Abstract
Stress-induced reinstatement of cocaine seeking requires corticotropin releasing factor (CRF) actions in the ventral tegmental area (VTA). However the mechanisms through which CRF regulates VTA function to promote cocaine use are not fully understood. Here we examined the role of GABAergic neurotransmission in the VTA mediated by GABA-A or GABA-B receptors in the reinstatement of extinguished cocaine seeking by a stressor, uncontrollable intermittent footshock, or bilateral intra-VTA administration of CRF. Rats underwent repeated daily cocaine self-administration (1.0 mg/kg/ing; 14 × 6 hrs/day) and extinction and were tested for reinstatement in response to footshock (0.5 mA, 0.5” duration, average every 40 sec; range 10–70 sec) or intra-VTA CRF delivery (500 ng/side) following intra-VTA pretreatment with the GABA-A antagonist, bicuculline, the GABA-B antagonist, 2-hydroxysaclofen or vehicle. Intra-VTA bicuculline (1, 10 or 20 ng/side) failed to block footshock- or CRF-induced cocaine seeking at either dose tested. By contrast, 2-hydroxysaclofen (0.2 or 2 µg/side) prevented reinstatement by both footshock and intra-VTA CRF at a concentration that failed to attenuate food-reinforced lever pressing (45 mg sucrose-sweetened pellets; FR4 schedule) in a separate group of rats. These data suggest that GABA-B receptor-dependent CRF actions in the VTA mediate stress-induced cocaine seeking and that GABA-B receptor antagonists may have utility for the management of stress-induced relapse in cocaine addicts.
Keywords: tress, relapse, reinstatement, GABA-B receptors, ventral tegmental area, cocaine
INTRODUCTION
Understanding the mechanisms that contribute to relapse to drug use in cocaine addicts is critical for the development of new and more effective treatment approaches for managing addiction. In rats, relapse can be studied using reinstatement models in which the ability of a stimulus to reestablish or reinstate cocaine seeking behavior in rats or mice following extinction is used as an indicator of its likelihood to promote relapse to use in human addicts (Mantsch et al. 2015). Many of the same stimuli that contribute to drug use in humans (stress, drug-associated cues, drug re-exposure), reinstate cocaine seeking following self-administration (SA) and extinction in rats.
Among the triggers for relapse, stress is particularly problematic due to its unpredictable and often uncontrollable nature. Reports from cocaine users that relapse is frequently related to the onset of stressful life events are supported by laboratory findings demonstrating that personalized scripts that relay stress-related imagery can precipitate craving in cocaine-dependent individuals (Sinha et al. 1999). In rats, it has been reported that a number of stressors can reinstate extinguished cocaine seeking following intravenous self-administration (see Mantsch et al. 2015 for review). However, most studies investigating stress-induced relapse in rats have used electric footshock delivered through the grid floor of the operant conditioning chambers as the reinstating stressor. Notably, we have previously reported that, like stress imagery in human addicts (Fox et al. 2005), the ability of footshock to reinstate cocaine seeking in rats increases according to the amount of prior drug intake (Mantsch et al. 2008).
Several studies have demonstrated that stress-induced reinstatement of cocaine seeking requires corticotropin-releasing factor (CRF) actions in the ventral tegmental area (VTA; Wang et al. 2005; Wang et al. 2007; Blacktop et al. 2011; Chen et al. 2014; Vranjkovic et al. 2014) and that intra-VTA CRF administration is sufficient to reinstate cocaine seeking following SA and extinction in rats (Wang et al. 2005; Blacktop et al. 2011). However, the downstream processes in the VTA through which CRF promotes drug use are unclear. Mechanistic studies have focused on CRF regulation of glutamatergic neurotransmission in the VTA (Wise and Morales, 2010). In the VTA, CRF has been reported to increase glutamate release as measured by both in vivo microdialysis (Wang et al. 2005) and spontaneous EPSCs in slice preparations (Hahn et al. 2010; Williams et al. 2014), as well as post-synaptic excitability through increases in AMPA:NMDA receptor ratios (Ungless et al. 2003; Hahn et al. 2009). Moreover, intra-VTA perfusion with kynurenic acid, which has a pharmacological profile that includes AMPA and NMDA receptor antagonism, prevents reinstatement in response to either footshock or intra-VTA CRF (Wang et al. 2005).
However, the actions of stress and CRF in the VTA are complex and appear to also include, under some circumstances, inhibition of neuronal activity (Ungless et al. 2004; Wanat et al. 2013; Twining et al. 2015) or terminal neurotransmitter release (Williams et al. 2014). The VTA receives dense GABAergic inputs from a number of regions, including the nucleus accumbens (Yim and Mogenson, 1980; Xia et al. 2011; Bocklisch et al. 2013) and bed nucleus of the stria terminalis (Kudo et al. 2012), both of which have been implicated in stress-induced cocaine seeking in rats (Erb and Stewart, 1999; McFarland et al. 2004; Vranjkovic et al. 2014). Additionally, the VTA contains GABAergic interneurons that regulate efferent neuronal projections from the region (Steffensen et al. 1998; Cruz et al. 2004) and dense GABAergic innervation from the rostromedial tegmental nucleus (i.e., tail of the VTA; Jhou et al. 2009; Matsui and Williams, 2011; Barrot et al. 2012). It has also been reported that CRF promotes GABAergic neurotransmission within the VTA. For example, CRF application to the VTA in ex vivo slice preparations promotes GABA-B regulation of G protein-coupled inwardly-rectifying potassium (GIRK) channels on dopamine cells (Beckstead et al. 2009). Despite these findings, the effects of GABA receptor antagonism in the VTA on stress-induced reinstatement have not been reported.
In the present study we investigate the potential contribution of GABAA and GABAB receptors in the VTA to the reinstatement of extinguished cocaine seeking in response to footshock or delivery of CRF into the VTA following self-administration. Rats with a history of daily self-administration that results in robust shock and intra-VTA CRF-induced reinstatement following extinction (14 × 6 hrs/day; 1 mg/kg/inf) received intra-VTA injections of the GABAA receptor antagonist, bicuculline, or the GABAB receptor antagonist, 2-hydroxysaclofen, prior to reinstatement testing. Notably, in contrast to previous work targeting VTA GABA receptors which used a cocktail of receptor agonists (baclofen/muscimol; McFarland et al. 2004), the present study uses receptor selective antagonists, thereby permitting 1) assessment of the contribution of GABAA vs. GABAB receptors and 2) the regulation of cocaine seeking through GABAergic signaling that is engaged by CRF and/or during stress.
METHODS
Subjects
Adult male Sprague–Dawley rats (Harlan Laboratories, St. Louis, MO) were housed individually under a 12 h/12 h reversed light/dark cycle (lights on at 7:00 PM) in a temperature and humidity controlled AAALAC-accredited animal facility. All procedures were approved by the Marquette University IACUC and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Catheter and cannula implantation
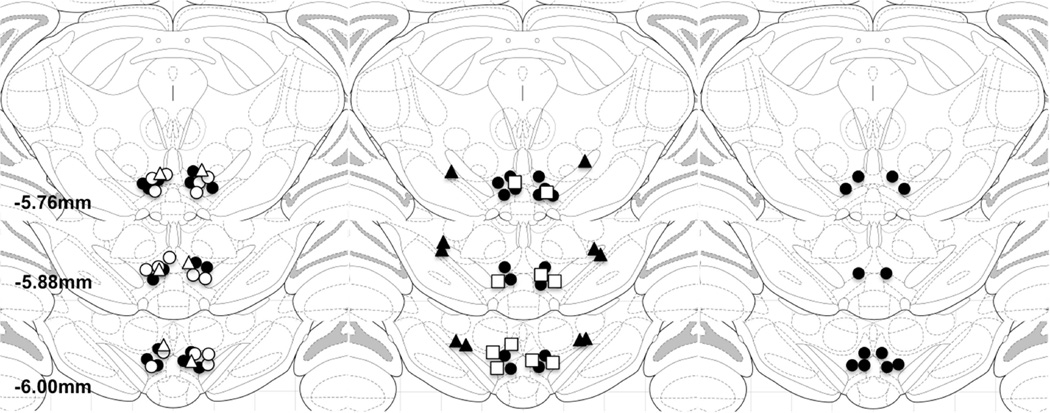
For the reinstatement studies, rats were implanted with chronic indwelling jugular catheters under ketamine HCl (100 mg/kg, ip) and xylazine (2 mg/kg, ip) anesthesia and with bilateral 2.1-cm 23 gauge guide cannulae aimed at the VTA for intracranial injections as previously described (Blacktop et al. 2011; Vranjkovic et al. 2014). The tips of the guide cannulae were aimed 0.5 mm above the target injection site (the posterior VTA) using the following coordinates determined from Paxinos and Watson (1998): 12° angle away from midline; A/P – 5.6 mm from bregma; M/L ± 2.2 mm from midline; and D/V – 6.7 mm from the skull surface. Placements for cannula targeting the VTA for rats from each of the experiments are depicted in Figure 1.
Figure 1. Intracranial injection sites.
Panels represent atlas diagrams (coronal sections −5.6, − 5.8 and −6.1 mm relative to Bregma; Paxinos and Watson, 2006) depicting injection sites from rats included in experiments in which effects of VTA injections of 1 ng/side (closed circles), 10 ng/side (open circles), or 20 ng/side (open triangles) bicuculline on CRF- and shock-induced cocaine seeking were tested (1A); rats included in experiments in which effects of VTA injections of 0.2 µg/side (closed circles) or 0.2 µg/side (open squares) 2-hydroxysaclofen on CRF- and shock-induced cocaine seeking were tested and rats with injections outside of the VTA used as anatomical controls (1B; closed triangles); and rats included in the experiments testing for effects on food-reinforced lever pressing (1C; closed circles).
Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse through the Drug Supply Program. CRF was purchased from Sigma-Aldrich and was administered at a concentration of 500 ng/side (Blacktop et al. 2011) dissolved in artificial cerebral spinal fluid (aCSF; 0.25 µl/side). The GABAA receptor antagonist (−)-bicuculline methiodide was purchased from Tocris Bioscience and was administered at doses of 1, 10 and 20 ng/side (0.002 and 0.02 and 0.04 nmol/side) dissolved in aCSF (0.25 µl/side). The doses used were selected based on prior reports assessing VTA effects (Sandner et al. 1996; Trojniar and Klejbor, 1999; Laviolette and van der Kooy, 2001; Grubb et al. 2002; Echo et al. 2002; Lavezzi et al. 2015). The occurrence of seizures prevented testing for effects of higher doses. The GABAB receptor antagonist 2-hydroxysaclofen was also purchased from Tocris Bioscience and was administered at doses of 0.2 and 2 µg/side (0.75 and 7.5 nmol/side) dissolved in aCSF (0.25 µl/side). The doses used were also selected based on prior reports assessing VTA effects (Xi and Stein, 1999; Echo et al. 2002; Ackerman et al. 2003; Miner et al. 2010).
Cocaine Self-Administration/Extinction
After recovery from surgery, rats were trained to self-administer cocaine (1.0 mg/kg/inf, iv) by pressing a lever under a fixed ratio 1 schedule during daily 2-h sessions, within which the active (i.e., front) lever was extended into the chamber and the corresponding stimulus light was illuminated. Pressing the lever resulted in an iv infusion of cocaine solution (200 µl over 5 s) followed by a 25-s time-out period during which the stimulus light was extinguished but the lever remained extended. Responding on a second, inactive (i.e., back) lever was recorded but had no programmed consequences. Response requirements were gradually increased until rats displayed stable responding (within 10% of the 3-session mean) under an FR4 schedule at which time they were allowed to self-administer cocaine six hours daily for 14 consecutive days. We have previously found that robust reinstatement of cocaine seeking in response to either footshock or intra-VTA CRF is only observed when rats are tested after self-administration under these “long-access” conditions (Mantsch et al 2008; Blacktop et al 2011). After the 14-day self-administration test period rats underwent extinction during ten consecutive 2-h sessions within which the cocaine solution was replaced by saline. As was the case during self- administration, the drug lever stimulus light was illuminated during the extinction session except during the post-infusion “time-out” period. Once rats displayed less than 20 responses/session over two consecutive sessions under extinction conditions, reinstatement testing was initiated.
Reinstatement Testing
After extinction, rats were tested for the effects of intra-VTA pretreatment with either bicuculline (GABAA antagonist) or 2-hydroxysaclofen (GABAB antagonist) on reinstatement in response to electric footshock or bilateral intra-VTA CRF injections. Separate groups of rats were tested for the effects of 1 ng, 10 or 20 ng/side bicuculline or 0.2 or 2 µg/side 2-hydroxysaclofen. Each rat was tested for reinstatement in response to shock and intra-VTA CRF following intra-VTA vehicle and drug pretreatment (four reinstatement tests total). The two-hour reinstatement sessions were otherwise identical to the extinction sessions (i.e., the drug lever stimulus light was illuminated except during the post-infusion “time-out” period). Consecutive reinstatement sessions were always separated by additional extinction training and rats were required to display less than 20 cocaine lever responses during an intervening extinction session in order to be tested again for reinstatement. The sequence of reinstatement testing (shock and CRF) was counterbalanced across rats such that some rats were tested for effects on shock-induced reinstatement first, while others were tested for CRF-induced reinstatement first. All microinfusions were delivered in a volume of 0.25 µl/side over a 1-min period with an additional 1-min period to allow for drug diffusion, ten minutes prior to the reinstatement session. In addition to testing for the effects of GABA receptor antagonists on shock- and CRF-induced cocaine seeking, the effects of intra-VTA administration of bicuculline and 2-hydroxysaclofen in the absence of shock or CRF were assessed in separate groups of rats.
Shock-Induced Reinstatement of Cocaine Seeking
To determine the contribution of VTA GABAA and GABAB receptors to stress-induced cocaine seeking, rats were tested for the ability of electric footshock, delivered though the stainless steel grid floors of the self-administration chambers, to reinstate cocaine seeking following pretreatment with bilateral antagonist drug injections into the VTA ten minutes prior to shock. During the 15-min footshock period, the houselight was illuminated and the levers were retracted and stimulus lights extinguished. Shocks (0.5 mA, 0.5” duration) were delivered an average of every 40 sec (range 10–70 sec). Immediately following the shock period, the houselight was extinguished and the active and inactive levers were extended into the chamber and the active lever stimulus light was illuminated (conditions identical to both self-administration and extinction). We have reported that these parameters produce robust footshock-induced reinstatement under the self-administration conditions used (Mantsch et al. 2008). Each rat was tested twice for shock-induced reinstatement in counter-balanced sequence: once following intra-VTA drug delivery and once following intra-VTA vehicle administration.
CRF-Induced Reinstatement of Cocaine Seeking
Rats were also tested for reinstatement in response to bilateral intra-VTA injections of CRF. Ten minutes after VTA administration of bicuculline, 2-hydroxysaclofen or vehicle, rats received bilateral injections with CRF (500 ng/side delivered a volume of 0.25 µl/side over a 1-min period ten minutes prior to reinstatement testing). Each rat was tested twice for CRF-induced reinstatement in counter-balanced sequence: once following intra-VTA drug delivery and once following intra-VTA vehicle administration. We have previously reported that this CRF concentration produces robust reinstatement under the self-administration conditions used (Blacktop et al. 2011). Moreover, CRF delivery via cannula placed into regions adjacent to the VTA failed to reinstate cocaine seeking (n=5; extinction responses/2-h session: 11.2 ± 1.59; CRF-induced responses/2-h session: 8.8 ± 1.39).
Testing for Effects on Food-Reinforced Lever Pressing
In order to confirm that the effects of intra-VTA injections on reinstatement were not attributable to non-specific motor impairments, separate groups of rats were tested for effects on sucrose pellet-reinforced lever pressing. These rats were maintained at 90% of their free-feeding body weights and trained to self-administer 45 mg sucrose-sweetened food pellets (BioServ) by pressing a response lever under a FR4 schedule of reinforcement during 30-min sessions. Once stable response patterns were observed (responding within 10% of the mean over 3 sessions), rats were tested for the effects of intra-VTA delivery of 2-hydroxysaclofen, as described above, on responding.
Histological Confirmation of Injection Sites
The accuracy of cannula implantation was confirmed postmortem in each rat after cardiac perfusion with 60-ml NaCl followed by 60-ml 2.5% buffered neutral formalin under sodium barbital anesthesia (55 mg/kg). Brains were removed and stored in 2.5% buffered formalin for at least one day. 200-µm sections were cut using a vibrotome, slide-mounted, and stained with cresyl violet for placement confirmation using a light microscope. Rats with injection sites outside of the VTA were excluded from the primary analysis and combined into a separate “missed placement” group for assessment of anatomical specificity of drug effects on shock-induced cocaine seeking.
Statistical Analyses
The effects of each drug/dose combination on CRF and shock-induced reinstatement were analyzed separately using 2-way repeated measure ANOVA with reinstatement (responding during the reinstatement session vs. the preceding extinction session) and intra-VTA drug treatment (drug vs. vehicle) as factors. A similar analysis was used to assess effects on food-reinforced responding (2-way repeated measures with reinforced lever pressing and drug treatment as factors). Analysis of drug effects alone consisted of comparison with the prior extinction session using paired t-tests. Statistical analyses were conducted using SPSS statistics software. For all analyses, statistical significance was defined as p<0.05.
RESULTS
Placements
The sites of injections for rats tested for the effects of intra-VTA drug injections and for anatomical control rats are depicted in Figure 1.
SA and extinction
Cocaine self-administration and extinction in rats used for each experiment are shown in Table 1 and were comparable across groups. Moreover the numbers of sessions required for acquisition of cocaine self-administration, total cocaine intake across all self-administration sessions and the numbers of sessions needed to reach the extinction criteria did not differ across groups (data not shown).
Table 1.
Cocaine self-administration and extinction in each group of rats that underwent reinstatement testing.
| Intra-VTA pretreatment group |
Infusions/6-h Session (±S.E.) |
Responses/2-h Session (±S.E.) |
|
|---|---|---|---|
| SA Day 1 | SA Day 14 | Last Ext Day | |
|
Bicuculline (1 ng/side) |
83.75 (±8.66) | 92.75 (±14.07) | 10.38 (±1.24) |
|
Bicuculline (10 ng/side) |
79.00 (±7.57) | 96.00 (±11.48) | 14.57 (±3.46) |
|
Bicuculline (20 ng/side) |
61.38 (±7.80) | 83.00 (±17.21) | 12.50 (±1.76) |
|
2-hydroxysaclofen (0.2 µg/side) |
70.80 (±3.81) | 86.4 (±5.56) | 10.20 (±1.56) |
|
2-hydroxysaclofen (2 µg/side) |
72.22 (±3.27) | 82.44 (±4.23) | 6.22 (±1.54) |
Data represent infusions/6-h session (±S.E.) on days 1 and 14 of self-administration and responses/2-h session (±S.E.) during the final extinction session prior to starting reinstatement testing in separate groups of rats tested for effects of intra-VTA bicuculline (1, 10, or 20 ng/side) or 2-hydroxysaclofen (0.2 or 2 µg/side) on shock and CRF-induced reinstatement.
Intra-VTA Injections of 2-hydroxysaclofen or bicuculline do not reinstate cocaine seeking
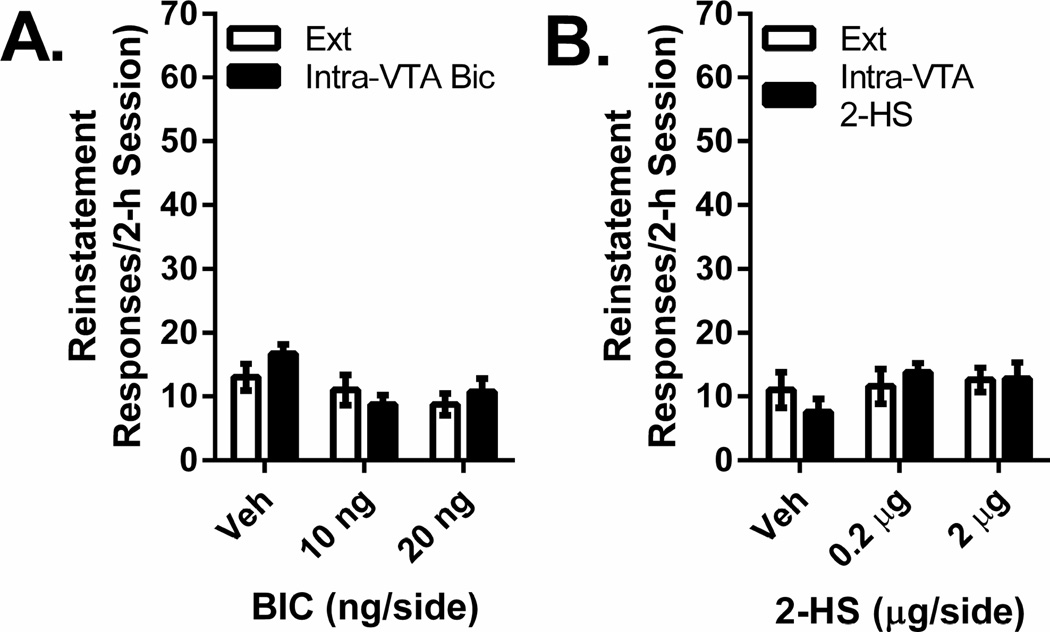
Neither intra-VTA 2-hydroxysaclofen nor intra-VTA bicuculline injections induced cocaine-seeking behavior. Cocaine seeking following intra-VTA injections of bicuculline (10 or 20 ng/side; n=4/dose) or 2-hydroxysaclofen (0.2 or 2 µg/side; n=5/dose) is shown in Figure 2. Separate 2-way ANOVAs failed to show overall increases in responding (i.e., reinstatement) relative to extinction, effects of intra-VTA drug dose or reinstatement × dose interactions.
Figure 2. Intra-VTA injections of bicuculline or 2-hydroxysaclofen do not induce cocaine seeking.
Data represent responding (lever presses/2-h session ±S.E.) recorded during reinstatement testing following intra-VTA injections of vehicle and bicuculline (BIC; 10 or 20 ng/side; Figure 2A) or 2-hydroxysaclofen (2-HS; 0.2 or 2 µg/side; Figure 2B) and responding during the corresponding previous extinction (Ext) sessions.
Effects of intra-VTA bicuculline injections on shock- and intra-VTA CRF-induced reinstatement
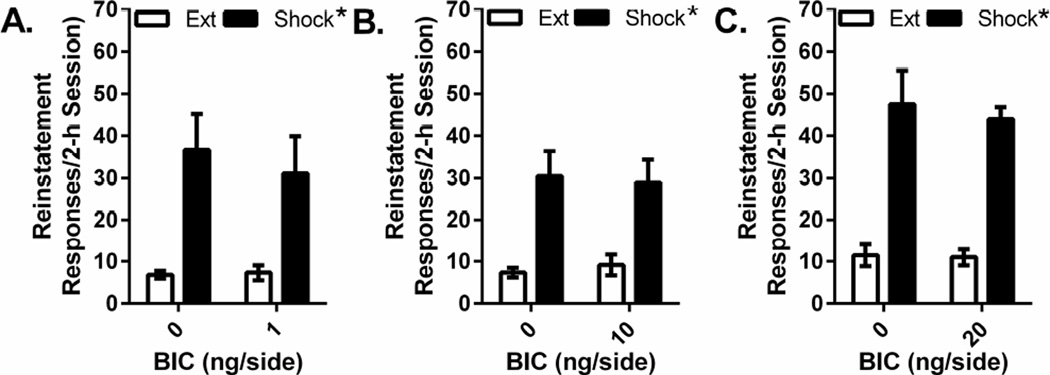
Antagonism of GABAA receptors in the VTA failed to alter stress- or intra-VTA CRF-induced cocaine seeking. The effects of intra-VTA injections of three bicuculline concentrations (1 ng/side, 10 ng/side, and 20 ng/side) on shock-induced cocaine seeking were tested and are shown in Figure 3. Consistent with other reports (e.g., Echo et al. 2002) we were unable to administer higher concentrations due to the emergence of seizures. Separate 2-way repeated measures (reinstatement × drug treatment) ANOVAs were conducted for each bicuculline dose. In all cases, shock-induced reinstatement was not altered by intra-VTA bicuculline. At each bicuculline concentration, significant shock-induced reinstatement was observed (1 ng/side: F1,5= 9.967, P<0.05, n=6; 10 ng/side: F1,4=42.631 P<0.01, n=5; 20 ng/side: F1,3=33.178 P=0.01, n=4). However significant effects of VTA bicuculline administration or significant bicuculline × reinstatement interactions were not found.
Figure 3. Effects of intra-VTA bicuculline injections on stress-induced reinstatement.
Data represent responding (lever presses/2-h session ±S.E.) recorded during reinstatement testing following electric footshock (0.5 mA, 0.5” shocks delivered an average of every 40 sec over a 15-min period) in rats pretreated with intra-VTA injections of vehicle or 1 ng/side (Figure 3A), 10 ng/side (Figure 3B), or 20 ng/side (Figure 3C) concentrations of the GABAA receptor antagonist, bicuculline (BIC), and responding during the previous extinction (Ext) session. Shock reinstated cocaine seeking regardless of whether rats were pretreated with vehicle or intra-VTA bicuculline (*P<0.05 overall shock reinstatement effect vs. Ext but no effect of BIC or shock × BIC interaction).
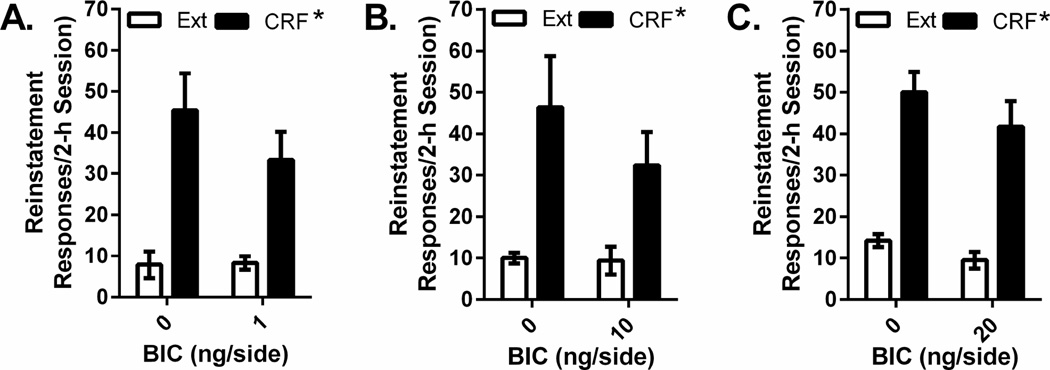
The effects of intra-VTA injections of the three bicuculline concentrations (1 ng/side, 10 ng/side, and 20 ng/side) on cocaine seeking in response to bilateral intra-VTA CRF injection (500 ng/side) are shown in Figure 4. Separate 2-way repeated measures (reinstatement × drug treatment) ANOVAs were conducted for each bicuculline concentration. As was the case with shock, CRF-induced reinstatement was not altered by intra-VTA bicuculline. At each bicuculline concentration, significant CRF-induced reinstatement was observed (1 ng/side: F1,5= 35.698, P=0.001, n=6; 10 ng/side: F1,4=15.896, P<0.05, n=5; 20 ng/side: F1,3=271.75, P<0.001, n=4). However significant effects of bicuculline administration or significant bicuculline × reinstatement interactions were not found.
Figure 4. Effects of intra-VTA bicuculline injections on CRF-induced reinstatement.
Data represent responding (lever presses/2-h session ±S.E.) recorded during reinstatement testing following bilateral intra-VTA administration of CRF (500 ng/side) in rats pretreated with intra-VTA injections of vehicle or 1 ng/side (Figure 4A), 10 ng/side (Figure 4B), or 20 ng/side (Figure 4C) concentrations of the GABAA receptor antagonist, bicuculline (BIC), and responding during the previous extinction (Ext) session. Intra-VTA CRF reinstated cocaine seeking regardless of whether rats were pretreated with vehicle or intra-VTA bicuculline (*P<0.05 overall CRF reinstatement effect vs. Ext but no effect of BIC or CRF × BIC interaction).
Effects of intra-VTA 2-hydroxysaclofen injections on shock- and intra-VTA CRF-induced reinstatement
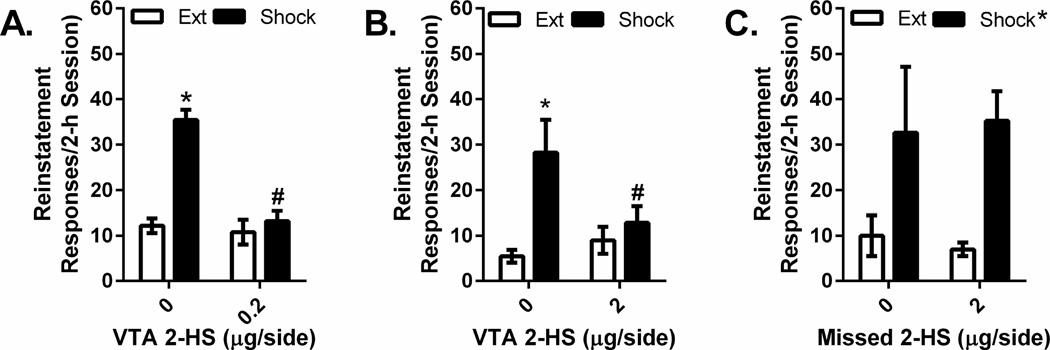
In contrast to bicuculline, bilateral injections of the GABAB receptor antagonist, 2-hydroxysaclofen, into the VTA prevented reinstatement in response to either shock delivery or intra-VTA CRF. The effects of intra-VTA injections of the two 2-hydroxysaclofen concentrations tested (0.2 µg/side and 2 µg/side) on shock-induced cocaine seeking are shown in Figure 5. Separate 2-way repeated measures (reinstatement × drug treatment) ANOVAs were conducted for each 2-hydroxysaclofen concentration. In both cases significant overall shock-induced reinstatement (0.2 µg/side: F1,4=153.84, P<0.001, n=5; 2 µg/side: F1,7=14.322, P<0.01, n=8) and significant interactions between 2-hydroxysaclofen and shock-induced reinstatement (0.2 µg/side: F1,4=91.274, P=0.001, n=5; 2 µg/side: F1,7=6.666, P<0.05) were observed. Post-hoc testing showed that there was significant shock-induced reinstatement in rats pretreated with intra-VTA vehicle (P<0.05) but not with either 2-hydroxysaclofen dose. Moreover, reinstatement following intra-VTA 2-hydroxysaclofen was significantly lower than following vehicle (0.2 µg/side, P<0.05; 2 µg/side, P=0.05).
Figure 5. Effects of intra-VTA 2-hydroxysaclofen injections on shock-induced reinstatement.
Data represent responding (lever presses/2-h session ±S.E.) recorded during reinstatement testing following electric footshock (0.5 mA, 0.5” shocks delivered an average of every 40 sec over a 15-min period) in rats pretreated with intra-VTA injections of vehicle or 0.2 µg/side (Figure 5A) or 2 µg/side (Figure 5B) of the GABAB receptor antagonist, 2-hydroxysaclofen (2-HS) and responding during the previous extinction (Ext) session. Shock reinstated cocaine seeking following vehicle (0 µg/side) pretreatment (*P<0.05 vs. Ext) but not following injections of 0.2 or 2 µg/side 2-hydroxysaclofen into the VTA. Moreover, lever pressing during the reinstatement session following shock delivery was significantly lower in rats that received intra-VTA injections of 0.2 or 2 µg/side 2-hydroxysaclofen relative to rats pretreated with vehicle (#P<0.05 vs. 0 µg/side). By contrast, in rats with misplaced cannula that received 2-hydroxysaclofen injections into brain regions adjacent to the VTA, 2-hydroxysaclofen (2 µg/side) failed to affects shock-induced reinstatement of cocaine seeking (Figure 5C).
To confirm anatomical localization of 2-hydroxysaclofen effects to the VTA, we also examined shock-reinstatement in a separate groups of rats that received 2 µg/side 2-hydroxysaclofen injections via guide cannula implanted into regions adjacent to the VTA (n=5; Figure 5c). 2-hydroxysaclofen had no effect on shock-induced reinstatement in these rats. A 2-way ANOVA showed a significant overall reinstatement effect (F1,4=109.606, P<0.001) but no effect of 2-hydroxysaclofen and no reinstatement × 2-hydroxysaclofen interaction.
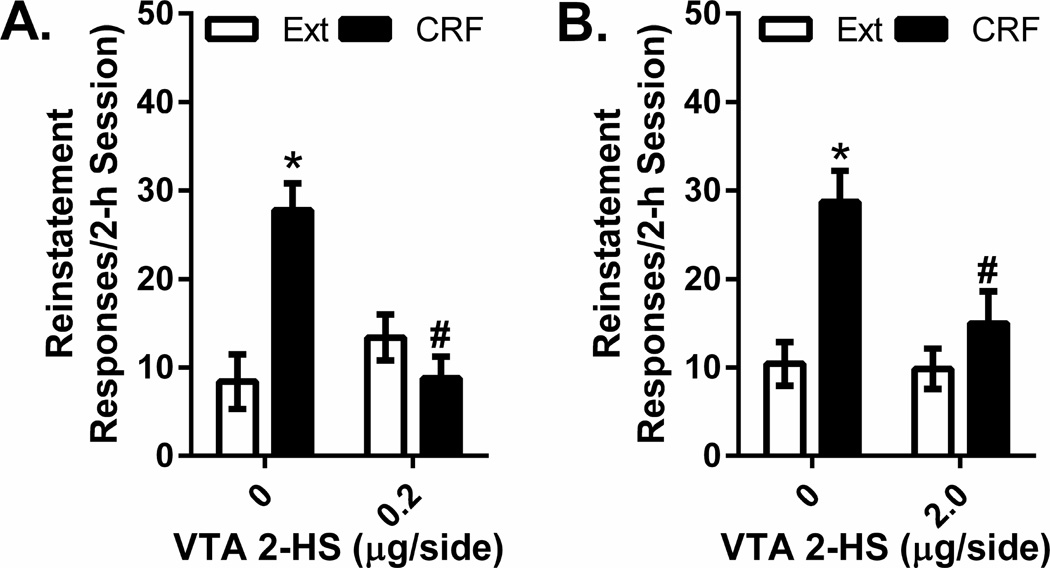
The effects of intra-VTA injections of the two 2-hydroxysaclofen concentrations (0.2 µg/side and 2 µg/side) on cocaine seeking in response to intra-VTA CRF are shown in Figure 6. As was the case with shock, significant overall intra-VTA CRF-induced reinstatement (500 ng/side: F1,4=, P<0.001, n=5; 2 µg/side: F1,7=, P<0.01, n=8) and significant interactions between 2-hydroxysaclofen and CRF-induced reinstatement (0.2 µg/side: F1,4= P=0.001, n=5; 2 µg/side: F1,7=, P<0.05) were observed. Post-hoc testing showed that there was significant CRF-induced reinstatement in rats pretreated with intra-VTA vehicle (P<0.05) but not but not with either 2-hydroxysaclofen dose. Additionally, reinstatement following intra-VTA 2-hydroxysaclofen was significantly lower than that observed following vehicle pretreatment (0.2 µg/side, P<0.05; 2 µg/side, P=0.05).
Figure 6. Effects of intra-VTA 2-hydroxysaclofen injections on CRF-induced reinstatement of extinguished cocaine seeking.
Data represent responding (lever presses/2-h session ±S.E.) following bilateral intra-VTA administration of CRF (500 ng/side) in rats pretreated with intra-VTA injections of vehicle or the 0.2 µg/side (Figure 6A) or 2 µg/side(Figure 6B) concentration of the GABAB receptor antagonist, 2-hydroxysaclofen (2-HS) and responding during the previous extinction (Ext) session. Intra-VTA CRF reinstated cocaine seeking following vehicle (0 µg/side) pretreatment (*P<0.05 vs. Ext) but not following pretreatment with 0.2 or 2 µg/side 2-hydroxysaclofen. Moreover, lever pressing during the reinstatement session following CRF administration was significantly lower in rats pretreated with 0.2 or 2 µg/side 2-hydroxysaclofen relative to rats pretreated with vehicle (#P<0.05 vs. 0 µg/side).
Effects of intra-VTA bicuculline or 2-hydroxysaclofen injections on food-reinforced responding
To determine if non-specific behavioral suppression contributed to effects on cocaine seeking, separate groups of rats were tested for effects of intra-VTA drug (10 or 20 ng/side bicuculline or 0.2 or 2 µg/side 2-hydroxysaclofen) or vehicle administration on lever-pressing reinforced by sucrose-sweetened food pellets during a 30-min session (Table 2; n=4–6/group). Two-way testing (baseline vs. test session preceded by VTA injection; within subjects) × drug (drug vs. vehicle; between subjects) ANOVAs failed to show effects of injection of any of the drugs into the VTA on food-reinforced responding (no significant effects of testing or drug or testing × drug interactions were observed.
Table 2.
Effects of intra-VTA bicuculline or 2-HS injections on food-reinforced lever pressing.
| Intra-VTA Treatment |
Vehicle | Bicuculline (10 ng/side) |
Bicuculline (20 ng/side) |
2-HS (0.2 µg/side) |
2-HS (2 µg/side) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bas | Veh | Bas | Bic | Bas | Bic | Bas | 2-HS | Bas | 2-HS | |
|
Responses/30 minutes |
304.4 (±17.5) |
307.3 (±17.9) |
330.5 (±28.7) |
340.0 (±17.9) |
327.2 (±28.7) |
340.0 (±27.6) |
307.5 (±15.1) |
303.0 (±13.7) |
293.0 (±25.44) |
286.0 (±34.89) |
Data represent the numbers of sucrose-sweetened food pellet-reinforced lever presses during the 30-minute session following bilateral intra-VTA injections of vehicle (n=6), 10 ng/side bicuculline (n=4), 20 ng/side bicuculline (n=4), 0.2 µg 2-hydroxysaclofen (n=4), or 2 µg 2-hydroxysaclofen (n=4) or during the preceding baseline session (Bas). Food-reinforced responding was not significantly altered by any of the VTA treatments.
DISCUSSION
Our results demonstrate that intra-VTA injections of the GABAB receptor antagonist, 2-hydroxysaclofen, prevent reinstatement in response to either footshock stress or intra-VTA delivery of the stressor-responsive neuropeptide, CRF. These effects were observed at 2-hydroxysaclofen doses that did not alter lever pressing reinforced by sucrose-sweetened food pellets and did not affect cocaine seeking in the absence of stress or CRF. By contrast, intra-VTA injections of the GABAA receptor antagonist, bicuculline across a range of doses, had no effects on shock- or CRF-induced reinstatement. Thus, the results suggest that 1) GABA release into the VTA and GABAB receptor activation are necessary for stress-induced cocaine seeking and 2) in the VTA, GABAB receptor activation is required for local CRF effects that underlie stress-induced cocaine seeking
The ventral tegmental area (VTA) is a key site at which inputs from a number of stress-responsive brain regions converge to regulate motivated behavior and reward and promote relapse to drug use in addicts. Understanding the mechanisms in the VTA through which stressors trigger cocaine use should facilitate the development of new and more effective treatment strategies. We and others have previously reported that CRF actions in the VTA are required for stress-induced reinstatement following cocaine self-administration and that CRF delivery into the VTA is sufficient induce cocaine seeking (Wang et al. 2005; 2007; Blacktop et al. 2011; Vranjkovic et al. 2014). These actions have been reported to involve both CRF-R1 (Blacktop et al. 2011; Chen et al. 2014) and CRF-R2 (Wang et al. 2005; 2007) receptors as well an interaction with the CRF binding protein (Wang et al 2007). As is the case with many neuropeptides, the cellular effects of CRF in the VTA appear to involve a complex coordination of cellular function through a wide array of actions at presynaptic, postsynaptic and extrasynaptic sites.
Much of the research examining CRF actions in the VTA has focused on its enhancement of excitatory neurotransmission (Wise and Morales, 2010). CRF directly excites dopamine and non-dopamine neurons in the VTA (Korotkova et al. 2005; Wanat et al. 2008) and indirectly facilitates excitatory synaptic transmission in the VTA through post-synaptic trafficking of NMDA and AMPA receptors (Ungless et al. 2003; Hahn et al. 2009) and promotion of glutamate release (Wang et al. 2005; Hahn et al. 2009). Stress-induced reinstatement following cocaine (Wang et al. 2005) or heroin SA (Wang et al. 2012) is associated with increased VTA glutamate levels, while intra-VTA delivery of kynurenic acid, an ionotropic glutamate receptor antagonist, prevents stress-induced reinstatement and corresponding increases in VTA dopamine levels (Wang et al. 2005; 2012).
Less attention has been given to the role of VTA GABAergic neurotransmission in stress-induced drug seeking. Delivery of a combination of the GABAA receptor agonist, muscimol, and the GABAB receptor agonist, baclofen, into the VTA attenuates cocaine seeking in response to a number of stimuli, including a cocaine priming injection (McFarland et al. 2001), a cocaine-paired conditioned stimulus (Di Ciano and Everitt, 2004), and footshock stress (McFarland et al. 2004). However, while these studies implicate the VTA in cocaine seeking, they provide limited mechanistic information regarding the precise role of GABA as they do not differentiate between the contributions of GABAA versus GABAB receptors and since, in contrast to antagonist-based approaches, they do not differentiate between GABAergic mechanisms that are active during stress and those that are not.
The actions of GABA in the VTA are complex. The VTA receives GABAergic inputs from a number of brain regions, including the nucleus accumbens (Yim and Mogenson, 1980; Bocklisch et al. 2013), periaqueductal gray (Omelchenko and Sesack, 2010), bed nuclei of the stria terminalis (Kudo et al. 2012), lateral septum (Luo et al. 2011), laterodorsal tegmentum (Omelchenko and Sesack, 2005), ventral pallidum (Mahler et al., 2014), and rostromedial tegmental area (Jhou et al. 2009; Matsui and Williams, 2011; Barrot et al. 2012). Moreover, VTA cell populations include GABAergic interneurons (Steffensen et al. 1998; Cruz et al. 2004) and GABAergic projection neurons (Van Bockstaele and Pickel, 1995; Carr and Sesack, 2000) that may provide collateral GABA mediated regulation within the region (Omelchenko and Sesack, 2009). Both GABAA and GABAB receptors are expressed on dopaminergic and non-dopaminergic cells in the VTA (Churchill et al. 1992; Wirtshafter and Sheppard, 2001; Okada et al. 2004; Ciccarelli et al. 2012) where they can regulate excitability, signaling and neurotransmitter release (see Creed et al. 2014 for review).
Stress-induced cocaine seeking is associated with altered GABAergic synaptic transmission in the VTA (Graziane et al. 2013). However, the mechanism through which GABAergic signaling contributes to cocaine seeking and its relationship to CRF has not been previously reported. It has been suggested that CRF-releasing terminals in the VTA, at least those that innervate dopamine neurons, are predominantly glutamatergic, based on synaptic morphology (Tagliaferro and Morales, 2008). However, as is the case with most neuropeptides, regional diffusion of CRF to sites that regulate GABAergic transmission is likely. CRF has been reported to promote inhibitory transmission in a number of other brain regions including the central amygdala (Nie et al. 2004; Bajo et al. 2008; Roberto et al. 2010), dorsal raphe nucleus (Waselus et al. 2005; Kirby et al. 2008) and bed nucleus of the stria terminalis (Kash and Winder, 2006). In the VTA, CRF promotes GABA release via presynaptic receptor actions (Williams et al. 2014) as well as excitation of intrinsic GABA interneurons (Korotkova et al. 2006) and, through a postsynaptic CRFR1/GABAB receptor interaction, promotes GABA-induced GIRK-mediated inhibitory postsynaptic currents (Beckstead et al. 2009). Thus, the blockade of stress-induced cocaine seeking by 2-hydroxysaclofen could be attributable to 1) preventing the activation of GABAB receptors by GABA released in response to CRF and/or 2) disruption of a post-synaptic interactions between CRF and GABAB receptors.
The exact mechanism through which CRF interacts with GABAB receptors in the VTA to promote cocaine seeking is unclear but likely involves one of several possibilities. First, GABAB receptor activation could inhibit VTA GABAergic interneurons, thereby disinhibiting dopamine neurons that project to terminal field regions involved in stress-induced drug seeking such as the prelimbic cortex (Capriles et al. 2003; McFarland et al. 2004) and nucleus accumbens (Shaham and Stewart, 1996; Xi et al. 2004). It has been reported that, in contrast to VTA dopamine neurons which are relatively insensitive to inhibition by the GABAB receptor agonist, baclofen, VTA GABA neurons are much more susceptible to baclofen inhibition (Bonci and Malenka, 1999; Cruz et al. 2004; but see Margolis et al. 2012), due in part to increased coupling of GABAB receptors to GIRK channels (Cruz et al. 2004). In fact, long-term potentiation at inhibitory synapses between descending D1 receptor-expressing nucleus accumbens GABAergic medium spiny neurons and VTA GABAergic interneurons, and the resulting downstream disinhibition of VTA dopamine neurons, have been shown to contribute to cocaine-induced increases in dopamine neuronal excitability (Bocklisch et al. 2013), while GABA-mediated interneuron suppression and the resulting of disinhibition of dopamine neurons have been proposed to underlie context-induced reinstatement of cocaine seeking (Luo et al. 2011). Thus, to the extent that CRF selectively regulates GABAergic synapses on local interneurons via GABAB receptors, CRF inhibition of local interneuron populations and disinhibition of dopaminergic neurons could account for its effects on cocaine seeking. While intriguing, this possibility is inconsistent with the finding that VTA GABAB receptor antagonism does not decrease but rather increases the nucleus accumbens dopamine response to stress (Doherty and Gratton, 2007) and tends to be inconsistent with our own finding that VTA GABAA receptor antagonism using bicuculline, which should reproduce any effects of 2-hydroxysaclofen-mediated inhibition of VTA GABAergic interneurons, does not reinstate cocaine seeking.
Alternatively, shock, via CRF, could promote GABA actions to decrease or alter the pattern of firing of VTA dopamine neurons, thereby promoting cocaine seeking. Indeed, Tan et al (2012) have reported that footshock excites VTA GABA interneurons while also inhibiting of a large majority of dopamine neurons, consistent with reports that, while footshock activates a subpopulation of dopamine neurons (Brischoux et al. 2009), most VTA neurons show a shock-induced reduction in firing (Ungless et al. 2004). These findings are paralleled by reports that aversive stimuli often produce time-locked decreases in nucleus accumbens dopamine release, as measured using voltammetry (Badrinarayan et al, 2012; Roitman et al, 2008; Oleson et al 2012). While the possibility that stressors can promote cocaine seeking via reductions in the activity of mesolimbic dopamine neurons is inconsistent with the assumption that stress-induced drug seeking is mediated by elevated dopamine in the nucleus accumbens (Shaham and Stewart, 1996; Xi et al. 2004; but see McFarland et al. 2004), we have recently reported that an aversive stimulus (intra-oral quinine delivery) that reduces dopamine levels in nucleus accumbens shell can reinstate cocaine seeking following self-administration in rats (Twining et al. 2015). Moreover, both quinine-induced cocaine seeking and quinine-induced reductions in nucleus accumbens dopamine levels are dependent on VTA CRF receptor activation (Twining et al. 2015).
Another possibility is that the effects 2-hydroxysaclofen on cocaine seeking are attributable to antagonism of presynaptic GABAB receptors on afferents into the VTA. It is well established that presynaptic GABAB receptors are important regulators of vesicular neurotransmitter release (see e.g., Takahashi et al., 1998; Sakaba and Neher, 2003; Padgett and Slesinger, 2010) and, in the VTA, GABAB receptors have been reported to regulate both GABA (Chen et al., 2015) and glutamate (Padgett et al., 2012) release. Cocaine seeking and dopamine neurons in the VTA are negatively regulated by GABAergic efferents from several brain regions, including the ventral pallidum (Mahler et al., 2014) and the rostromedial tegmental area (Huff and LaLumiere, 2015). Thus, it is possible that GABAB antagonism disinhibits these afferents, thereby promoting GABA release and GABAA receptor-mediated inhibition of dopaminergic neurons and cocaine seeking. As would be the case with interneuron inhibition, our finding that intra-VTA bicuculline does not increase cocaine seeking would suggest that, to the extent that this mechanism applies, GABAergic inputs are not providing baseline tonic inhibition of dopamine neurons in the VTA‥
Alternatively, CRF-induced GABA release may exert heterosynaptic effects via GABAB receptors to regulate glutamatergic transmission (Manzoni and Williams, 1999). Riegel and colleagues (Williams et al. 2014) have reported that CRF, via CRF-R2 receptor-mediated effects on GABA release, can promote GABAB receptor activation on glutamatergic terminals, thereby attenuating glutamate release and offsetting CRF-R1 receptor mediated increases in excitatory regulation of VTA dopamine neurons. It has been hypothesized that following cocaine this CRF-R2 receptor-driven inhibitory effect of GABA on excitatory transmission diminishes, resulting in a shift from inhibitory to excitatory regulation of dopamine neurons by CRF. While this represents an interesting mechanism through which CRF can regulate VTA dopamine neurons, according to this model, CRF effects on glutamatergic transmission in the VTA are no longer GABAB receptor-dependent after cocaine self-administration. Moreover, it is not clear how GABAB receptor attenuation of excitatory neurotransmission would result in reinstatement. Notably, an important distinction between that study and ours is the use of yohimbine in combination with non-extinguished response-contingent cocaine-associated cues as reinstating stimuli which may involve unique neurocircuitry and/or activate VTA inputs in addition to those that are stimulated by stress alone. Nonetheless, this proposed mechanism does not seem to account for our current findings.
When considering the possible contribution of VTA GABA to cocaine seeking it is also important to recognize that the regulation of neuronal firing patterns in the VTA by GABA is complex and does not necessarily result exclusively in inhibition of efferent projections. Transient GABA-mediated inhibition may coordinate or synchronize regional network activity and/or prime neurons for excitation and burst firing. Indeed, it has been reported that GABAergic transmission in the VTA is critical for nicotine-induced excitation of dopamine neurons and associated behavioral responses (Tolu et al., 2013).
Neither administration of bicuculline nor 2-hydroxysaclofen into the VTA alone was sufficient to induce cocaine seeking. Although reinstatement by intra-VTA bicuculline or 2-hydroxysaclofen has not, to our knowledge, been previously reported, these results were somewhat surprising to us, particularly in the case of bicuculline, which has been found to elevate nucleus accumbens dopamine levels (Ikemoto et al. 1997), increase locomotor activity (Grubb et al. 2002; Lavezzi et al. 2015), and produce conditioned place preference (Laviolette and van der Kooy, 2001) when injected into the VTA and has been reported to be self-administered directly into the VTA in mice (David et al. 1997). As elevated dopamine in the nucleus accumbens (Cornish and Kalivas, 2000) and intra-accumbens delivery of dopamine receptor agonists (Bachtell et al. 2005; Schmidt et al. 2006) have been reported to be sufficient for reinstatement of cocaine seeking, we predicted that intra-VTA GABA receptor antagonism would induce cocaine seeking, especially considering that GABA receptor agonist injections into the VTA suppress dopamine release (Yoshida et al. 1994), drug self-administration (Xi and Stein, 1999; Brebner et al. 2000; Backes and Hemby, 2008), and reinstatement (McFarland et al. 2001; Di Ciano and Everitt, 2004; McFarland et al. 2004). When considering the failure of GABA antagonists to induced cocaine seeking, the complexity of GABAergic regulation of the VTA and the understanding that distinct mechanisms contribute to reinforcement/reward vs. motivation/seeking should be taken into account. While it is possible that the antagonist doses used were insufficient to effectively target GABA receptors, we consider this unlikely, as the same 2-hydroxysaclofen doses attenuated reinstatement and we were unable to test higher intra-VTA bicuculline doses due to the emergence of seizure activity, as has been previously described (Echo et al. 2002).
To confirm that any GABA receptor antagonist effects on reinstatement were not attributable to non-specific behavioral disruption, separate groups of rats were tested for effects of intra-VTA antagonist injections on food-reinforced lever pressing. Neither bicuculline nor 2-hydroxysaclofen altered food-reinforced responding, suggesting that motor impairment likely did not contribute to 2-hydroxysaclofen-induced reductions in shock- and CRF-induced reinstatement. Considering that optogenetic stimulation of VTA GABA neurons impairs natural reward consumption (van Zessen et al. 2012), it is somewhat surprising that effects on food- reinforced responding were not observed. However, consistent with our findings, others have reported that GABAA and GABAB receptor antagonism in the VTA has minimal effects on feeding behavior (Echo et al. 2002; Ackerman et al. 2003; Miner et al. 2010). When interpreting these findings, it is important to note that the rates of responding during the food-reinforced sessions (approximately 30 responses/minute) were much higher than those during the reinstatement sessions. For this reason, less-pronounced disruption of motor performance that could have contributed to the observed effects on cocaine seeking or increases in responding attributable to enhancement of food reward may not have been detectable under these conditions.
Conclusion
To summarize, our results suggest that stress-induced reinstatement of extinguished cocaine seeking requires VTA GABAB receptor activation, likely via a CRF-regulated mechanism. While the precise process through which CRF interacts with GABAB receptors remains to be determined, these findings provide novel insight into mechanisms that contribute to stress-induced relapse and therefore may guide the development of new therapeutic approaches aimed at relapse prevention.
HIGHLIGHTS.
Administration of the GABA-B receptor antagonist, 2-hydroxysaclofen, into the ventral tegmental area (VTA) prevented reinstatement of cocaine seeking in response to electric footshock or intra-VTA corticotropin releasing factor (CRF) delivery following self-administration in rats.
The same intra-VTA doses of 2-hydroxysaclofen had no effect on food-reinforced lever pressing or cocaine seeking in the absence of shock or CRF administration
By contrast, intra-VTA administration of the GABA-A receptor antagonist, bicuculline, did not affect shock or intra-VTA CRF-induced reinstatement of cocaine seeking.
ACKNOWLEDGEMENTS
This work was supported by National Institute on Drug Abuse (NIDA) grant number DA15758 to John R. Mantsch. The authors acknowledge Chris Mueller for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ackerman TF, Lamonte N, Bodnar RJ. Lack of intersite GABA receptor subtype antagonist effects upon mu opioid receptor agonist-induced feeding elicited from either the ventral tegmental area or nucleus accumbens shell in rats. Physiol. Behav. 2003;79:191–198. doi: 10.1016/s0031-9384(03)00087-8. [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J. Neurosci. 2012;32:15779–15790. doi: 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacol. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Backes EN, Hemby SE. Contribution of ventral tegmental GABA receptors to cocaine self-administration in rats. Neurochem. Res. 2008;33:459–467. doi: 10.1007/s11064-007-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc. Natl. Acad. Sci. USA. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J. Neurosci. 2012;32:14094–14101. doi: 10.1523/JNEUROSCI.3370-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacol. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M, Tan KR, Lüscher C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341:1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Phelan R, Roberts DC. Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol. Biochem. Behav. 2000;66:857–862. doi: 10.1016/s0091-3057(00)00286-0. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Chen NA, Jupp B, Sztainberg Y, Lebow M, Brown RM, Kim JH, Chen A, Lawrence AJ. Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice. J. Neurosci. 2014;34:11560–11570. doi: 10.1523/JNEUROSCI.4763-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Zhao Y, Yang H, Luan W, Song J, Cui D, Dong Y, Lai B, Ma L, Zheng P. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. Elife. 2015;4 doi: 10.7554/eLife.09275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acidA receptors within the ventral tegmental area. Neurochem. Res. 1992;17:101–106. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- Ciccarelli A, Calza A, Panzanelli P, Concas A, Giustetto M, Sassoè-Pognetto M. Organization of GABAergic synaptic circuits in the rat ventral tegmental area. PLoS One. 2012;7:e46250. doi: 10.1371/journal.pone.0046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J. Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 2014;8:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bidirectional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Self-administration of the GABAA antagonist bicuculline into the ventral tegmental area in mice: dependence on D2 dopaminergic mechanisms. Psychopharmacol. 1997;130:85–90. doi: 10.1007/s002130050214. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur. J. Neurosci. 2004;19:1661–1667. doi: 10.1111/j.1460-9568.2004.03232.x. [DOI] [PubMed] [Google Scholar]
- Doherty M, Gratton A. Differential involvement of ventral tegmental GABA(A) and GABA(B) receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Res. 2007;1150:62–68. doi: 10.1016/j.brainres.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Echo JA, Lamonte N, Ackerman TF, Bodnar RJ. Alterations in food intake elicited by GABA and opioid agonists and antagonists administered into the ventral tegmental area region of rats. Physiol. Behav. 2002;76:107–116. doi: 10.1016/s0031-9384(02)00690-x. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinol. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron. 2013;77:942–954. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MC, Welch JR, Finn DA, Mark GP. Cocaine self-administration alters the locomotor response to microinjection of bicuculline into the ventral tegmental area of rats. Brain Res. 2002;952:44–51. doi: 10.1016/s0006-8993(02)03192-x. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J. Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, LaLumiere RT. The rostromedial tegmental nucleus modulates behavioral inhibition following cocaine self-administration in rats. Neuropsychopharmacology. 2015;40:861–873. doi: 10.1038/npp.2014.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J. Neurochem. 1997;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacol. 2006;51:1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J. Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J. Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzi HN, Parsley KP, Zahm DA. Modulation of locomotor activation by the rostromedial tegmental nucleus. Neuropsychopharmacol. 2015;40:676–687. doi: 10.1038/npp.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signaling between dopaminergic and non-dopaminergic neural motivational systems. Eur. J. Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor-and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacol. 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacol. 2015 doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J. Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-Sensitive GABA Inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J. Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner P, Borkuhova Y, Shimonova L, Khaimov A, Bodnar RJ. GABA-A and GABA-B receptors mediate feeding elicited by the GABA-B agonist baclofen in the ventral tegmental area and nucleus accumbens shell in rats: reciprocal and regional interactions. Brain Res. 2010;1355:86–96. doi: 10.1016/j.brainres.2010.07.109. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–154. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur. J. Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Matsushita N, Kobayashi K, Kobayashi K. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J. Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J. Neurosci. 2012;32:14804–14808. doi: 10.1523/JNEUROSCI.3087-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J. Comp. Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse. 2009;63:895–906. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J. Neurosci. Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martínez-Hernández J, Watanabe M, Moss SJ, Luján R, Lüscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73:978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat. Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABAB receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Sandner G, Bielajew C, Fouriezos G. Bicuculline microinjections into the ventral tegmental area of the rat: alteration of self-stimulation thresholds and of cytochrome oxidase activity in the brain. Behav Brain Res. 1996;79:145–151. doi: 10.1016/0166-4328(96)00009-5. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Pierce RC. Stimulation of D1-like or D2 dopamine receptors in the shell, but not the core, of the nucleus accumbens reinstates cocaine-seeking behaviour in the rat. Eur. J. Neurosci. 2006;23:219–228. doi: 10.1111/j.1460-9568.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacol. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacol. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J. Comp. Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolu S, Eddine R, Marti F, David V, Graupner M, Pons S, Baudonnat M, Husson M, Besson M, Reperant C, Zemdegs J, Pagès C, Hay YA, Lambolez B, Caboche J, Gutkin B, Gardier AM, Changeux JP, Faure P, Maskos U. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry. 2013;18:382–393. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- Trojniar W, Klejbor I. Facilitatory effect of unilateral lesion of the ventral tegmental area on locomotor response to stimulation of the contralateral ventral tegmental area: involvement of GABAergic transmission. Brain Res. 1999;842:419–430. doi: 10.1016/s0006-8993(99)01865-x. [DOI] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, Wheeler RA. Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol. Psychiatry. 2015;77:895–902. doi: 10.1016/j.biopsych.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 2014;34:12504–12514. doi: 10.1523/JNEUROSCI.0680-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A, Phillips PE. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacol. 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Heroin self-administration experience establishes control of ventral tegmental glutamate release by stress and environmental stimuli. Neuropsychopharmacol. 2012;37:2863–2869. doi: 10.1038/npp.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J. Comp. Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Sheppard AC. Localization of GABA(B) receptors in midbrain monoamine containing neurons in the rat. Brain Res. Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Williams CL, Buchta WC, Riegel AC. CRF-R2 and the heterosynaptic regulation of VTA glutamate during reinstatement of cocaine seeking. J. Neurosci. 2014;34:10402–10414. doi: 10.1523/JNEUROSCI.0911-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J. Pharmacol. Exp. Ther. 1999;290:1369–1374. [PubMed] [Google Scholar]
- Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacol. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus accumbens medium spiny neurons target non-dopaminergic neurons in the ventral tegmental area. J. Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Effect of picrotoxin and nipecotic acid on inhibitory response of dopaminergic neurons in the ventral tegmental area to stimulation of the nucleus accumbens. Brain Res. 1980;199:466–473. doi: 10.1016/0006-8993(80)90705-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Emoto H, Tanaka M. Opposite changes in the mesolimbic dopamine metabolism in the nerve terminal and cell body sites induced by locally infused baclofen in the rat. Brain Res. 1994;636:111–114. doi: 10.1016/0006-8993(94)90183-x. [DOI] [PubMed] [Google Scholar]