Abstract
Recent studies continue to support the proposition that non-neuronal components of the nervous system, mainly glial cells and associated chemical mediators, contribute to the development of neuronal hyperexcitability that underlies persistent pain conditions. In the event of peripheral injury, enhanced or abnormal nerve input is likely the most efficient way to activate simultaneously central neurons and glia. Injury induces phenotypic changes in glia and triggers signaling cascades that engage reciprocal interactions between presynaptic terminals, postsynaptic neurons, microglia and astrocytes. While some responses to peripheral injury may help the nervous system to adapt positively to counter the disastrous effect of injury, the net effect often leads to long-lasting sensitization of pain transmission pathways and chronic pain.
Introduction
Most recent studies continue to support the proposition that non-neuronal components of the nervous system, mainly glial cells and associated chemical mediators, contribute to the development of neuronal hyperexcitability that underlies persistent pain conditions. As microglia are considered innate immune cells of the so-called immune-privileged brain, their responses to peripheral injury with collaborative involvement of astroglia and cytokines are considered a type of neuroinflammation [1,2]. This type of neuroinflammation, however, is remote from the site of injury and characteristically distinct from the conventional meaning of “inflammation”. It is generally devoid of cardinal signs of inflammation in the brain and spinal cord, not necessarily deleterious to neurotransmission as seen in other degenerative neurological diseases, and most importantly, it depends upon enhanced afferent neuronal activity after peripheral injury. Thus, the central “neuroinflammation” induced by peripheral injury is deemed “neurogenic” [2-4], which consists of a plethora of mutual signaling between neurons and glia via chemical mediators and their receptors. While some of these responses to peripheral injury may help the central nervous system (CNS) to adapt positively to counter the disastrous effect of injury, the net effect often leads to long-lasting sensitization of pain transmission pathways and chronic pain. We will briefly discuss some recent literature on injury-induced central neuron-glial interactions and their significance in persistent pain.
Glial response to peripheral injury in humans
Despite ample evidence from animal studies, it has been a challenge to directly demonstrate the involvement of glia in human chronic pain conditions [see 5]. Indirect evidence suggests that humans also exhibit glial response to injury. Increased levels of astroglial marker glial fibrillary acidic protein (GFAP) and S100β were observed in postmortem spinal cord dorsal horn tissues from Human Immunodeficiency Virus (HIV) patients with chronic pain [6]. Increased CNS inflammatory cytokine levels in chronic pain patients have been observed [see 7].
Utilizing an improved in vivo marker of glial activation with integrated positron emission tomography (PET)-magnetic resonance imaging (MRI), Loggia et al [8**] have recently provided the first observation suggesting brain glial activation in chronic pain patients. They imaged the translocator protein (18kDa) (TSPO) through its specific binding to a newly developed PET radio ligand 11C-PBR28 in patients suffering from chronic low back pain. The TSPO was first described as the peripheral-type benzodiazepine receptor or recognition site [9]. It was found later that TSPO was also expressed in microglia, astrocytes and neurons [see 10, 11]. Interestingly, TSPO has been selectively upregulated in spinal astrocytes and microglia, but not in neurons, following L5 spinal nerve injury in rats [11] and has been used as a marker of increased glial activity after CNS injury in imaging studies [see 8**]. Peripheral immune challenge with lipopolysaccharide (LPS) induced an increased TSPO binding in the mouse brain with another TSPO PET ligand 18F-PBR06 [12].
In comparison between matched pairs controlling TSPO polymorphism, age and sex, the levels of TSPO, assessed by standardized uptake values for 11C-PBR28, was significantly increased in the thalamus and other regions including the insula, middle and posterior cingulate cortex, and ventromedial prefrontal cortex in patients with low back pain. These observations are consistent with previous report that limb denervation injury leads to long-lasting increase in binding of 11C(R)-PK11195, the earlier generation of the TSPO ligand, in the human thalamus [13]. The increased 11C-PBR28 tracer binding appeared somatotopically relevant in the somatosensory and motor cortices receiving input related to the pain in the lower back and leg, indicating correlation of increased glial activity with pain-related neuronal input [8**].
Activity-dependent microglial activity
Microglia are sensitive to their environment. In their resting state, microglia constantly scan and monitor their surroundings and respond to changes in homeostasis [see 14-16]. In a broader sense, changes in the extracellular milieu can also be induced by injury distant from the CNS, resulting from influx of chemical mediators and neurotransmitters released from afferent terminals activated by enhanced or abnormal input activity after injury. Studies have shown the contribution of primary afferent input to increased microglial activity after injury [17] and the development of pain-related behavior is delayed after peripheral nerve block [18]. In fact, peripheral nerve injury leads to increased activity of microglia in brain regions directly related to pain, mood and affect, as well as reward circuitry [19].
Microglia maintain a relationship with neurons, which positions microglia to perceive changes in neuronal activity. In the cortex, Baalman et al. [20] show that a subset of perineuronal microglia are specifically associated with the axon initial segment through a single process. Cilium-like protrusions of microglial processes closely appose or wrap around dendritic spines, presynaptic terminals, synaptic cleft and even perisynaptic astrocytic processes (Fig. 1A,B) [21]. It is interesting to note that microglia adjust their morphology and location in a dynamic fashion to sensory input. Tremblay et al. [21] demonstrate that altering light exposure induces changes in microglia processes in the visual cortex of juvenile mice, involving microglial contact/apposition with synaptic structures and motility of microglial processes. Visual deprivation reduced microglial process motility, which was reversed after light-reexposure. Evo et al. [22*] observed that microglial processes converge at dendritic spines in mouse brain slices in response to reduced extracellular calcium. They describe this phenomenon as “microglial process convergence”, which is apparently mediated by purinergic signaling. Conceivably, a reduction in extracellular calcium might mimic the effect of intense synaptic activity. Even in the resting state, the transient contacts of microglial processes with synaptic neural elements are modulated by neuronal activity [23]. Thus, microglia behavior is clearly regulated by sensory-driven neural activity.
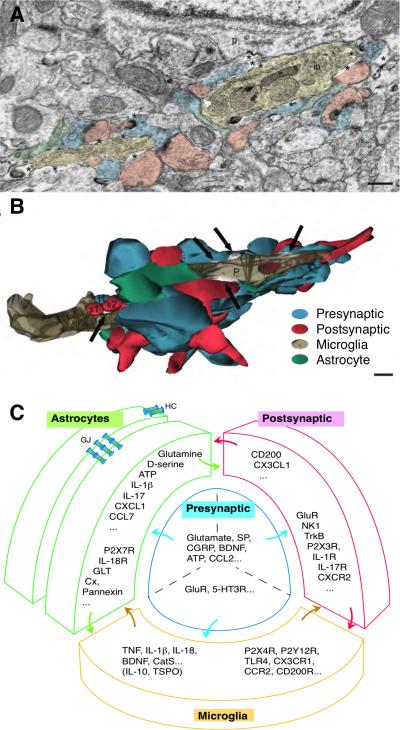
Fig. 1.
The tetrapartite model of the neuron-glial interactions. A. Electron microscope image showing oppositions between the presynaptic axon terminals (blue), postsynaptic dendritic spines (pink), microglia (beige) and perisynaptic astrocytes (green). Note a microglia contiguous to a neuronal perikaryon (p) with its associated extracellular space (asterisks) and contacted synapse-associated elements. in, cellular inclusion. Scale = 250 nm. (Adapted from [21] Tremblay et al. PLoS Biology, 2010, 8(11):e1000527, Fig. 2A.) B. Partial 3-D reconstruction of the microglial proximal process (P) cut in transverse. Note that microglial processes directly contact multiple presynaptic axon terminals (blue), postsynaptic dendritic spines (red), and perisynaptic astrocytic processes (green). Similar cellular relationship exists for axon terminals, dendrites and astrocytes. Black arrows indicate extracellular space pockets of various size and shape (white). s1, s2, two dendritic spines; t1, one axon terminal. Scale = 250 nm. (Adapted from [21] Tremblay et al. PLoS Biology, 2010, 8(11):e1000527, Fig. 2B.) C. The tetrapartite model of the neuron-glial interactions in spinal nociceptive processing. Note mutual contacts between all four components of the model. Example lists of neurotransmitters/modulators, cytokines/chemokines, growth factors, proteases, and their receptors/substrates involved in nociceptive processing are shown in respective compartments. Two astrocytes are shown connected by astrocytic gap junctions. Abbreviations: 5-HT, serotonin; BDNF, brain-derived neurotrophic factor; CatS, cysteine protease cathepsin S ; CCL2, chemokine (C-C motif) ligand 2; CCL7, Chemokine (C-C motif) ligand 7; CCR2, C-C chemokine receptor type 2; CGRP, calcitonin gene-related peptide; CX3CL1, chemokine (C-X3-C motif) ligand 1; CX3CR1, CX3C chemokine receptor 1; CXCL1, chemokine (C-X-C motif) ligand 1; Cx, connexon; CXCR2, CXC chemokine receptor 2; HC, connexin hemichannel; IL, interleukin; GJ, gap junctions; GLT, glutamate transporter; GluR, glutamate receptors; NK1, neurokinin 1; R, receptor; SP, substance P; TNF, tumor necrosis factor; TrkB, Tropomyosin receptor kinase B; TSPO, translocator protein; TLR4, Toll-like receptor 4;
The microglial cell membrane expresses a diversity set of receptors. What chemical signals do microglia pick up as a sign of injury? Among a handful of candidates, purines, as neurotransmitters, appear on the top of the list [15, 24-28*], although ATP could also be derived from other sources such as astrocytes [29]. Microglia-neuronal contact alters with changes in neuronal input. In P2Y12 receptor (P2Y12R) knock-out mice, microglial process convergence towards neuronal dendrites was eliminated [22*]. Tatsumi et al [30] recently show that microglial P2Y12R signaling following nerve injury involves downstream Rho-associated protein kinase (ROCK) that acts at actin. The ROCK inhibitor H1152 attenuated neuropathic pain behavior and reversed nerve injury and 2Me-SADP-induced retraction of microglial processes, implicating an effect on pain-related microglial motility.
The other top candidate that mediates neuronal input to microglia is the chemokine CX3CL1 [chemokine (C-X3-C motif) ligand 1], or fractalkine [31-35]. CX3CL1 is expressed in spinal cord dorsal horn and dorsal root ganglion neurons but not glia and its receptor CX3C chemokine receptor 1 (CX3CR1) is expressed exclusively in microglia [36]. Mice lacking CX3CR1 exhibited a delay in the development of allodynia following administration of the chemotherapeutic agent vincristine [37]. Direct application of CX3CL1 to spinal slices strengthens synaptic transmission between primary afferent C-fibers and dorsal horn lamina I neurons via microglial activity [38*]. Interestingly, this synaptic facilitatory effect of CX3CL1 does not depend on neural activity, which is consistent with a cascade related to CX3CL1/CX3CR1 signaling after peripheral injury. The functional chemokine domain of CX3CL1 is normally tethered to the membrane and released after cleaving by proteases. The cleavage of CX3CL1 is achieved by the cysteine protease cathepsin S (CatS) from microglia. Nerve injury induces CatS in spinal microglia in the region receiving damaged primary afferent terminals, followed by CX3CL1 cleavage [see 34]. Thus, direct application of CX3CL1 may bypass the cleavage step of the membrane-tethered CX3CL1 and produces an effect that is downstream to initial injury-related neuronal input.
There is still much to be learned on the nature of inputs that switch microglia from a surveying phenotype to an active state; and it is not fully understood how glial cells decode information encoded by multiple integrated chemical mediators. It is interesting to note that application of ATP alone promoted motility of microglia, while application of a list of neurotransmitters [glutamate, substance P, gamma-Aminobutyric acid (GABA), serotonin (5-HT) etc.], neurotrophic factors (nerve growth factor, brain-derived neurotrophic factor), and chemokines [chemokine (C-C motif) ligand 2 (CCL2), CX3CL1 etc.], even direct nerve stimulation, had no effect [22*,39]. To induce release of CatS in microglia, ATP alone was insufficient. Only with priming by LPS, ATP activates P2X7R on microglia and leads to release of CatS. It seems that to reproduce a full spectrum of ‘activated’ microglia phenotype after injury, a combination of signals that more closely mimic in vivo conditions are necessary. The factors that help to maintain microglia in the resting state are also worth attention. CD200R is expressed in microglia and interacts with neuronal CD200, the OX-2 membrane glycoprotein. Loss of CD200-CD200R signaling facilitates microglial activation in CNS after peripheral injury [see 32]. Down-regulation of CD200/CD200R1 in the knee synovium is associated with a painful condition [40].
Astroglia and neuronal activity after injury
Unlike microglia developed from macrophages of mesodermal hematopoietic cells, astrocytes are derived from the ectoderm and are involved in synaptic activity through their intimate anatomical relationship with neurons originating from the same germ layer. Ample evidence supports a role of astrocytes in the development of persistent pain hypersensitivity after injury [41-43]. Inhibition of astrocyte activity has been associated with the antihyperalgesic effect of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist [44]. Direct nerve stimulation induced astroglial activity and cytokine release [45] and prolonged local anesthesia block of afferent neurons delayed the onset of pain-related behavior and reduced nerve injury-induced astrocytic responses [46], indicating that astrocytic response is regulated by neural input. Studies have shown that activation of microglia usually preceded reactive astrocytosis after injury [47,48*]. However, this phenomenon is not universal. In chemotherapy-induced peripheral neuropathic pain, glial activation is only seen for astrocytes, but not microglia [49, also see 50]. In a female bone cancer pain model, reactive astrocytosis occurred earlier than microglial activation and microglia did not appear to be involved in the initiation, but was critical in the maintenance, of persistent pain hypersensitivity [28*].
Astrocytes release gliotransmitters that contribute to synaptic activity. D-serine as a coagonist of the NMDA (N-methyl-D-aspartate, GluN) receptor is released from astrocytes. From an activity-dependent point of view, a recent work shows that release of D-serine from astrocytes in culture can be induced by ATP through astrocytic P2X7 receptors [51]. This release is not calcium-dependent, consistent with growing awareness that astrocytic Ca2+ dynamics is not necessarily linked to synaptic activity [see 52]. The ATP-stimulated D-serine release from astrocytes, however, requires functional pannexin-1 channels [51]. Pannexin-1 is homologous to the invertebrate gap junction protein innexin, but does not structurally bridge neighboring cells [53]. The major function of pannexins is to facilitate communication between the extra- and intra-cellular compartments. Apparently, pannexin plays a role in neuron-astrocytic signaling after injury. Blocking pannexin-1 attenuated neuropathic pain behavior in rats [54].
A unique contribution of astrocytes to excitatory synaptic activity is removal of glutamate from the extracellular fluid through glutamate transporter-1 (GLT-1, EAAT2), which is a major determinant of glutamate receptor activation and related neuronal excitability. Peripheral nerve injury down-regulates GLT-1 in the spinal cord dorsal horn [55], which may lead to accumulation of glutamate in the synaptic cleft. Restoring GLT-1 with ceftriaxone relieved hyperalgesia [56]. The down-regulation of GLT-1 results in an enhanced extrasynaptic NMDA receptor activation, likely involving glutamate spillover to the extrasynaptic site [57].
It is important to appreciate that the activity of GLT-1 is not limited to glutamate recycling. Sodium is cotransported with glutamate into astrocytes so that neuronal sodium load can be reduced. Sodium imaging in brain slices shows that neuronal activity triggers a transient increase in Na+ in astrocytes [58, also see 59]. Interruption of glutamate transport and associated uptake of extracellular K+ via activity of astrocytic Na+-K+ ATPase led to elevated neuronal sodium and prolonged epileptiform burst activity in mouse hippocampal slices [58]. Thus, astrocytes are crucially important for neuronal sodium homeostasis particularly in the phase of intense activity.
Astrocytes form functional syncytium through gap junction proteins such as connexin43 (Cx43, GJ1). Six connexins assemble into a connexon and two connexons from adjacent cells face each other to form a gap junction channel. Gap junctions allow flow of ions and chemical mediators thus facilitating intercellular communication. Connexons that are not opposing a counterpart from other cells form hemichannels that provide an additional form of intra- and extra-cellular communication. Administration of carbenoxolone, a non-selective gap junction decoupler, has been shown to attenuate behavioral pain hypersensitivity in animal models [48*, 60-63], suggesting that astrocytic gap junctions are involved in persistent pain. Potential involvement of gap junctions in persistent pain gives a hint on the mechanisms underlying spread of excitation to a non-injured territory, possibly mirroring pain that occurs contralateral to the site of injury [60].
The proposed contribution of astrocytic gap junctions to persistent pain, however, requires further attention. In an in vitro astrocyte scratch injury model, the spread of injury involves an increase in Cx43 hemichannel, but not gap junction channel activity [64]. In mouse hippocampal slices, LPS induces astroglial Cx43 hemichannel opening without an effect on gap junction activity [65]. Chen et al. [48*] show that tumor necrosis factor (TNF)-activated astroctytes induce mechanical allodynia involving Cx43. However, the effect of TNF was on Cx43 hemichannel activity in astrocytes as shown by ethidium bromide uptake in cultures, but not on gap junction channels [48*]. While an involvement of astrocytic gap junctions cannot be ruled out, further studies should distinguish the role of Cx hemichannels in injury-induced pain hypersensitivity.
Integrated neuron-glial signaling in persistent pain
Studies on neuron-immune interactions in pain have led to a concept that the basic functional unit for spinal/trigeminal pain processing is tetrapartite, likely consisting of four components, presynaptic terminals (primary afferents), postsynaptic neurons/dendrites, and astrocyte and microglial processes [66-68]. Although there is no direct evidence at the spinal level, this concept is supported by functional anatomy data from brain that demonstrate mutual contacts between the four components in the brain and their dynamic sensitivity to sensory input and signaling molecules [20-22*] (Fig. 1).
Ample evidence has shown comprehensive reciprocal neuron-glial and glia-glial signaling following peripheral nerve or tissue injury, involving neurotransmitters/modulators, cytokines/chemokines, growth factors, proteases, and their receptors/substrates [69,70]. For example (Fig. 1C), ATP released from primary afferent central terminals can engage P2X3R on neurons [see 26], P2X4R/P2Y12R on microglia [see 24,30], and P2X7R on astrocytes [51]. Microglia release CatS that cleaves CX3CL1 tethered to spinal neurons and TNF/interleukin (IL)-18 that acts on astrocytes [48*, 71**]. Astrocytes can interact with C-C chemokine receptor type 2 (CCR2) on microglia through release of Chemokine (C-C motif) ligand 7 (CCL7) [72], and with chemokine (C-X-C motif) receptor 2 (CXCR2) on neurons through CXCL1 [48*,62]. Astrocytic IL-17 facilitates NMDA receptor phosphorylation through IL-17R on neurons [73]. Both microglia and astrocytes can release IL-1β that activates neuronal IL-1R [74,75]. Activation of IL-1R facilitates NMDA receptor phosphorylation in neurons [74], enhances glutamate release from primary afferents in spinal dorsal horn [76], increases endocytosis of GLT [77], and inhibits GABAergic neurotransmission (Fig. 2A) [78**]. All these signaling events can be triggered by peripheral injury or induced by inflammatory agents, and are mediated by cellular pathways involving multiple protein kinases and auxiliary factors, and importantly, linked to behavioral hyperalgesia.
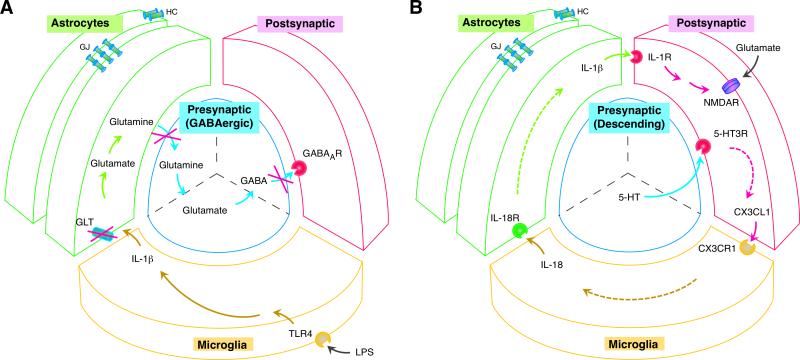
Fig. 2.
Four-way neuron-glial interactions in spinal nociceptive processing. A. Decreased GABAergic inhibition in the spinal dorsal horn induced by lipopolysaccharide (LPS) activation of microglia through TLR4, IL-1β release from microglia, suppressed astrocytic glutamate transporter activity after IL-1β-induced GLT endocytosis, and reduced glutamate-glutamine cycle-dependent GABA synthesis in presynaptic neurons [Adapted from 77,78**]. Reduced GABAergic inhibition unleashes postsynaptic excitatory neurons and contributes to pain hypersensitivity. B. Descending 5-HT-induced neuron-glial interactions and related signaling cascade that underlies pain hypersensitivity. Descending serotonin (5-HT) activates 5-HT3 receptors on spinal neurons, followed by a signaling cascade that involves release of neuronal CX3CL1, microglial IL-18, and astrocytic IL-1β and activation of their respective receptors CX3CR1 (microglia), IL18R (astrocytes) and IL-1R (neurons) [Adapted from 71]. IL-1R facilitates NMDA receptor activity that leads to neuronal hyperexcitability and behavioral hyperalgesia. Abbreviations: GABA, gamma-Aminobutyric acid; LPS, lipopolysaccharide; NMDAR, N-methyl-D-aspartate receptor; see Fig. 1 caption for other abbreviations.
The tetrapartite model of the spinal pain-processing unit should be understood in the context of different synaptic circuitry. In a GABAergic “inhibitory” synapse, decreased synaptic activity can be induced following LPS-induced IL-1β release from microglia, suppressed astrocytic GLT activity, and reduced GABA synthesis in presynaptic neurons (Fig.2A) [77,78**]. Spinal pain processing is subject to descending modulation. Descending serotonin produces facilitation by activating 5-HT3 receptors on spinal neurons, followed by upregulation of glial markers and behavioral hyperalgesia in rats [71**]. These effects of 5-HT are mediated by a signaling cascade that involves neuronal CX3CL1, microglial IL-18, astrocytic IL-1β and their respective receptors CX3CR1 (microglia), IL18R (astrocytes) and IL-1R (neurons) (Fig. 2B). Thus, pain-related neuron-glial signaling in the spinal cord can also be triggered or modulated by brainstem centrifugal input.
Neuroprotection, antinociception and glial activity
Further analysis of PET/MRI data indicated that the increased TSPO in the thalamus in human chronic pain patients is negatively associated with levels of pain and proinflammatory cytokine IL-1β [8**], suggesting a protective role of glial activation. Neuroprotection is in fact a major function of glia, although the net effect of increased glial activity after injury is often pronociceptive [3,79,80]. The TSPO agonist has been shown to attenuate persistent pain in animal models [10,11]. This raises the possibility of targeting TSPO for pain relief. Glia secrete anti-inflammatory cytokine IL-10 that enhances analgesia [81,82]. The A3 adenosine receptor agonist IB-MECA attenuated paclitaxel-induced pain-related behavior by reducing proinflammatory TNF and IL-1β and increasing anti-inflammatory IL-10. IB-MECA also attenuated nitration of astrocytic GLT-1 and glutamine synthetase [83], suggesting recovery of function of these proteins in synaptic glutamate homeostasis. Thus, in manipulating glia for pain relief, it is intuitive to selectively engage their protective mechanisms, not simply suppress glial activity.
Concluding remarks and additional comments
Neuron-glial interactions continue to attract major interest in searching for mechanisms and treatment of chronic pain. In the event of peripheral injury, the enhanced or abnormal nerve input is likely the most convenient and efficient way to alert simultaneously central neurons and glia, although infiltration of peripheral immune cells and immune mediators through a compromised blood-brain barrier/blood-spinal cord barrier may also play a role [50]. Injury-triggered signaling cascade engage reciprocal activation between presynaptic terminals, postsynaptic neurons, microglia and astrocytes, leading to long-lasting persistent pain.
It is unclear whether oligodentrocytes, the third major glia type in the CNS, interact similarly with neurons and other glial cells in response to peripheral injury. Gritsch et al. [84**] report that genetic ablation of oligodendrocytes induced cold and mechanical pain hypersensitivity in mice. It was found that the occurrence of pain hypersensitivity after diphtheria toxin-induced oligodendrocyte loss correlated with axon degeneration in the spinothalamic tract and upregulation of amyloid precursor proteins, a marker of axonal pathology. However, the microglial and astrocytic responses occurred about 3 weeks later except an early upregulation of one microglial marker, Iba-1 (ionized calcium-binding adapter molecule 1). It seems that interruption of interaction between oligodendrocytes and axons is sufficient to induce hyperalgesia and microglia/astrocytes do not contribute to the initiation of pain hypersensitivity in these mice. The dissociation of persistent pain hypersensitivity and glial responses has also been noticed in other models [85,86]. Further studies are expected to delineate a distinct role of neuron-glial interactions under specific pain conditions.
There has been a continuing effort in translating preclinical findings on neuron-glial interactions in pain. However, the clinical trials of agents targeting glia and pain-related chemokines/receptors have not been successful [see 1,8**,87 for further details]. For example, it is disappointing that a well-studied glial modulator propentofylline did not produce pain relief in post-herpetic neuralgia patients [88]. Minocycline, a microglial inhibitor, did not produce clinically meaningful pain relief [89,90]. AZD2423, a CCR2 antagonist, is ineffective against posttraumatic neuralgia [91]. To search for answers to negative clinical findings, Landry et al. [88] have provided some clues regarding potential relevant differences in function of microglia and macrophages between humans and rodents, which might explain observed failure in clinical trials. Finding explanations of failed trials is no easy task and there should be controlled direct comparisons between clinical and pre-clinical conditions [see 92]. Besides potential differences in biology and trial design issues, we need to rethink whether we have sufficient understanding of the mechanisms and to pursue coordinated preclinical and clinical studies in a disease-specific fashion.
Highlights.
-Brain glial activation is observed in patients suffering from chronic low back pain.
-Microglia maintain a dynamic relationship with neurons.
-Astrocytic connexin hemichannels play a role in injury-induced pain hypersensitivity.
-Neuron-glial signaling in the spinal cord can be triggered by descending input.
-Enhanced glial activity also offers neuroprotection as suggested by human studies.
Acknowledgements
The authors’ work was supported by NIH grants DE0212804, NS060735 and Maryland Stem Cell Foundation 2014-MSCRFI-0584.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure
The authors (KR, RD) declare no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period and scope of the review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 3.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svahn AJ, Becker TS, Graeber MB. Emergent properties of microglia. Brain Pathol. 2014;24:665–670. doi: 10.1111/bpa.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012;32:10833–10840. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjurstrom MF, Giron SE, Griffis CA. Cerebrospinal Fluid Cytokines and Neurotrophic Factors in Human Chronic Pain Populations: A Comprehensive Review. Pain Pract. 2014 doi: 10.1111/papr.12252. doi: 10.1111/papr.12252. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8**.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138(Pt 3):604–615. doi: 10.1093/brain/awu377. [Utilizing the novel technology of integrated positron emission tomography-magnetic resonance imaging and the recently developed radioligand 11C-PBR28, the authors show increased brain levels of the translocator protein, a marker of glial activation, in patients with chronic low back pain. This is the first observation on brain glial activation in chronic pain patients] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Hernstadt H, Wang S, Lim G, Mao J. Spinal translocator protein (TSPO) modulates pain behavior in rats with CFA-induced monoarthritis. Brain Res. 2009;1286:42–52. doi: 10.1016/j.brainres.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei XH, Wei X, Chen FY, Zang Y, Xin WJ, Pang RP, Chen Y, Wang J, Li YY, Shen KF, et al. The upregulation of translocator protein (18 kDa) promotes recovery from neuropathic pain in rats. J Neurosci. 2013;33:1540–1551. doi: 10.1523/JNEUROSCI.0324-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biesmans S, Acton PD, Cotto C, Langlois X, Ver Donck L, Bouwknecht JA, Aelvoet SA, Hellings N, Meert TF, Nuydens R. Effect of stress and peripheral immune activation on astrocyte activation in transgenic bioluminescent Gfap-luc mice. Glia. 2015;63:1126–1137. doi: 10.1002/glia.22804. [DOI] [PubMed] [Google Scholar]
- 13.Banati RB, Cagnin A, Brooks DJ, Gunn RN, Myers R, Jones T, Birch R, Anand P. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. Neuroreport. 2001;12:3439–3442. doi: 10.1097/00001756-200111160-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 15.Eyo UB, Wu LJ. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013;2013:456857. doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji RR. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology. 2007;107:312–321. doi: 10.1097/01.anes.0000270759.11086.e7. [DOI] [PubMed] [Google Scholar]
- 18.Okubo M, Castro A, Guo W, Zou S, Ren K, Wei F, Keller A, Dubner R. Transition to persistent orofacial pain after nerve injury involves supraspinal serotonin mechanisms. J Neurosci. 2013;33:5152–5161. doi: 10.1523/JNEUROSCI.3390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor AM, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci. 2015;35:8442–8450. doi: 10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN. Axon initial segment-associated microglia. J Neurosci. 2015;35:2283–2292. doi: 10.1523/JNEUROSCI.3751-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay MÈ, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Eyo UB, Gu N, De S, Dong H, Richardson JR, Wu LJ. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci. 2015;35:2417–2422. doi: 10.1523/JNEUROSCI.3279-14.2015. [Utilizing two-photon microscopy in mouse brain slices and in vivo, it was found that extracellular calcium reduction induced microglial processes to converge at distinct sites, a phenomenon the authors termed microglial process convergence (MPCs). MPCs target neuronal dendrites independent of neuronal action potential firing and is mediated by ATP release and microglial P2Y12 receptors. These results indicate that microglia monitor and interact with neurons during conditions of cerebral calcium reduction in the normal and diseased brain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beggs S, Salter MW. The known knowns of microglia-neuronal signalling in neuropathic pain. Neurosci Lett. 2013;557(Pt A):37–42. doi: 10.1016/j.neulet.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Barragán-Iglesias P, Pineda-Farias JB, Cervantes-Durán C, Bravo-Hernández M, Rocha-González HI, Murbartián J, Granados-Soto V. Role of spinal P2Y6 and P2Y11 receptors in neuropathic pain in rats: possible involvement of glial cells. Mol Pain. 2014;10:29. doi: 10.1186/1744-8069-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magni G, Ceruti S. The purinergic system and glial cells: emerging costars in nociception. Biomed Res Int. 2014;2014:495789. doi: 10.1155/2014/495789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying YL, Wei XH, Xu XB, She SZ, Zhou LJ, Lv J, Li D, Zheng B, Liu XG. Over-expression of P2X7 receptors in spinal glial cells contributes to the development of chronic postsurgicalpain induced by skin/muscle incision and retraction (SMIR) in rats. Exp Neurol. 2014;261:836–843. doi: 10.1016/j.expneurol.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 28*.Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N, Ji RR, Zhang YQ. Delayed Activation of Spinal Microglia Contributes to the Maintenance of Bone Cancer Pain in Female Wistar Rats via P2X7 Receptor and IL-18. J Neurosci. 2015;35:7950–7963. doi: 10.1523/JNEUROSCI.5250-14.2015. [The authors report an unconventional role of spinal microglia in the maintenance, but not initiation of advanced-phase bone cancer pain in a female rat model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Chun YE, Han KS, Lee J, Woo DH, Lee CJ. Ca(2+) Entry is Required for Mechanical Stimulation-induced ATP Release from Astrocyte. Exp Neurobiol. 2015;24:17–23. doi: 10.5607/en.2015.24.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia. 2015;63:216–228. doi: 10.1002/glia.22745. [DOI] [PubMed] [Google Scholar]
- 31.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kierdorf K, Prinz M. Factors regulating microglia activation. Front Cell Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiyomoto M, Shinoda M, Okada-Ogawa A, Noma N, Shibuta K, Tsuboi Y, Sessle BJ, Imamura Y, Iwata K. Fractalkine signaling in microglia contributes to ectopic orofacial pain following trapezius muscle inflammation. J Neurosci. 2013;33:7667–7680. doi: 10.1523/JNEUROSCI.4968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AK, Malcangio M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front Cell Neurosci. 2014;8:121. doi: 10.3389/fncel.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paolicelli RC, Bisht K, Tremblay MÈ. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 37.Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim KW, Mogg AJ, Perretti M, Malcangio M. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest. 2014;124:2023–2036. doi: 10.1172/JCI71389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Clark AK, Gruber-Schoffnegger D, Drdla-Schutting R, Gerhold KJ, Malcangio M, Sandkühler J. Selective activation of microglia facilitates synaptic strength. J Neurosci. 2015;35:4552–4570. doi: 10.1523/JNEUROSCI.2061-14.2015. [By recording from rat spinal cord slices, the authors show that selective activation of microglia CX3CR1 receptor by fractalkine is sufficient to rapidly facilitate synaptic strength between primary afferent C-fibers and lamina I neurons, the first synaptic relay in the nociceptive pathway. Triggering of this form of plasticity does not require enhanced neuronal activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T, Koga K, Li XY, Zhuo M. Spinal microglial motility is independent of neuronal activity and plasticity in adult mice. Mol Pain. 2010;6:19. doi: 10.1186/1744-8069-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren Y, Leng XM, Yang B, Zhang X. Aberrant CD200/CD200R1 expression contributes to painful synovium hyperplasia in a patient with primary hypertrophic osteoarthropathy. Rheumatol Int. 2013;33:2509–2512. doi: 10.1007/s00296-013-2732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang CY, Sessle BJ, Dostrovsky JO. Role of astrocytes in pain. Neurochem Res. 2012;37:2419–2431. doi: 10.1007/s11064-012-0801-6. [DOI] [PubMed] [Google Scholar]
- 42.Hansen RR, Malcangio M. Astrocytes--multitaskers in chronic pain. Eur J Pharmacol. 2013;716:120–128. doi: 10.1016/j.ejphar.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griggs RB, Donahue RR, Morgenweck J, Grace PM, Sutton A, Watkins LR, Taylor BK. Pioglitazone rapidly reduces neuropathic pain through astrocyte and non-genomic PPARγ mechanisms. Pain. 2015;156:469–482. doi: 10.1097/01.j.pain.0000460333.79127.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Guo W, Yang K, Wei F, Dubner R, Ren K. Contribution of Primary Afferent Input to Trigeminal Astroglial Hyperactivity, Cytokine Induction and NMDA Receptor Phosphorylation. Open Pain J. 2010;2010:144–152. doi: 10.2174/1876386301003010144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shankarappa SA, Tsui JH, Kim KN, Reznor G, Dohlman JC, Langer R, Kohane DS. Prolonged nerve blockade delays the onset of neuropathic pain. Proc Natl Acad Sci U S A. 2012;109:17555–17560. doi: 10.1073/pnas.1214634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193–2209. doi: 10.1093/brain/awu140. [The authors show that peripheral nerve injury upregulated connexin-43 to sustain late-phase neuropathic pain by releasing chemokines from spinal astrocytes. Spinal injection of TNF-activated astrocytes was sufficient to induce persistent mechanical allodynia and interestingly, TNF also increased connexin-43 expression and hemichannel activity, but not gap junction communication in astrocyte cultures from cortices and spinal cords. These findings demonstrate a novel mechanism of astrocytic connexin-43 to enhance spinal nociceptive transmission and maintain neuropathic pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson CR, Zhang H, Dougherty PM. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 2014;274:308–17. doi: 10.1016/j.neuroscience.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tenorio G, Kulkarni A, Kerr BJ. Resident glial cell activation in response to perispinal inflammation leads to acute changes in nociceptive sensitivity: implications for the generation of neuropathic pain. Pain. 2013;154:71–81. doi: 10.1016/j.pain.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Pan HC, Chou YC, Sun SH. P2X7 R-mediated Ca(2+) -independent d-serine release via pannexin-1 of the P2X7 R-pannexin-1 complex in astrocytes. Glia. 2015;63:877–893. doi: 10.1002/glia.22790. [DOI] [PubMed] [Google Scholar]
- 52.Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci. 2014;15:327–335. doi: 10.1038/nrn3725. [DOI] [PubMed] [Google Scholar]
- 53.Bond SR, Naus CC. The pannexins: past and present. Front Physiol. 2014;5:58. doi: 10.3389/fphys.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bravo D, Ibarra P, Retamal J, Pelissier T, Laurido C, Hernandez A, Constandil L. Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord. Pain. 2014;155:2108–2015. doi: 10.1016/j.pain.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholson KJ, Gilliland TM, Winkelstein BA. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J Neurosci Res. 2014;92:116–129. doi: 10.1002/jnr.23295. [DOI] [PubMed] [Google Scholar]
- 57.Nie H, Weng HR. Impaired glial glutamate uptake induces extrasynaptic glutamate spillover in the spinal sensory synapses of neuropathic rats. J Neurophysiol. 2010;103:2570–2580. doi: 10.1152/jn.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karus C, Mondragão MA, Ziemens D, Rose CR. Astrocytes restrict discharge duration and neuronal sodium loads during recurrent network activity. Glia. 2015;63:936–957. doi: 10.1002/glia.22793. [DOI] [PubMed] [Google Scholar]
- 59.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain. 2014;155:429–435. doi: 10.1016/j.pain.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Q, Cheong YK, Yang F, Tiwari V, Li J, Liu J, Raja SN, Li W, Guan Y. Intrathecal carbenoxolone inhibits neuropathic pain and spinal wide-dynamic range neuronal activity in rats after an L5 spinal nerve injury. Neurosci Lett. 2014;563:45–50. doi: 10.1016/j.neulet.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson CR, Dougherty PM. Spinal astrocyte gap junction and glutamate transporter expression contributes to a rat model of bortezomib-induced peripheral neuropathy. Neuroscience. 2015;285:1–10. doi: 10.1016/j.neuroscience.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rovegno M, Soto PA, Sáez PJ, Naus CC, Sáez JC, von Bernhardi R. Connexin43 hemichannels mediate secondary cellular damage spread from the trauma zone to distal zones in astrocyte monolayers. Glia. 2015;63:1185–1199. doi: 10.1002/glia.22808. [DOI] [PubMed] [Google Scholar]
- 65.Abudara V, Roux L, Dallérac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015;63:795–811. doi: 10.1002/glia.22785. [DOI] [PubMed] [Google Scholar]
- 66.De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 67.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;21(6):803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- 71**.Guo W, Miyoshi K, Dubner R, Gu M, Li M, Liu J, Yang J, Zou S, Ren K, Noguchi K, et al. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol Pain. 2014;10:35. doi: 10.1186/1744-8069-10-35. [The authors show that activation of spinal 5-HT3 receptors induced spinal glial hyperactivity, neuronal hyperexcitability and pain hypersensitivity in rats, which involves neuron-to-microglia signaling via the chemokine fractalkine, microglia to astrocyte signaling via cytokine IL-18, astrocyte to neuronal signaling by IL-1β, and enhanced activation of NMDA receptors in the spinal dorsal horn. The findings indicate that neuron-glial signaling in the spinal cord can also be triggered by descending 5-HT input.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imai S, Ikegami D, Yamashita A, Shimizu T, Narita M, Niikura K, Furuya M, Kobayashi Y, Miyashita K, Okutsu D, et al. Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain. 2013;136:828–43. doi: 10.1093/brain/aws330. [DOI] [PubMed] [Google Scholar]
- 73.Meng X, Zhang Y, Lao L, Saito R, Li A, Bäckman CM, Berman BM, Ren K, Wei PK, Zhang RX. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain. 2013;154:294–305. doi: 10.1016/j.pain.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Won KA, Kim MJ, Yang KY, Park JS, Lee MK, Park MK, Bae YC, Ahn DK. The glial neuronal GRK2 pathway participates in the development of trigeminal neuropathic pain in rats. J Pain. 2014;15:250–261. doi: 10.1016/j.jpain.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Yan X, Weng HR. Endogenous interleukin-1β in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J Biol Chem. 2013;288:30544–57. doi: 10.1074/jbc.M113.495465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62:1093–1109. doi: 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78**.Yan X, Jiang E, Weng HR. Activation of toll like receptor 4 attenuates GABA synthesis and postsynaptic GABA receptor activities in the spinal dorsal horn via releasing interleukin-1 beta. J Neuroinflammation. 2015;12:4. doi: 10.1186/s12974-014-0222-3. [The authors provide novel observation that LPS reduced GABAergic inhibition in the superficial spinal dorsal horn via mechanisms involving microglial TLR4 activation and IL-1β release and decreased astroglial glutamate transporter activity, followed by reduction of glutamate-glutamine cycle-dependent GABA synthesis in presynaptic neurons.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev. 2014;45:19–27. doi: 10.1016/j.neubiorev.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology. 2015;96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villacampa N, Almolda B, Vilella A, Campbell IL, González B, Castellano B. Astrocyte-targeted production of IL-10 induces changes in microglial reactivity and reduces motor neuron death after facial nerve axotomy. Glia. 2015;63:1166–84. doi: 10.1002/glia.22807. [DOI] [PubMed] [Google Scholar]
- 83.Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA, Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155:2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84**.Gritsch S, Lu J, Thilemann S, Wörtge S, Möbius W, Bruttger J, Karram K, Ruhwedel T, Blanfeld M, Vardeh D, et al. Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun. 2014;5:5472. doi: 10.1038/ncomms6472. [The authors report that genetic ablation of oligodendrocytes, the third glial type in CNS besides miscroglia and astroglia, rapidly triggers a pattern of sensory changes that closely resemble central neuropathic pain. Interestingly, both microglia and astroglia do not appear to contribute functionally to central pain after oligodendrocyte ablation. Axonal pathology in the spinal dorsal horn and spinothalamic tract are concurrent with the time course of pain hypersensitivity. These data reveal a role for oligodendrocytes in modulating central pain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leinders M, Knaepen L, De Kock M, Sommer C, Hermans E, Deumens R. Up-regulation of spinal microglial Iba-1 expression persists after resolution of neuropathic pain hypersensitivity. Neurosci Lett. 2013;554:146–150. doi: 10.1016/j.neulet.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 86.Ducourneau VR, Dolique T, Hachem-Delaunay S, Miraucourt LS, Amadio A, Blaszczyk L, Jacquot F, Ly J, Devoize L, Oliet SH, Dallel R, et al. Cancer pain is not necessarily correlated with spinal overexpression of reactive glia markers. Pain. 2014;155:275–291. doi: 10.1016/j.pain.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 87.Alfonso Romero-Sandoval E, Sweitzer S. Nonneuronal central mechanisms of pain: glia and immune response. Prog Mol Biol Transl Sci. 2015;131:325–358. doi: 10.1016/bs.pmbts.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Landry RP, Jacobs VL, Romero-Sandoval EA, De Leo JA. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp Neurol. 2012;234:340–350. doi: 10.1016/j.expneurol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Martinez V, Szekely B, Lemarié J, Martin F, Gentili M, Ben Ammar S, Lepeintre JF, Garreau de Loubresse C, Chauvin M, Bouhassira D, et al. The efficacy of a glial inhibitor, minocycline, for preventing persistent pain after lumbar discectomy: a randomized, double-blind, controlled study. Pain. 2013;234:1197–1203. doi: 10.1016/j.pain.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 90.Vanelderen P, Van Zundert J, Kozicz T, Puylaert M, De Vooght P, Mestrum R, Heylen R, Roubos E, Vissers K. Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology. 2015;122:399–406. doi: 10.1097/ALN.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 91.Kalliomäki J, Attal N, Jonzon B, Bach FW, Huizar K, Ratcliffe S, Eriksson B, Janecki M, Danilov A, Bouhassira D, et al. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain. 2013;154:761–767. doi: 10.1016/j.pain.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Watkins LR, Hutchinson MR, Johnson KW. Commentary on Landry et al.: “Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages”. Exp Neurol. 2012;234:351–353. doi: 10.1016/j.expneurol.2012.01.006. [DOI] [PubMed] [Google Scholar]