Abstract
Cough is a protective reflex to prevent aspiration and can be triggered by a multitude of stimuli. The commonest form of cough is caused by upper respiratory tract infection and has no benefit to the host. The virus hijacks this natural defence mechanism in order to propagate itself through the population. Despite the resolution of the majority of cold symptoms within 2 weeks, cough can persist for some time thereafter. Unfortunately, the mechanism of infectious cough brought on by pathogenic viruses, such as human rhinovirus, during colds, remains elusive despite the extensive work that has been undertaken. For socioeconomic reasons, it is imperative we identify the mechanism of cough. There are several theories which have been proposed as the causative mechanism of cough in rhinovirus infection, encompassing a range of different processes. Those of which hold most promise are physical disruption of the epithelial lining, excess mucus production and an inflammatory response to rhinovirus infection which may be excessive. And finally, neuronal modulation, the most convincing hypothesis, is thought to potentiate cough long after the original stimulus has been cleared. All these hypotheses will be briefly covered in the following sections.
Keywords: Cough/Mechanisms/Pharmacology, Respiratory Infection, Viral infection, Airway Epithelium
Introduction
Cough is a common symptom associated with upper respiratory tract infections (URTIs).1–4 In some patients, coughing can persist leading to a syndrome known as postviral or postinfectious cough which is arbitrarily defined as lasting from 3 to 8 weeks, with normal chest radiograph findings.5 6 In some individuals, cough persists even longer, when it is termed a chronic cough.7 While a normal cough is a vital protective reflex preventing aspiration, cough hypersensitivity is the mechanism thought to underlie almost all types of pathological cough.8 This has been demonstrated in URTI.9–11
Cough causes a plethora of complications affecting the cardiovascular, gastrointestinal and respiratory systems, with far-reaching psychological, neurological and musculoskeletal effects.6 7 12 While there are many agents on the market to reduce the frequency of cough and aid in the clearance of mucus, a systematic review of over-the-counter preparations failed to recommend any available treatment.13 For example, the use of codeine in respiratory tract infection-associated cough was found to be no more effective than its vehicle,14 and prescription-only medications are often unsuitable for certain groups of individuals.15
There are three metrics which are used to study cough: cough challenge, cough counting, and subjective end points such as visual analogue scale or quality of life. Cough challenge studies include the use of pro-tussive agents, such as capsaicin and citric acid, which stimulate transient receptor potential (TRP) ion channels to induce cough.16–18 TRP channels have been popularised as pro-tussive irritant receptors.19 20 However, on account of repeated clinical trial failures in patients with chronic cough using both cough counting and subjective measures, TRPV1 and A1 antagonists as anti-tussives have failed to reach the clinic,21 and an unpublished RCT of inhaled TRPA1 antagonist GRC 17536 (personal communication AHM, 2015). A recent shift of focus now proposes that other channels and receptors, such as P2X receptors, different TRP channels including TRPV4 and TRPM822–24 may be responsible for the observed hypersensitivity. It seems unlikely that one single channel, or receptor, is responsible for causing cough hypersensitivity in all participants in cases of postviral and chronic cough.
Research into URTI and cough faces many problems. Research which relies on natural infection of human volunteers is open to a range of uncontrollable variability including incubation time and causative agent (virus genus and serotype). There is also a lack of suitable animal models for studying HRV due to high host specificity of attachment receptors. Major group HRV requires human ICAM-1 receptor25 which is not present in guinea pigs, an animal classically used for studying the cough reflex. However, pathogens such as parainfluenza virus, a rarer cause of the common cold, can infect guinea pigs and produce a postviral cough with a hypersensitive airway response to capsaicin.26 As a result, studying the effects of HRV infection is often carried out in vitro using cell systems. There is a plethora of research into viral induced effects characterised from various respiratory cell lines, leading to a variety of proposed mechanisms for the induction of cough.
Mechanisms
Inflammatory mediators
HRV infection results in the production a broad profile of inflammatory mediators in the host. The primary inflammatory cytokines reported in HRV infection are interferon (IFN), interleukin (IL) 1, IL-6, IL-8, tumour necrosis factor (TNF) α, granulocyte-macrophage colony-stimulating factor and RANTES. The infection leads to massive upregulation,27 and, consequently, it is often described as a ‘cytokine disease’.28 Many symptoms are thought to occur as a result of the effects of inflammatory cytokines releasing of mediators. For example, sore throat may occur as a result of the release of bradykinin.29 The role of these endogeneous mediators is discussed below.
Bradykinin
The proinflammatory mediator bradykinin has been suggested as a potent tussive modulator of TRPA1 and TRPV1.30–32 It is thought to work through phospholipase C (PLC) causing channel phosphorylation and subsequent sensitisation.33 Elevated levels of bradykinin are found in the BAL fluid of patients with inflammatory airway conditions.34 Bradykinin has also been suggested to mediate ACE inhibitor cough32 which affects 15% of patients.35 Bradykinin and PGE2 possess the ability to sensitise the airways to cough stimulus in animal studies which can be effectively abolished on simultaneous application of antagonists to both TRPV1 and TRPA1.36
Tachykinins
Tachykinin peptides, neurokinin A and B, and substance P, are inflammatory neuropeptides, which collectively induce airway hyper-responsiveness, bronchial constriction,37 and increased vascular permeability.38 They also generate substantial mucus secretion39 and the secretion of inflammatory mediators from immune cells.40–42 It has been suggested that inhibition of tachykinin metabolism by ACE inhibitors is an alternate mechanism for ACE inhibitor cough.43 In HRV infection, tachykinins are released from neurons on TRPV1 activation.40 Unfortunately, the mechanism underlying this activity is currently unknown, and understanding such channel interaction may hold the key to modulating the development of viral and postviral cough. Reduction of degradation of tachykinins by neutral endopeptidases in respiratory viral infection44 are likely to enhance the noxious effects of tachykinins.
Despite its scarcity, substance P in humans has been found to be upregulated, both in nasal epithelium and plasma in chronic cough sufferers.45–47 The efficacy of substance P, mediated by tachykinin NK-1 receptors,48 is greatly enhanced by prior inflammation. Furthermore, when in excess, it is suggested to lower the threshold of pain perception to noxious stimuli, as demonstrated in several pain-associated disease states.49 50 In guinea pigs, the role of substance P in cough has been extensively investigated. Substance P results in bronchoconstriction but highly variable cough.51 Likewise, in healthy individuals, inhalation of substance P does not cause cough. However, at the same concentration, substance P has the ability to elicit cough in patients with common colds,52 suggesting a hypersensitive state induced by the virus. Additionally, the microvascular leakage of substance P is thought to activate rapidly adapting receptors (RARs)53 which may add to the irritant effect in common cold.
Calcitonin gene-related peptide
Evidence for the role of another neuropeptide calcitonin gene-related peptide (CGRP) is mixed. TRP channel (TRPV1) activation induces and controls the release40 54 from C-fibre terminals.55 CGRP has an inhibitory effect in substance P-induced bronchoconstriction,56 and when deficient, causes airway hyper-responsiveness.57 However, an increase in CGRP has been shown in many pain-associated conditions, including migraine and various forms of inflammation.58 59 Chronic cough sufferers have been shown to have increased neuronal levels of CGRP60 61 associated with the enhanced sensitivity to capsaicin.61 These effects appear to be mediated through the cytokines, IL-1β and TNF-α.59 In respiratory syncytial virus (RSV) infection, a rarer cause of URTI in the adult, the development of airway hyper-responsiveness appears to arise through a disruption of CGRP balance.57 62 However, this fails to explain the increased levels found in sufferers of chronic cough. Unlike substance P, CGRP does not directly induce mucus secretion,63 but may indirectly enhance through vasodilation.
The likelihood that substance P, CGRP or neurokinins have some role in cough hypersensitivity is high. Opiates have been shown to be highly effective in a subgroup of patients with cough.15 A possible mode of action is through prejunctional inhibition of peptide release-preventing neurotransmission either centrally, or from afferent nerves adjacent to inflammatory mediator receptors.40
Leukotrienes
Leukotrienes are potent inflammatory mediators of chemotaxis, bronchoconstriction and vascular permeation,64 which are predominately produced by leucocytes, but also by other inflammatory immune cells. Data is scarce on the extent leukotrienes play in HRV infection. However, Seymour et al65 identified a significant increase of precursor enzymes within the airways during HRV infection in healthy individuals, which can potentially increase the capacity of leukotriene B4 and C4 synthesis. Muscarinic receptor involvement has been implicated in the production of leukotriene B4.66 In cough-associated eosinophilic inflammation, blockade of leukotriene receptors has been shown to be efficacious.67 However, in a recent study, montelukast, a potent inhibitor of the receptor of leukotriene C4 and D4, has no effect on cough in the common cold.68
Eosinophils
Eosinophils are important mediators of cough in allergic disease but their role in viral infection is less clear. Eosinophils, if activated, during viral infection release a multitude of molecules including leukotrienes, growth factors, cytokines and major basic protein (MBP).69 MBP binds to as well as alters prejunctional M2 receptor function,70 71 and increases tachykinin release.44 MBP is cytotoxic72 and has been implicated in peripheral nerve remodelling.73 It is found in higher levels in nasal aspirates from children during HRV infection.74 Not only are eosinophils implicated in tachykinin modulation, but during degranulation they also release a potent peroxidase which generates reactive oxygen species (ROS) and reactive nitrogen species. These harmful oxidants are known potent TRPA1 agonists,75 76 which are receptors ascribed to cause cough in humans and guinea pigs.20
Muscarinic receptors
Muscarinic receptors are highly characterised in airway diseases such as asthma and chronic obstructive pulmonary disease with limited evidence of their involvement in respiratory infections. Of the five muscarinic receptors (M1-5), only M1-3 can be found in the respiratory system. M1 is expressed on epithelial cells and submucosal glands of pulmonary veins.77 M3 receptors are heavily expressed on smooth muscle, inflammatory and submucosal cells78 where they mediate bronchoconstriction, mucosal secretion and inflammatory responses.79 M2 receptors predominately regulate cardiac contraction, but can also be localised to respiratory smooth muscle.77 However, the most important role of M2 receptors in the airway is the prejunctional inhibition of acetylcholine release to limit the degree of bronchoconstriction.80 Respiratory viral agents, parainfluenza and RSV, have been shown to cause depletion and dysfunction of M2 receptors,81 thereby exaggerating cholinergic activity. This has been further shown in double-stranded (ds) RNA animal models, independent of inflammation,81 and mediated by IFN release.82 Therefore, viral infection-induced bronchoconstriction, airway hyper-responsiveness and mucus secretion causing cough may be indirectly mediated through M2 receptors.82 M2 dysregulation can be reversed with dexamethasone83 or pilocarpine.80 However, Lowry et al84 found anticholinergic bronchodilators to be ineffective against cough in natural URTI. The potent topical corticosteroid, fluticasone, was also ineffective in significantly reducing symptoms of viral URTI and, indeed, significantly increased the bacterial colonisation of the upper airway.85
Many of the inflammatory mediators discussed above do not directly evoke cough but work synergistically through other pulmonary fibres where the threshold for cough is lowered to provoke the urge to cough. Thus, this change to sensory nerve functionality secondary to these mediators may potentiate and prolong a cough response during and after respiratory viral infection.
Physical damage
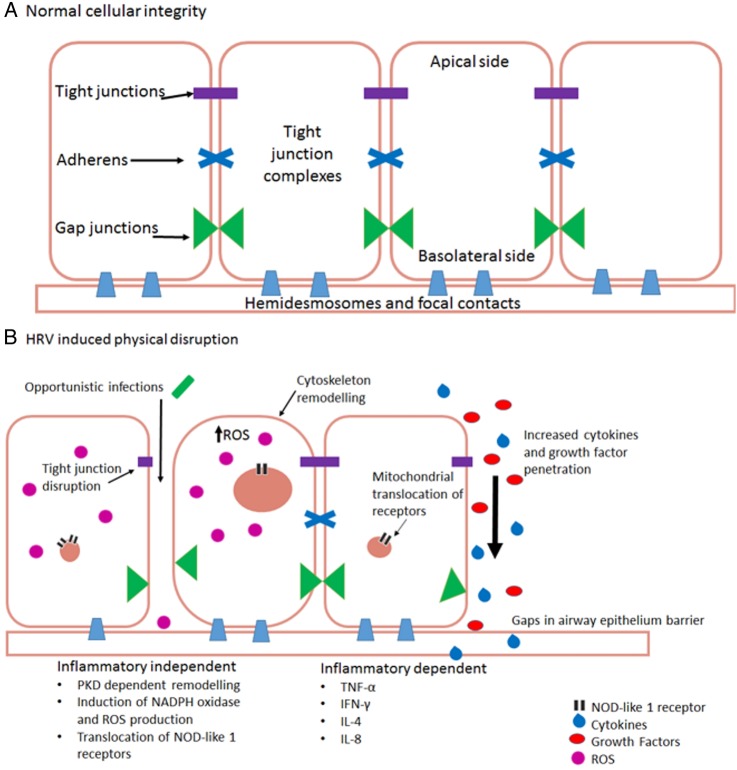
By comparison with other respiratory viruses such as influenza, HRV is renowned for its minimal cytopathic effects.86 It has been suggested that influenza has a higher incidence of cough than that seen with HRV infections.87 Thus, physical disruption of airway integrity may be a factor in a heightened cough response. HRV, or synthetic dsRNA stimuli polyinosinic:polycytidylic acid (poly(I:C)), is able to disrupt airway epithelial cells via disruption of tight junction complexes at apicolateral membranes through dissociation of zona occludin (ZO) 1, occludin, claudin-1, E-cadherin and β-catenin. This leads to a significant reduction in transepithelial resistance88–90 indicating a loss of epithelial integrity (figure 1A). Transepithelial resistance can also be decreased through respiratory-localised TRPV4 activation,91 and causes disruption of tight junction complexes leading to increased permeability. But whether these are related or not, are unknown. Whether this viral induced loss of integrity is dependent or independent of an inflammatory response is open to debate.
Figure 1.
(A) Normal healthy airway barrier. In a healthy airway, cells are connected together by tight junction complexes including tight junctions, adherens and gap junctions. Cells are attached to basement membranes by hemidesmosomes and focal contacts. Barrier permeability is minimal and tightly regulated to prevent the excessive release of essential molecules, ions and proteins. The barrier is protective against infection. (B) Human rhinovirus infection in airway epithelial cells. There are two main ways that HRV causes physical disruption of airway barriers, inflammatory-dependent and independent. Both replicating and non-replicating viruses can interfere with airway membrane integrity by disrupting tight junction complexes. This causes a reduction of transepithelial resistance with the potential consequence of contracting a secondary infection. Cytoskeletal remodelling mediated by protein kinase D (PKD) causes an actin reorganisation within infected cells, altering their structure and integrity, further allowing cells to lose their adjoining contacts. Replicating HRV produces a dsRNA intermediate structure which can interact and activate NOD-like receptor X-1 ultimately producing reactive oxygen species. These alone are capable of reducing transepithelial resistance and barrier disruption. Loss of gap junctions and cells leaves gaps within epithelial layers. These allow cytokines, growth factors, immune cells and further viral particles to penetrate deeper layers within the airways, causing dysregulation of cellular signalling. This dysregulation causes further upregulation of various molecules including growth factors, which, in turn, can lead to an increase of receptor expression, such as transient receptor potential channels which have a prolific effect to cause cough (TNF, tumour necrosis factor; IFN, interferon; IL, interleukin).
Inflammatory independent
ROS and other oxidants cause barrier disruption and affect permeability in tissues throughout the body92 through cytoskeletal and tight junction interruption. This effect is mirrored in polarised epithelial cells, such as those within the airways.88 93 During infection, HRV causes oxidative stress independent of viral replication or ICAM-1-mediated viral attachment.94 The mechanism is thought to be via a NOD-like receptor X-1 (NLRX-1) interaction with dsRNA causing a translocation of NADPH oxidase-1 (NOX-1) leading to the generation of ROS and oxidants.93 95 By contrast, ROS production is necessary for clearance of viral infections, but requires stringent regulation. Interaction with dsRNA receptor NLRX-1 induces mitochondrial ROS generation.96 97 This is likely a result of mitochondrial antiviral signalling protein interaction with dsRNA,98–100 an important component of HRV lifecycle.101 ROS are also potent agonists of TRPA1 and TRPV1,75 76 which poses a potential interaction route between HRV and TRP channels.
Inflammatory dependent
Other investigators propose that physical disruption is an inflammatory-dependent process caused by TNF-α, IFN-γ, IL-4 and IL-8 which mediate the tight junction dysregulation.102–104 A cytokine-induced effect may, however, be secondary to signalling pathways aforementioned, and which mechanism predominates may be dependent on specific cell type.
Consequently, varying degrees of barrier disruption and physical damage have the potential to cause a multitude of effects. A loss of integrity to airway barriers enables the transmigration of opportunistic bacteria causing secondary respiratory infection.90 It can also lead to dysregulation of intracellular signalling facilitating the upregulation of growth factors.105–107 Finally, in exposed animals, an enhanced activation of sensory nerve fibres leads to airway hyper-responsiveness108 and epithelial repair is delayed.109 Thus, there is a cycle of cytokine-induced barrier damage (figure 1B) leading to epithelial shedding. Airway epithelial lining begins to become permeable to larger molecules88 leading to a cycle of hypersensitivity and further damage.
Physical disruption to airways described above is a well-characterised part of the pathophysiology of lung diseases including asthma and cystic fibrosis, but the role it plays in URTI, such as HRV, has only recently begun to become clear, but may be crucially important in patients with pre-existing respiratory disease.
Mucus
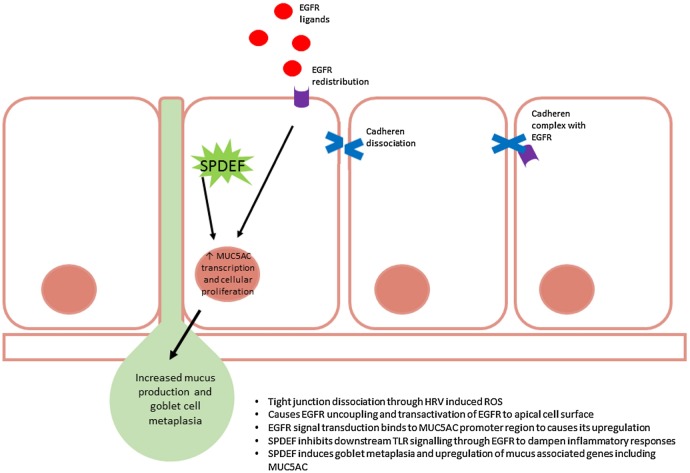
Excessive mucus production and secretion is common in URTI1 110 initiating symptoms such as a cough and sneezing, and thus facilitating transmission of infection.3 111 HRV, in particular, upregulates the transcription of various mucin genes including MUC5AC.112–114 This pathway is particularly involved in mucus production and release, but this complex process (figure 2) has yet to be completely characterised. Using nuclear factor (NF) κB and mitogen-activated protein kinase inhibitors, the pathway induced during HRV infection was originally identified. The mechanism is independent of serotype and genotype and is inducible by artificial genomic stimulus using poly(I:C).112 NFκB is upregulated as part of HRV lifecycle,115 116 so it is unsurprising that it plays a pivotal role in the production of symptoms during infection, and is essential for MUC5AC production. HRV is able to induce mucosal cell metaplasia through a novel TLR3-epidermal growth factor receptor (EGFR) coupling and the induction of EGFR ligands,113 including transforming growth factor α. This results in the production and secretion of mucins via MUC5AC promoter regions.112 The process of mucus secretion and tight junction disruption go hand in hand. A loss of epithelial integrity where a dissociation of E-cadherens from adherens tight junction complexes causes the uncoupling of EGFR where it becomes readily activated. However, an excess of EGFR activation promotes goblet metaplasia and, thus, excessive mucus secretion.117 Muscarinic receptors are also involved in mucus secretion, mediated predominately through M3 in cooperation with M1118 and are regulated by M2.119 Since stimulation of muscarinic receptors transactivate EGFR to stimulate goblet cell mucus secretion,120 121 it is possible that HRV possesses the ability to interact with muscarinic receptors to cause this process. The implications of this are far-reaching as not only may it begin to explain the aetiology of a viral mucosal cough but also chronic mucus secretion such as occurs in chronic bronchitis.
Figure 2.
Sterile-α-motif-pointed domain ETS-factor (SPDEF) and epidermal growth factor receptor (EGFR) regulation of mucus production and goblet cell metaplasia within the airways during upper respiratory tract infection (URTI). Reactive oxygen species (ROS)-induced uncoupling of EGFR permits its translocation to the apical membrane of cells which readily allows its activation. Excessive activation of EGFR promotes various cellular processes including goblet cell metaplasia and upregulation of mucus-associated genes including MUC5AC. Simultaneously, SPDEF is activated to dampen the inflammatory response mounted against the rhinovirus infection through blockade of TLR signal transduction. SPDEF also functions as a transcription modulator and causes upregulation of MUC5AC. Ultimately, increased MUC5AC and goblet metaplasia results in the hyperproduction of mucus, characteristic of rhinovirus infection.
A counter-regulatory mechanism to HRV-induced TLR-EGFR coupling is by induction of the transcription factor sterile-α-motif-pointed domain ETS-factor (SPDEF). SPDEF inhibits TLR signalling and type I IFN release to dampen the proinflammatory response induced by HRV.122 SPDEF is a transcriptional modulator with a wide variety of roles including endocrine and androgen interaction,123 124 however, recently this transcription modulator was found to regulate goblet cell metaplasia123 125 and upregulate genes associated with mucus production,126 including MUC5AC.125 It has been suggested that SPDEF initiates recovery and protects against excessive inflammatory damage during metaplasia. Dysregulation of this pathway may permit the cycle of cough and damage to persist, exposing basal membranes and nerve fibres, allowing HRV-induced physical damage and mucus overproduction to act synergistically.
Many patients have a dry or non-productive cough, and thus, an important cofactor mucus production alone is insufficient to explain coughing in URTI. Excessive mucus may exacerbate cough by several mechanisms including a stretch response and altered tonicity. Present within mucus are nucleosides and nucleotides, namely ATP and its breakdown products.127 ATP release occurs through cellular swelling mediated via pannexin-1128–130 and subsequent ATP-induced ATP release.131 Purinergic receptors are increasingly thought to be important mediators of cough hypersensitivity, and are discussed further in the section on neuronal mechanisms. In terms of mucus secretion, P2Y receptors enhance intracellular calcium concentrations and subsequent ciliary beat frequency.132 133 P2X7 is known to colocalise and interact with Pannexin-1.134–136 As part of the pore-forming complex of P2X7 receptor, pannexin-1 forms a death complex through extended pore dilation and increased permeability.135 137 138
In recent years, an increasing number of studies have begun to implicate TRPV4 in mucociliary clearance and airway defence, as it is essential for epithelial barrier function,91 and is highly expressed in ciliated tracheal cells.139–141 As well as arachidonic acid metabolites,142 TRPV4 can be activated through mechanical and osmotic stimulus,143 such as viscous and hypotonic mucus, to induce and regulate calcium release.144 Not only does this activate the channel but it also regulates ciliary beat frequency141 and mucus secretion, mediated by aquaporin 5.145
Neuronal modulation
Cough is clearly a neuronal reflex, so the hypothesis that neuronal modulation underlies the pathogenesis of viral cough is the most convincing. However, at present, there is no single comprehensive mechanism which explains cough induced by HRV or indeed any other respiratory pathogen. Theories include a cooperative role of pulmonary oxidative stress in vagal sensory nerves between TRPV1, TRPA1 and P2X receptors.146 Direct viral damage to mitochondria leading to ROS production may modulate or influence cough.
During URTI sensitivity to capsaicin, citric acid and histamine are transiently increased with a reduction in cough threshold,10 11 147 148 without concurrent hyper-responsiveness to methacholine.11 This suggests that HRV-induced cough is independent of bronchial smooth-muscle tone. This was originally proposed to occur through the sensitisation of RARs,149 but despite convincing evidence, RARs do not express TRPV1 receptors and are insensitive to chemical stimuli.150 As such, they are no longer proposed to be the primary fibre involved in the cough reflex,23 but may have a synergistic interaction with C-fibres.151 As a result of these findings, capsaicin-sensitive nerves are not the same nerves known to initiate pathological cough, despite the clear observation that inhaled aerosolised capsaicin produces a cough.18 These observed differences may be explained by phenotypic changes to nerve fibres induced during inflammation.152
Phenotypic changes imply altered gene expression and differentiation. In the guinea pig, low threshold mechanosensitive sensory nerves express TrkA,153 and application of other growth factors induce the functional expression of TRPV1 and TRPA1 de novo.152 In vivo research in the rat and guinea pig models have found inflammatory states through increased nerve growth factor (NGF) levels causing a phenotypic change of A-delta fibres. They now resemble C-fibres as shown by the coexpression of substance P and NGF.154 155 NGF is transported to sensory neurons via DRG and is able to alter transcription of various proteins and peptides.156 Further research into the effect of NGF on TRPV1 by Chuang et al33 and Ganju et al157 found that NGF activates the PLC pathway. TRPV1 associates with phosphatidylinositol 4,5-bisphosphate (PIP2) in its resting state, but PLC activation causes the hydrolysis of PIP2 to release TRPV1 from constitutive inhibition, thus increasing the opening probability of TRPV1.
Similarly, HRV infection in the human has been shown to cause the upregulation of a number of growth factors.105–107 Specifically, HRV can induce the upregulation of NGF158 and inhalation of aerosolised NGF can enhance cough in the guinea pig citric acid-induced cough model through TRPV1 and TrkA (NGF receptor) activation.159 160 Despite the differences between human and guinea pig airway innervation, it is these modifications in expression that may lead to a phenotypic change during URTI.
Interest into the role of TRPV4 and P2X receptors in cough has been growing exponentially. A recent abstract publication showed that the application of a TRPV4 agonist facilitated the subsequent activation of P2X3 receptor through sensitisation of airway sensory nerves.161 This is not the first time TRPV4 and P2X3 receptors have been hypothesised to play a cooperative role in pathophysiology.162 It has become apparent that there is significant overlap between TRP channel and purinergic receptor functionality, which have given rise to the persuasive theory that TRPV4 and purinergic receptors play a cooperative role in pathological cough. ATP, which has been shown to enhance cough reflex sensitivity24 in response to citric acid and histamine challenge,163 potentiates through P2X2/X3-mediated bronchoconstriction.164 165 A successful clinical trial using AF-219, a P2X3 antagonist, reduced the incidence of coughing in patients with chronic cough by 75%,166 provides more convincing evidence that cough sensitivity may be strongly upregulated by the P2X pathway.
Neurogenic inflammation from afferent sensory neurons167 is mediated mostly, but not exclusively, by neuropeptides CGRP, neurokinins and substance P. When present, it is thought to be a protective reflex, facilitating healing and modulation of local immunity. Unfortunately, there is wide interspecies and, in man, intersubject variability in afferent sensory innervation making interpretation of experimental findings difficult to translate into clinical relevance. However, neurogenic inflammation is well characterised in several diseases, including migraine,168 and more controversially in asthma169 and rhinitis, the latter of which is common in URTI,170 where it likely amplifies maladaptive responses. Neurogenic inflammation is essential for sensory neurons to prime and respond to noxious stimuli quickly. Consequently, afferent neurons are abundant in TRP channels, P2X, PAMPs and DAMP receptors. Only a limited set of TLRs, 3, 4, 7 and 9, are present within nociceptive neurons,171–173 therefore, not all pathogens are capable of directly causing neurogenic inflammation. Stimulation of these TLRs induce an inward depolarisation to elicit neuronal sensitisation to pain stimuli.171–173 A recent interesting finding identified TLR 7 stimulation leading to an itch-specific sensory pathway,173 through intracellular microRNA let-7b. This, in turn, induces a rapid inward current in neurons, coexpressing TLR7 and TRPA1 to generate pain.174 175 Extracellular ATP is a crucial damage-associated molecular pattern molecule ligand which is released during damage and injury. In nociceptive neurons, P2X3 receptors are key to ATP recognition and pain production.176 Purinergic receptor P2Y2 is also responsive to ATP, and is capable of TRPV1 sensitisation and activation in the absence of TRPV1 stimuli,177 a similar sensation previously ascribed to TRPV4 through HRV-induced mucus overproduction. Cytokines, namely IL-1β and TNF-α, produced as a result of infection cause TRP channel sensitisation and activation through membrane phosphorylation.178 179 The resultant effect means that TRP channels respond to innocuous stimuli as noxious stimuli causing allodynia and, perhaps, allotussia.
There is a surprising degree of overlap in the physiological location and functionality of thermo-TRP channels and P2X receptors. TRPA1 is reported to possess mechanotransductive properties likely attributable to their distinct characteristic ankyrin repeats180 in the inner ear to permit hearing,181 although this is disputed by other groups.182 However, despite this, an interesting notable finding was the identification of P2X receptors, also within the inner ear, and that their initial action potential firing is necessary for the maturation of hearing.183 Likewise in the bladder, TRPV1, TRPV2, TRPV4, TRPA1 and TRPM8 all have mechanosensory roles184 to interpret stretch and pain perception,185 whilest P2X2 and P2X3 play a major role in distension sensation to excite a micturition reflex.186 Most relevant to cough is their influence on pain. TRPA1 and TRPV1 are well characterised to elicit pain signals in response to noxious stimuli187 188 P2X2, P2X3, P2X4 and P2X7 having all been identified to play some form of role in various types of pain.189–192 Most importantly in neuropathic pain where some pharmacological agents which have shown promising results in clinical trials.193 Relevant to these observations is that P2X2 and P2X3 receptors expressed on afferent neurons are home to the classic pain sensation channel TRPV1.194
The role of TRP channels in HRV-induced cough has recently been explained in a first-of-its-kind study by Abdullah et al.195 A novel infection site of HRV was identified in neuronal cell lines with concomitant upregulation of expressed TRP channels TRPA1, TRPV1 and TRPM8 found on airway sensory nerves. HRV infection accounts for up to 50% of exacerbations in asthmatics,196 and both asthmatic197 and chronic cough sufferers198 199 have higher levels of TRP channels expressed in their airways. This finding adds to the mounting evidence that TRP channels play a major role in cough during HRV infections, but requires further investigation to definitively confirm this.
Summary
A postviral cough is generally unresponsive to conventional pharmacological intervention, but has shown limited responsiveness to the anticholinergic drug, tiotropium,200 which leaves a major hole in our therapeutic armamentarium. Currently, treatment merely consists of dampening the inflammatory response with the use of antiinflammatories (such as naproxen),201 and cough suppressants (codeine-containing products and dextromethorphan),15 in the hope of reducing the frequency, severity and transmission of cough. Opiates, although commonly prescribed, are not generally recommended for viral cough due to their poor efficacy and significant adverse effect profile.15
The multiple mechanisms described above provide a confusing and inter-related ‘soup’ of potential therapeutic targets, dissecting which, are the key players involved in this common affliction that will be challenging. We suggest that modulation of the afferent neuronal hypersensitivity will provide the most fruitful target in what is essentially a benign and self-limiting disease. Other strategies, such as systemic immune modulation, run the risk of generating unforeseen off-target effects. The rewards for understanding the mechanism of viral-induced cough will have enormous impact on human morbidity.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Witek TJ, Ramsey DL, Carr AN et al. . The natural history of community-acquired common colds symptoms assessed over 4-years. Rhinology 2015;53:81–8. doi:10.4193/Rhin14.149 [DOI] [PubMed] [Google Scholar]
- 2.Eccles R, Loose I, Jawad M et al. . Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Med 2003;4:118–24. doi:10.1046/j.1526-4637.2003.03019.x [DOI] [PubMed] [Google Scholar]
- 3.Curley FJ, Irwin RS, Pratter MR et al. . Cough and the common cold. Am Rev Respir Dis 1988;138:305–11. doi:10.1164/ajrccm/138.2.305 [DOI] [PubMed] [Google Scholar]
- 4.Reid DD, Williams RE, Hirch A. Colds among office workers an epidemiological study. Lancet 1953;262:1303–6. doi:10.1016/S0140-6736(53)91373-7 [DOI] [PubMed] [Google Scholar]
- 5.Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:138S–46S. doi:10.1378/chest.129.1_suppl.138S [DOI] [PubMed] [Google Scholar]
- 6.Irwin RS, Baumann MH, Bolser DC et al. . Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S–23S. doi:10.1378/chest.129.1_suppl.1S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morice AH, Fontana GA, Belvisi MG et al. . ERS guidelines on the assessment of cough. Eur Respir J 2007;29:1256–76. doi:10.1183/09031936.00101006 [DOI] [PubMed] [Google Scholar]
- 8.Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010;188(Suppl):S87–90. doi:10.1007/s00408-009-9185-z [DOI] [PubMed] [Google Scholar]
- 9.Dicpinigaitis PV, Tibb AS, Ramsey DL et al. . Stability of cough reflex sensitivity during viral upper respiratory tract infection (common cold). Pulm Pharmacol Ther 2014;28:154–7. doi:10.1016/j.pupt.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Dicpinigaitis PV, Bhat R, Rhoton WA et al. . Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 2011;105:615–18. doi:10.1016/j.rmed.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 11.O'Connell F, Thomas VE, Studham JM et al. . Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 1996;90:279–86. doi:10.1016/S0954-6111(96)90099-2 [DOI] [PubMed] [Google Scholar]
- 12.McGarvey LPA, Morice AH. Clinical cough and its mechanisms. Respir Physiol Neurobiol 2006;152:363–71. doi:10.1016/j.resp.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 13.Schroeder K, Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. BMJ 2002;324:329–31. doi:10.1136/bmj.324.7333.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 1992;17:175–80. doi:10.1111/j.1365-2710.1992.tb01289.x [DOI] [PubMed] [Google Scholar]
- 15.Morice AH, McGarvey L, Pavord I et al. . Recommendations for the management of cough in adults. Thorax 2006;61(Suppl 1):i1–24. doi:10.1136/thx.2006.065144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morice AH, Kastelik JA, Thompson R. Cough challenge in the assessment of cough reflex. Br J Clin Pharmacol 2001;52:365–75. doi:10.1046/j.0306-5251.2001.01475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laude EA, Higgins KS, Morice AH. A comparative study of the effects of citric acid, capsaicin and resiniferatoxin on the cough challenge in guinea-pig and man. Pulm Pharmacol 1993;6:171–5. doi:10.1006/pulp.1993.1023 [DOI] [PubMed] [Google Scholar]
- 18.Collier JG, Fuller RW. Capsaicin inhalation in man and the effects of sodium cromoglycate. Br J Pharmacol 1984;81:113–17. doi:10.1111/j.1476-5381.1984.tb10750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benemei S, Patacchini R, Trevisani M et al. . TRP channels. Curr Opin Pharmacol 2015;22:18–23. doi:10.1016/j.coph.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Birrell MA, Belvisi MG, Grace M et al. . TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med 2009;180:1042–7. doi:10.1164/rccm.200905-0665OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalid S, Murdoch R, Newlands A et al. . Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 2014;134:56–62. doi:10.1016/j.jaci.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 22.Morice AH. Developing antitussives the clinician's pipeline—what do we need? J Thorac Dis 2014;6:S735–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grace MS, Dubuis E, Birrell MA et al. . Pre-clinical studies in cough research: role of Transient Receptor Potential (TRP) channels. Pulm Pharmacol Ther 2013;26:498–507. doi:10.1016/j.pupt.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamei J, Takahashi Y, Yoshikawa Y et al. . Involvement of P2X receptor subtypes in ATP-induced enhancement of the cough reflex sensitivity. Eur J Pharmacol 2005;528:158–61. doi:10.1016/j.ejphar.2005.10.030 [DOI] [PubMed] [Google Scholar]
- 25.Tomassini JE, Graham D, DeWitt CM et al. . cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc Natl Acad Sci USA 1989;86:4907–11. doi:10.1073/pnas.86.13.4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye XM, Zhong NS, Liu CL et al. . Cough reflex sensitivity is increased in guinea pigs with parainfluenza virus infection. Exp Lung Res 2011;37:186–94. doi:10.3109/01902148.2010.540768 [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos NG, Johnston SL. Rhinoviruses as pathogens of the lower respiratory tract. Can Respir J 2000;7:409–14. [DOI] [PubMed] [Google Scholar]
- 28.Stöckl J, Vetr H, Majdic O et al. . Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10. J Clin Invest 1999;104:957–65. doi:10.1172/JCI7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Proud D, Reynolds CJ, Lacapra S et al. . Nasal provocation with Bradykinin induces symptoms of rhinitis and a sore throat. Am Rev Respir Dis 1988;137:613–16. doi:10.1164/ajrccm/137.3.613 [DOI] [PubMed] [Google Scholar]
- 30.Bandell M, Story GM, Hwang SW et al. . Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004;41:849–57. doi:10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 31.Carr MJ, Kollarik M, Meeker SN et al. . A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther 2003;304:1275–9. doi:10.1124/jpet.102.043422 [DOI] [PubMed] [Google Scholar]
- 32.Fox AJ, Lalloo UG, Belvisi MG et al. . Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough. Nat Med 1996;2:814–17. doi:10.1038/nm0796-814 [DOI] [PubMed] [Google Scholar]
- 33.Chuang HH, Prescott ED, Kong H et al. . Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001;411:957–62. doi:10.1038/35082088 [DOI] [PubMed] [Google Scholar]
- 34.Baumgarten CR, Lehmkuhl B, Henning R et al. . Bradykinin and other inflammatory mediators in BAL-fluid from patients with active pulmonary inflammation. Agents Actions Suppl 1992;38(Pt 3):475–81. [PubMed] [Google Scholar]
- 35.Yeo WW, Foster G, Ramsay LE. Prevalence of persistent cough during long-term enalapril treatment: controlled study versus nifedipine. Q J Med 1991;80:763–70. [PubMed] [Google Scholar]
- 36.Grace M, Birrell MA, Dubuis E et al. . Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax 2012;67:891–900. doi:10.1136/thoraxjnl-2011-201443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joos GF, Germonpre PR, Kips JC et al. . Sensory neuropeptides and the human lower airways: present state and future directions. Eur Respir J 1994;7:1161–71. [PubMed] [Google Scholar]
- 38.Lundberg JM, Brodin E, Hua X et al. . Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand 1984;120:217–27. doi:10.1111/j.1748-1716.1984.tb00127.x [DOI] [PubMed] [Google Scholar]
- 39.Rogers DF, Aursudkij B, Barnes PJ. Effects of tachykinins on mucus secretion in human bronchi in vitro. Eur J Pharmacol 1989;174:283–6. doi:10.1016/0014-2999(89)90322-1 [DOI] [PubMed] [Google Scholar]
- 40.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 1998;30:5–11. doi:10.1016/S0306-3623(97)00078-5 [DOI] [PubMed] [Google Scholar]
- 41.Numao T, Agrawal DK. Neuropeptides modulate human eosinophil chemotaxis. J Immunol 1992;149:3309–15. [PubMed] [Google Scholar]
- 42.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988;241:1218–21. doi:10.1126/science.2457950 [DOI] [PubMed] [Google Scholar]
- 43.Morice AH, Lowry R, Brown MJ et al. . Angiotensin-converting enzyme and the cough reflex. Lancet 1987;2:1116–18. doi:10.1016/S0140-6736(87)91547-9 [DOI] [PubMed] [Google Scholar]
- 44.Jacoby DB. Pathophysiology of airway viral infections. Pulm Pharmacol Ther 2004;17:333–6. doi:10.1016/j.pupt.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 45.Bae Y-J, Moon KA, Kim T-B et al. . The role of nitrosative stress in the pathogenesis of unexplained chronic cough with cough hypersensitivity. Am J Rhinol Allergy 2012;26:e10–14. doi:10.2500/ajra.2012.26.3730 [DOI] [PubMed] [Google Scholar]
- 46.Otsuka K, Niimi A, Matsumoto H et al. . Plasma substance P levels in patients with persistent cough. Respiration 2011;82:431–8. doi:10.1159/000330419 [DOI] [PubMed] [Google Scholar]
- 47.Cho Y, Park S, Lee CK et al. . Elevated substance P levels in nasal lavage fluids from patients with chronic nonproductive cough and increased cough sensitivity to inhaled capsaicin. J Allergy Clin Immunol 2003;112:695–701. doi:10.1016/S0091 [DOI] [PubMed] [Google Scholar]
- 48.Moore KA, Undem BJ, Weinreich D. Antigen inhalation unmasks NK-2 tachykinin receptor-mediated responses in vagal afferents. Am J Respir Crit Care Med 2000;161:232–6. doi:10.1164/ajrccm.161.1.9903091 [DOI] [PubMed] [Google Scholar]
- 49.Appelgren A, Appelgren B, Kopp S et al. . Substance P-associated increase of intra-articular temperature and pain threshold in the arthritic TMJ. J Orofac Pain 1998;12:101–7. [PubMed] [Google Scholar]
- 50.Evengard B, Nilsson CG, Lindh G et al. . Chronic fatigue syndrome differs from fibromyalgia. No evidence for elevated substance P levels in cerebrospinal fluid of patients with chronic fatigue syndrome. Pain 1998;78:153–5. doi:10.1016/S0304-3959(98)00134-1 [DOI] [PubMed] [Google Scholar]
- 51.El-Hashim AZ, Amine SA. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol 2005;513:125–33. doi:10.1016/j.ejphar.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 52.Katsumata U, Sekizawa K, Inoue H et al. . Inhibitory actions of procaterol, a beta-2 stimulant, on substance P-induced cough in normal subjects during upper respiratory tract infection. Tohoku J Exp Med 1989;158:105–6. doi:10.1620/tjem.158.105 [DOI] [PubMed] [Google Scholar]
- 53.Bonham AC, Kott KS, Ravi K et al. . Substance P contributes to rapidly adapting receptor responses to pulmonary venous congestion in rabbits. J Physiol 1996;493(Pt 1):229–38. doi:10.1113/jphysiol.1996.sp021378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kichko TI, Reeh PW. TRPV1 controls acid- and heat-induced calcitonin gene-related peptide release and sensitization by bradykinin in the isolated mouse trachea. Eur J Neurosci 2009;29:1896–904. doi:10.1111/j.1460-9568.2009.06747.x [DOI] [PubMed] [Google Scholar]
- 55.Mak JCW, Barnes PJ. Autoradiographic localization of calcitonin gene-related peptide (CGRP) binding sites in human and guinea pig lung. Peptides 1988;9:957–63. doi:10.1016/0196-9781(88)90073-3 [DOI] [PubMed] [Google Scholar]
- 56.Cadieux A, Monast NP, Pomerleau F et al. . Bronchoprotector properties of calcitonin gene-related peptide in guinea pig and human airways. Effect of pulmonary inflammation. Am J Respir Crit Care Med 1999;159:235–43. doi:10.1164/ajrccm.159.1.9711031 [DOI] [PubMed] [Google Scholar]
- 57.Dakhama A, Kanehiro A, Mäkelä MJ et al. . Regulation of airway hyperresponsiveness by calcitonin gene-related peptide in allergen sensitized and challenged mice. Am J Respir Crit Care Med 2002;165:1137–44. doi:10.1164/ajrccm.165.8.2109058 [DOI] [PubMed] [Google Scholar]
- 58.Lassen L, Haderslev P, Jacobsen V et al. . CGRP may play a causative role in migraine. Cephalalgia 2002;22:54–61. doi:10.1046/j.1468-2982.2002.00310.x [DOI] [PubMed] [Google Scholar]
- 59.Hua X-Y, Chen P, Fox A et al. . Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea: studies with interleukin-1beta and tumor necrosis factor-alpha. J Neurosci 1996;16:4742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang AB, Gibson PG, Ardill J et al. . Calcitonin gene-related peptide relates to cough sensitivity in children with chronic cough. Eur Respir J 2007;30:66–72. doi:10.1183/09031936.00150006 [DOI] [PubMed] [Google Scholar]
- 61.O'Connell F, Springall DR, Moradoghli-Haftvani A et al. . Abnormal intraepithelial airway nerves in persistent unexplained cough? Am J Respir Crit Care Med 1995;152:2068–75. doi:10.1164/ajrccm.152.6.8520777 [DOI] [PubMed] [Google Scholar]
- 62.Dakhama A, Park J-W, Taube C et al. . Alteration of airway neuropeptide expression and development of airway hyperresponsiveness following respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 2005;288:L761–70. doi:10.1152/ajplung.00143.2004 [DOI] [PubMed] [Google Scholar]
- 63.Webber SE, Lim JC, Widdicombe JG. The effects of calcitonin gene-related peptide on submucosal gland secretion and epithelial albumin transport in the ferret trachea in vitro. Br J Pharmacol 1991;102:79–84. doi:10.1111/j.1476-5381.1991.tb12135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hammarström S. Leukotrienes. Annu Rev Biochem 1983;52:355–77. doi:10.1146/annurev.bi.52.070183.002035 [DOI] [PubMed] [Google Scholar]
- 65.Seymour ML, Gilby N, Bardin PG et al. . Rhinovirus infection increases 5-lipoxygenase and cyclooxygenase-2 in bronchial biopsy specimens from nonatopic subjects. J Infect Dis 2002;185:540–4. doi:10.1086/338570 [DOI] [PubMed] [Google Scholar]
- 66.Profita M, Di Giorgi R, Sala A et al. . Muscarinic receptors, leukotriene B4 production and neutrophilic inflammation in COPD patients. Allergy 2005;60:1361–9. doi:10.1111/j.1398-9995.2005.00892.x [DOI] [PubMed] [Google Scholar]
- 67.Takemura M, Niimi A, Matsumoto H et al. . Clinical, physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma. Respiration 2012;83:308–15. doi:10.1159/000332835 [DOI] [PubMed] [Google Scholar]
- 68.Wang K, Birring SS, Taylor K et al. . Montelukast for postinfectious cough in adults: a double-blind randomised placebo-controlled trial. Lancet Respir Med 2014;2:35–43. doi:10.1016/S2213-2600(13)70245-5 [DOI] [PubMed] [Google Scholar]
- 69.Hogan SP, Rosenberg HF, Moqbel R et al. . Eosinophils: biological properties and role in health and disease. Clin Exp Allergy 2008;38:709–50. doi:10.1111/j.1365-2222.2008.02958.x [DOI] [PubMed] [Google Scholar]
- 70.Evans CM, Fryer AD, Jacoby DB et al. . Pretreatment with antibody to eosinophil major basic protein prevents hyperresponsiveness by protecting neuronal M2 muscarinic receptors in antigen-challenged guinea pigs. J Clin Invest 1997;100:2254–62. doi:10.1172/JCI119763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsen GL, Fame TM, Renz H et al. . Increased acetylcholine release in tracheas from allergen-exposed IgE-immune mice. Am J Physiol 1994;266:L263–70. [DOI] [PubMed] [Google Scholar]
- 72.Hisamatsu K, Ganbo T, Nakazawa T et al. . Cytotoxicity of human eosinophil granule major basic protein to human nasal sinus mucosa in vitro. J Allergy Clin Immunol 1990;86:52–63. doi:10.1016/S0091-6749(05)80123-X [DOI] [PubMed] [Google Scholar]
- 73.Pégorier S, Wagner LA, Gleich GJ et al. . Eosinophil-derived cationic proteins activate the synthesis of remodeling factors by airway epithelial cells. J Immunol 2006;177:4861–9. doi:10.4049/jimmunol.177.7.4861 [DOI] [PubMed] [Google Scholar]
- 74.Teran LM, Seminario MC, Shute JK et al. . RANTES, macrophage-inhibitory protein 1alpha, and the eosinophil product major basic protein are released into upper respiratory secretions during virus-induced asthma exacerbations in children. J Infect Dis 1999;179:677–81. doi:10.1086/314618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishio N, Taniguchi W, Sugimura YK et al. . Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating TRPA1 and TRPV1 channels. Neuroscience 2013;247:201–12. doi:10.1016/j.neuroscience.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 76.Andersson DA, Gentry C, Moss S et al. . Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008;28:2485–94. doi:10.1523/JNEUROSCI.5369-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mak JC, Barnes PJ. Autoradiographic visualization of muscarinic receptor subtypes in human and guinea pig lung. Am Rev Respir Dis 1990;141:1559–68. doi:10.1164/ajrccm/141.6.1559 [DOI] [PubMed] [Google Scholar]
- 78.Gosens R, Zaagsma J, Meurs H et al. . Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 2006;7:73 doi:10.1186/1465-9921-7-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rogers DF. Motor control of airway goblet cells and glands. Respir Physiol 2001;125:129–44. doi:10.1016/S0034-5687(00)00209-7 [DOI] [PubMed] [Google Scholar]
- 80.Fryer AD, Maclagan J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol 1984;83:973–8. doi:10.1111/j.1476-5381.1984.tb16539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bowerfind WML, Fryer AD, Jacoby DB. Double-stranded RNA causes airway hyperreactivity and neuronal M2 muscarinic receptor dysfunction. J Appl Physiol 2002;92:1417–22. doi:10.1152/japplphysiol.00934.2001 [DOI] [PubMed] [Google Scholar]
- 82.Jacoby DB, Xiao HQ, Lee NH et al. . Virus- and interferon-induced loss of inhibitory M2 muscarinic receptor function and gene expression in cultured airway parasympathetic neurons. J Clin Invest 1998;102:242–8. doi:10.1172/JCI1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacoby DB, Yost BL, Kumaravel B et al. . Glucocorticoid treatment increases inhibitory m(2) muscarinic receptor expression and function in the airways. Am J Respir Cell Mol Biol 2001;24:485–91. doi:10.1165/ajrcmb.24.4.4379 [DOI] [PubMed] [Google Scholar]
- 84.Lowry R, Wood A, Higenbottam T. The effect of anticholinergic bronchodilator therapy on cough during upper respiratory tract infections. Br J Clin Pharmacol 1994;37:187–91. doi:10.1111/j.1365-2125.1994.tb04259.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puhakka T, Mäkelä MJ, Malmström K et al. . The common cold: effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol 1998;101:726–31. doi:10.1016/S0091-6749(98)70301-X [DOI] [PubMed] [Google Scholar]
- 86.Winther B, Gwaltney JM, Hendley JO. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am Rev Respir Dis 1990;141:839–45. doi:10.1164/ajrccm/141.4_Pt_1.839 [DOI] [PubMed] [Google Scholar]
- 87.Monto AS, Gravenstein S, Elliott M et al. . Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000;160:3243–7. doi:10.1001/archinte.160.21.3243 [DOI] [PubMed] [Google Scholar]
- 88.Rezaee F, Meednu N, Emo JA et al. . Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. J Allergy Clin Immunol 2011;128:1216–24.e11 doi:10.1016/j.jaci.2011.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeo N-K, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope 2010;120:346–52. doi:10.1002/lary.20764 [DOI] [PubMed] [Google Scholar]
- 90.Sajjan U, Wang Q, Zhao Y et al. . Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 2008;178:1271–81. doi:10.1164/rccm.200801-136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reiter B, Kraft R, Günzel D et al. . TRPV4-mediated regulation of epithelial permeability. FASEB J 2006;20:1802–12. doi:10.1096/fj.06-5772com [DOI] [PubMed] [Google Scholar]
- 92.Yamaya M, Sekizawa K, Masuda T et al. . Oxidants affect permeability and repair of the cultured human tracheal epithelium. Am J Physiol 1995;268:L284–93. [DOI] [PubMed] [Google Scholar]
- 93.Comstock AT, Ganesan S, Chattoraj A et al. . Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 2011;85:6795–808. doi:10.1128/JVI.02074-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaul P, Biagioli MC, Singh I et al. . Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J Infect Dis 2000;181:1885–90. doi:10.1086/315504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Unger BL, Ganesan S, Comstock AT et al. . Nod-like receptor X-1 is required for rhinovirus-induced barrier dysfunction in airway epithelial cells. J Virol 2014;88:3705–18. doi:10.1128/JVI.03039-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnoult D, Soares F, Tattoli I et al. . An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci 2009;122:3161–8. doi:10.1242/jcs.051193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tattoli I, Carneiro LA, Jéhanno M et al. . NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-κB and JNK pathways by inducing reactive oxygen species production. EMBO Rep 2008;9:293–300. doi:10.1038/sj.embor.7401161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawai T, Takahashi K, Sato S et al. . IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol 2005;6:981–8. doi:10.1038/ni1243 [DOI] [PubMed] [Google Scholar]
- 99.Meylan E, Curran J, Hofmann K et al. . Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 2005;437:1167–72. doi:10.1038/nature04193 [DOI] [PubMed] [Google Scholar]
- 100.Seth RB, Sun L, Ea C-K et al. . Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005;122:669–82. doi:10.1016/j.cell.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 101.Hewson CA, Jardine A, Edwards MR et al. . Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol 2005;79:12273–9. doi:10.1128/JVI.79.19.12273-12279.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soyka MB, Wawrzyniak P, Eiwegger T et al. . Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol 2012;130:1087–96.e10 doi:10.1016/j.jaci.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 103.Bruewer M, Luegering A, Kucharzik T et al. . Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol 2003;171:6164–72. doi:10.4049/jimmunol.171.11.6164 [DOI] [PubMed] [Google Scholar]
- 104.Biagioli MC, Kaul P, Singh I et al. . The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic Biol Med 1999;26:454–62. doi:10.1016/S0891-5849(98)00233-0 [DOI] [PubMed] [Google Scholar]
- 105.Wang JH, Kwon HJ, Jang YJ. Rhinovirus upregulates matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor expression in nasal polyp fibroblasts. Laryngoscope 2009;119:1834–8. doi:10.1002/lary.20574 [DOI] [PubMed] [Google Scholar]
- 106.Leigh R, Oyelusi W, Wiehler S et al. . Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol 2008;121:1238–45.e4 doi:10.1016/j.jaci.2008.01.067 [DOI] [PubMed] [Google Scholar]
- 107.Psarras S, Volonaki E, Skevaki CL et al. . Vascular endothelial growth factor-mediated induction of angiogenesis by human rhinoviruses. J Allergy Clin Immunol 2006;117:291–7. doi:10.1016/j.jaci.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 108.Newcomb DC, Sajjan US, Nagarkar DR et al. . Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med 2008;177:1111–21. doi:10.1164/rccm.200708-1243OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bossios A, Psarras S, Gourgiotis D et al. . Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir Res 2005;6:114 doi:10.1186/1465-9921-6-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuta A, Doyle WJ, Gaumond E et al. . Rhinovirus infection induces mucus hypersecretion. Am J Physiol Lung Cell Mol Physiol 1998;274:L1017–23. [DOI] [PubMed] [Google Scholar]
- 111.Phipps RJ, Richardson PS. The effects of irritation at various levels of the airway upon tracheal mucus secretion in the cat. J Physiol 1976;261:563–81. doi:10.1113/jphysiol.1976.sp011574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hewson CA, Haas JJ, Bartlett NW et al. . Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-κB and EGFR pathways. Eur Respir J 2010;36:1425–35. doi:10.1183/09031936.00026910 [DOI] [PubMed] [Google Scholar]
- 113.Zhu L, Lee P-K, Lee W-M et al. . Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol 2009;40:610–19. doi:10.1165/rcmb.2008-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inoue D, Yamaya M, Kubo H et al. . Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir Physiol Neurobiol 2006;154:484–99. doi:10.1016/j.resp.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 115.Zhu Z, Tang W, Gwaltney JM et al. . Rhinovirus stimulation of interleukin-8 in vivo and in vitro: role of NF-kappaB. Am J Physiol 1997;273:L814–24. [DOI] [PubMed] [Google Scholar]
- 116.Zhu Z, Tang W, Ray A et al. . Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest 1996;97:421–30. doi:10.1172/JCI118431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Casalino-Matsuda SM, Monzón ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 2006;34:581–91. doi:10.1165/rcmb.2005-0386OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishihara H, Shimura S, Satoh M et al. . Muscarinic receptor subtypes in feline tracheal submucosal gland secretion. Am J Physiol 1992;262:L223–8. [DOI] [PubMed] [Google Scholar]
- 119.Ramnarine SI, Haddad EB, Khawaja AM et al. . On muscarinic control of neurogenic mucus secretion in ferret trachea. J Physiol 1996;494(Pt 2):577–86. doi:10.1113/jphysiol.1996.sp021515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iwase N, Sasaki T, Oshiro T et al. . Differential effect of epidermal growth factor on serous and mucous cells in porcine airway submucosal gland. Respir Physiol Neurobiol 2002;132:307–19. doi:10.1016/S1569-9048(02)00118-0 [DOI] [PubMed] [Google Scholar]
- 121.Kanno H, Horikawa Y, Hodges RR et al. . Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. AJP Cell Physiol 2003;284:C988–98. doi:10.1152/ajpcell.00582.2001 [DOI] [PubMed] [Google Scholar]
- 122.Korfhagen TR, Kitzmiller J, Chen G et al. . SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA 2012;109:16630–5. doi:10.1073/pnas.1208092109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park K-S, Korfhagen TR, Bruno MD et al. . SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007;117:978–88. doi:10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oettgen P, Finger E, Sun Z et al. . PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem 2000;275:1216–25. doi:10.1074/jbc.275.2.1216 [DOI] [PubMed] [Google Scholar]
- 125.Chen G, Korfhagen TR, Xu Y et al. . SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–24. doi:10.1172/JCI39731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bai J, Miao B, Wu X et al. . Enhanced expression of SAM-pointed domain-containing Ets-like factor in chronic rhinosinusitis with nasal polyps. Laryngoscope 2015;125:E97–103. doi:10.1002/lary.25008 [DOI] [PubMed] [Google Scholar]
- 127.Kreda SM, Seminario-Vidal L, van Heusden CA et al. . Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 2010;588:2255–67. doi:10.1113/jphysiol.2009.186643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seminario-Vidal L, Okada SF, Sesma JI et al. . Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem 2011;286:26277–86. doi:10.1074/jbc.M111.260562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ransford GA, Fregien N, Qiu F et al. . Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 2009;41:525–34. doi:10.1165/rcmb.2008-0367OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 2004;572:65–8. doi:10.1016/j.febslet.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 131.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 2006;580:239–44. doi:10.1016/j.febslet.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 132.Lieb T, Frei CW, Frohock JI et al. . Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 2002;538:633–46. doi:10.1113/jphysiol.2001.013222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Korngreen A, Priel Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J Physiol 1996;497(Pt 1):53–66. doi:10.1113/jphysiol.1996.sp021749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iglesias R, Locovei S, Roque A et al. . P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 2008;295:C752–60. doi:10.1152/ajpcell.00228.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Locovei S, Scemes E, Qiu F et al. . Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 2007;581:483–8. doi:10.1016/j.febslet.2006.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 2006;25:5071–82. doi:10.1038/sj.emboj.7601378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan Z, Khadra A, Li S et al. . Experimental characterization and mathematical modeling of P2X7 receptor channel gating. J Neurosci 2010;30:14213–24. doi:10.1523/JNEUROSCI.2390-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang L-H, Rassendren F, Mackenzie A et al. . N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol 2005;289:C1295–302. doi:10.1152/ajpcell.00253.2005 [DOI] [PubMed] [Google Scholar]
- 139.Alenmyr L, Uller L, Greiff L et al. . TRPV4-mediated calcium influx and ciliary activity in human native airway epithelial cells. Basic Clin Pharmacol Toxicol 2014;114:210–16. doi:10.1111/bcpt.12135 [DOI] [PubMed] [Google Scholar]
- 140.Lorenzo IM, Liedtke W, Sanderson MJ et al. . TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci USA 2008;105:12611–16. doi:10.1073/pnas.0803970105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andrade YN, Fernandes J, Vázquez E et al. . TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 2005;168:869–74. doi:10.1083/jcb.200409070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watanabe H, Vriens J, Prenen J et al. . Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003;424:434–8. doi:10.1038/nature01807 [DOI] [PubMed] [Google Scholar]
- 143.Fernandes J, Lorenzo IM, Andrade YN et al. . IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5ʹ-6ʹ-epoxyeicosatrienoic acid. J Gen Physiol 2008;131:i2 doi:10.1085/JGP1315OIA2 [DOI] [PubMed] [Google Scholar]
- 144.Fernández-Fernández JM, Andrade YN, Arniges M et al. . Functional coupling of TRPV4 cationic channel and large conductance, calcium-dependent potassium channel in human bronchial epithelial cell lines. Pflugers Arch 2008;457:149–59. doi:10.1007/s00424-008-0516-3 [DOI] [PubMed] [Google Scholar]
- 145.Liu X, Bandyopadhyay BC, Bandyopadhyay B et al. . A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 2006;281:15485–95. doi:10.1074/jbc.M600549200 [DOI] [PubMed] [Google Scholar]
- 146.Taylor-Clark TE, Undem BJ. Sensing pulmonary oxidative stress by lung vagal afferents. Respir Physiol Neurobiol 2011;178:406–13. doi:10.1016/j.resp.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grünberg K, Timmers MC, Smits HH et al. . Effect of experimental rhinovirus 16 colds on airway hyperresponsiveness to histamine and interleukin-8 in nasal lavage in asthmatic subjects in vivo. Clin Exp Allergy 1997;27:36–45. doi:10.1111/j.1365-2222.1997.tb00670.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Empey DW, Laitinen LA, Jacobs L et al. . Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis 1976;113:131–9. doi:10.1164/arrd.1976.113.2.131 [DOI] [PubMed] [Google Scholar]
- 149.Madison JM, Irwin RS. Pharmacotherapy of chronic cough in adults. Expert Opin Pharmacother 2003;4:1039–48. doi:10.1517/14656566.4.7.1039 [DOI] [PubMed] [Google Scholar]
- 150.Myers AC, Kajekar R, Undem BJ. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am J Physiol Lung Cell Mol Physiol 2002;282:L775–81. doi:10.1152/ajplung.00353.2001 [DOI] [PubMed] [Google Scholar]
- 151.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 2005;569:559–73. doi:10.1113/jphysiol.2005.093153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lieu TM, Myers AC, Meeker S et al. . TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 2012;302:L941–8. doi:10.1152/ajplung.00366.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lieu T, Kollarik M, Myers AC et al. . Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol 2011;300:L790–8. doi:10.1152/ajplung.00449.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hunter DD, Myers AC, Undem BJ. Nerve growth factor-induced phenotypic switch in guinea pig airway sensory neurons. Am J Respir Crit Care Med 2000;161:1985–90. doi:10.1164/ajrccm.161.6.9908051 [DOI] [PubMed] [Google Scholar]
- 155.Neumann S, Doubell TP, Leslie T et al. . Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 1996;384:360–4. doi:10.1038/384360a0 [DOI] [PubMed] [Google Scholar]
- 156.Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc B Biol Sci 1996;351:441–8. doi:10.1098/rstb.1996.0040 [DOI] [PubMed] [Google Scholar]
- 157.Ganju P, O'Bryan JP, Der C et al. . Differential regulation of SHC proteins by nerve growth factor in sensory neurons and PC12 cells. Eur J Neurosci 1998;10:1995–2008. doi:10.1046/j.1460-9568.1998.00209.x [DOI] [PubMed] [Google Scholar]
- 158.Othumpangat S, Regier M, Piedimonte G. Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression. Am J Physiol Lung Cell Mol Physiol 2012;302:L1057–66. doi:10.1152/ajplung.00365.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.El-Hashim AZ, Jaffal SM, Al-Rashidi FT et al. . Nerve growth factor enhances cough via a central mechanism of action. Pharmacol Res 2013;74:68–77. doi:10.1016/j.phrs.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 160.El-Hashim AZ, Jaffal SM. Nerve growth factor enhances cough and airway obstruction via TrkA receptor- and TRPV1-dependent mechanisms. Thorax 2009;64:791–7. doi:10.1136/thx.2009.113183 [DOI] [PubMed] [Google Scholar]
- 161.Bonvini SJ, Birrell MA, Grace MS et al. . TRPV4 and activation of airway sensory nerves: the role of ATP. Presented at Pharmacology 2014 Basic pharmacology oral communications (VII) 2014. [Google Scholar]
- 162.Aizawa N, Wyndaele J-J, Homma Y et al. . Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn 2012;31:148–55. doi:10.1002/nau.21212 [DOI] [PubMed] [Google Scholar]
- 163.Kamei J, Takahashi Y. Involvement of ionotropic purinergic receptors in the histamine-induced enhancement of the cough reflex sensitivity in guinea pigs. Eur J Pharmacol 2006;547:160–4. doi:10.1016/j.ejphar.2006.07.034 [DOI] [PubMed] [Google Scholar]
- 164.Basoglu OK, Barnes PJ, Kharitonov SA et al. . Effects of aerosolized adenosine 5ʹ-triphosphate in smokers and patients with Chronic Obstructive Pulmonary Disease. Chest 2015;148:430–5. doi:10.1378/chest.14-2285 [DOI] [PubMed] [Google Scholar]
- 165.Basoglu OK, Pelleg A, Essilfie-Quaye S et al. . Effects of aerosolized adenosine 5ʹ-triphosphate vs adenosine 5ʹ-monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest 2005;128:1905–9. doi:10.1378/chest.128.4.1905 [DOI] [PubMed] [Google Scholar]
- 166.Abdulqawi R, Dockry R, Holt K et al. . P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 2015;385:1198–205. doi:10.1016/S0140-6736(14)61255-1 [DOI] [PubMed] [Google Scholar]
- 167.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 2012;15:1063–7. doi:10.1038/nn.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache 2006;46(Suppl 1):S3–8. doi:10.1111/j.1526-4610.2006.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Barnes PJ. Neurogenic inflammation in the airways. Respir Physiol 2001;125:145–54. doi:10.1016/S0034-5687(00)00210-3 [DOI] [PubMed] [Google Scholar]
- 170.Gentile DA, Skoner DP. Viral rhinitis. Curr Allergy Asthma Rep 2001;1:227–34. doi:10.1007/s11882-001-0009-3 [DOI] [PubMed] [Google Scholar]
- 171.Diogenes A, Ferraz CCR, Akopian AN et al. . LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res 2011;90:759–64. doi:10.1177/0022034511400225 [DOI] [PubMed] [Google Scholar]
- 172.Qi J, Buzas K, Fan H et al. . Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J Immunol 2011;186:6417–26. doi:10.4049/jimmunol.1001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu T, Xu Z-Z, Park C-K et al. . Toll-like receptor 7 mediates pruritus. Nat Neurosci 2010;13:1460–2. doi:10.1038/nn.2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Park C-K, Xu Z-Z, Berta T et al. . Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014;82:47–54. doi:10.1016/j.neuron.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Winkler CW, Taylor KG, Peterson KE. Location is everything: let-7b microRNA and TLR7 signaling results in a painful TRP. Sci Signal 2014;7:pe14 doi:10.1126/scisignal.2005407 [DOI] [PubMed] [Google Scholar]
- 176.Souslova V, Cesare P, Ding Y et al. . Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 2000;407:1015–17. doi:10.1038/35039526 [DOI] [PubMed] [Google Scholar]
- 177.Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol 2005;25:819–32. doi:10.1007/s10571-005-4936-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Binshtok AM, Wang H, Zimmermann K et al. . Nociceptors are interleukin-1beta sensors. J Neurosci 2008;28:14062–73. doi:10.1523/JNEUROSCI.3795-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci 1997;17:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nagata K, Duggan A, Kumar G et al. . Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 2005;25:4052–61. doi:10.1523/JNEUROSCI.0013-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Corey DP, García-Añoveros J, Holt JR et al. . TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004;432:723–30. doi:10.1038/nature03066 [DOI] [PubMed] [Google Scholar]
- 182.Kwan KY, Allchorne AJ, Vollrath MA et al. . TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006;50:277–89. doi:10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 183.Tritsch NX, Yi E, Gale JE et al. . The origin of spontaneous activity in the developing auditory system. Nature 2007;450:50–5. doi:10.1038/nature06233 [DOI] [PubMed] [Google Scholar]
- 184.Araki I. TRP channels in urinary bladder mechanosensation. In: Islam S, ed.. Transient receptor potential channels: advances in experimental medicine and biology, 2011:861–79. [DOI] [PubMed] [Google Scholar]
- 185.Andersson K-E, Gratzke C, Hedlund P. The role of the transient receptor potential (TRP) superfamily of cation-selective channels in the management of the overactive bladder. BJU Int 2010;106:1114–27. doi:10.1111/j.1464-410X.2010.09650.x [DOI] [PubMed] [Google Scholar]
- 186.Kaan TKY, Yip PK, Grist J et al. . Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci 2010;30:4503–7. doi:10.1523/JNEUROSCI.6132-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Laing RJ, Dhaka A. ThermoTRPs and Pain. Neuroscientist 2015. Published Online First: 21 January 2015 doi:10.1177/1073858414567884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol 2013;29:355–84. doi:10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- 189.Ulmann L, Hatcher JP, Hughes JP et al. . Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 2008;28:11263–8. doi:10.1523/JNEUROSCI.2308-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chessell IP, Hatcher JP, Bountra C et al. . Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005;114:386–96. doi:10.1016/j.pain.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 191.McGaraughty S, Wismer CT, Zhu CZ et al. . Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol 2003;140:1381–8. doi:10.1038/sj.bjp.0705574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cockayne DA, Hamilton SG, Zhu QM et al. . Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000;407:1011–15. doi:10.1038/35039519 [DOI] [PubMed] [Google Scholar]
- 193.Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal 2012;8:41–56. doi:10.1007/s11302-011-9272-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guo A, Vulchanova L, Wang J et al. . Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 1999;11:946–58. doi:10.1046/j.1460-9568.1999.00503.x [DOI] [PubMed] [Google Scholar]
- 195.Abdullah H, Heaney LG, Cosby SL et al. . Rhinovirus upregulates transient receptor potential channels in a human neuronal cell line: implications for respiratory virus-induced cough reflex sensitivity. Thorax 2014;69:46–54. doi:10.1136/thoraxjnl-2013-203894 [DOI] [PubMed] [Google Scholar]
- 196.Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc 2005;2:150–6. doi:10.1513/pats.200502-018AW [DOI] [PubMed] [Google Scholar]
- 197.McGarvey LP, Butler CA, Stokesberry S et al. . Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol 2014;133:704–12.e4 doi:10.1016/j.jaci.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 198.Mitchell JE, Campbell AP, New NE et al. . Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res 2005;31:295–306. doi:10.1080/01902140590918803 [DOI] [PubMed] [Google Scholar]
- 199.Groneberg DA, Niimi A, Dinh QT et al. . Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 2004;170:1276–80. doi:10.1164/rccm.200402-174OC [DOI] [PubMed] [Google Scholar]
- 200.Dicpinigaitis PV, Spinner L, Santhyadka G et al. . Effect of tiotropium on cough reflex sensitivity in acute viral cough. Lung 2008;186:369–74. doi:10.1007/s00408-008-9114-6 [DOI] [PubMed] [Google Scholar]
- 201.Pratter MR. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest 2006;129:72S–4S. doi:10.1378/chest.129.1_suppl.72S [DOI] [PMC free article] [PubMed] [Google Scholar]