesis by RNA polymerase II, the primary transcripts (pre-mRNAs) of most eukaryotic genes are extensively processed through 5′ capping, 3′ polyadenylation, RNA editing and splicing [20]. These pre-mRNA processing steps are coupled spatially and temporally and determine the fate of the transcript, affecting the nuclear export, translation, localization and stability of the mature mRNAs and resulting in the rapid and efficient fine tuning of gene expression levels [20]. Although there are few reports demonstrating a functional role for RNA editing of myocardial K+ channel subunit transcripts, the expression levels of K+ channel subunit transcripts and the properties of the resulting proteins have been shown to be regulated by RNA editing. The inactivation properties of the channels formed by human Kv1.1, for example, are controlled by adenosine-to-inosine RNA editing mediated by human adenosine deaminase acting on RNA-2 [21].
In contrast, abnormal K+ channel subunit gene splicing has been implicated in human cardiac arrhythmias. Mutation-mediated splicing errors in KCNQ1, for example, lead to reduced IKs and type 1 Long QT syndrome (LQT1) [22, 23]. Similarly, abnormal splicing of KCNH2, resulting from intronic branch point [24] or 5′ splice site [25] mutations, impairs the functioning of the resulting HERG-encoded IKr channels, causing type 2 Long QT syndrome (LQT2).
MicroRNAs (miRs) (Figure 3) are small non-coding RNAs that regulate gene expression by targeting 3′ untranslated regions (3′ UTRs) to impede target protein translation or enhance target mRNA degradation [26]. Several miRs have been shown to contribute to post-transcriptional regulation of cardiac K+ channels. One of the muscle-specific miRs, miR-1, for instance, has been shown to regulate Ito,f by targeting the Kcnd2-repressing transcription factor Irx5 [27]. Targeted deletion of miR-1 in mouse heart leads to increased Irx5 expression, resulting in reduced Kcnd2 expression and impaired repolarization [27]. In addition, miR-1 is known to target Kcnj2 (Kir2.1) and the dysregulation of miR-1 has been shown to contribute to post-myocardial infarction (MI) arrhythmias (miR-1 up, Kir2.1 down) [28] and atrial fibrillation (miR-1 down, Kir2.1 up) [29]. Increased expression of another muscle-specific miR, miR-133a, has been demonstrated to prolong QT intervals by reducing KChIP2 levels and attenuating Ito,f [30]. Increased miR-212 expression is often observed with heart failure, and the upregulation of miR-212 has been reported to reduce Kir2.1 and IK1 density [31].
Post-Translational Regulation of Myocardial K+ Channels
K+ channel subunit proteins are subjected to post-translational modifications such as phosphorylation, sumoylation, palmitoylation and glycosylation, all of which have been implicated in the dynamic regulation of K+ channel trafficking, stability or function [32–34]. Of these, phosphorylation (Figure 3) is the most studied, and effects on K+ channel gating and trafficking have been described [32]. Both Kv4.2 and Kv4.3, the pore-forming α subunits underlying Ito,f, for example, are phosphorylated by calmodulin-dependent protein kinase II (CaMKII) in cardiomyocytes [35, 36], and the inhibition of CaMKII accelerates inactivation of human atrial Ito,f [37]. Kv4.2- and Kv4.3-encoded currents expressed in Xenopus oocytes and native Ito,f in adult rat epicardial myocytes are suppressed by PKC activation [38]. The activity of Kir6.2-encoded K+ channels is increased by PKC-dependent phosphorylation, through stabilization of the open state, in the presence and in the absence of the auxiliary subunit SUR2A [39]. Tyrosine kinase c-Src-mediated phosphorylation has also been shown to suppress Kv1.5-encoded currents [40], presumably by destabilizing channel complexes in the plasma membrane [41] and enhancing channel endocytosis [42].
Post-translational modification of Kv1.5 by small ubiquitin-like modifier (SUMO) proteins also modulates the properties of Kv1.5-encoded K+ channels; loss of Kv1.5 sumoylation, for example, results in a hyperpolarizing shift in the voltage-dependence of steady-state inactivation of Kv1.5-encoded currents [43]. SUMO modification has also been shown to shift the voltage-dependence of activation of currents encoded by other Kv α subunits, including Kv2.1 [33]. Palmitoylation also regulates Kv1.5 channel trafficking; S-palmitoylation occurs early during Kv1.5 biosynthesis and the inhibition of palmitoylation leads to accumulation of Kv1.5 in intracellular compartments, increased degradation, and diminished cell surface Kv1.5 protein expression [44]. Palmitoylation of KChIP2 appears to also be essential for its membrane localization and for augmentation of Kv4.2- and Kv4.3-encoded currents [34]. Glycosylation has also been shown to modulate the stability of K+ channel subunit proteins, including HERG [45] and Kv1.x [46].
Effects of Lipid Environment on Myocardial K+ Channels
In addition to the regulatory mechanisms affecting the expression levels and intrinsic properties of K+ channels, various extrinsic factors, such as membrane lipids and redox environment, also contribute to the modulation of functional K+ channels. Membrane stiffness, for example, which is determined by membrane lipid composition, has been shown to affect the open probabilities of Kv and Kir channels [47, 48]. Arachidonic acid, which is abundant in plasma membrane lipids, speeds inactivation of otherwise non-inactivating delayed rectifier Kv channels [49]. Cholesterol, on the other hand, directly inhibits Kir2.1 channels by stabilizing closed states [50]. In addition, depletion of membrane cholesterol increases the recruitment of Kv1.5 channels to the plasma membrane through Rab11-mediated channel recycling [51]. Lipid rafts and caveolae, two specialized membrane lipid structures, function as platforms for clustering of K+ channels and signaling molecules in macromolecular complexes, thereby modulating K channel properties [52, 53].
Epigenetic Regulation of Myocardial K+ Channels
Epigenetic mechanisms (Figure 3) modulate physiological trait variations without changes in DNA sequence and result from changes in DNA methylation, chromatin remodeling or histone modification [54]. Importantly, these changes can be heritable or acquired [54], and reflect interactions between the environment and gene expression. Considerable evidence suggests that epigenetic mechanisms are involved in the regulation of the expression of cardiac K+ channel subunit genes. Inducible cardiac ablation of PAX-interacting protein 1 (PTIP), a key component of the histone H3 lysine 4 (H3K4me) complex, for example, has been demonstrated to increase the transcript expression levels of the genes, Kcnip2, Kcnd2 and Kcnd3, encoding Ito,f channel subunits [55]. The protein expression levels of KChIP2 (Kcnip2), but not Kv4.2 (Kcnd2) or Kv4.3 (Kcnd3), were reported to be reduced significantly in PTIP−/− mouse hearts, leading to decreased Ito,f densities and action potential prolongation, as well as impaired calcium handling and contractility [55]. Promoter DNA methylation modulates the expression of the IKATP channel subunits, SUR1 and SUR2, in mouse cardiomyocytes [56]. The extent of promoter CpG methylation inversely correlates with the expression of SUR1 and SUR2, and treatment with the DNA methylation inhibitor 5′-Aza-2′ deoxycytidine significantly reduced both methylation at the SUR2 CpG island and SUR2A mRNA expression [56]. In addition, KCNQ1 is located in an imprinted gene cluster, where a paternally expressed anti-sense transcript, KCNQ1ot1, transcribed from a promoter located in intron 10 of the KCNQ1 gene, represses the expression of the KCNQ1 imprinted gene cluster [57]. The promoter region of KCNQ1ot1 contains a CpG island and is methylated on the maternal chromosome, thereby preventing KCNQ1ot1 expression and allowing the KCNQ1 gene cluster to be transcribed from the maternal allele [57]. These data suggest that variable KCNQ1 imprinting or mutations affecting the CpG island in the KCNQ1ot1 promoter could potentially contribute to LQT1 in the absence of mutations in the coding region of KCNQ1.
Myocardial K+ Channel Remodeling in Acquired Cardiac Disease
Marked changes in the densities and/or properties of myocardial K+ currents, typically referred to as K+ channel remodeling, are observed in association with both cardiac and systemic diseases, including cardiac hypertrophy, heart failure, atrial fibrillation, diabetes and in the ischemic myocardium (Table 2).
Table 2.
Remodeling of Myocardial K+ Currents in the Diseased Heart
| Ionic Current | Disease | Change | Observed Cardiac Effect(s) | Molecular Mechanism(s) |
|---|---|---|---|---|
| Ito,f | HF, MI, LVH | ↓ | APD prolongation; EADs | Transcriptional & post-transcriptional |
| AF | ↓ | APD shortening | Transcriptional & post-transcriptional | |
|
| ||||
| IK | HF, MI, LVH | ↓ | APD prolongation; EADs; DADs | Transcriptional & post-transcriptional |
| AF | Variable | APD shortening | Transcriptional & post-transcriptional | |
|
| ||||
| IK1 | HF, MI, LVH | ↓ | APD prolongation; EADs | Transcriptional & post-transcriptional |
| AF | ↑ | APD shortening; Vm ↓ | Transcriptional | |
|
| ||||
| IKATP | MI | ↑ | APD shortening; Conduction slowing | ATP depletion and acidosis ↑ P0 |
|
| ||||
| ISK | HF, MI | ↑ | APD shortening | Channel sensitivity to Ca2+ ↑ |
|
| ||||
| IKACh | AF | ↑ | APD shortening | ↑ P0 |
Ito,f: fast transient outward K+ current; IK: delayed rectifier K+ currents; IK1: inwardly rectifying K+ current; IKATP: ATP-sensitive K+ current; ISK: small conductance Ca2+-activated K+ currents; IKACh: acetylcholine activated K+ current; HF: heart failure; MI: myocardial infarction; LVH: pathological left ventricular hypertrophy; AF: atrial fibrillation; APD: action potential duration; Vm: membrane potential; EAD: early after-depolarization; DAD: delayed after-depolarization; P0: channel open probability.
K+ Channel Remodeling in Cardiac Hypertrophy
Cardiac hypertrophy, defined as ventricular wall thickening and enlargement of the heart, is associated with increased cardiomyocyte size secondary to pathological stresses (valvular heart disease, hypertension, myocardial infarction etc.), physiological loading (exercise training or pregnancy), and sarcomeric protein mutations [58]. Cardiac hypertrophy induced by pathological stresses (pathological hypertrophy) is associated with increased risk of ventricular arrhythmias and sudden death. In contrast, exercise training-induced physiological hypertrophy is not associated with electrical abnormalities or increased arrhythmia risk [59].
Pathological hypertrophy results in prolonged ventricular action potential durations and increased dispersion of repolarization, changes that are evident (prolonged QT internal and increased QT dispersion) in surface ECG recordings [60] and that reflect, at least in part, reduced repolarizing K+ current densities [61]. Impaired repolarization and increased dispersion are arrhythmogenic [61, 62]. Recent studies in a mouse model of pressure overload-induced left ventricular hypertrophy (LVH), produced by transverse aortic constriction, revealed that the repolarizing K+ current densities are reduced because K+ channel subunit expression levels are not increased in proportion with cellular hypertrophy, i.e., the increase in myocyte size [62]. We have recently demonstrated that, in marked contrast with pathological hypertrophy, physiological cardiac hypertrophy, induced by chronic exercise (swim) training or cardiac specific transgenic expression of constitutive active PI3Kα, is associated with increases in myocardial repolarizing K+, as well as depolarizing Na+ and Ca2+, currents, reflecting upregulation of the transcripts encoding the underlying channel subunits [63]. The transcriptional upregulation of ion channel subunits with physiological hypertrophy is in proportion to increased myocyte size and the global increases in RNA and protein expression, resulting in the normalization of current densities, action potential waveforms and myocardial functioning [63]. Interestingly, further experiments revealed that exercise training and enhanced PI3Kα signaling-mediated transcriptional upregulation of myocardial ion channel subunits is independent of cellular hypertrophy and Akt signaling [17].
K+ Channel Remodeling in Heart Failure
Heart failure, irrespective of the underlying etiology, is associated with increased risk of life-threatening arrhythmias and the incidence of sudden cardiac death in individuals with heart failure is estimated at a staggering 50% [64]. The increased incidence of lethal ventricular arrhythmias in heart failure is a consequence of complex pathological remodeling of cardiac structural [65], neurohumoral [66] and electrophysiological properties [67]. Electrical remodeling in the failing heart [67] reflects, at least in part, reductions in the densities of repolarizing K+ currents, including Ito,f [67, 68], IKs, IKr [69] and IK1 [67, 70], resulting in action potential prolongation, early afterdepolarizations and increased dispersion, all of which are arrhythmogenic. Activation of pathological signaling cascades in the failing heart results in decreased repolarizing K+ current amplitudes/densities, attributed to transcriptional and post-transcriptional downregulation of channel subunit expression [71]. Ca2+/calmodulin-dependent protein kinase II (CaMKII), for example, is chronically activated in heart failure; increased CaMKII activity has been shown to reduce functional Ito,f density through multiple mechanisms, including transcriptional downregulation of KCND3 (Kv4.3) [72] and changes in the kinetics of Ito,f inactivation [36]. Interestingly, the small conductance Ca2+-activated K+ current (ISK), which is not normally expressed in ventricular myocytes, has been shown to be unpregulated in the ventricles of failing hearts [73] and following myocardial infarction [74]. In addition, pharmacological blockade of ISK has been shown to suppress ventricular arrhythmias associated with both heart failure [73] and acute myocardial infarction [74].
K+ Channel Remodeling in Atrial Fibrillation
Atrial fibrillation (AF) is one of the most common arrhythmias seen in clinical practice, and the prevalence of AF increases with age, rising from the <1% in individuals < 60 years to ~20% among those 85 years or older [75]. AF dramatically impacts morbidity and mortality, and yet current therapeutic options are very limited. The rapid atrial rate during AF induces electrical remodeling that further potentiates the generation and maintenance of AF, a fact that led to the concept that “AF begets AF” [76]. Altered expression and functioning of multiple K+ channels are observed with AF-induced electrical remodeling. The transcript and protein expression levels of Kir2.1, for example, are increased in AF [77], accompanied by increased IK1 densities and hyperpolarized membrane potentials [78]. Another inwardly rectifying K+ current, IKACh (produced by Kir3.1 and Kir3.4), which is activated by muscarinic receptors in response to increased vagal input, is also enhanced in AF. The increase in IKACh in AF, however, is attributed to changes in channel open probability, not to alterations in channel subunit expression levels [79]. Increased IKACh contributes to action potential shortening and to reentrant rotor stabilization during AF [80]. The density of Ito,f and the expression of KCND3/Kv4.3 mRNA/protein are reduced with AF [78, 81]. There are also reports of remodeling of delayed rectifier K+ currents, such as IKur, IKr and IKs, although the effects described are variable and somewhat controversial.
K+ Channel Remodeling in Diabetes
Abnormal cardiac repolarization, QT-interval prolongation and T wave abnormalities are observed in some patients with diabetes [82]. Studies focused on defining the cellular and molecular mechanisms of diabetes-induced repolarization abnormalities have consistently shown downregulation of repolarizing Kv currents [83, 84]. Post-translational effects on Kv channels by increased levels of free fatty acid metabolites, such as palmitoylcarnitine and palmitoyl-CoA, has been described [85]. Acute treatment with insulin has been shown to reverse diabetes-induced Kv current remodeling, suggesting a critical role for insulin-dependent regulation of cardiac Kv channel functioning [83]. In addition, the peroxisome proliferator-activated receptor α (PPARα), a critical regulator of glucose/fatty acid metabolism, has been shown to be upregulated in the heart in diabetes and to result in the transcriptional downregulation of cardiac Kv channel subunit expression [86]. Consistent with this model, cardiac-specific over-expression of PPARα downregulates the transcript/protein expression levels of the Ito,f channel subunits Kcnd2/Kv4.2 and Kcnip2/KChIP2 [86], whereas targeted deletion of PPARα upregulates Ito,f [86].
K+ Channel Remodeling in Myocardial Ischemia and Infarction
Myocardial ischemia leads to ATP depletion and acidosis [87], and the activation of sarcolemmal IKATP channels. The opening of IKATP channels shortens action potential durations and decreases inward Ca2+ currents, thereby reducing Ca2+-mediated energy consumption and preventing Ca2+ overload-induced cell death. Increased IKATP channel opening, however, also results in cardiomyocyte hyperpolarization and renders cells inexcitable [88], creating a current sink that slows or blocks propagation and predisposes the heart to the development of ventricular arrhythmias [89, 90]. Pharmacological inhibition of IKATP channels in the ischemic heart reduces the incidence of ventricular arrhythmias in animal models and in humans [91, 92].
Myocardial infarction (MI) is associated with marked changes in K+ channel expression and functioning, and is also associated with increased arrhythmia risk. In a canine model of myocardial infarction, multiple K+ currents, Ito,f [93], IKr, IKs [94] and IK1 [95] are downregulated in cells in the infarct border zone. The downregulation of Ito,f is most noticeable during the acute phase (within days) following the infarct and appears to resolve over the course of 2 months [93]. The expression levels of the transcripts encoding IKr (HERG) and IKs (KvLQT1 and minK) channels are also decreased in day 2 post-infarct border-zone myocytes; the expression levels of HERG and KvLQT1, but not minK, recover by day 5 [94, 96]. The finding that KvLQT1, but not minK, expression has recovered may contribute to the observed rapid kinetics of activation of IKs that is observed in post-infarct border-zone myocytes [96]. In the myocardium distant to the infarct area (remote-zone), multiple K+ currents are also altered, likely reflecting the effects of myocyte hypertrophy or failure following the infarct. The densities of the currents, as well as the expression levels of subunits underlying Ito,f, IK1 and IK, for example, are consistently downregulated in remote-zone myocytes, leading to prolonged APD [97].
Perspective: Long non-coding RNAs as Potential Myocardial K+ Channel Regulators
It has become increasingly clear that the transcription of the eukaryotic genome is far more pervasive and complex than previously appreciated [100]. While the expression of mRNAs and miRNAs account for only ~1% of all transcribed species, up to 90% of the mammalian genome is transcribed as long non-coding RNAs (lncRNAs), a heterogeneous group of non-coding transcripts longer than 200 nucleotides [100]. LncRNAs have been shown to be functional and involved in specific physiological and pathological processes through epigenetic, transcriptional and posttranscriptional mechanisms. Although the roles of lncRNAs in various biological processes are beginning to emerge, our understanding of the functioning of lncRNAs in the cardiovascular system remains in its infancy.
Several antisense lncRNAs have been implicated in regulating genes that are critical for cardiac function, including cardiac troponin I [101] and myosin heavy/light chains [102]. A cardiac-specific lncRNA, Braveheart, for example, has been demonstrated to be essential for the epigenetic regulation of cardiac lineage commitment in mouse embryonic stem cells [103]. Recently, a cardiac-specific lncRNA Myheart (Mhrt), originating from MYH7 loci, was shown to be protective against stress-induced (pathological) cardiac hypertrophy in adult mouse heart by a mechanism involving ATP-dependent chromatin remodeling [104]. Aside from the anti-sense transcript of KCNQ1, KCNQ1ot1, which, as discussed above, represses the expression of the KCNQ1 imprinted gene cluster [57], there is little information available on lncRNA-mediated regulation of cardiac K+ channel expression or function. Indeed, there have been few, if any, studies focused on examining the role(s) of lncRNAs in the regulation of myocardial K+ (or other) channels. With more cardiac lncRNA expression profiling data becoming available and the advances in technologies to facilitate studies of lncRNA structure and functioning, it seems reasonable to expect that insights into lncRNA-mediated regulation of myocardial K+ channels will be forthcoming in the near future.
Conclusion
Electrophysiological and molecular studies have demonstrated that the K+ channels that underlie action potential repolarization in the mammalian heart are far more numerous and diverse than depolarizing Na+ or Ca2+ channels (Figure 1; Table 1). Accumulating evidence indicates that the expression and the functioning of myocardial K+ channels are regulated by transcriptional, post-transcriptional, post-translational, and epigenetic mechanisms (Figure 3). In addition to controlling repolarization, the molecular and functional diversity of myocardial K+ channels underlie regional differences in excitability, action potential propagation and the maintenance of normal cardiac rhythms. Maladaptive K+ channel remodeling and impaired channel function, associated with inherited and acquired cardiac and systemic diseases, can impair repolarization and increase the risk of potentially life-threatening arrhythmias. Advancing our understanding of the mechanisms involved in K+ channel regulation and modulation in cardiac physiology and pathology is the key to developing new therapeutic strategies to treat or prevent lethal arrhythmias associated with cardiac/systemic disease.
Acknowledgments
The authors wish to thank Rick Wilson for assistance with the generation of the Table and Figures included and this review and the US National Institutes of Health (HL034161 and HL066388 to JMN), the Taiwan Ministry of Science and Technology (MOST103-2320-B-002-068-MY2 to KCY) and the Taiwan National Health Research Institute (Career Development Grant NHRI-EX104-10418SC to KCY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–53. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 2.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38(3):305–18. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 3.Mahida S. Expanding role of SK channels in cardiac electrophysiology. Heart Rhythm. 2014;11(7):1233–8. doi: 10.1016/j.hrthm.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 4.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 5.Attali B, et al. Multiple mRNA isoforms encoding the mouse cardiac Kv1-5 delayed rectifier K+ channel. J Biol Chem. 1993;268(32):24283–9. [PubMed] [Google Scholar]
- 6.Nerbonne JM, Guo W. Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol. 2002;13(4):406–9. doi: 10.1046/j.1540-8167.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoogendijk MG, et al. The Brugada ECG pattern: a marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the Brugada syndrome. Circ Arrhythm Electrophysiol. 2010;3(3):283–90. doi: 10.1161/CIRCEP.110.937029. [DOI] [PubMed] [Google Scholar]
- 8.Costantini DL, et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005;123(2):347–58. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volders PG, et al. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99(2):206–10. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- 10.Luo X, et al. Genomic structure, transcriptional control, and tissue distribution of HERG1 and KCNQ1 genes. Am J Physiol Heart Circ Physiol. 2008;294(3):H1371–80. doi: 10.1152/ajpheart.01026.2007. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, et al. Transcriptional activation by stimulating protein 1 and post-transcriptional repression by muscle-specific microRNAs of IKs-encoding genes and potential implications in regional heterogeneity of their expressions. J Cell Physiol. 2007;212(2):358–67. doi: 10.1002/jcp.21030. [DOI] [PubMed] [Google Scholar]
- 12.Philip-Couderc P, et al. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res. 2008;102(2):e20–35. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- 13.Redell JB, Tempel BL. Multiple promoter elements interact to control the transcription of the potassium channel gene, KCNJ2. J Biol Chem. 1998;273(35):22807–18. doi: 10.1074/jbc.273.35.22807. [DOI] [PubMed] [Google Scholar]
- 14.Wiese C, et al. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res. 2009;104(3):388–97. doi: 10.1161/CIRCRESAHA.108.187062. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz IP, et al. A molecular pathway including Id2, Tbx5, and Nkx2-5 required for cardiac conduction system development. Cell. 2007;129(7):1365–76. doi: 10.1016/j.cell.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Rentschler S, et al. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation. 2012;126(9):1058–66. doi: 10.1161/CIRCULATIONAHA.112.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang KC, Tseng YT, Nerbonne JM. Exercise training and PI3Kalpha-induced electrical remodeling is independent of cellular hypertrophy and Akt signaling. J Mol Cell Cardiol. 2012;53(4):532–41. doi: 10.1016/j.yjmcc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong N, et al. Calcineurin increases cardiac transient outward K+ currents via transcriptional up-regulation of Kv4.2 channel subunits. J Biol Chem. 2006;281(50):38498–506. doi: 10.1074/jbc.M607774200. [DOI] [PubMed] [Google Scholar]
- 19.Dong D, et al. Overexpression of calcineurin in mouse causes sudden cardiac death associated with decreased density of K+ channels. Cardiovasc Res. 2003;57(2):320–32. doi: 10.1016/s0008-6363(02)00661-2. [DOI] [PubMed] [Google Scholar]
- 20.Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet. 2014;15(3):163–75. doi: 10.1038/nrg3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhalla T, et al. Control of human potassium channel inactivation by editing of a small mRNA hairpin. Nat Struct Mol Biol. 2004;11(10):950–6. doi: 10.1038/nsmb825. [DOI] [PubMed] [Google Scholar]
- 22.Murray A, et al. Splicing mutations in KCNQ1: a mutation hot spot at codon 344 that produces in frame transcripts. Circulation. 1999;100(10):1077–84. doi: 10.1161/01.cir.100.10.1077. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji K, et al. Mechanistic basis for the pathogenesis of long QT syndrome associated with a common splicing mutation in KCNQ1 gene. J Mol Cell Cardiol. 2007;42(3):662–9. doi: 10.1016/j.yjmcc.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Crotti L, et al. A KCNH2 branch point mutation causing aberrant splicing contributes to an explanation of genotype-negative long QT syndrome. Heart Rhythm. 2009;6(2):212–8. doi: 10.1016/j.hrthm.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Berthet M, et al. C-terminal HERG mutations: the role of hypokalemia and a KCNQ1-associated mutation in cardiac event occurrence. Circulation. 1999;99(11):1464–70. doi: 10.1161/01.cir.99.11.1464. [DOI] [PubMed] [Google Scholar]
- 26.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129(2):303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Yang B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13(4):486–91. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 29.Girmatsion Z, et al. Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart Rhythm. 2009;6(12):1802–9. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 30.Matkovich SJ, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106(1):166–75. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldoni D, et al. A novel dual-fluorescence strategy for functionally validating microRNA targets in 3′ untranslated regions: regulation of the inward rectifier potassium channel K(ir)2.1 by miR-212. Biochem J. 2012;448(1):103–13. doi: 10.1042/BJ20120578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park KS, et al. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology (Bethesda) 2008;23:49–57. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- 33.Plant LD, et al. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol. 2011;137(5):441–54. doi: 10.1085/jgp.201110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takimoto K, Yang EK, Conforti L. Palmitoylation of KChIP splicing variants is required for efficient cell surface expression of Kv4.3 channels. J Biol Chem. 2002;277(30):26904–11. doi: 10.1074/jbc.M203651200. [DOI] [PubMed] [Google Scholar]
- 35.Colinas O, et al. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2006;291(4):H1978–87. doi: 10.1152/ajpheart.01373.2005. [DOI] [PubMed] [Google Scholar]
- 36.Sergeant GP, et al. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2005;288(2):C304–13. doi: 10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- 37.Tessier S, et al. Regulation of the transient outward K(+) current by Ca(2+)/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85(9):810–9. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura TY, et al. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273(4 Pt 2):H1775–86. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- 39.Light PE, et al. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2000;97(16):9058–63. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holmes TC, et al. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274(5295):2089–91. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 41.Hattan D, et al. Tyrosine phosphorylation of Kv1.2 modulates its interaction with the actin-binding protein cortactin. J Biol Chem. 2002;277(41):38596–606. doi: 10.1074/jbc.M205005200. [DOI] [PubMed] [Google Scholar]
- 42.Nesti E, Everill B, Morielli AD. Endocytosis as a mechanism for tyrosine kinase-dependent suppression of a voltage-gated potassium channel. Mol Biol Cell. 2004;15(9):4073–88. doi: 10.1091/mbc.E03-11-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benson MD, et al. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci U S A. 2007;104(6):1805–10. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, et al. S-acylation regulates Kv1.5 channel surface expression. Am J Physiol Cell Physiol. 2007;293(1):C152–61. doi: 10.1152/ajpcell.00480.2006. [DOI] [PubMed] [Google Scholar]
- 45.Gong Q, et al. Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am J Physiol Heart Circ Physiol. 2002;283(1):H77–84. doi: 10.1152/ajpheart.00008.2002. [DOI] [PubMed] [Google Scholar]
- 46.Khanna R, et al. Glycosylation increases potassium channel stability and surface expression in mammalian cells. J Biol Chem. 2001;276(36):34028–34. doi: 10.1074/jbc.M105248200. [DOI] [PubMed] [Google Scholar]
- 47.Lundbaek JA, et al. Membrane stiffness and channel function. Biochemistry. 1996;35(12):3825–30. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- 48.Long SB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–82. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 49.Oliver D, et al. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304(5668):265–70. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- 50.Epshtein Y, et al. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci U S A. 2009;106(19):8055–60. doi: 10.1073/pnas.0809847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balse E, et al. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci U S A. 2009;106(34):14681–6. doi: 10.1073/pnas.0902809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98(2–3):149–60. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588(Pt 17):3169–78. doi: 10.1113/jphysiol.2010.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egger G, et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–63. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 55.Stein AB, et al. Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J Clin Invest. 2011;121(7):2641–50. doi: 10.1172/JCI44641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fatima N, et al. Promoter DNA methylation regulates murine SUR1 (Abcc8) and SUR2 (Abcc9) expression in HL-1 cardiomyocytes. PLoS One. 2012;7(7):e41533. doi: 10.1371/journal.pone.0041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mancini-DiNardo D, et al. A differentially methylated region within the gene Kcnq1 functions as an imprinted promoter and silencer. Hum Mol Genet. 2003;12(3):283–94. doi: 10.1093/hmg/ddg024. [DOI] [PubMed] [Google Scholar]
- 58.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102(4):470–9. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 59.Biffi A, et al. Relation between training-induced left ventricular hypertrophy and risk for ventricular tachyarrhythmias in elite athletes. Am J Cardiol. 2008;101(12):1792–5. doi: 10.1016/j.amjcard.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 60.Gillis AM, et al. Dispersion of ventricular repolarization and ventricular fibrillation in left ventricular hypertrophy: influence of selective potassium channel blockers. J Pharmacol Exp Ther. 2000;292(1):381–6. [PubMed] [Google Scholar]
- 61.Volk T, et al. Regional alterations of repolarizing K+ currents among the left ventricular free wall of rats with ascending aortic stenosis. J Physiol. 2001;530(Pt 3):443–55. doi: 10.1111/j.1469-7793.2001.0443k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marionneau C, et al. Distinct cellular and molecular mechanisms underlie functional remodeling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102(11):1406–15. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang KC, et al. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010;588(Pt 24):5015–32. doi: 10.1113/jphysiol.2010.197418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomaselli GF, et al. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90(5):2534–9. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 65.Akar FG, et al. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95(7):717–25. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 66.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50(6):404–19. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73(2):379–85. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 68.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42(2):270–83. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 69.Tsuji Y, et al. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res. 2000;48(2):300–9. doi: 10.1016/s0008-6363(00)00180-2. [DOI] [PubMed] [Google Scholar]
- 70.Li GR, et al. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283(3):H1031–41. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- 71.Nass RD, et al. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med. 2008;5(4):196–207. doi: 10.1038/ncpcardio1130. [DOI] [PubMed] [Google Scholar]
- 72.Xiao L, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103(7):733–42. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 73.Chua SK, et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108(8):971–9. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gui L, et al. Ventricular tachyarrhythmias in rats with acute myocardial infarction involves activation of small-conductance Ca2+-activated K+ channels. Am J Physiol Heart Circ Physiol. 2013;304(1):H118–30. doi: 10.1152/ajpheart.00820.2011. [DOI] [PubMed] [Google Scholar]
- 75.Heeringa J, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–53. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 76.Wijffels MC, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 77.Gaborit N, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112(4):471–81. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 78.Van Wagoner DR, et al. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80(6):772–81. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 79.Voigt N, et al. Changes in I K, ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res. 2008;77(1):35–43. doi: 10.1093/cvr/cvm051. [DOI] [PubMed] [Google Scholar]
- 80.Kneller J, et al. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002;90(9):E73–87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- 81.Yue L, et al. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84(7):776–84. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- 82.Veglio M, Chinaglia A, Cavallo-Perin P. QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest. 2004;27(2):175–81. doi: 10.1007/BF03346265. [DOI] [PubMed] [Google Scholar]
- 83.Xu Z, et al. Up-regulation of K(+) channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53(1):80–8. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- 84.Shimoni Y, et al. Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res. 1994;74(4):620–8. doi: 10.1161/01.res.74.4.620. [DOI] [PubMed] [Google Scholar]
- 85.Xu Z, Rozanski GJ. K+ current inhibition by amphiphilic fatty acid metabolites in rat ventricular myocytes. Am J Physiol. 1998;275(6 Pt 1):C1660–7. doi: 10.1152/ajpcell.1998.275.6.C1660. [DOI] [PubMed] [Google Scholar]
- 86.Marionneau C, et al. PPARalpha-mediated remodeling of repolarizing voltage-gated K+ (Kv) channels in a mouse model of metabolic cardiomyopathy. J Mol Cell Cardiol. 2008;44(6):1002–15. doi: 10.1016/j.yjmcc.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, et al. Allosteric modulation of the mouse Kir6.2 channel by intracellular H+ and ATP. J Physiol. 2002;543(Pt 2):495–504. doi: 10.1113/jphysiol.2002.025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bhatnagar A. Contribution of ATP to oxidative stress-induced changes in action potential of isolated cardiac myocytes. Am J Physiol. 1997;272(4 Pt 2):H1598–608. doi: 10.1152/ajpheart.1997.272.4.H1598. [DOI] [PubMed] [Google Scholar]
- 89.Akar FG, et al. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115(12):3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aon MA, et al. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol. 2009;41(10):1940–8. doi: 10.1016/j.biocel.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vajda S, Baczko I, Lepran I. Selective cardiac plasma-membrane K(ATP) channel inhibition is defibrillatory and improves survival during acute myocardial ischemia and reperfusion. Eur J Pharmacol. 2007;577(1–3):115–23. doi: 10.1016/j.ejphar.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 92.Cacciapuoti F, et al. Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non-insulin-dependent diabetes mellitus. Am J Cardiol. 1991;67(9):843–7. doi: 10.1016/0002-9149(91)90617-t. [DOI] [PubMed] [Google Scholar]
- 93.Dun W, et al. Dynamic remodeling of K+ and Ca2+ currents in cells that survived in the epicardial border zone of canine healed infarcted heart. Am J Physiol Heart Circ Physiol. 2004;287(3):H1046–54. doi: 10.1152/ajpheart.00082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang M, et al. Delayed rectifier K currents have reduced amplitudes and altered kinetics in myocytes from infarcted canine ventricle. Cardiovasc Res. 2000;48(1):34–43. doi: 10.1016/s0008-6363(00)00159-0. [DOI] [PubMed] [Google Scholar]
- 95.Pinto JM, Boyden PA. Reduced inward rectifying and increased E-4031-sensitive K+ current density in arrhythmogenic subendocardial purkinje myocytes from the infarcted heart. J Cardiovasc Electrophysiol. 1998;9(3):299–311. doi: 10.1111/j.1540-8167.1998.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 96.Dun W, Boyden PA. Diverse phenotypes of outward currents in cells that have survived in the 5-day-infarcted heart. Am J Physiol Heart Circ Physiol. 2005;289(2):H667–73. doi: 10.1152/ajpheart.00180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nattel S, et al. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87(2):425–56. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 98.Roden DM, Viswanathan PC. Genetics of acquired long QT syndrome. J Clin Invest. 2005;115(8):2025–32. doi: 10.1172/JCI25539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitcheson JS, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci U S A. 2000;97(22):12329–33. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mattick JS. The central role of RNA in human development and cognition. FEBS Lett. 2011;585(11):1600–16. doi: 10.1016/j.febslet.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Podlowski S, et al. Cardiac troponin I sense-antisense RNA duplexes in the myocardium. J Cell Biochem. 2002;85(1):198–207. [PubMed] [Google Scholar]
- 102.Haddad F, et al. Role of antisense RNA in coordinating cardiac myosin heavy chain gene switching. J Biol Chem. 2003;278(39):37132–8. doi: 10.1074/jbc.M305911200. [DOI] [PubMed] [Google Scholar]
- 103.Klattenhoff CA, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152(3):570–83. doi: 10.1016/j.cell.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han P, et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514(7520):102–6. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]