Abstract
Rationale
Platelets are known to participate in vascular pathologies; however, their role in neuroinflammatory diseases such as multiples sclerosis (MS) is unknown. Autoimmune CD4 T cells have been the main focus of studies of MS, although the factors that regulate T cell differentiation towards pathogenic Th1/Th17 phenotypes are not completely understood.
Objectives
We investigated the role of platelets in the modulation of CD4 T cell functions in MS patients and in mice with experimental autoimmune encephalitis (EAE), an animal model for MS.
Methods and Results
We found that early in MS and EAE platelets degranulated and produced a number of soluble factors serotonin (5HT), PF4 and PAF, which specifically stimulated differentiation of T cells towards pathogenic Th1, Th17 and IFN-γ/IL-17-producing CD4 T cells. At the later stages of MS and EAE platelets became exhausted in their ability to produce proinflammatory factors and stimulate CD4 T cells, but substantially increased their ability to form aggregates with CD4 T cells. Formation of platelet-CD4 T cell aggregates involved interaction of CD62P on activated platelets with adhesion molecule CD166 on activated CD4 T cells, contributing to downmodulation of CD4 T cell activation, proliferation and production of IFN-γ. Blocking of formation of platelet-CD4 T cell aggregates during progression of EAE substantially enhanced proliferation of CD4 T cell in the CNS and the periphery leading to exacerbation of the disease.
Conclusion
Our study indicates differential roles for platelets in the regulation of functions of pathogenic CD4 T cells during initiation and progression of CNS autoimmune inflammation.
Keywords: Platelets, MS/EAE, brain lipid rafts, Th1/Th17, platelet-CD4 T cell aggregates, serotonin
INTRODUCTION
Platelets play an important role in thrombosis and homeostasis, but their role in neuroinflammatory and neurodegenerative diseases is yet to be determined1–3. Recently it was demonstrated that platelets contributed to inflammation in several conditions including infection, rheumatoid arthritis and arthrosclerosis4–7. Since activated platelets secrete chemokines, cytokines and other proinflammatory mediators they have the capacity to initiate and regulate inflammation at the site of injury8. Platelet-derived factors have been shown to play a significant role in both inflammation and tissue repair, suggesting an important role of platelets in the initiation, progression and resolution of inflammation9. One of the proposed mechanisms of regulation of inflammation by platelets is their direct interaction with leukocytes1.
Platelet-leukocyte interactions have been a focus of investigations aimed at understanding the pathology of inflammatory diseases4, 10. Platelet-leukocyte aggregates were shown to occur in pathological processes associated with vascular abnormalities, thrombosis and inflammation10, 11. It was found that platelets interact with leukocytes and these interactions could modulate leukocyte function10, 12, 13. However, little is known about whether platelets affect the function of autoimmune CD4 T cells during neurologic diseases such as multiple sclerosis (MS).
MS is an inflammatory disease of the CNS that affects young adults and can lead to significant disability. MS and its animal model, experimental autoimmune encephalitis (EAE), involve pathogenic Th1, Th17 and recently discovered IFN-γ/IL-17double-positive CD4 T cells that recognize CNS self-antigens within myelin sheath such as myelin oligodendrocyte glycoprotein (MOG)14–16. MS usually begins as a relapsing remitting form (RRMS), which is characterized by unpredictable relapses followed by periods of remission. Within a 10–15 year period, many RRMS patients enter the stage of secondary progressive MS (SPMS), in which there is progressive neurologic decline without remission17. Current FDA-approved drugs for MS are primarily for relapsing forms of the disease. These drugs are only partially effective and many have side effects18. There are no treatments for MS that target platelets19; however, we recently showed that FDA-approved drug Copaxone affected platelet activation20. Thus, the study of interactions of platelets with CD4 T cells has the potential to identify new diagnostic and therapeutic targets for MS.
It was demonstrated several decades ago that platelets may become activated during MS21. Platelet abnormalities such as thrombocytopenia are 25 times more common among MS patients when compared to the general population22. A more recent study reported that in MS patients there are increased numbers of platelet-platelet aggregates, platelet-derived microparticles and increased expression of the activation marker CD62P on platelets23. Taken together with reports that platelet-specific αIIbβ3 and platelets themselves are found in CNS inflammatory lesions in MS24, 25, these data suggest that platelets may be actively involved in MS pathogenesis both in the CNS and the periphery. Our studies, along with the study by Langer et al., demonstrated that platelets accumulate in CNS inflammatory lesions of mice with EAE, and that depletion of platelets ameliorated CNS autoimmune inflammation in rodents at early stages of the disease25, 26. Our previous study also identified an important link between platelet activation and degranulation (i.e. secretion of the contents of a platelet’s granules, such as serotonin) and damaged neuronal tissue26. We found that platelets become activated by sialated brain-specific glycolipids integrated into neuronal and astroglial lipid rafts (brain lipid rafts)26, which become accessible to platelets due to alterations of blood brain barrier permeability during EAE and MS27, 28.
In the present study we investigated the functional ability of the platelets from MS patients vs. healthy control (HC) subjects to degranulate and secrete serotonin (5HT) in response to brain lipid rafts, as well as the interaction of platelets with CD4 T cells. We found that early in MS or EAE platelets exhibited distinct proinflammatory properties by secreting soluble factors 5HT, PAF, PF4 that stimulate proliferation and differentiation of pathogenic Th1, Th17, and IFN-γ/IL-17double-positive CD4 T cells. However, at more advanced stages of the disease platelets decreased 5HT content in the granules but increased the capacity to aggregate with CD4 T cells via interactions of CD62P on platelets with adhesion molecules ALCAM/CD166 on CD4 T cells resulting in downmodulation of T cell activation and suppression of CNS autoimmune inflammation.
METHODS
Subjects
Peripheral blood samples were obtained from HC subjects and MS patients (see Supplemental Methods for details).
Platelet and mononuclear cell isolation and analysis
For isolation and analysis of human platelets, peripheral blood samples were drawn using collecting tubes with EDTA. Whole blood and platelet rich plasma were analyzed by multicolor flow cytometry. For isolation of mononuclear cells from human subjects or mice, the Ficoll or Percoll density gradients were used according to the standard protocols (see Supplemental Methods)10, 26, 29, 30.
Platelet-T cell co-culture
Human CD4 T cells were isolated from PBMCs by negative selection using a magnetic separation, and stimulated for six days with anti-CD3/CD28 antibodies. Cytokine production in culture supernatants was measured by ELISA as described31. Activation of human or mouse platelets with TB or ADP was performed as described (see Supplemental Methods)26.
Mouse models
C57BL/6 mice were purchased from Jackson Laboratories. B6.MOG-TCR transgenic mice were maintained in our colony. For CD4 T cell recall response, MOG-TCR transgenic mice were immunized with MOG/CFA26. EAE was induced C57BL/6 mice as described earlier (see Supplemental Methods)26.
Flow cytometry analysis of expression of intracellular molecules
For intracellular detection of IL-17, IFN-γ, IL-4, or FoxP3, mouse or human CD4 T cells were activated with phorbol myristate acetate and ionomycin in the presence of GolgiStop for four hours. Cells were immediately stained for surface markers, fixed/permeabilized, and stained for intracellular antigens with proper antibodies directly conjugated with fluorophores. FACS analysis was performed as described previously (see Supplemental Methods)32–34.
Proliferation assays
Proliferation assays were performed as described in our earlier studies (see Supplemental Methods)32.
Statistical analysis
Unpaired Student’s t-test and Mann–Whitney U-test were used to determine significance. P values of less than 0.05 were considered significant (see Supplemental Methods for details).
RESULTS
Platelets from MS patients form aggregates with CD4 T cells
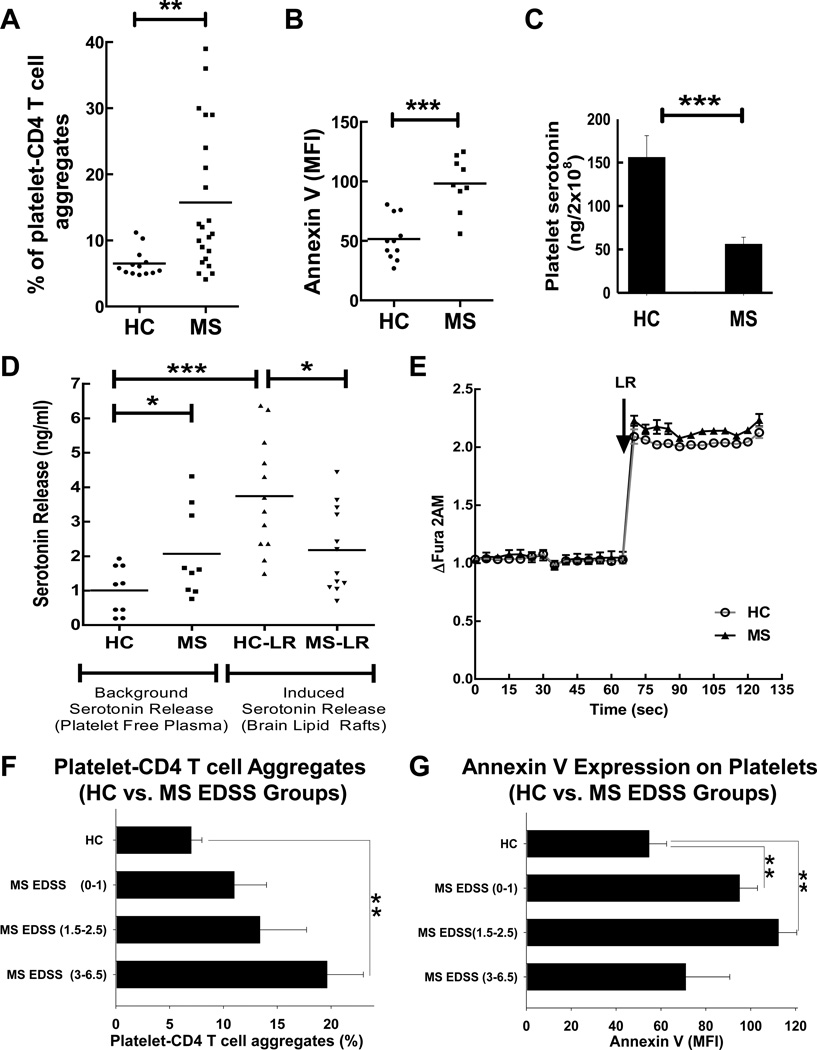
In our previous study we found that platelets promote neuroinflammation and communicate with immune cells26. Based on this, we hypothesized that platelets could directly interact with CD4 T cells and form platelet-CD4 T cell aggregates. We tested this hypothesis and found that the average percentage of CD4 T cells that were aggregated with platelets was significantly increased (2.4-fold) in the peripheral blood of MS patients when compared with healthy control subjects (Fig. 1A). These data demonstrate an active interaction of platelets with CD4 T cells during MS.
Figure 1. Analysis of platelet-CD4 T cell aggregates, expression of Annexin V on platelets, serotonin content in platelets, and serotonin secretion by platelets isolated from peripheral blood of MS patients or healthy controls (HC).
(A) Flow cytometry analysis of platelet-CD4 T cell aggregates. Whole blood preparations were stained for CD4 and the platelet marker CD42a and percentages of platelet-CD4 T cell aggregates were determined as co-expression of CD42a and CD4 of all CD4+ T cells. Percentages of CD42a+CD4+ T cells aggregates are shown for each individual. (B) Flow cytometry analysis of platelet surface staining for Annexin V on CD42a+ platelets from platelet rich plasma. The expression of Annexin V on CD42a+ gated platelets isolated from patients with untreated MS vs. healthy control subjects is presented as mean fluorescence intensity (MFI). (C) Comparison of the serotonin content in the platelets of HC vs. MS patients. Platelet rich plasma was obtained from the peripheral blood, platelets were counted and the volume was adjusted to have equal number of platelets in each sample. After that the platelets were isolated by centrifugation and the amount of serotonin was determined in platelet lysate as described in Methods. (D) Platelets were isolated from peripheral blood of HC or MS patients and the amount of spontaneous or brain lipid raft (LR)-induced serotonin release was measured in platelet-free plasma by ELISA as described in Methods. (E) Comparison of platelet Ca2+ influx in response to brain lipid rafts (LR) for platelets from MS patients (low serotonin producers in response to LR) vs. healthy control subjects (high serotonin producers in response to LR). One representative experiment of three is shown. The changes in the level of intracellular Ca2+ (y-axis) vs. time (x-axis) was assessed using fluorescent probe Fura 2AM as described in Methods.(F–G) Comparision of percentages of platelet-CD4 T cell aggregates (F) and Annexin V expression on platelets (G) in the peripheral blood of HC vs. three groups of MS patients with 1) low expanded disability status scale (EDSS) range (0–1), 2) intermediate EDSS range (1.5–2.5), and 3) high EDSS range (3–6.5).
In (A–D, F–G), mean with individual data points or mean ± S.E. is shown (*, p<0.05; **, p<0.01; ***, p<0.005). Unpaired Student’s tests and Mann–Whitney U tests were used to determine statistical significance as described in Methods.
Platelets degranulate during MS, but fail to secrete 5HT in response to stimulation by brain lipid rafts
In addition to increased adhesiveness to leukocytes, activated platelets have abilities to degranulate and secrete a number of proinflammatory substances, such as 5HT26, which are stored in their granules. It was reported that degranulating platelets produce microparticles during MS23 and EAE26 and become positive for Annexin V, which indicate ongoing processes of platelet activation and vesiculation35. We found that platelets from MS patients significantly increased (~2-fold increase for mean level) the Annexin V staining (Fig. 1B). In addition, we confirmed a reported increase23 in the proportion of platelet-derived microparticles in the peripheral blood of MS patients (Online Fig. I), indicating platelet degranulation during MS. We also found 2.8-fold decrease in 5HT level in the platelets of MS patients when compared to that of HC (Fig. 1C), indicating possible release of 5HT from platelet’s granules. Further analysis showed a 2-fold higher background level of 5HT in platelet-free plasma of MS patients vs. HC (Fig. 1D, left panel; Background Serotonin Release). Platelets are the main and the only source of serotonin in the peripheral blood, while 5HT originates from enterochromaffin cells in the gut and then is taken up by platelets36. Therefore 2.8-fold decrease in 5HT content in MS platelets, a 2-fold increase in the level of 5HT in platelet-free plasma, a 2-fold increase in Annexin V expression on circulating platelets, and an increase in the number of platelet-derived microparticles in the peripheral blood of MS patients indicate an active process of platelet degranulation during MS.
In our previous study we showed that brain lipid rafts (LR) play a role in the activation of mouse and human platelets and the initiation of neuroinflammation in the EAE model26. We thus compared the level of 5HT release in response to LR from platelets of MS patients vs. HC (Fig. 1D; right panel; Induced Serotonin Release). We found that platelets from HC released serotonin in response to LR leading to 3.8-fold higher 5HT level in platelet-free plasma when compared to background levels of 5HT in plasma (Fig. 1D; HC and HC-LR). At the same time, platelets from MS patients did not have increased levels of 5HT release above background level in response to LR (Fig. 1D; MS and MS-LR). Thus in contrast to platelets from HC, platelets from MS patients failed to secrete 5HT upon stimulation with LR.
Unresponsiveness of platelets from MS patients to LR could be due to desensitization of platelet signaling pathways or to exhaustion or depletion of substances stored in platelet granules (such as 5HT) due to chronic activation. We found that platelets from MS patients, which secreted lower amounts of 5HT in response to stimulation with LR, had a comparable level of activation of downstream signal pathways as measured by Ca2+ influx (Fig. 1E; HC and MS), demonstrating that the activation of signaling pathways in platelets from MS patients was unaltered. At the same time we found that 5HT was substantially decreased in platelets from MS patients (Fig.1C), which confirms previously reported study37. Thus, all of these results support the hypothesis that platelets become exhausted during MS in their ability to secrete 5HT, which will be further addressed. In summary, platelets from MS patients formed aggregates with CD4 T cells, demonstrated signs of degranulation (Annexin V staining, formation of platelet-derived microparticles, decrease in 5HT content in platelets), and failed to release 5HT in response to LR.
Platelets become Annexin V-positive during early stages of MS, but form platelet-CD4 T cell aggregates during advanced stages of the disease
Following the results showing broad distributions of percentages of platelet-CD4 T cell aggregates in the peripheral blood of MS patients (Fig. 1A), we decided to compare HC with three groups of MS patients with different expanded disability status scale (EDSS) levels (these serve as an indicator of the progression of the disease) for platelet-CD4 T cell aggregate formation. We also investigated the level of Annexin V staining in these three groups of MS patients. The group of MS patients with more advanced stages of the disease (with the highest EDSS level range of 3–6.5) had the highest level of platelet-CD4 T cell aggregates when compared to HC or two other groups of MS patients with lower EDSS levels (Fig. 1F). On the other hand, we found that the highest level of Annexin V staining was observed in two groups of MS patients with early stages of the disease, with no or mild neurological symptoms (EDSS level ranges of 0–1 and 1.5–2.5, respectively), when compared to HC or the third group of patients with the highest EDSS level (range of 3–6.5) (Fig. 1G). Thus, platelets become Annexin V-positive in MS patients with no or mild neurological symptoms while the formation of platelet-CD4 T cell aggregates was associated with progression of neurological clinical symptoms during more advanced stages of the disease.
Platelets from MS patients are exhausted in their ability to release contents from both dense and alpha- granules in response to activating stimuli
From our previous results, not only dense granule component 5HT was released during stimulation of mouse and human platelets with LR but also releases of alpha granule components PF4 and IL-1α were observed26, as well as upregulation of CD62P (not shown). The result of action of LR on many features of platelet activation and degranulation was comparable to that of thrombin (TB)26. Thus we hypothesized that we observe general phenomena of exhaustion of platelets from MS patients, which is characterized by inability to secrete 5HT and PF4 (i.e. both dense and alpha granule components) in response to various stimuli such as LR, TB or ADP. To test this, we analysed three HC individuals and three MS patients with impaired release of 5HT induced by LR and investigated PF4 release from the same individuals during the same experiment. Similarly to our previous study26, we found that platelets from HC released both 5HT and PF4. The releases of PF4 from HC platelets were less potent when compared to 5HT, but were observed for HC platelets stimulated with LR or ADP (Online Table II). At the same time platelets from MS patients failed to release both 5HT and PF4 in response to LR or ADP (Online Table II). Thus we found that platelets from MS patients failed to release products from both dense and alpha-granules upon stimulation with LR.
Normal platelets skew the differentiation of CD4 T cells towards Th1 and Th17 phenotypes
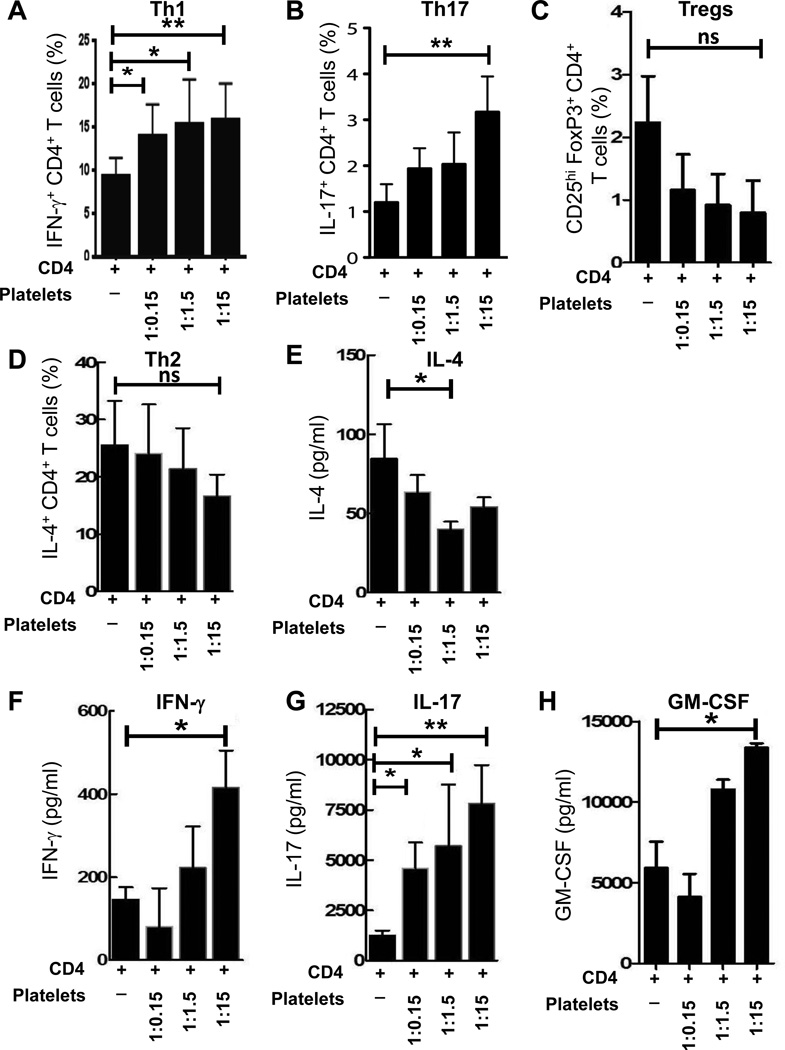
To investigate whether platelets affect the function of CD4 T cells, we performed co-culture experiments of CD4 T cells with platelets isolated from the same healthy subjects. We found that at optimal physiologic CD4 T cell-platelet ratio (1:15), platelets increased the percentages of IFN-γ (Fig. 2A) and IL-17 (Fig. 2B) producing CD4 T cells. Co-culture of CD4 T cells with platelets did not result in an increase of Tregs or Th2 cells: it was trend for decreased percentages of Tregs (Fig. 2C) and Th2 cells (Fig. 2D). When we investigated the production of Th2- (IL-4), Th1- (IFN-γ, GM-CSF), and Th17- (IL-17, GM-CSF) associated cytokines in culture supernatants by ELISA, we found that co-culture of CD4 T cells with platelets resulted in up-regulation of Th1- and Th17-associated cytokines IFN-γ, IL-17 and GM-CSF but not Th2-cytokine IL-4 (Fig. 2E–H).. Thus co-culture of human platelets with CD4 T cells promoted differentiation of human CD4 T cells towards Th1 and Th17 phenotypes.
Figure 2. Co-culture of human platelets with human CD4 T cells increases percentages of Th1 and Th17 cells and production of Th1- and Th17- associated cytokines, IFN-γ, IL-17 and GM-CSF.
Human CD4 T cells were isolated from PBMCs of healthy control (HC) subjects by negative selection. CD4 T cells were stimulated with anti-CD3 (1µg/ml) and anti-CD28 (1µg/ml) antibodies and cultured alone or co-cultured with various numbers of syngeneic platelets (CD4 T cell/platelet ratio 1:15, 1:1.5 and 1:0.15) for six days (see Methods). (A–B) Percentages of IFN-γ (A) and IL-17 (B) producing CD4 T cells were analyzed by flow cytometry using surface staining for CD4 and intracellular staining for IFN-γ and IL-17 as described in Methods. (C) Percentages of CD25hiFoxP3+CD4+ Tregs were determined using multicolor flow cytometry as described in Methods. (D) Percentages of IL-4+CD4+ Th2 cells were determined by flow cytometry as described in Methods. (E–H) The production of Th2-(IL-4 (E)), Th1- (IFN-γ (F), GM-CSF (H)) and Th17- (IL-17 (G), GM-CSF (H)) associated cytokines in culture supernatants were determined by ELISA (see Methods).
In (A–H), mean ± S.E. of 3–5 separate experiments is shown (*, p<0.05; **, p<0.01; ns, not significant).
Activated platelets from MS patients fail to differentiate CD4 T cells towards Th1 and Th17 phenotypes
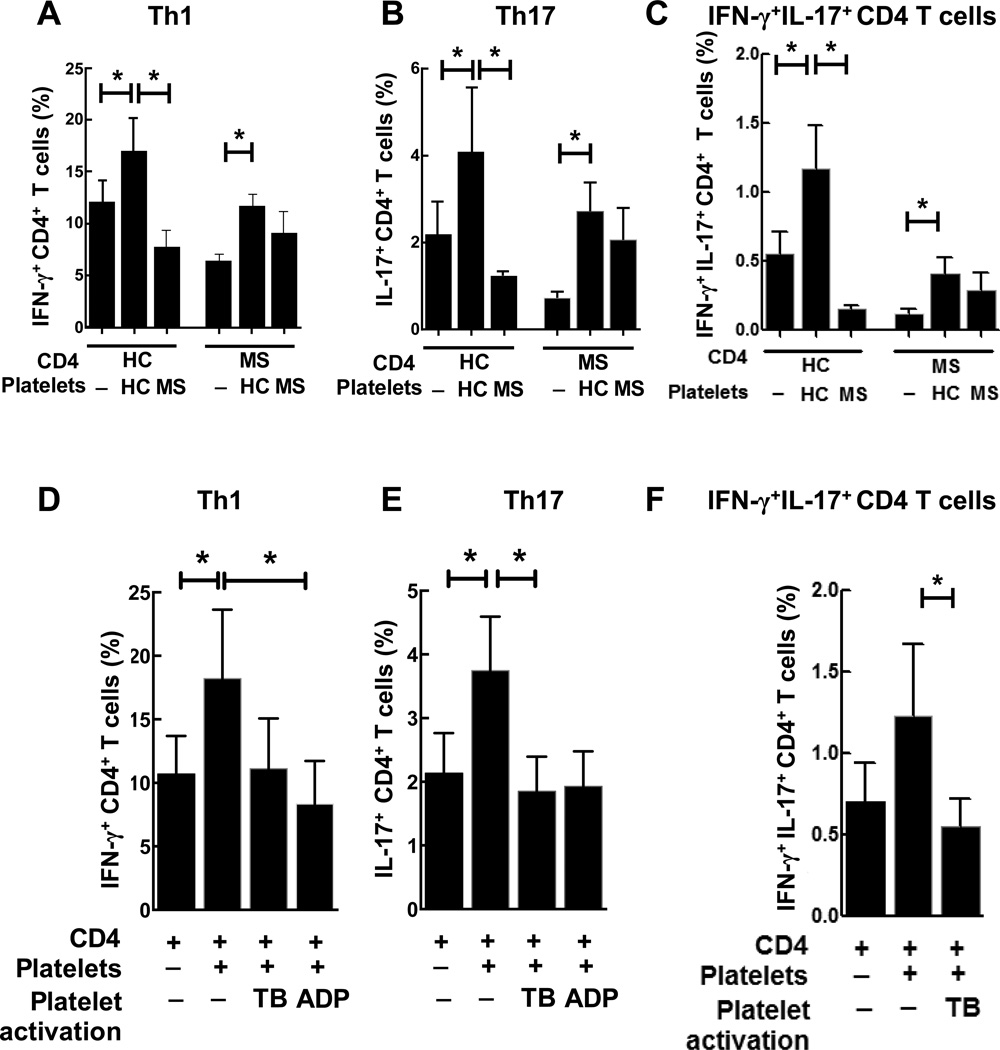
We next compared the ability of platelets isolated from MS patients or HC to skew the differentiation of CD4 T cells towards Th1 and Th17 phenotypes. We found that MS platelets had an impaired ability to skew the differentiation of CD4 T cells towards Th1 (Fig. 3A, Platelets), Th17 (Fig. 3B, Platelets), and IFN-γ/IL-17double-positive CD4 T cells (Fig. 3C, Platelets) when compared to HC platelets. Moreover, platelets from MS patients had impaired ability to stimulate proliferation of CD4 T cells and resulted in decrease in absolute numbers of Th1 and T17 cells in co-cultures (not shown). In addition, CD4 T cells from MS patients were less able to become differentiated towards Th1, Th17, and IFN-γ/IL-17 double-positive CD4 T cells when compared to HC (Fig. 3A–C, CD4).
Figure 3. Comparison of the ability of platelets from MS patients vs. healthy control (HC) subjects, or activated vs. non-activated platelets, to affect differentiation of CD4 T cells towards Th1 and Th17 phenotypes.
(A–C) Human CD4 T cells from healthy controls or MS patients were co-cultured with syngeneic platelets or with allogeneic platelets from MS or HC at ratio of 1:15 and the percentages of Th1 (A), Th17 (B), and IL-17/IFN-γ double-positive CD4 T cells (C) were determined by flow cytometry as described in Methods. (D–F) Human CD4 T cells from HC were cultured alone or co-cultured with syngeneic platelets as for Fig. 2 at 1:15 CD4 T cell/platelet ratio. Platelets were pre-incubated with buffer, thrombin (TB; 0.1 U/ml) or ADP (500 µM) for 30 min, washed, then added to CD4 T cells for co-culture. After six days of co-culture, the percentages of IFN-γ (C), or IL-17 (D), or IL-17/IFN-γ (F) producing CD4 T cells were determined by flow cytometry as described in Methods.
In (A–F), mean ± S.E. of 4–5 separate experiments is shown (*, p<0.05).
In keeping with the observation that platelets from MS patients were more activated ex vivo compared to HC (Fig. 1A,B), we hypothesized that chronic in vivo activation and exhaustion of secretary granules lead to a decrease in the ability of platelets from MS patients to activate CD4 T cells in vitro (Fig. 3A–C). To test this hypothesis, we activated in vitro platelets from HC with classic platelet activating agonists TB or ADP (or incubated platelets with buffer as a control), washed them, and added them to co-cultures. It was found that pre-activation of HC platelets with TB, or ADP, substantially reduced ability of platelets to enhance the production of IFN-γ (Fig. 3D), IL-17 (Fig. 3E), or both IFN-γ and IL-17 (Fig. 3F) when co-cultured with CD4 T cells. These results suggest that activated platelets lose their ability to stimulate Th1/Th17 differentiation.
Taken together, these data suggest that platelets from MS patients are much less potent in skewing differentiation of CD4 T cells towards Th1 and Th17, which correlates with their activation and decrease in ability to secrete proinflammatory factors such as 5HT (Fig 1C).
Platelet-derived soluble factors 5HT, PF4, and PAF contribute to Th1/Th17 differentiation
It was reported that platelet-derived soluble factors 5HT, PF4 and PAF could contribute to T cell proliferation and Th1/Th17 differentiation6, 12, 38–41. By using anti-5HT and anti-PF4 antibodies, and PAFR inhibitor, we found that platelet-derived 5HT contributed to increased proliferation of CD4 T cells and differentiation towards Th1, while PF4 and PAF contributed to Th17 differentiation (Online Fig. II).
Activated platelets have high capacity to form platelet-CD4 T cell aggregates, which leads to decrease in CD4 T cell proliferation and IFN-γ production
In the previous experiments we found that platelets became activated and the levels of platelet-CD4 T cell aggregates were increased during MS (Fig. 1A,B). Therefore we investigated the role of activated vs. not activated platelets in the formation of platelet-CD4 T cell aggregates and the influence of aggregation of CD4 T cell by platelets on T cell activation, proliferation and differentiation. We hypothesized that activated platelets had a higher capacity to form aggregates with T cells and downmodulate T cell activation in these aggregates. Two following findings supported our hypothesis. First, we found that human CD4 T cells that were aggregated with platelets had ~10-fold lower levels of proliferation and ~3-fold lower levels of IFN-γ production, when compared to CD4 T cells that were not aggregated with platelets in the same co-cultures (Online Fig. IIIA). Second, we observed that human TB-activated platelets had higher capacity to do both downmodulate CD4 T cell proliferation and IFN-γ production in platelet-T cell aggregates (Online Fig. IIIB,C) and form these platelet-CD4 T cell aggregates (Online Fig. IIID,E). Thus, the formation of aggregates of human CD4 T cells with activated platelets resulted in downmodulation of T cell proliferation and differentiation.
Platelets promote proliferation and differentiation of MOG-specific autoimmune T cells towards Th1 and Th17 in vivo
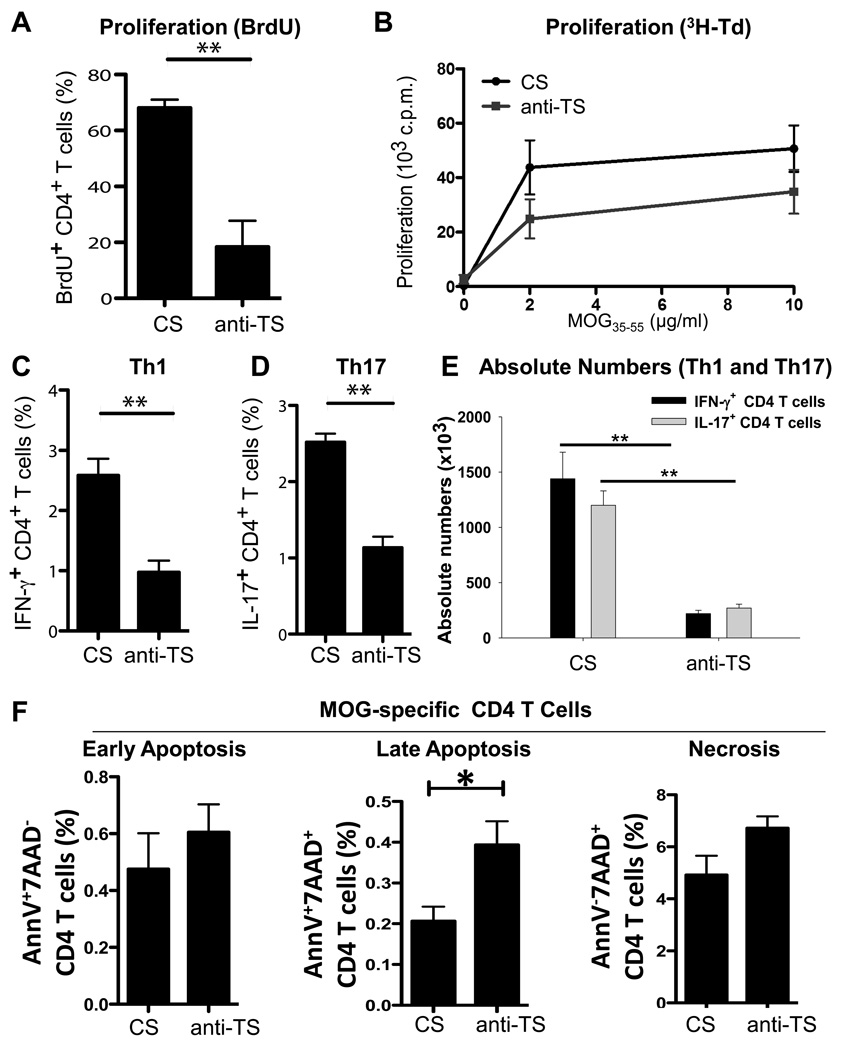
To confirm our in vitro results with human CD4 T cells, we used MOG-TCR transgenic mice where ~90% of T cells recognize myelin self-antigen MOG. Previously we found that depletion of platelets substantially ameliorated EAE induced by active immunization of mice with MOG26. In this study we investigated the role of platelets in activation of MOG-specific T cells in vivo. We immunized MOG-TCR transgenic mice with MOG35–55 peptide and found that depletion of platelets in vivo resulted in decreased proliferation of myelin-specific CD4 T cells as indicated by BrdU and 3H-thymidin incorporation assays (Fig. 4A,B), and decrease in the percentages and absolute numbers of and IFN-γ and IL-17 producing CD4 T cells (Fig. 4C–E). Furthermore, depletion of platelets increased apoptosis of myelin-specific CD4 T cells (Fig. 4F), suggesting that platelets also provide survival factors to pathogenic T cells during their activation. Thus we found that platelets contributed to the priming and activation of myelin-specific T cells in vivo and their differentiation towards Th1 and Th17.
Figure 4. Effect of platelet depletion on proliferation, Th1/Th17 differentiation, and cell death of myelin-specific autoimmune CD4 T cells in vivo.
(A–F) MOG-TCR transgenic mice were immunized with MOG35–55 peptide and CFA as described in Methods. On days 0, 2, 4, 6, and 8 post-immunization 30 µl of anti-thrombocyte (anti-TS) or control serum (CS) was injected i.p., and on day 10 post-immunization splenic CD4 T cells were restimulated in vitro with MOG35–55 peptide as described in Methods. (A–B) CD4 T cell proliferation was assessed on d3 after restimulation with MOG35–55 peptide (2–10 µg/ml) by BrdU (A) and 3H-Td (B) incorporation assays. In (A), percentages of BrdU+ CD4 T cells are shown. (C–F) On day 10 post-immunization splenic and draining lymph nodes CD4 T cells were collected and analyzed for expression of IFN-γ (C,E) and IL-17 (D,E) by intracellular staining using multicolour flow cytometry. Absolute numbers of IFN-γ and IL-17-producing CD4 T cells are shown in (E) (see Methods). (F) MOG TCR transgenic 2D2 mice were immunized with MOG/CFA. On day 0, 2, 4, 6 and 8 post-immunization 30 µl of anti-TS or CS was injected i.p., and on day 10 post-immunization splenic CD4 T cells were analyzed by flow cytometry for the expression of Annexin V (AnnV, apoptosis marker) and plasma membrane permeability using 7-Aminoactinomycine D (7AAD, necrosis marker, see Methods). Percentages of AnnV+7AAD− (early apoptosis), AnnV+7AAD+ (late apoptosis) and AnnV−7AAD+ (necrosis) cells of all CD4 T cells are shown.
In (A, C–F), mean ± S.E. of 6–8 individual mice is shown (*p<0.05; **, p<0.01). In (B) mean ± S.E. of triplicate is shown. Effectiveness of platelet depletion by i.p. administration of anti-TS was assessed by platelet counts in the peripheral blood as described in Methods.
Platelets become Annexin V positive early in EAE and form platelet-CD4 T cell aggregates during advanced stages of the disease
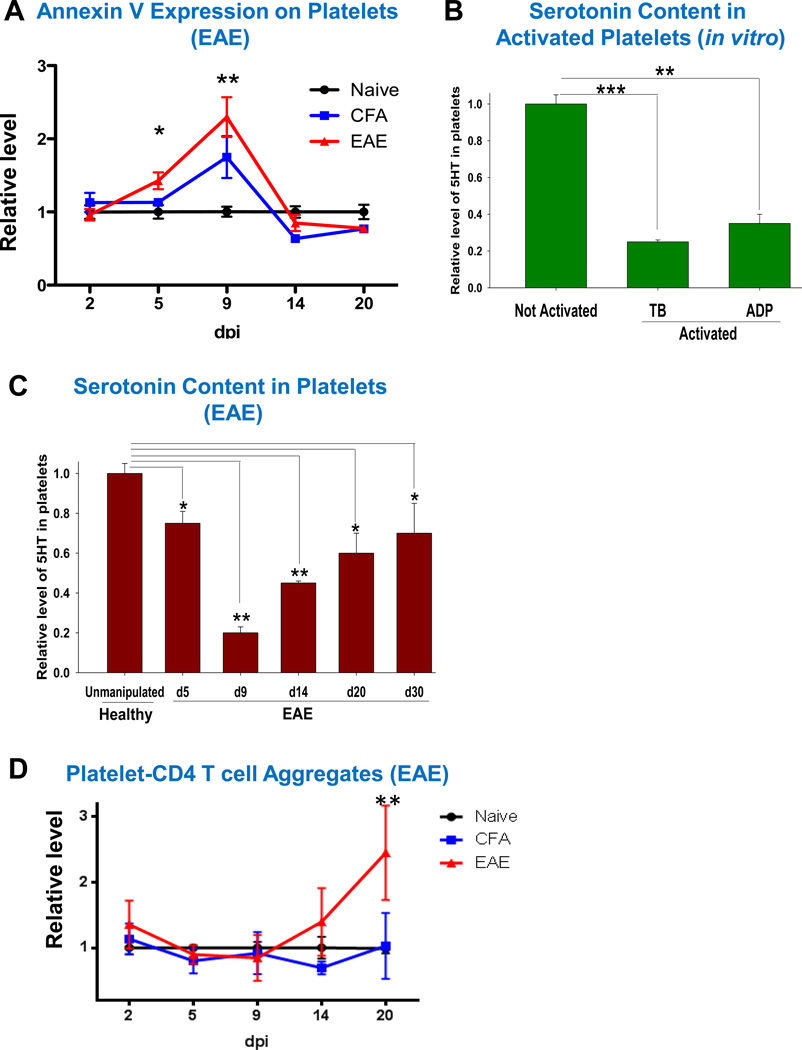
We further investigated Annexin V expression on platelets during EAE following our findings in MS (Fig. 1B). Similar to MS patients, we found that platelets from mice with EAE had 2.1-fold increase in the level of Annexin V staining (Fig. 5A). This was observed during the preclinical stages of the disease (Fig. 5A, day 5 and day 9). Starting from day 14 (EAE onset) the level of Annexin V staining returned to control levels of unmanipulated healthy animals (Fig.5A, day 14 and day 20), indicating that the process of platelet activation/degranulation was transient and occurred during the early stages of EAE. Since it is known that platelet activation/degranulation was accompanied by release of platelets’ granule constituents, we investigated the level of 5HT in the mouse platelets activated in vitro with TB, or ADP or investigated 5HT level in platelets during EAE. We found that TB- and ADP- activated platelets had significantly reduced 5HT content (Fig. 5B). We also found that the level of 5HT in platelets was decreased during EAE as early as d5 post-immunization. Serotonin content in the platelets reached its minimum level on d9, after which it was gradually increased on d14, d20, and d30, but still stayed below 5HT level in platelets of healthy mice (Fig 5C). In contrast to the level of Annexin V staining, the relative level of platelet-CD4 T cell aggregates began to increase on day 14 (disease onset) and was increased 2.3-fold during the peak of disease on d20 (Fig. 5D). Thus platelet activation/degranulation occurred in early preclinical stages of the disease, while formation of platelet-CD4 T cell aggregates occurred during the peak of the disease.
Figure 5. The role of platelets in the pathogenesis of EAE: expression of Annexin V, serotonin level in platelets and CD4 T cell-platelet aggregate formation at different stages of the disease.
Relative level of expression of platelet Annexin V on CD41+CD61+ gated mouse platelets (A), serotonin content in mouse platelets (B–C), and percentages of platelet-CD4 T cell aggregates (D) in the peripheral blood of unmanipulated (Naïve) B6 mice, mice immunized with CFA and i.p. Pertussis toxin (CFA), or MOG/CFA and i.p. Pertussis toxin (EAE).
In (B–C), platelet rich plasma was obtained from mouse peripheral blood, and platelets were isolated from platelet rich plasma of normal mice (B, C) or mice with EAE (C) by centrifugation, and the amount of serotonin was determined in platelet lysate as described in Methods. In (B), washed platelets were activated with TB or ADP in vitro as described in Methods and then level of 5HT was analyzed in platelet lysate. Relative levels of 5HT are shown in comparison with unmanupulated mice.
In (A–D), mean ± S.E. of total 4–10 individual mice for each group is shown for two separate experiments (*, p<0.05; **, p<0.01; ***, p<0.001).
CD62P expression on activated platelets plays an important role in the formation of platelet-CD4 T cell aggregates and downmodulation of T cell activation
Based on our previous results (Online Fig. III), we proposed that the ability of platelets to form aggregates with CD4 T cells imply that the platelets are activated and express CD62P. We tested this hypothesis in the mouse model and found that activated mouse platelets had high capacity to bind to CD4 T cells via CD62P-ALCAM contact interactions and interfere with the delivery of co-stimulatory signals from antigen presenting cells to T cells leading to a decreased level of CD4 T cell activation (Online Fig. IV). Contact interactions of activated mouse platelets with CD4 T cells were also evident in scanning electron microscopy images (Online Fig. V). Thus activated mouse platelets preferentially formed platelet-CD4 T cell aggregates and decreased CD4 T cell activation in these aggregates.
Formation of platelet-CD4 T cell aggregates resulted in amelioration of EAE
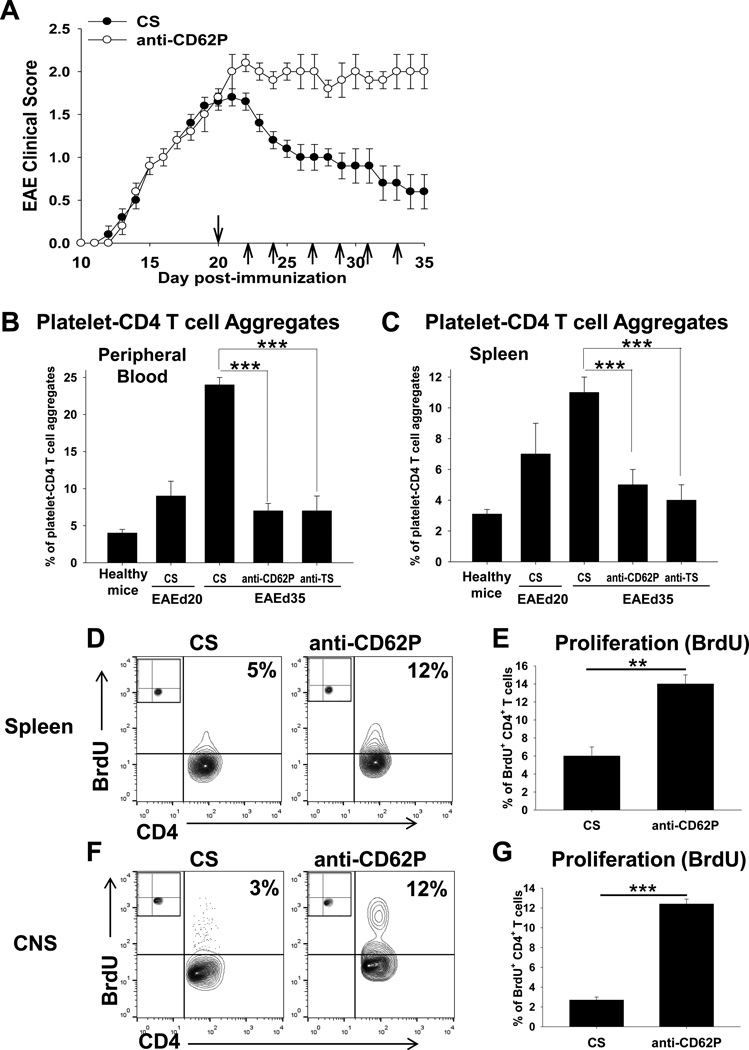
In the previous in vitro experiments we found that adhesion of activated platelets to CD4 T cells resulted in decreased level of T cell activation (Online Figs. III–V). In addition, we demonstrated that anti-CD62P antibodies significantly decreased the formation of platelet-CD4 T cell aggregates in vitro (Online Fig. IV). It was reported that CD62P, which is expressed on activated platelets, played an important role in the formation of platelet-leukocyte aggregates including CD4 T cells in humans and mice42–46. Based on this, we decided to investigate the role of platelet-CD4 T cell aggregates in vivo in the EAE model. We further investigated the level of platelet-CD4 aggregates starting from the peak of the disease at d20 when we initially observed an increase in the level of platelet-CD4 T cell aggregates (Fig. 5D). We found that the percentages of platelet-CD4 T cell aggregates in the peripheral blood reached the highest level on day 35, when mice spontaneously recovered from the disease (Fig. 6A,B; CS). When we isolated mononuclear cells from the spleen using the Ficoll gradient, we also found that platelets formed aggregates with CD4 T cells in the spleen during EAE with kinetics similar to that of peripheral blood (Fig. 6C; CS). When we injected anti-CD62P antibodies i.v. starting from d20 as indicated by arrows on Fig.6A, we found that the levels of platelet-CD4 T cell aggregates were substantially decreased by day 35 in peripheral blood and spleen (Fig. 6B,C; Anti-CD62P) while EAE was substantially exacerbated when compared with control group of mice (Fig. 6A). As an alternative method to decrease the level of CD4-T cell aggregates we performed the depletion of platelets with anti-TS, as we did in Fig. 4. Administration of anti-TS during EAE starting from d20 also resulted in a decrease in platelet-CD4 aggregates in peripheral blood and spleen, and the effect was similar to that of anti-CD62P antibodies (Fig. 6B,C; Anti-TS). In addition to exacerbation of EAE (Fig. 6A), administration of anti-CD62P antibodies resulted in ~2-fold and ~4-fold increases in the level of proliferation of CD4 T cells in the spleen and CNS on d35, respectively (Fig. 6D–G). Thus we found that formation of platelet-CD4 T cell aggregates in vivo decreased the level of proliferation of CD4 T cells in the CNS and periphery and contributed to recovery from EAE.
Figure 6. The role of platelet-CD4 T cell aggregates in the pathogenesis of EAE.
(A) EAE was induced and anti-CD62P antibodies (anti-CD62P) were injected i.v. starting from d20 as described in Methods. EAE clinical course (mean ± S.E.) of total 8–12 individual mice is shown for two separate experiments. Injection of anti-CD62P or CS is indicated by arrows.
(B–C) The flow cytometry analysis of platelet-CD4 T cell aggregates in the peripheral blood (B) or spleen (C) of the healthy mice, or mice with EAE treated with anti-CD62P, anti-TS (see Methods) or CS as on d20, 22, 24, 27, 29, 31 and 33. The cells from the peripheral blood or spleen of healthy mice or mice with EAE on day 20 or day 35 after EAE induction were stained for CD4 and CD41 and analyzed by flow cytometry as described in Methods. The percentages of CD4+CD41+ platelet-CD4 T cell aggregates are shown. (D–G) Comparison of the levels of proliferation of CD4 T cells in the CNS (D, E) and spleen (F,G) of mice with EAE treated with anti-CD62P or CS on day 35 after disease induction. Proliferation assay was performed as described in Methods. Representative contour-plots with percentages of BrdU+ CD4 T cells are shown. Isotype-matched control staining for CD41 is shown in upper left corner of each contour-plot.
In (B), (C), (E) and (G), mean ± S.E. of the group of 5–8 individual animals is shown (**, p<0.01; ***, p<0.005).
Depletion of platelets during late stages of EAE exacerbates the disease
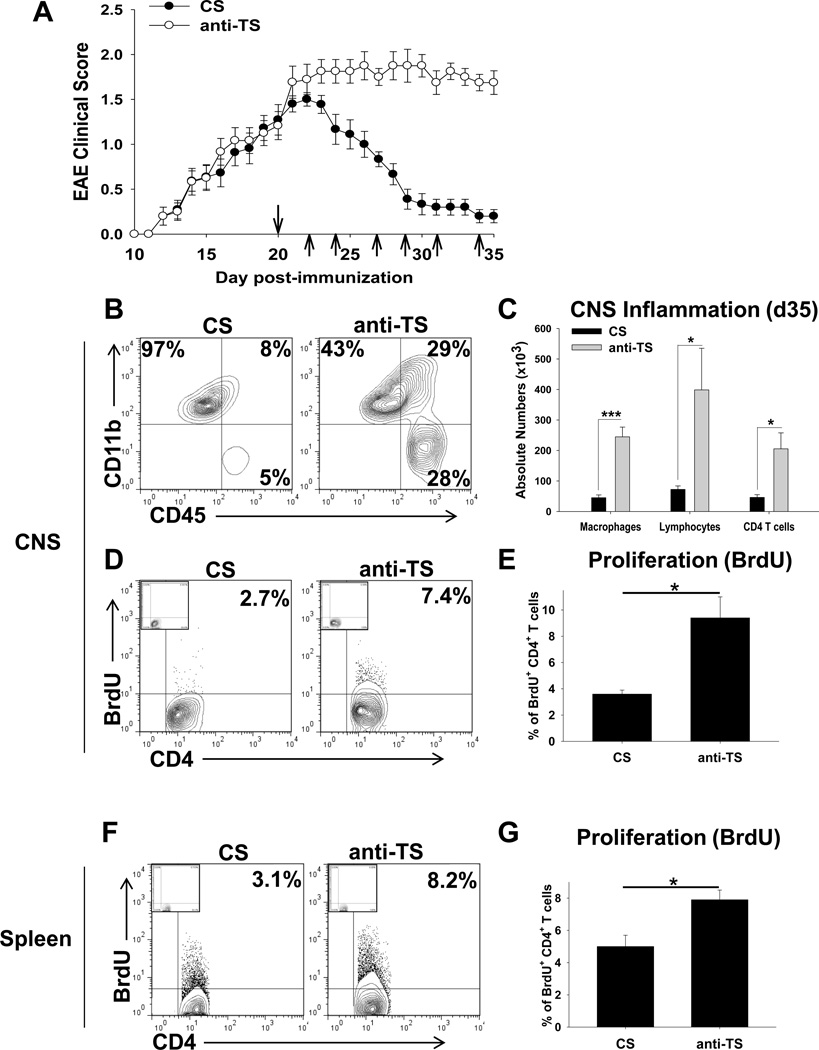
Since formation of platelet-CD4 T cell aggregates resulted in milder EAE disease symptoms (Fig. 6A), and depletion of platelets decreased the percentage of platelet-CD4 T cell aggregates in peripheral blood and spleen (Fig. 6B,C), we further investigated the role of platelets during the late stages of EAE by performing platelet depletion. We have previously found that during preclinical stages of the disease, the depletion of platelets substantially ameliorated EAE26, which was consistent with the present study demonstrating that normal (not activated) platelets stimulated Th1/Th17 cells (Fig. 2 and Fig. 4). Here we performed the depletion of platelets during the progression of EAE. We found that depletion of platelets starting from peak of EAE (d20) exacerbated the disease (Fig. 7A), resulting in an enhanced level of CNS inflammation, as determined by the level of infiltration of macrophages, lymphocytes and CD4 T cells into the CNS (Fig. 7B,C), and enhanced CD4 T cell proliferation levels in the CNS (Fig. 7D,E) and periphery (Fig. 7F,G) on d35. Similar to our observation for human CD4 T cells (Online Fig. III), we found that mouse CD4 T cells that were aggregated with platelets had 18-fold lower level of proliferation and 3.5-fold lower level of IFN-γ production during EAE (d35) when compared to CD4 T cells that were not aggregated with platelets (Online Fig. VI). Thus we further demonstrated that platelets play a regulatory role during progression of EAE and most likely during progression of MS.
Figure 7. The role of platelets in the pathogenesis of EAE during late stages of the diseases.
(A) EAE was induced and anti-thrombocyte (anti-TS) or control serum (CS) were injected i.p. starting from d20 as described in Methods. EAE clinical course (mean ± S.E.) of total 12–15 individual mice for each group is shown for three separate experiments. Injection of anti-TS or CS is indicated by arrows.
(B–C) The flow cytometry analysis of the CNS infiltrating cells isolated from the mice with EAE treated with anti-TS or CS. The mononuclear cells were isolated from the CNS on day 35 after EAE induction, stained for CD11b and CD45, or CD3 and CD4, and analyzed by flow cytometry as described in Methods. The percentages of populations of CD11b+CD45low microglia (left gates), CD11b+CD45hi macrophages (upper right gates) and CD11b−CD45hi lymphocytes (lower right gates) are shown in (B). The quantification of the absolute number of macrophages, lymphocytes and CD4 T cells (CD3+CD4+) in the CNS is shown in (C) (see Methods). (D–G) Comparison of the levels of proliferation of CD4 T cells in the CNS (D, E) and spleen (F,G) of mice with EAE treated with anti-TS or CS on day 35 after disease induction. Proliferation assay was performed as described in Methods. Representative contour-plots with percentages of BrdU+ CD4 T cells are shown. Isotype-matched control staining for BrdU is shown in upper left corner of each contour-plot.
In (C), (E) and (G), mean ± S.E. of the group of 5–8 individual animals is shown (*, p<0.05; **, p<0.01; ***, p<0.005).
DISCUSSION
In this article we have demonstrated a dual role for platelets in the stimulation and inhibition of pathogenic CD4 T cells in MS. Normal (not activated), but not in vitro or in vivo activated platelets, produced 5HT upon stimulation and contributed to Th1/Th17 differentiation. In both MS patients and the EAE model, signs of platelet activation/degranulation were observed during the early stages of the disease, whereas the levels of platelet-CD4 T cell aggregates were increased during progression of the disease at more advance stages. Activated platelets had higher capacity to form platelet-CD4 T cell aggregates during disease progression, and adhesion of activated platelets to CD4 T cells decreased T cell activation leading to downmodulation of EAE.
Serotonin is an important neurotransmitter that can directly affect functions of neurons in the CNS and can modulate the functions of the immune cells in the periphery, and thus may play a role in MS epidemiology47. In mammals, 5HT is found in the gastrointestinal tract, platelets, and in the CNS38. In the peripheral blood, platelets are the exclusive source of serotonin that is stored in platelet granules3, 8. It has been reported that elevated levels of 5HT in the cerebrospinal fluid (CSF) correlated with MS progression, and might thus indicate platelet degranulation and secretion of 5HT in the CNS in the areas of inflammatory lesions upon recognition of LR26. This was supported by the finding that during EAE and MS other platelet-derived PAF accumulated in the CNS and CSF48, 49. These platelet derived soluble factors could promote Th1/Th17 differentiation in the CNS and periphery. During advanced stages of the disease, secretory granules become depleted/exhausted and activated platelets form aggregates with T cells (Online Fig. V) and suppress them. At the final stage of platelet exhaustion, it is possible that activated platelets shed their surface receptors such as CD62P on budding microparticles (Online Figs. I and V) and stop interacting with T cells. The inhibitory effect of activated platelets was recently demonstrated for rheumatoid arthritis. It was shown for this disease that adhesion of platelets to CD4 T cells led to a decrease in their proliferation and expression of IL-17, IFN-γ, and an increase in IL-1010.
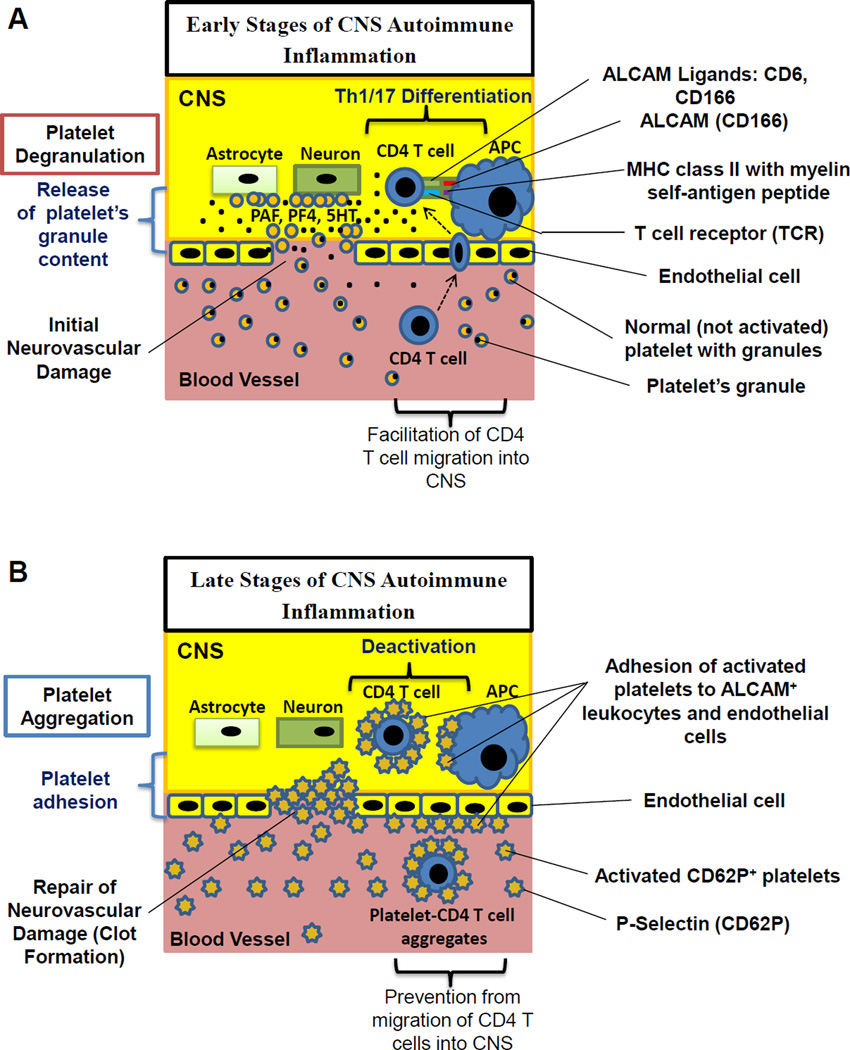
Thus the study suggests that degranulation and subsequent release of several platelet-derived soluble factors contributed to CD4 T cell proliferation and proinflammatory cytokine production, while formation of aggregates with platelets resulted in decrease in T cell proliferation and production of proinflammatory cytokines. Thus depending on the stage of MS or EAE disease, platelets could play proinflammatory and regulatory roles as summarized in Fig. 8. In early stages of MS platelets have high levels of proinflammatory mediators stored their granules such as 5HT, PAF, PF4, which are released when platelets become activated at the site of CNS inflammation by recognizing brain-specific LR or subendothelial matrix in damaged blood vessels (Fig.8A). In addition, degranulating platelets could produce a number of mediators that activate endothelial cells and promote migration of CD4 T cells into CNS (Fig. 8A). Late in MS the granule constituents of activated platelets become depleted, but platelets upregulate CD62P and adhere to activated CD4 T cells leading to their deactivation (Fig. 8B).
Figure 8. Model of differential regulation of CNS autoimmune inflammation by platelets during disease initiation and progression.
(A) In the early stages of the neuroinflammation platelets have non-activated phenotype with a low level of P-selectin (CD62P) expression and a high level of proinflammatory factors stored in platelet’s granules. During initial neurovascular damage in the early stages of CNS inflammation, normal platelets degranulate upon interaction with astroglial or neuronal glycolipids (brain lipid rafts), and/or subendothelal matrix, and the constituents of platelet granules PAF, PF4 and 5HT are released. In the CNS and the periphery platelet-derived PAF, PF4 and 5HT increased proliferation and differentiation of CD4 T cells into pathogenic Th1/Th17 cells upon interaction with antigen presenting cells (APCs). In addition, soluble platelet-derived mediators activated endothelial cells and facilitate the migration of CD4 T cells from blood vessels into the CNS. (B) In the late stages of the neuroinflammation platelets exhibited activated phenotype with low levels of platelet granule constituents such as 5HT and high levels of P-selectin (CD62) expression that resulted in adherence of platelets to activated CD4 T cells or APCs both of which express ALCAM. Formation of platelet-CD4 T cell aggregates prevented interaction of CD4 T cells with APCs in the CNS or periphery, leading to T cell deactivation and downmodulation of CNS inflammation. In addition, formation of platelet-CD4 T cell aggregates prevent migration of CD4 T cells into the CNS.
We found in our study that the formation of platelet-CD4 T cell aggregates was mediated at least in part via CD62P on activated platelets and CD166 on activated CD4 T cells. Besides CD166, CD62P on platelets can bind to other molecules on CD4 T cells such as P-selectin glycoprotein ligand-142 and TIM-150. In addition, activated platelets express a number of integrins that could also contribute to cell contact interactions of platelets with activated T cells51. We believe that binding of platelets to T cells resulted in perturbed interaction of CD4 T cell with antigen presenting cells to cause decrease in T cell activation and migration of T cells to the site of inflammation (Fig. 8B). In support of our hypothesis it was reported that ALCAM substantially contributed to human and mouse T cell activation and migration of CD4 T cells into CNS in EAE model52, 53.
In summary, our data indicate a role for platelets in the immunopathogenesis of CNS autoimmune demyelinating disease, which may provide new opportunities for diagnostics and the treatment of MS by monitoring and modulating the functions of platelets.
Supplementary Material
Novelty and Significance.
What Is Known?
Platelets can modulate inflammation by producing proinflammatory factors and interacting with immune cells.
Platelets become activated during autoimmune diseases such as multiple sclerosis, but the role of these cells in the pathogenesis of autoimmune diseases remains uncertain.
Although experimental autoimmune encephalitis (EAE) is widely used as a model for multiple sclerosis (MS), not all results obtained in EAE model could be directly translated to MS.
What New Information Does This Article Contribute?
In early stages of MS and EAE, platelets become activated and produced soluble proinflammatory factors that specifically stimulated the differentiation of CD4 T cells towards pathogenic Th1 and Th17 phenotypes and promoted neuroinflammation.
At the more advanced stages of neuroinflammation activated platelets became exhausted in their ability to secrete proinflammatory factors, but substantially increased their ability to form aggregates with CD4 T cells, contributing to downmodulation of T cell activation and resolution of inflammation.
The results obtained with the EAE model are in a very good agreement with the results in patients with MS
It is known that platelets regulate inflammation in many types of pathological conditions; however, the exact mechanism of such regulation remains uncertain. We found that at early stages of central nervous system autoimmune inflammation (also known as neuroinflammation) platelets stimulate adaptive immune response by promoting expansion and differentiation of CD4 T cells towards Th1 and Th17 phenotypes, which play pathogenic role in several types of autoimmune diseases including multiple sclerosis. During resolution of neuroinflammation activated platelets form aggregates with CD4 T cell leading to a decrease in T cell activation, which contributed to recovery from the disease. These results suggest that platelets play an important role in the regulation of adaptive immune response during inflammation, which might be viewed as a general mechanism by which platelets regulate immune responses during inflammatory diseases. Under such pathologic conditions platelets could be considered as “innate-like immune cells” that stimulate or inhibit CD4 T cells during initiation and resolution of inflammation. Hence, investigation of platelet-CD4 T cell interactions may have implications for diagnostics and treatment of several types of pathologic inflammatory conditions associated with neurovascular diseases such as multiple sclerosis, stroke, and atherosclerosis.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH RO1 NS071039 research grant (USA), the LCWIIM fund (Hong Kong), the LKS Biomedical Research fund (Chinese University of Hong Kong), and HMRF grant Ref. No. 02130636 (Hong Kong).
Nonstandard Abbreviations and Acronyms
- ADP
adenosine diphosphate
- ALCAM
activated leukocyte cell adhesion molecule (CD166)
- APCs
antigen presenting cells
- anti-TS
anti-thrombocyte serum
- BrdU
bromodeoxyuridine
- CFA
complete Freund's adjuvant
- CS
Control serum
- EAE
experimental autoimmune encephalitis
- FBS
fetal bovine serum
- FDA
Food Drug Administration
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HC
healthy control(s)
- IL
interleukin
- IFN
interferon
- LR
lipid rafts
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- PAF
platelet-activating factor
- PAFR
platelet-activating factor receptor
- PBMCs
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PF4
platelet factor 4
- RRMS
relapsing remitting multiple sclerosis
- SPMS
secondary progressive multiple sclerosis
- TCR
T-cell receptor
- Th
T helper (CD4 T cell)
- Tregs
regulatory T cells
- TB
Thrombin
- 5HT
5-hydroxytryptamine (serotonin)
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Langer HF, Chavakis T. Platelets and neurovascular inflammation. Thrombosis and haemostasis. 2013;110 doi: 10.1160/TH13-02-0096. [DOI] [PubMed] [Google Scholar]
- 2.Behari M, Shrivastava M. Role of platelets in neurodegenerative diseases: a universal pathophysiology. The International journal of neuroscience. 2013;123:287–299. doi: 10.3109/00207454.2012.751534. [DOI] [PubMed] [Google Scholar]
- 3.Horstman LL, Jy W, Ahn YS, Zivadinov R, Maghzi AH, Etemadifar M, Steven Alexander J, Minagar A. Role of platelets in neuroinflammation: a wide-angle perspective. Journal of neuroinflammation. 2010;7:10. doi: 10.1186/1742-2094-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 5.Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A, Nieswandt B, Massberg S, Zinkernagel RM, Hengartner H, Busch DH. A platelet-mediated system for shuttling blood-borne bacteria to CD8alpha+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- 6.Lang PA, Contaldo C, Georgiev P, El-Badry AM, Recher M, Kurrer M, Cervantes-Barragan L, Ludewig B, Calzascia T, Bolinger B, Merkler D, Odermatt B, Bader M, Graf R, Clavien PA, Hegazy AN, Lohning M, Harris NL, Ohashi PS, Hengartner H, Zinkernagel RM, Lang KS. Aggravation of viral hepatitis by platelet-derived serotonin. Nature medicine. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 7.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nature reviews Immunology. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 9.Stellos K, Kopf S, Paul A, Marquardt JU, Gawaz M, Huard J, Langer HF. Platelets in regeneration. Semin Thromb Hemost. 36:175–184. doi: 10.1055/s-0030-1251502. [DOI] [PubMed] [Google Scholar]
- 10.Zamora C, Canto E, Nieto JC, Ortiz MA, Diaz-Torne C, Diaz-Lopez C, Llobet JM, Juarez C, Vidal S. Functional consequences of platelet binding to T lymphocytes in inflammation. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.0213074. [DOI] [PubMed] [Google Scholar]
- 11.Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thrombosis research. 2012;129:263–266. doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, Hansson GK, Li N. Platelets regulate CD4(+) T-cell differentiation via multiple chemokines in humans. Thrombosis and haemostasis. 2011;106:353–362. doi: 10.1160/TH11-01-0020. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Huang Z, Stalesen R, Hansson GK, Li N. Platelets provoke distinct dynamics of immune responses by differentially regulating CD4(+) T-cell proliferation. J Thromb Haemost. 2014;12:1156–1165. doi: 10.1111/jth.12612. [DOI] [PubMed] [Google Scholar]
- 14.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 15.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 18.Ransohoff RM, Hafler DA, Lucchinetti CF. Multiple sclerosis-a quiet revolution. Nat Rev Neurol. 2015 doi: 10.1038/nrneurol.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman L. Platelets provide a bounty of potential targets for therapy in multiple sclerosis. Circulation research. 2012;110:1157–1158. doi: 10.1161/CIRCRESAHA.112.269050. [DOI] [PubMed] [Google Scholar]
- 20.Starossom SC, Veremeyko T, Dukhinova M, Yung AW, Ponomarev ED. Glatiramer acetate (copaxone) modulates platelet activation and inhibits thrombin-induced calcium influx: possible role of copaxone in targeting platelets during autoimmune neuroinflammation. PloS one. 2014;9:e96256. doi: 10.1371/journal.pone.0096256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright HP, Thompson RH, Zilkha KJ. Platelet adhesiveness in multiple sclerosis. Lancet. 1965;2:1109–1110. doi: 10.1016/s0140-6736(65)90069-3. [DOI] [PubMed] [Google Scholar]
- 22.Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4:2377–2383. doi: 10.1111/j.1538-7836.2006.02147.x. [DOI] [PubMed] [Google Scholar]
- 23.Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. Journal of neuroinflammation. 2008;5:27. doi: 10.1186/1742-2094-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 25.Langer HF, Choi EY, Zhou H, Schleicher R, Chung KJ, Tang Z, Gobel K, Bdeir K, Chatzigeorgiou A, Wong C, Bhatia S, Kruhlak MJ, Rose JW, Burns JB, Hill KE, Qu H, Zhang Y, Lehrmann E, Becker KG, Wang Y, Simon DI, Nieswandt B, Lambris JD, Li X, Meuth SG, Kubes P, Chavakis T. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circulation research. 2012;110:1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sotnikov I, Veremeyko T, Starossom SC, Barteneva N, Weiner HL, Ponomarev ED. Platelets recognize brain-specific glycolipid structures, respond to neurovascular damage and promote neuroinflammation. PloS one. 2013;8:e58979. doi: 10.1371/journal.pone.0058979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 28.Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. Journal of neuroimmunology. 2010;229:180–191. doi: 10.1016/j.jneuroim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PloS one. 2013;8:e81774. doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veremeyko T, Starossom SC, Weiner HL, Ponomarev ED. Detection of microRNAs in microglia by real-time PCR in normal CNS and during neuroinflammation. Journal of visualized experiments : JoVE. 2012 doi: 10.3791/4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 34.Ponomarev ED, Tarasenko TN, Sapozhnikov AM. Splenic cytotoxic cells recognize surface HSP70 on culture-adapted EL-4 mouse lymphoma cells. Immunol Lett. 2000;74:133–139. doi: 10.1016/s0165-2478(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 35.Tzima E, Poujol C, Nurden P, Nurden AT, Orchard MA, Walker JH. Annexin V relocates to the periphery of activated platelets following thrombin activation: an ultrastructural immunohistochemical approach. Cell Biol Int. 1999;23:629–635. doi: 10.1006/cbir.1999.0426. [DOI] [PubMed] [Google Scholar]
- 36.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. Journal of veterinary pharmacology and therapeutics. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 37.Baidina TV, Akintseva IV, Trushnikova TN. A chronic fatigue syndrome and blood platelet serotonin levels in patients with multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova. 2014;114:25–28. [PubMed] [Google Scholar]
- 38.Inoue M, Okazaki T, Kitazono T, Mizushima M, Omata M, Ozaki S. Regulation of antigen-specific CTL and Th1 cell activation through 5-Hydroxytryptamine 2A receptor. International immunopharmacology. 2011;11:67–73. doi: 10.1016/j.intimp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Drolet AM, Thivierge M, Turcotte S, Hanna D, Maynard B, Stankova J, Rola-Pleszczynski M. Platelet-activating factor induces Th17 cell differentiation. Mediators Inflamm. 2011;2011:913802. doi: 10.1155/2011/913802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh TP, Huettner B, Koefeler H, Mayer G, Bambach I, Wallbrecht K, Schon MP, Wolf P. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-beta1 transgenic mice. Am J Pathol. 2011;178:699–708. doi: 10.1016/j.ajpath.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards LJ, Constantinescu CS. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflammation & allergy drug targets. 2009;8:182–190. doi: 10.2174/187152809788681010. [DOI] [PubMed] [Google Scholar]
- 42.Schmalbach B, Stepanow O, Jochens A, Riedel C, Deuschl G, Kuhlenbaumer G. Determinants of Platelet-Leukocyte Aggregation and Platelet Activation in Stroke. Cerebrovasc Dis. 2015;39:176–180. doi: 10.1159/000375396. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama S, Ikeda H, Haramaki N, Yasukawa H, Murohara T, Imaizumi T. Platelet P-selectin plays an important role in arterial thrombogenesis by forming large stable platelet-leukocyte aggregates. J Am Coll Cardiol. 2005;45:1280–1286. doi: 10.1016/j.jacc.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Ji Q, Hjemdahl P. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J Thromb Haemost. 2006;4:874–881. doi: 10.1111/j.1538-7836.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy VR, Sardar MY, Ying Y, Song X, Haller C, Dai E, Wang X, Hanjaya-Putra D, Sun L, Morikis V, Simon SI, Woods RJ, Cummings RD, Chaikof EL. Glycopeptide analogues of PSGL-1 inhibit P-selectin in vitro and in vivo. Nat Commun. 2015;6:6387. doi: 10.1038/ncomms7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwig RJ, Schultz JE, Boehncke WH, Podda M, Tandi C, Krombach F, Baatz H, Kaufmann R, von Andrian UH, Zollner TM. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Invest Dermatol. 2004;122:830–836. doi: 10.1111/j.0022-202X.2004.22318.x. [DOI] [PubMed] [Google Scholar]
- 47.Gong X, Xie Z, Zuo H. A new track for understanding the pathogenesis of multiple sclerosis: from the perspective of early developmental deficit caused by the potential 5-HT deficiency in individuals in high-latitude areas. Medical hypotheses. 2008;71:580–583. doi: 10.1016/j.mehy.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Kihara Y, Yanagida K, Masago K, Kita Y, Hishikawa D, Shindou H, Ishii S, Shimizu T. Platelet-activating factor production in the spinal cord of experimental allergic encephalomyelitis mice via the group IVA cytosolic phospholipase A2-lyso-PAFAT axis. J Immunol. 2008;181:5008–5014. doi: 10.4049/jimmunol.181.7.5008. [DOI] [PubMed] [Google Scholar]
- 49.Callea L, Arese M, Orlandini A, Bargnani C, Priori A, Bussolino F. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. Journal of neuroimmunology. 1999;94:212–221. doi: 10.1016/s0165-5728(98)00246-x. [DOI] [PubMed] [Google Scholar]
- 50.Angiari S, Donnarumma T, Rossi B, Dusi S, Pietronigro E, Zenaro E, Della Bianca V, Toffali L, Piacentino G, Budui S, Rennert P, Xiao S, Laudanna C, Casasnovas JM, Kuchroo VK, Constantin G. TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity. Immunity. 2014;40:542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li N. Platelet-lymphocyte cross-talk. Journal of leukocyte biology. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 52.Hassan NJ, Barclay AN, Brown MH. Frontline: Optimal T cell activation requires the engagement of CD6 and CD166. Eur J Immunol. 2004;34:930–940. doi: 10.1002/eji.200424856. [DOI] [PubMed] [Google Scholar]
- 53.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.